Endometritis, Metaplasias, Polyps, and Miscellaneous Changes
Inflammatory and Infectious Processes
Infections of the gynecologic tract are relatively common, and cause an enormous health impact. It is estimated that there are 19 million cases of sexually transmitted diseases (STDs) in the United States, with a total healthcare cost of roughly $16.4 billion.1 These infections can be both symptomatic and asymptomatic, and it is believed that up to 24,000 cases of infertility in the United States are related to STDs. Symptomatic infections are most commonly diagnosed based on the clinical examination and basic laboratory studies, and, as such, a tissue biopsy is generally not needed. Approximately 20% of cases of STD can result in pelvic inflammatory disease (PID), which is inflammation of the uterus, fallopian tubes, or ovaries. An unknown fraction of STDs lead to asymptomatic infections, or to signs that are not recognized to be related to infection, such as dysfunctional uterine bleeding. Chronic endometritis has been shown to interfere with local expression of estrogen and progesterone receptors in the endometrium, which may also contribute to infertility.2 It is especially in the evaluation of women with bleeding that a diagnosis of infection is critical. STDs can be readily treated, and proper treatment can prevent later complications. In a minority of cases the pattern of inflammation in the endometrium is indicative of a specific organism (Table 16.1). In the majority of cases the pattern of inflammation is nonspecific.
Endometrial inflammation is recognized, as in other organs, primarily by the presence of inflammatory cells. However, as the endometrium may normally contain inflammatory cells, the diagnosis of endometritis is often not straightforward. Lymphocytes, even when focally collected around blood vessels, are not necessarily indicative of endometritis. As plasma cells are seldom found in a normal endometrium, their presence is indicative of chronic endometritis. Neutrophil polymorphonuclear leukocytes may also be found in the normal cycle, especially in appreciable numbers once menstruation is well established. Granulated lymphocytes, or CD56+ uterine natural killer cells, present in the stroma during the second half of the cycle, and especially in predecidua, are easily mistaken for neutrophils.3,4 Macrophages are always present in the normal endometrium, but in their usual appearance resemble endometrial stromal cells, rendering separation difficult.
Endometritis
Patients with endometritis often present with the clinical features of PID. They will have a history of fever and lower abdominal pain, with leukocytosis and bilateral adnexal or cervical motion tenderness on bimanual pelvic examination. If the infecting organism is less virulent or if the disease is early in its natural history, the symptoms, such as menstrual abnormalities, pelvic pain, or infertility, may not initially indicate the clinical diagnosis of endometritis. Although most cases of acute salpingitis are accompanied by endometritis, it is not known how often endometritis occurs in isolation, without involving the fallopian tubes. The diagnosis of endometritis is therefore often a histologic one that, particularly in the case of nonspecific endometritis, is not associated with a specific clinical presentation or cause.
Nonspecific Endometritis
Microscopic Features
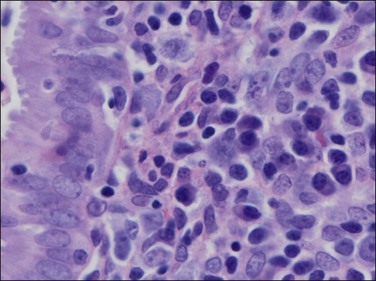
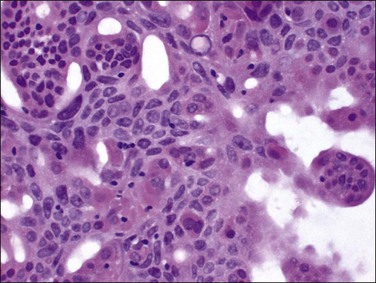
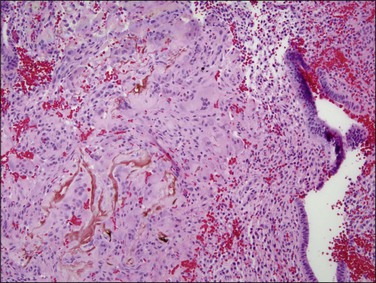
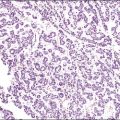
The histologic diagnosis of acute endometritis is made when significant numbers of neutrophils are present in the endometrial stroma at a time other than menstruation (Figure 16.1). Isolated or rare neutrophils are insufficient for a diagnosis of acute endometritis. The usual pattern is that of patchy distribution, with or without microabscesses. As with all forms of endometritis, it is not only the presence of a particular inflammatory cell that is important, but also the reaction pattern of the endometrium. A common appearance in the patient of reproductive age is glandular dyssynchrony in response to circulating sex hormones. In the case of acute endometritis it should be accompanied by other features of inflammation and repair, including edema, hemorrhage, venule ectasia, microabscesses, or pus-filled glands. In the case of menstrual endometrium other features of menstruation are typically seen, including secretory vacuoles in the glandular epithelium and predecidual change of the stroma with diffuse stromal breakdown. It may not always be possible to distinguish between acute endometritis and menstrual endometrium.
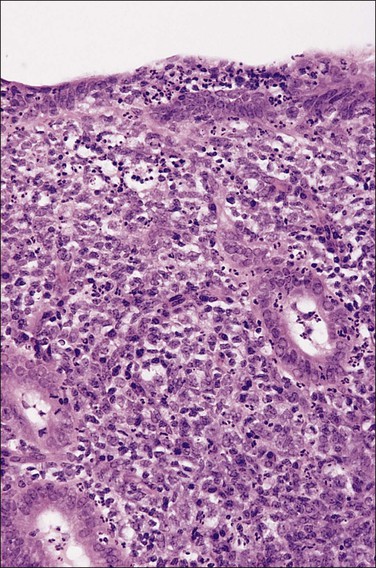
Figure 16.1 Acute endometritis. Polymorphs are present in the surface epithelium and throughout the stroma.
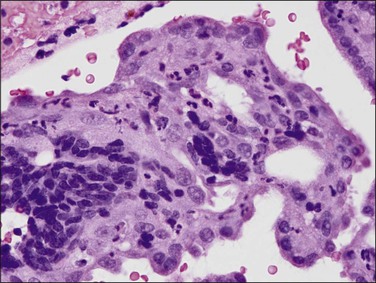
If plasma cells are present, often seen together with aggregates of lymphocytes and/or germinal centers, then a diagnosis of chronic endometritis is made (Figure 16.2). A not uncommon problem occurs when only a rare plasma cell is identified or it is a minor component of the infiltrate. If on a low-power magnification the endometrium looks ‘dirty’ with numerous lymphocytes (Figure 16.3), a careful search under higher magnification often shows scattered plasma cells that justify a diagnosis of chronic endometritis. Areas worthy of particular attention include glands engorged with inflammatory cells. As plasma cells may be a normal component within the stroma of the endocervix and lower uterine segment, care must be taken to ensure that only the functionalis is assessed.
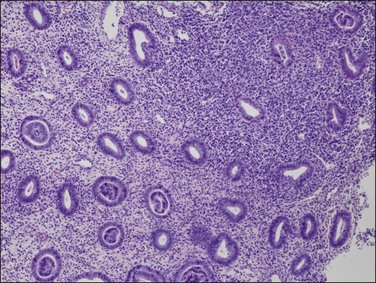
Figure 16.3 Chronic endometritis. At low magnification, the presence of a patchy infiltrate of inflammatory cells makes the stroma appear dirty.
Glands may be involved in the inflammatory process in two ways. There may be an infiltrate of inflammatory cells, predominantly neutrophils, affecting the epithelium of the glands and the lumina may contain pus. In addition, as the process becomes more advanced, the glandular response to hormonal stimulation becomes impaired, the glands appearing inactive, with neither proliferation nor secretion. Once an endometrium shows signs of endometritis, precise dating is not possible. It is probably not even reliable to categorize the endometrium as either proliferative or secretory. Dating is unnecessary in a patient who has significant endometritis. The histologic features of endometritis are summarized in Table 16.2.
Specific Forms of Endometritis
Chlamydia Trachomatis and Neisseria Gonorrhoeae
Many upper genital tract infections result from the sexually transmitted organisms Chlamydia trachomatis and Neisseria gonorrhoeae.5,6 Infection by C. trachomatis exhibits supranuclear, intracytoplasmic vacuoles containing inclusions, but these are not readily apparent by microscopic examination. Immunohistologic or molecular methods are usually required for confirmation of chlamydial infection. Polymerase chain reaction identifies C. trachomatis in one-fourth of plasma cell endometritis specimens, and these can also be cultured.7,8 Endometritis due to either Chlamydia or Neisseria has large numbers of plasma cells in the endometrial stroma, neutrophils in the endometrial surface epithelium, and lymphoid aggregates with transformed lymphocytes,9 thus providing clues to the diagnosis. Intraluminal neutrophils and subepithelial hemorrhages are also usually present, but these findings are less specific. Histopathology alone can suggest, but not reliably distinguish between, these organisms. Prominent lymphoid follicles with transformed lymphocytes points more to chlamydial infection while extensive stromal fragmentation favors gonococcal infection.5
Mycoplasma
Infection with the mycoplasma Ureaplasma urealyticum produces subtle but distinctive changes that may be easily missed. Mycoplasma is the second most common etiologic agent after common bacteria.10 The changes are focal, composed of lymphocytic clusters with histiocytes and occasional neutrophils. Plasma cells are not prominent. These focal collections often lie beneath the surface epithelium, around glands, and surrounding spiral arterioles. In many of these patients, U. urealyticum also has been cultured from the uterine cervix, suggesting an ascending infection. Mycoplasma genitalium is a common pathogen associated with acute endometritis.11
Cytomegalovirus
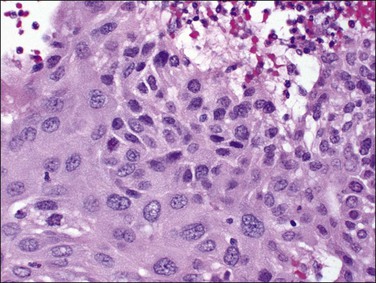
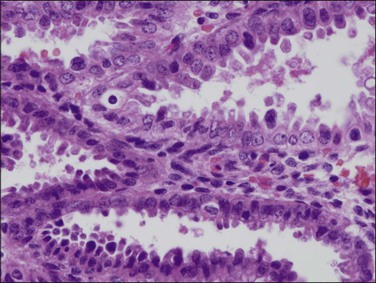
Cytomegalovirus (CMV) infection of the endometrium has been reported rarely and may be associated with pregnancy and immunosuppression as well as being found in women with no known underlying disorder. The affected epithelial and sometimes endothelial cells are grossly enlarged and each contains a usually single large, basophilic intranuclear inclusion that is surrounded by a narrow clear space separating it from the nuclear membrane (Figure 16.4).
Herpes Simplex Virus
Like CMV infection, herpes simplex virus (HSV) infection of the endometrium is rare. It may occur as an ascending process associated with cervical infection or result from disseminated virus in immunosuppressed patients. It may cause postpartum endometritis in cases where infants have died from disseminated HSV infection.5 Infection of the endometrium produces changes similar to those associated with HSV elsewhere, namely extensive acute inflammation with necrosis. Cowdry type A inclusions and, occasionally, multinucleated giant cells with ground-glass nuclei may be identified in glandular epithelium or in the stroma.
Tuberculous Endometritis
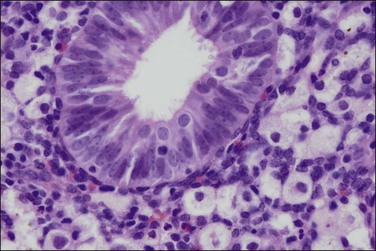
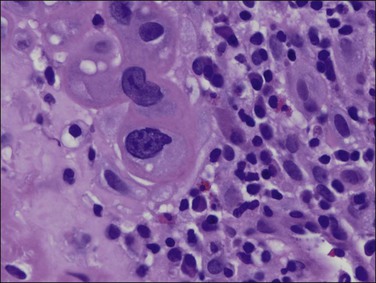
Although tuberculosis has become infrequent in the United States, it remains an endemic disease in some parts of the world12 and more may be seen in the future as a result of the emergence of drug-resistant strains of Mycobacterium tuberculosis and the immunosuppression of AIDS. Tuberculosis of the female genital tract is virtually always secondary to disease of the lungs or gastrointestinal tract, which often occurred many years previously. About three-fourths of women with tuberculosis of the genital tract have endometrial involvement. The fallopian tubes are almost always involved and the endometrium is affected secondarily by passage of the bacteria down the tube into the uterine cavity.
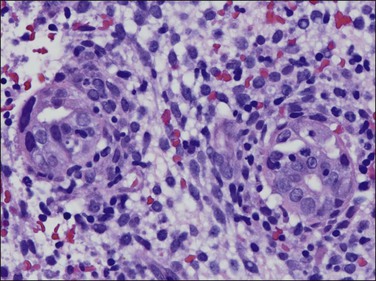
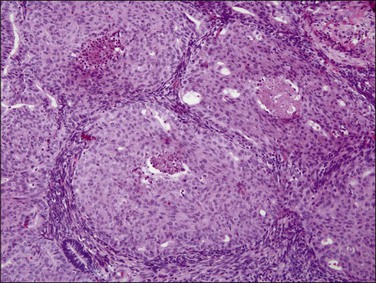
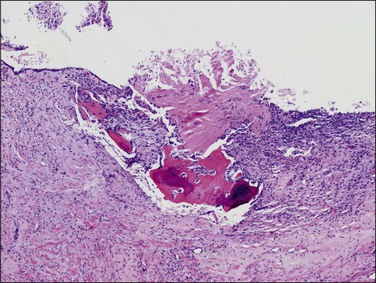
The granuloma of tuberculous endometritis contains a central collection of epithelioid cells with both Langhans and foreign body-type giant cells (Figure 16.5). There is usually a peripheral collar of lymphocytes. Because caseation is absent, the granulomas more closely resemble those of sarcoidosis, a feature reinforced because they are well circumscribed. Uterine sarcoidosis rarely presents without proven sarcoidosis elsewhere in the body.
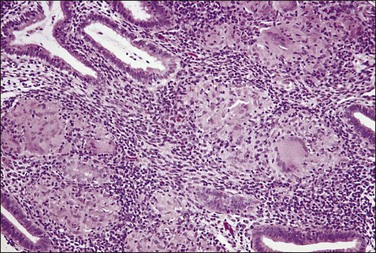
Figure 16.5 Tuberculous endometritis. Noncaseating granulomas are distributed throughout the stroma.
Culture of the endometrial tissue best confirms the diagnosis. In a classic study that has not been repeated, acid-fast bacilli were demonstrated in less than 2% of 1134 women with culture-proven organisms.13 More recently molecular methods have been developed that allow the specific detection, and strain classification, of M. tuberculosis in formalin-fixed paraffin-embedded materials at a level of sensitivity exceeding that of special stains.14–16
Actinomyces Israeilii
Infection by Actinomyces israeilii has long been associated with intrauterine contraceptive device (IUD) usage.17 This organism is a slow-growing, filamentous, Gram-positive anaerobe that is not normally found in the lower genital tract (Figure 16.6). When found in cervical smears or curettings, half the women lack symptoms. Microscopic examination of the curettings shows densely matted filaments of the Gram-positive organisms (Figure 16.7). Gomori methenamine silver stain is positive in Actinomyces, which is a useful means of identifying noninfectious pseudoactinomycotic radiate granules (negatively staining) that are even more common than actual organisms in women with an IUD.18,19 There is usually some degree of associated endometritis, with a dense infiltrate of neutrophils. The infection may progress to pelvic actinomycosis. The risk of infection by A. israeli relates less to the type of IUD in use than the length of time it has been in place in the uterine cavity; 85% of cases occur when the IUD has been worn for 3 or more years.
Other Forms of Endometritis
Granulomatous Endometritis
The finding of granulomas in an endometrial biopsy can be caused by a variety of diseases. In a recent review granulomas were identified in 0.15% of endometrial biopsies.20 Focal granulomas, that is the finding of a single granulomatous focus, were significantly associated with a prior history of cesarean section or dilatation and curettage, and none of the women had any signs of a systemic disorder or infection upon follow-up. In terms of infection, granulomas can be caused by tuberculosis (as described previously), atypical mycobacteria, endemic mycosis, actinomycosis, and parasites.20,21 Noninfectious causes can include a reaction to foreign material, Crohn disease, sarcoidosis, or lymphoma. Granulomas can also be seen postsurgically. Sarcoidosis is associated with well-formed, clearly demarcated epithelioid cell granulomas lacking central necrosis. It occurs usually, but not always, in women with known systemic disease. However, as necrosis is unusual in tuberculous endometritis, endometrial sarcoid should only be diagnosed if the disease is present elsewhere in the body. An effort should be made to identify a treatable, infective cause if it is present. Fungi may be identified using periodic acid–Schiff (PAS) or Grocott stains, but it is questionable whether a time-consuming search for acid-fast bacilli is worth undertaking because of the very low chance of identifying the organism, even in culture-proven cases.13 The identification of pigment or birefringence under polarized light points to a foreign body reaction. A clinical history may also be useful in coming to a conclusion.
Histiocytic Endometritis
On rare occasions, the endometrium discloses a massive infiltrate of histiocytes that contain abundant lipid, together with multinucleated giant cells, hemosiderin, cholesterol clefts, plasma cells, lymphocytes, and occasional neutrophils (Figure 16.8). This may occur in association with pyometra, due to postmenopausal cervical stenosis, or in association with an endometrial glandular neoplasm. The terms ‘pseudoxanthoma’ and ‘xanthogranuloma’ have also been used but the term ‘histiocytic endometritis’ is preferred. The condition bears no relation to the focal clusters of foamy macrophages sometimes found in the stroma of endometrial carcinomas. Appearances similar to histiocytic endometritis may be seen in the fallopian tube. Malakoplakia is a rare variant of histiocytic endometritis. The appearances are generally similar to those already described but in addition Michaelis–Gutmann bodies are present. These are small, round, laminated calcospherules that may be found both in the cytoplasm of the histiocytes and also extracellularly.
Postpartum and Postabortal Endometritis
Endometritis occurs in 2–5% of women following vaginal delivery, 20–55% of women following cesarean section, and 1–8% of women having termination of pregnancy in a hospital setting.22 The risk of endometritis is even higher in women with retained products of conception. Plasma cell infiltrates, which are not a normal postpartum response, are characteristic of postpartum chronic endometritis (Figure 16.9). Microbiologic examination shows that the infection is caused by two or more microorganisms, the most frequently cultured being U. urealyticum and Gardnerella vaginalis. The latter is most commonly associated with bacterial vaginosis and clinical evidence of bacterial vaginosis is associated with a sixfold increase in the risk of post-cesarean endometritis.23 Furthermore, recent studies have shown that bacterial vaginosis is associated with a higher risk of endometritis9 and upper genital tract infection in the nonpregnant state.24
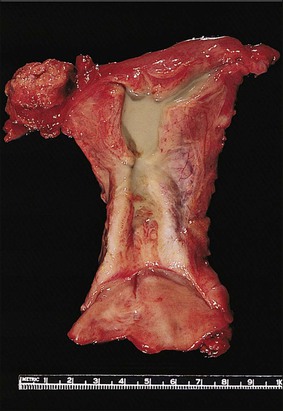
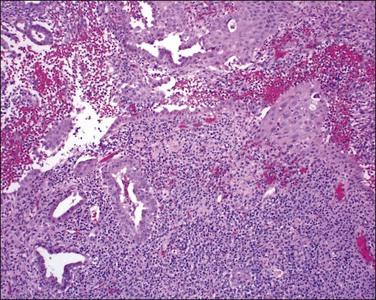
Pyometra
Inflammation of the endometrium is most dramatic when pyometra, pus filling the uterine cavity, develops (Figure 16.10). This is often due to a blocked cervical canal in conditions such as cervical carcinoma or, more frequently, postmenopausal stenosis. Introduction of foreign material through fistulous tracts is another common cause. As the term implies, the cavity of the distended uterus is filled with pus and the endometrium usually shows the features of severe acute and chronic inflammation (Figure 16.11). Squamous metaplasia may develop in the surface epithelium and glands, and at its most extreme is known as ichthyosis uteri.25 Squamous cell carcinoma, rarely, may also occur.
Endometrial Metaplasias
Endometrial metaplasia is a change in cellular differentiation to a type that is not present in the normal endometrium. Terms such as metaplasia, differentiation, and ‘change’ are used, often interchangeably, to reflect the wide variety of cell types that can be seen in the endometrium. These can include alterations in cytoplasm, nuclei, and even architecture. Metaplasia most commonly involves the epithelial compartment but can also involve the stromal compartment. Because altered differentiation may be seen in benign, premalignant, or malignant endometria, a simple diagnosis of endometrial metaplasia is rarely sufficient to fully convey the lesion’s clinical significance. Endometrial metaplasias are best considered a spectrum of altered differentiation states in which the pathologist should attempt to determine the underlying mechanism, and use terminology that clearly communicates clinical significance to the referring physician.
Epithelial Metaplasias
Etiology
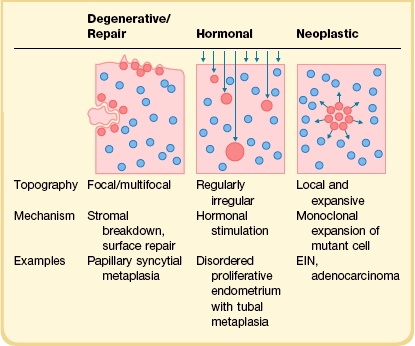
The term metaplasia applies to a broad range of cytologic appearances caused by degenerative, hormonal, or neoplastic processes (Table 16.3). The overall low-magnification topography of metaplastic changes within the endometrial compartment, combined with the local histologic context, are extremely useful in ascertaining a likely cause.26,27 Degenerative changes can be localized to areas of inflammation, stromal breakdown, or recent surface repair. Hormonally induced changes tend to be diffusely and randomly scattered throughout the upper endometrial compartment (functionalis), whereas premalignant and malignant processes are monoclonal expansions that begin as localizing lesions offset by the normal background. The genetic underpinnings of müllerian metaplasia are just now emerging. Genetic mediators of müllerian differentiation include members of the homeobox gene superfamily, HOX, where coordinate expression of HOX genes determines differentiation state.28 Altered HOX gene expression, through hormonal29,30 or epigenetic mechanisms,31,32 is capable of re-determining differentiation state. For example, HOXA11 expression can be induced by progestins and in müllerian epithelium is associated with acquisition of mucinous differentiation.33
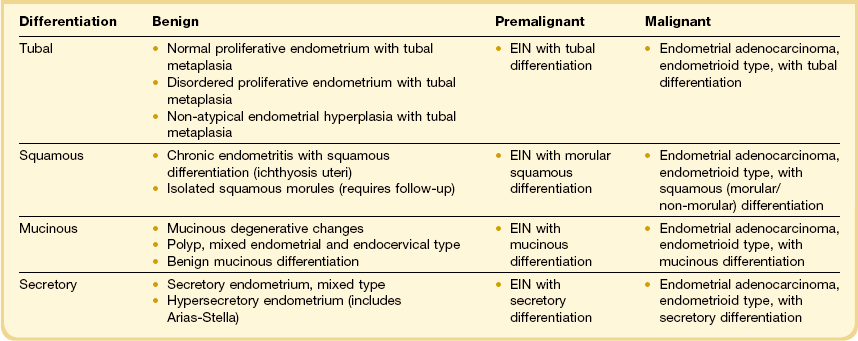
A particular pitfall in dealing with endometrial neoplasms with altered (metaplastic) differentiation is the incorrect assumption that metaplasia and neoplasia are mutually exclusive processes. Squamous, mucinous, tubal, or secretory differentiation may be acquired features intrinsic to a premalignant (endometrial intraepithelial neoplasia; EIN) or malignant (adenocarcinoma) neoplastic clone.34–37 In these instances, altered differentiation is best noted descriptively appended to a primary diagnosis of EIN or adenocarcinoma (Table 16.4). Individual examples of EIN or adenocarcinoma can demonstrate quite unstable differentiation states in which admixed cytologies line individual glands or populate different regions of the neoplastic field.34,38,39 In the case of metaplastic premalignant lesions, all EIN criteria must be met (Chapter 17),40 with the observed change in cellular differentiation sometimes contributing to the altered cytology requirement. Overtly malignant processes, especially mucinous and secretory adenocarcinomas, may have unusually bland nuclei as part of their altered differentiation state.
Tubal Metaplasias
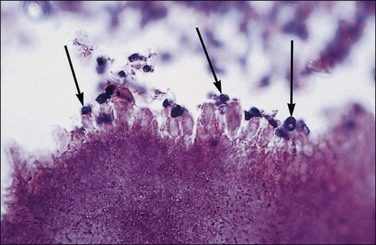
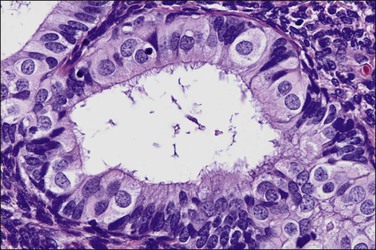
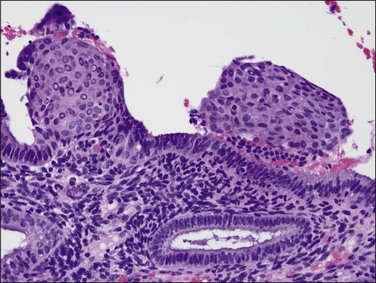
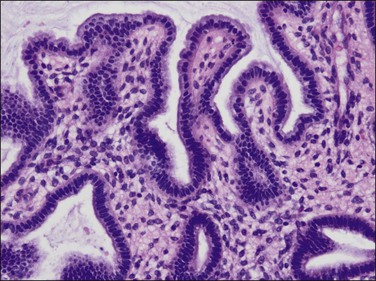
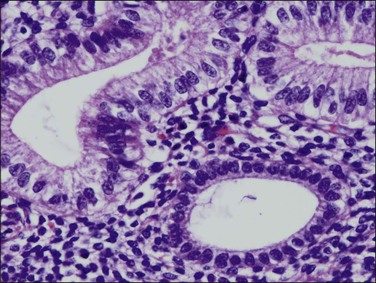
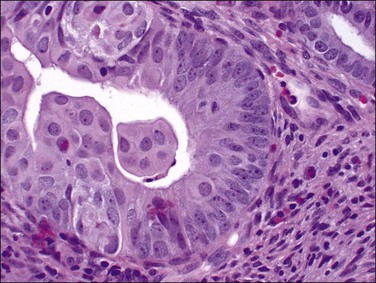
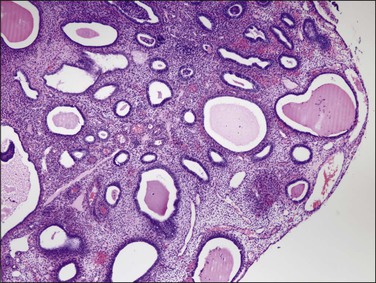
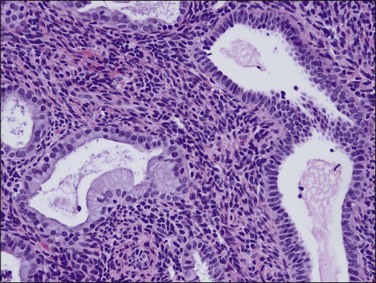
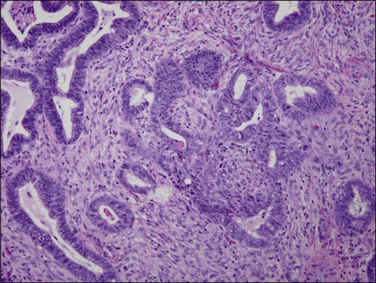
Tubal differentiation is the most commonly encountered form of endometrial metaplasia, accounting for 60% of examples of metaplasia in the endometrium. Cilia are the most distinctive manifestation, but changes evident even at low magnification are increased cytoplasmic volume and eosinophilic staining. Interspersed between cuboidal ciliated cells are occasional round secretory cells in a pattern resembling that of the normal fallopian tube. The density of cilia varies greatly, and even a cilia-poor epithelium that demonstrates characteristic cytoplasmic properties of admixed round and columnar cells may be considered serous like (‘seroid’) or tubal in character (Figure 16.12). Some cilia-depleted tubal metaplasias display such prominent micropapillary or eosinophilic features that they may be considered mixed-type metaplasias.
Tubal differentiation may be induced by estrogen exposure or be a manifestation of a neoplastic clone. It is frequent in the normal proliferative endometrium, especially the uterine lining, suggesting that this can be a normal physiologic finding indicative of an underlying estrogenic milieu. An endometrial field altered by unopposed estrogens demonstrates tubal metaplasia in irregularly scattered occasional glands rather than in tight clusters. It also occurs in half of endometrial adenocarcinomas and 20% of precancerous EIN lesions demonstrating localized clonal architecture (Figure 16.13).37,41 When tubal glands are present within an EIN lesion, cilia formation is usually variable (Figure 16.14), admixed with micropapillary or endometrioid cytologies (Figure 16.15) in flanking glands. Some EIN lesions with tubal differentiation occur within an inactive or even atrophic background, implying that estrogens, while common, are not essential for tubal differentiation within neoplastic clones.
Squamous Metaplasia
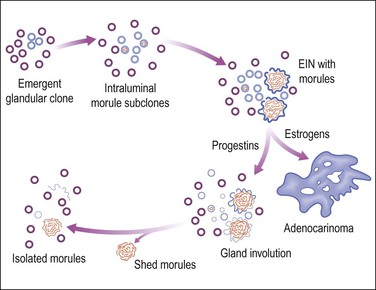
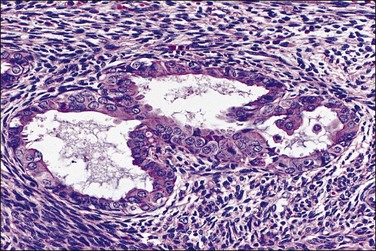
Squamous metaplasia may be seen as a stratified epithelium lining the uterine cavity, or as expansile round morules within individual glands. When there is an isolated finding, the former typically results from chronic irritation or infection. Squamous morules have a rather unique appearance and have not been described outside of the uterus. These round balls of somewhat immature epithelium are quite distinct, though, and are usually easily identified when present. Squamous morules are an interesting form of metaplasia, because they are quite frequently associated with neoplasia but are not malignant.
Squamous differentiation within endometrial glands occurs in 18% of premalignant EIN34 lesions and approximately 25% of adenocarcinomas.37,40 Not uncommonly, one or several foci of isolated squamous morules is found in a small biopsy specimen, but proves to be located at the periphery of an EIN lesion. This has prompted the clinical recommendation that follow-up sampling be performed when such unexplained morules are found.
Ichthyosis Uteri
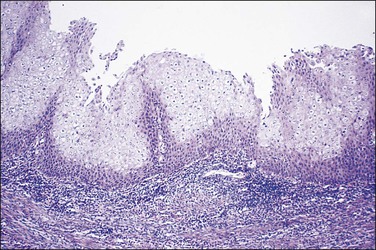
Ichthyosis uteri defines a stratified squamous uterine lining epithelium (Figure 16.16) that extends downward to individual glands and demonstrates intercellular bridges and even keratin pearls.25,42 Chronic endometritis of several years’ duration is almost always the antecedent condition (Figure 16.17). Much more common in the pre-antibiotic era, ichthyosis uteri is now rare and most commonly encountered in women with anatomic abnormalities favoring recurrent infection, such as fistulous tracts into the uterine cavity. We have encountered cases in which primary squamous cell carcinoma of the endometrium has been associated with ichthyosis uteri of the adjacent, non-malignant endometrium, although there is no evidence that ichthyosis itself is premalignant.
EIN with Squamous Morules
Even though morules may be an intimate component of a premalignant EIN lesion, it is the glandular element that has biologic potential to progress to carcinoma (Figure 16.18).43 Conserved molecular markers such as β-catenin mutation in both the squamous and glandular elements of EIN as well as the adenocarcinoma suggests all are derived from a common clone.44 Loss of estrogen and progesterone receptors and reduced mitotic activity occurs specifically in the squamous but not glandular elements44,45 (Figures 16.19–16.21), suggesting that squamous differentiation confers a terminal differentiation state with behavior and biologic potential quite different than that of the accompanying glands. Progestin-treated EIN lesions may demonstrate response within the glandular component, with relative sparing of the progesterone receptor-deficient morules (Figures 16.22 and 16.23).
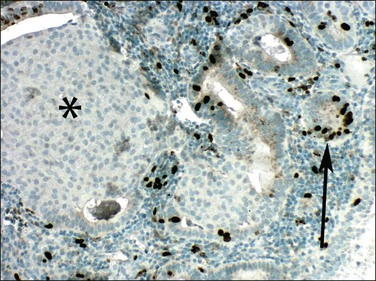
Figure 16.19 Squamous morules in EIN are mitotically quiescent. Mitotic activity shown by Ki-67 (MIB1) immunohistochemistry is high in glands (arrow) but not in squamous morules (asterisk).
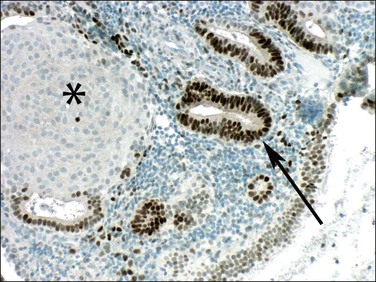
Figure 16.20 Squamous morules in EIN lose estrogen responsiveness. Immunohistochemistry showing estrogen receptors in glands (arrow) but not in squamous morules (asterisk).
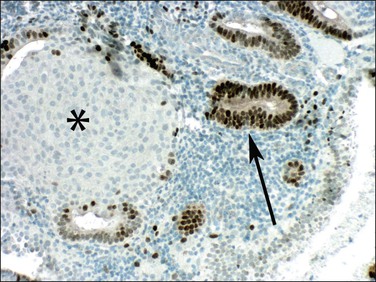
Figure 16.21 Squamous morules in EIN lose progesterone responsiveness. Immunohistochemistry showing progesterone receptors in glands (arrow) but not in squamous morules (asterisk).
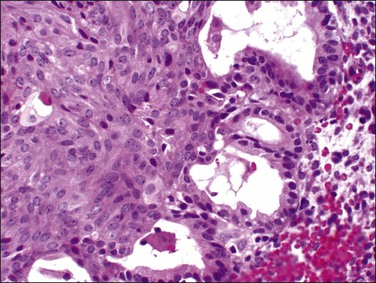
Figure 16.23 Re-biopsy of Figure 16.22 case after 4 months of therapy with progestins. The squamous components are unchanged, but cells of the glandular epithelium demonstrate reduction of epithelial thickness and nuclear size.
A diagnosis of EIN is made exclusively by careful evaluation of the glandular cytology and architecture, which must meet all criteria specified in Chapter 17, including size greater than 1 mm, cytologic change, and area of glands exceeding that of stroma. Intermingled morules, which are hormonally unresponsive and lack mitotic activity, should not be incorporated into the size and area estimates. This can be accomplished either by focusing upon a morule-depleted area of the glandular lesion or by mentally subtracting the interposed squamous areas.
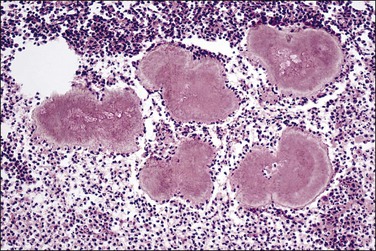
Squamous morules within the gland tracts of EIN lesions are not always easily recognizable as well-differentiated squamous cells. The morules form central, rounded epithelial aggregates that are usually continuous at the periphery with the endometrial glandular epithelium (Figure 16.24). The morules may appear to enlarge into sheets with multiple glands arranged radially around the periphery (Figure 16.25). These should not be confused with cribriform adenocarcinoma. Key features are peripheral glands displaced by an expansile solid central component, and transition from glandular to squamous cytology within individual lumens. Central comedo necrosis of squamous morules is common (Figure 16.26) in the context of EIN, but of no clinical or diagnostic significance.
Isolated Squamous Morules
Not uncommonly, the presence of isolated squamous morules is the only clue that something more serious may be present elsewhere in the endometrium (Figures 16.27 and 16.28). The endometrium may be proliferative or secretory; the glands small and uniform with little nuclear atypia and a low mitotic count. Possible mechanisms for presentation of isolated squamous morules in an otherwise unremarkable endometrial biopsy include morule persistence following involution of a previous glandular lesion (Figure 16.18), or morule shedding from a coexisting glandular lesion missed due to sampling error. Isolated morules should always be clearly described, with diagnosis of the underlying glandular pattern and careful clinical correlation or follow-up.
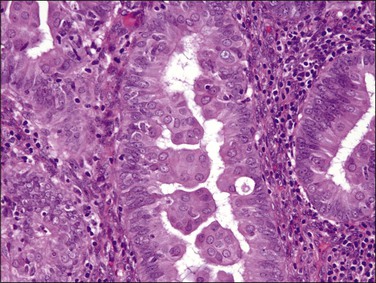
Mucinous Metaplasia
Mucinous metaplasia is a relatively common form of metaplasia that can vary in appearance from extracellular mucin to extensive intracellular mucin droplets. Mucinous metaplasia is almost uniformly of endocervical type. It can be seen in benign and degenerative conditions, and as a result of hormone therapy. Additionally, mucinous differentiation can be frequently seen in intraepithelial neoplasia and adenocarcinoma. Evaluation of mucinous epithelium is made more difficult by the fact that mucinous epithelium, whether part of a benign or malignant process, almost never shows any cytologic atypia. In general, it is the cases with prominent or regimented intracellular mucinous vacuoles that are most problematic. These are separated by architecture into different clinical outcome, and thus diagnostic, categories.46,47
Benign Mucinous Changes
Mucin droplets may be seen in association with degenerative changes or syncytial repair associated with stromal breakdown. In these cases, there are disorganized cellular aggregates of cuboidal to columnar eosinophilic cells with mucinous vacuoles or entrapped extracellular mucin droplets (Figure 16.29). A minor degree of architectural complexity as evidenced by multilayering of the cells and irregular slit-like spaces can be seen in association with reparative changes and should be distinguished from the more worrisome architectural features associated with mucinous metaplasia as outlined later.
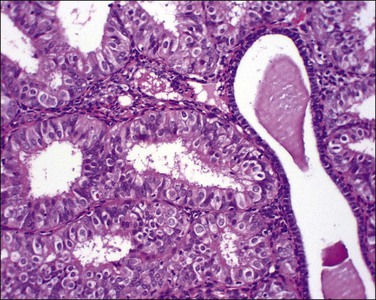
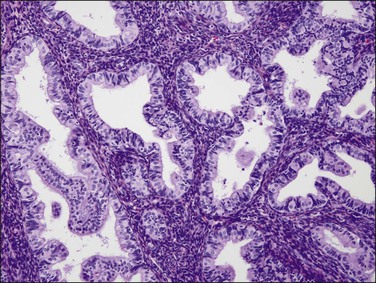
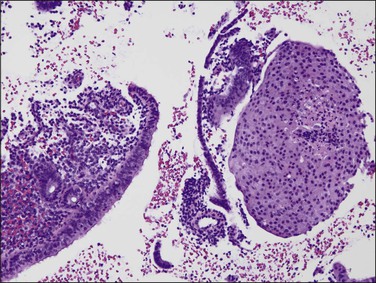
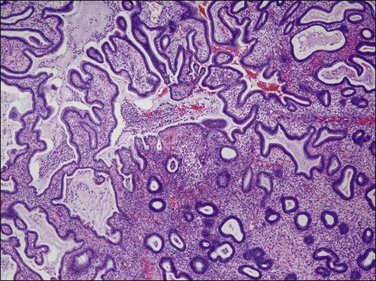
‘Complex hyperplastic papillary proliferations of the endometrium’ are a special type of papillary mucinous lesion composed of blunt stromal cores covered by a single layer of bland flat endometrioid to mucinous epithelium. Papillary components may anastomose at their base with underlying tubal glands (Figures 16.30 and 16.31). Most are within endometrial polyps. These have been shown in a limited series to have a benign course.48
EIN and Adenocarcinoma with Mucinous Differentiation
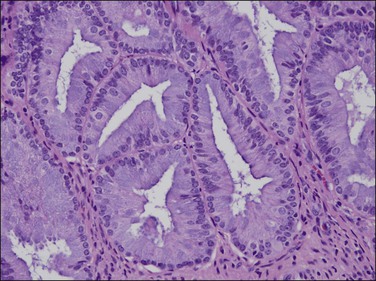
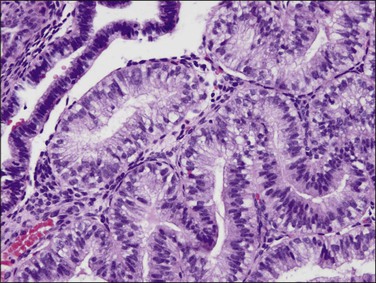
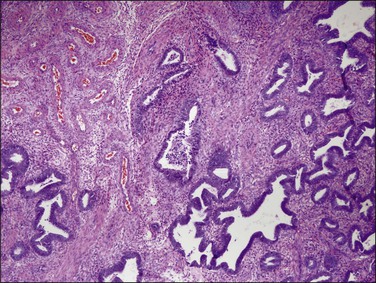
EIN may demonstrate mucinous metaplasia within the crowded glands that define the lesion (Figure 16.32). The extent of mucinous differentiation varies between adjacent, and even within individual, EIN glands. Intermediate extents of vacuole formation are common, phasing in and out between areas of mucinous and endometrioid differentiation. When gland-lining epithelium is simple, and architecture restricted to tubular or slightly branching forms, these are unlikely to be confused with carcinoma. Papillary redundant folds within mucinous EIN lesions are typically intraglandular, allowing them to be distinguished from the more complex folded sheets or meandering gland lumens of carcinoma.
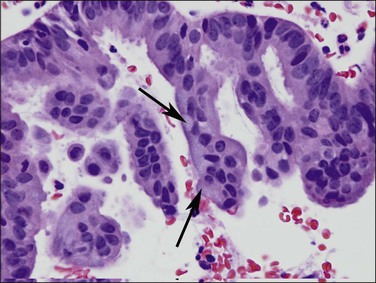
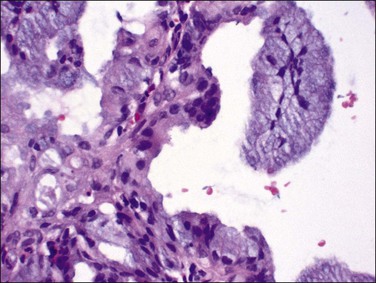
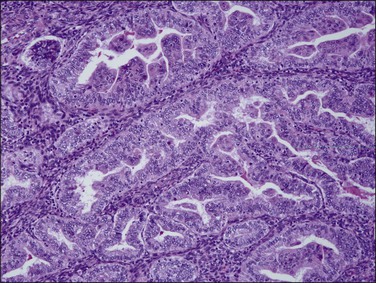
Very well differentiated endometrial adenocarcinomas with mucinous differentiation are most readily distinguished from EIN by the presence of a conspicuous exophytic surface papillary (Figure 16.33) or microglandular cribriform architectural component.49 Cytology may be bland, and alternating endometrioid and mucinous differentiation within individual surfaces (Figure 16.34) is a clue to its nonendocervical origin. The papillary fronds of well-differentiated adenocarcinoma are supported by a complicated delicate branching stromal support network, and may appear in a biopsy or curettage as detached ‘free-floating’ filiform fragments, often admixed with a microglandular component. These microglandular mucinous proliferations show small glands with rigid, ‘punched out’ lumens superficially resembling microglandular hyperplasia of the endocervix (Figure 16.35). Very small or highly fragmented pieces of tissue with this appearance may or may not represent a well-differentiated mucinous adenocarcinoma of the endometrium, the diagnosis of which depends on the amount of tissue, its preservation, and fragmentation. In scanty specimens, we diagnose a ‘complex mucinous epithelial proliferation’ and advise a follow-up endometrial sampling.
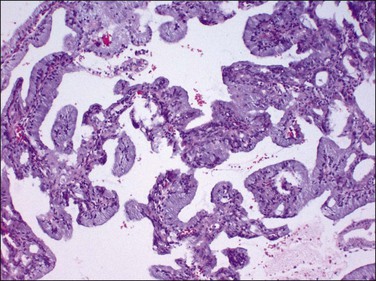
Figure 16.33 Well-differentiated endometrial adenocarcinoma, endometrioid type, with mucinous differentiation. Filiform pattern.
Secretory Metaplasia
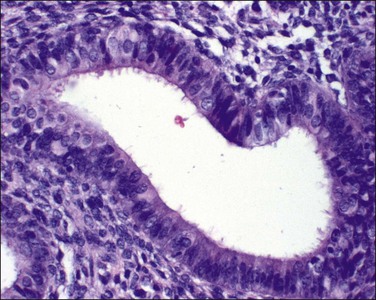
Secretory change, a normal response of endometrial glands to circulating progesterone, is accompanied also by a characteristic change in the stromal cells to a cell resembling decidua. These changes, strictly speaking, are physiologic and not metaplastic. Normal secretory phase glands can focally have little intervening stroma and can show a variety of artifacts that can lead to an erroneous diagnosis of neoplasia. Hypersecretory change, also referred to as Arias-Stella phenomenon, is a result of high levels of progesterone accompanying pregnancy (Figure 16.36). Because the secretory glands can have irregular hyperchromatic nuclei they can be mistaken for neoplastic epithelium (Figure 16.37). This mistake is most easily made in sites other than the endometrium; for example, in the cervix or fallopian tube. However, the change is entirely benign.
Neoplastic glands of EIN and adenocarcinoma may be capable of mounting a secretory response to progestins, and this may appear as subnuclear vacuoles in patients who have recently ovulated or received hormonal therapy (Figures 16.38 and 16.39). Not all secretory changes, however, are caused by progestational stimulation.69 A subset of neoplastic lesions develop prominent cytoplasmic secretory vacuoles as an integral feature of their neoplastic growth, independent of ambient progestational exposure (Figure 16.40). In those instances the stroma is free of pseudodecidual changes, and secretory change is confined to architecturally and cytologically abnormal glands. These can be diagnosed as EIN or adenocarcinoma, with secretory differentiation.
Metaplasias to Nonspecific Cell Types
Eosinophilic Metaplasia
An eosinophilic change can be associated with a variety of endometrial alterations such as ciliated cell metaplasia, squamous metaplasia, papillary metaplasia, and adenocarcinoma, and, as such, it is fair to question its status as a true metaplasia. Many eosinophilic metaplasias contain features overlapping with other altered differentiation states, such as mucin expression (85% of cases),50 or micropapillary architecture. The metaplasia can take several forms. When focal and associated with a simple lining epithelium, the change is always benign. This change is most commonly encountered within endometrial polyps, but occurs within the endometrial glands of the functionalis, usually in an inflammatory setting.
More commonly, the term ‘eosinophilic metaplasia’ refers to a change that is also known as oxyphil or oncocytic metaplasia. The cells showing eosinophilic metaplasia, which may be either on the surface or in the glands, are of a tall columnar type with abundant eosinophilic cytoplasm that ranges from homogeneous to granular and a bulging surface membrane similar to the oncocytes seen in other organs such as the salivary glands, pancreas, thyroid, and kidney. The nuclei are centrally placed, large, and round to oval with a delicate chromatin pattern. The eosinophilia corresponds to myriads of mitochondria as shown by electron microscopy.48 Like most other metaplasias, this change can be associated with a spectrum of endometrial changes ranging from benign to malignant.
Micropapillary Metaplasia
Papillary metaplasia is a descriptive term without any specific cellular basis. It involves glandular tissue thrown into papilla and is principally used to reflect degenerative processes that may involve endometrial glands and epithelial surfaces,47 in addition to a tufted pattern of intraglandular differentiation in EIN (Figure 16.41) and adenocarcinoma. It lacks the pleomorphic nuclear cytology and coarse chromatin seen in serous adenocarcinomas, which also show papillary tufting. It is of practical diagnostic significance because endometria with this histologic feature may present an unusually broad differential diagnosis, including degenerative, reactive, and neoplastic processes.
EIN lesions may show micropapillary differentiation where a crowded focus of glands is distinguished by small intraluminal tufts protruding from a flat simple epithelium. These can be confused with adenocarcinoma when tufting defines a complicated luminal architecture mimicking a maze-like or cribriform architecture (Figure 16.42). Tangential sectioning artifacts of the papillary areas create numerous clefts communicating to the central lumen, and redundant ‘saw-tooth’ folds contained within the boundaries of an encasing basement membrane. Nuclear cytology is round and bland or monomorphic, unlike the highly atypical exfoliative nuclei characteristic of the papillary branching typifying an adenocarcinoma as serous. Micropapillary areas are often admixed with, or blend into, other EIN glands with tubal or endometrioid differentiation (Figure 16.43).
Papillary Syncytial Metaplasia
This epithelial change is a misnomer because the papillary areas are not a true syncytium, nor do they resemble any specific normal epithelium. Rather, papillary syncytial metaplasia is a degenerative process in which collapsed sheets of epithelium with abundant pink cytoplasm resemble squamous cells, but lack true squamous differentiation (Figure 16.44). This has also been called ‘surface syncytial change’ and ‘eosinophilic syncytial metaplasia.’ It is found in nearly one-fifth of curettage specimens and appears as aggregates of eosinophilic, cuboidal to spindly epithelial cells.50 The underlying stroma is nondescript to tight balls, indicative of menstruation or anovulatory bleeding (Figure 16.45). Supportive fibrovascular cores are not seen.
Micropapillary ‘Hobnail’ Metaplasia
This form of metaplasia is rare, and likely represents a variety of etiologies including degenerative changes associated with underlying necrosis, artifacts, pseudo-Arias-Stella-like changes and changes associated with endometrial polyps. On morphologic grounds, hobnail cell metaplasia exhibits glandular cells with rounded nuclear protrusions into the gland lumina. These can be artificially created by exfoliation in response to nonphysiologic fluid instillation during hysteroscopy or placement of a specimen in hypotonic medium (Figure 16.46). When the cytoplasm is abundant and clear, the differential diagnosis is with clear cell carcinoma. Lack of mitotic activity and atypia, and the fact that the change is focal, assists in recognizing it as a benign process.
Mesenchymal Metaplasias
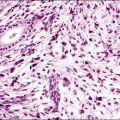
Osseous Metaplasia
Osseous tissue in the endometrial stroma is a rarely encountered condition that is often associated with a previous history of abortion or instrumentation (Figure 16.47). Some may be residual fetal bone incorporated into the endometrium. Others are doubtless genuine metaplasia, albeit sometimes induced by the retention of fetal bones.51,52 Most cases occur in women aged between 20 and 40 years and are associated with infertility.53 It occurs also in postmenopausal women.54
Endometrial Polyps
Etiology and Natural History
Polyps arise as monoclonal overgrowths of genetically altered endometrial stromal cells with secondary induction of polyclonal benign glands through as yet undefined stromal–epithelial interactive mechanisms.56 Chromosomal analysis of polyp stroma shows in the majority of cases clonal translocations, involving 6p21–p22, 12q13–15, or 7q22 regions.57 One breakpoint gene, the HMGIC gene at 12q15, is also rearranged in uterine leiomyomas, lipomas, pleomorphic adenomas, and pulmonary chondroid hamartomas.58
Endometrial polyps variably respond to circulating estrogens and progesterone. Most commonly the polyp is nonfunctional, lacking those normal cyclical changes seen in the adjacent normal endometrium. The glands are frequently entirely inactive, showing neither proliferative nor secretory activity. Some or many of the glands may be dilated, slightly branching, or irregularly distributed. Functioning polyps are less common than those already described and are composed of glands that show secretory activity during the secretory phase. When present, the secretory changes in the polyp either lag behind or are less well developed and dyssynchronous relative to the normal endometrium. An irregular gland pattern is common and should not give cause for concern or be separately diagnosed as an endometrial hyperplasia, especially when it is uniformly distributed throughout the entire polyp (Figure 16.48).
There have been numerous reports of endometrial polyps occurring in women treated with tamoxifen for breast cancer. It is clear that women on tamoxifen have a higher incidence of endometrial polyps than the rest of the population and that their polyps are likely to be much larger, more fibrotic, more likely to show mucinous metaplasia, and more likely to contain hyperplasia or carcinoma than women not on tamoxifen treatment.59,60
Clinical Features
Endometrial polyps may be found at any age, but are most frequent around and shortly after menopause. They are present in about 13–17% of women.61 Polyps may cause abnormal bleeding, a finding that may be explained when hemorrhage or necrosis is demonstrated on pathologic examination. Use of a rigid curette to completely shear away the polyp may be both diagnostic and therapeutic at the same time, although diagnostic material is often rendered by flexible sampling devices (Pipelle). Because they are typically removed in fragments and difficult to orient, surgical margins are difficult to assess within most polyp specimens. If EIN or adenocarcinoma is found in what is thought to be a polyp at curettage, the patient should generally be managed in exactly the same way as would have been done had the changes been in a nonpolypoid area.
Gross Features
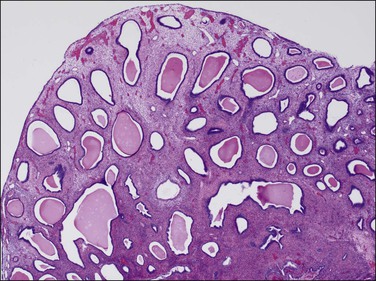
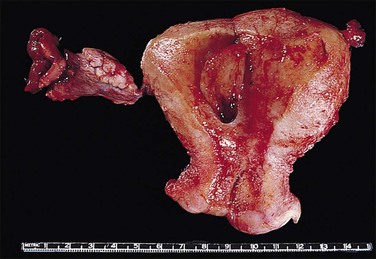
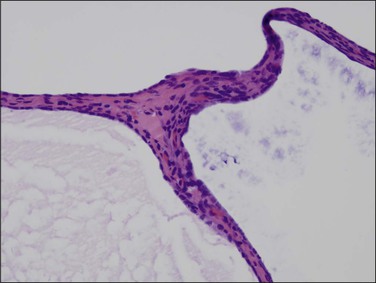
Early in their development, clonal stromal proliferations destined to become bulky endometrial polyps may not be grossly evident. These can be seen microscopically as a localized stromal component with interposed distorted glands within the endometrial functionalis (Figure 16.49). More commonly, at the time of diagnosis endometrial polyps range in size from a slightly rounded protuberance to a large, broad-based or pedunculated, oval structure filling the uterine cavity. They may also expand the cervical canal and present at the external cervical os. Pedunculated polyps are the easiest to recognize and often attract the most attention (Figure 16.50). Many polyps are sessile and have a broad base of attachment to the internal surface of the uterus. Some sessile polyps are only slightly raised and are easy to miss on gross examination. About 20% of polyps are multiple. The surface may be smooth and shiny and there is often hemorrhage, particularly at the tip. Necrosis is sometimes present, although this is uncommon in benign polyps. The cut surface may be uniform or it may show cysts, hemorrhage, and necrosis.
Microscopic Features
The diagnosis of a polyp in a curetting depends upon the finding of at least two of three particular histologic features, in addition to exclusion of mimics. These include: (1) irregularly shaped and positioned glands, (2) stroma altered by fibrosis or excessive collagen, and (3) thick-walled blood vessels (Figures 16.51 and 16.52). In addition, as polyps elongate, the glands often get stretched such that parallel glands with intervening fibrous stroma becomes a useful clue to the diagnosis (Figure 16.53).62 There are two polyp mimics that should be excluded before the diagnosis of an endometrial polyp can be made. The first is fragments of basalis, which can be ruled out if at least one of the polyp fragments shows surface epithelium. The second is lower uterine segment, which typically has a lower gland density, a more collagenous stroma, mucinous and tubal differentiation, and a lack of endometrial stroma. It is important to note that the identification of polypoid endometrial mucosa with surface epithelium on three sides is not sufficient for the diagnosis of an endometrial polyp if the previously mentioned features are lacking. Many fragments of normal epithelium can be artifactually cut in such a way as to have a ‘polypoid’ appearance.
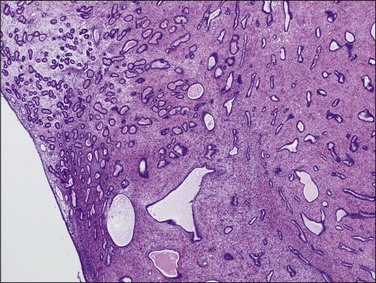
Figure 16.53 Endometrial polyp. Irregular gland density within the polyp and at the interface with adjacent endometrium. Some compressed glands are parallel to the surface.
Admixtures of mucinous and endometrial-type glands can be seen within endometrial polyps (Figure 16.54). The mucinous component demonstrates cytoplasmic mucin resembling that of the normal endocervix. When mucinous glands are prominent, occasional interspersed endometrial glands, commingled endometrial stroma, and lack of overlying squamous metaplasia are useful in distinguishing them from cervical polyps.
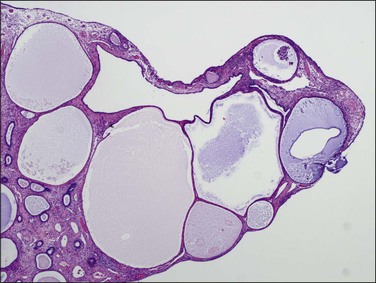
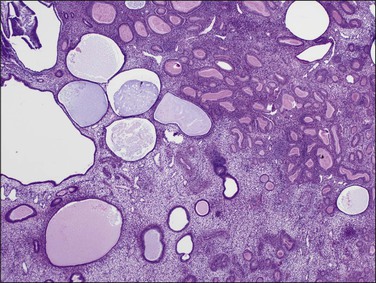
In postmenopausal women, the polyps are usually composed of dilated cysts (Figure 16.55) lined by atrophic epithelium (Figure 16.56). They are sometimes called ‘senile’ polyps. These are clearly a remnant of the time when the endometrium was active, and the pattern described represents a regressed state following a period of growth, perhaps many years previously.
EIN, when seen in polyps, may be recognized as a discrete geographic focus of crowded glands with a cohesive cytology that differs from the background polyp (Figure 16.57). Polyps with EIN need to be distinguished from a carcinoma arising in a pre-existing polyp and a polypoid carcinoma, which depends on finding a benign fibrous stalk or benign glandular elements in the polypoid mass.
Atypical Polypoid Adenomyoma
Atypical polypoid adenomyomas are admixtures of smooth muscle and atypical endometrial glands which form an exophytic, usually broad-based, projection into the uterine cavity.63 These tumors occur primarily in reproductive age women, and are only rarely diagnosed in patients after menopause. When encountered within a fragmented endometrial sample, the disordered arrangement of atypical glands among a dense muscular stroma can easily be confused as myoinvasive adenocarcinoma. The presence of characteristic and extensive morular squamous metaplasia (present in >90% of examples; Figure 16.58), lack of cribriform or solid architecture, and absence of stromal desmoplasia are helpful in excluding adenocarcinoma. Approximately half persist or recur locally after conservative treatment by curettage.63
Atypical polypoid adenomyomas have been associated with endometrioid adenocarcinoma and its precursor lesions (EIN or atypical endometrial hyperplasia), either of which may be present within the main polypoid mass or elsewhere in the endometrium.64,65 It is therefore important to look carefully for coexisting carcinoma, and examine the native endometrium for EIN. If either are present, they should be separately diagnosed.
Miscellaneous Conditions
Asherman’s Syndrome
Asherman’s syndrome, defined as synechiae within the endometrial cavity, often causes menstrual disorders and infertility (62% and 43% of patients, respectively).66 The most common causes are curettage after a missed abortion or during the puerperium. Genital tuberculosis, in countries where this disease is still common, is a principal causative factor. Hysterosalpingography and hysteroscopy are the usual methods of definitive diagnosis.
There are no gross features as the specimens usually consist of fragments of fibrous tissue with varying amounts of entrapped residual endometrial glands. The rare hysterectomy specimen discloses massive fibrosis of the residual ‘endometrium.’
Treatment usually consists of lysing the adhesions followed by immediate insertion of an IUD, together with a course of estrogens. The usual measure of successful treatment is the ability to achieve a term delivery.67,68 Placenta accreta is not an uncommon complication during the subsequent pregnancy, usually because the endometrium is absent and the placental villi have implanted directly into the myometrium.
Radiation Effect
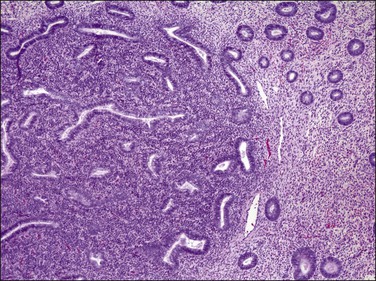
Radiation delivered for therapy of cervical or other pelvic malignancies may alter the histology of what initially was normal endometrium. Grossly, no changes are seen, unless the absence of lush endometrium 2–3 mm thick is considered abnormal. The endometrium is usually paper thin, and more akin to an atrophic endometrium. The chief microscopical features are glands in which the nuclei are irregular in size and shape, but in which the chromatin is usually smudged (Figure 16.59). Mitoses are absent. The glands are either normal or slightly irregular in shape, but lack any evidence of any usual features of carcinoma with which it is commonly mistaken. Normal glands affected by radiotherapy lack signs of invasion or a cribriform arrangement of the cells. The glands are also usually spaced as normal glands.
The Effects of the IUD on the Endometrium
Often, the IUD (Figure 16.60) appears to have no demonstrable effect on the endometrium, although subtle, local changes are almost invariable. Focally compressed endometrium may be seen at the site of contact, which may vary between a slight indentation of the surface to deep implantation almost through the endometrium or even into the myometrium (Figure 16.61). Rarely, an IUD may penetrate the uterine wall and even lie in the peritoneal cavity. Commonly, near the point where the IUD is in contact with the endometrium, a fibroblastic overgrowth will develop to isolate the IUD as if it were a foreign body. The surface epithelium at the point of contact with the IUD may also show atypical features, with loss of cellular polarity and nuclear enlargement and pleomorphism. Squamous metaplasia of the surface epithelium has been seen on occasion. The stroma may exhibit focal edema and dilated blood vessels. Small foci of an exaggerated decidual-like response to progesterone may also be evident.
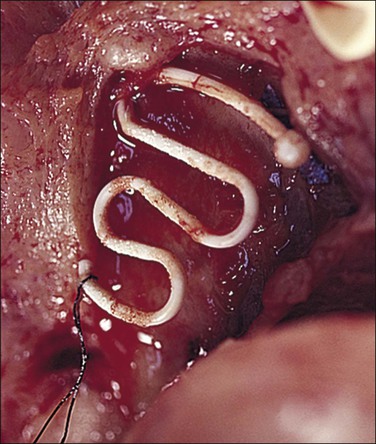
Figure 16.60 IUD. A Lippes loop in position in an opened uterus. Adhesions are attached to the loop.
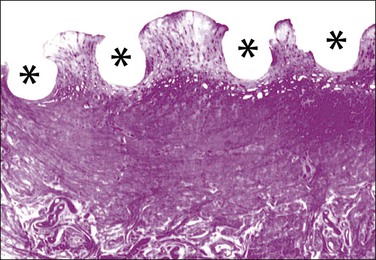
Figure 16.61 IUD. Imprint of a Lippes loop in the endometrium. The endometrium is normal between the depressions (asterisks) caused by the IUD itself.
Inflammation is the most important complication of an IUD in the endometrium, particularly with the noncopper-containing types. It is common to observe a sparse, focal scattering of neutrophils in the superficial stroma close to the contact point with the device, as well as neutrophils and nuclear debris in gland lumina. These minor local inflammatory changes are thought more often to result from irritation rather than infection, as the uterine cavity becomes sterile again within 48 hours after the device has been inserted. Even so, some organisms are probably introduced with it. More widespread and severe inflammatory changes, however, result from infection. In these circumstances, the full picture of acute or chronic endometritis may be seen with a stromal infiltrate of neutrophils, plasma cells and lymphocytes, pus in the gland lumina, and suppression of the glandular hormonal cyclical response.
Endometrial Ablation
Procedures for endometrial ablation have grown in popularity as a method to treat benign symptomatic disease. Diffuse coagulative necrosis lacking any granulomatous component dominates the histologic picture for the first few weeks. It closely resembles the artifact commonly seen on the periphery of surgical specimens obtained by cauterizing sampling devices, but is more extensive and uniform. As collagen replaces the necrotic tissue, the appearance varies from well-formed noncaseating granulomas, which are difficult to distinguish from those of infective origin, to surrounding foreign-body giant cells and debris (Figure 16.62). Not all endometrial epithelium is destroyed during ablation, and residual glands that regrow may be inaccessible to future biopsy attempts because of obscuring adhesions. For this reason endometrial ablation is not advised for treatment of premalignant disease, where complete sampling during follow-up surveillance biopsies is desired.
References
1. Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2009. http://www.cdc.gov/std/stats09/default.htm, 2009. [[accessed 2012]].
2. Mishra, K, Wadhwa, N, Guleria, K, Agarwal, S. ER, PR and Ki-67 expression status in granulomatous and chronic non-specific endometritis. J Obstet Gynaecol Res. 2008; 34:371–378.
3. Disep, B, Innes, BA, Cochrane, HR, et al. Immunohistochemical characterization of endometrial leucocytes in endometritis. Histopathology. 2004; 45:625–632.
4. Searle, RF, Jones, RK, Bulmer, JN. Phenotypic analysis and proliferative responses of human endometrial granulated lymphocytes during the menstrual cycle. Biol Reprod. 1999; 60:871–878.
5. Kiviat, NB, Wolner-Hanssen, P, Eschenbach, DA, et al. Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am J Surg Pathol. 1990; 14:167–175.
6. Peeling, RW, Brunham, RC. Chlamydiae as pathogens: new species and new issues. Emerg Infect Dis. 1996; 2:307–319.
7. Mount, S, Mead, P, Cooper, K. Chlamydia trachomatis in the endometrium: can surgical pathologists identify plasma cells? Adv Anat Pathol. 2001; 8:327–329.
8. Paukku, M, Puolakkainen, M, Paavonen, T, Paavonen, J. Plasma cell endometritis is associated with Chlamydia trachomatis infection. Am J Clin Pathol. 1999; 112:211–215.
9. Korn, AP, Bolan, G, Padian, N, et al. Plasma cell endometritis in women with symptomatic bacterial vaginosis. Obstet Gynecol. 1995; 85:387–390.
10. Cicinelli, E, De, ZD, Nicoletti, R, et al. Chronic endometritis: correlation among hysteroscopic, histologic, and bacteriologic findings in a prospective trial with 2190 consecutive office hysteroscopies. Fertil Steril. 2008; 89:677–684.
11. Haggerty, CL. Evidence for a role of Mycoplasma genitalium in pelvic inflammatory disease. Curr Opin Infect Dis. 2008; 21:65–69.
12. Kumar, P, Shah, NP, Singhal, A, et al. Association of tuberculous endometritis with infertility and other gynecological complaints of women in India. J Clin Microbiol. 2008; 46:4068–4070.
13. Nogales-Ortiz, F, Tarancon, I, Nogales, FF, Jr. The pathology of female genital tuberculosis. A 31-year study of 1436 cases. Obstet Gynecol. 1979; 53:422–428.
14. Patzina, RA, de Andrade, HF, Jr., de Brito, T, et al. Molecular and standard approaches to the diagnosis of mycobacterial granulomatous lymphadenitis in paraffin-embedded tissue. Lab Invest. 2002; 82:1095–1097.
15. Selva, E, Hofman, V, Berto, F, et al. The value of polymerase chain reaction detection of Mycobacterium tuberculosis in granulomas isolated by laser capture microdissection. Pathology. 2004; 36:77–81.
16. Salian, NV, Rish, JA, Eisenach, KD, et al. Polymerase chain reaction to detect Mycobacterium tuberculosis in histologic specimens. Am J Respir Crit Care Med. 1998; 158:1150–1155.
17. Valicenti, JF, Jr., Pappas, AA, Graber, CD, et al. Detection and prevalence of IUD-associated Actinomyces colonization and related morbidity. A prospective study of 69,925 cervical smears. JAMA. 1982; 247:1149–1152.
18. Boyle, DP, McCluggage, WG. Combined actinomycotic and pseudoactinomycotic radiate granules in the female genital tract: description of a series of cases. J Clin Pathol. 2009; 62:1123–1126.
19. Pritt, B, Mount, SL, Cooper, K, Blaszyk, H. Pseudoactinomycotic radiate granules of the gynaecological tract: review of a diagnostic pitfall. J Clin Pathol. 2006; 59:17–20.
20. Almoujahed, MO, Briski, LE, Prysak, M, et al. Uterine granulomas: clinical and pathologic features. Am J Clin Pathol. 2002; 117:771–775.
21. Knuth, KR, Fraiz, J, Fisch, JA, Draper, TW. Pinworm infestation of the genital tract. Am Fam Physician. 1988; 38:127–130.
22. Zaino, RJ. Endometritis. In: Silverberg S, ed. Interpretation of endometrial biopsies and curettings. Philadelphia: Lippincott-Raven; 1996:241–261.
23. Watts, DH, Krohn, MA, Hillier, SL, Eschenbach, DA. Bacterial vaginosis as a risk factor for post-cesarean endometritis. Obstet Gynecol. 1990; 75:52–58.
24. Peipert, JF, Montagno, AB, Cooper, AS, Sung, CJ. Bacterial vaginosis as a risk factor for upper genital tract infection. Am J Obstet Gynecol. 1997; 77:1184–1187.
25. Bewtra, C, Xie, QM, Hunter, WJ, Jurgensen, W. Ichthyosis uteri: a case report and review of the literature. Arch Pathol Lab Med. 2005; 129:e124–e125.
26. Mutter, GL, Ince, TA. Molecular pathogenesis of endometrial cancer. In: Fuller A, Seiden MV, Young R, eds. Uterine cancer: American Cancer Society atlas of clinical oncology. Hamilton, ON Canada: B.C. Decker; 2004:10–21.
27. Mutter, GL. Endometrial carcinogenesis: an integrated molecular, histologic, and functional model of a dualistic disease. In: Giordano A, Bovicelli A, Kurman R, eds. Molecular pathology of gynecologic cancer. Totowa, NJ, USA: Humana, 2006.
28. Deutchman, ME, Hartman, KJ. Postpartum pyometra: a case report. J Fam Pract. 1993; 36:449–452.
29. Naora, H. Developmental patterning in the wrong context: the paradox of epithelial ovarian cancers. Cell Cycle. 2005; 4:1033–1035.
30. Samuel, S, Naora, H. Homeobox gene expression in cancer: insights from developmental regulation and deregulation. Eur J Cancer. 2005; 41:2428–2437.
31. Block, K, Kardana, A, Igarashi, P, Taylor, HS. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. FASEB J. 2000; 14:1101–1108.
32. Taylor, HS, Arici, A, Olive, D, Igarashi, P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998; 101:1379–1384.
33. Yoshida, H, Broaddus, R, Cheng, W, et al. Deregulation of the HOXA10 homeobox gene in endometrial carcinoma: role in epithelial-mesenchymal transition. Cancer Res. 2006; 66:889–897.
34. Jovanovic, AS, Boynton, KA, Mutter, GL. Uteri of women with endometrial carcinoma contain a histopathologic spectrum of monoclonal putative precancers, some with microsatellite instability. Cancer Res. 1996; 56:1917–1921.
35. Cheng, W, Liu, J, Yoshida, H, et al. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med. 2005; 11:531–537.
36. Taylor, HS, Igarashi, P, Olive, DL, Arici, A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab. 1999; 84:1129–1135.
37. Carlson, JW, Mutter, GL. Endometrial intraepithelial neoplasia is associated with polyps and frequently has metaplastic change. Histopathology. 2008; 53:325–332.
38. Mutter, GL. Histopathology of genetically defined endometrial precancers. Int J Gynecol Pathol. 2000; 19:301–309.
39. Mutter, GL. Diagnosis of premalignant endometrial disease. J Clin Pathol. 2002; 55:326–331.
40. Silverberg, SG, Mutter, GL, Kurman, RJ, et al. Tumors of the uterine corpus: epithelial tumors and related lesions. In: Tavassoli FA, Stratton MR, eds. WHO classification of tumors: pathology and genetics of tumors of the breast and female genital organs. Lyon, France: IARC Press; 2003:221–232.
41. Kaku, T, Silverberg, SG, Tsukamoto, N, et al. Association of endometrial epithelial metaplasias with endometrial carcinoma and hyperplasia in Japanese and American women. Am J Pathol. 1993; 12:297–300.
42. Crum, CP, Richart, RM, Fenoglio, CM. Adenoacanthosis of endometrium: a clinicopathologic study in premenopausal women. Am J Surg Pathol. 1981; 5:15–20.
43. Lin, MC, Lomo, L, Baak, JPA, et al. Squamous morules are functionally inert elements of premalignant endometrial neoplasia. Mod Pathol. 2009; 22:167–174.
44. Saegusa, M, Okayasu, I. Frequent nuclear beta-catenin accumulation and associated mutations in endometrioid-type endometrial and ovarian carcinomas with squamous differentiation. J Pathol. 2001; 194:59–67.
45. Brachtel, EF, Sanchez-Estevez, C, Moreno-Bueno, G, et al. Distinct molecular alterations in complex endometrial hyperplasia (CEH) with and without immature squamous metaplasia (squamous morules). Am J Surg Pathol. 2005; 29:1322–1329.
46. Vang, R, Tavassoli, FA. Proliferative mucinous lesions of the endometrium: analysis of existing criteria for diagnosing carcinoma in biopsies and curettings. Int J Surg Pathol. 2003; 11:261–270.
47. Nucci, M, Crum, CP, Prasad, C, Mutter, GL. Mucinous endometrial epithelial proliferations: a morphologic spectrum of changes with diverse clinical significance. Mod Pathol. 2000; 12:1137–1142.
48. Lehman, MB, Hart, WR. Simple and complex hyperplastic papillary proliferations of the endometrium—a clinicopathologic study of nine cases of apparently localized papillary lesions with fibrovascular stromal cores and epithelial metaplasia. Am J Surg Pathol. 2001; 25:1347–1354.
49. Bergeron, C, Ferenczy, A. Oncocytic metaplasia in endometrial hyperplasia and carcinoma. Int J Gynecol Pathol. 1988; 7:93–95.
50. Zaman, SS, Mazur, MT. Endometrial papillary syncytial change. A nonspecific alteration associated with active breakdown. Am J Clin Pathol. 1993; 99:741–745.
51. Basu, M, Mammen, C, Owen, E. Bony fragments in the uterus: an association with secondary subfertility. Ultrasound Obstet Gynecol. 2003; 22:402–406.
52. Bolaji, I, Saridogan, E, Hasan, N, et al. Prolonged retention of fetal bones with osseous metaplasia of the endometrium. Int J Gynaecol Obstet. 1995; 50:65–66.
53. Bahceci, M, Demirel, LC. Osseous metaplasia of the endometrium: a rare cause of infertility and its hysteroscopic management. Hum Reprod. 1996; 11:2537–2539.
54. Shimizu, M, Nakayama, M. Endometrial ossification in a postmenopausal woman. J Clin Pathol. 1997; 50:171–172.
55. Creagh, TM, Bain, BJ, Evans, DJ, et al. Endometrial extramedullary haemopoiesis. J Pathol. 1995; 176:99–104.
56. Fletcher, J, Pinkus, J, Lage, J, et al. Clonal 6p21 rearrangement is restricted to the mesenchymal component of an endometrial polyp. Genes Chromosomes Cancer. 1992; 5:260–263.
57. Dal Cin, P, Vanni, R, Marras, S, et al. Four cytogenetic subgroups can be identified in endometrial polyps. Cancer Res. 1995; 55:1565–1568.
58. Bol, S, Wanschura, S, Thode, B, et al. An endometrial polyp with a rearrangement of HMGI-C underlying a complex cytogenetic rearrangement involving chromosomes 2 and 12. Cancer Genet Cytogenet. 1996; 90:88–90.
59. Cohen, I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol. 2004; 94:256–266.
60. Deligdisch, L, Kalir, T, Cohen, CJ, et al. Endometrial histopathology in 700 patients treated with tamoxifen for breast cancer. Gynecol Oncol. 2000; 78:181–186.
61. Kim, KR, Peng, R, Ro, JY, Robboy, SJ. A diagnostically useful histopathologic feature of endometrial polyp: the long axis of endometrial glands arranged parallel to surface epithelium. Am J Surg Pathol. 2004; 28:1057–1062.
62. Reslova, T, Tosner, J, Resl, M, et al. Endometrial polyps. A clinical study of 245 cases. Arch Gynecol Obstet. 1999; 262:133–139.
63. Longacre, TA, Chung, MH, Rouse, RV, Hendrickson, MR. Atypical polypoid adenomyofibromas (atypical polypoid adenomyomas) of the uterus. A clinicopathologic study of 55 cases. Am J Surg Pathol. 1996; 20:1–20.
64. Ota, S, Catasus, L, Matias-Guiu, X, et al. Molecular pathology of atypical polypoid adenomyoma of the uterus. Hum Pathol. 2003; 34:784–788.
65. Clement, PB, Young, RH. Endometrioid carcinoma of the uterine corpus: a review of its pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2002; 9:145–184.
66. Fernandez, H, Al-Najjar, F, Chauveaud-Lambling, A, et al. Fertility after treatment of Asherman’s syndrome stage 3 and 4. J Minim Invasive Gynecol. 2006; 13:398–402.
67. Roy, KH, Mattox, JH. Advances in endometrial ablation. Obstet Gynecol Surv. 2002; 57:789–802.
68. Mencaglia, L, Tonellotto, D. Endometrial ablation: a review. In: Blanc B, Marty R, DeMontgolfier R, eds. Office and operative hysteroscopy. Paris, France: Springer-Verlag; 2002:197–202.
69. Parra-Herran, CE, Monte, NM, Mutter, GL. Endometrial intraepithelial neoplasia with secretory differentiation: Diagnostic features and underlying mechanisms. Mod Pathol. 2013; 26:868–873.