CHAPTER 64 Endophthalmitis
Diagnosis, clinical findings, and management
Introduction
Infectious endophthalmitis is defined as inflammation of intraocular fluids and tissues caused by microbial organisms. It may result in severe visual loss1–3. In this chapter the epidemiology, diagnosis, and clinical findings are discussed and medical and surgical management issues for infectious endophthalmitis are reviewed.
Epidemiologic consideration and terminology
Infectious endophthalmitis is classified by the events leading to the infection and by the timing of the clinical diagnosis1–3. The broad categories include postoperative endophthalmitis (acute onset, chronic or delayed-onset, conjunctival filtering bleb associated), post-traumatic endophthalmitis, intravitreal injection-associated endophthalmitis, and endogenous endophthalmitis (Table 64.1). Rare categories include cases associated with microbial keratitis or suture removal. These categories are important in predicting the causative organisms and guiding therapeutic decisions before microbiological confirmation of the clinical diagnosis.
Table 64.1 Classification of endophthalmitis – most frequent organisms in various clinical settings
Postoperative endophthalmitis is the most frequent category, accounting for greater than 70% of cases3. In a nosocomial survey (2002–2009) of a university-based hospital reviewing over 56 672 intraocular surgical procedures with or without intraocular lens implantation, acute-onset endophthalmitis occurred in 14 cases (0.025%)4. In this survey of intraocular surgical cases, the rates of endophthalmitis were highest after penetrating keratoplasy (0.108% of 2788 cases) and lowest after pars plana vitrectomy (0.01% or 2 of 18 492 cases). Despite concerns that clear corneal sutureless temporal incision cataract surgery may increase the risk of endophthalmitis, this and most other studies do not substantiate this5. Comparing the rate of endophthalmitis at the same university-based hospital to studies from prior time periods, there has been a steady decline in the incidence of endophthalmitis6. Another study reviewed 440 000 consecutive cataract surgeries in Canada from 2002 to 2006 and determined the rate of endophthalmitis to be 0.14%. They found the rate to be higher in men, cases with capsular rupture, and older patients7. There is an increased incidence of endophthalmitis in patients with diabetes mellitus that can possibly be explained by known immune compromise in diabetic patients8. Endophthalmitis from an intravitreal injection is a more recently recognized category of endophthalmitis due to the increased utilization of intravitreal medications to treat retinal disease. Intravitreal injections are currently the most common procedure in the Medicare system. Vascular endothelial growth factor inhibitors (VEGF) (bevacizumab, ranibizumab) and depot-steroids (triamcinolone acetonide) are the two main drug groups injected intravitreally for treatment purposes. The VEGF inhibitors are mainly used for treating exudative macular degeneration, but have also been shown to be effective for treatment of macular edema resulting from retinal vascular diseases and retinal and iris neovascularization resulting from retinal ischemia in diabetic retinopathy and retinal vein occlusions. Intravitreal triamcinolone acetonide is used to treat macular edema from various causes, as well as intraocular inflammatory diseases. In a meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor(VEGF) agents, endophthalmitis occurred in 52 of 105 536 injections (0.049%)9. 52% of these were culture positive. Of the culture positive cases, coagulase-negative Staphylococcus accounted for 65.4%, Streptococcus species for 30.8% and Bacillus cereus for 3.8%. The frequency of Streptococcus was approximately 3 times higher than that reported after cataract surgery related endophthalmitis. The authors recommended strategies to minimize oropharyngeal droplet transmission including avoiding talking, coughing, and sneezing or wearing surgical masks during injections. In another large study from a single medical center and satellite clinics, the rate of endophthalmitis following intravitreal injection of anti VEGF agents was 0.02% (12/60 322). Streptococcus species was the most common organism isolated and was associated with a poorer visual outcome9a. The infectious endophthalmitis incidence data from triamcinolone acetonide also suggests that it is an uncommon complication. It is reported to occur in from 0.05–0.87% of cases. Combining data from the diabetic retinopathy clinical research network diabetic macular edema trial and the standard care vs. triamcinolone acetonide for retinal vein occlusion (SCORE) there was one case of endophthalmitis in 2009 injections (0.05%)9b. In 2004, a panel of experienced researchers and clinicians met and created a “Best Practice” guide for intravitreal injections to minimize the incidence infection. Although there was some disagreement among panel members, a list of guidelines accepted by most of the panel members has been published (Table 60.5). Use of a lid speculum and application of topical povidone-iodine are highly recommended for all injections. The needle should avoid contact with the patient’s eyelid prior to entering the eye. There is no evidence that pre or postoperative antibiotics reduce the rate of infectious endophthalmitis following an intravitreal injection. Endophthalmitis may also occur infrequently in the setting of a conjunctival filtering bleb10–12, wound dehiscence, or vitreous wick13. Chronic or delayed-onset endophthalmitis may be caused by less virulent bacteria (e.g. Propionibacterium acnes, Staphylococcus epidermidis) or by fungi14–17.
In reported large clinical series18–20, endophthalmitis after penetrating ocular trauma represents approximately 25% of all endophthalmitis cases. In one large study of penetrating ocular trauma, endophthalmitis occurred in 10.7% of cases with a retained intraocular foreign body and 5.2% of cases without a retained intraocular foreign body21. The National Eye Trauma System Registry reported an endophthalmitis incidence of 6.9% (34 of 492 cases) after penetrating ocular injuries with retained intraocular foreign bodies22. Metallic intraocular foreign bodies were as likely to be associated with infectious endophthalmitis (7.2%) as non-metallic foreign bodies (7.3%) and organic matter (6.3%) foreign bodies22. Rupture of the crystalline lens capsule is also a risk for endophthalmitis in open-globe injuries23. More recent data indicate that the incidence of endophthalmitis after penetrating trauma may be declining24.
Compared with the postoperative and post-trauma categories, endogenous endophthalmitis cases occur with the least frequency and more often occur in debilitated or immunocompromised patients or in patients with a history of intravenous drug abuse25–31. The incidence of intravitreal injection-associated endophthalmitis is less than 0.1% in most reported series32.
Clinical features, diagnosis, and differential diagnosis
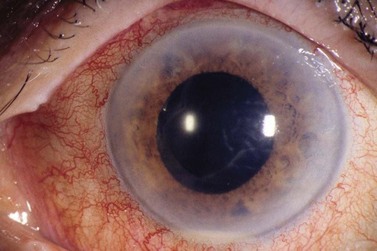
The diagnostic features of infectious endophthalmitis can be divided into two aspects: clinical recognition and microbiological confirmation. The clinical signs of endophthalmitis vary depending on the preceding events or surgery, the infecting organism, the associated inflammation, and the duration of the disease prior to diagnosis. In acute-onset postoperative endophthalmitis when bacteria are the etiologic agents, the hallmark of the clinical diagnosis is marked intraocular inflammation with hypopyon (Fig. 64.1)1,2. Other signs of acute postoperative bacterial endophthalmitis include fibrin in the anterior chamber and on the intraocular lens (IOL), corneal edema, marked conjunctival congestion, lid edema, and vitreitis. Retinal periphlebitis is another clinical sign that is diagnostically more helpful in eyes with relatively clear media33. The clinical features of endophthalmitis after clear cornea cataract surgery are similar to those in cases following scleral incision cataract surgery, except the time of diagnosis may be later with clear corneal surgery cases34. Endophthalmitis cases caused by fungal organisms generally have less inflammation, a more indolent course, and less ocular pain. Endogenous Candida cases often manifest as isolated white infiltrates in the formed vitreous overlying a focal area of chorioretinitis27.
The clinical diagnosis of endophthalmitis is confirmed by obtaining intraocular (aqueous and vitreous) specimens. A vitreous specimen is much more likely to yield a positive culture result than is a simultaneously acquired aqueous specimen35. The vitreous specimen can be obtained either by needle biopsy or by the use of an automated vitrectomy instrument. A needle biopsy or limited vitrectomy approach can be performed in a treatment room, but a three-port pars plana vitrectomy usually requires the use of the operating room and ancillary equipment. One report of 138 culture-proven endophthalmitis cases showed a positive culture result in 34.8% of anterior chamber specimens, 58.2% of vitreous tap specimens, and 80% of vitrectomy fluid specimens35.
The technique for culturing intraocular specimens depends on the volume of the specimen and the suspected clinical diagnosis2,35,36. Direct inoculation of the intraocular fluid specimen onto culture media is a traditional approach and remains a very practical technique. The specific media used for direct inoculation are listed in Table 64.2. This approach is especially important when limited specimens (such as a needle vitreous or aqueous aspiration) are obtained. These specimens can be directly inoculated onto the appropriate media, including anaerobic media in cases of suspected P. acnes endophthalmitis. Specimens obtained by the use of automated vitrectomy instruments are diluted by the infusion fluid but can be processed by two methods. One method for processing the vitrectomy specimen uses a membrane filter system in which the vitrectomy specimen is passed through 0.45 mm filter paper that concentrates the microorganisms and particulate matter. This filter paper is then sectioned and distributed on the appropriate media. An alternative method involves direct inoculation of the initially aspirated vitrectomy specimen into standard blood culture bottles36. The latter technique is particularly useful at night or on the weekend when the microbiology laboratory staff are not available to assist in processing the vitrectomy specimen. In a retrospective review of 83 cases, this blood culture bottle method for processing vitrectomy specimens yielded a 91% incidence of positive culture results36. This rate of positive culture resulting from clinically diagnosed endophthalmitis cases was similar to simultaneously processed specimens using the membrane filter system. In another study this method yielded positive cultures in 34 of 48 vitreous specimens (70.8%)37. Direct inoculation of vitreous onto the BancTec Peds Plus F broth has also been shown to be a simpler and effective alternative culture method38.
Immunologic as well as molecular genetic technologies enable rapid and specific identification of infectious agents. These techniques have been used in both clinical and experimental settings, and their future use in this area appears promising39,40. Molecular genetic technology has made available specific DNA probes that will interact with the unique DNA sequence for a particular pathogen39. Polymerase chain reaction (PCR) uses a primer set and DNA polymerase to amplify small amounts of DNA. It shows clinical potential as a rapid and sensitive diagnostic technique to aid in the confirmation of clinical observations and as an adjunct to conventional culture techniques41. Clinical application of PCR techniques for the more rapid diagnosis of bacterial endophthalmitis is currently under investigation40.
The differential diagnosis of marked intraocular cellular inflammation after ocular surgery includes sterile inflammation related to retained lens fragments, iris trauma, pre-existing uveitis, and foreign material introduced during surgery1,4–6. Retained cortical lens remnants have been reported to cause more inflammation than nuclear remnants42. Retained lens fragments may occasionally cause a marked inflammatory reaction with hypopyon, which may clinically resemble infectious endophthalmitis43,44. Blood in the anterior chamber or vitreous cavity may also be confused with endophthalmitis, especially when the blood is long-standing and associated with anterior segment trauma during preceding surgery. Similarly, difficult or prolonged surgery, which often includes vitreous loss or vitreous incarceration in the cataract incision, may increase postoperative inflammation.
Toxic anterior segment syndrome (TASS) can mimic infectious endophthalmitis and the incidence of this has risen in recent years. It is probably caused by multiple factors but, as the name implies, is presumed to be due to a toxin entering the eye at the time of surgery. The American Society of Cataract and refractive Surgeons found many different possible causes of TASS45. The use of toxic single use materials and instruments as well as the sterilization and processing of reusable instruments are possible causes. The inflammatory response is usually seen within the first 24 hours after surgery. It can be intense and associated with a hypopyon and fibrin formation in the anterior chamber. Corneal edema can be severe. The vitreous is typically not involved, as is the case with an infectious endophthalmitis. In addition other differentiating features between TASS and infectious endophthalmitis include minimal to no pain, lid edema or purulent discharge with TASS, and onset within 24 hours as opposed to 4–7 days with infection46.
Goals of management
Antibiotics
The management goal for endophthalmitis is first and foremost to eradicate the infection and sterilize the intraocular contents. Antibiotics are the mainstay of therapy. Antibiotics can be delivered to the eye by several routes, including direct intravitreal injection, periocular injection, and topical application (Table 64.3). Endophthalmitis management, like the management of infections elsewhere in the body, requires selection of safe and effective antimicrobial agents. The antibiotics selected should cover the broad range of Gram-positive and Gram-negative organisms causing infectious endophthalmitis. Systemic antibiotics may be used to supplement local ocular treatment in selected cases. Injection of intravitreal antibiotics is the primary means of achieving this goal. Of all the available antimicrobial agents evaluated for intravitreal injection, only a few are used regularly in clinical practice. In the Endophthalmitis Vitrectomy Study (EVS), intravitreal vancomycin 1 mg in combination with amikacin 0.4 mg was used for the initial empiric treatment of acute-onset endophthalmitis47–58. An alternative to the aminoglycosides for coverage of Gram-negative organisms is the use of intravitreal ceftazidime 2.25 mg, a third-generation cephalosporin59,60. Although ceftazidime may precipitate when used with vancomycin, this has not been shown to alter its effectiveness in the clinical setting58. These two antibiotics should not be mixed in the same syringe. These antibiotic combinations provide broad coverage for nearly all of the organisms causing bacterial endophthalmitis, including staphylococci, streptococci, Bacillus species, and the Gram-negative organisms.
Table 64.3 Antibiotics considered for endophthalmitis treatment: concentration and dosages of principal agents used for treatment of endophthalmitis*
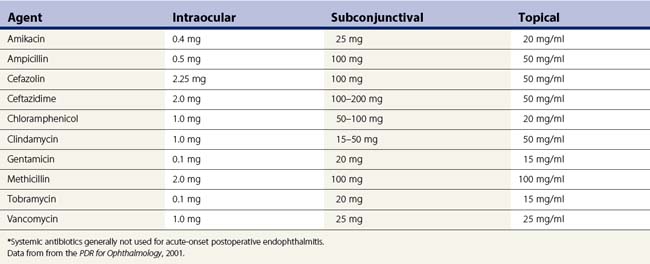
Vancomycin is generally considered to be the drug of choice for Gram-positive bacterial coverage. Vancomycin has excellent activity against coagulase-negative staphylococci and other Gram-positive organisms. In the EVS, coagulase-negative micrococci were the most commonly cultured bacteria in acute-onset post-cataract surgery cases47–57. Vancomycin has been reported to be consistently effective for the broad range of streptococcal organisms as well as nearly all Gram-positive organisms.
Repetitive injections of intravitreal antibiotics may cause significant retinal toxicity. In a rabbit model, eyes treated with a second or third intraocular vancomycin/aminoglycoside injection at 48-hour intervals showed progressive toxicity61. In view of the low rate of persistent infection after initial combination therapy, repeat injection of intravitreal antibiotics are considered only in those cases with progressive inflammation caused by virulent organisms62. Based on the initial culture report, a single intravitreal antibiotic may be selected for this repeat injection.
Vitrectomy
Disadvantages of vitrectomy include the requirement for more sophisticated instrumentation, possibly available only in an operating room, a setting that can be associated with a delay in initiating treatment. The view of the posterior segment is frequently obscured by fibrin and inflammatory debris on the surface of the IOL or in the anterior chamber, making vitrectomy surgery difficult and potentially hazardous. The view of the posterior segment can frequently be improved by either aspirating or peeling the inflammatory material from the anterior segment or surface of the IOL63.
Another disadvantage of vitrectomy is its effect on reducing the half-life of injected intravitreal antibiotics64. Doft and associates studied the ocular clearance of amphotericin B injected into the vitreous in a rabbit model of unmodified phakic eyes, Candida-infected phakic eyes, aphakic eyes, and aphakic vitrectomized eyes. The half-lives of drug disappearance after a single amphotericin B 10 µg intravitreal injection were 9.1, 8.6, 4.7, and 4.1 days, respectively. The authors summarized that this rapid disappearance of amphotericin B from vitrectomized eyes must be considered in the clinical management of patients with fungal endophthalmitis.
Vitrectomy for endophthalmitis can be performed using either a standard 20-gauge three-port system or a transconjunctival cannulated 23 or 25-gauge system depending on the surgeon’s preference and the clinical circumstances. Possible advantages of the small gauge technique over the 20-gauge approach include shorter operating time, reduced postoperative inflammation from sutures in an already inflamed eye, and possibly reduced incidence of postoperative retinal tears and detachments65. In the setting of post-cataract surgery endophthalmitis, a pars plana vitrectomy is generally recommended for endophthalmitis cases with light perception vision. The inflammatory response is usually severe and, in such cases, preoperative echography should be performed to rule out retinal detachment and to document the presence or absence of a posterior vitreous detachment. When there is a posterior vitreous detachment, the vitrectomy surgeon can remove more opaque vitreous near the posterior pole and have greater confidence in avoiding contact with the retina.
Indications for surgery
The indications for performing vitreoretinal surgery for endophthalmitis as opposed to vitreous tap and intravitreal antibiotics were defined in the Endophthalmitis Vitrectomy Study (EVS). The EVS was a multicenter, National Eye Institute (NEI)-sponsored trial that evaluated pars plana vitrectomy and systemic antibiotics in acute postoperative endophthalmitis47–57. The EVS also evaluated a variety of clinical and microbiologic factors relating to endophthalmitis. The study enrolled 420 patients with symptoms and signs of endophthalmitis occurring within 6 weeks of cataract extraction or secondary IOL implantation. Patients were randomized to treatment with pars plana vitrectomy or to vitreous tap/biopsy, and to treatment with or without systemic antibiotics. All patients in the study received intravitreal antibiotic therapy (vancomycin 1 mg and amikacin 0.4 mg), and topical and systemic corticosteroids. Patients who appeared clinically worse after 36–60 hours after presentation underwent re-injection of intravitreal antibiotics. Similarly, patients who were initially randomized to tap/biopsy and had worsening also underwent vitrectomy. The main endpoint of the study was best-corrected visual acuity at 9 to 12 months after presentation. A secondary endpoint was media clarity.
Visual acuity results
The EVS demonstrated that immediate pars plana vitrectomy was beneficial for patients who presented with visual acuity of light perception only47. In this subgroup of patients, vitrectomy was associated with a threefold increase in the frequency of achieving 20/40 or better acuity (33% vs. 11%), approximately a twofold chance of achieving 20/100 or better acuity (56% vs. 30%), and a 50% decrease in the frequency of severe visual loss to worse than 5/200 acuity (20% vs. 47%). There was no difference in outcome between immediate pars plana vitrectomy and tap/biopsy for patients with an initial visual acuity of hand motions or better. In this subgroup, patients had about the same chance of achieving 20/40 or better acuity (66% vs. 62%) and 20/100 or better acuity (86% vs. 84%), and a similar risk for severe visual loss to worse than 5/200 acuity (5% vs. 3%), whether they had immediate three-port pars plana vitrectomy or vitreous tap/biopsy. However, there was a possible exception. Diabetic patients with initial visual acuity of hand motions or better obtained somewhat better visual acuity outcome with vitrectomy compared with tap/biopsy. Final visual acuity of 20/40 or better was obtained in 57% of vitrectomy patients and 40% of tap biopsy patients. The difference was not statistically significant. It was suggested that either vitrectomy or tap/biopsy could be considered reasonable for diabetic patients48.
At 9–12 months after presentation, clear media, as judged by a 20/40 view of the fundus by indirect ophthalmoscopy, was achieved slightly less frequently in the tap/biopsy eyes (83%) than in the vitrectomized eyes (90%), but this difference was not statistically significant. In no cases were vitreous opacities judged to be a principal cause of impaired vision at the final examination52.
Systemic antibiotics (amikacin, ceftazidime) were observed to have no effect on visual outcome or media clarity in the EVS, even when subgroup analysis that considered microbiologic susceptibilities was performed47,57. The study concluded that systemic antibiotics provided no additional benefit to intravitreally administered antibiotics.
Recent studies have confirmed the relevance of the EVS to similar cases in the modern era of clear corneal surgery51. In spite of newer systemic antibiotics, the traditional intravitreal antibiotics remain effective and are the mainstay of therapy.
Surgical technique
Tap and inject
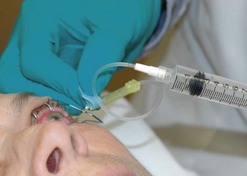
A sterile prep of the eye and eyelids is performed using povidone iodine and a sterile lid speculum is placed. A syringe attached to a 23-gauge butterfly needle is preferred for the aspiration. A 23-gauge butterfly needle is usually readily available in the clinic and works well for this purpose. The needle is inserted approximately 3 mm from the limbus into the mid vitreous and moderate aspiration is applied (Fig. 64.2). If vitreous does not aspirate initially the needle tip can be carefully moved in an attempt to locate a pocket of liquid vitreous. If after several attempts a vitreous specimen cannot be obtained the procedure should be terminated as excessive aspiration can lead to development of iatrogenic retinal tears. Another option if vitreous aspiration is not successful is the use of a portable battery operated small gauge vitrector.
Vitrectomy
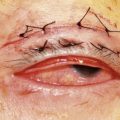
A traditional ocular sterile prep is performed using povidone iodine antiseptic and surgical drapes (Fig. 64.3). An infusion canula is inserted but infusion should not begin until one can visualize the canula in the vitreous cavity. This may require clearing inflammatory material from the anterior chamber by placing the vitrector into the anterior chamber through a limbal incision. Usually the material can be cut and aspirated but in some cases removal of fibrin is facilitated by twirling the material around the vitrectomy probe, similar to twirling spaghetti around a fork. An anterior chamber specimen can be obtained at this time.
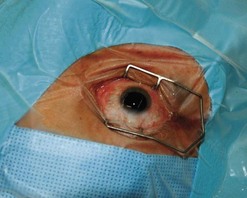
Fig. 64.3 An eye prepped for surgery with povidone iodine scrub and plastic eye drape covering the lashes.
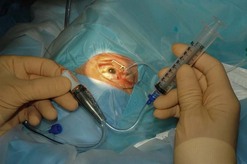
Once visualization of the vitreous is possible a more concentrated vitreous specimen is acquired. This can be done by ‘breaking’ the aspiration line from the vitrector and inserting a 10 ml syringe. As the vitreous cutter is activated an assistant can slowly aspirate approximately 0.5 ml of concentrated vitreous into the syringe (Fig. 64.4). The infusion can be turned on after the specimen is obtained and a complete vitrectomy performed. The extent of vitreous removal may depend on adequacy of visualization during the vitrectomy. An inadequate view to the posterior segment because of corneal edema, or residual inflammatory material in the anterior segment may limit the extent of vitrectomy that can be performed.
Postoperative care, additional procedures, and results of treatment (EVS data)
Early and late additional procedures
The EVS also evaluated the frequency of additional intervention following initial treatment52. Within 1 week of presentation, additional procedures were required in 8% of vitrectomized eyes versus 13% of those treated with tap/biopsy. Of 44 eyes (10% overall) that required repeat procedures, most (9%) underwent such procedures for worsening inflammation, and the remainder (1.4%, six eyes) for other complications after the initial treatment procedure. These complications included glaucoma, wound leak, and retinal detachment. Eyes that required additional procedures soon after initial presentation had a worse visual outcome, with only 15% of eyes achieving 20/40 or better visual acuity compared with 57% of eyes that did not require such procedures. The poorer outcome in eyes requiring secondary procedures could be attributed to the worse early course in such eyes, rather than to the secondary procedures themselves.
Clinical presentation and visual outcome
Clinical factors on presentation can be correlated with final visual outcome in the EVS. The single most important predictor of visual outcome was presenting visual acuity. Patients with LP visual acuity at presentation had twice the risk of decreased vision compared with those with hand motions or better. Overall, 23% of patients with light perception acuity achieved 20/40 or better final acuity, compared with 64% of patients who had hand motions or better acuity47. The data confirmed that early treatment of endophthalmitis prior to severe visual loss is critical to maximize visual outcome, and that such treatment is more important in influencing outcome than any other factor, including vitrectomy. Other clinical factors that independently predicted decreased final visual acuity were older age, history of diabetes, corneal infiltrate or ring ulcer, abnormal intraocular pressure, rubeosis, an absent red reflex, and an open posterior capsule55.
The European Society of Cataract and Refractive Surgeons (ESCRS) Endophthalmitis Study
Although the Endophthalmitis Vitrectomy Study (EVS) provided valuable information from the 1990s, the ESCRS added additional and updated data regarding the management of post-cataract endophthalmitis69. The study was conducted at 24 centers in nine European countries from 2003 to 2006. It was a partially masked randomized placebo-controlled study evaluating the prophylactic effect of intracameral cefuroxime injection and/or perioperative levofloxacin eye drops on the incidence of endophthalmitis after cataract surgery. Data on 16 211 patients were published in June of 2007. The rate of endophthalmitis was 4.92 times higher in the control group than the cefuroxime treated group. Although perioperative topical levofloxacin was associated with a reduced incidence of endophthalmitis, it did not achieve statistical significance. Other identified risk factors for proven infective endophthalmitis in the study were silicone IOL material and male sex.
Currently intracameral cefuroxime has not become the standard practice for endophthalmitis prevention in the United States or in Europe70.
Systemic antibiotics
Controversy has existed in the literature over whether systemic antibiotics are necessary for successful treatment of infectious endophthalmitis. Intraocular inflammation and/or performance of a vitrectomy may alter the blood–retina barrier in a manner to allow better intravitreal penetration of systemically administered antibiotics. Prior to the EVS, successful treatment results in culture-positive cases of exogenous bacterial endophthalmitis without the use of systemic antibiotic therapy were published71. One series included 16 endophthalmitis cases caused by S. epidermidis or more virulent organisms. Repeat intravitreal antibiotic injections were performed in seven cases because of suspected initial treatment failure, but in all patients the infection was ultimately cured without the use of systemic antibiotics. Three arguments against the use of intravenous antibiotics are the potential systemic toxicity, the variable intravitreal penetration, and the high cost of some antibiotics. Certain limitations of the EVS with respect to systemic antibiotic therapy should be recognized. First amikacin and ceftazidime were the only systemic antibiotics evaluated in the EVS. Second, whereas patients with acute-onset endophthalmitis following cataract surgery derived no additional benefit from the EVS systemically administered antibiotics, the study made no recommendations regarding systemic antibiotics for endophthalmitis prophylaxis, or for chronic, traumatic, bleb-related, fungal, or endogenous endophthalmitis.
Since the conclusion of the EVS, oral antibiotics in the fluoroquinolone class with a broad spectrum of activity and excellent intravitreal penetration have become available. These oral fluoroquinolones, include gatifloxacin (Tequin) (no longer commercially available) and moxifloxacin (Avelox; Bayer Pharmaceuticals Corp, West Haven, Conn). Moxifloxacin hydrochloride (Avelox; Bayer Pharmaceuticals Corp, West Haven, Conn) was shown to achieve therapeutic levels in the aqueous and vitreous for a wide variety of Gram-positive and Gram-negative pathogens following oral administration in non-inflamed eyes72. Although resistant organisms causing endophthalmitis are becoming more prevalent73, this drug may be considered in the management of endophthalmitis as a supplement to local ocular therapy. Although the fluoroquinolones are overall a relatively safe class of drugs, side effects must be considered and weighed against the benefit of the drug. Tendon rupture can occur with the fluoroquinolones, particularly in patients over 60 and those on corticosteroids74.
Periocular antibiotic therapy
Conflicting data regarding the intravitreal penetration after periocular antibiotic injection have been reported75. Causes for the variability in these experiments include the inflammatory status of the eye and, possibly, sampling technique. The physiochemical properties of the drug may affect transscleral and transcorneal permeability. Of the antibiotics now in use, the third generation cephalosporins (ceftazidime and ceftriaxone) achieve the highest vitreous levels. A study comparing the effectiveness of treating endophthalmitis cases with periocular antibiotic injection in addition to intravitreal antibiotic with cases treated without periocular antibiotics demonstrated no statistical difference in outcomes76.
Topical antibiotic therapy
Most studies concerning the efficacy of topically applied antibiotics pertain to corneal infections and cataract surgery prophylaxis. Significant intraocular levels of antibiotics can be achieved with frequent administration77. Alternative topical antibiotics include fortified antibiotics (vancomycin, and ceftazidime or amikacin) versus standard commercially available fourth generation fluoroquinolones.
Corticosteroid therapy
Corticosteroids can be administered to the eye by several routes (intravitreal, systemic, periocular, and topical). Clinical studies have reported no adverse effect when using intravitreal dexamethasone in conjunction with intraocular antibiotics78–80. Intravitreal triamcinolone acetonide has also been shown to reduce inflammation without exacerbating the infection in a small series of endophthalmitis cases81. A study using a rabbit model of endophthalmitis demonstrated better clinical outcomes, post-treatment ERGs, and less histopathologic evidence of tissue destruction when intravitreal dexamethasone was used compared with antibiotics alone82.
In addition to intravitreal corticosteroids, periocular corticosteroids are also commonly used in the treatment of endophthalmitis. The periocular dosage may include dexamethasone 12 mg or more, administered together with periocular antibiotics1. Topical corticosteroids are usually started on the first morning after the initial treatment of endophthalmitis. These drops may be alternated on an hourly basis with the use of topical antibiotics.
Systemic corticosteroids were used in the EVS in the treatment of all study patients with postoperative endophthalmitis. In one study, a combination of topical and systemic corticosteroids gave better results than no corticosteroids or only topical corticosteroid administration83. Because many patients with endophthalmitis also have diabetes mellitus8, caution is advised when using higher dosages of systemic corticosteroids.
Management of complications from endophthalmitis
In the EVS, major adverse events included retinal detachment in 5%, phthisis in 3%, significant elevation of intraocular pressure (30 mmHg or more) in 1%, and enucleation or evisceration in 1%. Compared with vitreous tap/biopsy, vitrectomy was associated with a slightly lower rate of complications. It is important to remember that the EVS excluded patients with opaque corneas blocking a satisfactory view for vitrectomy or patients with NLP visual acuity at presentation. In the EVS, retinal detachment and phthisis occurred in 2.7% and 2% of vitrectomy eyes, respectively, compared with 7% and 4% of tap/biopsy eyes47. Enucleation was performed in three tap/biopsy eyes and in no vitrectomy eyes. The EVS treatment recommendations were based on visual outcome and not small differences in complication rates between treatment modalities.
Macular abnormalities were the most common cause of visual loss in the EVS. These included macular edema, pigmentary degeneration, epiretinal membrane, and ischemia. Such abnormalities were more common with worse presenting visual acuity, occurring in up to 17% of patients with hand motions or better acuity and up to 40% of patients presenting with light perception acuity. In light perception eyes that did not receive vitrectomy (the subgroup that did most poorly), excess visual loss was due to anterior segment media opacification (15%), and phthisis or enucleation (23%). These events were observed much less frequently (0.7%–7%) in the remaining treatment groups47.
Two adverse events during or after endophthalmitis treatment may markedly influence visual acuity outcomes. Antibiotic toxicity and retinal detachment are significant because further visual loss may occur in spite of successful treatment of the infections. Macular infarction after the use of intraocular aminoglycosides is a clinically recognized complication manifesting as a relatively well-defined area of retinal whitening, often in the macula84. Reported cases of macular infarction secondary to administration of intraocular aminoglycosides have been observed after excessive intraocular doses, and others after apparent injection of a recommended safe dose. A localized increase in the drug concentration in dependent areas of the retina may play a role in aminoglycoside toxicity. If some of the perifoveal capillaries are spared, retention of some central vision is possible.
1 American Academy of Ophthalmology. Basic and Clinical Science Course. Section 9. Intraocular Inflammation and Uveitis, 2001–2002. San Francisco: American Academy of Ophthalmology; 2009.
2 Fan JC, Niederer RL, Lany HVL, et al. Infectious endophthalmitis: clinical features, management and visual outcomes. Clin Exp Ophthalmol. 2008;36:631-636.
3 Maguire JI. Postoperative endophthalmitis: optimal management and the role and timing of vitrectomy surgery. Eye. 2008;22:1290-1300.
4 Wykoff CC, Flynn HWJr, Shi W, et al. Nosocomial acute-postoperative endophthalmitis at a university teaching hospital (2002–2009). Am J Ophthalmol. 2010;150:392-398.
5 Taban M, Behrens A, Newcomb RL, et al. Acute endophthalmitis following cataract surgery. A systematic review of the literature. Arch Ophthalmol. 2005;123:613-620.
6 Miller JJ, Scott IU, Flynn HWJr, et al. Acute-onset endophthalmitis after cataract surgery (2000–2004): incidence, clinical settings, and visual acuity outcomes after treatment. Am J Ophthalmol. 2005;139:983-987.
7 Hatch WV, Cernat G, Wong D, et al. Risk factors for acute endophthalmitis after cataract surgery: a population-based study. Ophthalmology. 2009;116:425-430.
8 Doft BH, Wisniewski SR, Kelsey SF, et al. Endophthalmitis vitrectomy study Group. Diabetes and postoperative endophthalmitis in the endophthalmitis vitrectomy study. Arch Ophthalmol. 2001;119(5):650-656.
9 McCannel CA. Meta-analysis of endophthalmitis after intrvitreal injection of anti-vascular endothelial growth factor antagonists. Causative organisms and possible preventive strategies. Retina. 2011;31:654-661.
Moshfeghi AA, Rosenfeld PJ, Flynn HWJr. Endophthalmitis after intravitreal anti-vascular endothelial growth factor antagonists. A six year experience at a university referral center. Retina. 2011;31:662-668.
Bhavsar AR, Ip MS, Glassman AR, DRCRnet and the SCORE Study Groups. The risk of endophthalmitis following intravitral triamcinolone acetonide injection in the DRCRnet and SCORE clinical trials. Am J Ophthalmol. 2007;144:454-456.
10 Mandelbaum S, Forster RK, Gelender H, et al. Late onset endophthalmitis associated with filtering blebs. Ophthalmology. 1985;92:964.
11 Wolner B, Liebmann JM, Sassani JW, et al. Late bleb-related endophthalmitis after trabeculectomy with adjunctive 5-fluorouracil. Ophthalmology. 1991;98:1053.
12 Brown RH, Yang LH, Walker SD, et al. Treatment of bleb infection after glaucoma filtering surgery. Arch Ophthalmol. 1994;112:57.
13 Ruiz RS, Teeters VW. The vitreous wick syndrome: a late complication following cataract extraction. Am J Ophthalmol. 1970;70:483.
14 Fox GM, Joondeph BC, Flynn HWJr, et al. Delayed onset pseudophakic endophthalmitis. Am J Ophthalmol. 1991;111:163.
15 Clark WL, Kaiser PK, Flynn HWJr, et al. Treatment strategies and visual acuity outcomes in chronic postoperative P. acnes endophthalmitis. Ophthalmology. 1999;106:1665-1670.
16 Aldave AJ, Stein JD, Deramo VA, et al. Treatment strategies for postoperative Propionibacterium acnes endophthalmitis. Ophthalmology. 1999;106:2395-2401.
17 Ciulla TA. Update on acute and chronic endophthalmitis. Ophthalmology. 1999;106:2237-2238.
18 Puliafito CA, Baker AS, Haaf J, et al. Infectious endophthalmitis: review of 36 cases. Ophthalmology. 1982;89:921.
19 Rowsey JJ, Newsom MS, Sexton DJ, et al. Endophthalmitis: current approaches. Ophthalmology. 1982;89:1055.
20 Bohigian GM, Olk RJ. Factors associated with a poor visual result in endophthalmitis. Am J Ophthalmol. 1986;101:332.
21 Brinton GS, Topping TM, Hyndiuk RA, et al. Posttraumatic endophthalmitis. Arch Ophthalmol. 1984;102:547.
22 Thompson JT, Parver LM, Enger C, et al. Endophthalmitis after penetrating ocular injuries with retained intraocular foreign bodies. Ophthalmology. 1993;100:1468.
23 Thompson WS, Rubsamen PE, Flynn HWJr, et al. Endophthalmitis after penetrating trauma. Risk factors and visual acuity outcomes. Ophthalmology. 1995;102:1696-1701.
24 Anderoli CM, Anderoli MT, Kloek CE, et al. Low rate of endophthalmitis in a large series of open globe injuries. Am J Ophthalmol. 2009;147:601-608.
25 Weichel ED, Colyer MH, Ludlow SE, et al. Combat ocular trauma visual outcomes during operations Iraqi and enduring freedom. Ophthalmology. 2008;115:2235-2245.
26 Greenwald MJ, Wohl LG, Sell CH. Metastatic bacterial endophthalmitis: a contemporary reappraisal. Surv Ophthalmol. 1986;31:81.
27 Brod RD, Flynn HWJr, Clarkson JG, et al. Endogenous Candida endophthalmitis: management without intravenous amphotericin B. Ophthalmology. 1990;97:666.
28 Weishaar P, Flynn HWJr, Murray TG, et al. Endogenous Aspergillus endophthalmitis. Clinical features and treatment outcomes. Ophthalmology. 1998;105:57-65.
29 Okada AA, Johnson RP, Liles C, et al. Endogenous bacterial endophthalmitis. Ophthalmology. 1994;101:832-838.
30 Smith SR, Kroll AJ, Lou PL, et al. Endogenous bacterial and fungal endophthalmitis. Int Ophthalmol Clin. 2007;47:173-183.
31 Jackson TL, Eykyn SJ, Graham EM, et al. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48:403-423.
32 Pilli S, Kotsolis A, Spaide RF, et al. Endophthalmitis associated with intravitreal anti-vascular growth factor therapy injections in an office setting. Am J Ophthalmol. 2008;145:879-882.
33 Packer AJ, Weingeist TA, Abrams GW. Retinal periphlebitis as an early sign of bacterial endophthalmitis. Am J Ophthalmol. 1983;96:66.
34 Lalwani GA, Flynn HWJr, Scott IU, et al. Acute-onset endophthalmitis after clear corneal cataract surgery (1996–2005) Clinical features, causative organisms, and visual acuity outcomes. Ophthalmology. 2008;115:473-476.
35 Donahue SP, Kowalski RP, Jewart BH, et al. Vitreous cultures in suspected endophthalmitis: biopsy or vitrectomy? Ophthalmology. 1993;100:452.
36 Joondeph BC, Flynn HWJr, Miller D, et al. A new culture method for infectious endophthalmitis. Arch Ophthalmol. 1989;107:1334.
37 Kapran EI, Altan T, Eren H, et al. The use of blood culture bottles in endophthalmitis. Retina. 2007;27:971-973.
38 Kratz A, Levy J, Belfair N, et al. Broth culture vs. traditional approach in the work-up of endophthalmitis. 2006;141:1022–6.
39 Rao NA. A laboratory approach to rapid diagnosis of ocular infections and prospects for the future. Am J Ophthalmol. 1989;107:283.
40 Goldschmidt P, Degeorge S, Benallaoua D, et al. New test for the diagnosis of bacterial endophthalmitis. Br J Ophthalmol. 2009;10:1-16.
41 Anand AR, Madhavan HN, Neela V, et al. Use of polymerase chain reaction in the diagnosis of fungal endophthalmitis. Ophthalmology. 2001;108:326-330.
42 Chandler PA. Problems in the diagnosis and treatment of lens-induced uveitis and glaucoma. Arch Ophthalmol. 1958;60:828.
43 Irvine WD, Flynn HWJr, Murray TG, et al. Retained lens fragments after phacoemulsification manifesting as marked intraocular inflammation with hypopyon. Am J Ophthalmol. 1992;114:610.
44 Schaal S, Barr CC. Management of retained lens fragments after cataract surgery with and without pars plana vitrectomy. J Cataract Refract Surg. 2009;35:863-867.
45 Werner L, Sher JH, Taylor JR, et al. Toxic anterior segment syndrome and possible association with ointment in the anterior chamber following cataract surgery. J Cataract Refract Surg. 2006;32:227-235.
46 Holland SP, Morck DW, Lee TL. Update on toxic anterior segment syndrome. Curr Opin Ophthalmol. 2007;18(1):4-8.
47 Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479-1496.
48 Doft DH, Wisnisky SR, Kellsy SF, et al. Diabetes and postoperative endophthalmitis in the Endophthalmitis Vitrectomy Study. Arch Ophthalmol. 2001;119:650-656.
49 Endophthalmitis Vitrectomy Study Group. Microbiologic factors and visual outcome in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122:830-846.
50 Bannerman TL, Rhoden DL, McAllister M, et al. The source of coagulase-negative staphylococci in the Endophthalmitis Vitrectomy Study. A comparison of eyelid and intraocular isolates using pulsed-field gel electrophoresis. Arch Ophthalmol. 1997;115:357-361.
51 Flynn HFJr, Scott IU. Legacy of the Endophthalmitis Vitrectomy Study. Arch Ophthalmol. 2008;126:559-561.
52 Doft BH, Kelsey SF, Wisniewski SR, et al. Additional procedures after the initial vitrectomy or tap-biopsy in the Endophthalmitis Vitrectomy Study. Ophthalmology. 1998;105:707-716.
53 Han DP, Wisniewski SR, Wilson LA, et al. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study [published erratum appears in Am J. Ophthalmol 1996 Dec 122:920]. Am J Ophthalmol. 1996;122:1-17.
54 Han DP, Wisniewski SR, Kelsey SF, et al. Microbiologic yields and complication rates of vitreous needle aspiration versus mechanized vitreous biopsy in the Endophthalmitis Vitrectomy Study. Retina. 1999;19:98-102.
55 Johnson MW, Doft BH, Kelsey SF, et al. The Endophthalmitis Vitrectomy Study. Relationship between clinical presentation and microbiologic spectrum. Ophthalmology. 1997;104:261-272.
56 Doft BH. Treatment of post cataract extraction endophthalmitis. A summary of the results from the Endophthalmitis Vitrectomy Study. Arch Ophthal. 2008;126:554-556.
57 Durand M. Microbiologic factors and visual outcome in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1997;124:127-130.
58 Kwok AK, Hui M, Pang CP, et al. An in vitro study of ceftazidime and vancomycin concentrations in various fluid media: implications for use in treating endophthalmitis. Invest Ophthalmol Vis Sci. 2002;43(4):1182-1188.
59 Jay WM, Fishman P, Aziz M, et al. Intravitreal ceftazidime in a rabbit model: dose and time-dependent toxicity and pharmacokinetic analysis. J Ocul Pharmacol. 1987;3:257.
60 Campochiaro PA, Green WR. Toxicity of intravitreous ceftazidime in primate retina. Arch Ophthalmol. 1992;110:1625.
61 Oum BS, D’Amico DJ, Wong KW. Intravitreal antibiotic therapy with vancomycin and aminoglycoside: an experimental study of combination and repetitive injections. Arch Ophthalmol. 1989;107:1055.
62 Olson JC, Flynn HWJr, Forster RK, et al. Results in the treatment of postoperative endophthalmitis. Ophthalmology. 1983;90:692.
63 Friberg TR. En bloc removal of inflammatory fibrocellular membranes from the iris surface in endophthalmitis. Arch Ophthalmol. 1991;109:736.
64 Doft BH, Weiskopf J, Nilsson-Ehle I, et al. Amphotericin clearance in vitrectomized versus nonvitrectomized eyes. Ophthalmology. 1985;92:1601.
65 Nagpal M, Wartikar S, Nagpal K. Comparison of clinical outcomes and wound dynamics of sclerotomy ports of 20, 25, and 23 gauge vitrectomy. Retina. 2009;29:225-231.
66 Campochiaro PA, Lim JL. Aminoglycoside Toxicity Study Group. Aminoglycoside toxicity in the treatment of endophthalmitis. Arch Ophthalmol. 1994;112:48.
67 Doft BM, Kelsey SF, Wisniewski SR. EVS study group. Retinal detachment in the endophthalmitis vitrectomy study. Arch Ophthalmol. 2000;118:1661-1665.
68 Chen E. 25 gauge transconjunctival sutureless vitrectomy. Curr Opin Ophthalmol. 2007;18:188-193.
69 Endophthalmitis Study Group, European Society of Cataract and Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery. results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978-988.
70 Chang DF, Braga-Mele R, Mamalis N, et al. Prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2007 ASCRS member survey. J Cataract Refract Surg. 2007;33:1801-1805.
71 Pavan PR, Brinser JH. Exogenous bacterial endophthalmitis treated without systemic antibiotics. Am J Ophthalmol. 1987;104:121.
72 Hariprasad SM, Shah GK, Mieler WF, et al. Vitreous and aqueous penetration of orally administered moxifloxacin in humans. Arch Ophthalmol. 2006;124:178-182.
73 Miller D, Flynn PM, Scott IU, et al. In vitro fluoroquinolone resistance in staphylococcal endophthalmitis isolates. Arch Ophthalmol. 2006;124:479-483.
74 Kuehn BM. Fluoroquinolone warning. JAMA. 2008;300:891.
75 Smiddy WE, Smiddy RJ, Ba’Arath B, et al. Subconjunctival antibiotics in the treatment of endophthalmitis managed without vitrectomy. Retina. 2005;25:751-758.
76 Iyer MN, Han DP, Yun HJ, et al. Subconjunctival antibiotic injection for acute post-cataract extraction endophthalmitis – is it necessary? Am J Ophthalmol. 2004;137:1120-1121.
77 Holland EJ, Lane SS, Kim T, et al. Ocular penetration and pharmacokinetics of topical gatifloxacin 0.3% and moxifloxacin 0.5% ophthalmic solutions after keratoplasty. Cornea. 2008;27:3141-3149.
78 Das T, Jaleli S, Gothwal V, et al. Intravitreal dexamethasone in exogenous bacterial endophthalmitis: result of a prospective randomized study. Br J Ophthalmol. 1999;83:1050-1055.
79 Irvine WD, Flynn HWJr, Miller DA, et al. Endophthalmitis caused by Gram negative organisms. Arch Ophthalmol. 1992;110:1450-1454.
80 Mao LK, Flynn HWJr, Miller D, et al. Endophthalmitis caused by Staphylococcus aureus. Am J Ophthalmol. 1993;116:584.
81 Pathengay A, Shah GY, Das T, et al. Intravitreal triamcinolone acetonide in the management of exogenous bacterial endophthalmitis. Am J Ophthalmol. 2006;141:938-940.
82 De Kaspar HM, Ta CN, Engelbert M, et al. Effects of intravitreal corticosteroid in the treatment of Staphylococcus aureus-induced experimental endophthalmitis. Retina. 2008;28:326-332.
83 Koul S, Philipson BT, Philipson A. Visual outcome of endophthalmitis in Sweden. Acta Ophthalmol. 1989;67:504.
84 Doft BM, Kelsey SF, Wisniewski SR. EVS study group. Retinal detachment in the endophthalmitis vitrectomy study. Arch Ophthalmol. 2000;118:1661-1665.