Chapter 49 Endometriosis
INTRODUCTION
The prevalence of endometriosis in the general population is unknown and will remain so until a reliable, noninvasive test can be applied to a large unselected population. Because the standard method of diagnosing endometriosis is an invasive surgical procedure, any studied population will be symptomatic and will represent a biased segment of the overall population. Within these symptomatic populations, the reported prevalence has been increasing over the past two decades. This is likely due to the recognition of more subtle forms of endometriosis and the more liberal use of diagnostic laparoscopy.1 This observed increased prevalence might also represent a real increase in the incidence of endometriosis due to sociological, environmental, and genetic factors.
TYPES OF ENDOMETRIOSIS
The microscopic definition of endometriosis implies the presence of endometrial glands and stroma outside the endometrial cavity and uterine musculature. After the introduction of laparoscopy in the late 1960s, black puckered peritoneal lesions were the first lesions to be recognized as representing endometriosis. In the mid-1980s, we recognized that different forms of nonpigmented lesions also contained the required histologic features of endometriosis. Deep infiltrating endometriosis2,3 is recognized as a separate clinical entity that might be easily missed at laparoscopy and may have a different etiology than the classic peritoneal and ovarian lesions.
Typical brown/black lesions are now believed to be the end result of subtle lesions that have evolved over a number of years.4 New lesions appear red and then turn white as they shed and grow, and they ultimately turn black as a result of both the hemosiderin deposition and intraluminal debris. This theory of lesion evolution is supported by the average age at which these lesions are found in patients. The red lesions are found mainly in younger patients; the black and white lesions are generally observed in older patients.5 The black lesions may be of different dimension and depth of penetration than the red and white lesions. The red lesions are the most metabolically active, whereas the white lesions are the least active.
Ovarian Endometriosis
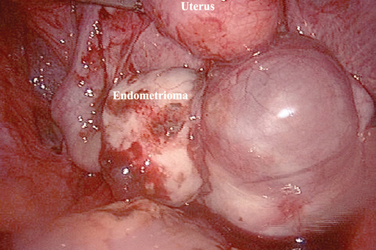
Ovarian endometriosis, or endometriomas, increase with age and are generally associated with a more advanced stage of the disease.6 This form of endometriosis can be diagnosed with a high level of accuracy by serial ultrasounds.7 The main differential diagnosis is a hemorrhagic corpus luteum, which will disappear over the course of a few months. These ovarian forms of endometriosis often have associated peritoneal implants, although only a small percentage of patients with peritoneal implants will eventually develop an endometrioma. Endometriomas can be uniloculated or multiloculated. They are more commonly localized in the left ovary8 because peritoneal implants are more common in the left hemipelvis.
Deep Endometriosis
Invasion of endometriosis deeper than 5 mm has been associated with increased pain.9 These lesions have been grouped with the following classification: large area surrounded by sclerotic white lesions (Type I), area of retraction of the bowel (Type II), a nodule of the rectovaginal septum (Type III), and sclerosing endometriosis invading the sigmoid colon (Type IV). These lesions can be difficult to classify during laparoscopy due to the retroperitoneal nature of the disease. A rectovaginal examination during the menstrual period in the office setting or a thorough examination under anesthesia before laparoscopy may alert the surgeon to the presence of these types of lesions.
PREVALENCE OF ENDOMETRIOSIS
Over the past three decades, the incidence of endometriosis in patients who present with either pelvic pain or infertility has been increasing and has been reported to be as high as 80%.10 This increase has been proportional to the surgeon’s awareness of subtle lesions and deep endometriosis. If microscopic endometriosis is included, virtually all women with pelvic pain or infertility have some form of endometriosis. The classic lesions generally appear later in life and will have an incidence as high as 70% in patients with pelvic pain or infertility.11
The incidence of ovarian cystic endometriosis (endometriomas) increases with age,6 but its incidence and progression seem to stabilize in patients who are older than age 35. The prevalence of endometriosis in the general population is estimated to range from 1% to 3%. Approximately 10% to 20% of patients undergoing laparoscopy for pelvic pain or infertility are found to have deep endometriosis.6
CLASSIFICATION OF ENDOMETRIOSIS
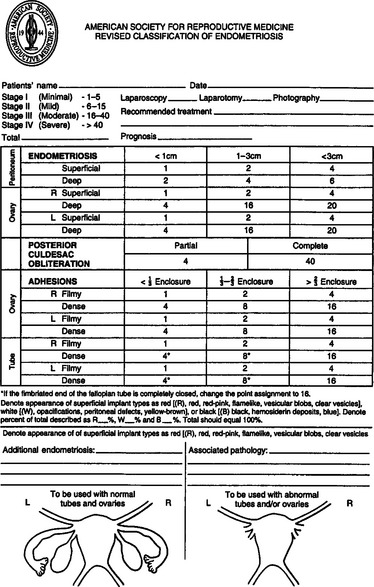
The classification of endometriosis has been an evolving process. The first classification system to gain widespread acceptance was that of Acosta and colleagues in 1973,12 which classified endometriosis as mild, moderate, or severe and showed an inverse relationship to pregnancy rates. In 1978, the American Fertility Society (AFS, now called the American Society for Reproductive Medicine [ASRM]) convened a committee to design a classification system, which was published in 1979.13 This classification included four stages and used a weighted pain score that included assessment of the extent of endometriosis in two dimensions and the presence and extent of adhesions in the peritoneum, ovaries, and tubes. It also took into account whether the endometriosis was unilateral or bilateral. The size of the endometrioma was also taken into account, along with the presence of filmy versus dense adhesions.
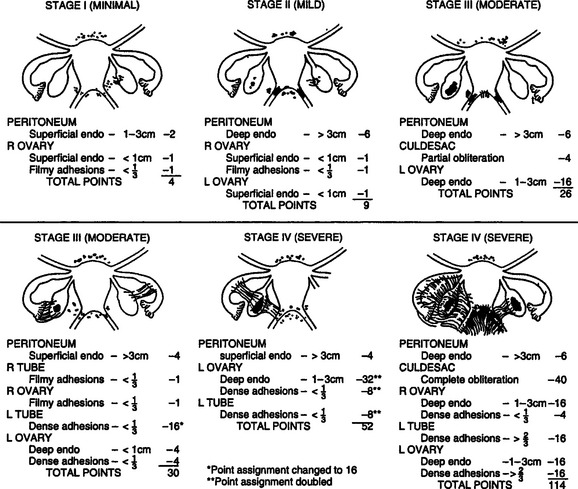
In 1985,14 the original AFS classification was revised because of its inability to predict pregnancy. It now includes a three-dimensional assessment of the disease differentiating superficial from invasive disease (Figs. 49-1 and 49-2). It also quantifies the extent of adhesion around the tubes and ovaries and distinguishes between incomplete and complete enclosure of the fimbria, the latter of which automatically places the disease in the moderate category. Cul-de-sac adhesions are weighted heavily, with complete obliteration, which is characteristic of severe disease, resulting in a stage IV assignment. An endometrioma that is larger than 3 cm in diameter is at least stage III disease.
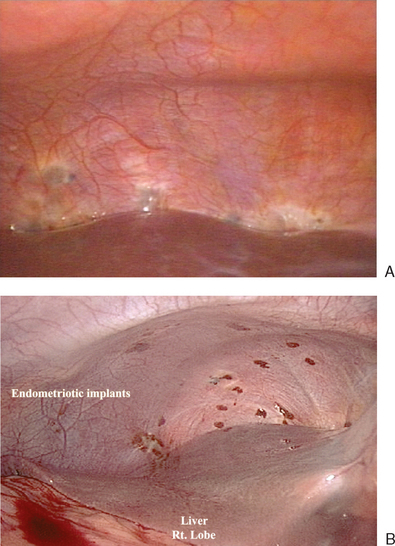
In 1996,15 a further revision was introduced in the AFS classification (ASRM) in an attempt to correlate the results with pain. This included a description and photography of the lesions. The lesions are categorized as red (red, red-pink, clear, white, yellow-brown, and peritoneal defects) and black (blue and black) (Figs. 49-3 and 49-4). The percentage of each of these lesions is also documented. Despite these improvements, the correlation between the stage of endometriosis and the likelihood of pregnancy is poor, and future improvement in the classification is likely.16
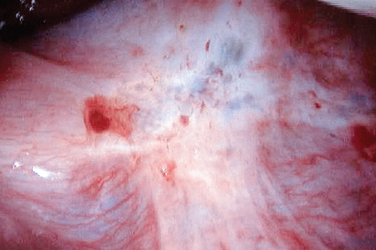
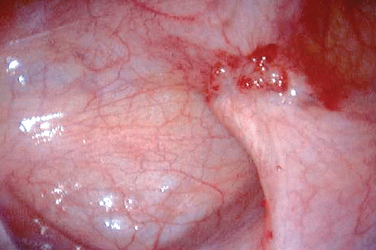
Figure 49-3 Typical black and white lesions of the pelvic peritoneum with dense fibrosis and retraction.
Another limitation of the ASRM classification stems from the fact that this system was mainly designed to assess a patient’s fertility potential and correlates poorly with the pain level. Many authors17 have found no correlation between the degree of pain and the ASRM classification system. However, a correlation between pain and the depth of penetration (5 mm or more) does seem to exist.9
PREDISPOSING FACTORS FOR ENDOMETRIOSIS
There is a correlation between the volume of menstrual flow and the prevalence of endometriosis. This may lead to an increase in menstrual tubal reflux. Clinical conditions that are associated with an increase in menstrual reflux—a noncommunicating uterine horn, for example—are associated with a virtually 100% incidence of endometriosis.2 Increased exposure to menstrual flow, which occurs with early menarche and nulliparity, has been correlated with a high incidence of the disease.18 In a recent study of 2777 women with endometriosis,19 the authors reported that the proportion of patients who had a uterine fibroid was significantly higher than those without fibroids. This finding suggests a causal relationship between endometriosis and the presence of uterine fibroids, which might be due to an increased menstrual flow reflux in these patients.
There appears to be some correlation between a higher degree of education, social stress, and the prevalence of disease.19 The Nurses’ Health Study prospectively assessed predisposing factors for endometriosis and observed an association with early age of menarche, shorter length of menstrual cycles during late adolescence, and nulliparity. In parous women, a decreased risk was found with the number of liveborn children and duration of lactation.20 In the best animal model for endometriosis, the stress of captivity was associated with a higher incidence of endometriosis.21
Heredity is an important predisposing factor for endometriosis because the prevalence is increased sevenfold among first-degree relatives. In monozygotic twins, the prevalence increased 15-fold.22 Exposure to pollutants, especially endocrine-disrupting compounds such as dioxins or polychlorinated biphenyls, might also play a role in the predisposition to endometriosis. In the baboon, for instance, exposure to increasing amounts of dioxin correlated with the incidence and extent of endometriosis.23 In vitro data has also shown that these dioxins affect the expression of stromal progesterone receptor expression.24 Some human data also support a role for these toxins in the pathophysiology of endometriosis.25
There is a correlation between infertility, pelvic pain, dysmenorrhea, dyspareunia, and endometriosis. Although stage I endometriosis may not be more common in infertile women than in fertile women, stage II is significantly more common in infertile patients.26
Although some authors have found a correlation between alcohol use and endometriosis, others have not been able to confirm these results. The use of oral contraception has been linked to an increased risk27 of endometriosis, but other studies either found a protective effect28 or no correlation at all.19 The use of an intrauterine contraceptive device is not associated with increased incidence of endometriosis.19 Body mass index has not been correlated with the incidence of endometriosis. It is unknown whether diet and exercise can halt or limit the evolution of endometriosis. Early exposure to pregnancy may help protect against endometriosis. Indeed, symptoms associated with endometriosis such as pelvic pain are often decreased for a number of years after pregnancy.
The difficulty with assessing risk is often due to the common presence of endometriosis in the pelvis that may not be pathologic. Based on a study that involved repetitive laparoscopy in baboons, we know that endometriosis appears spontaneously29 and that new lesions seem to appear and disappear spontaneously. Thus, endometriotic lesions in humans most likely are in continuous evolution as well.
PATHOPHYSIOLOGY
Transplantation Theory
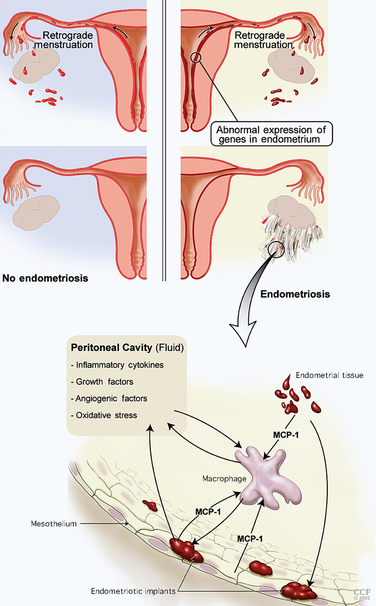
The basic components of the disease process are an abnormal endometrium, invasion of the peritoneum, angiogenesis, and an immune response and inflammation (Table 49-1; Fig. 49-5). The most accepted general concept of the etiology of this disease is the transplantation theory of Sampson. In 1927, Sampson proposed that endometrial cells regurgitated through the fallopian tubes and implanted on different peritoneal surfaces, leading to endometriosis. However, three major elements must be present to support the Sampson theory: first, endometrial cells must be refluxed through the fallopian tubes at the time of menstruation; second, these cells must be viable; and third, they should implant anatomically based on the distribution of reflux cells in the peritoneal cavity.
Table 49-1 Important Components for Implantation of Endometrium
MMP = matrix metalloproteinases; VEGF = vascular endothelial growth factor
An intact peritoneal surface does not act as a barrier to endometriosis formation.30 However, certain conditions need to be present for endometrial tissue to invade and persist. Recent data from the literature suggest that the endometrium of patients who are in the process of developing endometriosis is intrinsically different from that of patients without endometriosis.
Intrinsic Abnormalities of the Endometrium
Apoptosis
Apoptosis plays a critical role in the normal function of the endometrium.31 This process helps eliminate senescent cells from the functional layer of the late secretory and menstrual endometrium. Apoptosis in the eutopic and ectopic endometrium is controlled by the Bcl-2 and Fas/FasL systems. The Bcl-2 system diminishes apoptosis, whereas the Fas/FasL system promotes apoptosis. These processes are altered in the endometrium of patients with endometriosis in a way that decreases apoptosis, leading possibly to an increase of viable regurgitated endometrial cells.
Decreased Progesterone Sensitivity
Matrix metalloproteinases promote tumor-cell invasion of tissues. Alterations in these enzymes are therefore likely to increase the invasive potential of refluxed endometrium.32,33 Furthermore, altered expression of these enzymes during the luteal phase may be associated with implantation disorders in patients with endometriosis.
For endometrial cells to attach to the peritoneal surface, they must bind to the extracellular matrix (ECM). After attachment of endometrial cells to the peritoneal surface, endometrial glands must invade the peritoneal surface. This tissue degrades the ECM between the mesothelial cells, possibly via the persistent expression of MMP in refluxed endometrial tissue. MMP expression has been found in red lesions of endometriosis32 and endometriomas independent of the menstrual cycle phase. Type III procollagen—a byproduct of ECM metabolism—has been detected in the peritoneal fluid of patients with endometriosis.34
Angiogenic Factors
Angiogenic factors may play a role in the survival of the implants. These angiogenic factors are present in the peritoneal fluid of 58% of patients with endometriosis.35 Vascular endothelial growth factors (VEGF) can be produced by activated peritoneal macrophages, refluxed endometrial cells, and endometriotic lesions. VEGF36 is responsible for creating a new blood supply to the newly implanted endometrial cells. VEGF levels are elevated in the peritoneal fluid of women with endometriosis, and they correlate with the stage of the disease. Levels are higher around red lesions than around more mature black lesions. Other angiogenic factors such as interleukin 1 (IL-1) and IL-6 are also expressed by endometriotic tissue. Once scar tissue forms around the implants, neovascularization is limited.37
Abnormalities of the Immune System
Immune Cells
Natural killer cells are an important component of the immune system. Because they are cytotoxic, they have antitumor effects. In patients with endometriosis, this intraperitoneal cytotoxicity is decreased.38 T lymphocytes, which are responsible for cellular immunity, are increased in the peritoneal fluid of patients with endometriosis.39 Both helper and suppressor subtypes of T lymphocytes seem to be increased as well. Their role in the development of endometriosis remains unclear. Considerable evidence also suggests that endometriosis is associated with an increased incidence of autoantibodies of the immunoglobulin G (IgG) and IgA class. Autoantibodies to ovarian and endometrial cells have been reported in patients with endometriosis.40
Inflammatory Cells and Cytokines
A complex cascade of immune events results in an impaired peritoneal environment (Table 49-2). Macrophages, which are the most abundant nucleated cells in the peritoneal fluid, are increased in patients with endometriosis. The increase in number does not correlate with the stage of endometriosis.41 Increased activation of these macrophages is associated with increased secretion of cytokines but not phagocytic activity. The scavenging capacity of these macrophages appears to be impaired, resulting in decreased phagocytosis of endometrial cells.42 A protein similar to haptoglobin on endometriotic epithelial cells may decrease macrophage phagocytic capacity. Impaired clearance supports the pathogenetic hypothesis of implantation. The cytokines that are secreted play a role in the implantation of endometrial tissue as well as in the resulting infertility and pain. Macrophages and monocytes in peritoneal fluid of patients with endometriosis seem to increase the growth and maintenance of endometriotic implants.43
| Increased activated macrophages |
The observed increase in peritoneal fluid macrophages in patients with endometriosis can be linked to the increased expression of monocyte chemoattractant protein-1 (MCP-1).44 MCP-1 is produced by endometrial cells, mesothelial cells, macrophages, and endometriotic implants. The presence of endometrial tissue from patients with endometriosis in the peritoneal cavity increases expression of MCP-1 by the mesothelium.45 Oxidative stress is an activator of MCP-1 expression through nuclear factor κB (NFκB). MCP-1 is the most critical cytokine in the macrophage-related pathophysiology of endometriosis. The concentration of MCP-1 in the peritoneal fluid correlates well with the stage of the disease.
Cytokines and growth factors are found in higher concentrations in patients with endometriosis.46 Cytokines are small proteins that act as communicators between cells, especially those involved in inflammation and the immune system. These cytokines act in autocrine or paracrine loops to magnify their effect. They promote implantation, proliferation, and survival of the endometriotic tissue and are thought to be mediators in the clinical manifestation of the disease. Levels of several interleukins have been reported to be elevated in the peritoneal cavity of patients with endometriosis. They are secreted by the endometriotic lesions and recruited macrophages. Most interleukins are linked to stimulation of prostaglandins, fibroblast proliferation, and altered immune function. IL-1, which originates from activated macrophages and endometriosis implants, stimulates VEGF47 and IL-6. Although IL-6 has not consistently been shown to be increased in peritoneal fluid, the serum level has been shown to be more predictably elevated. IL-6 is produced by endothelial cells, fibroblasts, and monocytes as well as by eutopic and ectopic endometrium. It has a large number of physiologic reproductive functions, such as a promoter of endometrial proliferation and macrophage activator.
Interleukin-8 has also been found to be elevated in peritoneal fluid of patients with endometriosis. IL-8 can promote the implantation and growth of ectopic endometrium in the peritoneal cavity. Because it can also stimulate the adhesion of endometrial stromal cells to fibronectin, it can increase endometrial cell attachment to the mesothelium. IL-8 can also promote the invasion of endometrial cells by increasing the secretion of metalloproteinase,48 and it has been shown to stimulate endometrial cell proliferation in vitro and angiogenesis.
Another cytokine chemoattractant for monocytes called RANTES (Regulated on Activation, Normal T-cell Expressed and Secreted)49 is expressed in high concentrations in the peritoneal fluid of patients with endometriosis, and its level correlates with the stage of the disease. It seems to be produced in increased amounts by ectopic endometrial implants in response to chemokines. Tumor necrosis factor (TNF) is another cytokine that is produced in increased amounts and found in higher concentrations in the peritoneal fluid of patients with endometriosis.50 It is recognized as the most commonly elevated cytokine in peritoneal fluid.50 It causes an increase in the adherence of endometrial cells to mesothelial cells and may be involved in infertility because it adversely affects sperm motility and is toxic to embryos.36
The soluble form of intercellular adhesion molecule-1 (sICAM-1) is shed in higher amounts from the endometrium of patients with endometriosis, and this has been shown to correlate with the number of endometriotic implants in the pelvis.51 A prospective cohort study found that sICAM-1, when used in combination with CA-125, was a good marker for deep peritoneal endometriosis.52
Leptin is an adipocyte hormone that is produced by endometriotic tissues.53 Its peritoneal concentration is inversely correlated with the stage of endometriosis.54
Macrophage migration inhibitory factor is a pro-inflammatory cytokine produced by macrophages and endometriotic stromal and glandular cells. Its production is increased by TNF-α. Macrophage migration inhibitory factor was found recently to be significantly elevated in the peritoneal fluid of patients with endometriosis, but its levels did not correlate with the extent of disease or depth of penetration.55
Oxidative Stress in Peritoneal Endometriosis
Macrophages in the peritoneal fluid of patients with endometriosis produce large amounts of reactive oxygen species,56 which may lead to the expression of genes that allow cell adhesion.57 Oxidative stress occurs when levels of reactive oxygen species overwhelm the body’s antioxidant defense system. The concept of peritoneal cavity oxidative stress in patients with endometriosis is an extension of the chronic inflammatory nature of the disorder. Several studies have shown that antioxidant defenses may be altered in endometriosis, leading to an increased risk of oxidative stress. The peritoneal fluid from endometriosis patients may contain low levels of vitamin E (an antioxidant). Mifepristone (RU 486) has been showed to inhibit endometrial cell growth through its antioxidant effects.58 Oxidative stress may contribute in part to infertility through its effects on gametes or gamete interaction.36
Genetics of Endometriosis
Comparing the prevalence of the disease between monozygotic and dizygotic twins, it is estimated that 51% of the prevalence of the disease is attributed to genetic factors. The influence of specific environmental factors in a population of women make it difficult to separate out the genetic contribution to the disease. Therefore, the use of animal models such as the monkey may allow a more controlled assessment of such factors. In monkey colonies, there was evidence of familial aggregation63—the risk for full siblings was higher than that for half siblings (maternal or paternal). Population studies in Iceland64 from all identified cases of endometriosis showed a higher degree of common relatives than a control group of unaffected women, which confirms that the endometriosis group originated from a small number of founder women. A Norwegian study65 showed a greater familial association with women having severe endometriosis. Thus, the genetic influence may be more pronounced in relatives with severe disease. This was also demonstrated in a UK study.61 These findings are typical of diseases that are inherited as complex genetic traits, such as diabetes, asthma, and hypertension.
Researchers have suggested that many genes are altered with a higher frequency in patients with endometriosis, including the GALT gene and ICAM-1—an adhesion molecule encoding gene. Genes involved in ovarian steroidogenesis have also been implicated, including the gene encoding for the estrogen receptor and the gene encoding for the progesterone receptor. Aromatase enzyme gene expression seems to be increased within endometriosis lesions. This enzyme is usually not expressed in eutopic endometrium, and increased expression may lead to higher local concentrations. This might explain rare cases of postmenopausal women with active endometriotic lesions. Some of these rare cases have been reported to respond to the administration of aromatase inhibitors. A higher prevalence of the 4 G allele of the plasminogen activator inhibitor-1 (PAI-1) is found in patients with endometriosis.62 This results in hypofibrinolysis and persistence of the fibrin matrix that could support the initiation of endometriotic lesions.
Potential Alternative Hypothesis: Coelomic Metaplasia Theory
Endometriomas
The etiology of endometrioma formation is still the cause of many debates (Fig. 49-6). After analyzing serial sections of ovaries with endometriomas, Hughesdon66 suggested that 90% of endometriosis resulted from the adhesion of ovaries to the surrounding structures, which leads to progressive invagination of the ovarian cortex after repetitive bleeding from the endometrial implants on the surface of the ovaries. They would, in fact, constitute a pseudocyst. Some investigators also suggested that endometriomas might be the result of infiltration of functional cysts by endometriosis. Donnez and colleagues67 have suggested an alternative pathophysiology, which is based on coelomic metaplasia of invaginated epithelial inclusions.
Rectovaginal Endometriosis
Endometrial glands and stroma are often found in the retrocervical or rectovaginal space. The histology of these lesions differs from that of superficial endometriotic lesions.67 Smooth muscle proliferation and fibrosis are constantly present, are similar to adenomyotic nodules, and are responsible for the nodular aspect of these lesions often found on palpation of the rectovaginal wall. These lesions will typically lead to long-term pain and deep dyspareunia. They can be found in the uterosacral ligament, the posterior vaginal wall, or sometimes in the anterior rectal wall.68 They can even extend laterally and affect the ureters. Their histology suggests that they do not originate from deep infiltrating lesions but are more likely to be the result of metaplasia of the müllerian rest, according to Donnez and colleagues.69 Once these nodules are removed, they are not likely to recur.70,71 This suggests that these lesions originate from müllerian rest metaplasia rather than from infiltration of superficial endometriosis. Secretory changes are frequently absent in these nodules during the luteal phase, suggesting that they do not respond to physiologic levels of progesterone. The coexpression of vimentin and cytokeratin in these lesions is characteristic of a mesodermal müllerian origin.69
ANATOMIC SITES OF ENDOMETRIOSIS
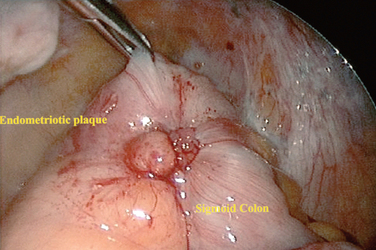
Endometriosis is most commonly found in the pelvis and near the tubal fimbria.72 The most common locations, in descending order, are the ovaries, the anterior and posterior cul-de-sac, the broad ligament, and the uterosacral ligaments. The left hemipelvis is the most common location—64% compared to the right hemipelvis. More endometriosis is found on the left ovary, possibly because the sigmoid colon alters intraperitoneal movement of fluid. Tubal endometriosis is relatively uncommon, constituting approximately 5% of all cases.73 However, minimal tubal adhesions of the fimbriae are relatively common. The bowel is the most common extragenital location of endometriosis.74
The most common bowel locations, in decreasing frequency, are the rectum, sigmoid colon, cecum, and terminal ileum. Most bowel lesions are superficial and limited to the serosa. Occasionally, transmural involvement of the bowel occurs, which may cause cyclic diarrhea, rectal bleeding, abdominal distension and, rarely, bowel obstruction. The urinary tract is involved in only 1% of cases,75 and it most often affects the bladder. Symptoms are similar to those associated with recurrent cystitis.76 Endometriosis of the urinary tract should be suspected if cyclical urinary symptoms occur. The ureter can be affected in 0.14% to 1% of cases, more commonly on the left side (63%).
Extrapelvic Endometriosis
After surgical procedures, endometrial cells can implant in the scar; therefore, endometriosis has been reported in abdominal scars after hysterectomy, appendectomy, and laparoscopy.77 Perineal endometriosis is also occasionally found in episiotomy scars. Many cases of endometriosis of cesarean scars have also been reported. Rare lung cases of endometriosis leading to cyclic hemoptysis or even pneumothorax78 have been reported and imply that hematogeneous or lymphatic dissemination of endometrial cells is possible. This mechanism can also explain the possible spread to rare locations, such as the brain. Endometriosis may also exceptionally affect the liver, pancreas, kidney, urethra, vertebra, and bones.
PATHOPHYSIOLOGY OF INFERTILITY ASSOCIATED WITH ENDOMETRIOSIS
Even though the association between infertility and endometriosis is well established,79 the mechanism of this association remains complex and is not completely understood.80 Many women with endometriosis remain fertile, and fertility decreases as the ASRM score increases.81 The following factors may explain why:
Anatomic Changes
Endometriosis, when moderate or severe, will often lead to adhesion formation between different pelvic or abdominal organs. Peritubal or peri-ovarian adhesions81 and tubal wall fibrosis can decrease tubal motility and access to the ovulated oocytes.82 Minor adhesions can also be found inside the tubal lumen and may impair transport of the oocyte.
Immunologic Factors
The peritoneal environment of women with endometriosis is greatly altered, with abnormal levels of cytokines, growth factors, and inflammatory cells, which are likely to participate in the etiology of endometrial implants.83 These alterations negatively affect sperm motility, oocyte maturation, fertilization, embryo survival, and tubal function. The increased number of peritoneal macrophages observed in the peritoneal fluid of patients with endometriosis will lead to higher phagocytic activity83 against sperm and higher nitric oxide synthetase activity. Higher nitric oxide levels may be toxic to the embryo and may negatively affect sperm function and reduce embryonic implantation.84 The peritoneal fluid of women with endometriosis reduces sperm–oocyte interaction in a dose-dependent fashion.85
Oxidative stress has been associated with gamete and embryo toxicity. The addition of antioxidants such as vitamin C and E to the embryo culture media, for example, is associated with an increased blastocyst development rate in oxidative-stressed embryos.86 Oxidative stress is also associated with increased DNA damage in patients with male factor infertility.87
Oocyte Maturation
Levels of transferrin are higher in the peritoneal fluid of women with endometriosis,88 and such levels have been found to decrease follicle-stimulating hormone (FSH)-regulated granulosa cell differentiation, which may lead to ovarian dysfunction. The granulosa cells of patients with endometriosis have been shown to have lower levels of VEGF,89 which is an important angiogenic and mutagenic factor. This may therefore lead to a decrease in oocyte maturation. Levels of TNF90 in granulosa cells of patients with endometriosis have been found to be increased, and this may affect oocyte maturation.
Effect on Embryo Development and Implantation
Patients with stage I and II endometriosis have high levels of antiendometrial antibodies,91 which may reduce implantation. IL-1 and IL-692 are elevated in the peritoneal fluid of patients with endometriosis and are embryotoxic. They may also contribute to the observed decrease in fertility. Luteal phase defects were reported to be more common in patients with endometriosis by earlier studies,46 but these findings have not been confirmed by others. Expression of the HOXA1 and HOXA11 genes,93 which is usually up-regulated during the secretory phase of the menstrual cycle, is not up-regulated in patients with endometriosis. These genes were recently reported to regulate the expression of integrin,94 which plays a crucial role in the embryo’s ability to attach to the endometrium.
A decrease in β3 integrin expression has been reported in patients with endometriosis, which might explain the decrease in implantation.95 The initial attachment of the embryo may involve cell adhesion molecules such as β3 integrin. Researchers have, in fact, documented over 100 aberrant regulated genes in eutopic endometrium of patients with endometriosis during embryo implantation.96 These genes affect cell adhesion molecules, secreted proteins, transporters, and immune modulators. It is not known which of these aberrant genes causes the decrease in fertility.
The presence of free radicals in the endometrium might also decrease oocyte and embryo quality.97 The expression of enzymes involved in the balance of free radical formation and clearing have been shown to be altered in the eutopic endometrium of patients with endometriosis.
MECHANISM OF PAIN RELATED TO ENDOMETRIOSIS
Pain associated with endometriosis is quite complex and is not directly correlated with the extent of disease by the ASRM classification.15,98 The depth of endometriosis lesions is associated with the presence of pain, especially dysmenorrhea. Pain associated with advanced disease can be caused by extensive adhesions, ovarian cysts, or deeply infiltrating endometriosis. Expression of nerve growth factor is associated with endometriosis pain. However, there was no relationship between nerve fibers in these adhesions and pelvic pain.
Even patients with early-stage disease (few scattered implants) can experience pain. This pain can be explained in part by the increase in prostaglandins found in these patients. In contrast to the normal endometrium, ectopic endometrium (endometriosis) is the site of at least two molecular aberrations that result in the accumulation of increasing quantities of estradiol and prostaglandin PGE2.99 With the first aberration, the activation of the gene that encodes aromatase (CYP19) increases, leading to an increase in aromatase activity in the endometriotic tissue. This activation is stimulated by PGE2, which is the most potent inducer of aromatase activity in the endometriotic stromal cells. The second important molecular aberration in the endometriotic tissue is the increased stimulation of cyclo-oxygenase-2 (COX-2) by estradiol, which leads to increased production of PGE2. This establishes a circular event leading to an important accumulation of PGE2 in the endometriotic tissue.
DIAGNOSIS OF ENDOMETRIOSIS
The definitive diagnosis of endometriosis can only be made surgically. However, several noninvasive methods have been proposed. The classic symptoms are dysmenorrhea, noncyclic pelvic pain, dyspareunia, and infertility. Specific physical signs indicative of advanced endometriosis are listed in Table 49-3. Unfortunately, most patients have only nonspecific signs on physical examination. Physical examination during menstruation is more sensitive in the detection of pelvic disease.
If any of the classic symptoms of endometriosis are present, the positive predictive value is 56% and the negative predictive value is 78%. In addition, the sensitivity is 76% and the specificity is 58%. However, this includes patients with ovarian endometriomas as well. If ovarian disease is excluded, these symptoms would have less favorable statistical parameters for the purposes of diagnosis. Findings on physical examination would improve these parameters.100 The more severe symptoms of dysmenorrhea are associated with the presence of deeply infiltrating disease.
There are no laboratory tests that can identify patients with endometriosis with a high degree of sensitivity and specificity. CA-125 levels can be elevated with advanced endometriosis but are typically normal in early-stage disease. Overall, a sensitivity between 24% and 94% and a specificity between 83% and 93% have been reported.101 A meta-analysis on the value of CA-125 has confirmed these observations.102 The search for biomarkers of this disease is quite active. The diagnostic accuracy of serum IL-6 and peritoneal TNFα was shown to be excellent (sensitivity, 90% to 100%; specificity, 67% to 89%).50 Further studies are required before clinical use can be recommended. Other potential markers are endometrial genetic markers such as P450 aromatase or placental protein 14.
Ovarian endometriosis (see Fig. 49-6) can be predicted with a high degree of accuracy with ultrasonography. However, nonovarian disease cannot. Furthermore, other imaging modalities such as computed tomography (CT) scan and MRI do not substantially improve the diagnostic accuracy of ultrasound for nonovarian endometriosis. There may be a role for identification of deeply infiltrating lesions that involve the cul-de-sac or lesions in uncommon locations, such as the sciatic nerve. Results of diagnostic tests for patients with gastrointestinal symptoms, such as colonoscopy or barium enema radiography, are typically normal or may occasionally show stricture. Patients with significant urinary symptoms should have a urologic evaluation to rule out interstitial cystitis or potentially endometriosis of the bladder.
Surgical Diagnosis
The typical lesions of endometriosis are referred to as pigmented (powder-burn, brown, blue-black, brown, and yellow) and nonpigmented (clear, white, red polypoid, or flamelike) lesions (see Figs. 49-3 and 49-4). Defects in the peritoneum or peritoneal windows may contain these lesions. Adhesions can be subtle, as seen around the fimbriae, or severe dense adhesions that are quite vascular and include the tubes and ovaries. The scoring system for endometriosis, the ASRM classification, is widely used clinically but has a significant intraobserver and interobserver variation (between 38% and 52%).15,103
The positive predictive value of laparoscopic visualization of endometriosis is considered to be approximately 50%. Classic lesions, such as red or black lesions, have a visual accuracy between 90% and 100%. White lesions are associated with endometriosis less often.104 The main pathologies that can be confused with endometriosis are endosalpingiosis, fibrosis, mesothelial hyperplasia, carbon deposits from previous surgery, and malignancy. Hemangiomas, adrenal rests, and splenosis can also rarely be confused with endometriosis. For this reason, diagnostic laparoscopy should be accompanied with biopsy for lesions that are not clearly endometriosis. Clinical trials for endometriosis management should always be performed with biopsy confirmation.
Associated Disease Processes
Surveys of endometriosis patients report increased incidence of atopic disease and other autoimmune phenomena such as thyroid disease. Fibromyalgia and chronic fatigue syndrome are often reported. Endometriosis is associated with increased incidence of ovarian cancer (especially endometrioid and clear-cell type), non-Hodgkin lymphoma, dysplastic nevi, and melanoma. Approximately 75% of neoplasms complicating endometriosis arise in the ovary. Most are endometrioid type (90%); 5% are clear-cell. Extrapelvic malignancies associated with endometriosis tend to be adenocarcinomas. However, sarcomas have also been reported.105
TREATMENT OF ENDOMETRIOSIS IN THE INFERTILE COUPLE
The cumulative spontaneous pregnancy rate over 3 years in women with unexplained infertility (i.e., normal pelvis) is significantly higher than in women with untreated minimal or mild endometriosis (54.6 % versus 35.5%; P = 0.048).106 It is estimated that in an infertile couple with stage 1 or 2 endometriosis, the monthly fecundity rate is 3% per cycle. Smoking decreases the chance of pregnancy and live birth in both groups. Medical suppressive therapy with an oral contraceptive agent or gonadotropin-releasing hormone (GnRH) agonist does not improve the pregnancy rate.
Surgical Treatment
Surgical treatment of minimal or mild endometriosis improves the spontaneous pregnancy rate. However, the absolute amount is open to debate. Two studies have investigated this question with randomized clinical trials: a multicenter Canadian trial and an Italian trial.107,108 The Canadian trial showed statistically significant improvement. The ongoing pregnancy rate was 29% in the treated group and 17% in the nontreated group. The number needed to treat—that is, the number of persons that would need to be treated surgically to achieve an extra pregnancy—was 9 (95 CI, 5–33). The Italian trial did not show any improvement in pregnancy rates. A meta-analysis of these studies showed an improvement but at a reduced rate from that of the Canadian study. Postoperative suppressive medical therapy after surgical treatment does not improve fertility. The only value of medical suppressive therapy appears to be before in vitro fertilization (IVF) in moderate to severe cases.
If surgical therapy with early-stage disease fails or if the patient does not wish surgical therapy, the next step is treatment with clomiphene citrate and intrauterine insemination (IUI)109 followed by gonadotropin and IUI. Each is typically attempted for three cycles.
In advanced disease, surgical management improves fertility. However, this surgery is complex and requires meticulous dissection. If an initial surgery for advanced disease fails, subsequent surgery is less successful than IVF in establishing a pregnancy and should be reserved for patients who require management of pain.110 The use of clomiphene citrate or gonadotropin and IUI in patients with advanced disease should be reserved for patients with normal tubes and minimal adhesive disease. The use of GnRH agonists before a cycle of gonadotropin and IUI has not been clearly established as beneficial.
In Vitro Fertilization
In a meta-analysis of observational studies, the diagnosis of endometriosis was associated with diminished IVF pregnancy rates111 compared with tubal factor infertility, especially with advanced disease. It is unclear whether the markedly reduced pregnancy rates with IVF in patients with advanced disease are due to previous surgery or simply to the advanced disease itself.112
Endometriomas
Endometriomas (see Fig. 49-6) should be removed to confirm they are benign. Removal can improve spontaneous fertility. IVF outcomes have been reported to be similar in patients with and without endometriomas. However, the number of oocytes, fertilization rates, and number of embryos obtained were decreased in women with previously-treated endometriomas compared with those without endometriomas. In relation to IVF, there are several questions that arise about ovarian endometriomas:
The surgical technique used to remove an endometrioma has been evaluated in several trials in an attempt to define what approach results in the least damage to the ovary. Excision by “stripping” the cyst off the ovary will result in ovarian tissue removal with the specimen in more than 50% of cases. In cysts with true capsules, this occurs in only 6% of cases.113 However, few follicles were found in the ovarian tissue removed with the endometrioma. In a randomized trial, excision of the endometrioma was associated with less recurrence and higher fertility rates than fenestration and bipolar coagulation.114 Simple drainage of an endometrioma has been shown to be ineffective.
TREATMENT OF PATIENTS WITH CHRONIC PELVIC PAIN
Medical Management
Oral contraceptives and nonsteroidal anti-inflammatory drugs (NSAIDs) are used as first-line therapy for chronic pain associated with endometriosis. However, a recent Cochrane review concluded that there is little data that supports their efficacy in these patients.115
Several classes of drugs have traditionally been used to manage pain associated with endometriosis (Table 49-4): GnRH agonists, progestins such as norethindrone acetate and medroxyprogesterone acetate, and danazol. Empiric treatment with GnRH agonists without laparoscopy is considered an acceptable approach for the management of chronic pelvic pain after exclusion of other common causes.116 Therefore, response to this treatment does not make the diagnosis of endometriosis. In a study by Ling,117 more than 80% of patients treated with the GnRH agonist had pain relief, but 39% of patients treated with placebo also had pain relief. This placebo response rate is also seen with surgical treatment for endometriosis as well.
Progestins
The levonorgestrel intrauterine system (Mirena) has been successfully used for symptomatic endometriosis. A trial also demonstrated pain relief and decreased menstrual blood loss. The continuation rate dropped to 68% after 1 year of use and stabilized over the remaining years. Patients discontinued the drug because of irregular menstrual bleeding and persistent pain. However, in patients who continued the device, pain was well-controlled.118
Gonadotropin-Releasing Hormone Agonists
This class of drugs is highly effective in relieving the pain associated with endometriosis. The drug is given via an intramuscular (leuprolide acetate), subcutaneous (goserelin), or nasal (nafarelin) route. After an initial increase in gonadotropins during the first 10 days, there is a decrease in pituitary secretion secondary to GnRH receptor down-regulation. These drugs are typically given for an initial 6-month course. The majority of patients (75% to 80%) in all clinical trials responded. However, many patients will have recurrence of pain. In a study by Dlugi and colleagues, 54% of patients with moderate to severe pain before treatment reported recurrence of pain within 3 months of a 6-month treatment course.119 Only 37% of patients still had relief after 1 year. Similar results have been reported by many other centers. Miller and colleagues reported a median time to recurrence of pain of 5.2 months.120 About 60% of patients that have recurrence of pain will respond to another six-month trial of GnRH agonists. However, the main concern is the progressive loss in BMD. Menstrual periods return between 2 and 3 months after the last monthly injection, but recovery of BMD takes more time.
Androgens
Danazol is a 17-ethinyl-testosterone derivative that is as effective as GnRH agonists in relieving the pain associated with endometriosis. The side effects from this class of drugs are related to their androgenicity and include weight gain, edema, acne, hirsutism, and myalgia. The drug can cause androgenic changes in the lipid profile and raise liver enzymes. Danazol suppresses luteinizing hormone and FSH and induces amenorrhea at the traditional doses used. Typically, 400 to 800 mg daily are used for 6 months. Recurrence of pain occurs with the same frequency and within the same time period as with the GnRH agonists. A new vaginal formulation may have fewer systemic side effects with the same efficacy of pain relief.
Drugs that are currently under investigation include TNFα blockers,121 inhibitors of MMP,122 antiangiogenic agents,123 and selective estrogen receptor-B (ERB) agonists.124 The ERB agonists probably have their primary action on the immune system. Aromatase inhibitors have been successfully used in a limited number of cases of persistent disease after hysterectomy and bilateral oophorectomy, as well as in patients with intact pelvic organs. The putative mechanism is by suppression of locally produced estrogens from aromatase activity expressed by the endometriotic lesions. Typically, an aromatase inhibitor such as anastrozole 1 mg daily is given with a low-dose oral contraceptive (20μg estradiol) to prevent ovarian cyst formation and a possible decrease in BMD.125 The addition of anastrozole to a GnRH agonist seems to have prolonged the time to symptom recurrence as well as frequency of recurrence. However, BMD decreased more with the addition of the aromatase inhibitor.126
Surgical Management
Two prospective randomized, controlled studies have clearly shown that surgical therapy is superior to no treatment for relief of pain from endometriosis.127,128
There are several observations that can be surmised from these studies:
Neurectomy
Interruption of the cervical and uterine sensory nerves by transection of the uterosacral ligaments, a uterosacral nerve ablation, has been shown not to have any long-term benefit.129 A presacral neurectomy has been shown to be beneficial in patients with chronic pelvic pain and endometriosis when performed with surgical treatment of the endometriosis lesions.130 The procedure is associated with greater relief of midline pain rather than lateral pain. The success of the procedure is dependent on excision of the superior hypogastric plexus (presacral nerve) before extensive branching has occurred. The dissection is actually prelumbar. The landmark on the right is the ureter and right common iliac artery; on the left, it is the sigmoid colon and inferior mesenteric vessels. The floor of the prelumbar space contains the left common iliac vein. The most common postsurgical complications are constipation and urinary urgency.
Posthysterectomy Recurrence
Endometriosis can recur in 5% to 10% of patients after hysterectomy and bilateral salpingo-oophorectomy.131 The role of hormone replacement therapy after surgical castration is controversial. There is a possibility of symptom or disease recurrence. On the other hand, there is the real possibility of severe vasomotor symptoms and osteoporosis. No high-quality data support a particular recommendation. The recurrence rate of endometriosis in hormone replacement therapy was assessed in a randomized, nonplacebo-controlled trial in women who had undergone bilateral salpingo-oophorectomy (90% of patients had also undergone hysterectomy). Hormone replacement therapy consisted of transdermal estradiol delivering 50μg per day and cyclic micronized progesterone. Patients were followed for a median of 45 months. The risk of recurrence in the treated and nontreated group was 3.5% and 0%, respectively.132 The cases of recurrent endometriosis were observed in women who may not have had total removal of the disease. Hormone replacement therapy is not contraindicated, and the risks and benefits should be discussed with the patient.
Rectovaginal Endometriosis
However, some patients with rectovaginal endometriosis are asymptomatic. These patients do not need treatment. Follow-up studies on a cohort of untreated patients with asymptomatic cul-de-sac disease have shown that most patients do not develop symptoms or further growth of the disease.133 Many patients achieved pregnancy without surgical resection.
MANAGEMENT OF ENDOMETRIOSIS ON EXTRAGENITAL ORGANS
Gastrointestinal Endometriosis
Although gastrointestinal symptoms are quite common in women with endometriosis, the overall incidence of bowel involvement is reported to be between 5% and 10%. Endometriosis of the gastrointestinal system typically involves the rectum or rectosigmoid colon (Fig. 49-7). Recurrence of disease after hysterectomy and oophorectomy more commonly involves the bowel. Complete obliteration of the cul-de-sac is a common operative finding. These patients typically present with gastrointestinal symptoms in addition to the typical chronic pelvic pain complex. Excision of disease or intestinal resection can be performed by laparotomy or laparoscopy.134 Rectovaginal fistula and abscess formation are the most serious complications reported.
Respiratory System
Diaphragmatic endometriosis can be asymptomatic and noted incidentally at diagnostic laparoscopy (Fig. 49-8). Symptomatic patients often report right-sided chest pain or shoulder pain in association with menstruation that occasionally radiates into the neck or arm and dyspnea. Sometimes these symptoms occur during the nonmenstrual phase of the cycle. These patients seem to have associated pelvic endometriosis that ranges from mild to severe. The right hemidiaphragm is more often involved. Typically, there are smaller sentinel lesions that predict the occurrence of more significant lesions on the posterior diaphragm. Many significant lesions of the right diaphragm will not be seen from a laparoscope placed at the umbilicus, and a view from a right upper quadrant port is necessary. Electrosurgery, laser excision, or surgical excision should be performed carefully because the thickness of the diaphragm ranges between 1 and 5 mm. Medical treatment with GnRH agonists has been reported to have mixed results. Surgical castration has also been attempted, with mixed results in symptomatic patients. Asymptomatic diaphragmatic lesions do not need treatment.
Thoracic endometriosis most commonly presents as a right-sided catamenial pneumothorax but can also be manifested by hemothorax, hemoptysis, or pulmonary nodules. The typical symptoms are chest pain and dyspnea. Not all of these patients have pelvic endometriosis at the time of surgical management of the thoracic disease. Pleural or diaphragmatic endometriosis can be found in less than 25% of cases.135 A chest CT scan may demonstrate pulmonary or pleural nodules, especially if performed during a menstrual period. Chemical pleurodesis is associated with a lower recurrence rate of catamenial pneumothorax than hormonal treatment. However, initial treatment with hormonal therapy is indicated. Catamenial chest pain may persist after pleurodesis and require endocrine manipulation to induce a hypoestrogenic state.
Genitourinary System
Endometriosis often involves the peritoneum over the ureter. However, direct ureter involvement is uncommon and has been reported in less than 1% of patients. In patients with rectovaginal endometriosis, the prevalence is higher. Ureteral involvement can be the result of extrinsic compression from extensive endometriosis that surrounds the ureter with significant fibrosis. Intrinsic endometriosis involves the presence of glands and stroma within the wall of the ureter without extension from the surrounding tissues. These lesions can be associated with loss of kidney function. These patients usually have severe pelvic pain, dysmenorrhea, and dyspareunia. Some patients have associated urinary symptoms such as hematuria or dysuria. The majority of the patients have significant involvement of the rectovaginal septum with nodules that are often larger than 3 cm. Preoperative imaging studies such as MRI with contrast should be used to evaluate the renal system preoperatively in patients with rectovaginal disease. Ultrasonography will usually not detect these lesions. Medical therapy has been used successfully in a limited number of patients. Most cases of ureteral endometriosis can be treated with excision of the periurethral fibrosis and active lesions without ureter resection.
Sciatic Nerve Involvement
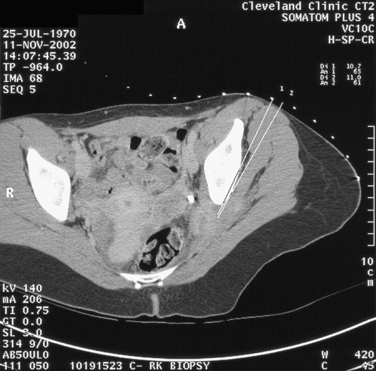
Patients with endometriosis of the sciatic nerve can present with hip pain, which is usually localized to the buttock. The pain radiates down the back of the leg, and numbness occurs in areas innervated by the sciatic nerve. The symptoms typically occur in association with a menstrual period but then extend into other times of the cycle. Progressive leg and foot muscle weakness, with electromyogram studies showing denervation, can be demonstrated. MRI typically shows a lesion infiltrating the sciatic nerve. CT-guided biopsy can be used to confirm the diagnosis (Fig. 49-9). Two thirds of cases are localized to the right side. Most cases have pelvic endometriosis associated with this disease. Catamenial sciatica can occur without characteristic imaging results. Treatment with a GnRH agonist (leuprolide acetate 3.7 mg every 28 days) with add-back therapy (transdermal estradiol 25μg) has been shown to reverse the neurologic abnormalities. If symptoms persist, electromyogram studies show worsening of the syndrome; if recurrence occurs after medical therapy, surgery is indicated. In patients who have completed their family, surgical castration with hormone replacement is an option. If this fails or if patients have not yet completed their family, excision of the lesion off the sciatic nerveis suggested. However, this surgery is complex because the lesions often start on the lumbosacral plexus on the piriformis muscle and wind through the sciatic notch.
1 Janson RPS, Russel P. Nonpigmented endometriosis: Clinical, laparoscopic, and pathologic definition. Am J Obstet Gynecol. 1986;155:1154-1159.
2 Cornillie FJ, Oosterlynck D, Lauweryns JM, Koninckx PR. Deeply infiltrating pelvic endometriosis: Histology and clinical significance. Fertil Steril. 1990;53:978-983.
3 Koninckx PR, Meuleman C, Oosterlynck D, Cornillie FJ. Diagnosis of deep endometriosis by clinical examination during menstruation and plasma CA-125 concentration. Fertil Steril. 1990;65:280-287.
4 Brosens IA. Is mild endometriosis a progressive disease? Hum Reprod. 1994;9:2209-2211.
5 Redwine DB. Age-related evolution in color appearance of endometriosis. Fertil Steril. 1987;48:1062-1063.
6 Koninckx PR, Meuleman C, Demeyere S, et al. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril. 1991;55:759-765.
7 Alcazar JL, Laparte C, Jurado M, Lopez GG. The role of transvaginal ultrasonography combined with color velocity imaging and pulsed Doppler in the diagnosis of endometrioma. Fertil Steril. 1997;67:487-491.
8 Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164-166.
9 Koninckx PR, Martin DC. Deep endometriosis: A consequence of infiltration or retraction or possibly adenomyosis externa? Fertil Steril. 1992;58:924-928.
10 Wheeler JM. Epidemiology of endometriosis-associated infertility. Med Obstet Gynecol. 1989;34:41-46.
11 Walter AJ, Hentz JG, Magtibay PM, et al. Endometriosis: Correlation between histologic and visual findings at laparoscopy. Obstet Gynecol. 2001;184:1407-1411.
12 Acosta AA, Buttram VC, Besch PK, et al. A proposed classification of pelvic endometriosis. Obstet Gynecol. 1973;42:19-25.
13 American Fertility Society. Classification of endometriosis. Fertil Steril. 1979;32:631-634.
14 American Fertility Society. Revised American Fertility Society classification. Fertil Steril. 1985;43:351.
15 American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;65:817-821.
16 Guzick DS, Silliman NP, Adamson GD, et al. Prediction of pregnancy in infertile women based on the American Society for Reproductive Medicine’s revised classification of endometriosis. Fertil Steril. 1997;67:822-829.
17 Vercellini P, Bocciolone L, Vendola N, et al. Morphologic appearance in women with chronic pelvic pain. Peritoneal endometriosis. J Reprod Med. 1991;36:533-536.
18 Moen MH, Schei B. Epidemiology of endometriosis in a Norwegian county. Acta Obstet Gynecol Scand. 1997;76:559-562.
19 Hemmings R, Rivard M, Olive D, et al. Evaluation of risk factors assicated with endometriosis. Fertil Steril. 2004;81:1513-1521.
20 Missmer SA, Hankisson SE, Spiegelman D, et al. Reproductive history and endometriosis among premenopausal women. Obstet Gynecol. 2004;104:965-974.
21 D’Hooghe TM, Bambra CS, De JI, et al. The prevalence of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) increases with the duration of captivity. Acta Obstet Gynecol Scand. 1996;75:98-101.
22 Zondervan KT, Cardon LR, Kennedy SH. The genetic basis of endometriosis. Curr Opin Obstet Gynecol. 2001;13:309-314.
23 Rier SE, Martin DC, Bowman RE, et al. Endometriosis in azsa Rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7, 8-tetrachlorodibenzo-p-dioxin. Fund Appl Toxicol. 1993;21:433-441.
24 Igarashi TM, Bruner-Tran KL, Yeaman GR, et al. Reduced expression of progesterone receptor-β in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7, 8-tetrachlorodibenzo-p-dioxin. Fertil Steril. 2005;84:67-74.
25 Heilier JF, Nackers F, Verougstraete V, et al. Increased dioxin-like compounds in the serum of women peritoneal endometriosis and deep endometriotic (adenomyotic) nodules. Fertil Steril. 2005;84:305-312.
26 Matorras R, Rodriguez F, et al. Women who are not exposed to spermatozoa and infertile women have similar rates of stage I endometriosis. Fertil Steril. 2001;76:923-928.
27 Parazzini F, Ferraroni M, Bocciolone L, et al. Contraceptive methods and risk of pelvic endometriosis. Contraception. 1994;49:47-55.
28 Sangi-Haghpeykar H, Poindexter AN. Epidemiology of endometriosis amoung parous women. Obstet Gynecol. 1995;85:983-992.
29 D’Hooghe TM, Bamra CS, Isahakia M, Koninckx PR. Evolution of minimal endometriosis in the baboon (Papio anubis, Papio cynocephalus) over a 12 month period. Fertil Steril. 1992;5:409-412.
30 Witz CA, Allsup KT, Montoya-Rodriguez IA, et al. Pathogenesis of endometriosis—current research. Hum Fertil. 2003;6:34-40.
31 Harada T, Kaponis A, Iwabe T, et al. Apoptosis in human endometrium and endometriosis. Hum Reprod. 2004;19:29-38.
32 Kokorine I, Nisolle M, Donnez J, et al. Expression of interstitial collagenase (matrix metalloproteinase-1) is related to the activity of human endometriotic lesions. Fertil Steril. 1997;68:246-251.
33 Osteen KG, Igarashi TM, Yeaman GR, Bruner-Tran KL. Steroid and cytokine regulation of matrix metalloproteinases and the pathophysiology of endometriosis. Gynecol Obstet Invest. 2003;21:155-163.
34 Spuijbroek MD, Dunselman GA, Menheere PP, Evers JL. Early endometriosis invades the extracellular matrix. Fertil Steril. 1922;58:929-933.
35 Halme PR. Angiogenic activity of peritoneal fluid from women with endometriosis. Fertil Steril. 1993;59:778-782.
36 Said TM, Agarwal A, Falcone T, et al. Infliximab may reverse the toxic effects induced by tumor necrosis factor α in human spermatozoa: An in vitro model. Fertil Steril. 2005;83:1665-1673.
37 Nisolle M, Casanas-Roux F, Anaf V, et al. Morphometric study of the stromal vascularization in peritoneal endoemtriosis. Fertil Steril. 1993;59:681-684.
38 Oosterlynck DJ, Cornillie FJ, Waer M, et al. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil Steril. 1991;56:45-51.
39 Dmowski WP, Gebel HM, Braun DP. The role of cell-mediated immunity in pathogenesis of endometriosis. Acta Obstet Gynecol Scand. 1994;159:7-14.
40 Mathur S, Peress MR, Wiliamson HO, et al. Autoimmunity to endometrium and ovary in endometriosis. Clin Exp Immunol. 1982;50:25-26.
41 Olive DL, Winberg JB, Haney AF. Peritoneal macrophages and infertility: The association between cell number and pelvic pathology. Fertil Steril. 1985;44:772-777.
42 Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann NY Acad Sci. 2002;655:159-173.
43 Lebovic DI, Mueller MD, Taylor R. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10.
44 Arici A, Oral E, Attar E, et al. Monocyte chemotactic protein-1 concentration in peritoneal fluid of women with endometriosis and its modulation of expression in mesothelial cells. Fertil Steril. 1997;67:1065-1072.
45 Rong R, Ramachandran S, Santanam N, et al. Induction of monocyte chemotactic protein-1 in peritoneal mesothelial and endometrial cells by oxidized low-density lipoprotein and peritoneal fluid from women with endometriosis. Fertil Steril. 2002;78:843-848.
46 Fakih H, Bagget B, Holtz G, et al. Interleukin-1: Possible role in the infertility associated with endometriosis. Fertil Steril. 1987;47:213-217.
47 Lebovic DI, Bentzien F, Chao VA, et al. Induction of an angiogenic phenotype in endometriotic stromal cell cultures by interleukin-1β. Mol Hum Reprod. 2000;6:269-275.
48 Arici A, Head JR, MacDonald PC, Casey ML. Regulation of interleukin-8 gene expression in human endometrial cells in culture. Mol Cell Endocrinol. 1993;94:195-204.
49 Khorram O, Taylor RN, Ryan IP, et al. Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis. Am J Obstet Gynecol. 1993;169:1545-1549.
50 Bedaiwy MA, Falcone T, Sharma RK, et al. Prediction of endometriosis with serum and peritoneal fluid markers: A prospective controlled trial. Hum Reprod. 2002;17:426-431.
51 Vigano P, Somigliana E, Gaffuri B, et al. Endometrial release of soluble intercellular adhesion molecule 1 and endometriosis: Relationship to the extent of the disease. Obstet Gynecol. 2000;95:115-118.
52 Somigliana E, Vigano P, Candiani M, et al. Use of serum-soluble intercellular adhesion molecule-1 as a new marker of endometriosis. Fertil Steril. 2002;77:1028-1031.
53 Wu MH, Chuang PC, Chen HM, et al. Increased leptin expression in endometriosis cells is associated with endometrial stromal cell proliferation and leptin gene up-regulation. Mol Hum Reprod. 2002;8:456-464.
54 Bedaiwy MA, Falcone T, Goldberg JM, et al. Peritoneal fluid leptin is associated with chronic pelvic pain but not infertility in endometriosis patients. Hum Reprod. 2006;21:788-791.
55 Mahutte NG, Matalliotakis IM, Goumenou AG, et al. Elevations in peritoneal fluid macrophage migration inhibitory factor are independent of the depth of invasion or stage of endometriosis. Fertil Steril. 2004;82:97-101.
56 van Langendonckt A, Casanas-Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril. 2002;5:861-870.
57 Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the preimplantation embryo and its surroundings. Hum Reprod Update. 2001;7:175-189.
58 Murphy A, Zhou MG, Malkapuram S, et al. RU486-induced growth inhibition of human endometrial cells. Fertil Steril. 2000;74:1014-1019.
59 Kennedy S, Mardon H, Barlow D. Familial endometriosis. J Assist Reprod Genet. 1995;12:32-34.
60 Hadfield RM, Mardon HJ, Barlow DH, Kennedy SH. Endometriosis in monozygotic twins. Fertil Steril. 1997;68:941-942.
61 Coxhead D, Thomas EJ. Familial inheritance of endometriosis in a British population: A case control study. J Obstet Gynaecol. 1993;13:42-44.
62 Bedaiury MA, Falcone T, Mascha ED, Casper RF. Genetic polymorphism in the fibrinolytic system and endometriosis. Obstet Gynecol. 2006;108:162-168.
63 Hadfield RM, Yudkin PL, Coe CL, et al. Risk factors for endometriosis in the rhesus monkey (Macaca mulatta): A case-control study. Hum Reprod. 2002;3:109-115.
64 Stefansson H, Geirsson RT, Steinthorsdottir V, et al. Genetic factors contribute to the risk of developing endometrisis. Hum Reprod. 2002;17:3-9.
65 Moen MH, Magnus P. The familial risk of endometriosis. Acta Obstet Gynecol Scand. 1993;72:560-564.
66 Hughesdon PE. The structure of endometrial cysts of the ovary. J Obstet Gynecol Br Empire. 1957;44:69-84.
67 Donnez J, Nisolle M, Gillet N, et al. Large ovarian endometriomas. Hum Reprod. 1996;11:641-646.
68 Donnez J, Donnez O, Squifflet J, Nisolle M. The concept of “adenomyotic disease of the retroperitoneal space” is born. Gynaecol Endosc. 2001;10:91-94.
69 Donnez J, Nisolle M, Smoes P, et al. Peritoneal endometriosis and “endometriotic” nodules of the rectovaginal septum are two different entities. Fertil Steril. 1996;66:362-368.
70 Adamyan L. Gynecologic and obstetrics surgery. Additional international perspectives. In: Nichols DH, editor. Vaginal Surgery. St-Louis: Mosby Year Book; 1993:1043-1046.
71 Donnez J, Nisolle M, Casanas-Roux F, et al. Rectovaginal septum, endometriosis or adenomyosis: Laparoscopic management in a series of 231 patients. Hum Reprod. 1995;2:230-235.
72 Jenkins S, Olive DL, Haney AF. Endometriosis: Pathogenetic implications of the anatomic distribution. Obstet Gynecol. 1986;67:335.
73 Lyons R, Djahanbakhch O, Saridogan E, et al. Peritoneal fluid, endometriosis, and ciliary beat in the human fallopian tube. Lancet. 2002;360:1221-1222.
74 Bergqvist A. Different types of extragenital endometriosis: A review. Gynecol Endocrinol. 1993;3:207.
75 Vercellini P, Meschia M, De Giorgi O, et al. Bladder detrusor endometriosis: Clinical and pathogenetic implactions. J Urol. 1995;155:84-86.
76 Vercellini P, Pisacteta A, Pesole S, et al. Is ureteral endometriosis an asymptomatic disease? BJOG. 2000;107:559-561.
77 Chatterjee SK. Scar endometriosis: A clinicopathologic study of 17 cases. Obstet. 1980;56:81-84.
78 Moffatt S. Mitchell J: Massive pleural endometriosis. Eur J Cardiothorac Surg. 2002;22:321-323.
79 Decker WH, Lopez H. Conservative surgical treatment of endometriosis and infertility. Infertil. 1979;2:155-164.
80 Olive DL, Schwartz LB. Endometriosis. Infertil. 1979;328:1759-1769.
81 Thornton JG, Morley S, Lilleyman J, et al. The relationship between laparoscopic disease, pelvic pain and infertility: An unbiased assessment. Eur J Obstet Gynecol Reprod Biol. 1997;74:57-62.
82 Frackiewicz EJ, Zarotsky V. Diagnosis and treatment of endometriosis. Expert Opin Pharmacother. 2003;4:67-82.
83 Halme J. Role of peritoneal inflammation in endometriosis-associated infertility. Ann NY Acad Sci. 1991;622:266-274.
84 Osborn BH, Haney AF, Misukonis MA, Weinberg JB. Inducible nitric oxide synthase expression by peritoneal macrophages in endometriosis-associated infertility. Fertil Steril. 2002;77:46-51.
85 Coddington CC, Oehninger S, Cunningham DS, et al. Peritoneal fluid from patients with endometriosis decreases sperm binding to the zona pellucida in the hemizona assay: A preliminary report. Fertil Steril. 1987;57:783-786.
86 Wang X, Falcone T, Attaran M, et al. Vitamin C and vitamin E supplementation reduces oxidative stressed induced embryo toxicity and improves blastocyst development rates. Fertil Steril. 2002;78:1272-1277.
87 Wang X, Sharma RK, Sikka SC, et al. Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male-factor infertility. Fertil Steril. 2003;80:531-535.
88 Treloar SA, Hadfield RM, Montgomery G, et al. The International Endogene Study: A collection of families for genetic research in endometriosis. Fertil Steril. 2002;78:679-685.
89 Mathur SP. Autoimmunity in endometriosis: Relevance to infertility. Am J Reprod Immunol. 2000;44:89-95.
90 Yamashita Y, Ueda M, Takehara M, et al. Influence of severe endometriosis on gene expression of vascular endothelial growth factor and interleukin-6 in granulosa cells from patients undergoing controlled ovarian hyperstimulation for in vitro fertilization-embryo transfer. Fertil Steril. 2002;78:865.
91 Carlberg M, Nejaty J, Froysa B, et al. Elevated expression of tumour necrosis factor α in cultured granulosa cells from women with endometriosis. Hum Reprod. 2000;15:1250-1255.
92 Ulcova-Gallova Z, Bouse V, Svabek L, et al. Endometriosis in reproductive immunology. Am J Reprod Immunol. 2002;47:269-274.
93 Pittaway DE, Maxson W, Daniell J, et al. Luteal phase defects in infertility patients with endometriosis. Fertil Steril. 1983;39:712-713.
94 Taylor HS, Bagot C, Kardana A, et al. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14:1328-1331.
95 Lessey BA, Castelbaum AJ, Sawin SW, et al. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79:643-649.
96 Ota H, Igarashi S, Sato N, et al. Involvement of catalase in the endometrium of patients with endometriosis and adenomyosis. Fertil Steril. 2002;78:804-809.
97 Daftary GS, Troy PJ, Bagot CN, et al. Direct regulation of β3-integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol. 2002;16:571-579.
98 Whiteside JL, Falcone T. Endometriosis-related pelvic pain: What is the evidence? Clin Obstet Gynecol. 2003;46:824-830.
99 Bulun SE, Zeitoun KM, Takayama K, et al. Aromatase as a therapeutic target in endometriosis. Trends Endocrinol Metab. 2000;11:22-26.
100 Eskenazi B, Warner M, Bonsignore L, et al. Validation study of nonsurgical diagnosis of endometriosis. Fertil Steril. 2001;76:395-929.
101 Bedaiwy MA, Falcone T. Laboratory testing for endometriosis. Clinic Chim Acta. 2004;340:41-56.
102 Mol BWJ, Bayram N, Lijmer JG, et al. The performance of CA-125 measurement in the detection of endometriosis: A meta-analysis. Fertil Steril. 1998;70:1101-1108.
103 Hornstein MD, Gleason RE, Orav J, et al. The reproducibility of the revised AFS classification of endometriosis. Fertil Steril. 1993;59:1015-1021.
104 Mettler L, Schollmeyer T, Lehmann-Willenbrock E, et al. Accuracy of laparoscopic diagnosis of endometriosis. JSLS. 2003;7:15-18.
105 Jelovsek JE, Brainard J, Winans C, Falcone T. Endometriosis of the liver containing Müllerian adenosarcoma. Am J Obstet Gynecol. 2004;191:1725-1727.
106 Akande VA, Hunt LP, Cahill DJ, Jenkins JM. Differences in time to natural conception between women with unexplained infertility and infertile women with minor endometriosis. Hum Reprod. 2004;19:96-103.
107 Marcoux S, Maheux R, Berube S. Laparoscopic surgery in infertile women with minimal and mild endometriosis. NEJM. 1997;337:217-222.
108 Gruppo Italiano per lo Studion dell’endometriosi. Ablation of lesions or no treatment in minimal–mild endometriosis in infertile women: A randomized trial. Hum Reprod. 1999;14:1332-1334.
109 Deaton J, Gibson M, Blackmer K, et al. A randomized, controlled trial of clomiphene citrate and intrauterine insemination (IUI) in couples with unexplained infertility or surgically corrected endometriosis. Fertil Steril. 1990;54:1083-1089.
110 Pagidas K, Falcone T, Hemmings R, Miron P. Comparison of reoperation for moderate (stage III) and severe (stage IV) endometriosis infertility with in vitro fertilization–embryo transfer. Fertil Steril. 1996;65:791-795.
111 Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on IVF. Fertil Steril. 2002;77:1148-1155.
112 Pal L, Shifren JL, Isaacson KB, et al. Impact of varying stages of endometriosis on the outcome of IVT–ET. J Assist Reprod Genet. 1998;15:27-31.
113 Muzii L, Bianchi A, Croce C, et al. Laparoscopic excision of ovarian cysts: Is the stripping technique a tissue-sparing procedure? Fertil Steril. 2002;77:609-614.
114 Beretta P, Franchi M, Ghezzi F, et al. Randomized clinical trial of two laparoscopic treatments of endometriomas: Cystectomy versus drainage and coagulation. Fertil Steril. 1998;70:1176-1180.
115 Moore J, Kennedy S, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2004. CD00474.
116 ACOG: However patients without endometriosis also obtain relief from pain with a GnRH agonist. Washington, DC, ACOG Practice Bulletin no. 51, March 2004.
117 Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93:51-58.
118 Lockhat FB, Emembolu O, Konje JC. The efficacy, side effects and continuation rates in women with symptomatic endometriosis undergoing treatment with an intra-uterine administered progestogen (levonorgestrel): A 3 year follow up. Hum Reprod. 2005;20:789-793.
119 Dlugi AM, Miller JD, Knittle J, for the Lupron Study Group. Lupron Depot (leuprolide acetate for depot suspension) in the treatment of endometriosis: A randomized, placebo controlled, double blind study. Fertil Steril. 1990;54:419-427.
120 Miller JD, Shaw RW, Casper RF, et al. Historical prospective cohort study of the recurrence of pain after discontinuation of treatment with danazol or a GnRH agonist. Fertil Steril. 1998;70:293-296.
121 Nothnick WB, D’Hooghe TM. Medical management of endometriosis: Novel targets and approaches towards the devlopment of future treatment regimens. Gynecol Obstet Invest. 2003;55:189-198.
122 Osteen KG, Yeaman GR, Bruner-Tran KL. Matrix metalloproteinases and endometriosis. Semin Reprod Med. 2003;21:155-163.
123 Hull ML, Charnock-Jones DS, Chan CLK, et al. Antiangiogenic agents are effective inhibitors of endometriosis. J Clin Endocrinol Metab. 2003;88:2889-2899.
124 Harris HA, Bruner-Tran KL, Zhang X, et al. A selective estrogen receptor B (ERB) agonist causes lesion regression in an experimentally induced model of endometriosis. Hum Reprod. 2005;20:936-941.
125 Amsterdam LL, Gentry W, Jobanputra S, et al. Anastrozole and oral contraceptives: A novel treatment for endometriosis. Fertil Steril. 2005;84:300-304.
126 Soysal S, Soysal ME, Ozer S, et al. The effects of post-surgical administration of goserelin plus anastrozole compared to goserelin alone in patients with severe endometriosis: A prospective randomized trial. Hum Reprod. 2004;19:160-167.
127 Sutton CJG, Ewen SP, Whitela N, Haines P. Prospective randomized double blind controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild and moderate endometriosis. Fertil Steril. 1994;62:696-700.
128 Abbott J, Hawe J, Hunter D, et al. Laparoscopic excision of endometriosis: A randomized, placebo controlled trial. Fertil Steril. 2004;82:878-884.
129 Proctor ML, Farquhar CM, Sinclair OJ, Johnson NP. Surgical interruption of pelvic nerve pathways for primary and secondary dysmenorrhea. Cochrane Satabase Syst Rev. 2003. CD01317.
130 Zullo F, Palomba S, Zupi E, et al. Effectiveness of presacral neurectomy in women with severe dysmenorrhea caused by endometriosis who were treated with laparoscopic conservative surgery: A 1 year prospective randomized double-blind controlled trial. Am J Obstet Gynecol. 2003;189:5-10.
131 Clayton RD, Hawe JA, Love JC, et al. Recurrent pain after hysterectomy and bilateral salpingo-oophorectomy for endometriosis: Evaluation of laparoscopic excision of residual endometriosis. BJOG. 1999;106:740-744.
132 Matorras R, Elorriaga MA, Pijoan JI, et al. Recurrence of endometriosis in women with bilateral adnexectomy (with or without total hysterectomy) who received hormone replacement therapy. Fertil Steril. 2002;77:303-308.
133 Fedele L, Bianchi S, Zanconato G, et al. Is rectovaginal endometriosis a progressive disease? Am J Obstet Gynecol. 2004;191:1539-1542.
134 Duepree HJ, Senagore AJ, Delaney CP, et al. Laparoscopic resection of deep pelvic endometriosis with rectosigmoid involvement. J Am Coll Surg. 2002;195:754-758.
135 Joseph J, Sahn SA. Thoracic endometriosis syndrome: New observations from an analysis of 110 cases. Am J Med. 1996;100:164-170.