Chapter 22 Endoscopic Endonasal Pituitary and Skull Base Surgery
Neuroendoscopy was first implemented almost a century ago for choroid plexus surgery in a patient with hydrocephalus. General enthusiasm for ventricular endoscopy experienced an initial decline with the advent of ventricular shunt systems but was later revived for third ventriculostomies in selected patients. The first reported use of the endoscope specifically for trans-sphenoidal surgery was in a sublabial approach by Guiot and colleagues in 1963.1 However, the general advancement of intracranial and spinal neuroendoscopy continued to be limited, in part trumped by historical developments in neuroimaging and microneurosurgery. Yet in the past three decades, a few pioneering endoscopic neurosurgeons continued to expand and refine the use of neuroendoscopy in endoscope-assisted microsurgery, endonasal trans-sphenoidal surgery, ventricular tumor surgery, extra-axial intracranial surgery, intra-axial brain surgery with stereotactic guidance, and spinal surgery. These advances were accompanied by concurrent technological developments in endoscopic optics, video-imaging systems, endoscopic accessory attachments for neurosurgical applications, specialized neuroendoscopic surgical instruments, radiologic imaging, and compatible frameless stereotactic image-guided systems. As neuroendoscopic surgical techniques and equipment have co-evolved, the addition of neuroendoscopy to the repertoire of the modern neurosurgeon has become increasingly practical. Of note, general interest has noticeably grown for the use of neuroendoscopy in endonasal trans-sphenoidal pituitary surgery, and the common use of neuroendoscopy in pituitary surgery became truly practical in recent years with the development of commercially-available neuroendoscopic equipment.
Although the removal of pituitary tumors completely through endoscopic visualization via an endonasal route has been a relatively recent development, the use of the endonasal pathway itself was initially reported in 1909 by Hirsch who performed his first pituitary surgery in Vienna by approaching the sella through an endonasal route using multiple-staged sinonasal operations with naked-eye visualization. Despite his first endonasal trans-sphenoidal surgery having reported success, Hirsch subsequently converted to a trans-septal submucosal approach, possibly due to fear of surgical infection through such a wide communication made between the nasal and the cranial cavity. Griffith and Veerapen revisited the endonasal approach in 1987, with insertion of a trans-sphenoidal retractor through the natural nasal airway to the sphenoid rostrum for microscopic pituitary surgery.2 In 1994, Cooke and Jones reported the lack of sinonasal and dental complications when an endonasal route was adopted for microscopic pituitary surgery.3 But the most significant progression of the traditional sublabial and transfixional-trans-septal approaches to the direct endonasal route was highly facilitated by the neuroendoscope, with the initial use of sinonasal endoscopy in Europe four decades ago. In the field of Otolaryngology, the introduction of endoscopic sinus surgery to the United States kindled an evolution in surgical techniques such that endoscopic sinus surgery rapidly replaced many forms of conventional sinus surgery, with radical changes in concepts of sinonasal pathophysiology and associated treatments aided by endoscopic exploration. Rather than stripping the infected sinus mucosa as was done in conventional sinus surgery, endoscopic sinus surgery aimed to restore physiologic mucous drainage merely by eliminating obstructive pathoanatomy at sinus ostia via the endonasal route and became popularized as functional endoscopic sinus surgery (FESS). Successful advances in sinonasal endoscopy then enhanced interest in the use of endoscopy for trans-sphenoidal surgery. Endoscopic trans-sphenoidal surgery started with guidance during simple biopsy of a sellar lesion, and then evolved to assist visualization during insertion of trans-sphenoidal retractors or during microscopic removal of pituitary adenomas, and eventually the pure form of endoscopic endonasal pituitary tumor surgery emerged.4–12
This chapter describes endoscopic endonasal trans-sphenoidal surgery (EE-TS) along with related endoscopic endonasal approaches to the midline skull base such as the anterior cranial fossa (EE-ACF), optic nerve or cavernous sinus (EE-CS), pterygoid fossa or petrous apex (EE-Pterygoid or EE-Petrous), clivus or posterior fossa (EE-PFossa), and craniocervical junction (EE-CC junction). EE-TS is not merely endoscope-assisted microscopic surgery but is rather an operation done completely with an endoscope without any trans-sphenoidal retractor or nasal speculum, eliminating the need for postoperative nasal packing or other adjuncts. The physical nature of an endoscope with its optics at the tip and slender shaft allows simple access to the sella through the natural nasal air pathway via a nostril. EE-TS uses an endonasal route to the rostrum of the sphenoid sinus with an anterior sphenoidotomy about 1 to 1.5 cm in diameter. The wide-angled panoramic view, angled-lens views, and a close-up zoom-in view provide optical advantages with distinct visualization at the surgical target site. The application of principles and anatomy in EE-TS was extended to the surgical treatment of midline skull base pathologies (from the anterior cranial fossa to the clivus and posterior fossa along with the craniocervical junction) and paramedian skull base pathologies (from the optic nerve and cavernous sinus regions to the pterygoid fossa and petrous tip). Endoscopic endonasal techniques can potentially be applied to nearly any lesion within approximately 2-cm width of the midline skull base from the crista galli at the anterior-superior skull base to the foramen magnum and atlantoaxial region at the posterior-inferior skull base.13–23
Pertinent Sinonasal Anatomy
The nasal cavity itself is bordered medially by the nasal septum (comprised of the septal cartilage, perpendicular plate of the ethmoid bone, and the vomer); superiorly by the cribriform plate of the ethmoid bone and bridge of the nose (consisting of the nasal portion of the frontal bone, nasal bone, and frontal process of the maxilla); inferiorly by the floor of the nasal cavity (involving the palatine process of the maxilla and the horizontal plate of the palatine bone); and conchae or turbinates laterally (inferior, middle, superior, and sometimes supreme turbinates). The superior and middle conchae (along with the occasional supreme concha) are components of the ethmoid bone, whereas the inferior concha is a separate bone. The EE-TS procedure traverses the region medial to the middle turbinate, between the middle turbinate and the nasal septum, on the way to the sphenoid sinus then the pituitary fossa at the sella turcica. Nasal septal deviation is not an uncommon phenomenon, such that often the larger nasal cavity is selected as the route for EE-TS based on preoperative imaging and intraoperative visualization.
As mentioned, there can be significant variability in the structure of the ethmoid sinus, which is sometimes termed the ethmoid labyrinth, with the ethmoid air cells having some surgically pertinent variations for EE-TS. The agger nasi is a mound or prominence on the anterior-lateral aspect of the nasal cavity formed by mucous membrane covering the ethmoidal crest of the maxilla near the anterior aspect of the middle turbinate. The agger nasi cells are the most anterior ethmoidal cells located just anterior and lateral to the nasofrontal recess and can sometimes be involved in frontal sinus outflow obstruction. For EE-TS, the surgeon should be aware that a hyperpneumatized agger nasi cell can occasionally present as a bulge that mimics the anterior view of a turbinate. Haller cells (infraorbital ethmoid air cells or maxilloethmoidal cells) are closely related to the ethmoid infundibulum along the medial roof of the maxillary sinus, inferior-lateral to the ethmoid bulla, and extend into the inferomedial orbital floor at the inferior margin of the lamina papyracea. Since Haller cells are quite lateral, these usually do not present a problem during EE-TS, although their proximity to the ethmoid infundibulum can result in inadvertent orbital entry during FESS if not recognized. Onodi cells (sphenoethmoidal cells) are posterior ethmoidal air cells that can project superiorly into the sphenoid sinus towards the lateral side and can potentially be confused with a septated region of the sphenoid sinus. The optic nerve and/or internal carotid artery can bulge into Onodi cells instead of the sphenoid sinus proper, or occasionally may have either partial or complete bony dehiscence at the sphenoid sinus, presenting risk for injury during surgery. It is also possible to have extensive pneumatization of the anterior clinoid process at the optico-carotid recess (OCR), which can also expose the optic nerve and internal carotid artery to additional risk. It is also possible for patients who have undergone previous trans-sphenoidal surgery to have a mucocele at the region of the sphenoid sinus, which can also potentially lead to intraoperative confusion, although this is usually recognizable on the preoperative MRI with the mucocele usually having a spherical type of shape and bright T2 signal such that it can be anticipated intraoperatively.
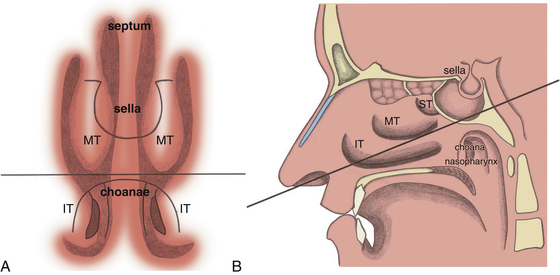
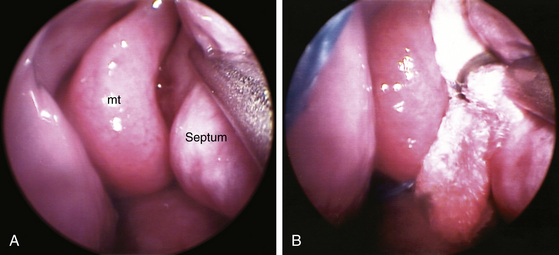
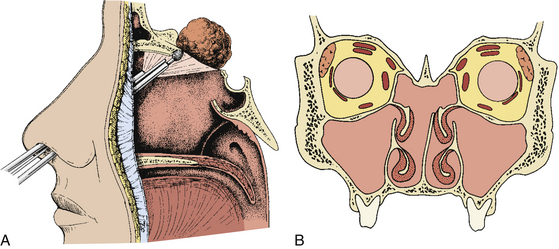
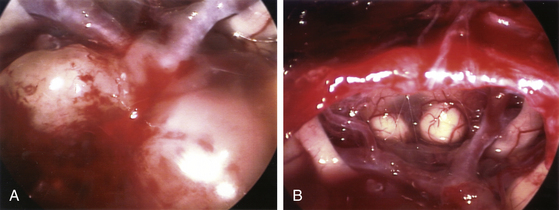
When the sphenoid sinus is entered with an endoscope, the complex anatomy is visualized in a panoramic fashion. The clival indentation is seen at the bottom midline, the bony protuberances covering the internal carotid arteries are lateral to the clival indentation, the sella is at the center, the cavernous sinuses are seen lateral to the sella, the tuberculum sella is at the top, and the bony protuberances of the optic nerves are seen laterally. Surgical landmarks for endoscopic endonasal pituitary surgery consist of the choanae and nasopharynx along with the inferior margin of the middle turbinate. The choana at the anterior-superior entry of the nasopharynx is always a useful landmark in order to confirm the middle turbinate. The extended line along the inferior margin of the middle turbinate leads to the region approximately 1 cm inferior to the sellar floor. Although the sphenoid sinus ostium at the sphenoethmoidal recess may also be visible under the endoscope, it may not always be easily identifiable or precisely consistent in its relationship to the sella. Thus, the sphenoid sinus ostium can be regarded as an inconsistent surgical landmark, whereas the choana and inferior margin of the middle turbinate tend to be quite consistent. Anterior sphenoidotomy measuring approximately 1.5 cm in diameter is performed at the rostrum of the sphenoid sinus at a location rostral to an extended line from the inferior margin of the middle turbinate (Fig. 22-1A and B).
The nasal mucosa is predominantly supplied by the sphenopalatine artery, with contributions from the greater palatine artery, branches of the facial artery, and the anterior and posterior ethmoidal arteries. The posterior septal artery arises from the sphenopalatine branch of the internal maxillary artery and passes to the posterior nasal septum at the inferior-medial aspect of the middle turbinate posterior margin. When surgical access is obtained between the middle turbinate and nasal septum for an anterior sphenoidotomy, the posterior septal artery often requires coagulation and division to prevent unwanted intraoperative or postoperative nasal bleeding. Delayed copious nasal bleeding after trans-sphenoidal surgery usually arises from rebleeding of the posterior septal artery.
For endoscopic endonasal approach to the anterior cranial fossa (EE-ACF), the region of the ethmoid roof with the cribriform plate and the fovea ethmoidalis should be studied on preoperative imaging. Keros categorized three types of skull base conformations along a spectrum depending on the depth of the olfactory sulcus and corresponding height of the lateral lamella, with Type 1 having 1-3 mm depth, Type 2 having 3-7 mm depth, and Type 3 having 7-16 mm depth such that the ethmoid roof is significantly higher than the cribriform plate.24 The Keros classification is more pertinent for ethmoid sinus surgery to avoid inadvertent entry through the thin lateral lamella of the cribriform plate, especially for a deep olfactory sulcus. However, for EE-ACF or EE-CS (via the middle meatal approach with posterior ethmoidectomy), one should be aware of the potential individual variations in depth of the olfactory sulcus and the specific anatomic configuration should be noted preoperatively to assist with optimal intraoperative maneuvering. In the same region, the ethmoidal sulcus is the groove in the lateral lamella for the anterior ethmoidal artery, which branches from the ophthalmic artery and enters from the orbit in a bony canal in the ethmoid roof (anterior ethmoidal canal) or just below the level of the ethmoid roof to course anteromedially with the anterior ethmoidal nerve and to penetrate the lateral lamellae to supply the dura in the region of the olfactory sulcus. The posterior ethmoidal artery traverses the posterior ethmoidal canal within a 3 mm planar region above the cribriform plate. The anterior and posterior ethmoidal arteries usually provide the major blood supply for olfactory groove or planum sphenoidale meningiomas at the skull base.
Optical Advantages of an Endoscope
Wide-Angled Panoramic View
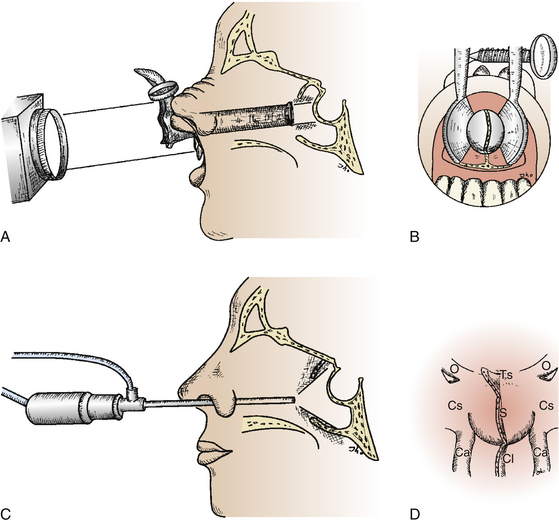
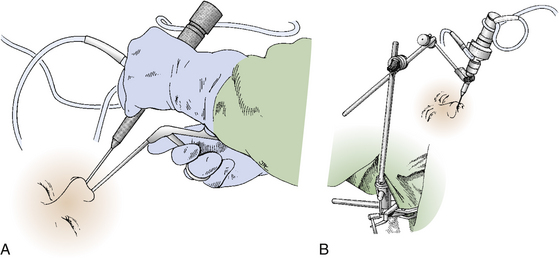
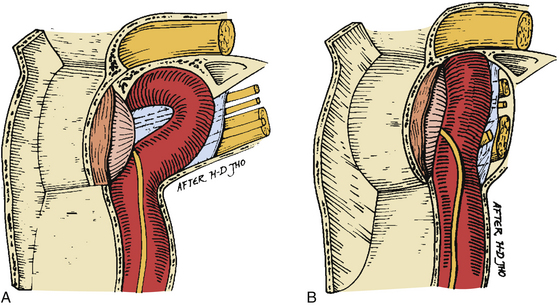
As trans-sphenoidal pituitary surgery began its evolvement in the 19th century, one major advance was the adoption of the operating microscope in the 1960’s. The use of the endoscope for pituitary tumor resection represents another significant advancement. Whereas the operating microscope provides a magnified view of a limited portion of the sella through a narrow corridor revealed by the trans-sphenoidal retractor, an endoscope can physically enter into the sphenoid sinus and provide a wide-angled panoramic view with zooming capability. An operating microscope renders a tubular parallel beam view, but an endoscope shows a diverging flask-shaped wide-angled view (Fig. 22-2A to D). This wide-angled panoramic view is particularly useful for pituitary tumor surgery because it allows excellent anatomical visualization at the posterior wall of the sphenoid sinus. However, it must be recognized that the endoscopic view renders a fish-eye effect with maximum magnification at the center and relative contraction at the periphery with visualization of a wide anatomical area. In the well-pneumatized sphenoid sinus, the sella is readily recognizable at the center of the surgical view, and a panoramic image of the surrounding anatomy at the posterior wall of the sphenoid sinus is revealed under direct endoscopic view. Unless the region of the sella is not pneumatized or the patient is a complicated reoperation case, the use of fluoroscopic roentgenogram is not necessary since endoscopic visualization can adequately reveal the distinct surgical anatomy.
Angled-Lens View
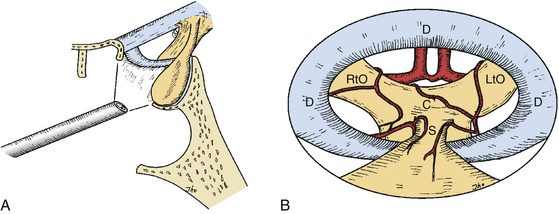
The angled-lens endoscopic view provides direct visualization of the anatomical corners such as the suprasellar area or towards the cavernous sinus. These views can be of great assistance even if an endoscope is only used as an adjunctive tool during conventional microscopic surgery. Operating under an angled-lens endoscopic view requires specially designed surgical tools and advanced endoscopic surgical skills, particularly for the 70-degree-lens endoscope. As an angled-lens endoscope is rotated towards the surgical target, various anatomical corners can be visualized from the floor of the sella to the medial wall of the cavernous sinus and towards the suprasellar region. A fiberoptic endoscope can sometimes be used to inspect anatomical corners involving curved routes. This angled view is advantageous when large suprasellar macroadenomas are to be removed. It also allows clear visualization at the medial wall of the cavernous sinus when the lateral margin of a sellar tumor abutting the cavernous sinus is dissected away or when tumor tissue invading the cavernous sinus is to be removed under direct visualization. Although pituitary adenomas with significant invasion of the cavernous sinus were once generally regarded as inoperable, EE-TS allows safe access to the cavernous sinus for tumor removal. Angled views allow for direct surgical access to the pterygoid fossa, anterior cranial fossa, clivus, and posterior cranial fossa in addition to the cavernous sinus (Fig. 22-3A and B).
Surgical Procedure
Surgical Instruments
Appropriate surgical equipment is necessary to perform optimal endoscopic pituitary surgery. Attempting an endoscopic operation of this nature with a borrowed otolaryngologic endoscope can potentially result in frustration. Endoscopic surgical techniques are quite different from those of microscopic surgery. Being well-trained in microscopic surgery does not preclude the need for practice in endoscopy. The required surgical instruments are endoscopes with 0-, 30-, and 70-degree lenses (Fig. 22-4A) and their appendages, including a video-imaging system and light source connections, an endoscope lens-cleansing device, a rigid endoscope holder, and various other surgical instruments specifically designed for endoscopic pituitary surgery. The length of an endoscope must be 18 cm or longer. When an 18 cm-long endoscope was used for removal of a posterior fossa tumor through an endonasal transclival approach, it proved to be marginally short and restricted the surgeon’s operating space between the endoscopic appendages and the patient’s face. Fluoroscopic guidance, which was used in earlier patients, is no longer used in routine pituitary surgery. It is rarely but occasionally used for anterior or posterior cranial fossa surgery, and for pituitary tumor patients in which complexity of the sinonasal anatomy is anticipated.
An endoscopic lens-cleansing device is required to cleanse the lens so that the surgeon can operate without interruption (Fig. 22-4B). The device consists of a disposable irrigation tube, which has a loop of tubing passed through a battery-powered rotary device. The irrigation tube is connected into a warmed saline bag (the temperature prevents lens fogging), which is hung on a pole, and the motor-powered irrigation device is controlled by a foot pedal to flush saline forward. When the foot pedal is released, the motor reverses its rotary direction and draws the saline back for 1 to 2 seconds. The forward flow of irrigation saline cleans the lens, and the reverse flow clears water bubbles at the tip of the endoscope. Although this device is not yet perfect, it helps the surgeon significantly in the task of keeping the endoscope lens clean in order to preserve the optimal technical continuity and flow of the procedure.
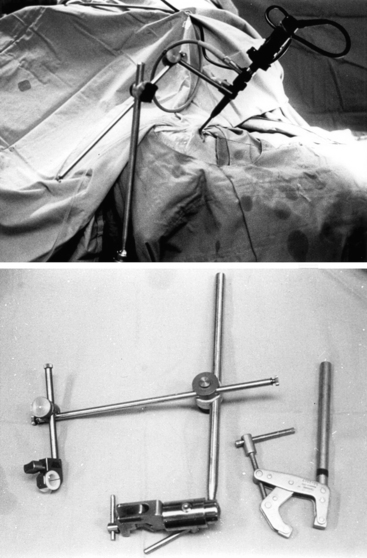
Appropriate endoscope holders help provide stability of the visual image and bimanual instrument use during portions of the case during which this is optimal, such as drilling and the majority of tumor removal (Fig. 22-5). Although there are portions of surgery during which dynamic visualization is important, such as the initial approach to the sphenoid sinus rostrum and final visualization of any tumor remnants at anatomic corners of the sella, endoscope holders provide camera stability akin to a video camera tripod during appropriate segments of surgery and does not require the presence of a trained assistant to constantly drive or hold the endoscope camera. The endoscope holder should also provide rigid fixation of the endoscope while also allowing easy transition between stable fixation and manual dynamic steering. The holding terminal is necessarily compact and slender so as to render adequate operating space around the endoscope shaft for the surgeon to maneuver surgical instruments. We routinely use a customized manual endoscope holder specifically designed for EE-TS, in contrast to a nitrogen-powered holder called the Mitaka Point Setter (Mitaka USA; Park City, Utah) for our endoscopic transcranial approaches, and a nitrogen-powered holder called Unitrac (Aesculap; Tuttlingen, Germany) for our endoscopic spine surgery. Manual holders have multiple joints that are tightened by hand to set the final position, but only a single joint has to be loosened during a case to transition from endoscope fixation to manual driving or back. Manual holders are highly stable without significant constraints in range-of-motion or disadvantage of the settling phenomenon, but the configuration can purposefully be set such that the range-of-motion of the final joint is constrained to maintain the trajectory of the endonasal route for EE-TS and depth adjustments only require the simple untightening of a single joint. Nitrogen-powered holders are more expensive but conveniently provide release and tightening using a single button. The Mitaka Point Setter provides excellent stability and minimal settling phenomenon but is limited by the constrained range-of-motion to cases that require only minor maneuvering of the endoscope holder. The Unitrac is stable and highly maneuverable but displays significant settling phenomenon such that the final endoscope position sinks somewhat with gravity before locking in final position. Other endoscope holders are still in development to attempt to maximize the strengths and minimize the weaknesses of the various endoscope holders.
Myths in Sinonasal Surgery
Since the nasal cavity cannot truly be conventionally prepared with extensive antiseptic solutions, we prescribed postoperative oral antibiotic regimens empirically for 5 days in our early patients. Subsequently, postoperative antibiotic treatment has been completely abandoned without any evidence of increased infection. We still swab the nasal cavity with an antiseptic solution during surgical preparation, but even this practice may not be clearly necessary.
Positioning
The patient is positioned supine with the torso elevated about 15 to 20 degrees, and the head is positioned with the forehead-chin line set horizontally. The level of the head is placed a little higher than that of the heart with the intent that the cavernous sinus venous pressure stays low to minimize venous bleeding. The horizontally leveled head positioning allows the surgeon to access the region of the middle turbinate easily and naturally for EE-TS when the endoscope is inserted at approximately 25 degrees cephalad.11 When the anterior cranial fossa is to be explored as in EE-ACF, the head is extended approximately 15 degrees.19 Conversely, the head is flexed by 15 degrees when the clival or posterior fossa region is to be explored in EE-PFossa.22 The head is rotated toward the surgeon at 10 to 20 degrees. A head pin fixation device can optionally be used but is not necessary. The hip and knee joints are gently flexed for patient comfort, and a soft pillow is placed underneath the knee joints. A Foley catheter is not routinely used but is inserted occasionally in selective patients who are expected to undergo longer operating hours or for the possible anticipated occurrence of diabetes insipidus. Fluoroscopic C-arm imaging is not used except in patients with anterior fossa or posterior fossa tumors or in patients with complex sinonasal anatomy. Ophthalmic eye ointment is placed on the cornea and conjunctiva, and the eyelids are taped closed with double-layers of soft vinyl adhesives to prevent corneal abrasions and infiltration by prep solutions. The oropharynx is packed with a 2-inch gauze roll to prevent the accumulation of blood at the perilaryngeal area during surgery, which may cause aspiration at the time of extubation.
Surgical Approaches
An endoscopic endonasal approach to the sella can be made by a paraseptal, middle meatal, or middle turbinectomy approach.10,19 The paraseptal approach is the least invasive and basic workhorse among these three approaches, and the surgical technique described here for EE-TS involves the paraseptal approach. The paraseptal approach is made between the nasal septum and the middle turbinate with temporary displacement of the middle turbinate laterally and contralateral displacement of the posterior nasal septum by fracturing at the sphenoid rostrum. The fractured nasal septum is pushed away by submucosal dissection at the contralateral side of the sphenoid rostrum.
Surgical Technique of Endoscopic Endonasal Trans-Sphenoidal Surgery
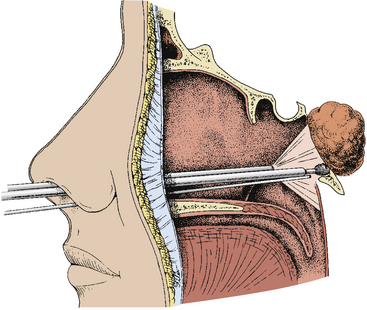
The endoscopic endonasal trans-sphenoidal (EE-TS) procedure can now be thought of as four basic steps: manual endoscope navigation to the sphenoid sinus, opening of sphenoid and sella, tumor removal, and closure. As an overview, the initial step of endonasal navigation to the rostrum of the sphenoid sinus uses the pathway between the nasal septum medially and the middle turbinate laterally while steering the endoscope with one hand and using a suction in the other hand to help lead the endoscope under direct visualization (Fig. 22-6A). Once the target site of the sphenoid sinus rostrum is exposed, the endoscope can be placed in its holder and the drill is used in one hand with the suction in the other to make the anterior sphenoidotomy and to access the sellar target (Fig. 22-6B). When the anterior sphenoidotomy is complete, the endoscope can be adjusted to an optimal surgical position in the endoscope holder for sellar entry and tumor removal. Finally, after tumor removal, the sellar bone is replaced and the middle turbinate pushed back into position during closure of the operation.
When the endoscope is held in the hand, the palm and last three digits are generally used to stabilize the endoscope shaft, and the index finger and thumb are used to steer the video camera to maintain anatomic orientation of the video image. This endoscope grip will naturally keep the endoscope at a 25-degree incline and allows for continuous steering of the video camera for maintaining correct orientation of the video image. When the patient’s head is positioned horizontally with the forehead-chin line parallel to the floor, the middle turbinate is easily visualized at the tip of the endoscope when it is inserted into the nasal cavity. If the space between the nasal septum and middle turbinate is limited, several half- by three-inch cotton patties are inserted between the septum and middle turbinate to gently widen the space with minimal trauma (Fig. 22-7A and B). The cotton patties are pushed down to the rostrum of the sphenoid sinus. Care must be taken not to traumatize the mucosa in the nasal cavity while surgical instruments are being inserted and removed. The degree of surgical trauma to the nasal mucosa is a crucial factor in determining the amount of postoperative nasal bleeding. When a sharp-edged surgical instrument is inserted, the instrument tip must be guided by direct endoscopic visualization. The insertion of the surgical instrument should ideally be impelled by the gravity of the surgical instrument itself rather than surgeon’s mechanical push. If cotton patties are required to develop the middle turbinate-septal space, the cotton patties are removed after a few minutes and the developed space will remain, although there may be some mild mucosal bleeding. Mucosal bleeding from the rostrum of the sphenoid sinus in the middle turbinate-septal space is controlled with electrocoagulation using the malleable monopolar suction coagulator. The mucosa at the rostrum of the sphenoid sinus is visualized with the manual-driven endoscope and completely coagulated before being divided vertically approximately 1.5 cm in length rostrally from the level of the inferior margin of the middle turbinate. The posterior septal artery arises from the sphenopalatine artery and passes at the inferolateral corner of the sphenoethmoidal recess, which is approximately the posteromedial corner of the inferior margin of the middle turbinate. The posterior septal artery must often be coagulated and divided to prevent intraoperative and postoperative nasal bleeding. The site for an anterior sphenoidotomy is again confirmed with reference to the surgical landmarks, which are the inferior margin of the middle turbinate and the choana of the nasopharynx (Fig. 22-8).
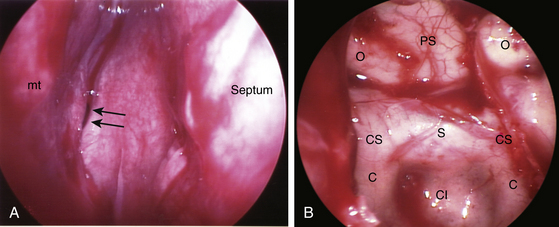
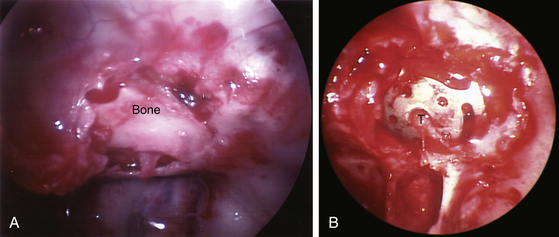
The second part of EE-TS involves use of the customized endoscope holder, which is an L-shaped connection from the operating table that connects to a reverse L-shaped segment running parallel to the trajectory of the endonasal pathway and a distal clamp that holds the endoscope itself. The endoscope is placed back into the nasal cavity following the same originally established trajectory along the endoscope holder with the coagulated sphenoid rostrum target reidentified at the sphenoid sinus and vomer interface. The endoscope is fixed in the endoscope holder, and a 4 mm diamond drill bit is used to drill the posterior nasal septum at the vomer and sphenoid rostrum to allow displacement towards the contralateral side. The floor of the sphenoid sinus is drilled along with only the underlying focal portion of mucosa (with the remaining sphenoid sinus mucosa left intact). The floor of the sella turcica presents as a bulge or mound with the prominences of the carotid arteries and cavernous sinuses bilaterally visualized and correlated with any anticipated anatomic variations from the preoperative MRI. The prominences of the optic nerves, the opticocarotid recess (OCR), and the tuberculum sella are often anterior to the pituitary target location and may not necessarily need to be exposed. The malleable coated suction monopolar is turned down to a setting of 10V (from 25V used for mucosal coagulation at the outer face of the sphenoid sinus during the first part of EE-TS) and the bipolar is routinely set at 35V (with adjustment as needed for any intradural use). The anterior sphenoidotomy to be made ranges from the inferior margin of the middle turbinate to the sphenoid sinus ostia, which is typically about 1 to 1.5 cm rostral from the inferior margin of the middle turbinate. However, the plane of the inferior margin of the middle turbinate is the consistent anatomic landmark. Although the sphenoid sinus ostia may be visualized directly (Fig. 22-9A), its identification is not necessary for performing an anterior sphenoidotomy. When the sphenoid sinus is entered near the level of the inferior margin of the middle turbinate, any further rostral exposure can be easily made relative to the exposed sellar floor. When an anterior sphenoidotomy is first attempted rostrally, the surgeon may erroneously enter the anterior cranial fossa because the superior turbinate can sometimes mimic the middle turbinate. Therefore, the middle turbinate must be confirmed in reference to the choana of the nasopharynx. The inferior margin of the middle turbinate is located just rostral to roof of the choana at the nasopharynx in a sagittal plane. Following the inferior margin of the middle turbinate posteriorly then leads to the clival indentation at approximately 1 cm inferior to the level of the sellar floor.
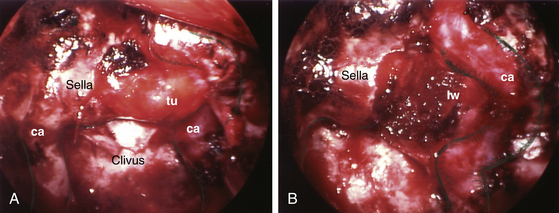
Anterior sphenoidotomy can be made with either of two different surgical techniques: power-drilling or rongeuring with fracture. In the first technique, use of a power-drill creates a clean opening at the anterior wall of the sphenoid sinus. The endoscope is placed on the endoscope holder, and the power-drill is inserted along with a suction cannula next to the endoscope shaft. The vomer is drilled first, and then the nasal septum is pushed to the contralateral side when it loosens. At the contralateral rostrum of the sphenoid sinus, submucosal dissection is carried-out to expose the bilateral rostrum of the sphenoid sinus. Then the exposure at the rostrum of the sphenoid sinus is very much similar to the exposure through a conventional trans-septal approach. Drilling is performed along the lateral gutter at the anterior wall of the sphenoid sinus, and the sphenoidal mucosa is exposed bilaterally. Using Kurze scissors, an opening is made at the sphenoid sinus mucosa. Then the posterior wall of the sphenoid sinus can be directly visualized demonstrating the tuberculum sella, clivus, cavernous sinus, and optic system. The second technique involves rongeuring after mechanical fracture of the nasal septum and vomer from the rostrum of the sphenoid sinuses using the septal breaker, which is a special instrument designed for this endoscopic operation. The rostral nasal septum is relatively easy to break; however, the caudally located vomer is often too thick to break without using the specially made septal breaker. The fractured nasal septum is pushed contralaterally, and the contralateral rostrum of the sphenoid sinus is dissected submucosally. The anterior aspect of the sphenoid rostrum is exposed bilaterally. With Kerrison rongeurs, the anterior wall of the sphenoid sinus is first opened along the lateral gutter. Then the bony opening of an anterior sphenoidotomy is completed using Kerrison rongeurs. The sinus mucosa is opened in the same manner as previously mentioned. Attention is paid not to strip the sphenoid sinus mucosa since inadvertent stripping can cause unwanted oozing of blood from the bony sinus wall. The anterior wall of the sphenoid sinus is removed performing an anterior sphenoidotomy about 1 to 1.5 cm in size. The sphenoid sinus septum is trimmed with a power-drill or rongeurs. Further rostral extension of the anterior sphenoidotomy is performed accordingly relative to the sella. Often the sella is exposed from the tuberculum sella to the clival indentation in the vertical dimension and from one cavernous sinus to the other cavernous sinus in the transverse dimension. At this point, endoscopic view demonstrates the tuberculum sella rostrally, optic protuberances at 11- and 1-o’clock positions, the bony wall covering the cavernous sinus and carotid artery laterally, clival indentation caudally, and the internal carotid arteries at 7- and 5-o’clock locations (Fig. 22-9B). The endoscope is adjusted in the endoscope holder as the endoscope tip is advanced for a close-up view of the sella.
In the case of a microadenoma covered by normal pituitary tissue, the pituitary tissue is sliced or split with a Jannetta 45-degree microdissector to locate the tumor tissue. The identified tumor tissue is removed by curettage; a thin layer of the normal pituitary tissue is then shaved-off at the margin of the resection site, especially to optimize the chance of endocrine cure for functional microadenomas. For macroadenomas, the tumor tissue often spills out when the dura mater is opened. Care has to be taken not to lose the tumor specimen by suctioning since the tumor tissue should be sampled for pathologic examination. Once enough of the tumor specimen is collected, the tumor is removed with a suction cannula at the central portion of the sella for debulking. A pituitary ring curette in one hand with suction in the other hand or dual suction cannulas (No. 5 or 7 French) can be used for soft tumor removal. When the tumor is fibrotic, either from previous medical and surgical treatments or by its intrinsic nature, the tumor tissue is gently curetted with a pituitary ring curette held in one hand, in addition to being suctioned with a suction cannula held in the other hand. When the tumor resection cavity is created at the central portion of the pituitary fossa, a 45-degree angled curette is first used followed by a 90-degree angled curette to remove the lower portion of the tumor from the floor of the sella. An inferiorly angled pituitary ring curette is used in one hand and an inferiorly curved suction cannula in the other hand for removal of the lower portion of the tumor. The dura mater at the floor of the sella is exposed directly when the lower portion of the tumor is removed. Next the lateral portion of the tumor is removed with a superiorly angled suction cannula and a superiorly angled pituitary ring curette, 45-degrees as well as 90-degrees. The medial wall of the cavernous sinus is directly exposed when the lateral portion of the tumor is removed. The rostral portion of the tumor is removed circumferentially with various superiorly curved and angled suction cannulas and pituitary ring curettes. When normal pituitary gland tissue is identified, it is preserved as much as possible. When the diaphragm sella is identified along the peripheral edge of the rostral portion of the tumor, the tumor is continuously removed circumferentially. When the tumor is removed along the edge of the diaphragma sella, the suprasellar portion of the tumor progressively descends through the central opening of the diaphragma sella. The suprasellar portion of the tumor that progressively descends is continuously removed with either two superiorly curved suction cannulas or a suction cannula and a pituitary ring curette, both angled superiorly.
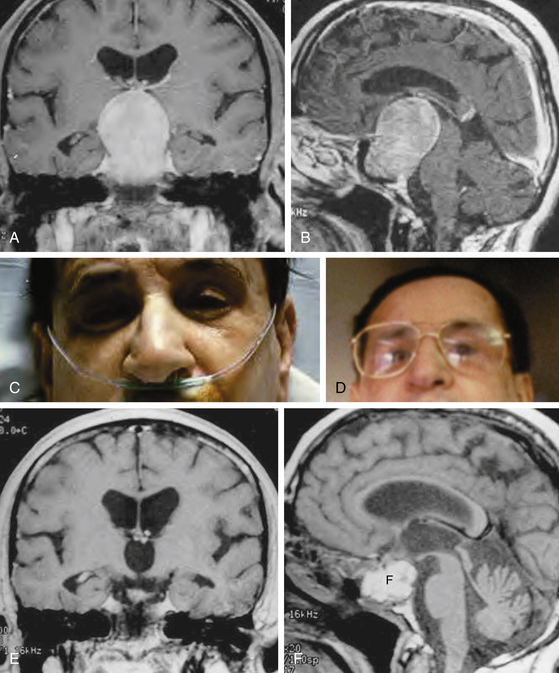
When the tumor is so solid and fibrotic that the suprasellar portion of the tumor does not descend spontaneously, the suprasellar portion can be exposed directly using a 30-degree lens endoscope or by further removal of the bone at the planum sphenoidale or tuberculum sella. When the arachnoid membrane is ruptured, the optic nerves and chiasm, anterior cerebral artery system, and inferior aspect of the hypothalamus are under direct view with a 30-degree lens endoscope. When CSF leakage does not occur intraoperatively and the tumor is a microadenoma, an abdominal fat graft is unnecessary. After removal of a large macroadenoma, the tumor resection cavity is supported with an abdominal fat graft (Fig. 22-10A to F). Occasionally a piece of Gelfoam sponge is used instead of an abdominal fat graft when a sufficient amount of the pituitary tissue still remains rostrally. An abdominal fat graft is harvested using a 1- to 2-cm transverse skin incision just inferior to the umbilicus. The anterior wall of the sella is reconstructed using autogenous bone saved at the time of the anterior sphenoidotomy (Fig. 22-11A). When autogenous bone is not available, a piece of thin titanium mesh is placed (Fig. 22-11B). No foreign material is placed in the sphenoid sinus or nasal cavity.
Endoscopic Endonasal Approach to the Anterior Cranial Fossa
For meningiomas located in the olfactory groove, planum sphenoidale, or tuberculum sella and for repair of CSF leakage at the anterior cranial fossa, the endoscopic endonasal approach to the anterior cranial fossa skull base (EE-ACF) has been employed (Fig. 22-12A).16,17,19 This approach is also useful for suprasellar craniopharyngiomas or large suprasellar pituitary tumors with fibrotic solid consistency. For tumor removal, a paraseptal approach is used (Fig. 22-12B). For CSF leak repair, either a paraseptal or a middle meatal approach can be used. Fluoroscopic C-arm imaging or frameless stereotactic image-guidance system can be used for optimizing the trajectory to the target lesion during EE-ACF.
For EE-ACF tumor surgery, a paraseptal approach is often used. For tumors located at the olfactory groove, planum sphenoidale, or tuberculum sella, the surgical approach is similar to the aforementioned EE-TS pituitary operation except that further rostral exposure is required. The middle turbinate is laterally displaced, and the nasal septum is fractured contralaterally. A rostral anterior sphenoidotomy is performed to enter the sphenoid sinuses, and then the sella and tuberculum sella are identified. Under fluoroscopic guidance, further rostral exposure is made at the anterior skull base removing the posterior ethmoid sinuses. This approach itself interrupts anterior or posterior ethmoidal arteries during exposure, resulting in major devascularization of the meningioma. Approximately a 2-cm wide portion of the midline anterior skull base can be exposed with this technique. Bone of the skull base can be removed with a high-speed drill or Kerrison punch. The dura mater is opened, and the tumor is removed with central debulking followed by peripheral dissection. When the central portion of the tumor is excised, peripheral dissection is carried out to inspect the posterior aspect of the tumor. The remaining tumor is gradually flipped, and dissection along the posterior wall of the tumor is carried out until the remaining tumor is excised (Fig. 22-13A to D). Prudent selection of meningiomas should be considered for EE-ACF approaches versus transcranial approaches (whether minimally invasive endoscopic or open microscopic) because the inability to achieve at least a Simpson grade 2 resection due to inadequate exposure may result in suboptimal recurrence rates.
The main potential problem related to this surgery is postoperative CSF leak and subsequent meningitis. Therefore adequate skull base reconstruction is essential. When active CSF leak is noted at the time of surgery, a small piece of fat graft is inserted at the various corners intracranially in order to obstruct the active CSF inflow. Then a large dural graft is placed intradurally with enough margin overlapping, dural suture is performed with titanium clips, and another layer of dural graft is laid extradurally. There are other similar methods of dural graft reconstruction described such as composing the double-layer graft with a central suture prior to placement at the skull base defect or using alternative methods of dural suturing such as nitinol U-clips. The skull base defect reconstruction is then supported with autogenous bone graft or titanium mesh placement. Abdominal fat grafts are used at the ethmoidectomy site as additional support (Fig. 22-14A to C). When fat graft material is placed at the tumor resection cavity, care must be given not to cause unintended compression to the optic nerve system. Nasoseptal flaps have been reported but may not be routinely necessary depending on the skull base defect. The middle turbinate is placed back in its normal position to complete the operation.
Endoscopic Endonasal Approach to the Optic Nerve or Cavernous Sinus
Although surgical indication for decompression of the optic nerve is a controversial issue, the optic nerve at the optic canal can be easily exposed using this endoscopic endonasal approach.13 The surgical approach to the cavernous sinus is similar to that of the optic nerve.14,15,20,21 This endoscopic approach to the cavernous sinus is best suited for pituitary adenomas invading the cavernous sinus. Biopsy for the histologic diagnosis of cavernous sinus lesions can be performed with this technique. Tough fibrotic tumors in this region, such as some types of meningiomas, can be challenging to remove using endoscopic technique. The endoscopic endonasal cavernous sinus (EE-CS) procedure is an anteromedial approach, and the fact that cavernous cranial nerves are located towards the lateral wall of the cavernous sinus makes this approach advantageous.
The optic nerves or cavernous sinuses can generally be approached through a paraseptal approach, with increasing exposure as needed by creating a middle meatal approach or even further exposure by using a middle turbinectomy approach.15 In the paraseptal approach, the endoscope visualizes the contralateral optic nerve or cavernous sinus better than it does the ipsilateral one, and this technique exposes the anteromedial aspect of the cavernous sinus (Fig. 22-15A). The anteromedial exposure of the optic nerve or cavernous sinus through the contralateral nostril using the paraseptal approach is similar to that of the previously described EE-TS for pituitary adenomas except that the trajectory is adjusted by entry through the contralateral nasal passage from the lesion. The middle meatal approach with a posterior ethmoidectomy provides a straight anterior approach to the cavernous sinus (Fig. 22-15B). The largest corridor to the optic nerve or cavernous sinus is provided by the middle turbinectomy approach, but overall the paraseptal approach is generally sufficient for approaching the optic nerve or cavernous sinus for the majority of lesions.
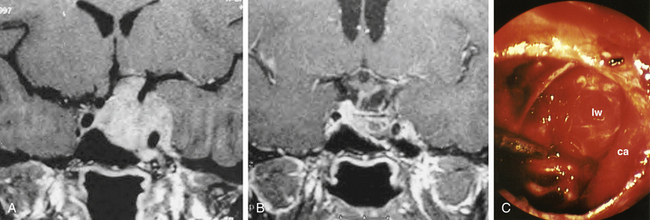
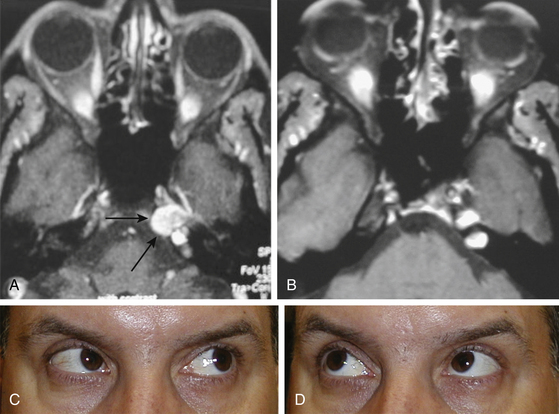
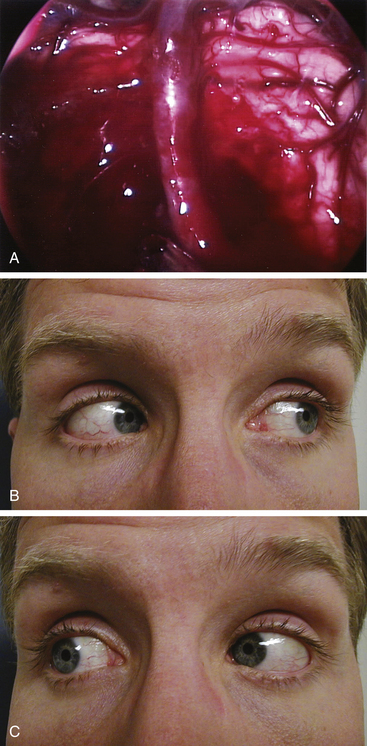
During the anterior sphenoidotomy, submucosal dissection at the contralateral side of the sphenoid rostrum is extended further laterally, and the anterior sphenoidotomy can be performed generously at the contralateral side of the sphenoid sinus. This exposes the contralateral cavernous sinus laterally up to the medial anterior temporal fossa. The bone is removed from the anterior aspect of the sella as well as from the cavernous sinus, and unroofing the internal carotid artery may be completed if necessary. The sellar portion of the pituitary tumor is removed first before attacking the portion of tumor in the cavernous sinus. For an isolated cavernous sinus tumor, the tumor is approached directly by opening the dura mater medial to the carotid siphon (Fig. 22-16A and B). The tumor is removed with various pituitary ring curettes and suction cannulas. Attention must be taken during tumor removal not to traumatize the lateral wall of the cavernous sinus since this can cause ocular cranial nerve dysfunction. The carotid artery pulsation is directly visible, and tumor resection is performed medially and posteriorly to the carotid siphon, which arcs in a C-shape for the patient’s right carotid artery or a reverse C-shape for the left carotid artery with the convexity directed anterolaterally. When the tumor is completely removed, the lateral wall of the cavernous sinus can be completely visualized (Fig. 22-17A to C). The cavernous carotid artery is surrounded using an abdominal fat graft to protect the artery postoperatively. If necessary, the sphenoid sinus can additionally be filled with an abdominal fat graft for further protection of the exposed carotid artery. In contrast with pituitary surgery, the sphenoid sinus mucosa should be removed completely before the fat graft is placed in this case.
Endoscopic Endonasal Approach to the Pterygoid Fossa or Petrous Apex
Tumors involving the pterygoid fossa can be approached with a middle meatal or middle turbinectomy approach. The posteromedial wall of the maxillary sinus can be removed if necessary, and then the pterygoid fossa is fully exposed. The vidian nerve or sphenopalatine ganglion in the pterygoid fossa can also be approached with this technique. The petrous apex can be approached with a paraseptal approach. This endoscopic endonasal approach is particularly useful for drainage of cholesterol granulomas. Approach through the contralateral nostril gives a bit more lateral angulation in the trajectory to the petrous apex. When the sphenoid sinus is entered, the sellar floor and carotid artery protuberance provide anatomical orientation, and a stereotactic image-guidance device may assist with further anatomical orientation. The internal carotid artery is exposed at the medial margin, and the petrous apex is approached through the clivus inferior to the sellar floor and medial to the paraclival segment of the cavernous carotid. When the cholesterol granuloma is entered, xanthochromic fluid and material can be drained (Fig. 22-18A to D). Placement of fat graft material over the carotid artery for arterial protection is not necessary unless the aforementioned segment of the carotid artery is fully exposed by the exposure. Once the cholesterol granuloma is drained, the operation is complete.
Endoscopic Endonasal Approach to the Clivus or Posterior Fossa
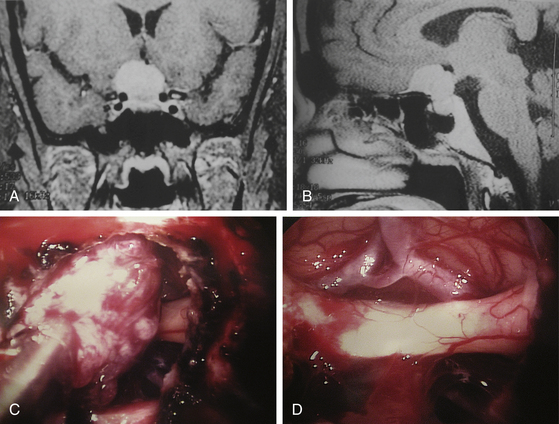
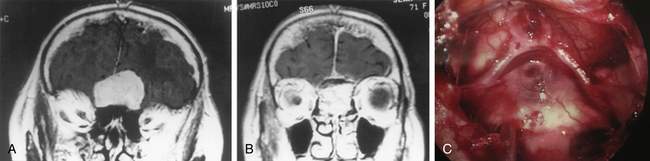
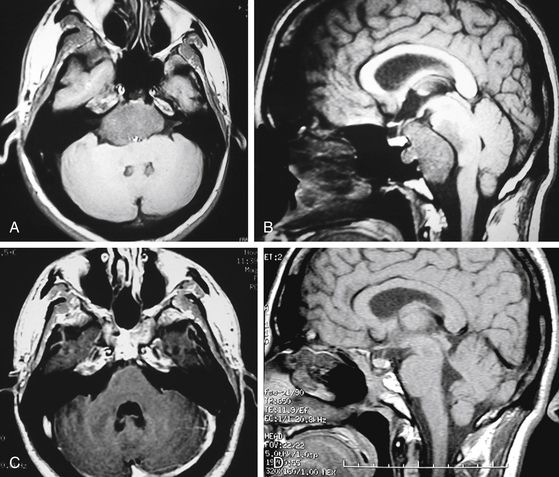
The advantage of the endoscopic technique in the endonasal surgical approach to the clivus and posterior fossa (EE-PFossa) is the flexibility and range of endoscopic visualization, which spans from the crista galli at the anterior cranial fossa to the foramen magnum at the posterior cranial fossa.22,23 This endoscopic approach exposes the entire clivus from the floor of the sella to the foramen magnum at a midline width of approximately 2 cm (Fig. 22-19), with the internal carotid arteries serving as the lateral limits of this exposure. This technique has been used for the radical resection of clival chordomas and midline clival meningiomas. A paraseptal approach is most commonly used, but the middle turbinectomy approach can be used when a larger surgical corridor is required. Fluoroscopic C-arm imaging or frameless stereotactic system is used for guidance to the vertical dimension. The clival bone is removed with a high-speed drill between the internal carotid arteries in the transverse dimension and from the floor of the sella to the foramen magnum or to the lower clival level as needed. The chordoma is then removed with suction cannulas and pituitary rongeurs. Bone is shaved at the tumor resection margin until normal bone is clearly documented. Intradural tumor is then resected, during which the pons and medulla can be visualized (Fig. 22-20A to D and Fig. 22-21A to C). A 30- or 70-degree lens endoscope visualizes the further rostral aspect of the brain stem or cranial nerves laterally (Fig. 22-22A and B). Dural defects are repaired with an abdominal fat graft, but once appropriate surgical instruments are fully developed, direct repair by a dural graft will be the ideal method for reconstruction. The dural titanium microclip applicator is currently available but is too short to be used effectively for this purpose.
Endoscopic Endonasal Approach to the Craniocervical Junction (EE-CC Junction)
This endoscopic endonasal approach provides simple access to the craniocervical junction and the C1–C2 (atlanto-axial) area.18 Pathologies such as chordoma, os odontoidum, basilar impression or invagination, rheumatoid basilar settling, or anterior lesions compressing the ventral aspect of the cervicomedullary junction can be approached with these techniques. Lateral fluoroscopic guidance or stereotactic image guidance can assist to access the surgical target. It can be done through one nostril or two nostrils. Under the endoscopic visualization, the nasopharyngeal mucosa and posterior pharyngeal muscles are incised at the midline with electrocoagulation. The clivus at the ventral foramen magnum and the C1–C2 area can be exposed as indicated, and the clivus at the ventral foramen magnum, anterior rim of the C1, and odontoid can be drilled to the tectorial membrane. The dura mater can be exposed by incising the tectorial membrane. Lateral image using fluoroscopic guidance or frameless strereotactic image-guidance can help indicate the extent of surgical exposure. Additional posterior fusion surgery is necessary when stabilizing structures such as the C1 anterior arch and the transverse ligament are traversed to access the lesion. When the odontoid tip protrudes rostrally beyond the upper edge of C1 anterior arch, the protruding portion of the odontoid can sometimes be removed without removing the ventral C1 rim. In that case, C1–C2 stability can be preserved.
Postoperative Management
Patients are kept in a regular hospital room overnight and are discharged home the next day if they do well. Postoperative discomfort is minimal and often does not require any strong analgesics. Postoperative nasal bleeding has also been very minimal since the techniques of meticulous hemostasis and eliminating intraoperative use of vasoconstrictors were adopted. Patients may note a few drops of bloody discharge for a day or two when they get up early in the morning or from a prolonged lying position. This minimal nasal drainage is usually accumulated bloody discharge at the sellar area in the sphenoid sinus during a recumbent position. Watery nasal discharge may indicate a postoperative CSF leakage, which is a potential complication that can delay hospital discharge. A few drops of fluid leakage are often benign, usually stopping in a day or so.
Surgical Results
Among the 200 patients in our early series, 160 had pituitary adenomas, 10 had anterior skull base meningiomas, eight had clival chordomas, and 22 had other skull base pathologies. Fifty-five patients had undergone previous surgical and/or radiation treatments. Among the 160 patients with pituitary adenomas, 37 (23%) patients had microadenomas and 123 (77%) patients had macroadenomas. Among the 90 patients with nonfunctioning adenomas, 71 (79%) patients had gross total removal. Among the 38 patients with prolactinomas, 25 (66%) patients normalized their postoperative prolactin levels. Thirteen (72%) out of 18 patients with Cushing’s disease had normal postoperative cortisol levels. Among the 13 patients with acromegaly, 11 (85%) had normalized postoperative IGF-1 levels. The length of hospital stay was less than 1 day in 75% of patients. One night of hospital stay has been routine for uncomplicated cases, although same-day surgery has occasionally been performed upon specific request and discussion with appropriate patients. Although our early surgical results are comparable to those reported with microscopic trans-sphenoidal surgery, we have continued to improve our surgical outcome with further increasing EE-TS experience. Of note, Tabaee et al. reported in a meta-analysis combining nine studies (totaling 821 patients) undergoing endoscopic pituitary surgery, including one of our early series of patients, gross total tumor removal in 78% and resolution of various hormone-secreting tumors ranging 81%-84%, along with complication rates of 2% CSF leak and 1% diabetes insipidus.25
Potential Complications and their Avoidance
There is a risk of delayed massive epistaxis occurring a few days to 2 weeks postoperatively. When patients go to an emergency room with significant epistaxis following EE-TS, intraoperative internal carotid artery injury with delayed pseudoaneurysm rupture is a diagnosis that emergency physicians may contemplate. However, the most common cause of such delayed nasal hemorrhage after trans-sphenoidal surgery is bleeding from the stump of the posterior septal artery, which can often be controlled acutely with direct pressure and can be addressed definitively with endoscopic electrocoagulation. But despite precautions, there is also rare risk of potential internal carotid artery injury with any technique of trans-sphenoidal surgery, and its occurrence requires tamponade with packing followed by endovascular intervention.
Alfieri A., Jho H.D., Tschabitscher M. Endoscopic endonasal approach to the ventral cranio-cervical juncture: anatomical study. Acta Neurochir (Wien). 2002;144:219-225.
Alfieri A., Jho H.D. Endoscopic endonasal approach to the cavernous sinus. An anatomic study. Neurosurgery. 2001;48:827-837.
Alfieri A., Jho H.D. Endoscopic endonasal approach to the cavernous sinus. Surgical approaches. Neurosurgery. 2001;49:354-362.
Carrau R.L., Jho H.D., Ko Y. Transnasal-transsphenoidal endoscopic surgery of the pituitary gland. Laryngoscope. 1996;106:914-918.
Ceylan S., Koc K., Anik I. Endoscopic endonasal transsphenoidal approach for pituitary adenomas invading the cavernous sinus. Clinical article. J Neurosurg. 2010;112:99-107.
Cooke R.S., Jones R.A.C. Experience with the direct transnasal transsphenoidal approach to the pituitary fossa. Br J Neurosurg. 1994;8:193-196.
de Divitiis E., Cavallo L.M., Cappabianca P., Esposito F. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumor: part 2. Neurosurgery. 2007;60:46-59.
Griffith H.B., Veerapen R. A direct transnasal approach to the sphenoid sinus: technical note. J Neurosurg. 1987;66:140-142.
Guiot G., Rougerie J., Fourestler A., et al. Une nouvelle technique endoscopique: exploration endoscopiques intracraniennes. Presse Med. 1963;71:1225-1228.
Jho H.D., Carrau R.L., Ko Y., Daly M. Endoscopic pituitary surgery: an early experience. Surg Neurol. 1997;47:213-223.
Jankowski R., Auque J., Simon C., et al. Endoscopic pituitary tumor surgery. Laryngoscope. 1992;102:198-202.
Jho H.D. Endoscopic transsphenoidal surgery. J Neuro-oncology. 2001;54:187-195.
Jho H.D. Endoscopic endonasal approach to the optic nerve: a technical note. Minim Invas Neurosurg. 2001;44:190-193.
Jho H.D., Alfieri A. Endoscopic endonasal pituitary surgery: evolution of surgical technique and equipment in 150 operations. Minim Invas Neurosurg. 2001;44:1-12.
Jho H.D., Alfieri A. Endoscopic transsphenoidal pituitary surgery: various surgical techniques and recommended steps for procedural transition. Br J Neurosurg. 2000;14:424-432.
Jho H.D., Carrau R.L. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg. 1997;87:44-51.
Jho H.D., Carrau R.L. Endoscopy assisted transsphenoidal surgery for pituitary adenoma: technical note. Acta Neurochir (Wien). 1996;138:1416-1425.
Jho H.D., Carrau R.L., Ko Y.. Endoscopic pituitary surgery, Wilkins R.H., Rengachary S.S., editors, Neurosurgical Operative Atlas, Baltimore, Williams & Wilkins, 1996;vol 5:1-12.
Jho H.D., Carrau R.L., Mclaughlin M.L., Somaza S.C. Endoscopic transsphenoidal resection of a large chordoma in the posterior fossa. Acta Neurochir (Wien). 1997;139:343-348.
Jho H.D., Ha H.G. Endoscopic endonasal skull base surgery: part 1—The midline anterior fossa skull base. Minim Invas Neurosurg. 2004;47:1-8.
Jho H.D., Ha H.G. Endoscopic endonasal skull base surgery: part 2—The cavernous sinus. Minim Invas Neurosurg. 2004;47:9-15.
Jho H.D., Ha H.G. Endoscopic endonasal skull base surgery: part 3—The clivus and posterior fossa. Minim Invas Neurosurg. 2004;47:16-23.
Keros P. Uber die praktische beteudung der Niveau-Unterschiede der lamina cribosa des ethmoids. In: Naumann H.H., editor. Head and neck surgery, vol 1. Face and facial skull. Philadelphia: WB Saunders; 1980:392.
Laufer I., Anand V.K., Schwartz T.H. Endoscopic endonasal extended transsphenoidal transplanum transtuberculum approach for resection of suprasellar lesions. J Neurosurg. 2007;106:400-406.
Tabaee A., Anand V.K., Barron Y., et al. Endoscopic pituitary surgery: a systemic review and meta-analysis. J Neurosurg. 2009;111:545-554.
1. Guiot G., Rougerie J., Fourestler A., et al. Une nouvelle technique endoscopique: exploration endoscopiques intracraniennes. Presse Med. 1963;71:1225-1228.
2. Griffith H.B., Veerapen R. A direct transnasal approach to the sphenoid sinus: technical note. J Neurosurg. 1987;66:140-142.
3. Cooke R.S., Jones R.A.C. Experience with the direct transnasal transsphenoidal approach to the pituitary fossa. Br J Neurosurg. 1994;8:193-196.
4. Jho H.D., Carrau R.L., Ko Y., Daly M. Endoscopic pituitary surgery: an early experience. Surg Neurol. 1997;47:213-223.
5. Carrau R.L., Jho H.D., Ko Y. Transnasal-transsphenoidal endoscopic surgery of the pituitary gland. Laryngoscope. 1996;106:914-918.
6. Jankowski R., Auque J., Simon C., et al. Endoscopic pituitary tumor surgery. Laryngoscope. 1992;102:198-202.
7. Jho H.D., Carrau R.L., Ko Y.. Endoscopic pituitary surgery, Wilkins R.H., Rengachary S.S., editors, Neurosurgical Operative Atlas, Baltimore, Williams & Wilkins, 1996;vol 5:1-12.
8. Jho H.D., Carrau R.L. Endoscopy assisted transsphenoidal surgery for pituitary adenoma: technical note. Acta Neurochir (Wien). 1996;138:1416-1425.
9. Jho H.D., Carrau R.L. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg. 1997;87:44-51.
10. Jho H.D., Alfieri A. Endoscopic transsphenoidal pituitary surgery: various surgical techniques and recommended steps for procedural transition. Br J Neurosurg. 2000;14:424-432.
11. Jho H.D., Alfieri A. Endoscopic endonasal pituitary surgery: evolution of surgical technique and equipment in 150 operations. Minim Invas Neurosurg. 2001;44:1-12.
12. Jho H.D. Endoscopic transsphenoidal surgery. J Neuro-oncology. 2001;54:187-195.
13. Jho H.D. Endoscopic endonasal approach to the optic nerve: a technical note. Minim Invas Neurosurg. 2001;44:190-193.
14. Alfieri A., Jho H.D. Endoscopic endonasal approach to the cavernous sinus. An anatomic study. Neurosurgery. 2001;48:827-837.
15. Alfieri A., Jho H.D. Endoscopic endonasal approach to the cavernous sinus. Surgical approaches. Neurosurgery. 2001;49:354-362.
16. de Divitiis E., Cavallo L.M., Cappabianca P., Esposito F. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumor: part 2. Neurosurgery. 2007;60:46-59.
17. Laufer I., Anand V.K., Schwartz T.H. Endoscopic endonasal extended transsphenoidal transplanum transtuberculum approach for resection of suprasellar lesions. J Neurosurg. 2007;106:400-406.
18. Alfieri A., Jho H.D., Tschabitscher M. Endoscopic endonasal approach to the ventral cranio-cervical juncture: anatomical study. Acta Neurochir (Wien). 2002;144:219-225.
19. Jho H.D., Ha H.G. Endoscopic endonasal skull base surgery: part 1 —The midline anterior fossa skull base. Minim Invas Neurosurg. 2004;47:1-8.
20. Jho H.D., Ha H.G. Endoscopic endonasal skull base surgery: part 2—The cavernous sinus. Minim Invas Neurosurg. 2004;47:9-15.
21. Ceylan S., Koc K., Anik I. Endoscopic endonasal transsphenoidal approach for pituitary adenomas invading the cavernous sinus. Clinical article. J Neurosurg. 2010;112:99-107.
22. Jho H.D., Ha H.G. Endoscopic endonasal skull base surgery: part 3—The clivus and posterior fossa. Minim Invas Neurosurg. 2004;47:16-23.
23. Jho H.D., Carrau R.L., Mclaughlin M.L., Somaza S.C. Endoscopic transsphenoidal resection of a large chordoma in the posterior fossa. Acta Neurochir (Wien). 1997;139:343-348.
24. Keros P. Uber die praktische beteudung der Niveau-Unterschiede der lamina cribosa des ethmoids. In: Naumann H.H., editor. Head and neck surgery, vol 1. Face and facial skull. Philadelphia: WB Saunders; 1980:392.
25. Tabaee A., Anand V.K., Barron Y., et al. Endoscopic pituitary surgery: a systemic review and meta-analysis. J Neurosurg. 2009;111:545-554.