CHAPTER 387 Endovascular Management of Arteriovenous Malformations for Cure
Early results with endovascular embolization of AVMs were disappointing. Selective access to most cerebral AVMs was not possible with the equipment available. Untargeted embolization was attempted with the use of Silastic beads or other objects.1 High flow, it was hoped, would carry most of the embolic material to the AVM vessels.2 Off-target occlusion occurred frequently, and procedural complication rates were significant. Since then, increased understanding of AVM morphology and significant refinements in endovascular technique have improved the risk-to-benefit ratio. Endovascular therapy has now become an important component in the therapeutic management of many brain AVMs. Currently, embolization is used in a variety of manners, including (1) as adjuvant therapy before definitive microsurgery or radiosurgery, (2) as palliative therapy for inoperable or otherwise incurable AVMs, and (3) as curative therapy (i.e., without surgical resection or radiotherapy). It is the curative use of embolization that we now consider.
However, before discussing curative embolization, it should be noted that no decision for treatment of any lesion as complex and heterogeneous as a brain AVM should be made in isolation. Rather, a multidisciplinary team made up of physicians with expertise in not only endovascular embolization but also microsurgical resection and radiosurgery should consider all available treatment options carefully. When decisions are made in this manner, definitive cure of brain AVMs by endovascular embolization as the sole treatment modality, however, is found to be desirable in only a select minority of cases. The reason is that the angioarchitecture of the AVM must permit solid casting of the AVM nidus with permanent embolic material in such a way that the draining vein is occluded only after the nidus is completely occluded. Failure to completely occlude the nidus first can lead to disastrous bleeding complications. This fact, combined with the frequently availability of less risky treatment alternatives, usually results in another treatment modality being chosen even when the anatomy is permissive.3–7
Interventional Planning
Architecture of the Arteriovenous Malformation
Patient characteristics must be used in conjunction with AVM architecture to formulate a treatment plan. Along with a thorough clinical examination, all AVM patients require detailed preoperative radiographic clarification of AVM anatomy and architecture and associated aneurysms. Magnetic resonance imaging is beneficial in elucidating the overall structure of the AVM and is the best modality to determine its anatomic location. The size of the nidus, proximity to eloquent parenchyma, and frequently deep venous drainage can also be determined to calculate the Spetzler-Martin grade. In addition, it can provide information about the best approach for endovascular management and the history of hemorrhage. Superselective digital subtraction angiography is then necessary to fully disclose the angioarchitecture of the AVM. A number of radiologic variables have been associated with an increased risk for hemorrhage, including small AVM size, elevated feeding artery pressure, periventricular or intraventricular location, basal ganglia location, deep venous drainage, impaired venous drainage, single draining vein, intranidal aneurysm, multiple aneurysms, and vertebrobasilar blood supply.8–16 These parameters also influence the possible success of endovascular therapy because they determine the accessibility of the lesion with the microcatheter system, the number and type of feeding arteries, and the size of the AVM nidus and its hemodynamic properties.
Feeding Vessel Morphology
The vascular supply to an AVM can be derived from pial and dural arteries. In some cases, this dual vascular supply can lead to confusion of brain AVMs for dural-based arteriovenous fistulas. Selective arteriography of intracranial and extracranial vessels can help distinguish between these dural- and pial-based shunts. Dural arteries contribute to AVMs in approximately 30% of cases.17,18 Perforating arteries and choroidal feeders can supply deep-seated brain AVMs and lesions in the vicinity of the ventricular system. Care must be taken during endovascular occlusion of choroidal arteries, which frequently also supply important neural structures. Data on endovascular occlusion as the sole modality for the treatment of AVMs are limited. In one study of 307 patients undergoing 513 embolization sessions for curative, adjuvant, and palliative embolization of brain AVMs, accessibility was possible in only 415 embolization sessions (80%).19 Additionally, 10 of 62 complications (16%) occurred during microcatheter exploration before embolization and led to 1 death and 1 permanent neurological deficit. Similarly, in a study of patients undergoing embolization as the primary treatment modality for intraventricular or paraventricular lesions, 12 of 14 patients (86%) had lesions accessible to embolization.20 In a series of 387 patients, Valavanis and Yasargil reported complete obliteration of 60% of sulcal AVMS but just 12.5% of gyral AVMs with endovascular therapy.21 The number of feeding arteries is a crucial determinant of the success of both delineation of the AVM and treatment. In one study in which the goal was primary curative embolization, the predefined criteria adopted for attempted curative embolization were based on radiologic interpretation of the technical feasibility and likelihood of complete embolization. AVMs with an increased likelihood of curative embolization had (1) a nidus that was accessible with the tip of the catheter, (2) three or fewer arterial feeders, (3) and a nidus that was not larger than 3 cm.22 In patients with angioarchitecture that makes vascular isolation of the AVM from surrounding brain particularly difficult, the risk-to-benefit ratio must be reassessed with regard to the expected therapeutic benefits of embolization. Although preoperative endovascular occlusion of AVMs is desirable in many cases, microsurgery or radiosurgery alone has a superior risk-to-benefit ratio.
Size
In 1971, Doppman and coworkers introduced the term nidus, which refers to the area between the distal segment of the feeding arteries and the proximal segment of the draining veins where the arteriovenous shunt occurs.23 According to Yasargil, the AVM nidus can be categorized into seven different types primarily based on size: (1) the nidus may be occult (not seen angiographically and not located at surgery); (2) the nidus may cryptic (not visible with angiography or at surgery but recognized histologically); (3) the nidus may be microscopic (visible as a site of arteriovenous shunting on angiography but not apparent on direct inspection of the brain); and (4) the nidus may small (1 to 2 cm), (5) moderate (2 to 4 cm), (6) large (4 to 6 cm), or (7) giant (>6 cm).24 Improvements in high-resolution angiography25,26 have led to increased delineation of AVM angioarchitecture and most likely an increase in the number of AVMs in the microscopic category diagnosed.
The size of an AVM nidus influences the difficulty of surgical resection and radiosurgical obliteration and has been included in most classifications of AVMs.6,27 Size is also a critical determinant in planning primary curative embolization. Smaller brain AVMs are often more amenable to complete endovascular occlusion without increasing the rate of procedure-related complications. Studies have found that primary curative embolization was predominantly possible with brain AVMs less than 3 cm in diameter or smaller than 4 cm3.19–22,28–33 Future developments in embolic material and delivery catheters may change these assertions over time.
Hemodynamic Properties
In addition to the angioarchitecture of the nidus and feeders, the hemodynamic properties of an individual AVM nidus are crucial for successful endovascular embolization. The number, morphology, and velocity of the arteriovenous shunt determine whether embolic material can be deposited selectively and safely in the nidus. The nidus of a brain AVM can be compact or diffuse, although the diffuse angiographic appearance of a perinidal hypervascularity is often related to high-flow arterial angiopathy rather than diffuse segments of the AVM nidus. An AVM nidus can have multiple compartments and can be plexiform, fistulous, or mixed. In a plexiform nidus, selective angiography discloses a multitude of intranidal arteriovenous shunts. The diameter of these vessels is usually small, and the velocity of the shunt is generally low. In contrast, fistulous lesions lack the plexiform area of the nidus but typically involve a single-hole, high-flow arteriovenous fistula shunting directly into draining veins. This pattern of rapid arteriovenous shunting can be found in conjunction with a plexiform nidus in larger, mixed lesions and has a bearing on the method of treatment. The angioarchitecture of the AVM nidus and the velocity of the arteriovenous shunt are crucial determinants of whether lesions can be embolized totally and permanently. Haw and coauthors reported that a pure fistula or a nidus with a fistulous component was a significant predictor of complications after endovascular therapy.19 This stands in contrast to Valavanis and Christoforidis, who reported a dominant fistulous component in the nidus as being predictive of endovascular cure.34 In mixed fistulous and plexiform lesions, an unrecognized fistulous component can result in embolization and occlusion of the draining vein with catastrophic hemorrhagic complications.19 When a single pathologic fistula is present, the lesion may be best suited for endovascular therapy with a high cure rate,33 but these lesions result in a number of technical difficulties. According to Haw and colleagues, a wedged microcatheter position may not be possible, which decreases the accuracy of glue placement and may require increased glue concentrations and injection pressures. Rapid blood flow may increase the risk of placement of glue into the draining vein or reflux into normal arteries. Finally, these fistulas often require stiffer and larger diameter over-the-wire microcatheters, which increases the risk for arterial injury. An adequate distance between the microcatheter tip and the arteriovenous fistula is critical for safe embolization of these high-flow lesions.19
Technical Aspects
Catheter Selection
Early attempts at catheterization and occlusion of blood vessels were made in aneurysm patients via an open surgical approach, which was associated with many of the same risks and morbidity as open neurosurgical procedures.1 The first microcatheters that allowed access to AVM feeders were flow directed. Mounted calibrated leak balloons helped control flow and allowed the release of contrast material and cyanoacrylate embolic material, but these balloons were easy to overinflate and had a high rate of vessel perforation.35
Subsequently, a torqueable guidewire system that allowed passage of a catheter was developed (Advanced Cardiovascular System, Santa Clara, CA). Modifications of this new generation of microcatheters, including softer distal segments, improved their versatility.36,37 A thick-walled proximal catheter segment controls torque and transmits longitudinal movements, the intermediate catheter segment is flexible but still transmits torque, and the distal segment is soft and thin walled. There are now many vendors of microcatheters designed for intracranial navigation. However, the greater tortuosity and fragility of cerebral arteries than extracranial systemic arteries requires specialized training for safe navigation.
Flow Control
When a microcatheter has been positioned in the feeding artery or nidus of an AVM, an individual compartment can be cast if surrounding blood flow can be controlled. As discussed, high-velocity arteriovenous shunting, unsuitable nidal architecture (i.e., fistulous single-hole compartments), or both can increase the risk for off-target embolization. If the angiogram shows congestion of the nidus (i.e., slow or stationary dye) after injection of embolic material, downstream (i.e., venous outflow) occlusion may have occurred. High-grade stenosis of draining veins is a risk factor for AVM rupture.21 Small amounts of glue passing through the shunt can occlude the stenotic portion of the draining vein. In multicompartmental AVMs with multiple feeders and one draining vein, casting of one compartment, including the venous outlet, can obstruct the draining system for the other compartments. Because there is no significant stromal support for pial arteries, particularly in and around the AVM nidus, hemorrhage is likely to occur. For this reason, venous occlusion must be avoided.
To overcome these difficulties, several techniques were historically proposed. First, some microcatheter designs permitted control of flow in the feeder during embolization (calibrated leak balloon catheter). Second, soft platinum wires (available in straight and coiled forms) were designed to be injected through the microcatheter in high-flow fistulas before glue embolization to slow the torrential blood flow.38,39 These liquid coils (Boston Scientific/Target Therapeutics, Fremont, CA) remain in the fistula site and prevent a large amount of glue from migrating through the fistula. Finally, embolization can be performed with the patient under induced systemic arterial hypotension. Although liquid coils are no longer manufactured and systemic hypotension is rarely if ever used, these techniques remain historically significant.
Embolic Agents
Cyanoacrylates
Cyanoacrylate polymers are the most widely used of the liquid agents. Zanetti and Sherman first introduced polymerizing cyanoacrylates as liquid embolic agents for endovascular embolization.40 Because of their low viscosity, cyanoacrylates can be delivered through small, flexible, flow-directed catheters that can easily be manipulated to allow penetration deep into the AVM nidus. They can also be delivered quickly with infusion in generally less than 1 minute. Cyanoacrylates have high resistance to in vivo biodegradation.41,42 An intensive inflammatory response helps maintain vessel occlusion and stimulates fibrosis. Although recanalization can occur, it is rare after complete embolization. The risk for recanalization is increased if there is proximal occlusion with minimal or no penetration of the nidus.31 In these cases in which the nidus remains active, collateral changes commonly develop and revascularize the nidus; such changes may be unfavorable for future endovascular occlusion and present additional challenges during surgical resection of the AVM.
The first cyanoacrylate used was isobutyl-2-cyanoacrylate, but it was discontinued after studies demonstrated that the agent possessed some carcinogenic potential in animals.32 Histoacryl-blue (N-butyl-2-cyanoacrylate [NBCA]) is now the embolization material of choice. NBCA is mixed with low-viscosity oily contrast media (e.g., Ethiodol ultrafluid), with or without additional tantalum or tungsten powder for radiologic visualization. NBCA polymerizes immediately on contact with free hydrogen ion, but the catheter is rinsed with dextrose to prevent premature initiation of such polymerization. The main disadvantage of NBCA relates to its adhesive properties and the risk of adherence of the catheter to the adjacent blood vessel wall, which can cause injury to the vessel or inhibit removal. Modifications of the cyanoacrylate formula endeavor to reduce its adhesiveness and increase its cohesiveness for AVM embolization.
EVOH Copolymer-DMSO Solvent
In the late 1980s, ethylene vinyl alcohol polymer dissolved in dimethyl sulfoxide (EVOH-DMSO) was first used for the embolization of AVMs.43 This liquid precipitate is similar to NBCA and is currently available in the United States as Onyx (ev3, Plymouth, MN). This agent is viscous and precipitates in the biologic environment as the DMSO solvent diffuses into the lipid-rich environment. Although Onyx can be used to fill AVM vessels, it does not specifically adhere to the delivery catheter. This property permits more time for operator control over the volume and rate of delivery, yet ongoing fluoroscopic observation and periodic control angiography to assess the status of obliteration of the AVM are still necessary.
Preliminary studies found that DMSO induced vasospasm and angionecrosis.44,45 Further studies found that decreasing the volume of DMSO and its rate of introduction could reduce these effects.46 In a study of 23 AVM patients, Jahan and coauthors reported one complication related to distal vasospasm that developed during injection and histopathologic evidence of angionecrosis in two AVM specimens resected 24 hours after embolization.47 A recent study reported angionecrosis in 42.9% of patients, but subsequent studies have not commented on this effect.48–52 Although long-term data are lacking, Murayama and associates demonstrated no recanalization in swine after 6 months of follow-up,46 and Jahan and colleagues reported no recanalization in a limited number of patients imaged up to 20 months after embolization.47 In a series of 47 patients, Weber and coworkers reported an initial 84% nidal reduction, with recanalization seen in 11% on angiography at 3 months.49 A study of patients undergoing microsurgical resection after Onyx embolization reported recanalization of embolized vessels in 14.3% on pathologic inspection,53 but angiographic or pathologic recanalization rates have not been reported in other recent studies.48,50–52 The use of Onyx is not without complications. Authors have reported cerebral artery perforation because relatively stiff-walled microcatheters must be used that can resist the high pressure needed to inject Onyx.49 Catheters tend to become lodged in the Onyx cast as the polymer precipitates around the shaft, even though the precipitate is nonadhesive.49 Embolization with Onyx requires increased fluoroscopy and procedure times in comparison to NBCA, and further investigation is needed to justify the increased radiation exposure and procedure time associated with Onyx.54 Because Onyx remains relatively new at the time of this writing, its role in the treatment of cerebral AVMs remains to be defined.
Polyvinyl Alcohol
Polyvinyl alcohol (PVA) is the most commonly used particulate agent historically for the embolization of AVMs. PVA particles (50 to 1000 µm) can be used in various size ranges at the discretion of the operator. A microcatheter with a diameter large enough to accommodate the particle size selected is necessary to prevent occlusion of the microcatheter. The use of larger catheters decreases flexibility in the tortuous cerebral vasculature and makes superselective angiography of small or distal vessels more difficult.55
Recanalization is also more likely to occur with particulates than with cyanoacrylates,56–58 and therefore embolization with particulates is indicated only for preoperative embolization when surgical resection will follow closely. In a randomized clinical trial comparing embolization with NBCA versus PVA, the trial investigators found no difference in the degree of nidal reduction, number of vessels embolized, surgical resection times, transfusions, fluid replacement, or patient outcomes. There was a significant increase in the incidence of postsurgical hematomas in the PVA group.55 This study led to approval of NBCA by the Food and Drug Administration for preoperative occlusion of brain AVMs.
Alternative Embolysates
A number of other agents have been used for the embolization of AVMs, including Silastic pellets, blood clots, dura, silk sutures, and Gelfoam.59–62 Undiluted absolute ethyl alcohol (98% dehydrated alcohol injection—U.S. Pharmacopeia) has also been used for the embolization of AVMs. Of the 17 patient treated by embolization, 7 patients were cured by embolization alone, 3 patients after microsurgery, and 1 additional patient after radiosurgery. Despite these promising results, 2 patients with partially treated lesions died and 8 patients experienced complications related to therapy. Absolute ethanol also results in significant brain edema and can precipitate vasospasm. These significant side effects have not led to the widespread use of ethanol for embolization of brain AVMs.
Risk Assessment
Complications
In the literature there has been a wide range of reported morbidity (0% to 50%) and mortality (1% to 4%) after embolization of AVMs.7,28–31,47,63–85 Rates are dependent on many factors: patient selection, embolic agents, means of delivering the occlusive agent, preexisting neurological morbidity, the goals of embolization (i.e., primary curative or adjuvant therapy), and the time of outcome assessment.* Typically, neurological deficits occur in 10% to 14% of patients, disabling deficits in 2% to 5% of patients, and death in approximately 1%.19,20,22,29,55,56,65,66,87–93 Recent studies using EVOH-DMSO have reported similar results.47,49–53 When embolization is used as primary treatment, adjuvant therapy, or palliative therapy, the following factors are predictive of postembolization deficits: number of embolization procedures, number of arterial feeders embolized, eloquent location, location in the basal ganglia, deep venous drainage, size smaller that 3 cm, size larger than 6 cm, Spetzler-Martin grade III to V, venous penetration of the glue cast, and AVMs with a pure fistula or a nidus with a fistulous component.19,88–90,94 We have found that that a significant number of patients experiencing deficits from embolization of brain AVMs will exhibit improvement over time.94
The most common and serious complication of embolization is intraprocedural or delayed hemorrhage. Hemorrhagic complications associated with embolization occur in 1% to 2% of procedures (3% to 15% of patients).73,92,94,95 Hemorrhage during or after embolization may be due to perforation of the vessel during catheterization, rupture of the AVM, rupture of an intranidal aneurysm, or hemodynamic changes caused by alterations in feeder pressure, normal perfusion pressure breakthrough, or obstruction of venous outflow.92,96–99 We have found that intraembolization or postembolization hemorrhage is more likely with AVMs smaller than 3 cm in diameter or larger than 6 cm in diameter.94 Small AVMs may be technically more difficult to embolize than medium-sized AVMs. The increased propensity of small, untreated AVMs to bleed has been demonstrated in many studies and may be due to increased pressure within small AVMs.14–16 Deficits and hemorrhage are more likely to occur after the embolization of larger AVMs because it is more difficult to achieve complete embolization. Picard and coauthors reported that a volume of glue injected greater than 1 mL, venous embolization, and postembolization venous stagnation in or around the nidus were predictive of hemorrhage after embolization.95
Complications rates when embolization is used as a primary modality have been similar in patients achieving complete obliteration.19–22 These AVMs are more likely to be accessible and small and have fewer feeding vessels. Although accessibility and decreased number of feeding vessels are associated with decreased complications, we have found small (<3 cm) versus medium (3-6 cm) size to be predictive of neurological deficits, intraprocedural hemorrhage, and infarction after embolization used as adjuvant therapy to microsurgery or radiosurgery.94
Outcomes
In most published series, the number of totally and permanently embolized AVMs is small. In overall cohorts of patients treated by embolization, success rates of curative embolization are mainly in the range of 5% to 20% but vary greatly from 0% to 70%.*
In an analysis of 1246 cases from 1960 to 1995, the overall cure rate was 5%. There was no significant difference between cure rates of AVMs published before 1990 (n = 708, 4%) and those published after 1990 (n = 538, 5%). In a study by Fournier and associates, 7 (14%) of the 49 patients exhibited morphologic cure by embolization alone as shown on 2-year follow-up angiograms.31 In a series of 101 patients, Vinuela and coauthors reported a 9.7% cure rate with embolization alone. Cures were achieved in patients with small AVMs and few feeding pedicles.29 In a series of patients in whom curative embolization was primarily used for high-grade AVMs, Deruty and colleagues achieved a cure rate of just 5%.63 In a series of 125 patients undergoing embolization before radiosurgery, Gobin and collaborators reported a cure rate of 11.2%. Increasing feeding pedicles and AVM volume were also inversely related to complete obliteration.30 In a selective referral group of 150 patients, Wikholm and coauthors reported a cure rate of 13.3%. Again, size was the critical determinant of occlusion; 71% of the AVMs smaller than 4 cm3 were obliterated versus 15% of AVMs 4 to 8 cm3.84 Willinsky and coworkers reported the results of embolization of small brain AVMs. Although only a minority of these patients had deep-seated brain AVMs, the cure rate with embolization alone was 27%.33
Curative embolization rates have been similar in patients treated with Onyx and NBCA and range between approximately 15% and 50%.49–52102 In a study of 93 patients treated with Onyx, Weber and associates reported that embolization was curative in 20% of patients. Onyx resulted in high occlusion rates (volume reduction >90%) when the AVM was in a supratentorial and cortical location, the nidus was compact and plexiform, and there was a small number of supplying (direct) feeders and one superficial draining vein. Onyx did not achieve a high occlusion rate (<70%) in AVMs with multiple compartmental draining veins, in AVMs with multiple supplying arteries (especially leptomeningeal, en passant, or perforating feeders), and in AVMs with a diffuse nidus.49 In a study of 44 patients treated with Onyx, van Rooij and coauthors reported curative embolization rates of 16%.51 In a study by Mounayer and colleagues of 94 patients treated with Onyx over a span of 210 sessions, treatment was concluded in 53 patients at the time of publication. In this select cohort, curative embolization was achieved in 26 of 53 patients (49%).50 In a study of 101 patients treated with Onyx by Katsaridis and associates, only 52 patients had received complete treatment and further embolizations were planned in 49 patients. In the select number of patients receiving complete treatment, embolization was curative in 28 of 52 patients (54%).52
In locations that make microsurgery particularly difficult, embolization may be an alternative in carefully selected patients. AVMs of the basal ganglia and thalamus may have a higher annual hemorrhage rate103 and worse clinical course.104 In a study by Hurst and colleagues of 14 AVMs located in the thalamus or basal ganglia, cure by embolization was achieved in 15% of cases.72 In a study by Paulsen and collaborators of 38 AVMs in the thalamus or basal ganglia, 1 AVM was cured by embolization alone and 14 of 37 lesions were cured by combined modalities.79
It is important to note that in the series just described, patients were often treated with embolization as an adjuvant to radiosurgery or microsurgery. The patients typically had significant reductions in size that facilitated microsurgery or radiosurgery. The rate of complete obliteration may be different in patients specifically selected for curative embolization. In a study by Yu and colleagues, 27 patients underwent embolization. Ten patients were specifically treated with the goal of curative embolization because they preferred the reduced invasiveness of embolization to surgical excision. These patients had AVMs in which the nidus was not larger than 3 cm, the number of feeders did not exceed three, and the nidus was accessible with the tip of the catheter. The success rate of endovascular embolization in patients treated with curative intent was 60% (6 of 10), and the overall cure rate was 22% (6 of 27). Follow-up angiography performed at 17 to 32 months showed complete obliteration of the AVM nidus in the 6 patients after initial embolization, and these patients remained asymptomatic 5 to 7 years after treatment.22
In a study of 306 patients undergoing 513 embolizations, embolization was curative in 29 patients (9%). In the 415 sessions in which the AVM was accessible to deposition of embolic material, the rate of overall curative embolization was 7%. In 64 patients in whom the primary goal was curative embolization, the AVM was accessible in 55 patients and 17 AVMs were cured by embolization, for curative embolization rates of 26% in the total group and 31% in those with accessible lesions.19
Valavanis and Yasargil reported a 40% cure rate in a series of 387 embolized patients. In a subgroup of 182 AVMs that were embolized with curative intent, they reported a 75% cure rate.21 Direct, dominant feeding arteries, a monocompartmental nidus, and a dominant fistulous component of the nidus, without perinidal angiogenesis, were the chief variables predictive of endovascular obliteration. These authors did not find size or number of feeding pedicles to be an important determinant of the potential for endovascular cure.
In well-selected patients, curative embolization may be beneficial for AVMs located in regions with a poor response to microsurgery or radiosurgery. In a study of 14 patients with AVMs in intraventricular or paraventricular locations, curative embolization was achieved in 6 of 12 lesions that could be approached. In carefully selected patients, curative embolization may be appropriate because surgical treatment of AVMs in these locations is more difficult105 and decreased rates of obliteration106 and poorer outcomes107 have been observed with radiosurgery.
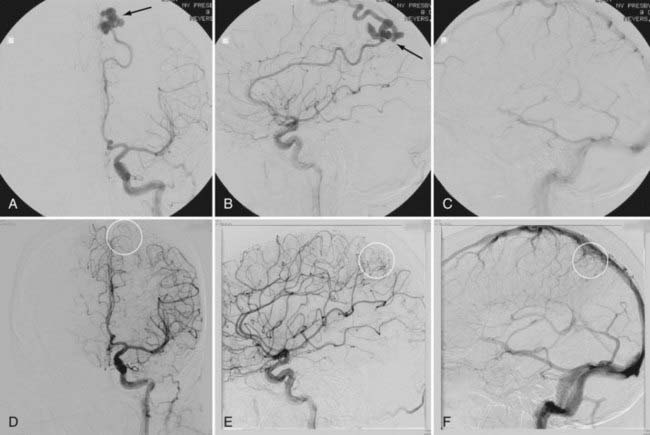
In a select number of patients with a low risk profile, primary embolization may be an acceptable form of treatment. The patient in Figure 387-1A to C had a small AVM that was easily accessible with only one arterial feeding vessel from the left pericallosal artery. This high-flow lesion had varicoid venous drainage, which placed the patient at increased risk for hemorrhage. This lesion was successfully cured after embolization with NBCA under monitored anesthetic care and provocative neurological testing. The patient returned at 3 months and recently at 6 months (Fig. 387-1D to F) for angiography, which confirmed complete obliteration of the lesion.
Recanalization
In any AVM treated by curative embolization, complete angiographic embolization of an AVM does not guarantee that future hemorrhage will not occur because the nidus of the AVM remains and delayed recanalization through collateral channels is a possibility.108 There is some evidence that patients with incompletely embolized AVMs are at increased risk for future morbidity and mortality from hemorrhage.84,109,110 A study by Heindenreich and colleagues demonstrated that an incomplete reduction of greater than 60% resulted in a 19-fold increased risk for hemorrhage.111 Complete endovascular cure is considered possible with the use of cyanoacrylate, but the degree of permanent obliteration is probably high yet remains unknown.108
Conclusion
Interventional planning must determine the modality or combination of modalities that can achieve the greatest success rate consistent with the patient’s characteristics and goals, AVM architecture, and the capabilities of the treatment option to fulfill the goals of treatment.3–7 With these goals in mind, patients should be assessed by a multidisciplinary team with expertise in endovascular embolization, microsurgical resection, and radiosurgery.
The prerequisite to curing an AVM definitively with endovascular embolization techniques alone is angioarchitecture that permits solid casting of the AVM nidus with permanent embolizing material before occlusion of venous outflow. If curative therapy is the intended goal, permanent material must be used (e.g., NBCA or Onyx) instead of particles. Complete and permanent occlusion is achieved in only selected cases. AVMs cured by endovascular therapy tend to be small with relatively simple architecture. It is these precise lesions that most often can be cured by microsurgery or radiosurgery. Microsurgery results in immediate abatement of hemorrhage risk. For patients with small lesions in noneloquent locations, microsurgery has demonstrated excellent results.6,8,112,113 However, microsurgery is not indicated for all AVM patients. Complete resection of AVMs in deep or eloquent areas may result in high morbidity and mortality.114 These lesions may be cured by radiosurgery with little morbidity and mortality, but obliteration is often delayed by 2 to 5 years.115–117 Nevertheless, endovascular embolization is a less invasive alternative for patients who are disinclined to undergo microsurgery or are unable to wait for the delayed benefits of radiosurgery. It may also be an alternative therapy in patients whose comorbid conditions inhibit general anesthesia and open surgery and in patients with an increased hemorrhage risk profile that prohibits radiosurgery. For small, deep-seated AVMs with one or two feeding arteries, especially those in the basal ganglia or thalamus, there is some evidence that endovascular embolization offers an appropriate option with immediate protection from new or recurrent hemorrhage.
, 1999 Arteriovenous malformations of the brain in adults. N Engl J Med. 1999;340:1812-1818.
Berenstein A, Lasjaunias P. Surgical Neuroangiography, Vol 4. New York: Springer. 1991.
Cronqvist M, Wirestam R, Ramgren B, et al. Endovascular treatment of intracerebral arteriovenous malformations: procedural safety, complications, and results evaluated by MR imaging, including diffusion and perfusion imaging. AJNR Am J Neuroradiol. 2006;27:162-176.
Hartmann A, Stapf C, Hofmeister C, et al. Determinants of neurological outcome after surgery for brain arteriovenous malformation. Stroke. 2000;31:2361-2364.
Kim LJ, Albuquerque FC, Spetzler RF, et al. Postembolization neurological deficits in cerebral arteriovenous malformations: stratification by arteriovenous malformation grade. Neurosurgery. 2006;59:53-59.
Merland JJ, Rufenacht D, Laurent A, et al. Endovascular treatment with isobutyl cyano acrylate in patients with arteriovenous malformation of the brain. Indications, results and complications. Acta Radiol Suppl. 1986;369:621-622.
Mounayer C, Hammami N, Piotin M, et al. Nidal embolization of brain arteriovenous malformations using onyx in 94 patients. AJNR Am J Neuroradiol. 2007;28:518-523.
, 2002 N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi-center trial. AJNR Am J Neuroradiol. 2002;23:748-755.
Ogilvy CS, Stieg PE, Awad I, et al. AHA scientific statement: Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Stroke Association. Stroke. 2001;32:1458-1471.
Pollock BE, Brown RDJ. Use of the modified Rankin scale to assess outcome after arteriovenous malformation radiosurgery. Neurology. 2006;67:1630-1634.
Shin M, Maruyama K, Kurita H, et al. Analysis of nidus obliteration rates after gamma knife surgery for arteriovenous malformations based on long-term follow-up data: The University of Tokyo experience. J Neurosurg. 2004;101:18-24.
Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
Spetzler RF, Wilson CB, Weinstein P, et al. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651-672.
Starke RM, Komotar RJ, Otten ML, et al. Adjuvant embolization with n-butyl cyanoacrylate in the treatment of cerebral arteriovenous malformations: outcomes, complications, and predictors of neurologic deficits. Stroke. 2009.
Vinuela F, Dion JE, Duckwiler G, et al. Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: experience with 101 cases. J Neurosurg. 1991;75:856-864.
Wikholm G, Lundqvist C, Svendsen P. The Goteborg cohort of embolized cerebral arteriovenous malformations: a 6-year follow-up. Neurosurgery. 2001;49:799-805.
1 Bristol RE, Albuquerque FC, McDougall CG. The evolution of endovascular treatment for intracranial arteriovenous malformations. Neurosurg Focus. 2006;20(6):E6.
2 Luessenhop AJ, Spence WT. Artificial embolization of cerebral arteries. Report of use in a case of arteriovenous malformation. JAMA. 1960;172:1153-1155.
3 Richling B, Killer M, Al-Schameri AR, et al. Therapy of brain arteriovenous malformations: multimodality treatment from a balanced standpoint. Neurosurgery. 2006;59(5 suppl):S148-S157.
4 Ogilvy CS, Stieg PE, Awad I, et al. AHA scientific statement: Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Stroke Association. Stroke. 2001;32:1458-1471.
5 Batjer HH, Devous MDSr, Seibert GB, et al. Intracranial arteriovenous malformation: relationship between clinical factors and surgical complications. Neurosurgery. 1989;24:75-79.
6 Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
7 Hartmann A, Stapf C, Hofmeister C, et al. Determinants of neurological outcome after surgery for brain arteriovenous malformation. Stroke. 2000;31:2361-2364.
8 Hartmann A, Mast H, Mohr JP, et al. Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke. 1998;29:931-934.
9 Batjer H, Suss RA, Samson D. Intracranial arteriovenous malformations associated with aneurysms. Neurosurgery. 1986;18:29-35.
10 Kader A, Young WL, Pile-Spellman J, et al. The influence of hemodynamic and anatomic factors on hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1994;34:801-807.
11 Miyasaka Y, Yada K, Ohwada T, et al. An analysis of the venous drainage system as a factor in hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76:239-243.
12 Turjman F, Massoud TF, Vinuela F, et al. Correlation of the angioarchitectural features of cerebral arteriovenous malformations with clinical presentation of hemorrhage. Neurosurgery. 1995;37:856-860.
13 Marks MP, Lane B, Steinberg GK, et al. Hemorrhage in intracerebral arteriovenous malformations: angiographic determinants. Radiology. 1990;176:807-813.
14 Duong DH, Young WL, Vang MC, et al. Feeding artery pressure and venous drainage pattern are primary determinants of hemorrhage from cerebral arteriovenous malformations. Stroke. 1998;29:1167-1176.
15 Waltimo O. The change in size of intracranial arteriovenous malformations. J Neurol Sci. 1973;19:21-27.
16 Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331-337.
17 Willinsky R, Lasjaunias P, Terbrugge K, et al. Brain arteriovenous malformations: analysis of the angio-architecture in relationship to hemorrhage (based on 152 patients explored and/or treated at the Hôpital de Bicôtre between 1981 and 1986). J Neuroradiol. 1988;15:225-237.
18 Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology. 1969;93:1071-1078.
19 Haw CS, terBrugge K, Willinsky R, et al. Complications of embolization of arteriovenous malformations of the brain. J Neurosurg. 2006;104:226-232.
20 Oran I, Parildar M, Derbent A. Ventricular/paraventricular small arteriovenous malformations: role of embolisation with cyanoacrylate. Neuroradiology. 2005;47:287-294.
21 Valavanis A, Yasargil MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg. 1998;24:131-214.
22 Yu SC, Chan MS, Lam JM, et al. Complete obliteration of intracranial arteriovenous malformation with endovascular cyanoacrylate embolization: initial success and rate of permanent cure. AJNR Am J Neuroradiol. 2004;25:1139-1143.
23 Doppman JL, Zapol W, Pierce J. Transcatheter embolization with a silicone rubber preparation. Experimental observations. Invest Radiol. 1971;6:304-309.
24 Yasargil MG. Microneurosurgery. Vol IIIA, AVM of the Brain. New York: Thieme; 1987.
25 Yao TL, Eskioglu E, Ayad M, et al. Improved image interpretation with combined superselective and standard angiography (double injection technique) during embolization of arteriovenous malformations. Neurosurgery. 2008;62:140-141.
26 Warren DJ, Hoggard N, Walton L, et al. Cerebral arteriovenous malformations: comparison of novel magnetic resonance angiographic techniques and conventional catheter angiography. Neurosurgery. 2001;48:973-982.
27 Pollock BE, Flickinger JC. A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg. 2002;96:79-85.
28 Wikholm G, Lundqvist C, Svendsen P. Embolization of cerebral arteriovenous malformations: part I—technique, morphology, and complications. Neurosurgery. 1996;39:448-457.
29 Vinuela F, Dion JE, Duckwiler G, et al. Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: experience with 101 cases. J Neurosurg. 1991;75:856-864.
30 Gobin YP, Laurent A, Merienne L, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996;85:19-28.
31 Fournier D, TerBrugge KG, Willinsky R, et al. Endovascular treatment of intracerebral arteriovenous malformations: experience in 49 cases. J Neurosurg. 1991;75:228-233.
32 Fiorella D, Albuquerque FC, Woo HH, et al. The role of neuroendovascular therapy for the treatment of brain arteriovenous malformations. Neurosurgery. 2006;59(5 suppl):S163-S177.
33 Willinsky R, Goyal M, TerBrugge K, et al. Embolization of small (<3 cm) brain arteriovenous malformations. Correlation of angiographic results to a proposed angioarchitecture grading system. Intervent Neuroradiol. 2001;7:19-27.
34 Valavanis A, Christoforidis G. Endovascular management of cerebral arteriovenous malformations. Neurointerventionist. 1999;1:34-40.
35 Kerber C. Balloon catheter with a calibrated leak. A new system for superselective angiography and occlusive catheter therapy. Radiology. 1976;120:547-550.
36 Berenstein A. Technique of catheterization and embolization of the lenticulostriate arteries. J Neurosurg. 1981;54:783-789.
37 Latchaw RE, Gold LH. Polyvinyl foam embolization of vascular and neoplastic lesions of the head, neck, and spine. Radiology. 1979;131:669-679.
38 Berenstein A, Lasjaunias P. Surgical Neuroangiography, vol 4. New York: Springer. 1991.
39 Richling B, Knosp E. Ein fuhrungsdrahtgesteuertes Kathersystem zur begehung und embolisation intrazerebraler Arterien. In: Schneider GH, Vogler E, editors. Digitale dildgenbende Verfahren, interventionelle Verfahren, integrierte digitale Radiologie. Berlin. Springer, 1988.
40 Zanetti PH, Sherman FE. Experimental evaluation of a tissue adhesive as an agent for the treatment of aneurysms and arteriovenous anomalies. J Neurosurg. 1972;36:72-79.
41 Richling B. Homologous controlled-viscosity fibrin for endovascular embolization. Part II: Catheterization technique, animal experiments. Acta Neurochir (Wien). 1982;64:109-124.
42 Richling B. Homologous controlled-viscosity fibrin for endovascular embolization. Part I. Experimental development of the medium. Acta Neurochir (Wien). 1982;62:159-170.
43 Taki W, Yonekawa Y, Iwata H, et al. A new liquid material for embolization of arteriovenous malformations. AJNR Am J Neuroradiol. 1990;11:163-168.
44 Chaloupka JC, Vinuela F, Vinters HV, et al. Technical feasibility and histopathologic studies of ethylene vinyl copolymer (EVAL) using a swine endovascular embolization model. AJNR Am J Neuroradiol. 1994;15:1107-1115.
45 Sampei K, Hashimoto N, Kazekawa K, et al. Histological changes in brain tissue and vasculature after intracarotid infusion of organic solvents in rats. Neuroradiology. 1996;38:291-294.
46 Murayama Y, Vinuela F, Ulhoa A, et al. Nonadhesive liquid embolic agent for cerebral arteriovenous malformations: preliminary histopathological studies in swine rete mirabile. Neurosurgery. 1998;43:1164-1175.
47 Jahan R, Murayama Y, Gobin YP, et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery. 2001;48:984-995.
48 Jankowitz BT, Vora N, Jovin T, et al. Treatment of pediatric intracranial vascular malformations using Onyx-18. J Neurosurg Pediatr. 2008;2:171-176.
49 Weber W, Kis B, Siekmann R, et al. Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery. 2007;61:244-252.
50 Mounayer C, Hammami N, Piotin M, et al. Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. AJNR Am J Neuroradiol. 2007;28:518-523.
51 van Rooij WJ, Sluzewski M, Beute GN. Brain AVM embolization with Onyx. AJNR Am J Neuroradiol. 2007;28:172-177.
52 Katsaridis V, Papagiannaki C, Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50:589-597.
53 Natarajan SK, Ghodke B, Britz GW, et al. Multimodality treatment of brain arteriovenous malformations with microsurgery after embolization with Onyx: single-center experience and technical nuances. Neurosurgery. 2008;62:1213-1225.
54 Velat GJ, Reavey-Cantwell JF, Sistrom C, et al. Comparison of n-butyl cyanoacrylate and Onyx for the embolization of intracranial arteriovenous malformations: analysis of fluoroscopy and procedure times. Neurosurgery. 2008;63:ONS73-78.
55 N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi-center trial. AJNR Am J Neuroradiol. 2002;23:748-755.
56 Sorimachi T, Koike T, Takeuchi S, et al. Embolization of cerebral arteriovenous malformations achieved with polyvinyl alcohol particles: angiographic reappearance and complications. AJNR Am J Neuroradiol. 1999;20:1323-1328.
57 Mathis JA, Barr JD, Horton JA, et al. The efficacy of particulate embolization combined with stereotactic radiosurgery for treatment of large arteriovenous malformations of the brain. AJNR Am J Neuroradiol. 1995;16:299-306.
58 Standard SC, Guterman LR, Chavis TD, et al. Delayed recanalization of a cerebral arteriovenous malformation following angiographic obliteration with polyvinyl alcohol embolization. Surg Neurol. 1995;44:109-112.
59 Rosch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1972;102:303-306.
60 Porstmann W, Wierny L, Warnke H, et al. Catheter closure of patent ductus arteriosus. 62 cases treated without thoracotomy. Radiol Clin North Am. 1971;9:203-218.
61 Deveikis JP, Manz HJ, Luessenhop AJ, et al. A clinical and neuropathologic study of silk suture as an embolic agent for brain arteriovenous malformations. AJNR Am J Neuroradiol. 1994;15:263-271.
62 Luessenhop AJ, Rosa L. Cerebral arteriovenous malformations. Indications for and results of surgery, and the role of intravascular techniques. J Neurosurg. 1984;60:14-22.
63 Deruty R, Pelissou-Guyotat I, Mottolese C, et al. The combined management of cerebral arteriovenous malformations. Experience with 100 cases and review of the literature. Acta Neurochir (Wien). 1993;123:101-112.
64 Arteriovenous malformations of the brain in adults. N Engl J Med. 1999;340:1812-1818.
65 Hartmann A, Mast H, Mohr JP, et al. Determinants of staged endovascular and surgical treatment outcome of brain arteriovenous malformations. Stroke. 2005;36:2431-2435.
66 Hartmann A, Pile-Spellman J, Stapf C, et al. Risk of endovascular treatment of brain arteriovenous malformations. Stroke. 2002;33:1816-1820.
67 Morgan MK, Zurin AA, Harrington T, et al. Changing role for preoperative embolisation in the management of arteriovenous malformations of the brain. J Clin Neurosci. 2000;7:527-530.
68 Taylor CL, Dutton K, Rappard G, et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004;100:810-812.
69 Debrun GM, Aletich V, Ausman JI, et al. Embolization of the nidus of brain arteriovenous malformations with n-butyl cyanoacrylate. Neurosurgery. 1997;40:112-120.
70 Deruty R, Pelissou-Guyotat I, Amat D, et al. Complications after multidisciplinary treatment of cerebral arteriovenous malformations. Acta Neurochir (Wien). 1996;138:119-131.
71 Frizzel RT, Fisher WS3rd. Cure, morbidity, and mortality associated with embolization of brain arteriovenous malformations: a review of 1246 patients in 32 series over a 35-year period. Neurosurgery. 1995;37:1031-1039.
72 Hurst RW, Berenstein A, Kupersmith MJ, et al. Deep central arteriovenous malformations of the brain: the role of endovascular treatment. J Neurosurg. 1995;82:190-195.
73 Jafar JJ, Davis AJ, Berenstein A, et al. The effect of embolization with n-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg. 1993;78:60-69.
74 Mathis JA, Barr JD, Horton JA, et al. The efficacy of particulate embolization combined with stereotactic radiosurgery for treatment of large arteriovenous malformations of the brain. AJNR Am J Neuroradiol. 1995;16:299-306.
75 Meisel HJ, Mansmann U, Alvarez H, et al. Effect of partial targeted n-butyl-cyano-acrylate embolization in brain AVM. Acta Neurochir (Wien). 2002;144:879-887.
76 Merland JJ, Rufenacht D, Laurent A, et al. Endovascular treatment with isobutyl cyano acrylate in patients with arteriovenous malformation of the brain. Indications, results and complications. Acta Radiol Suppl. 1986;369:621-622.
77 Pasqualin A, Scienza R, Cioffi F, et al. Treatment of cerebral arteriovenous malformations with a combination of preoperative embolization and surgery. Neurosurgery. 1991;29:358-368.
78 Paulsen RD, Steinberg GK, Norbash AM, et al. Embolization of rolandic cortex arteriovenous malformations. Neurosurgery. 1999;44:479-484.
79 Paulsen RD, Steinberg GK, Norbash AM, et al. Embolization of basal ganglia and thalamic arteriovenous malformations. Neurosurgery. 1999;44:991-996.
80 Purdy PD, Samson D, Batjer HH, et al. Preoperative embolization of cerebral arteriovenous malformations with polyvinyl alcohol particles: experience in 51 adults. AJNR Am J Neuroradiol. 1990;11:501-510.
81 Schmutz F, McAuliffe W, Anderson DM, et al. Embolization of cerebral arteriovenous malformations with silk: histopathologic changes and hemorrhagic complications. AJNR Am J Neuroradiol. 1997;18:1233-1237.
82 Vinuela F, Fox AJ, Debrun G, et al. Progressive thrombosis of brain arteriovenous malformations after embolization with isobutyl 2-cyanoacrylate. AJNR Am J Neuroradiol. 1983;4:1233-1238.
83 Wikholm G, Lundqvist C, Svendsen P. Transarterial embolization of cerebral arteriovenous malformations: improvement of results with experience. AJNR Am J Neuroradiol. 1995;16:1811-1817.
84 Wikholm G, Lundqvist C, Svendsen P. The Goteborg cohort of embolized cerebral arteriovenous malformations: a 6-year follow-up. Neurosurgery. 2001;49:799-805.
85 Lundqvist C, Wikholm G, Svendsen P. Embolization of cerebral arteriovenous malformations: part II—aspects of complications and late outcome. Neurosurgery. 1996;39:460-467.
86 Debrun G, Vinuela F, Fox A, et al. Embolization of cerebral arteriovenous malformations with bucrylate. J Neurosurg. 1982;56:615-627.
87 Cronqvist M, Wirestam R, Ramgren B, et al. Endovascular treatment of intracerebral arteriovenous malformations: procedural safety, complications, and results evaluated by MR imaging, including diffusion and perfusion imaging. AJNR Am J Neuroradiol. 2006;27:162-176.
88 Jayaraman MV, Marcellus ML, Hamilton S, et al. Neurologic complications of arteriovenous malformation embolization using liquid embolic agents. AJNR Am J Neuroradiol. 2008;29:242-246.
89 Kim LJ, Albuquerque FC, Spetzler RF, et al. Postembolization neurological deficits in cerebral arteriovenous malformations: stratification by arteriovenous malformation grade. Neurosurgery. 2006;59:53-59.
90 Ledezma CJ, Hoh BL, Carter BS, et al. Complications of cerebral arteriovenous malformation embolization: multivariate analysis of predictive factors. Neurosurgery. 2006;58:602-611.
91 Spetzler RF, Martin NA, Carter LP, et al. Surgical management of large AVM’s by staged embolization and operative excision. J Neurosurg. 1987;67:17-28.
92 Purdy PD, Batjer HH, Samson D. Management of hemorrhagic complications from preoperative embolization of arteriovenous malformations. J Neurosurg. 1991;74:205-211.
93 Purdy PD, Batjer HH, Samson D, et al. Intraarterial sodium Amytal administration to guide preoperative embolization of cerebral arteriovenous malformations. J Neurosurg Anesthesiol. 1991;3:103-106.
94 Starke RM, Komotar RJ, Otten ML, et al. Adjuvant embolization with n-butyl cyanoacrylate in the treatment of cerebral arteriovenous malformations: outcomes, complications, and predictors of neurologic deficits. Stroke. 2009.
95 Picard L, Da Costa E, Anxionnat R, et al. Acute spontaneous hemorrhage after embolization of brain arteriovenous malformation with n-butyl cyanoacrylate. J Neuroradiol. 2001;28:147-165.
96 Jafar JJ, Rezai AR. Acute surgical management of intracranial arteriovenous malformations. Neurosurgery. 1994;34:8-12.
97 Massoud TF, Hademenos GJ, Young WL, et al. Can induction of systemic hypotension help prevent nidus rupture complicating arteriovenous malformation embolization? Analysis of underlying mechanism achieved using a theoretical model. AJNR Am J Neuroradiol. 2000;21:1255-1267.
98 Sorimachi T, Takeuchi S, Koike T, et al. Blood pressure monitoring in feeding arteries of cerebral arteriovenous malformations during embolization: a preventive role in hemodynamic complications. Neurosurgery. 1995;37:1041-1047.
99 Spetzler RF, Wilson CB, Weinstein P, et al. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651-672.
100 Wilms G, Goffin J, Plets C, et al. Embolization of arteriovenous malformations of the brain: preliminary experience. J Belg Radiol. 1993;76:299-303.
101 Cockroft KM, Hwang SK, Rosenwasser RH. Endovascular treatment of cerebral arteriovenous malformations: indications, techniques, outcome, and complications. Neurosurg Clin N Am. 2005;16:367-380. x
102 Florio F, Lauriola W, Nardella M, et al. Endovascular treatment of intracranial arterio-venous malformations with onyx embolization: preliminary experience. Radiol Med (Torino). 2003;106:512-520.
103 Sasaki T, Kurita H, Saito I, et al. Arteriovenous malformations in the basal ganglia and thalamus: management and results in 101 cases. J Neurosurg. 1998;88:285-292.
104 Lawton MT, Hamilton MG, Spetzler RF. Multimodality treatment of deep arteriovenous malformations: thalamus, basal ganglia, and brain stem. Neurosurgery. 1995;37:29-36.
105 Miyasaka Y, Yada K, Ohwada T, et al. Choroid plexus arteriovenous malformations. Neurol Med Chir (Tokyo). 1992;32:201-206.
106 Nataf F, Meder JF, Oppenheim C, et al. [Radiosurgery of choroidal and cisternal cerebral arteriovenous malformations.]. Neurochirurgie. 2001;47:283-290.
107 Flickinger JC, Kondziolka D, Maitz AH, et al. Analysis of neurological sequelae from radiosurgery of arteriovenous malformations: how location affects outcome. Int J Radiat Oncol Biol Phys. 1998;40:273-278.
108 Connors JJ, Wojak JC. Interventional Neuroradiology: strategies and Practical Techniques. Philadelphia: Saunders; 1999.
109 Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg. 2003;98:3-7.
110 Miyamoto S, Hashimoto N, Nagata I, et al. Posttreatment sequelae of palliatively treated cerebral arteriovenous malformations. Neurosurgery. 2000;46:589-594.
111 Heidenreich JO, Hartlieb S, Stendel R, et al. Bleeding complications after endovascular therapy of cerebral arteriovenous malformations. AJNR Am J Neuroradiol. 2006;27:313-316.
112 Sisti MB, Kader A, Stein BM. Microsurgery for 67 intracranial arteriovenous malformations less than 3 cm in diameter. J Neurosurg. 1993;79:653-660.
113 Heros RC, Korosue K, Diebold PM. Surgical excision of cerebral arteriovenous malformations: late results. Neurosurgery. 1990;26:570-578.
114 Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994;34:2-6.
115 Pollock BE, Brown RDJ. Use of the modified Rankin scale to assess outcome after arteriovenous malformation radiosurgery. Neurology. 2006;67:1630-1634.
116 Pollock BE, Lunsford LD, Kondziolka D, et al. Patient outcomes after stereotactic radiosurgery for “operable” arteriovenous malformations. Neurosurgery. 1994;35:1-8.
117 Shin M, Maruyama K, Kurita H, et al. Analysis of nidus obliteration rates after gamma knife surgery for arteriovenous malformations based on long-term follow-up data: the University of Tokyo experience. J Neurosurg. 2004;101:18-24.