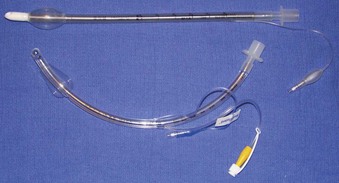
Chapter 47 Endotracheal Tube and Respiratory Care
II. Properties of the Endotracheal Tube
III. Endotracheal Tubes and Other Airway Adjuncts
IV. Proper Safeguarding of the Airway
V. Maintenance of the Endotracheal Tube
I Introduction
The earliest recorded use of airway manipulation with an artificial device dates back to early Roman civilization when Asclepiades performed a tracheostomy for laryngeal edema. Today it is clear that the role of the endotracheal tube (ETT) in medicine is as invaluable as that of any other medical device created to date. The establishment of a definitive airway via the ETT in both elective and emergency situations has allowed for the delivery of immediate life-sustaining therapies during resuscitation, the maintenance of oxygenation and ventilation in prolonged illness, and the (temporary) delivery of inhaled anesthesia.1 This chapter begins with a brief history of the development of the ETT. It describes the various ETTs available along with their indications for use and respective limitations. It reviews basic airway anatomy with regard to ETT placement, proper positioning and stabilization of the ETT, and complications attributed to its use. Finally, it addresses respiratory care of the intubated and mechanically ventilated patient.
II Properties of the Endotracheal Tube
A Anatomy of the Endotracheal Tube
Between the time of Asclepiades and the present, ETTs have been constructed of a variety of materials, including reed, brass, and steel. Eventually, in 1917, Magill and Rowbotham manufactured them from rubber for the purpose of administering anesthesia.2 In 1928, when Guedel and Waters added a protective cuff to prevent aspiration, the modern ETT was born. Rubber, however, had limitations in this application, such as increased stiffness with rising temperature and limited adhesive properties with different polymers, which required the cuffs to be manufactured from the same polymer as the tube.3 These shortcomings led to the search for alternative materials. In 1967, polyvinyl chloride (PVC) was popularized by Dr. S. A. Leader, and it has since been the material most commonly used. One property that makes PVC attractive is that it provides stiffness to an ETT at room temperature to assist with intubation yet becomes more malleable with the increased temperature in situ. Other properties include the ability to embed radiopaque lines in the material to assist with positioning and recognition on a radiograph. Because it accepts many materials, the addition of an exteriorized inflation line to connect the pilot balloon to the cuff can allow for varied cuff materials. Finally, it simply is much lower in cost than other available materials.3
The 15-mm adapter allows for universality between ventilating devices such as a bag-mask ventilation system, anesthesia circuit, or ventilator circuit. The adapter fits ETTs as large as 12 mm internal diameter and as small as 3 mm, thereby providing further commonality among multiple ETTs and ventilating devices. Having one standard size also allows for interchange between devices made for tracheostomies or ETTs. The adapter is removable to allow for passage of intraluminal devices (e.g., bronchoscope, suction catheter) or to allow passage of the ETT via a supraglottic airway device such as a laryngeal mask airway (LMA, LMA North America, San Diego, CA). Adapter removal may facilitate the extraction of extensive biofilm accumulation or mucus plugs. Additionally, some clinicians choose to resize (shorten) the ETT.4
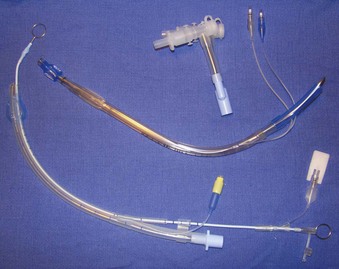
The cuffs on the early ETTs were, like the tubes themselves, composed of rubber. The rubber ETT cuffs had limitations such as the need for elevated inflation pressures (high pressure, low volume [HPLV]) to fill the cuff and occlude the airway surrounding the ETT. These high inflation pressures result in the transmission of high lateral pressures to the tracheal wall, albeit in a very minimal contact area, to maintain a seal. The trachea is not circular but rather D–shaped. HPLV cuffs inflate in a circular manner, thereby altering the structure of the trachea; the high pressure exerted on the tracheal wall impairs capillary pressure and possibly results in greater mucosal ischemia.5 The most commonly used HPLV cuffs in today’s practice are the reusable silicone ETTs found with intubating LMAs (ILMAs) (Fig. 47-1). Caution should be exercised when using these ETTs for prolonged periods, given their inherent risk of tracheal mucosal damage. The introduction of PVC-based cuffs reduced this problem because the cuff wall was more supple and thinner, allowing the cuff to accommodate high volume and low pressure (HVLP) and thus providing an adequate seal with lower lateral wall pressures.3,5,6 The main value of the HVLP cuff is its ability to conform to the irregular borders of the trachea.7–9 Polyurethane is even thinner and more pliable, with increased tensile strength, allowing for higher volumes, larger contact areas, and minimal mucosal pressures.10 Foam-based cuffs exist and provide maximal conformation to the tracheal walls, but they do little for the prevention of microaspiration.9
Remodeling of the cuff has been particularly driven by the desire to improve prevention of ventilator-associated pneumonia (VAP), and the shape of the cuff has also been altered. The Mallinckrodt TaperGuard Evac ETT (Covidien, Boulder, CO) is a new option that has been demonstrated in randomized, controlled trials to reduce microaspiration by as much as 83%, compared with traditional HVLP barrel shaped cuffs (Fig. 47-2).8 It is postulated that a barrel-shaped tube tends to wrinkle and fold in an attempt to conform to the tracheal wall, allowing small channels for potential microaspiration, whereas the bulbous, conical shape of the TaperGuard ETT may reduce wrinkling and thus decrease the incidence of microaspiration. Continued work in this area may lead to improved tracheal wall sealing capabilities at safe levels of pressure while minimizing potential pathways for the translocation of oronasal and gastric secretions, which is thought to be the prime etiologic pathway for VAP.11
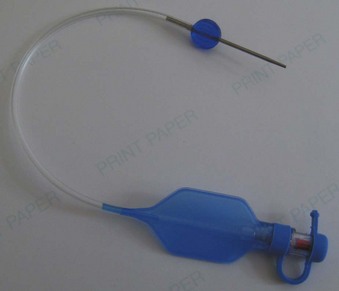
The pilot balloon of an ETT functions as an indirect volume gauge for the ETT cuff, relative to the amount of air located in the cuff (inflated or deflated). The pilot balloon does not provide information about the absolute volume insufflated or the pressure exerted on the tracheal mucosa. When a pilot balloon fails or is an impediment to an intubation, options are generally limited to a tracheal tube exchange.12–14 Pilot balloon failures have multiple causes. Shearing along the ETT connection (usually due to contact with dentition), cracked inflation valves (from syringe manipulation or trauma), material aging, and pilot tubing laceration due to biting all cause the ETT cuff to lose air over time.15–18 Simple techniques have been described to replace a pilot balloon in a variety of clinical situations using equipment readily available in the operating room. Needles or intravenous catheters with stopcocks or claves, epidural clamp connectors, and commercially available repair kits (Fig. 47-3) provide reliable substitutions for incompetent pilot balloons when they are connected to the pilot-cuff inflation line.19,20 The procedure for replacing an incompetent valve is as follows: cut the inflation tube distal to the pilot balloon; insert a needle or intravenous catheter into the cut end (or affix the hub of an epidural catheter to the cut end); and use a stopcock or clave (an item capable of stemming the entrance of air) on the needle or catheter after insufflation, paying careful attention not to overinflate.
B Development and Properties of the Endotracheal Tube
The purpose of the ETT has always been the same, and it has always had the same inherent problems. Technology continues to advance the standard ETT for improved function and decreased physiologic insult. Rather than compensate for the resistance produced by a rubber or PVC ETT, a newly designed ETT has been produced of ultrathin polyurethane that is reinforced with wire to resist collapsing and kinking. This wire is unique in that it has an elastic shape memory to prevent deformation. The internal diameter is increased without compromising the rigid shape of the ETT. The result is a tube with a resistance similar to that of the upper airway that is lighter, offers less airflow resistance, and, when compressed, forms an egg shape rather than an oval.21 Experimentally, this new design has been shown to decrease inspiratory and expiratory resistance by 60% each and the inspiratory, expiratory, and total work of breathing (WOB) by 70%, 47%, and 45%, respectively.22,23
The use of the ETT continues to expand. No longer is it expected to be simply a conduit for ventilation. As the technology has advanced, the original ETT has steadily been outfitted with a host of successful innovations to improve patient care, whether for convenience or necessity. For example, modifications to the cuff to improve occlusion of the trachea in an effort to prevent microaspiration, coupled with an extra subglottic suctioning port (and other patient care maneuvers), have served to vastly reduce the incidence of VAP.7–9 Another example is the modification of the surfaces of the ETT to minimize bacterial adhesion and thereby minimize biofilm accumulation.24 As for bells and whistles, there are ETTs with fiberoptic cameras distally, allowing for ease of placement and the possibility of continued intratracheal surveillance. Another example is the addition of multiple sensors, for so-called bioimpedance cardiography, that are capable of monitoring stroke volume variation, cardiac output, systemic vascular resistance, and arterial pressures (due to the close proximity of the ETT and the aorta) and thereby, at least theoretically, preventing the need for further invasive technologies. Continued study of these modifications may provide justification to adapt these technologies to patient care.
D Complications of Endotracheal Tube Placement
Complications associated with ETT placement should be grouped into three major subcategories: those that occur at intubation, those that occur with the ETT in situ, and postextubation sequelae.25 The problems associated with placement are numerous and can be worsened in emergencies, with multiple attempts, use of a variety of devices, or inexperience of the operator.26 Problems at placement include dental and oral problems, maxillofacial damage, displacement of the arytenoid cartilages, vocal cord ulceration or dysfunction, airway perforation, autonomic hyperactivity, and, of course, failed intubation. Structural damage is unlikely to be repaired until the patient no longer requires intubation, unless the damage interferes with ventilation and oxygenation. Problems that occur as a result of an in situ ETT include those related to the ETT, such as aspiration, vocal cord paralysis, or transient nerve palsy; ulceration and granuloma formation in the trachea and on the cords; tracheal synechiae; subglottic stenosis; laryngeal webbing; tracheomalacia; tracheoesophageal, tracheoinnominate, or tracheocarotid fistula; and recurrent and superior laryngeal nerve damage.27 Other complications are related to mechanical ventilation facilitated by the ETT and include aspiration, barotrauma (pneumothorax, pneumomediastinum), VAP, and dislodgement.28 Finally, postextubation complications can lead to long-term morbidity or the urgent need for reintubation. Many of the postextubation culprits have already been encountered as complications of ETT placement or presence, particularly subglottic stenosis, vocal cord injury, and hoarseness.28,29
III Endotracheal Tubes and Other Airway Adjuncts
A Choice of Endotracheal Tube Size
In selecting an ETT, consideration must be given to the functional reason for placement as well as patient-specific factors such as body height, gender, airway integrity, airway pathology, and previous airway manipulation or instrumentation. Theoretically, short-term placement for anesthesia should be different than placement for prolonged support with mechanical ventilation or for fiberoptic bronchoscopy to aid therapy. Generally, the trachea of an adult female accepts a tube of 7.5 to 8.0 mm and that of a male accepts one of 8.5 to 9.0 mm, but typically a 7.0-mm ETT is used for females and an 8.0-mm tube for males, at least in the United States. It is also generally accepted that an ETT of at least 8.0 mm is needed for competent use of an adult-based bronchoscopic investigation.30
1 Small Tubes and Airway Resistance
The physics of laminar gas flow through a conduit are described by the Hagen-Poiseuille equation, which reflects the relationship of resistance varying inversely with the fourth power of tube radius. Despite the fact that gas flow through ETTs is often turbulent rather than laminar, the effect on resistance to gas flow represented by each millimeter decrease in tube size is considerable, ranging from 25% to 100%.31 Airway resistance is affected by more than tube diameter: the presence of secretion within the tube, ETT kinking, and positioning of the head and neck can also increase the tendency for turbulent flow.32,33 The fundamental principle of which to be mindful is that airway resistance induced by an ETT is inversely proportional to the tube size—hence, the mantra that the largest tube is usually the best size.34
Airway resistance increases with decreasing ETT diameter, whether due to internal occlusion, smaller size, or external compression. As airway resistance increases, WOB also increases.31 The increase in WOB associated with a 1-mm reduction in ETT diameter varies in accordance with tidal volume and respiratory rate at a given minute ventilation and can range from 34% to 154%.31 When ventilation is controlled, the increase in WOB related to ETT resistance is seldom of any consequence, because it is overcome by ventilator adjustments. However, small-diameter tubes create greater difficulty for patients in weaning from ventilatory support due to the higher levels of resistance encountered when attempting to breathe spontaneously.35,36 It has been suggested that an inability to spontaneously ventilate due to the increased WOB imposed by a 7-mm ETT might indicate that extubation will fail regardless of tube size.37,38
Increased airway resistance associated with a smaller-diameter ETT may also be associated with inadvertent PEEP. Patients with high oxygen consumption, increased carbon dioxide production, or ventilation-perfusion relationships that produce high dead space ventilation often require higher minute ventilations to achieve appropriate ventilation and oxygenation. The gas flows necessary to maintain such a minute ventilation are also quite high, and the resistance imposed by a smaller-diameter ETT further prohibits the completion of expiratory flow before initiation of the subsequent inspiration. This breath stacking results in air trapping and unwanted PEEP, magnifying the risk of mechanical ventilation because barotrauma and subsequent intrathoracic overpressure could result in circulatory compromise.39
The restriction to gas flow through any ETT increases dramatically when devices such as a suction catheter or bronchoscope are placed in the lumen. The cross-sectional area of the tube is effectively reduced by an amount equal to the cross-sectional area of the device inserted into the tube. The limitation of gas flow has consequences for both the inspiratory and expiratory phases: inspiratory flow may be inadequate to maintain oxygenation and ventilation during the procedure, and retarded expiratory flow may lead to overdistention resulting in barotrauma or circulatory compromise.40
2 Large Tubes and Trauma
Whereas smaller-diameter ETTs have disadvantages related to gas flow and airway resistance, larger tubes are more frequently associated with traumatic placements and damage to both the laryngeal structures and the tracheal mucosa.37,38,41 Larger ETTs are associated with a higher incidence of sore throat after general anesthesia, compared with smaller-diameter tubes, but this difference is relatively negligible with long-term intubation.42 With prolonged intubation, laryngeal trauma is more likely. Women, because of the inherently smaller size of their airway, are more susceptible to injury than men.43,44
Laryngeal structures at particular risk for trauma are the arytenoid cartilages and the cricoid cartilage. Trauma results not only from the shape discrepancy between the round ETT and the angular, wedge-shaped glottic opening but also from direct contact and pressure on these structures and from repetitive tube movement, which leads to ulceration or erosion of the protective mucosa.44–46 Tracheal mucosal injury can also occur because of the irregular surfaces created by wrinkling and folding of the ETT cuff or the externalized pilot tube used to fill the ETT cuff. If the tracheal lumen is “overcrowded,” airway injury is more likely to occur when large tubes are used and little cuff volume is required to seal the airway.47
B Potentially Beneficial Alternatives to the Standard Endotracheal Tube
1 Preformed and Reinforced Tubes
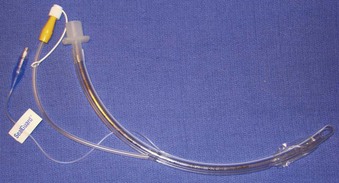
Rigid, preformed tubes such as those developed for long-term use in tracheostomy were known to maintain their patency despite the need for angulation. Preformed tubes have been developed for specific application in anesthesia practice as well. The Ring-Adair-Elwyn (RAE) tubes (Mallinckrodt Inc., Pleasanton, CA), both oral and nasal (Fig. 47-4), maintain a fixed contour similar to the average facial profile, allowing for head and neck surgery while minimizing surgical field interference. Their contour also reduces the risk of pressure injury to the posterior pharynx when repositioning is desired. The intra-airway length is tied to the size of the ETT, with a relatively appropriate depth based on the average size of a patient for whom the tube might be selected.48,49
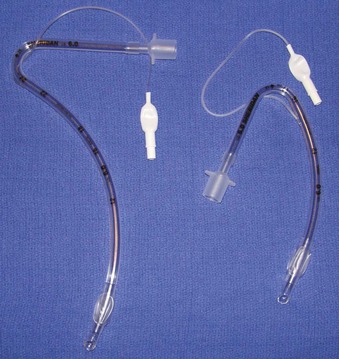
Figure 47-4 Nasal (left) and oral (right) Ring-Adair-Elwyn (RAE) preformed endotracheal tubes.
(Courtesy Covidien, Mansfield, MA.)
The embedded wire concept of the armored ETT has also been developed for long-term tracheostomy use. Although the armored tracheostomy tube is not free of risks, one advantage is that its flexibility allows its length and intratracheal depth to be adjusted, which may be beneficial if tracheomalacia at the level of the cuff develops.50 These tracheostomy tubes are also popular for use in morbidly obese patients, in whom, because of the depth of tissue, preformed tracheostomy tubes may not have the shape required to fit an individual patient. One major consequence of this type of reinforced ETT may occur when external pressure is applied to the wire-reinforced component (i.e., by patient biting). Once a compression threshold is reached, the luminal support provided by the wire may be compromised, and a permanent, irreparable dent remains that can significantly endanger ventilation and suctioning capabilities.
2 Laser Tubes
Progress in laser technology has advanced surgical capabilities, particularly for airway surgery. To protect patients and health care providers from laser-induced injury to eyes and airways, special precautions are required. Fire is the most serious danger associated with the use of lasers in the operating room, especially when a laser is used in airway surgery.30,51–53 A major complication related to the use of lasers for laryngeal surgery is ignition of the ETT.54 The laser beam may ignite the tube by direct penetration or indirectly if burning tissue is inhaled into the tube.30,51,52,55 The ease of ignition is related to the ETT material, the concentration of oxygen in use, and any other adjunctive materials or gases that could support combustion.30,52,55 Most ETTs are constructed of PVC, which is highly flammable. Ideally, PVC tubes should not be used for airways when a laser is employed.51,55,56
ETTs can be laser-proofed or protected from the laser beam by wrapping them with either reflective metal tape or muslin. Ideally, they should be constructed from noncombustible materials. In particular, the ETT cuff is vulnerable to puncture by the laser beam and should be filled with saline or water, which allows more energy to be absorbed before disruption.30,52,55 One trick to enhance appreciation of a penetrated, defective cuff, is to place a dye indicator, such as methylene blue, into the solution that is instilled into the cuff. Any leakage will clearly mark the airway and alert the provider to the potential dangers.57 Protecting the tube from the laser beam by wrapping it with a foil tape has proved effective (commercial devices are available) (Fig. 47-5).58 Tubes made of materials such as metal and silicone and those with special double cuffs also reduce the risk of airway fires and injury during laser airway surgery.51,58
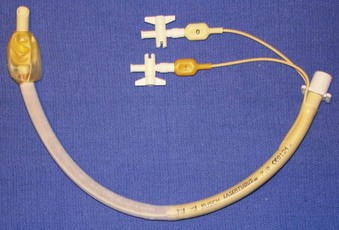
3 Subglottic Suctioning Evac Endotracheal Tubes
Hospitalized patients who require mechanical ventilation are susceptible to the development of aspiration pneumonia. VAP is known to increase hospital length of stay, health care costs, and mortality.59 Organisms that grow in pooled subglottic secretions above the inflated cuff of the ETT, but beneath the glottis, have previously been unmeasurable with any reliability and are now demonstrated to be a major impetus for VAP. Several nursing care measures may be taken to reduce the incidence of VAP caused by this route, including improved oral care, patient positioning by elevating the head of the bed past 30 degrees, frequent suctioning, and ensuring postpyloric tube feedings, but none of these measures completely stops the production.60
The presence of these pooled collections has led to the development of specific ETTs that possess a dedicated suction system capable of emptying this area of debris. Drainage of subglottic secretions has been shown to prevent VAP.61–64 The currently available subglottic drainage ETTs have a suction lumen that opens on the external (posterolateral) surface of the ETT immediately above the cuff (Fig. 47-6). The lumen is attached to constant or intermittent suction for active drainage of the space. Although these ETTs are beneficial, their efficacy is not 100%, and therefore all of the aforementioned nursing care actions remain vital to good hygiene and prevention of VAP. The subglottic drainage tubes have been further developed to include variations in cuff construction (materials, shapes, volumes, locations) that help to prevent aspiration of the subglottic debris.
Subglottic secretions are not the only recognized cause for VAP. Biofilm is an accumulation of debris adhered to the internal circumference of the ETT that is composed of tissue, secretions, mucus, and undetermined bacteria load. Biofilm can be aspirated, leading to a nidus for infection or causing an area of obstruction to airflow. Biofilm removal and reduction by hygiene care are currently better researched than prevention. However, there is a growing interest in the reduction of biofilm through construction of ETTs impregnated with antimicrobial agents.65–67 The ability of such developments to affect the incidence of VAP has not yet been proved.
4 Double-Lumen Endotracheal Tubes
The uses of a double-lumen endotracheal tube (DLT) (Fig. 47-7) can be separated into relative and absolute indications. The absolute indications are isolation, to avoid soilage or contamination of the contralateral lung tissue when dealing with infections or frank hemoptysis from a unilateral location, bronchoalveolar lavage, and one-lung ventilation (OLV). The most common reason for placement is OLV for surgical exposure, but OLV can also be important in cases of bronchopleural or bronchocutaneous fistula, unilateral pulmonary hemorrhage, giant unilateral bulla or cyst, and severe unilateral ventilation-perfusion mismatch. The relative indications all deal with surgical exposure. Complementing the DLT as another option for lung isolation, particularly if a DLT cannot be placed, are bronchial blocking devices. However, the DLT has an advantage because of the ability to pass suctioning catheters or fiberoptic devices into the area on collapse without drastically jeopardizing OLV or contaminating the contralateral side.
Relative contraindications to the placement of a DLT are fairly minimal. They include patient refusal (likely due to risk of trauma secondary to the large size), a known difficult airway, and the speed with which an isolated airway must be established. In patients with difficult airways, specially designed airway exchange catheters (Cook Medical, Bloomington, IN) (Fig. 47-8) can be used after placement of a conventional ETT to facilitate DLT placement. Additionally, some makers of video laryngoscopic technologies have developed specific DLT devices. The time required for placement is usually the biggest detractor to their use. Situations such as frank hemoptysis may be better served by a rapid-sequence induction and placement of a contralateral, main stem, single-lumen ETT for stabilization.
5 Supraglottic Airways
Supraglottic devices are continually coming onto the market. Largely designed for shorter surgical procedures to deliver general anesthesia, their use seems to be growing past their original intent. Supraglottic airways (Fig. 47-9) such as the LMA, the esophageal-tracheal Combitube (ETC, Tyco Healthcare, Mansfield, MA), the King LT (King Systems, Noblesville, IN), and other variants have provided valuable means of establishing an (unsecured) airway in an emergency. Equally important is their ability to function as a conduit for endotracheal intubation. For example, the ILMA is a blind passage device that is designed to place an ETT through the LMA. Recently, use of the LMA as a conduit for fiberoptic bronchoscopy and subsequent ETT placement has also been demonstrated in emergency situations.68 Although these devices do not provide classically definitive airways, their utility is unsurpassed in helping to manage the difficult airway.
IV Proper Safeguarding of the Airway
A Airway Evaluation: Predicting the Difficult Airway
However, a difficult airway should be assumed when one approaches the patient outside the controlled setting of the operating room. An airway physical examination is paramount to predicting a successful attempt. Criteria such as dental status, mouth opening, thyromental distance, cervical range of motion, Mallampati score, and neck circumference are all standard examination points. However, the emergent intubation presents a host of new, potentially detrimental issues not seen in operating room intubations. For example, hemodynamics may not allow for the controlled process typically seen in the operating room. Trauma patients may have actively unstable facial fractures or cervical vertebral injuries that merit inline stabilization and modified techniques for tube placement. Neurosurgical patients may have external fixators to stabilize injuries or intracranial monitoring devices that make it difficult to position the head. The type of bed a patient is in can also create access issues with intubation. A bariatric bed with an inflatable mattress can be very difficult to properly ramp, thereby making positioning suboptimal. Last, but not least, is the patient with failed extubation who must be reintubated. Issues with anxiety, hypoxemia, decreased functional residual and closing capacities, copious secretions, residual airway edema, and subglottic stenosis all make a repeat attempt more difficult than the first pass.28
B Identifying Proper Position of the Endotracheal Tube
1 Detection of Esophageal Intubation
Once the clinician has deemed intubation necessary and has performed the intervention, confirmation of proper ET placement must be provided expeditiously. An incorrectly positioned ETT can produce adverse effects, especially in an already apneic patient. Unrecognized esophageal intubation can have disastrous consequences with a reported incidence as high as 8% in critical patients.69 Therefore, a brief discussion of verification of ETT placement is warranted.
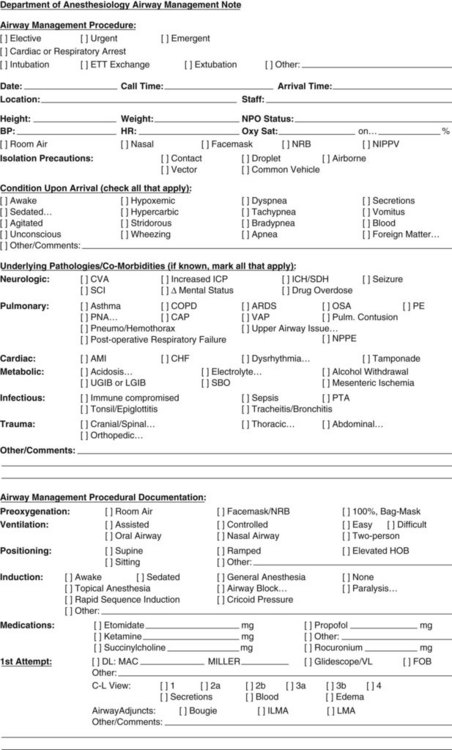
Once intubation has been accomplished, confirmation of proper placement of the ETT in the trachea needs to be achieved, ideally by the detection of end-tidal carbon dioxide (EtCO2) in expired gases using capnography or other capnometric (colorimetric) methods.70 The detection of EtCO2 is not fail-safe and does not guarantee that the ETT is positioned within the tracheal lumen (e.g., the tip may lie above the vocal cords). After three to five breaths, the absence of EtCO2 suggests a nontracheal placement. Blockage or soilage of the EtCO2 detection device can hamper efforts. EtCO2 detection should be complemented with indirect maneuvers such as auscultation, ETT misting or fogging, bag compliance, chest wall excursions, lack of phonation, and improved oxygen saturation. The dependence of any EtCO2 detection device on adequate cardiac output has spurred utilization of an esophageal detector device (Fig. 47-10). It is essentially an air-filled bulb placed on the end of an in situ ETT. After a vacuum has been created by squeezing the bulb, immediate re-expansion should occur when the bulb is placed on an ETT in the trachea (any column of air). If the ETT is esophageal, the suction created by the bulb will draw the pliable esophageal tissue into the distal lumen of the ETT, preventing full expansion of the bulb on top of the ETT (column of soft tissue).71–73
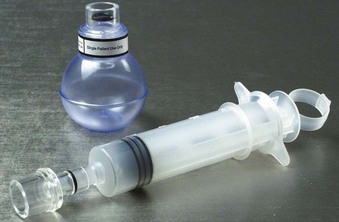
Figure 47-10 Esophageal bulb detector and a homemade syringe device.
(Courtesy Wolfe-Tory Medical, Inc.)
If intubation is to occur in a patient with cardiac arrest, capnometry may be fallible given the amount of down time and the lack of any life-sustaining cardiac output. If cardiopulmonary resuscitation (CPR) is adequate, this technicality is likely to be moot. However, esophageal bulb detectors can be helpful in this situation, although false-positives and false-negatives do occur and confound ETT verification. Still other methods that are independent of carbon dioxide and cardiac output exist, such as a tracheal whistle to verify correct placement (Box 47-1), but not one is without limitations.72–77 Ideally, two methods are to be considered fail-safe: direct or indirect visualization of the ETT traversing the vocal cords and fiberoptic verification via the ETT lumen. The limitation of direct laryngoscopy is the operator’s line of sight to the glottic opening. The key is to identify the ETT positioned in the glottis, not simply to see it go in. An indirect method such as video laryngoscopy does improve the validity of ETT passage. Fiberoptic verification is hampered by equipment availability at the bedside, any airway or ETT soilage, time constraints, and operator skill. Chest rise and condensation from expired gas found in the ETT have also been used, but these signs may occur in esophageal intubations as well.77 Therefore, the gold standard used today is the presence of EtCO2 in expired gases as demonstrated by capnography or other colorimetric techniques.70
Box 47-1
Methods Used to Verify Endotracheal Tube Placement
Sustainable, exhaled CO2 by capnography or colorimetric methods
Fiberoptic visualization of carina
Videolaryngoscopic visualization of translaryngeal endotracheal tube position
Direct laryngoscopic visualization of translaryngeal position
Positive response with esophageal bulb detector device or syringe method
Auscultation of breath sounds in bilateral lung fields and absence of same in epigastric area
Visualization of chest wall movement with spontaneous patient efforts
Reservoir bag synchrony with spontaneous patient efforts
Palpable ballottement of cuff in suprasternal notch
Condensation in endotracheal tube
Lack of phonation in the nonparalyzed, semiconscious patient
Bougie passage to distinguish between tracheobronchial tree and esophagus
Capnography yields quantifiable measurements of inspired and expired gases in addition to a waveform generated with each tidal volume. Although not without limitations (e.g., lack of portability, need for a power source), capnography reliably identifies initial proper placement of the ETT (nonesophageal) and provides a continuous verification of ETT security. If esophageal intubation has occurred, a gradual reduction in height of the capnograph waveforms is observed with successive breaths. False-positive results during esophageal intubations may occur in situations of ingested CO2-containing or -liberating substances (e.g., carbonated beverages) before intubation, bag-mask ventilation with inflation of expired air into the stomach, or intubation of the supralaryngeal hypopharynx.78 Inappropriate extubations may occur due to misinterpretation of a false-negative situation because an EtCO2 waveform is lacking despite proper placement. This error may occur with unrecognized circuit disconnections, an obstructed or kinked ETT, a disconnected or contaminated gas sampling line (water, secretions, entrainment of room air), equipment failure, severe bronchospasm, or inadequate cardiac output. Unquestionably, capnography is dependent on pulmonary blood flow. In the absence of perfusion (e.g., cardiac arrest), the utility of capnography can be limited; however, even in very low-flow states (e.g., CPR, separation from cardiopulmonary bypass), it has been shown to provide effective detection. Ornato and colleagues used an animal model to evaluate the relationship between cardiac output (CO) and EtCO2. Through manipulation of CO, with inotropes or controlled hemorrhage, a logarithmic relationship between CO and EtCO2 was demonstrated.50 This finding shows that capnography is useful in cardiac resuscitation to assist with evaluation of low-flow states and adequacy of perfusion.
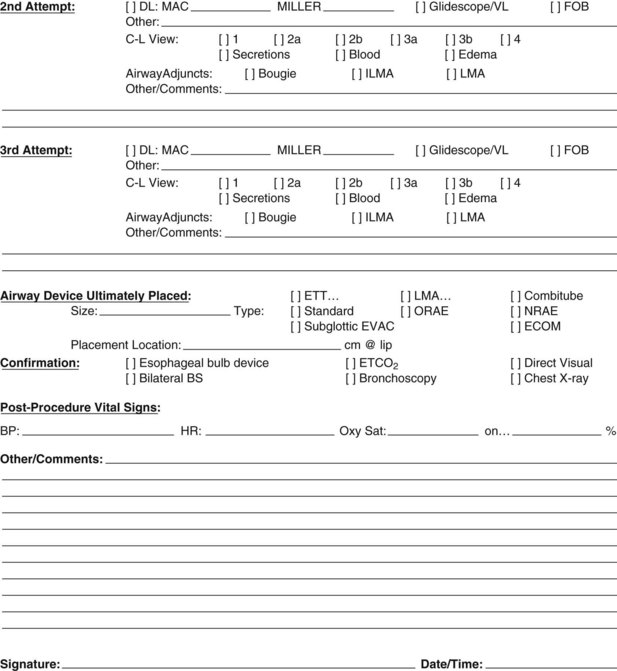
To alleviate the logistic concerns with capnography in emergency situations—mainly the lack of portability and the need for a power source—a portable and reliable means of detecting EtCO2 was developed. Colorimetric EtCO2 uses a detector impregnated with metacresol purple (Fig. 47-11). This indicator is pH sensitive and changes color, from purple to yellow, in the presence of CO2. The devices are disposable, attach between the ETT and the circuit or bag, and provide a rapid and reliable indication of CO2 concentration on a graded scale: A (purple) corresponds to an EtCO2 level of 0.5%, B (tan) to a level of 0.5% to 2%, and C (yellow) to a level greater than 2%.70,77 Limitations of this method are that it is ineffective with exposure to humidified gases, vomitus, or secretions and in cases of prolonged cardiac arrest or low-perfusion states. False-positive results can occur as well, just as in capnography. Delays in recognition of esophageal intubation with colorimetric capnometry have been reported far more often than with capnography, particularly in patients with prolonged bag-mask ventilation or ingestion of EtCO2-containing substances before intubation. Therefore, capnography simply is the best method for detection of esophageal intubation.79

Figure 47-11 Example of a colorimetric capnometric device.
(Courtesy Mercury Medical, Clearwater, FL.)
As mentioned previously, the esophageal bulb detector capitalizes on the physical characteristics of the esophagus, which, unlike the trachea, collapses when negative pressure is applied. Commercial devices exist, but a homemade version can be improvised using a syringe that attaches to the end of the ETT. When the plunger is withdrawn, resistance is appreciated if an esophageal intubation has occurred, because the walls of the esophagus collapse around the ETT. Unencumbered aspiration of the plunger occurs with proper tracheal placement.71 Bulb detector devices are reported to be reliable and effective, with one study demonstrating a sensitivity of 100% and a specificity of 99%.72 However, other studies have reported limitations and false-negative results in patients with copious or aspirated secretions, gastric distention, vomitus in the airway, morbid obesity, or reduced functional residual capacity.71,80–83
2 Confirmation of Appropriate Depth of Insertion
After correct tracheal placement of the ETT has been verified, it is imperative to identify the correct depth of the ETT to ensure adequate ventilation of each lung.49 Before addressing the various methods that assist in confirmation of appropriate ETT depth, a brief discussion of what is considered to be the correct depth is warranted. Malpositioning of ETTs occurs frequently, with unrecognized right main stem intubation occurring in approximately 4% of chest radiographs.49,69,84 The generally accepted depth of insertion of the ETT is between 2 and 7 cm above the carina, optimally between 4 and 7 cm above the carina with the head and neck in a neutral position.49,77,85–87 It is important to realize this, because flexion-extension movements of the neck can displace the ETT upward or downward with resultant extubation or main stem intubation, respectively. Typically the right main stem bronchus is entered, given its straighter trajectory in relation to the trachea. If endobronchial intubation remains undetected, an inadvertent hyperinflation of the ipsilateral lung can occur with subsequent pneumothorax and concomitant atelectasis of the hypoventilated contralateral lung. Indeed, it has been reported that up to 15% of chest radiographs reveal malpositioned ETTs in intubated patients.84,88,89 This is more frequently seen after difficult airway management.
In the operating room, chest radiographs are not used to confirm proper position; rather, indirect clinical assessment methods are used (i.e., auscultation of bilateral breath sounds, visualization of equal chest expansion, and direct visualization of the ETT tip placed just below the vocal cords). The ETT is also manufactured with distance measurements to aid with the depth of insertion. In orally intubated patients, a depth of 23 cm at the teeth or corner of the mouth has been advocated for men and 21 to 22 cm for women.90,91 In nasotracheal intubations, a depth of 26 cm at the nares in women and 28 cm in men should be sufficient for proper tracheal position.92 Other methods used include direct visualization of the ETT tip in reference to the carina with a fiberoptic scope or catheter,69 transtracheal illumination, and ballottement of the ETT cuff in the suprasternal notch.93–96
3 Cuff Pressure Monitoring
Probably the most often overlooked parameter in daily airway care is cuff pressure.97,98 Almost universally, this measurement is neglected in operating room intubations. However, it is well documented that excessive forces applied to the tracheal mucosa can cause necrosis and ulceration.9,99,100 Cuff pressures of 30 cm H2O for 4 hours have been shown to damage ciliary motility for at least 3 days.100–102 In addition, animal studies have revealed diminished circulation in the tracheal mucosa with a pressure of just 20 cm H2O, exaggerated greatly in the presence of hypotension.103
Normal occlusive pressures should be between 20 and 30 cm H2O to avoid complications while maintaining an adequate seal in the tracheal lumen circumferentially around the cuff to prevent microaspiration and ultimately VAP.100 Depending on the type of cuff used, the same pressure range seems to be effective at accomplishing this task both in vitro and during in vivo animal studies. For example, standardized HVLP cuffs have been demonstrated to be ineffective at preventing microaspiration with pressures as high as 60 cm H2O,7 whereas polyurethane tubes seem to be effective down to 15 cm H2O.7,10,104
As was previously discussed, ETTs are not without risks. However, it appears that most of the morbidity is related to either inappropriate inflation of an ETT cuff or a defective ETT cuff.98–100 An often dreaded scenario that leads to increased morbidity is frequent ETT exchanges for suspected cuff leaks. Trended values for cuff pressure could help to alleviate some of these unnecessary procedures. Most importantly, it has been demonstrated that manual palpation of the cuff or instillation of a standard volume of air often underestimates the actual occlusive pressure delivered, leading to unrecognized complications.97,98,100 Therefore, it is recommended that frequent examination of the cuff pressure be documented and trended using manometry.
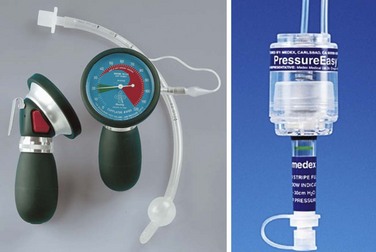
Reusable aneroid manometers can be onerous to calibrate and are often difficult to locate; in addition, they pose a recurring risk of cross-contamination for each patient. The manometers currently in use in most ICUs provide only a single data point at the time of collection. A commercially available disposable, precalibrated device that constantly measures airway cuff pressures is available. The PressureEasy device (Smiths Medical, St. Paul, MN), among others, attaches to the pilot balloon and exhibits a mark in a fixed window when the measured cuff pressure is in the optimal range of 20 and 30 cm H2O (Fig. 47-12).97–100
4 Evaluation of an Audible Cuff Leak
The various sources of a suspected cuff leak require investigation because each necessitates a different solution. Any one solution, incorrectly applied, may lead to morbidity or mortality. For example, a cuff leak resulting from a herniated cuff above the vocal cords may prompt the team to order a chest radiograph. Further displacement may take place during lifting and repositioning of the patient for the examination. Conversely, if an ETT is malpositioned in the hypopharynx (intact pilot balloon) but is assumed to be a properly positioned ETT with a cuff perforation, an exchange catheter passed via the ETT (presumably into the trachea) may exit the supraglottic ETT and enter the esophagus, leading to airway compromise. Table 47-1 highlights the possible causes and potential solutions, with relevant risk assessments for an apparent cuff leak evaluation.
| Problem | Solution | Risk Level |
|---|---|---|
| Cuff perforation (pilot balloon deflation) | Exchange ETT (only feasible choice) | High |
| Incompetent pilot valve | Exchange ETT | High |
| Clamp line (Kelly, hemostat)—short term solution | Low | |
| Place stopcock or cap on valve | Low | |
| Replace pilot balloon–line assembly | Low | |
| Broken pilot line | Exchange ETT | High |
| Clamp line (Kelly, hemostat)—short-term solution | Low | |
| Replace pilot balloon line assembly (homemade vs. commercial) | Low | |
| Displaced ETT (intact pilot balloon) | Perform fiberoptic evaluation—diagnostic | Low-moderate |
| Blindly advance ETT | Very high | |
| Videolaryngoscopy evaluation | Low-moderate | |
| Blindly pass airway exchange catheter | High | |
| Perform direct laryngoscopy (suboptimal line of sight) | Low-moderate |
ETT, Endotracheal tube.
5 Documentation of Placement
As an ICU course progresses, the details surrounding the original ETT placement may prove less relevant because the patient’s clinical status and airway are dynamic, not static. A previously easy airway may remain so, but difficulty often increases as edema, trauma, secretion management, and patient status decline to confound an airway intervention. Anatomic abnormalities may be hidden or exaggerated by excessive fluid administration or a capillary leakage phenomenon in the critically ill patient, and examination of the airway may become impossible. Appreciation of the airway adjuncts that have been attempted is imperative for the incoming airway team. Immediate availability of pertinent historical airway management details may prove helpful in delivering better care. Documentation of airway interventions and procedures on a specific sheet (Fig. 47-13) may provide the incoming airway team perspective on what to expect in a single concise location.
C Stabilization of the Endotracheal Tube
After verification and confirmation of proper tracheal placement and position have been achieved, care should be focused on securing the ETT in its proper position, and frequent assessments should be made to recognize malpositioning.105,106
Securing and surveillance of the ETT are important not only to ensure proper depth and positioning but also to reduce the incidence of inadvertent extubation.105 Unplanned extubation is primarily a problem in the ICU. It has a reported incidence of approximately 2% to 16%, with 80% of those extubated requiring reintubation, and contributes to a higher rate of airway-related complications, hemodynamic alterations, patient morbidity, and mortality.48,107–109 The most frequently identified cause is inadequate sedation of a mechanically ventilated patient, and only a few studies have specifically addressed techniques used to secure ETTs. In a study comparing four such techniques, Levy and Griego concluded that the use of simple adhesive tape split at both ends and secured to both the ETT and patient’s face was more effective than proprietary methods and allowed more effective nursing care, improved oral hygiene, and greater comfort for the patient.106 However, Barnason and colleagues found no statistical difference between two methods studied in preventing unplanned extubation, allowing oral hygiene, or maintaining facial skin integrity.110
Attention to proper ETT stabilization primarily focuses on the reduction of unplanned extubation, improved comfort for the patient with prolonged ventilatory requirements, minimization of iatrogenic complications related to the method of fixture, and ease of nursing and respiratory care. One study addressed massive air leaks and contributing factors. The authors defined a massive air leak as one that requires extubation. Over a 2-year period, 18 ETTs were removed for massive air leaks, of which 61% were found to be free of mechanical defects (intact cuff). Fourteen of the 18 patients required reintubation; 2 of them aspirated gastric contents on replacement, and 1 suffered severe epistaxis from a blind nasal reintubation, resulting in a 21% complication rate.111 The authors concluded that malpositioning was the most plausible explanation for the apparent air leaks. This study reinforces the importance of securing the ETT and daily vigilance to ensure that proper depth and positioning are confirmed and maintained.
Despite these studies, no consensus exists concerning the best method of securing the ETT.
1 Taping
The classically described methods are a “barber’s pole” technique for operating room intubations, a simple split tape technique (Fig. 47-14), and a more secure “four-point” technique for intubations anticipated to last for 24 hours or longer. In each of these methods, tape is used to anchor the ETT to the face. Moisture, in the form of sweat, secretions, or vomitus, can jeopardize the integrity of the bond between adhesive and skin, putting the security of the ETT at risk.105 Additionally, patient comfort is an issue when tape is used for this purpose. Skin breakdown due to allergic reactions, pressure necrosis, or repetitive trauma has been documented as a potential problem from the use of this method. The four-point method is more secure than the barber’s pole technique because the tape encircles the patient’s head and is not as susceptible to moisture because it is anchored to itself as well as to the patient’s skin.
2 Commercially Available Devices
Although several options are available, a proprietary device has been developed to help secure the ETT and provide increased patient comfort while facilitating airway care. This device, the AnchorFast (Hollister Inc., Libertyville, IL) (Fig. 47-15), incorporates an ergonomically designed frame to minimize well-known pressure points, a latex-free adhesive on a pad, and a padded Velcro-style retaining strap that encircles the head. Preliminary studies comparing this device against classic adhesive tapes showed reduced skin breakdown, fewer lip ulcers, and improved patient comfort.106,112
D Rapid Response Cart for Airway Emergencies
Areas in which care for intubated patients is provided need to have a dedicated set of supplies to establish emergency airways in the event of primary respiratory failure or cardiac arrest to reestablish lost, previously secured airways. The responding airway team must be knowledgeable and competent in advanced management techniques and provided with immediate access to such equipment. Infrequent or casual airway managers should ask for assistance early in the management process or even immediately, before starting. The American Society of Anesthesiologists difficult airway algorithm (Fig. 47-16) should be close at hand to guide less experienced individuals who encounter problems.113 Supplies on the cart may differ based on personal preference and facility purchase, but there are some items that must be present to help save a life.
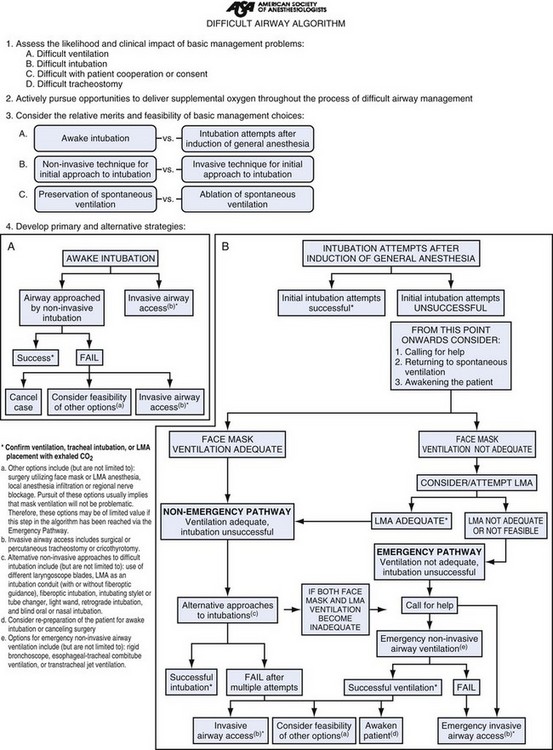
Figure 47-16 American Society of Anesthesiologists difficult airway algorithm. LMA, Laryngeal mask airway.
(Courtesy of American Society of Anesthesiologists, Park Ridge, IL.)
Laryngoscopy, ETT exchange, pulmonary toilet, and a “cannot intubate, cannot ventilate” (CICV) scenario must all be covered in the equipment selection. Laryngoscopy includes both conventional (direct) and video laryngoscopy; it is used to assist the team with replacing the ETT after an inadvertent extubation, augmenting the exchange of a damaged ETT over an airway exchange catheter, improving the ability to assess ETT location within the airway, and interrogating the physiologic integrity of an airway slated for extubation.114
An airway exchange catheter, coupled with video laryngoscopy, greatly reduces the complication rates for necessary airway exchanges in ICU patients, thereby earning its place in an airway response cart within the ICU.114 A fiberoptic bronchoscope should be available for confirmation purposes, for awake intubation procedures, and for deeper pulmonary toilet needs to improve ventilation and oxygenation.115 Supraglottic airway devices should be available for situations in which mask ventilation is difficult or as a conduit to facilitate the placement of an endotracheal airway.116 Lastly, equipment for the placement of a surgical airway must be provided. Despite all of the options available, a scalpel may be the only device capable of establishing the airway. In cases of a known or suspected difficult airway, it is helpful to immediately contact someone with the ability to place a surgical airway should other conventional or advanced noninvasive means fail.
Box 47-2 delineates the contents of a proposed difficult airway cart, which should be readily available in case of any airway catastrophe.
Box 47-2 Contents of a Difficult Airway Cart
Fiberoptic bronchoscope and video monitoring system (optional), adult and pediatric sizes
Ovassapian and Williams airways
Video laryngoscopy (various products available) blades, sizes for neonate to large adult
Direct laryngoscopy Miller and Macintosh blades, sizes 1-4
Intubating laryngeal mask airways, sizes 3-5 (or equivalent)
Standard laryngeal mask airways, sizes 3-5 (or equivalent)
Esophageal-tracheal Combitube (or King LT or EasyTube by Rüsch)
Gum elastic bougies (various manufacturers)
Airway exchange catheters, multiple sizes
Endotracheal tubes, sizes 6-9, standard and Evac types (pediatric sizes if appropriate)
Cricothyroidotomy kit (purchased or hospital standardized)
Retrograde intubation kit (Cook Critical Care)
Medications kit (etomidate, propofol, ketamine, succinylcholine, rocuronium, intravenous and viscous lidocaine, atropine)
V Maintenance of the Endotracheal Tube
The presence of the ETT bypasses the host defenses of the upper airway, eliminates the humidification of inspired gases, increases the WOB, limits the administration of medications, and prevents prophylactic oral hygiene. All of these changes promote bacterial colonization, inflammation, and sputum production. Inability to actively clear secretions because of a poor cough or increased difficulty of passive removal by health care personnel can lead to plugging of proximal and distal airways as well as the ETT. These retained secretions may result in the formation of atelectasis, ventilation-perfusion mismatching in the form of a shunt or dead space, hypoxemia, and increased respiratory load, thereby prolonging the duration of mechanical ventilation.117,118 Therefore, aggressive respiratory care must be provided for the intubated patient to avoid these complications and further morbidity.
A Heat and Humidity of Inspired Gas
During normal breathing, the air is delivered to the carina at a temperature of 32° C and an absolute humidity of 30.4 mg H2O/L.119 The insertion of an ETT via the nose, mouth, or trachea bypasses the upper airway and causes the natural ability to heat and humidify inspired gas to be lost. The American Association for Respiratory Care states that devices should provide a minimum of 30 mg of H2O/L of delivered gas at 30° C.120 If inspired air is not warmed and humidified, the result is a dry, cool gas that is damaging to the respiratory tract and impedes mucociliary function. Secretions may become dry and inspissated, possibly leading to partial or complete occlusion of the ETT lumen. If left unrecognized, this occlusion may lead to barotrauma and death.93 The most common method to protect against this situation is the use of an active heated humidifier or a passive heat and moisture exchanger (HME) (Fig. 47-17).121
HMEs are typically cylindrical devices that are fitted to the ventilator circuit, usually just proximal to the ETT connector and the Y-piece, with limited changes on airway mechanics.122,123 This is the most effective HME placement for maximizing humidity and temperature retention in the patient circuit.121,124 The materials provide heat, humidification, and filtering properties, earning the device the nickname, “artificial nose.”125 They are lightweight and inexpensive, require no power source, and reduce circuit condensation, making them attractive alternatives to the more expensive heated humidifiers.
Use of heated humidifiers is associated with the production of almost 100% humidity in the inspiratory gas and is thus more effective than use of HMEs. These units require an external power source and additional circuitry, increasing cost. Accidental overheating can occur and may create additional damage to the airway if temperatures are not frequently monitored. There is no consensus about the proper duration of use of these implements. Multiple studies have failed to show a correlation of increased incidence of pneumonia with heated humidifiers versus HMEs or with frequent changes of HMEs or heated humidifiers. Therefore, frequent changes (i.e., more often than every 7 days) of ventilator circuits, unless they are visually soiled, is neither cost-effective nor medically efficacious.73,126–129
B Suctioning
Perhaps the simplest and most logical means of assisting with secretion clearance is direct suctioning. This modality is safe, but when it is performed carelessly, complications may occur, including soft tissue or airway trauma, aspiration, laryngospasm, increased intracranial pressure, bronchospasm, hypoxemia, and cardiac dysrhythmias.130 Hypoxemia can be minimized with preoxygenation using a fraction of inspired oxygen (FIO2) of 100%. In patients with intracranial hypertension, mild hyperventilation or blunting of the cough reflex with instilled intravenous lidocaine just before suctioning may reduce the risks of additional increases in intracranial pressure. The evacuation procedure should be brief and intermittent. The vacuum should be applied only after the suction catheter has been advanced to its distal position. After each pass of the catheter, lung re-expansion with a few gentle manual breaths should be administered. Suctioning can be applied by a single-use open system in which the catheter is unprotected and open to the environment or by a closed system (Fig. 47-18) that sheathes the catheter in a sterile protective covering.
Closed systems are usually incorporated into the ventilator breathing circuit at the junction of the Y-piece and the ETT or tracheostomy tube, allowing continued ventilation during suctioning with no need to disconnect the circuit. The advantage of not having to disconnect is important for patients who require aggressive ventilator management (e.g., high PEEP therapy), making them less susceptible to alveolar derecruitment compared with open suctioning. The retractable catheter does not add any additional restriction to airflow (when not deployed). There are concerns that colonization of these devices with aspiration of bacterial particles and cross-contamination may predispose to VAP.131 Such concerns have led to differing opinions on the appropriate timing of any system changes. Kollef and coworkers randomly assigned patients to scheduled changes every 24 hours or to no change except when there was a malfunction or visible soiling.132 In both groups, 15% of patients developed VAP. The only difference was in total cost: $11,016 USD in the group with scheduled changes and $837 USD in the group with no scheduled changes.132,133
C Subglottic Care
Secretion management has, by necessity, moved beyond the ETT lumen. Suctioning of secretions that are pooled above the ETT cuff, in the subglottic space, is an important step in good tracheal care and is paramount in the prevention of VAP.111,132–137 Subglottic suctioning has been shown to decrease the incidence of VAP in the ICU from 16% to 4%.135 Specialized ETTs with dedicated subglottic suctioning ports are more expensive ($15 compared with $1 for a standard ETT).138 An interesting cost analysis done in 2003 demonstrated that despite this increased cost, the estimated cost benefit of an ETT with a subglottic suction port was $4,992 per case of VAP saved.138 The U.S. Centers for Disease Control and Prevention (CDC) universally recommends the use of subglottic suctioning tubes in the ICU to help reduce the rate of VAP.139
Subglottic suctioning can be done with small suction catheters that are advanced down the trachea until resistance is met from the ETT cuff. Another option is using ETTs with a subglottic port positioned just above the cuff. These specialized ETTs are certainly not infallible and do not replace vigilant airway care. It has been demonstrated that dysfunction of the suction lumen can occur almost 50% of the time.140 In 43% of cases, the cause of the suction loss was determined to be prolapse of the tracheal mucosa into the subglottic suction port.140
D Bronchoscopy
The use of fiberoptic bronchoscopy for routine secretion management is not advocated. It is expensive, requires proficient training, and may produce complications such as barotrauma secondary to a marked reduction or cessation in expiratory airflow (depending on the relative airway caliber) in intubated patients.4 It should be reserved for assisting with lobar collapse caused by mucus plugging or inspissated secretions not amenable to conventional mucolysis, to perform so-called pulmonary toilet bronchoscopy that may be needed after an inhalation injury to evaluate for tracheobronchial injury, to aid in the diagnosis of significant hemoptysis, or to assist with specimen procurement when clinical suspicion merits sampling.
E Biofilm Management
Biofilm and adherence of secretions (Fig. 47-19) within the ETT have been implicated in the development of VAP, increased WOB, delays in extubation, and other complications.141–144 Biofilm can easily be identified by bronchoscopy on insertion of the scope into the ETT. Newer technologies using acoustic reflectometry have also been developed to help with monitoring the accumulation of biofilm and evaluating the integrity of the intubated airway. The SonarMed airway monitoring device (SonarMed, Indianapolis, IN) employs this technology to assess ETT positioning and movement as well as ETT patency hampered by either internal or external agents.
Traditional methods used to manage biofilm include catheter suctioning, bronchoscopic lavage, and ETT exchange. Catheter suctioning is often ineffective for biofilm removal, because its presence frequently evades detection as the suction catheter navigates the patent channel formed by the biofilm concretions, thereby leaving the impression that the lumen is patent. Bronchoscopic lavage and ETT exchange are hampered by significant costs and hazards for both practitioners and patients. Another option is a device that essentially scrapes the biofilm from the luminal surface of the ETT. The Complete Airway Management (CAM) Rescue Cath by Omneotech (Tavernier, FL) (Fig. 47-20) resembles a Fogarty catheter used to remove a thrombus. This product has an inflatable balloon at the distal end of a catheter that is encased in a latticed netting to provide “traction” and atraumatic “abrasion” of the lumen. It is introduced into the ETT and advanced to the distal end (based on ETT and CAM depth markings); the balloon is then inflated, and the catheter is fully withdrawn back out through the proximal end of the catheter, along with any luminal biofilm. This device and procedure may prove to be a useful option in already hypoxic or PEEP-dependent patients in whom time-consuming bronchoscopies could be hazardous. The CAM Rescue Cath is also a good option for patients with a potentially difficult airway, in whom ETT exchange can pose a considerable risk.145
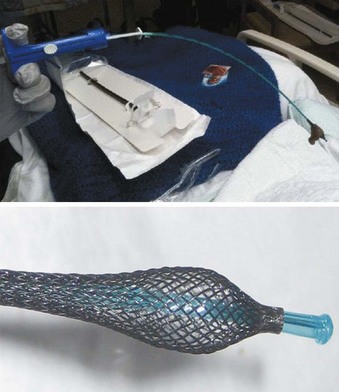
Figure 47-20 The Omneotech Complete Airway Management (CAM) Rescue Cath catheter.
(Courtesy of Omneotech, Tavernier, FL.)
Information on the prevention of biofilm formation is rife with conflicting data. One study showed no difference in rate of Pseudomonas aeruginosa and Staphylococcus epidermidis biofilm formation among different ETT materials including PVC, silicone, stainless steel, and sterling silver.146 More recently, however, ETTs coated in silver and chlorhexidine showed significantly reduced rates of biofilm colonization in a nonclinical setting.23,147 The preventive management of biofilm may not be far in the future, but chlorhexidine-coated ETTs are currently not available. For now, the best strategy to manage biofilm is increased vigilance, fastidious respiratory care, and new technologies aimed at minimization and safe clearance.
VI Respiratory Therapies for the Intubated Patient
A Secretion Clearance and Control Therapies
1 Mucolytic Agents
Agents used to decrease the viscosity of tracheobronchial secretions and assist with their reduction and clearance have been used for decades. The primary agent in use is N-acetylcysteine (NAC, Mucomyst). NAC is a sulfhydryl-containing compound; therefore, it is classified as a thiol. It has extensive first-pass metabolism in the gastrointestinal tract and liver when administered orally and is almost completely absorbed; only minimal amounts are excreted in the feces. The plasma half-life is approximately 2 hours, with virtually no detectable NAC at approximately 12 hours.148
Most of NAC’s biochemical effects appear to be related to its sulfhydryl group, which reduces the production hydroxyl radicals.148,149 This effect has provided many uses for NAC beyond that of a mucolytic agent, including hepatic protection in acetaminophen overdose and renal protection against contrast-induced nephropathy.52,150 NAC’s effects on mucus viscosity result from its ability to disrupt the disulfide bridges and render them more liquid.133,148 NAC is usually delivered by nebulizer in combination with a β2-adrenergic agonist because it can induce bronchospasm.151 Clinically, its effects have been variable in patients with chronic bronchitis, for whom oral NAC is used to assist with exacerbations and symptomatic relief.152–154 Direct instillation of NAC during bronchoscopy may assist in secretion removal.
Another important factor in mucus viscosity is DNA content. DNA contributes to secretion viscosity because it accumulates from the degradation of bacteria and neutrophils. An agent that is considered mostly a mucokinetic agent, recombinant deoxyribonuclease (DNase, Pulmozyme), has been used in nebulized form in patients with bronchiectasis caused by cystic fibrosis with good results; however, it is expensive, and its use beyond this population of patients is not indicated.117,155,156
One interesting therapy used in the population of burn patients who have an associated inhalation injury is nebulized heparin. Heparin assists with decreasing and removing bronchial casts that form with inhalation injury. Heparin’s anticoagulant effects assist with removal of casts, and it may act as a free radical scavenger with anti-inflammatory effects. Although studies have not consistently shown a significant change in pulmonary function, cast formation and removal are favorably altered.157,158
2 Chest Physiotherapy
a Percussion and Postural Drainage
Used extensively in patients with cystic fibrosis, the technique of percussion with postural drainage utilizes external percussion of the chest wall overlying the affected lung region. Percussion can be applied manually with a cupped hand or by an automated, usually pneumatic, device. The application of percussion or vibration, or both, to the chest wall functions to loosen the secretions in the bronchi and facilitate their mobilization.94,159 A steep Trendelenburg position of 25 degrees or more is employed—less if the patient cannot tolerate that angle—to facilitate the gravitational effects on mucus clearance.77,160
Relative contraindications to the postural component of this therapy are the presence of increased intracranial pressure; the possibility of an unprotected airway and the potential for aspiration; recent esophageal, ophthalmic, or intracranial surgery; congestive heart failure; and uncontrolled hypertension.120 As for the application of percussion or vibration, placement of the technique over recent surgical sites (e.g., split-thickness skin grafts, rib fractures or chest trauma, pulmonary contusions, burns, unstable spine fractures) or in the presence of coagulopathies, subcutaneous emphysema, or bronchospasm are all relative contraindications.160 Hazards include hypoxemia and accidental extubation.
Clinically and experimentally, the use of percussion with postural drainage in cystic fibrosis patients is well supported.161–163 However, patients’ compliance remains a concern, because the technique is burdensome for patients and caregivers.
b Positive End-Expiratory Pressure Therapy
PEEP therapy, as a secretion clearance technique, creates a restriction to expiratory flow by means of a face mask or mouthpiece. The resistance is adjusted to 10 to 20 cm H2O of back pressure during expiration, which allows airflow to move into distal airways and associated lung units, forcing past secretions and causing them to move toward the larger airways, where suctioning is more feasible. The maneuver is used with gentle and forceful coughs lasting up to 20 minutes and aerosolized medications that can be administered concurrently. Patients with an increased WOB or severe dyspnea may have difficulty performing this technique due to temporary lapses in ventilation. PEEP therapy is at least as effective for secretion clearance as percussion with postural drainage, if not more effective, and patient satisfaction is markedly more favorable.125,164,165
c Intrapulmonary Percussive Ventilation
Intrapulmonary percussive ventilation (IPV) can be delivered through a mouthpiece or to the end of the ETT. Its high-frequency percussive oscillations function to loosen retained secretions, expand airways and lungs, and reduce atelectasis. Conceptualized and designed by Dr. Forrest Bird, IPV uses a “phasetron”—a sliding venturi device capable of providing 5 to 35 cm H2O pressure during oscillations of 2 to 5 Hz.166 Aerosolized medications may also be delivered during IPV treatments. Favorable results have been reported for secretion clearance and lung expansion in patients with cystic fibrosis, as well as in other disorders with an increased incidence of thickened secretions.167,168 IPV offers an advantage to patients who lack the ability to perform percussion with postural drainage or high PEEP therapies.
d High-Frequency Chest Wall Compression
Therapy with high-frequency chest wall compression entails the wearing of an inflatable vest around the chest. Air is instilled into the vest bladder and then rapidly withdrawn in a cyclic manner, essentially creating an artificial cough. The high-frequency oscillations that are produced range from 5 to 25 Hz and can generate pressures as high as 50 cm H2O. These oscillations create a gentle “squeezing” of the patient’s chest that mimics small coughs. The frequency of the oscillations can be adjusted, and sensors in the vest can reduce the pressure delivered when the patient’s chest expands (as with a sigh breath or a deep cough).141 Secretion clearance and improvement in mucus rheology have also been reported.169,170 Perhaps the biggest drawback of this method is its cost, estimated at $15,900 USD for each unit.
B Overcoming Work of Breathing Imposed by Endotracheal Tubes, Tracheostomy Tubes, and Ventilator Circuits
With any translaryngeal intubation, the upper airway is bypassed and the resistance it imparts is thereby removed; however, there is still a substantial amount of work performed by the patient in an effort to ventilate. WOB is minimal during normal, quiet breathing, accounting for about 5% of the total oxygen consumption at rest. With increases in WOB, oxygen consumption can be markedly increased to as much as 30% or more.155 This newly acquired increased demand may not be well tolerated by the critically ill patient. The additional WOB (WOBadd) imposed by the artificial airway and ventilator apparatus not only hinders weaning and liberation from mechanical ventilation but also impairs tissue oxygenation and alters critical blood flow, which may lead to worsened organ dysfunction.86 To initiate a “breath” from the ventilator, a pressure differential across the ETT and circuit must be produced. The patient must overcome this resistance to initiate the demand flow needed for ventilation to occur. It has been shown experimentally that the ETT, the ventilator circuit, and the ventilator itself all add varying degrees of additional work for the patient to overcome, on top of the problems that initially necessitated intubation and mechanical ventilation.32,171
1 Pressure Support
Various modalities have been designed to overcome WOBadd imposed by the artificial airway and ventilator.155 One is pressure support (PS), which is used as either an adjunct or as a mode of ventilation to help the spontaneously breathing patient overcome the WOBadd imposed by an artificial airway.21,168,172 A preset, flow-triggered inspiratory pressure chosen by the clinician is added to the airway opening pressure when inspiration is triggered by the patient. Because it is flow cycled, the patient can control the duration and depth of inspiration, and the PS ceases when some preset gas flow has diminished, usually about 25% of the maximal peak flow achieved.173 PS has been shown to decrease WOBadd even with normal lungs.173,174 However, in patients with obstructive pulmonary disease and expiratory flow limitation, breath stacking, or auto-PEEP, flow may not decelerate quickly enough, and active exhalation may be necessary to terminate the PS, thereby creating additional WOB.175 The amount of inspiratory pressure added is usually 4 to 15 cm H2O. Currently, it is recommended that PS be applied for all spontaneously breathing, intubated patients to assist with overcoming the resistance of the ETT or tracheostomy tube.176 Additional PS may be necessary if tidal volumes or respiratory rates, or both, are inadequate to support oxygenation. Most patients tolerate PS well, but decreased tolerance in patients susceptible to expiratory flow limitation must be appreciated and accounted for in any management strategy.
2 Continuous Positive Airway Pressure
Continuous positive airway pressure (CPAP) is applied at end-exhalation in spontaneously breathing patients. Much like PEEP, CPAP is designed to offset the degree of atelectasis that occurs inherently in the intubated, supine patient. CPAP ranging from 4 to 10 cm H2O should be provided for all spontaneously breathing patients in an effort to compensate for the loss of expiratory lung volumes and further promote oxygenation. So-called physiologic PEEP, an amount thought to rectify the aforementioned atelectasis, is theoretical but is estimated to be equivalent to 4 cm H2O in the normal lung. The reduction in WOB seen with CPAP has been appreciated primarily in the setting of expiratory airflow reductions; in such cases, CPAP offsets the auto-PEEP, thereby reducing the work required to generate the next inspiratory effort.177 The type of flow-triggered mechanism and the location at which the flow differential is measured (typically at the tracheal end of the ETT) have been shown experimentally to decrease the inspiratory WOB as well.178 CPAP is usually applied in combination with added PS.
3 Automatic Tube Compensation
As stated previously, the ETT imposes a substantial degree of resistance to inspiration. The modalities described thus far assist with decreasing some of the work needed to overcome this burden. Added PS and PS ventilation help compensate for the resistance primarily encountered during inhalation but not during exhalation, and they are not consistently provided because of the varying flow across the ETT during normal breathing.179 Much resistance to exhalation is also produced by the presence of an ETT. Indeed, the internal diameter of the ETT greatly affects this phenomenon, as do other factors such as gas flow rates, gas density and viscosity, and luminal secretions adherent to the ETT wall. Automatic tube compensation (ATC) is a feature on some newer ventilators. It is designed to assist with the resistance imposed by the ETT or tracheostomy tube during both the inspiratory and expiratory phases of the respiratory cycle. By altering the PS delivered—raising it during inspiration and lowering it during expiration according to the pressure-flow characteristics of the ETT—ATC adjusts for the resistance and the pressure drop across the ETT during spontaneous breathing. A computer assists by calculating the pressure difference across the ETT (ΔPETT) based on ETT size, measuring gas flow and airway pressure, and selecting the resistive properties of the ETT.179 Unlike PS, ATC cannot be used as a ventilatory mode; it is merely an adjunct component to mechanical ventilation.
One drawback to ATC is the inability to correct for the reductions in airway diameter that can occur with secretions or kinking. This limitation results in an inaccurate measurement of ΔPETT such that ATC undercompensates for the pressure difference across the airway. A high index of suspicion is necessary to monitor for this possibility. Clinically, ATC has been shown to decrease the WOBadd encountered with ETTs and tracheostomy tubes.28 When these modalities were used to assist with weaning and extubation of patients in a T-piece trial, there was no difference in the workload encountered with ATC and T-piece alone, whereas adding PS to the T-piece trial at 7 cm H2O unloaded this additional work.180
C Pharmacologic Treatments
1 Inhalation Drug Delivery
The presence of an ETT does not limit drug delivery to the lungs and may actually enhance it. Many clinicians take advantage of this route of administration. The two predominant methods used to deliver agents are metered-dose inhalers (MDIs) and nebulizers. The drugs delivered by these devices are most commonly bronchodilators, mucolytics, corticosteroids, and antibiotics. For pulmonary ailments, inhaled drugs achieve efficacy comparable to or exceeding that of systemically delivered drugs with a smaller dose.181–183 Tracheal administration of some traditionally systemic drugs often requires much higher doses to ensure absorption.
Inhalation drug delivery has other advantages over systemic administration. Systemic side effects can be reduced, because systemic absorption is markedly decreased. Variable reports regarding penetration and distribution of an aerosol to the lower respiratory tract range from 0% to 42% with nebulizers and 0.3% to 98% with MDIs. However, when the delivery method was standardized, the amount delivered in either method was similar, about 15%.184–186
Particle size also plays an important role in delivery. The larger the particle, the less likely it is to be delivered distally to the alveoli. Aerosol particles ranging between 1 and 5 µm are optimal for proper deposition.181,182,185,187 The density of the gas carrying the aerosol also influences the delivery in an inverse relationship. Improvement in delivery has been reported when a mixture of helium and oxygen was used in the ventilator circuits of both MDIs and nebulizers.183,188
a Nebulizers
The performance of a nebulizer depends on multiple factors including the model, operating pressure, flow rate, and volume of diluent utilized. Nebulizers are capable of generating aerosols with particle sizes of 1 to 3 µm, and the size produced is inversely influenced by the flow rate or pressure used: the greater the flow rate, the smaller the particle.181,182 Nebulizers may be used continuously or intermittently. Intermittent use appears to be more efficient than continuous delivery, with less waste of aerosol demonstrated.189 Placing a nebulizer upstream from the Y-piece and ETT also increases drug delivery.187,189,190 Interestingly, the use of continuous drug nebulization may impair the ability of the patient to initiate a negative-pressure inspiratory effort in the PS mode of ventilation, thereby leading to hypoventilation.168,191
b Metered-Dose Inhalers
An MDI delivers medication in combination with a mixture of pressurized propellants, preservatives, flavoring agents, and surfactants. The final concentration of active drug constitutes about 1% of the total volume in the canister.181 When the stem on the MDI canister is depressed, a finite amount of drug is released at a certain velocity, and a spray cloud develops. Various adapters are available that fit in line with the ventilator circuit or on the end of the ETT as so-called elbow adapters to aid in the administration of inhalational therapies. Chambers or spacers appear to provide better delivery of aerosol compared with the more commonly used elbow adapters.192 MDIs typically cause more aerosol deposition on the ETT than nebulizers do, decreasing the amount of drug delivered. These particles, in turn, adhere to the ETT. This problem can be reduced by using a spacer and performing the administration with meticulous attention to timing of the ventilatory cycle: it is most effective during inspiration and when synchronized with the patient’s spontaneous effort. Dhand and Tobin reported excellent results with their technique of MDI delivery.189
When comparing the overall efficacy of nebulizers versus that of MDIs, several factors favor the use of MDIs in mechanically ventilated patients. Nebulizers may become colonized with bacteria and help to deliver an aerosolized inoculum. Bowton and colleagues reported a potential saving of $300,000 USD annually with the use of MDIs compared with nebulizers.193
2 Inhaled Bronchodilators
β2-agonists also have a beneficial effect on respiratory cilia in that they cause an increase in ciliary beat frequency.194 This phenomenon is mediated by β-adrenergic receptors and can be attenuated with nonselective beta-blocking agents. An increase in the frequency of ciliary beating promotes mucus clearance over the respiratory epithelium. Other effects include increased water secretion onto the airway surface, which facilitates mucus clearance.195 Indeed, the beneficial effects of β-agonists on bronchial reactivity and mucociliary clearance are evident. However, there are data suggesting a more robust effect in healthier airways than in chronically diseased airways such as those seen in patients with chronic bronchitis, possibly due to downregulation and chronic attenuation.196 Newer formulations of inhaled β-agonists such as levalbuterol may have reduced side effect profiles and possibly improved outcomes.
3 Anticholinergics
Although inhaled β-agonists are pivotal in the reduction of airway reactivity, the use of inhaled anticholinergics such as ipratropium bromide or the newer tiotropium bromide needs to be emphasized, given their obvious synergistic effect with β-agonists. It is well appreciated that many of the mechanisms of airway reactivity and inflammation associated with bronchospastic disease are cholinergically mediated. In patients with chronic obstructive pulmonary disease, the use of these agents alone or in combination with β-agonists is the foundation for rescue therapy and a mainstay in chronic management.183
4 Corticosteroids
Inhaled glucocorticoid therapy has become a mainstay of treatment in various obstructive respiratory ailments, including chronic obstructive pulmonary disease and asthma. This class of medicines unquestionably has disease-specific effects at the target organ. Equally interesting is the fact that administration in an aerosol preparation magnifies their effects while markedly reducing their side effect profile, leading to a lower risk-benefit value. Targeted efficacy with minimal adverse effects helps to quantify an appropriate risk-benefit value.197,198 High lung deposition or targeting, high receptor binding, longer pulmonary retention, and high lipid conjugation are among the pharmacokinetic parameters that lead to improved efficacy of these compounds and should be considered. A low or negligible oral bioavailability, smaller particle size leading to a relatively inactive drug at the oropharynx, higher plasma protein binding, increased metabolism rates, higher clearances, and lower systemic concentrations are associated with lower risks for adverse effects.197–199
For individuals who require long-term care with inhaled glucocorticoids during ventilator dependency, therapy should be continued to minimize the underlying disease process and thereby decrease the number of new variables, including adrenal insufficiency, in the treatment equation with inhaled or intravenous formulations.200 Despite all of these perceived benefits, inhaled glucocorticoids do not seem to reduce mortality.201 Acute exacerbations tend to be treated with intravenous or oral preparations due to the higher doses required.
5 Inhaled Antibiotics
Inhaled antibiotics have been used for decades, falling in and out of favor over the years. They are used primarily for treatment and suppression of chronic airway bacterial colonization. Their theoretical advantages are improved drug delivery and higher concentrations at the site of infection, leading to improved efficacy and better bacterial eradication compared with systemic administration.90,202–204 The primary concern with this therapy is development of bacterial resistance.
Results differ on efficacy. Inhaled antibiotics, mainly aminoglycosides, have been used extensively in cystic fibrosis patients with good results.90,202–205 Palmer and colleagues reported a marked reduction in the volume of airway secretions and a decrease in the laboratory markers of inflammation in a prospective study of mechanically ventilated patients with chronic respiratory failure.90,205 However, their study lacked power and was not randomized.
Other studies have failed to show similar benefits but rather have demonstrated poor, unpredictable drug delivery.206 Unequal ventilation, atelectasis, lobar collapse, and consolidation impair even drug distribution. Bronchospasm with chest tightness has also been reported.134 Use of inhaled antibiotics should be limited to selected patients, such as those with cystic fibrosis. Routine use to assist with secretion reduction and clearance in the mechanically ventilated patient is not recommended.
D Positioning of the Patient
The appropriate position in which to maintain the patient requiring mechanical ventilation has been debated. Current recommendations from the CDC state that elevating the head 30 to 40 degrees reduces the risk of VAP. A study examining head elevation and the rate of VAP concluded early after interim analysis showed an incidence of microbiologically confirmed pneumonia of 5% in semirecumbent patients versus 23% in supine patients.207 The semirecumbent position has also been supported with regard to facilitating nursing care and decreasing gastric reflux and resultant aspiration. It has not been shown to have an effect on the hemodynamic status of the patient, although this remains a common theoretical concern.208
Special beds that provide continuous lateral rotation have been used for patients who cannot be repositioned easily, such as those with severe head or traumatic brain injury, bariatric patients, and those who are pharmacologically paralyzed (e.g., patients with acute respiratory distress syndrome [ARDS]). These beds are advertised to enhance skin care, reduce thrombotic events, and improve pulmonary function. Their use is proposed to reduce atelectasis and, hence, pneumonia formation; however, studies’ results have remained conflicting.209,210
Occasionally, in severe cases of ARDS, alternative positioning (prone) may be necessary to facilitate ventilation and oxygenation. This method attempts to combat the physiologic shunt that is responsible for the observed hypoxemia. The prone position improves oxygenation by increasing lung volume, recruiting posterior lung fields, and redistributing perfusion.211 No statistical difference was shown in a meta-analysis of the data for prone positioning of patients, although a small subset of patients with severe ARDS has been shown to benefit.212,213 However, prone positioning is not without risks, particularly an increased risk for bed sores and ETT complications such as dislodgement.212 Recently, a bed has become commercially available that possesses the ability to fully prone the patient, making use of this potentially beneficial intervention more dependent on necessity than caregiver feasibility. (Rotoprone, KCI Therapeutic Support Systems, San Antonio, TX.)
VIII Clinical Pearls
• Polyurethane endotracheal tube (ETT) cuffs that have high-volume, low-pressure (HVLP) cuffs are capable of conforming to the irregular borders of the tracheal lumen and therefore are more effective at preventing microaspiration.
• ETT placement has mechanical and physiologic consequences. Vigilant surveillance of skin hygiene, airway patency, cuff integrity, and ventilatory support must be realized to minimize injury and maximize support.
• Confirmation of ETT placement is necessary to aid in proper resuscitation efforts. Verification by the presence of end-tidal carbon dioxide, whether by capnography or capnometry or by direct or indirect visualization, is mandatory to ensure appropriate placement.
• Cuff leak evaluation is a multifaceted endeavor requiring vigilance, diligence, and skill. An appropriate analysis of the potential cause, scrutinized against the risks and benefits of ETT exchange, must occur with limited interference to homeostasis.
• ETT exchange, whether for biofilm accumulation and luminal obstruction, ETT cuff damage (cuff leak), or other ETT mechanical failure (e.g., kinking) is a high-risk ordeal. The decision to exchange an ETT should be assessed against newer, currently available technologies, such as biofilm extraction, that are designed to salvage damaged ETTs. Should the decision to perform an exchange arise, airway adjuncts such as an airway exchange catheter and video laryngoscopy have proved invaluable for achieving higher success rates.
• Once an ETT is in place, efforts must be aggressive and perpetual to decrease the risk of ventilator-associated pneumonia (VAP); these efforts range from acid-suppression therapies to use of specially designed ETTs to advanced nursing care regimens. VAP appears to be more multifaceted than previously believed. Subglottic suctioning and biofilm management are just the beginning steps.
• Any area of a facility that deals with intubated patients should have a readily accessible difficult airway cart. The cart should be well outfitted but tailored to the types of airways managed and familiar as well as specific for the providers who respond to such emergencies.
• As with pulmonary artery catheters, it is not the difficult airway cart that manages the airway but the personnel who utilize it.
• It is better to investigate any perceived ETT problem electively than to deal with its consequences after it becomes an acute emergency.
• The landscape of ETT design, construction, and maintenance has changed and will continue to change over the next decade. Not all variations will prove effective, but improved patient care will take place.
All references can be found online at expertconsult.com.
9 Spiegel JE. Endotracheal tube cuffs: Design and function. Anesthesiology News Guide to Airway Management. New York: McMahon Publishing; 2010.
21 Kuhlen R, Max M, Dembinski R, et al. Breathing pattern and workload during automatic tube compensation, pressure support and T-piece trials in weaning patients. Eur J Anaesthesiol. 2003;20:10–16.
25 Divatia JV, Bhowmick K. Complications of endotracheal intubation and other airway management procedures. Indian J Anaesth. 2005;49:308–318.
26 Mort TC. Emergency tracheal intubation: Complications associated with repeated laryngoscopic attempts. Anesth Analg. 2004;99:607–613.
61 Berra L, De Marchi L, Panigada M, et al. Evaluation of continuous aspiration of subglottic secretion in an in vivo study. Crit Care Med. 2004;32:2071–2078.
62 Dezfulian C, Shojania K, Collard HR, et al. Subglottic secretion drainage for preventing ventilator-associated pneumonia: A meta-analysis. Am J Med. 2005;118:11–18.
91 Salem MR. Verification of endotracheal tube position. Anesthesiol Clin North Am. 2001;19:813–839.
100 Sengupta P, Sessier DI, Maglinger P, et al. Endotracheal tube cuff pressure in three hospitals and the volume required to produce appropriate cuff pressure. BMCAnesthesiol. 2004;4(1):8.
104 Lorente L, Lecuona M, Jiménez A, et al. Influence of an endotracheal tube with polyurethane cuff and subglottic secretion drainage on pneumonia. Am J Respir Crit Care Med. 2007;176:1079–1183.
113 American Society of Anesthesiologists Task Force on Difficult Airway Management. Practice guideline for management of the difficult airway. Anesthesiology. 2003;98:1269–1277.
135 Smulders K, van der Hoeven H, Weers-Pothoff I, et al. A randomized clinical trial of intermittent subglottic secretion drainage in patients receiving mechanical ventilation. Chest. 2002;121:858–862.
137 Coffin SE, Klompas M, Classen D, et al. Strategies to prevent ventilator associated pneumonia in acute care hospitals. Supplement Article SHEA/ISDA Practice Recommendation. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S31–S40.
139 Tablan OC, Anderson LJ, Besser R, et al. Guidelines for preventing health-care associated pneumonia, 2003. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53(RR-3):1–36.
140 Dragoumanis CK, Vretzakis GI, Papaloannou VE, et al. Investigating the failure to aspirate subglottic secretions with the Evac endotracheal tube. Anesth Analg. 2007;105:1083–1085.
141 Kapadia FN. Factors associated with blocked tracheal tubes. Intensive Care Med. 2001;27:1679–1681.
145 Mort T, Aldo F, Kopp GW. Managing the unusual airway—Case studies in complexity: Clearing luminal occlusions. Anesthesiology News. 2010;36(8):64–65.
146 Jarrett WA, Ribes J, Manaligod JM. Biofilm formation on tracheostomy tubes. Ear Nose Throat J. 2002;81:659–661.
164 Shah C, Kollef MH. Endotracheal tube intraluminal volume loss among mechanically ventilated patients. Crit Care Med. 2004;32:120–125.
212 Kopterides P, Siempos II, Armaganidis A. Prone positioning in hypoxemic respiratory failure: Meta-analysis of randomized controlled trials. J Crit Care. 2009;24:89–100.
1 Macewan W. Report. Br Med J. 1880;2:122.
2 Condon HA, Gilchrist E. Stanley Rowbotham: Twentieth century pioneer anaesthetist. Anaesthesia. 1986;41:46–52.
3 Wong J, Keens T, Wannamaker E, et al. Effects of gravity in tracheal transport rates in normal subjects and in patients with cystic fibrosis. Pediatrics. 1977;60:146–152.
4 Matsushima Y, Jones R, King E, et al. Alterations in pulmonary mechanics and gas exchange during routine fiberoptic bronchoscopy. Chest. 1984;86:184.
5 Lotano R, Gerber D, Aseron C, et al. Utility of postintubation radiographs in the intensive care unit. Crit Care. 2000;4:50–53.
6 Sim WS, Chung IS, Chin JU, et al. Risk factors for epistaxis during nasotracheal intubation. Anaesth Intensive Care. 2002;30:449–452.
7 Dullenkopf A, Gerber A, Weiss M. Fluid leakage past tracheal tube cuffs: Evaluation of the Microcuff endotracheal tube. Intensive Care Med. 2003;29:1849–1853.
8 Mulier J, Van den Brande F, Dilleman B, et al. Tracheal cuff leak in morbidly obese patients intubated with a TaperguardTM, Hi-LoTM Cuffed and Hi-LoTM Cuffed and Lubricated Tracheal Tube. Abstract P-9108. Presented at the 63rd PostGraduate Assembly, New York, December 12. 2009.
9 Spiegel JE. Endotracheal tube cuffs: Design and function. Anesthesiology News Guide to Airway Management. New York: McMahon Publishing; New York; 2010. pp 51–58
10 Poelart J, Depuydt P, De Wolf A, et al. Polyurethane cuffed endotracheal tubes to prevent early postoperative pneumonia after cardiac surgery: A pilot study. J Thorac Cardiovasc Surg. 2008;135:771–776.
11 TaperGuard Evac Endotracheal Tube FDA 510(k) application K090352, U.S. Food and Drug Administration, 2009.
12 Ho AM, Contardi LH. What to do when an endotracheal tube cuff leaks. J Trauma. 1996;40:486–487.
13 Short JA. An unusual cause of tracheal tube cuff damage. Anaesthesia. 1997;52:93–94.
14 Verborgh C, Camu F. Management of cuff incompetence in an endotracheal tube. Anesthesiology. 1987;66:441.
15 Chua WL, Ng AS. A defective endotracheal tube. Singapore Med J. 2002;43:476–478.
16 Gettelman TA, Morris GN. Endotracheal tube failure: Undetected by routine testing. Anesth Analg. 1995;81:1313.
17 Heusner JE, Viscomi CM. Endotracheal tube cuff failure due to valve damage. Anesth Analg. 1991;72:270.
18 Mesa A, Miguel R. Hidden damage to a reinforced LMA-Fastrach endotracheal tube. Anesth Analg. 2000;90:1250–1251.
19 Kovatsis PG, Fiadjoe JE, Stricker PA. Simple, reliable replacement of pilot balloons for a variety of clinical situations. Pediatr Anesth. 2010;20:490–494.
20 Sprung J, Bourke DL, Thomas P, et al. Clever cure for an endotracheal tube cuff leak. Anesthesiology. 1994;81:790–791.
21 Kuhlen R, Max M, Dembinski R, et al. Breathing pattern and workload during automatic tube compensation, pressure support and T-piece trials in weaning patients. Eur J Anaesthesiol. 2003;20:10–16.
22 Adair CG, Gorman SP, O’Neill FB, et al. Selective decontamination of the digestive tract (SDD) does not prevent the formation of microbial biofilms on the endotracheal tubes. J Antimicrob Chemother. 1993;31:689–697.
23 Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903.
24 Balazs DJ, Triandafillu K, Wood P, et al. Surface modification of PVC endotracheal tube surfaces to reduce Pseudomonas aeruginosa adhesion: Plasma processing and chemical methods. Eur Cells Mater. 2003;6(Suppl 1):86.
25 Divatia JV, Bhowmick K. Complications of endotracheal intubation and other airway management procedures. Indian J Anaesth. 2005;49:308–318.
26 Mort TC. Emergency tracheal intubation: Complications associated with repeated laryngoscopic attempts. Anesth Analg. 2004;99:607–613.
27 Gelman JJ, Aro M, Weiss SM. Tracheo-innominate artery fistula. J Am Coll Surg. 1994;179:626–634.
28 Hawkins DB. Glottic and subglottic stenosis from endotracheal intubation. Laryngoscope. 1977;87:339.
29 Weber S. Traumatic complications of airway management. Anesth Clin North Am. 2002;20:503–512.
30 Sois M, Dillon F. What is the safest foil tape for endotracheal tube protection during Nd-YAG laser surgery? A comparative study. Anesthesiology. 1990;72:553.
31 Bolder PM, Healy TE, Bolder AR, et al. The extra work of breathing through adult endotracheal tubes. Anesth Analg. 1986;65:853–859.
32 Bersten AD, Rutten AJ, Vedig AE, et al. Additional work of breathing imposed by endotracheal tubes, breathing circuits, and intensive care ventilators. Crit Care Med. 1989;17:671–677.
33 Gal TJ, Suratt PM. Resistance to breathing in healthy subjects following endotracheal intubation under topical anesthesia. Anesth Analg. 1980;59:270–274.
34 Demers RR, Sullivan MJ, Paliotta J. Airflow resistance of endotracheal tubes. JAMA. 1977;237:1362.
35 Schwartz DE, Lieberman JA, Cohen NH. Women are at a greater risk than men for malpositioning of the endotracheal tube after emergent intubation. Crit Care Med. 1994;22:1127–1131.
36 Tanigawa K, Takeda T, Goto E, et al. The efficacy of esophageal detector devices in verifying tracheal tube placement: A randomized cross-over study of out-of-hospital cardiac arrest patients. Anesth Analg. 2001;92:375–378.
37 Sahn SA, Lakshminarayan S, Petty TL. Weaning from mechanical ventilation. JAMA. 1976;235:2208–2212.
38 Stout DM, Bishop MJH, Dwersteg JF, et al. Correlation of endotracheal tube size with sore throat and hoarseness following general anesthesia. Anesthesiology. 1987;67:419–421.
39 Shapiro BA. Chest physical therapy administered by respiratory therapists. Respir Care. 1981;26:655–656.
40 Miller AB, Pavia D, Agnew JE, et al. Effect of oral N-acetylcysteine on mucus clearance. Br J Dis Chest. 1985;79:262–266.
41 Bryce DP, Briant TD, Pearson FG. Laryngeal and tracheal complications of intubation. Ann Otol Rhinol Laryngol. 1968;77:442–461.
42 Sullivan M, Paliotta J, Saklad M. Endotracheal tube as a factor in measurement of respiratory mechanics. J Appl Physiol. 1976;41:590–592.
43 Henschke CI, Yankelevitz DF, Wand A, et al. Accuracy and efficacy of chest radiography in the intensive care unit. Radiol Clin North Am. 1996;34:21–31.
44 Hilding AC. Laryngotracheal damage during intratracheal anesthesia. Ann Otol Rhinol Laryngol. 1971;80:565–581.
45 Bishop MJ, Weymuller EA, Fink BR. Laryngeal effects of prolonged intubation. Anesth Analg. 1984;63:335–342.
46 Stone DJ, Bogdonoff DL. Airway considerations in the management of patients requiring long-term endotracheal intubation. Anesth Analg. 1992;74:276–287.
47 Stenqvist O, Sonander H, Nilsson K. Small endotracheal tubes: Ventilator and intratracheal pressures during controlled ventilation. Br J Anaesth. 1979;51:375–381.
48 Carrion MI, Ayuso D, Marcos M, et al. Accidental removal of endotracheal and nasogastric tubes and intravascular catheters. Crit Care Med. 2000;28:63–66.
49 Roberts JR, Spadafora M, Cone DC. Proper depth placement of oral endotracheal tubes in adults prior to radiographic confirmation. Acad Emerg Med. 1995;2:20–24.
50 Ornato JP, Garnett AR, Glauser FL. Relationship between cardiac output and the end-tidal carbon dioxide tension. Ann Emerg Med. 1990;19:1104–1106.
51 Hermens JM, Bennett MJ, Hirschman CA. Anesthesia for laser surgery. Anesth Analg. 1983;62:218–229.
52 Sosis MB, Dillon FX. Saline-filled cuffs help prevent laser-induced polyvinylchloride endotracheal tube fires. Anesth Analg. 1991;72:187–189.
53 Stamm AM. Ventilator-associated pneumonia and frequency of circuit changes. Am J Infect Control. 1998;26:71–73.
54 Cozine K, Rosenbaum LM, Askanazi J, et al. Laser-induced endotracheal tube fire. Anesthesiology. 1981;55:553–583.
55 Sosis MB. Hazards of laser surgery. Semin Anesth. 1990;9:90.
56 Smilkstein MJ, Knapp GL, Kulig KW, et al. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose: Analysis of the national multicenter study (1976–1985). N Engl J Med. 1988;319:1557–1562.
57 Barash PG, Cullen BF, Stoelting RK. Clinical anesthesia, ed 6, Philadelphia: Lippincott-Raven, 2009.
58 Sole ML, Poalillo FE, Byers JF, et al. Bacterial growth in secretions and on suctioning equipment of orally intubated patients: A pilot study. Am J Crit Care. 2002;11:141–149.
59 Baughman RP. Diagnosis of ventilator-associated pneumonia. Microbes Infect. 2005;7:262–267.
60 Grap MJ, Cantley M, Munro CL, et al. Use of backrest elevation in critical care: A pilot study. Am J Crit Care. 1999;8:475–480.
61 Berra L, De Marchi L, Panigada M, et al. Evaluation of continuous aspiration of subglottic secretion in an in vivo study. Crit Care Med. 2004;32:2071–2078.
62 Dezfulian C, Shojania K, Collard HR, et al. Subglottic secretion drainage for preventing ventilator-associated pneumonia: A meta-analysis. Am J Med. 2005;118:11–18.
63 Kollef MH, Shapiro SD, Boyd V, et al. A randomized clinical trial comparing an extended-use hygroscopic condenser humidifier with heated-water humidification in mechanically ventilated patients. Chest. 1998;113:759–767.
64 Manthous CA, Chatila W, Schmidt GA, et al. Treatment of bronchospasm by a metered-dose inhaler albuterol in mechanically ventilated patients. Chest. 1995;107:210–213.
65 Berra L, De Marchi L, Yu ZX, et al. Endotracheal tubes coated with antiseptics decrease bacterial colonization of the ventilator circuits, lungs, and endotracheal tube. Anesthesiology. 2004;100:1446–1456.
66 Pacheco-Fowler V, Gaonkar T, Wyer PC, et al. Antiseptic impregnated endotracheal tubes for the prevention of bacterial colonization. J Hosp Infect. 2004;57:170–174.
67 Pai VB, Nahata MC. Efficacy and safety of aerosolized tobramycin in cystic fibrosis. Pediatr Pulmonol. 2001;32:314–327.
68 Keck JP, Mort TC. Supraglottic airway devices for rescue during emergency airway management in the remote location. Abstract 476. Presented at the 40th Annual Congress of the Society for Critical Care Medicine, San Diego, CA, January. 2011.
69 Seegobin RD, van Hasselt GL. Endotracheal cuff pressure and tracheal mucosal blood flow: Endoscopic study of effects of four large volume cuffs. Br Med J. 1984;288:965–968.
70 Goldberg JS, Rawle PR, Zehnder JL, et al. Colorimetric end-tidal carbon dioxide monitoring for tracheal intubation. Anesth Analg. 1990;70:191–194.
71 Kasper CL, Deem S. The self-inflating bulb to detect esophageal intubation during emergency airway management. Anesthesiology. 1998;88:898–902.
72 Lang DJ, Wafai Y, Salem MR, et al. Efficacy of the self-inflating bulb in confirming tracheal intubation in the morbidly obese. Anesthesiology. 1996;85:246–253.
73 Tanigawa K, Takeda T, Goto E, et al. Accuracy and reliability of the self-inflating bulb to verify tracheal intubation in out-of-hospital cardiac arrest patients. Anesthesiology. 2000;93:1432–1436.
74 Cook RT, Moglia BB, Consevage NW, et al. The use of the Beck Airway Airflow Monitor for verifying intratracheal endotracheal tube placement in patients in the pediatric emergency department and intensive care unit. Pediatr Emerg Care. 1996;12:331–332.
75 Cook RT, Stene JK, Marcolina B. Use of a Beck Airway Airflow Monitor and controllable-tip endotracheal tube in two cases on nonlaryngoscopic oral intubation. Am J Emerg Med. 1995;13:180–183.
76 Cook RT, Stene JK. The BAAM and endotrol endotracheal tube for blind oral intubation. Beck Airway Air Flow Monitor. J Clin Anesth. 1993;5:431–432.
77 Schwartz DE, Matthay MA, Cohen NH. Death and other complications of emergency airway management in critically ill adults: A prospective investigation of 297 tracheal intubations. Anesthesiology. 1995;82:367–376.
78 Andersen KH, Hald A. Assessing the position of the tracheal tube: The reliability of different methods. Anaesthesia. 1989;44:984–985.
79 Rao S, Wilson DW, Brooks RA. Acute effects of nebulization of N-acetylcysteine on pulmonary mechanics and gas exchange. Am Rev Respir Dis. 1970;102:17–22.
80 Andres AH, Langenstein H. The esophageal detector device is unreliable when the stomach has been ventilated. Anesthesiology. 1999;91:566–568.
81 Leigh JM, Maynard JP. Pressure on the tracheal mucosa from cuffed tubes. Br Med J. 1979;1(6172):1173–1174.
82 Tasaki O, Mozingo DW, Dubick MA, et al. Effects of heparin and lisofylline on pulmonary function after smoke inhalation injury in an ovine model. Crit Care Med. 2002;30:637–643.
83 Tepel M, Van Der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–184.
84 Goodman LR, Conrardy PA, Laing F, et al. Radiographic evaluation of endotracheal tube position. AJR Am J Roentgenol. 1976;127:433–434.
85 Grenvik A, Ayres SM, Holbrook PR, et al. Textbook of critical care, ed 4, Philadelphia: WB Saunders, 2000.
86 Haberthur C, Fabry B, Stocker R, et al. Additional inspiratory work of breathing imposed by tracheostomy tubes and non-ideal ventilator properties in critically ill patients. Intensive Care Med. 1999;25:514–519.
87 Reed DB, Clinton JE. Proper depth of placement of nasotracheal tubes in adults prior to radiographic confirmation. Acad Emerg Med. 1997;4:1111–1114.
88 Hill BB, Zweng TN, Maley RH, et al. Percutaneous dilational tracheostomy. J Trauma. 1996;41:238–244.
89 Scott LR, Benson MS, Bishop MJ. Relationship of endotracheal tube size to auto-PEEP at high minute ventilation. Respir Care. 1986;31:1080–1082.
90 Palmer LB, Smaldone GC, Simon SR, et al. Aerosolized antibiotics in mechanically ventilated patients: Delivery and response. Crit Care Med. 1998;26:31–39.
91 Salem MR. Verification of endotracheal tube position. Anesthesiol Clin North Am. 2001;19:813–839.
92 Ricard JD, Le Miere E, Markowicz P, et al. Efficiency and safety of mechanical ventilation with a heat and moisture exchanger changed only once a week. Am J Respir Crit Care Med. 2000;16:1104–1109.
93 Mehta S. Transtracheal illumination for optimal tracheal tube placement: A clinical study. Anaesthesia. 1989;44:970–972.
94 Mortensen J, Falk M, Groth S, et al. Effects of postural drainage and positive expiratory pressure physiotherapy on tracheobronchial clearance in cystic fibrosis. Chest. 1991;100:1350–1357.
95 Pattnaik SK, Bodra R. Ballottability of cuff to confirm the correct intratracheal position of the endotracheal tube in the intensive care unit. Eur J Anaesthesiol. 2000;17:587–590.
96 Puntervoll SA, Søreide E, Jacewicz W, et al. Rapid detection of oesophageal intubation: Take care when using colorimetric capnometry. Acta Anaesthesiol Scand. 2002;46:455–457.
97 Curiel-Garcia JA, Guerrero-Romero F, Rodriguez-Moran M. Cuff pressure in endotracheal intubation: Should it be routinely measured. Gac Mex Med. 2001;137:179–182.
98 Stewart S, Secrest JA, Norwood BR, et al. A comparison of endotracheal tube cuff pressures using estimation techniques and direct intracuff measurement. AANA J. 2003;71:443–447.
99 Hoffman RJ, Parwani V, Hahn IH. Experienced emergency medicine physicians cannot inflate or estimate endotracheal tube cuff pressure using standard techniques. Am J Emerg Med. 2006;24:139–143.
100 Sengupta P, Sessler DI, Maglinger P, et al. Endotracheal tube cuff pressure in three hospitals and the volume required to produce appropriate cuff pressure. BMC Anesthesiol. 2004;4(1):8.
101 Habib MP. Physiologic implications of artificial airways. Chest. 1989;96:180–184.
102 Levine SA, Niederman MS. The impact of tracheal intubations on host defenses and risks for nosocomial pneumonia. Clin Chest Med. 1991;12:523–543.
103 Dobrin P, Canfield T. Cuffed endotracheal tubes: Mucosal pressures and tracheal wall blood flow. Am J Surg. 1977;133:562–568.
104 Lorente L, Lecuona M, Jiménez A, et al. Influence of an endotracheal tube with polyurethane cuff and subglottic secretion drainage on pneumonia. Am J Respir Crit Care Med. 2007;176:1079–1183.
105 Clarke T, Evans S, Way P, et al. A comparison of two methods of securing an endotracheal tube. Aust Crit Care. 1998;11:45–50.
106 Levy H, Griego L. A comparative study of oral endotracheal tube securing methods. Chest. 1993;104:1537–1540.
107 Chevron V, Menard JF, Richard JC, et al. Unplanned extubation: Risk factors of development and predictive criteria for reintubation. Crit Care Med. 1998;26:1049–1053.
108 Coppolo DP, May JJ. Self-extubations: A 12-month experience. Chest. 1990;98:165–169.
109 Tomkiewicz RP, App EM, De Sanctis GT, et al. A comparison of a new mucolytic N-acetylcysteine L-lysinate with N-acetylcysteine: Airway epithelial changes and mucus changes in dog. Pulm Pharmacol. 1995;8:259–265.
110 Barnason S, Graham J, Wild MC, et al. Comparison of two endotracheal tube securement techniques on unplanned extubation, oral mucosa, and facial skin integrity. Heart Lung. 1998;27:409–417.
111 Kollef MH, Prentice D, Shapiro SD, et al. Mechanical ventilation with or without daily changes of in-line suction catheters. Am J Respir Crit Care Med. 1997;156:466–472.
112 Kaplow R, Bookbinder M. A comparison of four endotracheal tube holders. Heart Lung. 1994;23:59–66.
113 American Society of Anesthesiologists Task Force on Difficult Airway Management. Practice guideline for management of the difficult airway. Anesthesiology. 2003;98:1269–1277.
114 Keck JP, Mort TC. Video laryngoscopy vs. direct laryngoscopy for airway evaluation prior to extubation. Abstract A906. Presented at the American Society of Anesthesiologists Annual Meeting, New Orleans, LA, October. 2009.
115 Peruzzi WT, Smith B. Bronchial hygiene therapy. Crit Care Clin. 1995;11:79–96.
116 Pollack CV, Jr. The laryngeal mask airway: A comprehensive review for the emergency medicine physician. J Emerg Med. 2001;20:53–66.
117 Barker AF. Bronchiectasis. N Engl J Med. 2002;346:1383–1393.
118 Bartlett RH. Postoperative pulmonary prophylaxis: Breathe deeply and read carefully. Chest. 1982;81:1–3.
119 McFadden ER, Pichurko BM, Bowman HF, et al. Thermal mapping of the airways in humans. J Appl Physiol. 1985;58:564–570.
120 American Association for Respiratory Care. AARC clinical practice guideline: Postural drainage therapy. Respir Care. 1991;36:1418–1425.
121 Chiaranda M, Verona L, Pinamonti O, et al. Use of heat and moisture exchangers filters in mechanically ventilated ICU patients: Influence on airway flow resistance. Intensive Care Med. 1993;19:462–466.
122 Emergency Care Research Institute. Heat and moisture exchangers. Health Devices. 1983;12:155–166.
123 Villafane MC, Cinnella G, Lofaso F, et al. Gradual reduction of endotracheal tube diameter during mechanical ventilation via different humidification devices. Anesthesiology. 1996;85:1341–1349.
124 Inui D, Oto J, Nishimura M. Effect of heat and moisture exchanger (HME) positioning on inspiratory gas humidification. BMC Pulm Med. 2006;6:19.
125 Martin C, Papazian L, Perrin G, et al. Performance evaluation of three vaporizing humidifiers and two heat and moisture exchangers in patients with minute ventilation 10L/min. Chest. 1992;102:1347–1350.
126 Dreyfuss D, Djedaini K, Gros I, et al. Mechanical ventilation with heated humidifiers or heat and moisture exchanges: Effects on patient colonization and incidence of nosocomial pneumonia. Am J Respir Crit Care Med. 1995;151:986–992.
127 Kolobow T, Tsuno K, Rossi N, et al. Design and development of ultra-thin walled, nonkinking endotracheal tubes of a new “no-pressure” laryngeal seal design: A preliminary report. Anesthesiology. 1994;81:1061–1067.
128 Constant M, Stern R, Doershuk C. Efficacy of the Flutter device for airway mucus clearance in patients with cystic fibrosis. J Pediatr. 1994;124:689–693.
129 Ring WH, Adair JC, Elwyn RA. A new pediatric endotracheal tube. Anesth Analg. 1975;54:273–274.
130 Demers RR. Complications of endotracheal suctioning procedures. Respir Care. 1982;27:453–457.
131 Steen JA. Impact of tube design and materials on complications of tracheal intubation. In: Bishop MJ, ed. Problems in anesthesia, Vol 2. Philadelphia: JB Lippincott; 1988:211–223. Physiology and consequences of tracheal intubation
132 Kollef MH, Skubas NJ, Sundt TM. A randomized clinical trial of continuous aspiration of subglottic secretions in cardiac surgery patients. Chest. 1999;116:1339–1346.
133 Kollef MH, Shapiro SD, Fraser VJ, et al. Mechanical ventilation with or without 7-day circuit changes. Ann Intern Med. 1995;123:168–174.
134 Mahul P, Auboyer C, Jospe R, et al. Prevention of nosocomial pneumonia in intubated patients: Respective role of mechanical subglottic secretions drainage and stress ulcer prophylaxis. Intensive Care Med. 1992;18:20–25.
135 Smulders K, van der Hoeven H, Weers-Pothoff I, et al. A randomized clinical trial of intermittent subglottic secretion drainage in patients receiving mechanical ventilation. Chest. 2002;121:858–862.
136 Valles J, Artigas A, Rello J, et al. Continuous aspiration of subglottic secretions in preventing ventilator-associated pneumonia. Ann Intern Med. 1995;122:179–186.
137 Coffin SE, Klompas M, Classen D, et al. Strategies to prevent ventilator associated pneumonia in acute care hospitals. Supplement Article SHEA/ISDA Practice Recommendation. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S31–S40.
138 Shorr AF, O’Malley PG. Continuous subglottic suctioning for the prevention of ventilator-associated pneumonia: Potential economic implications. Chest. 2001;119:228–235.
139 Tablan OC, Anderson LJ, Besser R, et al. Guidelines for preventing health-care associated pneumonia, 2003. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53(RR-3):1–36.
140 Dragoumanis CK, Vretzakis GI, Papaloannou VE, et al. Investigating the failure to aspirate subglottic secretions with the Evac endotracheal tube. Anesth Analg. 2007;105:1083–1085.
141 Kapadia FN. Factors associated with blocked tracheal tubes. Intensive Care Med. 2001;27:1679–1681.
142 Kapadia FN, Bajan KB, Raje KV. Airway accidents in intubated ICU patients: An epidemiological study. Crit Care Med. 2000;28:659–664.
143 Kirton OC, DeHaven CB, Morgan JP, et al. Elevated imposed work of breathing, masquerading as ventilator weaning intolerance. Chest. 1995;108:1021–1025.
144 Puntervoll SA, Soreide E, Jacewicz W, et al. Rapid detection of oesophageal intubation: Take care when using colorimetric capnometry. Acta Anaesthesiol Scand. 2002;46:455–457.
145 Mort T, Aldo F, Kopp GW. Managing the unusual airway—Case studies in complexity: Clearing luminal occlusions. Anesthesiology News. 2010;36(8):64–65.
146 Jarrett WA, Ribes J, Manaligod JM. Biofilm formation on tracheostomy tubes. Ear Nose Throat J. 2002;81:659–661.
147 Mohamed JA, Reitzel R, Hachem R, et al. Activity of antimicrobial-coated endotracheal tubes (ETT) in preventing the biofilm colonization of resistant bacteria in a biofilm model with neutralizing broth. Abstracts 66, Presentation 467: Healthcare- and Community-Acquired Infections and Infection Control. Presented at the 48th Annual Meeting of the Infectious Diseases Society of America, Vancouver, Canada, October 22. 2010.
148 Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998;3:114–127.
149 DeVries N, DeFlora S. N-Acetyl-1-cysteine. J Cell Biochem. 1993;17F:S270–S277.
150 Tomkiewicz RP, App EM, Coffiner M, et al. Mucolytic treatment with N-acetylcysteine L-lysinate metered dose inhaler in dogs: Airway epithelial changes. Eur Respir J. 1994;7:81–87.
151 Rasmussen JB, Glennow C. Reduction in days of illness after long-term treatment with N-acetylcysteine controlled-release tablets in patients with chronic bronchitis. Eur Respir J. 1988;1:351–355.
152 British Thoracic Society Research Committee. Oral N-acetylcysteine and exacerbation rates in patients with chronic bronchitis and severe airway obstruction. Thorax. 1985;40:832–835.
153 Mukhopadhyay S, Staddon GE, Eastman C, et al. The quantitative distribution of nebulized antibiotic in the lung in cystic fibrosis. Respir Med. 1994;88:203–211.
154 Reisman J, Rivington-Law B, Corey M, et al. Role of conventional therapy in cystic fibrosis. J Pediatr. 1988;113:632–636.
155 Fabry B, Hapeerthur C, Zappe D, et al. Breathing pattern and additional work of breathing in spontaneously breathing patients with different ventilatory demands during inspiratory pressure support and automatic tube compensation. Intensive Care Med. 1997;23:545–552.
156 Warwick W, Hansen L. Long-term effect of high-frequency chest compression therapy on pulmonary complications of cystic fibrosis. Pediatr Pulmonol. 1991;11:265–271.
157 Cox CS, Zwischenberger JB, Traber DL, et al. Heparin improves oxygenation and minimizes barotraumas after severe smoke inhalation in an ovine model. Surg Gynecol Obstet. 1993;176:339–349.
158 Tindol GA, DiBenedetto RJ, Kosciuk L. Unplanned extubation. Chest. 1994;105:1804–1807.
159 Gondor M, Nixon PA, Mutich R, et al. Comparison of Flutter device and chest physical therapy in the treatment of cystic fibrosis pulmonary exacerbation. Pediatr Pulmonol. 1999;28:255–260.
160 Ranieri VM, Grasso S, Fiore T, et al. Auto-positive end-expiratory pressure and dynamic hyperinflation. Clin Chest Med. 1996;17:379–395.
161 Desmond K, Schwenk F, Thomas E, et al. Immediate and long term effects of chest physiotherapy in cystic fibrosis. J Pediatr. 1983;103:538–542.
162 Homnick D, Shite F, deCatro C. Comparison of effects of an intrapulmonary percussive ventilator to standard aerosol and chest physiotherapy in treatment of cystic fibrosis. Pediatr Pulmonol. 1995;20:50–55.
163 Ricard JD, Markowicz P, Djedaini K, et al. Bedside evaluation of efficient airway humidification during mechanical ventilation of the critically ill. Chest. 1999;115:1646–1652.
164 Shah C, Kollef MH. Endotracheal tube intraluminal volume loss among mechanically ventilated patients. Crit Care Med. 2004;32:120–125.
165 Owens RL, Cheney F. Endobronchial intubation: A preventable complication. Anesthesiology. 1987;67:255–257.
166 Wright PE, Marini JJ, Bernard GR. In vitro versus in vivo comparison of endotracheal airflow resistance. Am Rev Respir Dis. 1989;140:10–16.
167 Birnkrant D, Pope J, Lewarski J, et al. Persistent pulmonary consolidation treated with intrapulmonary percussive ventilation: A preliminary report. Pediatr Pulmonol. 1996;21:246–249.
168 Jubran A, Van de Graaff WB, Tobin MJ. Variability of patient-ventilator interaction with pressure support ventilation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152:129–136.
169 Wanner A, Salathe M, O’Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902.
170 White G. Equipment theory for respiratory care. Albany, NY: Delmar; 1996.
171 Skinner MW, Waldron RJ, Anderson MB. Normal laryngoscopy and intubation. In: Hanowell LH, Waldron RJ. Airway management. Philadelphia: Lippincott-Raven; 1996:81–96.
172 MacIntyre NR. Respiratory function during pressure support ventilation. Chest. 1986;89:677–683.
173 Mahlmeister M, Fink J, Hoffman G, et al. Positive-expiratory-pressure mask therapy: Theoretical and practical considerations and a review of the literature. Respir Care. 1991;36:1218–1229.
174 Tomkiewicz RP, Biviji A, King M. Rheologic studies regarding high-frequency chest compressions (HFCC) and improvement of mucus clearance in cystic fibrosis [abstract]. Am J Respir Crit Care Med. 1994;149:A669.
175 Kearl RA, Hooper RG. Massive airway leaks: An analysis of the role of endotracheal tubes. Crit Care Med. 1993;21:518–521.
176 Brochard L, Rua F, Lorino H, et al. Inspiratory pressure support compensates for the additional work of breathing caused by the endotracheal tube. Anesthesiology. 1991;75:739–745.
177 Raphael DT. Acoustic reflectometry profiles of endotracheal and esophageal intubation. Anesthesiology. 2000;92:1293–1299.
178 Banner MJ, Blanch PB, Kerby RR. Imposed work of breathing and methods of triggering a demand-flow, continuous positive airway pressure system. Crit Care Med. 1993;21:183–190.
179 Harrison GA, Tonkin JP. Prolonged (therapeutic) endotracheal intubation. Br J Anaesth. 1968;40:241–249.
180 Langenderfer B. Alternatives to percussion and postural drainage. J Cardiopulm Rehabil. 1998;18:283–289.
181 Duarte AG, Dhand R, Reid R, et al. Serum albuterol levels in mechanically ventilated patients and healthy subjects after metered-dose inhaler administration. Am J Respir Crit Care Med. 1996;154:1658–1663.
182 Duarte AG, Fink JB, Dhand R. Inhalation therapy during mechanical ventilation. Respir Care Clin North Am. 2001;7:233–260.
183 Fink JB, Dhand R, Duarte AG, et al. Deposition of aerosol from metered-dose inhaler during mechanical ventilation: An in vitro model. Am J Respir Crit Care Med. 1996;154:382–387.
184 Diot P, Morra L, Smaldone GC. Albuterol delivery in a model of mechanical ventilation: Comparison of metered-dose inhaler and nebulizer efficiency. Am J Respir Crit Care Med. 1995;152:1391–1394.
185 Fernandez A, Lazaro A, Garcia A, et al. Bronchodilators in patients with chronic obstructive pulmonary disease on mechanical ventilation: Utilization of metered-dose inhalers. Am Rev Respir Dis. 1990;141:164–168.
186 Frederiksen B, Koch C, Hoiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis patients. Pediatr Pulmonol. 1997;23:330–335.
187 Dhand R, Tobin MJ. Inhaled bronchodilator therapy in mechanically ventilated patients. Am J Respir Crit Care Med. 1997;156:3–10.
188 Goode ML, Fink JB, Dhand R, et al. Improvement in aerosol delivery with helium-oxygen mixtures in mechanical ventilation. Am J Respir Crit Care Med. 2001;163:109–114.
189 Dhand R, Tobin MJ. Bronchodilator delivery with metered-dose inhalers in mechanically-ventilated patients. Eur Respir J. 1996;9:585–595.
190 Dhand R. Special problems in aerosol delivery: Artificial airways. Respir Care. 2000;45:636–645.
191 Beaty CD, Ritz RH, Benson MS. Continuous in-line nebulizers complicate pressure support ventilation. Chest. 1989;96:1360–1363.
192 Martin C, Perrin G, Gevaudan M, et al. Heat and moisture exchangers in the intensive care unit. Chest. 1990;97:144–149.
193 Bowton DL, Goldsmith WM, Haponik EF. Substitution of metered-dose inhalers for hand-held nebulizers: Success and cost savings in a large, acute-care hospital. Chest. 1992;101:305–308.
194 Watson WF. Development of the PVC endotracheal tube. Biomaterials. 1980;1:41–46.
195 Davis B, Marin MG, Yee JW, et al. Effect of terbutaline on movement of Cl− and Na+ across the trachea of the dog. Am Rev Respir Dis. 1979;120:547–552.
196 Bennett WD. Effect of beta-adrenergic agonists on mucociliary clearance. J Allergy Clin Immunol. 2002;110:S291–S297.
197 Rohatagi S, Derendorf H, Zech K. Risk-benefit value of inhaled corticosteroids: A pharmacokinetic/pharmacodynamic perspective. Chest. 2003;123:430s–431s.
198 Rohatagi S, Appajosyula S, Derendorf H, et al. Risk-benefit value of inhaled glucocorticoids: A pharmacokinetic/pharmacodynamic perspective. J Clin Pharmacol. 2004;44:37–47.
199 Hubner M, Hochhaus G, Derendorf H. Comparative pharmacology, bioavailability, pharmacokinetics, and pharmacodynamics of inhaled glucocorticosteroids. Immunol Allergy Clin North Am. 2005;25(3):469–488.
200 Todd GRG, Acerini CL, Ross-Russell R, et al. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child. 2002;87:457–461.
201 Barnes PJ. Inhaled corticosteroids are not beneficial in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:342–344.
202 Fuller HD, Dolovich MB, Posmituck G, et al. Pressurized aerosol versus jet aerosol delivery to mechanically ventilated patients: Comparison of dose to the lungs. Am Rev Respir Dis. 1990;141:440–444.
203 Maddison J, Dodd M, Webb AK. Nebulized colistin causes chest tightness in adults with cystic fibrosis. Respir Med. 1994;88:145–147.
204 Pappas JN, Goodman PC. Predicting proper endotracheal tube placement in underexposed radiographs: Tangent line of the aortic arch. AJR Am J Roentgenol. 1999;173:1357–1359.
205 Itokazu GS, Weinstein RA. Aerosolized antibiotics: Another look. Crit Care Med. 1998;26:5–6.
206 Oberwalder B, Evans JC, Zach MS. Forced expiration against a variable resistance: A new chest physiotherapy method in cystic fibrosis. Pediatr Pulmonol. 1986;2:358–367.
207 Drakulovic MB, Torres A, Bauer TT, et al. Supine position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: A randomized trial. Lancet. 1999;354:1851–1858.
208 Guttmann J, Haberthur C, Mols G. Automatic tube compensation. Respir Care Clin North Am. 2001;7:475–501.
209 Anzueto A, Peters JI, Seidner SR, et al. Effects of continuous bed rotation and prolonged mechanical ventilation on healthy, adult baboons. Crit Care Med. 1997;25:1560–1564.
210 Clemmer RP, Green S, Ziegler B. Effectiveness of the kinetic treatment table for preventing and treating pulmonary complications in severely head-injured patients. Crit Care Med. 1990;18:614–617.
211 Pelosi P, Brazzi L, Gattinoni L. Prone position in acute respiratory distress syndrome. Eur Respir J. 2002;20:1017–1028.
212 Kopterides P, Siempos II, Armaganidis A. Prone positioning in hypoxemic respiratory failure: Meta-analysis of randomized controlled trials. J Crit Care. 2009;24:89–100.
213 Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: Systematic review and meta-analysis. Intensive Care Med. 2010;36:585–599.