CHAPTER 377 Endovascular Hunterian Ligation
Hunterian ligation refers to one of the oldest successful interventions for arterial aneurysms—ligation of the femoral artery to treat a popliteal aneurysm by John Hunter in 1785.1 Recently, the development of endovascular techniques has made it possible to completely occlude an artery intra-arterially, without the need for surgical access. Because refinements in the coiling technology used to treat cerebral aneurysms have continued to broaden the scope of intracranial aneurysms that can be repaired by coiling, hunterian ligation is more frequently relied on as a last resort to treat only the most surgically inaccessible and difficult aneurysms. Historically, hunterian ligation has referred to the permanent sacrifice of a parent artery to prevent access of blood to the aneurysm. This technique has also been referred to as “deconstructive” therapy, in contrast to “reconstructive” therapy, which refers to the targeted occlusion of a vascular abnormality without impairment of blood flow in the parent vessel. Hunterian ligation most often targets complex aneurysms, but sacrifice of the parent artery has also been used for the treatment of a wide range of neurosurgical entities, including hemorrhagic stroke, vascular tumors, arteriovenous malformations, fistulas, and arterial dissections.
History
John Hunter’s arterial sacrifice was first applied to the cerebral vasculature in 1804 by Abernethy as he unsuccessfully attempted to ligate the carotid artery in a case of posttraumatic dissection of the carotid artery. His pioneering attempt resulted in stroke, thus leaving the first successful carotid ligation to Astley Cooper in 1808. Only a year later, Victor Horsley successfully ligated the common carotid to treat a giant internal carotid artery (ICA) aneurysm. Carotid ligation, either common or internal, was a common form of treatment of ruptured aneurysms until the late 1960s.1 Various methods of occlusion were used (gradual or abrupt). In addition, special clamps were developed that allowed treatment in the awake state so that if neurological impairment occurs with gradual occlusion, flow could be restored rapidly.1–4 In retrospect, by today’s standards, carotid ligation appears to be an inelegant treatment. Nevertheless, it was found to be effective in preventing rebleeding over the short term (6 months) in a randomized trial comparing abrupt common carotid occlusion with bed rest in patients with a ruptured posterior communicating aneurysm.3 Subsequently, long-term (≈10 years) follow-up of these patients revealed that the protection against rebleeding was lost; both ligated and bed-rested patients had the same rebleeding rate.5
Intracranial access would have to wait, however, until neurosurgical techniques dramatically improved, and it was not until 1945 that Poppen became the first to document ligation of the vertebral artery to treat an aneurysm. In 1962, the basilar artery was ligated proximally by Mount and Taveras.6 Without the ability to predict which patients could tolerate proximal arterial occlusion, however, favorable outcome was a matter of chance. Development of the Drake tourniquet permitted the surgeon to temporarily occlude an artery the day after surgery, when the patient had fully recovered from anesthesia, and therefore observe whether sacrifice of the parent vessel could be tolerated. Drake’s original study of occlusion of the basilar or vertebral artery in 14 patients was strikingly successful in that 7 of these patients did well.7 Surgical hunterian ligation, however, is limited by surgical access and was slowly replaced by reconstructive techniques for most intracranial aneurysms. Since the development of endovascular balloons in the early 1990s, parent vessel occlusion has become possible through transfemoral access, ironically the same vessel that Hunter first ligated in 1785. Further development of endovascular coils has increased the efficacy and expanded the indications for endovascular permanent occlusion (PO).
Indications
Endovascular hunterian ligation is reserved primarily for giant and fusiform aneurysms. Certain traumatic pseudoaneurysms and infectious aneurysms in which the risk associated with endovascular intervention is high may also be good candidates.8 The most complex aneurysms cannot be treated either by conventional surgical clipping or by endovascular coiling, which makes the technique, as well as the evidence supporting its practice, based predominantly on observational data from case series and individual reports.8 Although it would be ideal to exclude the lesion from the cerebral circulation and maintain blood flow through the parent artery, such reconstructive methods cannot be used for all aneurysms because of their shape and location. Surgeons have observed that as many as two thirds of giant intracranial aneurysms may not be amenable to clip reconstruction or endovascular treatment as a result of the location of the aneurysm or its morphology.7 This number, however, continues to shrink because of improving endovascular technology. The major treatment question when considering endovascular hunterian ligation is whether to perform an arterial bypass before permanent vessel occlusion. The current standard of care requires preoperative evaluation with balloon test occlusion (BTO) followed by distal perfusion.
Approaches to the Occlusion of Specific Cerebral Vessels
The Internal Carotid Arteries
As described earlier, the carotid arteries were the first cerebral vessels to be intentionally and successfully ligated in 1808 by the English surgeon Astley Cooper. Because of the surgical inaccessibility of portions of its course and the robust circulation of the circle of Willis, the ICA is the vessel most commonly treated with PO. PO is predominantly performed for lesions of the ICA itself, but it may be done for complex lesions of the anterior cerebral artery (ACA) or middle cerebral artery (MCA) as well. O’Shaughnessy and colleagues, in their description of 58 PO procedures performed on the ICA over a period of 15 years, included aneurysms in multiple locations: 40 intracavernous, 5 petrous carotid, 3 cervical carotid, and 10 ophthalmic segment aneurysms.8 BTO was used in all cases, and EC/IC bypass was performed when deemed necessary after testing. Outcomes were reported at a mean follow-up of 76 months, with three patients dying during treatment, transient ischemia developing in six, delayed infarction developing in two, and the aneurysms of one patient enlarging after endovascular occlusion and requiring surgical clipping.
Certain types of aneurysms generally elude coiling by current technology and require PO as a last resort. In 2007, Park and coauthors reported 12 patients with blood blister aneurysms of the ICA, 7 of whom were treated with conventional endovascular coiling or stent-assisted coiling and 5 treated with BTO and endovascular trapping, which consists of permanent parent vessel occlusion both proximally and distally to prevent arterial backflow.9 The 7 patients treated with coiling demonstrated aneurysm regrowth, and 3 suffered rebleeding with severe morbidity. All 5 patients treated with PO had an excellent neurological outcome. With appropriate neurological testing, PO of the ICA can be an important treatment modality that may even be the preferred intervention in selected cases.
Recent studies have evaluated a new endovascular device, the Amplatzer vascular plug, for performing ICA parent vessel occlusion.10–12 The vascular plug is a self-expanding nitinol wire mesh that can be used in conjunction with coiling to act as an anchor on which the coil can be deployed. Preliminary studies have shown success in treating patients with this device for aneurysms in the cavernous portion of the ICA, cavernous carotid fistulas, and vertebral artery aneurysm at the origin of the posterior inferior cerebellar artery (PICA). Further studies are needed to evaluate this device and others in development that facilitate endovascular vessel occlusion.
The Vertebrobasilar Circulation
Ligation of the vertebrobasilar circulation for a known vertebral artery lesion appears to have first been performed by Poppen in 1945.13,14 Vertebral artery ligation, both unilateral and bilateral, was used by Drake in 1975 to treat large vertebral or basilar artery aneurysms in 14 patients.7 With stricter patient selection and the development of endovascular techniques, patient outcomes have improved significantly enough to justify vertebral artery PO in the proximal posterior circulation.
In 1991, Aymard and associates reported unilateral or bilateral endovascular hunterian ligation of the vertebral artery in 21 patients: 13 showed complete neurological recovery, 6 had partial aneurysm thrombosis, 1 had no thrombosis, 1 died, and 1 suffered a transient stroke.15 Because of this high rate of morbidity and mortality, the authors advocated strict preoperative CRT. An important contribution of this study was that the authors noted that in most cases, occlusion of the vertebral artery was most effective at the level of C1 because antegrade collateral flow is possible through the external carotid artery.16 It is important to note, however, that too much collateral flow can prevent aneurysmal thrombosis. Halbach and coauthors published a series of 15 patients who underwent proximal PO for vertebral artery vascular pathology and had improved outcomes.17
Collateral backflow from the opposite vertebral artery and the circle of Willis makes endovascular trapping an attractive treatment option for vertebral artery lesions.18,19 Double microcoil trapping after BTO of the vertebral artery proximal to the aneurysm was performed on 11 patients suffering SAH from dissecting vertebral artery aneurysms. As previously mentioned, this technique simultaneously occludes the parent vessel proximal and distal to the vascular lesion. The case series of 11 patients by Kai and colleagues reported good neurological outcome with only one transient adverse result.19
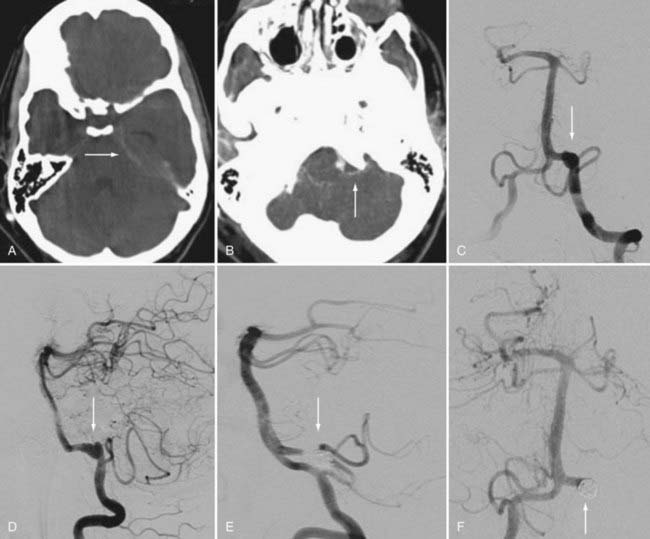
Aneurysms involving the origin of the PICA raise important concerns that can guide favorable management options (Fig. 377-1). A treatment rubric was proposed by Iihara and coworkers in which it was suggested that certain strategies could be used depending on the relationship between the origin of the PICA and the aneurysm sac.18 They proposed that if the aneurysm is separate from the origin of the PICA, internal occlusion should be the recommended treatment. For an aneurysm involving the origin of PICA and manifested as SAH, the suggested treatment is proximal occlusion and internal trapping. If the aneurysm incorporates the origin of PICA and does not involve SAH, BTO should be performed followed by occipital artery–PICA bypass. For the authors of the case series of 18 patients, the algorithm resulted in a favorable morbidity rate of 17% with no instances of PICA infarction and no deaths.
The Distal Circulation
Although the ACA, MCA, and posterior cerebral artery (PCA) are distal to the collateral circulation of the circle of Willis, collateral flow through leptomeningeal vessels makes PO in the distal circulation possible. In their anterior circulation case series published in 1994, Drake and coworkers described 29 cases of surgical hunterian ligation and bypass as a treatment strategy for distal aneurysms involving the ACA and MCA.20 In 4 of the 29 patients, the bypass failed, but none of these patients suffered postoperative morbidity because of the robust collateral flow that filled the M2 segment. In 1 patient, these collaterals were demonstrated on angiography to provide retrograde flow to the thrombosed aneurysm. In 2 of the patients treated for ACA aneurysms, complete thrombosis of the ACA resulted in death or severe neurological morbidity.
Efforts to perform endovascular hunterian ligation in these vessels have not been sparse. In 1991, Hodes and coworkers described five successful attempts in the distal circulation, including three M1 aneurysms, one M3 aneurysm, and one A1 aneurysm.21 At least two case series have emerged describing the distal posterior circulation. Xavier and coauthors reported three cases of P2 fusiform aneurysms treated by endovascular PO with no morbidity, and Arat and colleagues reported eight endovascular procedures performed for PCA aneurysms with a 12.5% morbidity rate.22,23 Mycotic aneurysms have a predilection for the distal circulation and have also been treated with endovascular PO; in one study, 12 patients underwent the procedure with no postoperative morbidity or mortality related to the operation.24,25
Cerebral Reserve Testing
The first test occlusion was performed by Allen and Matas in 1911, who advocated 20 to 30 minutes of carotid occlusion percutaneously or after surgical exposure under local anesthesia.26 This preliminary testing became known as the Matas test, but it was widely thought to lack sensitivity because it was difficult to effectively fully occlude the carotid artery for such a long time when it was attempted percutaneously. The modern endovascular version of this test has been extensively evaluated in the literature. In 2000, van Rooij and colleagues showed that of 17 patients who successfully passed BTO and subsequently underwent endovascular hunterian ligation, none experienced complications.27
There are many variations of the BTO protocol ranging from brief balloon inflation to an array of CRT and imaging under various conditions. Initially, a subset of patients were found to experience morbidity after PO even though BTO had produced no symptoms. Additional testing was then developed that included a hypotensive chanllenge.28 In this procedure, BTO is performed for 20 to 30 minutes with neurological examination every 5 minutes, and then mean arterial blood pressure is pharmacologically reduced to 66% of the patient’s baseline and maintained at that level for 20 minutes. The addition of a hypotensive challenge improved the sensitivity of CRT for failure; Standard and associates showed that this testing reduced the morbidity and mortality associated with PO to less than 5%.29
A wide variety of adjunctive testing is currently available to assist BTO. These techniques include encephalography, neurophysiologic monitoring, stump pressure evaluation, near-infrared spectroscopy, transcranial Doppler ultrasonography, 99mTc-hexamethylpropyleneamine oxime (HMPAO) single-photon emission computed tomography, positron emission tomography, stable xenon-labeled computed tomography (Xe-CT), and patient-specific computer modeling.30–35 Synchronous venous filling (<0.5-second delay) on angiography and on Xe-CT has also been correlated with increased success after endovascular hunterian ligation.
Complications
Lesions requiring hunterian ligation are the most difficult to treat with common treatment modalities and therefore lead to high rates of complications. The most common complication after endovascular PO is, of course, ischemic stroke. Postocclusion thromboembolism, coil migration, secondary aneurysm development, and perforator occlusion can also occur.14,36,37 As described earlier, enormous effort is being invested in minimizing these complications through extensive preoperative hemodynamic testing. Nishioka in 1966 reported a complication rate of 10% in 160 patients and cited ischemic infarction in only 2 of those patients.38 Surgical hunterian ligation, however, may carry a higher risk for thromboembolic stroke than seen with endovascular PO. Advances in preoperative testing, patient selection, and endovascular occlusion techniques continue to improve endovascular PO outcomes. In 2000, van Rooij and coauthors reported no complications in 17 patients,27 and in 2005, Abud and colleagues reported no complications in 60 patients who underwent endovascular PO of the ICA.39
Arat A, Islak C, Saatci I, et al. Endovascular parent artery occlusion in large-giant or fusiform distal posterior cerebral artery aneurysms. Neuroradiology. 2002;44:700.
Aymard A, Gobin YP, Hodes JE, et al. Endovascular occlusion of vertebral arteries in the treatment of unclippable vertebrobasilar aneurysms. J Neurosurg. 1991;74:393.
Charbel FT, Zhao M, Amin-Hanjani S, et al. A patient-specific computer model to predict outcomes of the balloon occlusion test. J Neurosurg. 2004;101:977.
Ciceri EF, Klucznik RP, Grossman RG, et al. Aneurysms of the posterior cerebral artery: classification and endovascular treatment. AJNR Am J Neuroradiol. 2001;22:27.
Drake CG. Ligation of the vertebral (unilateral or bilateral) or basilar artery in the treatment of large intracranial aneurysms. J Neurosurg. 1975;43:255.
Drake CG, Peerless SJ, Ferguson GG. Hunterian proximal arterial occlusion for giant aneurysms of the carotid circulation. J Neurosurg. 1994;81:656.
Eckard DA, Purdy PD, Bonte FJ. Temporary balloon occlusion of the carotid artery combined with brain blood flow imaging as a test to predict tolerance prior to permanent carotid sacrifice. AJNR Am J Neuroradiol. 1992;13:1565.
Eckert B, Thie A, Carvajal M, et al. Predicting hemodynamic ischemia by transcranial Doppler monitoring during therapeutic balloon occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 1998;19:577.
Giller CA, Mathews D, Walker B, et al. Prediction of tolerance to carotid artery occlusion using transcranial Doppler ultrasound. J Neurosurg. 1994;81:15.
Gralla J, Schroth G, Kickuth R, et al. Closing the gap between coil and balloon in the neurointerventional armamentarium? Initial clinical experience with a nitinol vascular occlusion plug. Neuroradiology. 2008;50:709.
Hodes JE, Aymard A, Gobin YP, et al. Endovascular occlusion of intracranial vessels for curative treatment of unclippable aneurysms: report of 16 cases. J Neurosurg. 1991;75:694.
Iihara K, Sakai N, Murao K, et al. Dissecting aneurysms of the vertebral artery: a management strategy. J Neurosurg. 2002;97:259.
Kai Y, Hamada JI, Morioka M, et al. Endovascular coil trapping for ruptured vertebral artery dissecting aneurysms by using double microcatheters technique in the acute stage. Acta Neurochir (Wien). 2003;145:447.
Liu AY, Lopez JR, Do HM, et al. Neurophysiological monitoring in the endovascular therapy of aneurysms. AJNR Am J Neuroradiol. 2003;24:1520.
Lorberboym M, Pandit N, Machac J, et al. Brain perfusion imaging during preoperative temporary balloon occlusion of the internal carotid artery. J Nucl Med. 1996;37:415.
Matas R. I. Testing the efficiency of the collateral circulation as a preliminary to the occlusion of the great surgical arteries. Ann Surg. 1911;53:1.
Mount LA, Taveras JM. Ligation of basilar artery in treatment of an aneurysm at the basilar-artery bifurcation. J Neurosurg. 1962;19:167.
Nishioka H. Results of the treatment of intracranial aneurysms by occlusion of the carotid artery in the neck. J Neurosurg. 1966;25:660.
O’Shaughnessy BA, Salehi SA, Mindea SA, et al. Selective cerebral revascularization as an adjunct in the treatment of giant anterior circulation aneurysms. Neurosurg Focus. 2003;14(3):e4.
Polevaya NV, Kalani MY, Steinberg GK, et al. The transition from hunterian ligation to intracranial aneurysm clips: a historical perspective. Neurosurg Focus. 2006;20(6):E3.
Ratnam LA, Walkden RM, Munneke GJ, et al. The Amplatzer vascular plug for large vessel occlusion in the endovascular management of aneurysms. Eur Radiol. 2008;18:2006.
Standard SC, Ahuja A, Guterman LR, et al. Balloon test occlusion of the internal carotid artery with hypotensive challenge. AJNR Am J Neuroradiol. 1995;16:1453.
van Rooij WJ, Sluzewski M, Slob MJ, et al. Predictive value of angiographic testing for tolerance to therapeutic occlusion of the carotid artery. AJNR Am J Neuroradiol. 2005;26:175.
Vishteh AG, Smith KA, McDougall CG, et al. Distal posterior cerebral artery revascularization in multimodality management of complex peripheral posterior cerebral artery aneurysms: technical case report. Neurosurgery. 1998;43:166.
Winn HR, Richardson AE, Jane JA. Late morbidity and mortality of common carotid ligation for posterior communicating aneurysms. A comparison to conservative treatment. J Neurosurg. 1977;47:727-736.
1 Polevaya NV, Kalani MY, Steinberg GK, et al. The transition from hunterian ligation to intracranial aneurysm clips: a historical perspective. Neurosurg Focus. 2006;20(6):E3.
2 Selverstone B, White JC. A new technique for gradual occlusion of the carotid artery. J Nerv Ment Dis. 1952;115:87.
3 McKissock W, Richardson A, Walsh L. Posterior-communicating aneurysms: a controlled trial of the conservative and surgical treatment of ruptured aneurysms of the internal carotid artery at or near the point of origin of the posterior communicating artery. Lancet. 1960;1:1203.
4 Crutchfield WG. Instruments for use in the treatment of certain intracranial vascular lesions. J Neurosurg. 1959;16:471.
5 Winn HR, Richardson AE, Jane JA. Late morbidity and mortality of common carotid ligation for posterior communicating aneurysms. A comparison to conservative treatment. J Neurosurg. 1977;47:727.
6 Mount LA, Taveras JM. Ligation of basilar artery in treatment of an aneurysm at the basilar-artery bifurcation. J Neurosurg. 1962;19:167.
7 Drake CG. Ligation of the vertebral (unilateral or bilateral) or basilar artery in the treatment of large intracranial aneurysms. J Neurosurg. 1975;43:255.
8 O’Shaughnessy BA, Salehi SA, Mindea SA, et al. Selective cerebral revascularization as an adjunct in the treatment of giant anterior circulation aneurysms. Neurosurg Focus. 2003;14(3):e4.
9 Park JH, Park IS, Han DH, et al. Endovascular treatment of blood blister–like aneurysms of the internal carotid artery. J Neurosurg. 2007;106:812.
10 Scott DA, Keston P, White P, et al. Vascular plug for ICA occlusion in cavernous carotid aneurysms: technical note. Neuroradiology. 2008;50:795.
11 Hoit DA, Schirmer CM, Malek AM. Use of the Amplatzer vascular plug as an anchoring scaffold for coil-mediated parent vessel occlusion: technical case report. Neurosurgery. 2006;59:ONSE171.
12 Gralla J, Schroth G, Kickuth R, et al. Closing the gap between coil and balloon in the neurointerventional armamentarium? Initial clinical experience with a nitinol vascular occlusion plug. Neuroradiology. 2008;50:709.
13 Fager CA, Poppen JL. Observations on controlled ligation of the internal carotid artery. Surg Clin North Am. 1956;36:567.
14 Poppen JL. Ligation of the internal carotid artery in the neck; prevention of certain complications. J Neurosurg. 1950;7:532.
15 Aymard A, Gobin YP, Hodes JE, et al. Endovascular occlusion of vertebral arteries in the treatment of unclippable vertebrobasilar aneurysms. J Neurosurg. 1991;74:393.
16 Cellerini M, Mangiafico S, Ammannati F, et al. Ruptured, dissecting posterior inferior cerebellar artery aneurysms: endovascular treatment without parent vessel occlusion. Neuroradiology. 2008;50:315.
17 Halbach VV, Higashida RT, Dowd CF, et al. Endovascular treatment of vertebral artery dissections and pseudoaneurysms. J Neurosurg. 1993;79:183.
18 Iihara K, Sakai N, Murao K, et al. Dissecting aneurysms of the vertebral artery: a management strategy. J Neurosurg. 2002;97:259.
19 Kai Y, Hamada JI, Morioka M, et al. Endovascular coil trapping for ruptured vertebral artery dissecting aneurysms by using double microcatheters technique in the acute stage. Acta Neurochir (Wien). 2003;145:447.
20 Drake CG, Peerless SJ, Ferguson GG. Hunterian proximal arterial occlusion for giant aneurysms of the carotid circulation. J Neurosurg. 1994;81:656.
21 Hodes JE, Aymard A, Gobin YP, et al. Endovascular occlusion of intracranial vessels for curative treatment of unclippable aneurysms: report of 16 cases. J Neurosurg. 1991;75:694.
22 Arat A, Islak C, Saatci I, et al. Endovascular parent artery occlusion in large-giant or fusiform distal posterior cerebral artery aneurysms. Neuroradiology. 2002;44:700.
23 Xavier J, Vasconcelos C, Cruz R, et al. [Endovascular treatment of dissecting aneurysms of the posterior cerebral artery.]. Acta Med Port. 2001;14:65.
24 Quisling SV, Mawn LA, Larson TC3rd. Blindness associated with enlarging mycotic aneurysm after cavernous sinus thrombosis. Ophthalmology. 2003;110:2036.
25 Watanabe A, Hirano K, Ishii R. Cerebral mycotic aneurysm treated with endovascular occlusion—case report. Neurol Med Chir (Tokyo). 1998;38:657.
26 Matas R. I. Testing the efficiency of the collateral circulation as a preliminary to the occlusion of the great surgical arteries. Ann Surg. 1911;53:1.
27 van Rooij WJ, Sluzewski M, Metz NH, et al. Carotid balloon occlusion for large and giant aneurysms: evaluation of a new test occlusion protocol. Neurosurgery. 2000;47:116.
28 Domaratskaya EI, Tsetlin VV, Bueverova EI, et al. Continuous gamma and neutron irradiation at low doses can increase the number of stromal progenitor cell (CFU-F) in mouse bone marrow. Adv Space Res. 2005;36:1334.
29 Standard SC, Ahuja A, Guterman LR, et al. Balloon test occlusion of the internal carotid artery with hypotensive challenge. AJNR Am J Neuroradiol. 1995;16:1453.
30 van Rooij WJ, Sluzewski M, Slob MJ, et al. Predictive value of angiographic testing for tolerance to therapeutic occlusion of the carotid artery. AJNR Am J Neuroradiol. 2005;26:175.
31 Eckert B, Thie A, Carvajal M, et al. Predicting hemodynamic ischemia by transcranial Doppler monitoring during therapeutic balloon occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 1998;19:577.
32 Kaminogo M, Ochi M, Onizuka M, et al. An additional monitoring of regional cerebral oxygen saturation to HMPAO SPECT study during balloon test occlusion. Stroke. 1999;30:407.
33 Keller E, Ries F, Urbach H, et al. [Endovascular balloon occlusion test of the internal carotid artery with increased hemodynamic monitoring for determination of circulatory reserve before planned carotid occlusion]. Rofo. 1996;164:324.
34 Liu AY, Lopez JR, Do HM, et al. Neurophysiological monitoring in the endovascular therapy of aneurysms. AJNR Am J Neuroradiol. 2003;24:1520.
35 Marshall RS, Lazar RM, Young WL, et al. Clinical utility of quantitative cerebral blood flow measurements during internal carotid artery test occlusions. Neurosurgery. 2002;50:996.
36 Donmez H, Mavili E, Ikizceli T, et al. Stroke secondary to aseptic meningitis after endovascular treatment of a giant aneurysm with parent artery occlusion. Cardiovasc Intervent Radiol. 2009;32:801.
37 Chang SD, Marks MP, Steinberg GK. Recanalization and rupture of a giant vertebral artery aneurysm after hunterian ligation: case report. Neurosurgery. 1999;44:1117.
38 Nishioka H. Results of the treatment of intracranial aneurysms by occlusion of the carotid artery in the neck. J Neurosurg. 1966;25:660.
39 Abud DG, Spelle L, Piotin M, et al. Venous phase timing during balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR Am J Neuroradiol. 2005;26:2602.