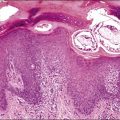
Endometrial Adenocarcinoma
An Oversimplified View of Endometrial Adenocarcinoma: Types 1 and 2
Classification of Endometrial Adenocarcinoma
Risk Factors in Endometrial Carcinoma
Endometrioid Adenocarcinoma and Its Variants
Other Types of Endometrial Carcinoma
Synchronous Endometrial and Ovarian Carcinoma
Tumors Metastatic to the Endometrium
Introduction
Adenocarcinoma of the endometrium is the most common gynecologic cancer in the United States, having a lifetime occurrence risk of 2.5%1 with 44,000 new cases and 7950 deaths annually.2 The median age at presentation is 63 years, of which 90% of cases are found in women past menopause and only 1% are under age 40 years. The absolute prevalence of endometrial cancer is affected by the background hysterectomy rate,3 which varies greatly between populations, and is 40% by age 60 in the United States.4
An Oversimplified View of Endometrial Adenocarcinoma: Types 1 and 2
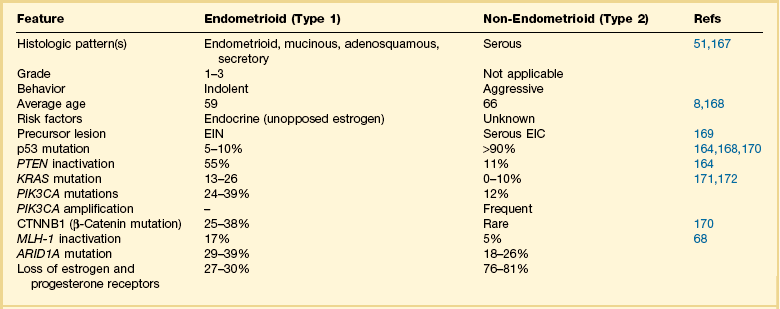
A two-type view of endometrial adenocarcinoma combines epidemiologic, clinical, histologic, and molecular genetic data, and provides a useful pathogenetic model supported by multiple lines of evidence (Figure 18.1). The two types are: endometrioid carcinomas and their variants (type 1) and the non-endometrioid (type 2) carcinomas.
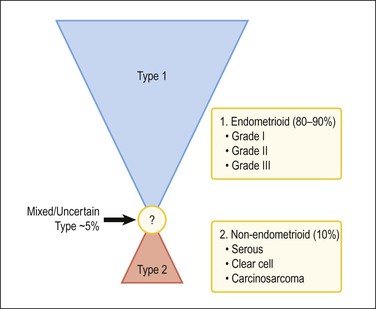
Figure 18.1 Types of endometrial carcinoma, and their clinically significant subdivisions. Endometrioid (type 1) tumors include many histologic variants, but it is tumor grade that is most important in risk prognostication. Non-endometrioid tumors (type 2) are ungraded, but histotypes should be diagnosed separately as each has a distinctive natural history.
For many years endometrial cancers were subdivided according to histologic grade into well, moderately, and poorly differentiated groups. This was the case until serous carcinoma, the most aggressive type of endometrial cancer, was recognized in 1982,5 and soon found to be unassociated with unopposed estrogens, the risk factor tightly linked to other endometrial carcinomas. Based on this finding, a proposal was made to separate endometrial adenocarcinomas into two distinct groups designated as types 1 and 2 (Table 18.1).6 It was a division into either indolent estrogen-induced or aggressive estrogen-independent tumors, respectively. Type 1 was further designated as ‘endometrioid’ and type 2 ‘non-endometrioid’ to reflect their divergent histologies, thereby suggesting that the noted functional differences might be extrapolated to their histologic classification.7
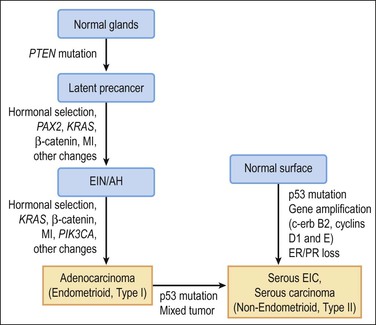
Molecular genetic analyses of well-differentiated endometrioid and serous carcinomas support this dualistic model of endometrial tumorigenesis (Figure 18.2).8 Studies using cDNA microarrays confirm that these two tumor types have distinctively different gene expression profiles.9 Increasing genetic damage can be seen in precursor lesions within the endometrioid pathway, beginning with PTEN or PAX2 inactivation in normal-appearing glands (latent precancers), followed by positive hormonal selection and clonal outgrowth as endometrial intraepithelial neoplasia (EIN; also known as atypical hyperplasia or AH), and then cancer (Figure 18.3).10 Comparable gradations are less evident in the serous carcinoma pathway, where a noninvasive form of disease, serous endometrial intraepithelial carcinoma (serous EIC), is genetically identical to its invasive counterpart and also capable of metastasizing.
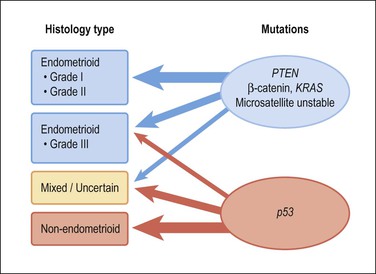
Figure 18.2 Type 1 and 2 endometrial carcinoma differ in the frequency of specific mutations, but there is substantial crossover especially in the grade III endometrioid and mixed categories. p53 inactivation is present in about 90% of serous carcinomas, and much less in clear cell carcinomas and carcinosarcomas.
Although the dualistic model is applicable to a high proportion of endometrial carcinomas, not all tumors fit in. In fact, a gray zone exists between the two broad types, with a significant number of tumors showing overlapping clinical, morphologic, and molecular features (Figures 18.1 and 18.2). Moreover, there is an ongoing debate about whether a histologic subset of endometrioid carcinomas (those that are poorly differentiated or have high nuclear grade) should be assigned to the type 2 group. Furthermore, it is now accepted that a non-endometrioid component may emerge from a pre-existing endometrioid carcinoma. The probable mechanism for this is development of genetic heterogeneity within elements of a type 1 tumor, and progressive expansion of a particularly aggressive tumor subclone with genetic and behavioral features resembling those of type 2 tumors9 (Figure 18.2).
Some unique tumor histotypes, such as clear cell carcinomas and carcinosarcomas, demonstrate clinical and molecular features that do not fit into either of the prototype endometrioid and non-endometrioid categories.133 These other histologic types may achieve even greater significance if and when specific therapeutic agents are developed to their peculiar molecular genetic alterations.
Molecular Pathology of Type 1 and 2 Cancers
Estrogen-related type 1 tumors frequently demonstrate one or more of the following: microsatellite instability, inactivated PTEN tumor suppressor gene, KRAS mutations, and activation of the β-catenin gene (CTNNB1).11 In contrast, the estrogen-independent type 2 tumors show loss of heterozygosity at different loci, altered p53, and abnormalities in genes regulating mitotic checkpoints.9 However, p53 mutations are found in approximately 10% of endometrioid carcinomas, most frequently in grade 3 and occasionally in grade 2 tumors. Overall, p53 mutations occur in 50% of grade 3 tumors, but not in grade 1 tumors or EIN/AH. This finding suggests that p53 is involved in the progression, but not the initiation, of endometrioid carcinoma.
Our understanding of synergies between multiple independent genetic events that produce endometrioid (type 1) carcinoma centers upon the PI3K pathway, and its ability when perturbed to activate AKT (Figures 18.2–18.4). It is estimated that more than 80% of endometrioid carcinomas have an abnormality in the PI3K pathway,12 which act to increase levels of PIP3, an activator of AKT. Thus PTEN inactivation (60% of cases), or constitutive activation of KRAS (10–30%) or PIK3CA (30%) act in concert to accumulate PIP3, which in turn activates AKT by phosphorylation.13 Once activated, pAKT initiates a cascade of tumorigenic events that includes stimulation of the mTOR pathway, deregulation of cell cycle control, blocking of apoptosis, and prolonged cell survival.13 This model presents new downstream targets for suppression by pharmacologic inhibitors, such as the mTOR pathway.
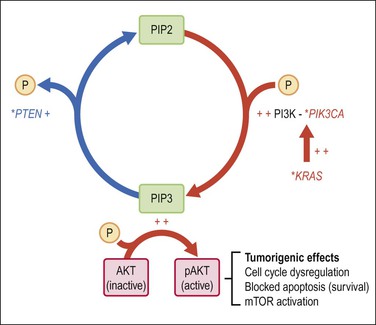
Figure 18.4 Activation of the AKT pathway in endometrioid (type 1) tumorigenesis. Activation of AKT by PIP-mediated phosphorylation is augmented by primary mutations (*), which activate the KRAS and/or PIK3CA genes, or inactivate PTEN. The combination of increased PIP3 production (red) with diminished PIP3 dephosphorylation (blue) has the net effect of increasing PIP3. pAKT has numerous downstream effects including activation of the mTOR pathway, changes in cell cycle regulation, blockade of apoptosis, and prolonged cell survival.
Classification of Endometrial Adenocarcinoma
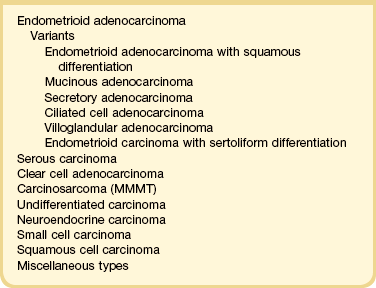
Recognition of histologic subtypes is an important factor in planning treatment and predicting clinical outcome. A histologic classification of endometrial carcinomas is given in Table 18.2, and this chapter is organized according to these diagnostic categories.
Risk Factors in Endometrial Carcinoma
Estrogens and Estrogen-Associated Conditions
The overriding stimulus behind the development of EIN/AH and the endometrioid type of endometrial carcinoma is the effect of estrogens, both endogenous and exogenous. These are not mirrored in non-endometrioid tumors, with the exception of carcinosarcomas. Beginning in 1969, a notable rise in the number of endometrial cancers occurred, which coincided with a fourfold increase in the use of estrogens for the alleviation of perimenopausal and postmenopausal symptoms in women. The relative risk of developing endometrial carcinoma in women taking unopposed estrogens is elevated 3- to 6-fold,14 rising to 9.5-fold if unopposed estrogen has been used for 10 years or longer.15 The increased risk persists for several years after the estrogen is discontinued.16 The risk is roughly similar whether the estrogens are taken continuously or cyclically. The additional administration of progestins for several days of each month reduces the risk of carcinoma to baseline population levels. Although progestins are usually prescribed for either 7 or 10 days per month in women taking estrogen-replacement therapy, the protection from endometrial cancer is much greater if progestins are used for at least 10 days or given continuously as combined estrogen-progestin therapy.
Obesity
The overall relative risk for an obese woman to develop endometrial cancer increases proportionally with increasing body mass index, up to sixfold for the morbidly obese (BMI > 40).17–19 This risk can be reduced to near baseline levels with bariatric surgery and successful weight reduction.20 Endometrial cancer is only one of the many comorbidities seen in the obese patient. The association in women past menopause is commonly explained in terms of increased aromatization of androgens to estrogens (estrone and estradiol) in adipose tissue, this being the major source of estrogens in women of this age group. A woman who completes surgical therapy for stage 1 endometrial cancer is more likely to die of cardiovascular disease than any other cause including her cancer.21
Diabetes
The magnitude of endometrial cancer risk in patients with type 2 diabetes has been difficult to measure because of a high frequency of coexisting risk factors such as obesity and polycystic ovarian disease. However, improved quantitative markers have confirmed a positive association of diabetes with endometrial cancer. Insulin resistance, as measured by the (inversely proportional) serum surrogate marker adiponectin, correlates highly with endometrial cancer risk even when corrected for obesity.22,23
Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is a constellation of endocrine disorders expressing at least two of the following features: anovulation or infrequent ovulation, androgen excess, and polycystic ovaries.24 Ovarian cysts typically are theca–lutein follicles with prominent luteinization of the theca interna and an inconspicuous granulosa cell layer. Primary endocrine defects in PCOS are peripheral insulin resistance and excess ovarian production of androgens.25 The population of affected women is enriched for risk factors such as obesity, with endometrial findings indicating a hyperestrogenic state.26 Endometrial carcinoma occurs at 2.7-fold increased risk in women with PCOS,27 but, as the women are all young, this number comprises a significant proportion of endometrial carcinomas in women under age 45 years.
Ovarian Sex Cord–Stromal Tumors
Granulosa cell tumor is a relatively uncommon tumor that mainly affects women shortly after menopause. Most tumors produce increased estrogens and about half the women affected present with postmenopausal bleeding, one-third having proliferative endometrium. Endometrial carcinomas occur in 9–13% of women with granulosa cell tumors.28
Other Risk Factors
Heritable Risk
Women with hereditary non-polyposis colorectal carcinoma syndrome (HNPCC), Lynch syndrome, a condition affecting about 1% of the population, have a 70% lifetime risk of endometrial adenocarcinoma.29 They tend to develop disease 15 years earlier than sporadic occurrences and the prognosis is favorable.30 Their tumors show many of the histopathologic and genetic features of endometrioid endometrial adenocarcinomas,31 including transit through a premalignant EIN/AH phase,32 and genetic alterations in mismatch repair genes (mainly MSH-2, MSH-6, and MLH-1)33,34 and PTEN.35 Immunohistochemistry for DNA mismatch repair proteins is often abnormal in endometrial cancers of Lynch syndrome patients, and has been suggested as a screening test for this heritable condition. Immunohistochemistry is nonspecific, however, because it is a somatically acquired non-heritable feature in 18% of all sporadic endometrial carcinomas. One cost–benefit study has recommended restricting immunotesting of primary endometrial tumors to those patients having additional clinical risk factors, such as one first-degree relative with a Lynch-associated carcinoma.36
Germline BRCA mutation, which increases breast and ovarian cancer occurrences, does not significantly alter endometrial cancer risk.37 There may be a small indirect effect on endometrial cancer incidence in those patients managed by prophylactic tamoxifen administration.38
Tamoxifen
The antiestrogen tamoxifen is widely used as an adjuvant therapy for women with breast cancer. Tamoxifen is a nonsteroidal compound that competes with estrogen for estrogen receptors (ERs). In women of childbearing age it antagonizes endogenous estrogens and induces endometrial inactivity or atrophy, but in postmenopausal women, who are normally hypoestrogenic, it may have a weak estrogenic effect. Tamoxifen administration is associated with an overall slightly increased risk (2–3 times) of endometrial adenocarcinoma.39 Carcinoma occurrences are mainly of early stage and low grade, but a small subset of aggressive high-grade endometrioid carcinomas, clear cell carcinomas, or carcinosarcomas are disproportionately increased.40,41 Placement of a levonorgestrel-impregnated intrauterine device in tamoxifen-treated patients has not yet demonstrated statistically significant protection from tamoxifen-related endometrial cancers.42
In addition to the increased risk of endometrial carcinoma, women treated with tamoxifen are particularly prone to developing endometrial polyps, especially ones of gigantic size (see Chapter 15).
Reproductive Factors
Most studies have shown an association between early age at menarche, late age at natural menopause, and total length of ovulation span,43 although these findings are not universal. The use of oral contraceptives reduces the risk of endometrial cancer, in some studies by half.
Nulliparity is a strong independent risk factor for endometrial carcinoma.43 Women with endometrial carcinoma are less likely to have had children than normal controls and, if they are parous, they will have had fewer children. Infertility, particularly that associated with anovulation and progesterone insufficiency, is also associated with the risk of developing endometrial carcinoma.45 Nulliparity is significant only when the endometrial carcinoma develops before menopause and not after.46 This suggests that the hormonal disturbances that prevent conception also encourage malignant change in the endometrium. The protective effect of pregnancy applies only to full-term pregnancy.47
Cigarette Smoking
Cigarette smoking reduces the risk of endometrial carcinoma. The effect is limited primarily to women whose disease is detected after menopause and, among these women, current smokers show the greatest reduction in risk, and former smokers are less affected.48 The mechanism whereby cigarette smoking reduces risk is not clear. One study has shown that serum estrogen levels were unaffected but that androstenedione levels were slightly higher in smokers.49 Paradoxically, women with advanced stage endometrial carcinoma (stages II–IV) were more likely to be smokers than women with early stage disease (stages 0–I).50
Endometrioid Adenocarcinoma and Its Variants
Clinical Features
Most tumors develop slowly in the setting of hyperestrogenism against a background of non-atypical endometrial hyperplasia, although some arise in atrophic endometrium.51 Endometrioid carcinoma is predominantly a disease of the sixth and seventh decades and 75% of cases occur after the menopause. Only 5% occur in women less than 40 years old. They are low-grade, non-myoinvasive, associated with a good prognosis and often develop after a long history of anovulatory cycles or estrogen therapy. Endometrial carcinoma rarely occurs in pregnancy.52
Women with endometrioid carcinoma most often present with abnormal vaginal bleeding, which means in the majority of cases postmenopausal bleeding. The fact that they bleed, however, simply means that the tumor is often large or advanced. Smaller carcinomas may be asymptomatic. Surprisingly frequently, asymptomatic tumor is documented in women who have an endometrial biopsy before instituting hormone replacement therapy, or had the tumor discovered initially at autopsy.53 Patients with advanced disease may complain of pelvic pain, which reflects tumor spread.
The diagnosis is made by endometrial biopsy or curettage, but imaging techniques and hysteroscopy are being used more and can play an important role. Outpatient endometrial sampling techniques (Pipelle biopsy) have an excellent diagnostic rate for endometrial carcinoma, similar to that for curettage.54
Gross Features
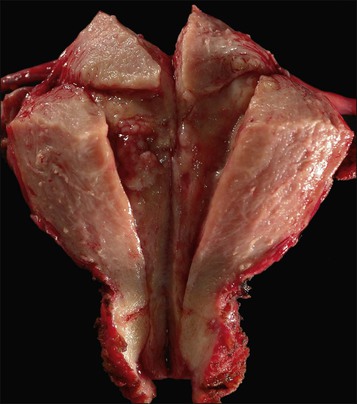
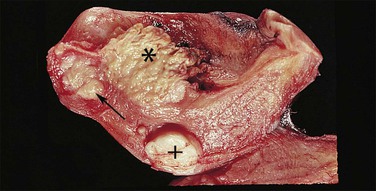
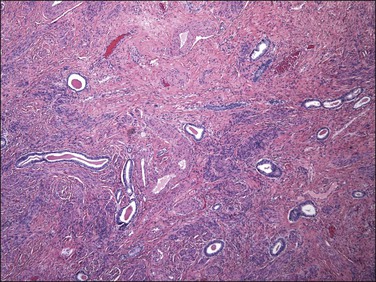
Endometrioid carcinoma can present variously to the naked eye when the uterus is opened. The uterus may be slightly or grossly enlarged but it may be of normal size or even small and atrophic, particularly in a postmenopausal woman. Most tumors arise in the corpus but some originate in the lower uterine segment. The tumor may present as a single mass or there may be multiple separate masses (Figure 18.5) or a diffuse thickening of the endometrium. Carcinomas are situated more frequently on the posterior than the anterior wall. The most common appearance is of an exophytic, rough, perhaps papillary area of the endometrium with a shaggy surface and ulceration (Figure 18.6). Sometimes the tumor is polypoid, with a fairly narrow base. When this is the case, its surface may be smooth and hemorrhagic and the uterine cavity distended, with concomitant thinning of the uterine wall. When the tumor is polypoid, the remaining endometrium usually appears thin. Myometrial invasion may be obvious to the naked eye (Figure 18.6), with either pushing or infiltrating borders, but frequently it is difficult to appreciate the degree of myometrial invasion grossly. There seems to be no correlation between the degree of exophytic growth of the tumor within the uterine cavity and the presence of myometrial invasion.55 However, a tumor diameter of more than 2 cm generally is associated with poorer prognosis and a higher frequency of distant failure.57
Microscopic Features
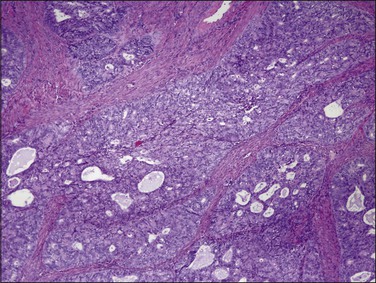
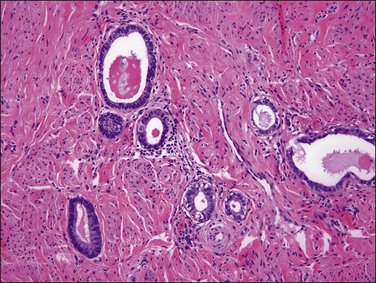
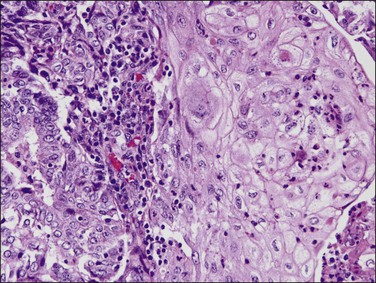
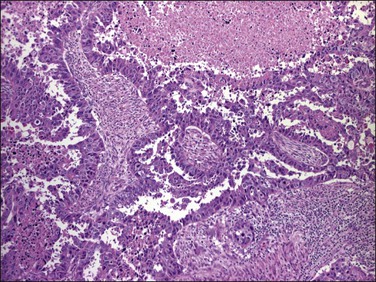
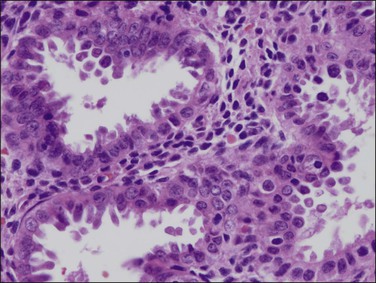
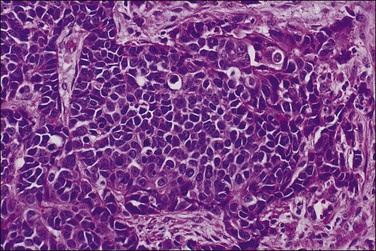
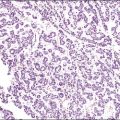
The glandular pattern and cellular features generally resemble the proliferative endometrium. Carcinoma is recognized within the endometrial compartment by the presence of at least one of the following: meandering interconnected lumens formed by folded sheets of neoplastic epithelium (Figure 18.7), irregular angulated and tapering glandular contours (Figure 18.8), a cribriform pattern of the glands (Figures 18.9 and 18.10), or a solid area of glandular epithelium (Figures 18.10 and 18.11). Several features may be present together. These points are summarized in Figure 18.12.
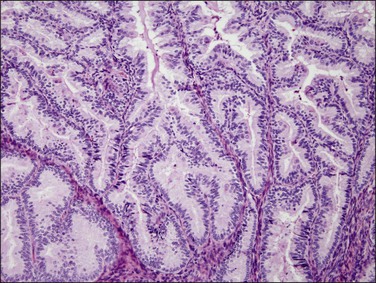
Figure 18.7 Endometrial adenocarcinoma, endometrioid type, grade 1 (well differentiated). The branching glands with maze-like lumens indicate an architecture of folded sheets of neoplastic epithelium, rather than dense packing of tubular structures.
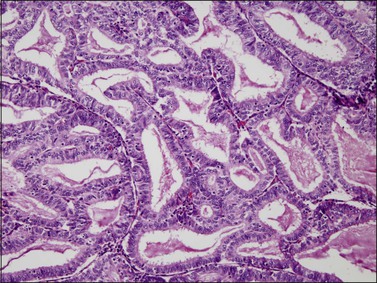
Figure 18.8 Endometrial adenocarcinoma, endometrioid type. Well-differentiated (grade 1) tumor with confluent glands.
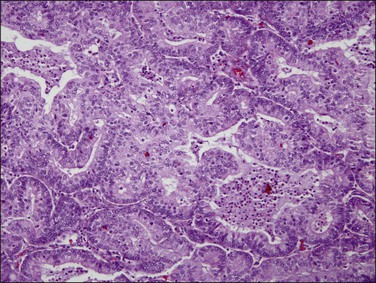
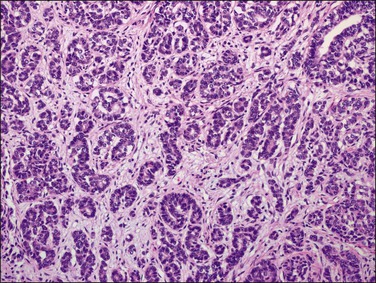
Figure 18.9 Endometrial adenocarcinoma, endometrioid type. Moderately differentiated (grade 2) tumor, with solid and cribriform areas.
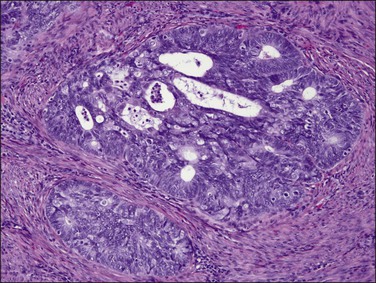
Figure 18.10 Endometrial adenocarcinoma, endometrioid type. Moderately differentiated (grade 2) tumor, with solid and cribriform areas.
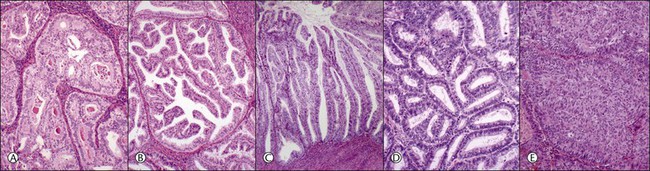
Figure 18.12 (A–E) Intraendometrial adenocarcinoma. Glandular architectural patterns that may be seen in non-myoinvasive areas. (A) Cribriform glands; (B) Maze-like or meandering lumens; (C) Villoglandular; (D) Confluent polygonal molded glands; (E) Solid growth.
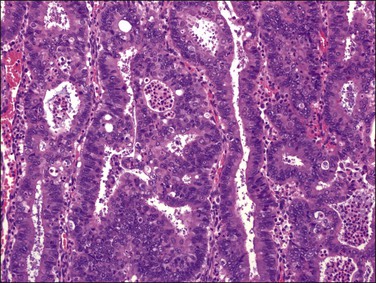
Stratification of epithelial cells is almost always seen. Occasionally, cribriform fragments have a microglandular appearance easily confused with a cervical lesion (Figure 18.13). The individual epithelial cells are larger than would be expected in the proliferative phase. Compared with the normal endometrium, the carcinoma cells have a distinctly altered cytology that varies between cases and even within areas of a single tumor, but may include rounded nuclei, clumped chromatin, and prominent nucleoli. Individual tumors frequently show patchy changes in differentiation to mucinous, squamous, tubal, or other cytologies, and in these cases cytoplasmic as well as nuclear features stand out from the normal background. Some endometrioid adenocarcinomas secrete abundant mucin (‘mucin-rich’ variant), and these may or may not have identifiable intracytoplasmic mucin. Mitotic figures are usually present but may be scanty in well-differentiated tumors.
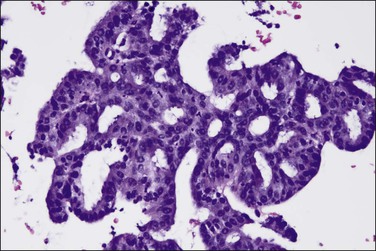
Figure 18.13 Endometrial adenocarcinoma, endometrioid type, with microglandular cribriform architecture. These delicate lesions tend to be exophytic, and may appear highly fragmented in biopsy specimens.
Endometrioid adenocarcinomas, like their precursor EIN lesions, spread within the endometrial compartment by extension of newly formed, well-differentiated neoplastic glands into the adjacent stroma (centripetal growth). In turn, the adjacent endometrial stroma responds by remodeling, rarely showing a desmoplastic change. For this reason, qualitatively assessing the character of the endometrial stroma within the endometrial compartment itself is noncontributory in distinguishing noninvasive from invasive carcinoma.
Foamy histiocytes are commonly seen in the endometrial stroma of patients with a carcinoma. Nearly one-fifth of cases contain stromal cells laden with lipid (Figure 18.14), but there is no correlation between the presence of these cells and the grade of the tumor or the survival of the patient.56 This change is simply a reactive response to tumor cells. The presence of such histiocytic cells in endometrial biopsies showing EIN should always lead to further diagnostic work-up for coexistent carcinoma.
Histologic Grading
The most commonly used histologic grading system is that of the International Federation of Gynecology and Obstetrics (FIGO) and is recommended by the World Health Organization (WHO; Table 18.3).57 This three-tiered grading system is applied to endometrioid adenocarcinomas which are classified, as well differentiated (grade 1), moderately differentiated (grade 2), or poorly differentiated (grade 3). The grading procedure is based on the amount of solid non-squamous areas within a tumor. Squamous components are excluded. Grade 1 tumors (Figure 18.15) show 5% or less solid growth and often there is no solid tumor at all. Grade 2 tumors (Figure 18.16) show solid growth in 6–50% of the tumor. More than 50% solid growth is considered grade 3 (Figure 18.17). Most endometrioid adenocarcinomas are grade 1 or 2.
Table 18.3
Grading of Endometrioid Endometrial Carcinoma (Note: Serous, Clear Cell, and Carcinosarcoma Tumors Are Not Graded)
| Grade 1 | 5% or less of non-squamous solid growth |
| Grade 2 | 6–50% of non-squamous solid growth |
| Grade 3 | More than 50% of non-squamous solid growth |
In tumors with squamous differentiation, grading is based on the glandular component.
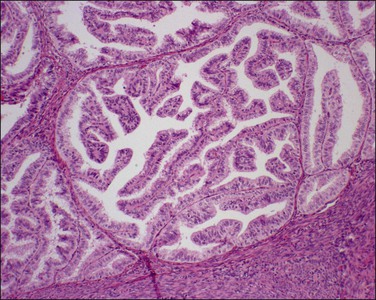
Figure 18.15 Endometrial adenocarcinoma, endometrioid type, grade 1 (well differentiated). Grade 1 tumors have less than 5% solid growth.
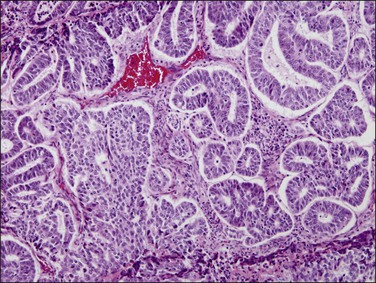
Figure 18.16 Endometrial adenocarcinoma, endometrioid type, grade 2 (moderately differentiated). Grade 2 tumors have between 5% and 50% solid areas.
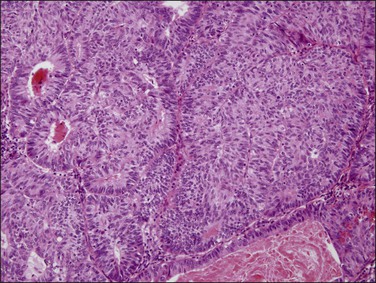
Figure 18.17 Endometrial adenocarcinoma, endometrioid type, grade 3 (poorly differentiated). Grade 3 tumors have more than 50% (non-squamous) solid growth.
Although the FIGO grading system of endometrioid carcinoma relies first and foremost on the architectural pattern of the glands, the histologic grade should be raised by one level beyond that determined by architecture alone in those cases exhibiting severe nuclear atypia (Table 18.458 and Figure 18.18). Usually the architectural and nuclear grades correspond, but, when at variance, the nuclear grade is often the more reliable indicator of prognosis.57 When the final grade is elevated due to severe nuclear atypia, a note should appear in the report so that the clinician is alerted that the patient falls into this special category.
Table 18.4
Nuclear Grading of Endometrial Carcinoma
| Low Grade | High Grade (Severe Atypia) |
| Little variation in shape | Marked variation in shape |
| Little variation in size | Marked variation in size (some markedly enlarged) |
| Hypochromasia | Marked hyperchromasia (may be focal) |
| No variation in staining intensity | Marked variation in staining intensity |
| Evenly distributed chromatin | Coarsely clumped chromatin |
| Nucleoli not prominent | Prominent nucleoli |
| Sparse mitoses | Frequent mitoses with abnormal forms |
Myoinvasion
Myoinvasion is diagnosed when the malignant glands have transgressed the endomyometrial junction and extend into the underlying myometrium. The normal junction, however, is not a straight line, but a rather vague and irregular border. Thus, in many cases, tumors confined to the endometrium cannot be distinguished microscopically from tumors invading the superficial myometrium. In fact, myoinvasion is overdiagnosed in routine practice in as much as 25% of cases; in contrast, failure to diagnose true myoinvasion is extremely rare.59 This is supported by recent FIGO data, which indicate almost identical 5 year survival rates for women with noninvasive tumors and those with tumors invading less than half of the myometrial thickness.59 In contrast, the distinction of inner half invasion from outer half invasion is usually straightforward, and the probability of recurrence is markedly increased for women with deeply invasive tumors. Thus, in the 2009 FIGO staging classification of endometrial carcinoma, tumors with no myometrial invasion and tumors with less than 50% invasion are combined under stage IA.60
Occasionally, endometrial carcinoma appears confined to foci of adenomyosis. Adenomyosis is really a ‘diverticulum’ of endometrium deep into the myometrium, and carcinoma can extend into these foci without invading the myometrium.59 Tumor involvement of adenomyotic foci occurs in about 25% of cases and is not associated with an adverse prognosis.61,62 When tumor cells extend outward into the myometrium, depth of invasion is measured not from the point of transgression in adenomyosis, but from the superficial endomyometrial junction.
The decision to perform pelvic and para-aortic lymph node dissection is largely based on depth of myometrial invasion (also on cell type and histologic grade). Intraoperatively, the pathologist may be requested to assess these features by frozen section.
Maximum depth of myoinvasion is measured in millimeters from the endomyometrial junction, and expressed as the percentage of the total myometrial thickness. Problems arise, however, when the boundary is distorted by the tumor itself or other lesions such as leiomyomas. Residual areas of normal endometrium (Figure 18.19) or overrun normal glands are informative landmarks, when available. Bulky exophytic tumors can be difficult to orient in a single histologic section and, in such cases, assessment of the endomyometrial boundary at the site of deepest invasion requires a combination of gross and histologic evaluation.
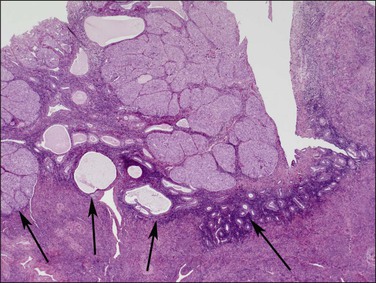
Figure 18.19 This tumor is entirely intraendometrial, but the irregular junction between the endometrium and myometrium imparts a false impression of myoinvasion. Endometrial stroma (arrows) surrounds the tumor.
Myoinvasion is assessed by evaluation of the topographical distribution of glands and the appearance of adjacent stroma.63 Invasion across a broad front (21% of cases) may be difficult to distinguish from an irregular endomyometrial interface unless it can be compared to the adjacent uninvolved endomyometrium. This is sometimes evident by a ‘shoulder’ formed at the juncture of a zone of myoinvasion with an area of surface involvement (Figure 18.20, arrow). Most common (67%) are irregular groups of glands with or without a stromal response (Figure 18.21). ‘Adenomyosis-like’ invasion (7% of cases) (Figure 18.22) can be distinguished from non-myoinvasive tumor extending into foci of adenomyosis (Figures 18.23 and 18.24) by its lack of accompanying endometrial stroma. Unfortunately, CD10 immunoreaction occurs focally in the cells surrounding tumor clusters in the myometrium of women without adenomyosis, and this limits utility. A distinctive pattern of microcystic, elongated or slit-like, and fragmented (MELF) invasive glands is often accompanied by individual invading cells and a loose stromal response with marked inflammation (Figures 18.25 and 18.26). The MELF pattern of invasion has some features of epithelial–mesenchymal transition, including a greatly reduced proliferative activity and acquisition of a cytokeratin (CK)7-positive immunophenotype.64 Least common of all (1%) are widely spaced individual glands lacking any stromal response, akin to adenoma malignum of the endocervix (Figures 18.27 and 18.28).
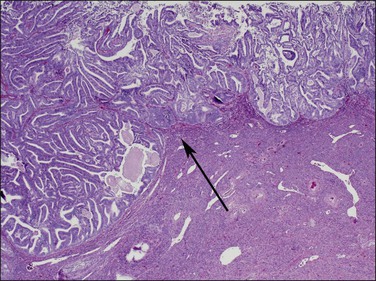
Figure 18.20 Broad front myoinvasion, showing a ‘shoulder’ (arrow) between myoinvasive (left) and surface (right) involvement.
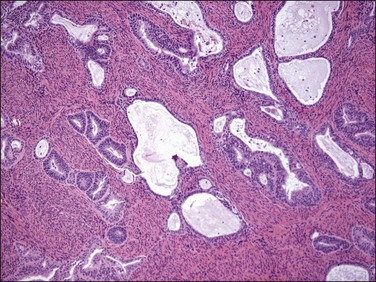
Figure 18.21 Irregular infiltrating glands with no stromal response. (Courtesy of Dr. Marisa R. Nucci.)
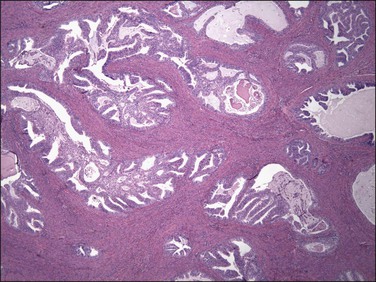
Figure 18.22 Myoinvasion by blunt tumor structures in an ‘adenomyosis-like’ pattern. (Courtesy of Dr. Marisa R. Nucci.)
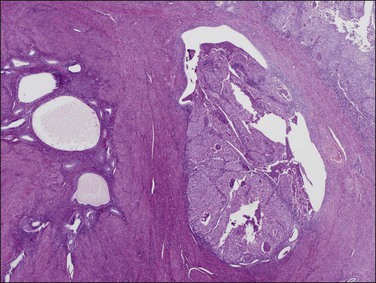
Figure 18.23 Tumor extension into adenomyosis, non-myoinvasive. Two areas of adenomyosis are seen, one with only benign glands (left), and one with extension of adenocarcinoma from the surface (right).
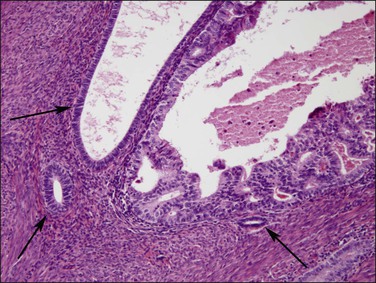
Figure 18.24 Tumor extension into adenomyosis, non-myoinvasive. Detail showing focus of carcinoma and benign glands. A thin layer of intervening stroma is seen.
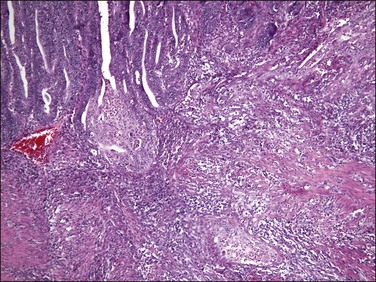
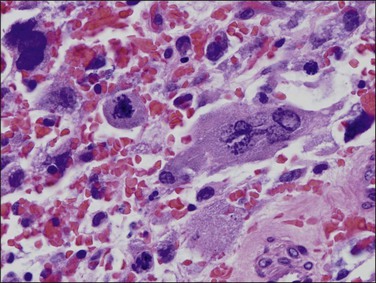
Figure 18.25 Myoinvasion by microcystic elongated (slit-like) glands (MELF). (Courtesy of Dr. Marisa R. Nucci.)
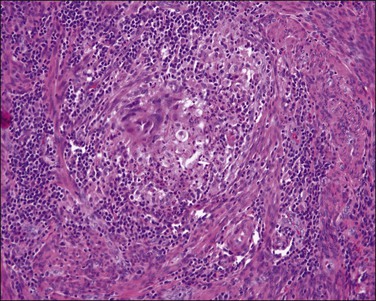
Figure 18.26 Individual invasive cells in MELF-type invasion are eosinophilic or squamous like, and somewhat obscured by the combined stromal and inflammatory response. (Courtesy of Dr. Marisa R. Nucci.)
Precursor Lesions: Endometrial Intraepithelial Neoplasia/Atypical Hyperplasia
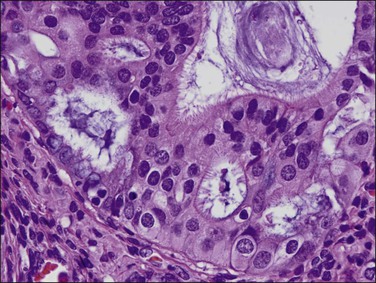
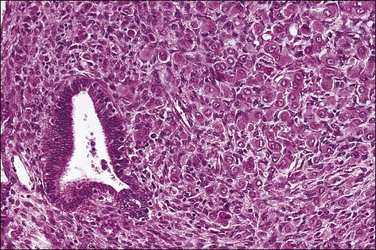
Endometrial intraepithelial neoplasia (also known as atypical hyperplasia or AH) is an immediate precursor to endometrioid endometrial adenocarcinoma, which is discussed at length in Chapter 17. It is a clonal expansion of mutated glands, which can be seen as cytologically altered crowded areas of endometrial glands lacking the architectural (solid, cribriform, maze-like, myoinvasive) characteristics of adenocarcinoma (Figure 18.12). Often residual EIN is present within the endometrium at the time of presentation with carcinoma (Figures 18.29 and 18.30).
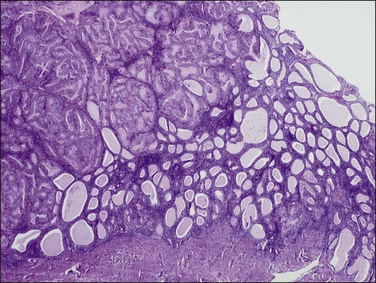
Figure 18.29 Endometrial adenocarcinoma, intraendometrial (left). The endometrium on the right is a premalignant EIN composed of packed round glands.
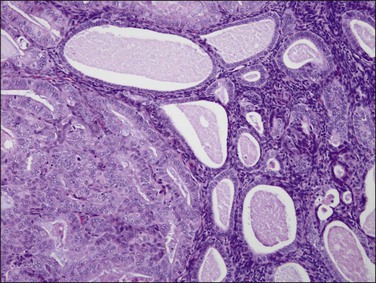
Figure 18.30 Endometrial adenocarcinoma, intraendometrial. Individual tubular glands of EIN are present on the right and adenocarcinoma, with cribriform glands, on the left.
Cytologic Correlation
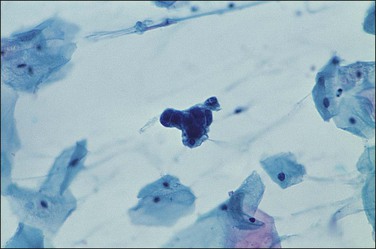
There are no established guidelines for routine endometrial cytologic screening, but exfoliated neoplastic endometrial cells are sometimes encountered in cervical Pap smears. Up to two-thirds of women with endometrial adenocarcinoma have malignant cells in their cervical cytology specimen and high-grade as well as high-stage tumors seem to be detected more frequently.65 In cervical smears, malignant endometrial cells characteristically appear as small clusters with darkly stained nuclei (Figure 18.31) or perhaps as single discrete cells that are easily overlooked. On rare occasions following the recognition of malignant glandular cells in a cervical Pap smear, the endocervical curettage and endometrial biopsy may be negative. The possibility of an ovarian lesion should be considered and excluded.
Transcervical cytology sampling devices such as the Tao brush have been designed to directly access the endometrium.66 With this device malignant cells in an endometrial brushing are freshly removed and rapidly fixed so the features are well preserved. Appearance is widely variable, from large single cells to small clusters as well as sheets (Figure 18.32). Although superior to a cervical Pap smear specimen, transcervical instrumentation with a brush introduces many of the potential morbidities of the Pipelle biopsy while yielding less tissue.
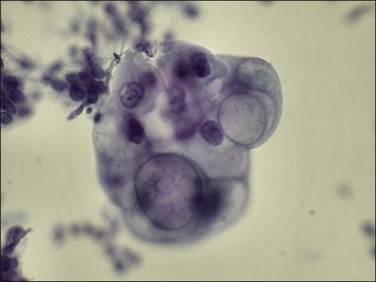
Figure 18.32 Transcervical brush cytology of endometrioid endometrial adenocarcinoma. Carcinoma showing bubble-like mucin-filled cytoplasm. (Courtesy of John Maksem.)
Shed tumor DNA incidentally collected by liquid cervical Pap smear may be sequenced to demonstrate mutations known to be present in a coincident endometrial carcinoma.67 Because the DNA may be shed from anywhere above it is not possible to localize a lesion, nor is the sensitivity and specificity of such a screen known when large numbers of mutations are screened in the asymptomatic patient.
Variants of Endometrioid Carcinoma
Several histologic variants of endometrioid adenocarcinoma are recognized.
Endometrioid carcinoma with squamous differentiation (one-fourth of endometrial adenocarcinomas) display focal squamous differentiation (Figures 18.33 and 18.34). Formerly, the distinction was made between tumors where the squamous component was well or poorly differentiated. The former tumors were called ‘adenoacanthomas’ and the latter ‘adenosquamous carcinomas.’ Several studies have confirmed that prognosis relates largely to the grade of the glandular component. In fact, the glandular component is much easier to grade in a reproducible fashion, and superior in predicting lymph node metastasis and 5 year survival. Therefore, it is recommended that endometrioid carcinomas with squamous epithelium are classified as endometrioid carcinoma with squamous differentiation and graded, on the basis of the glandular component, as well, moderately, or poorly differentiated (grade 1, 2, or 3, respectively). Besides, the clinical features of adenocarcinoma containing squamous epithelium and those of endometrioid adenocarcinoma are essentially the same.
Well-differentiated tumors (grade 1) are composed of glands and squamous nests but usually the glandular component predominates. Squamous epithelium can appear in strips or sheets (Figure 18.34), and when present as oval nests within gland lumens it is referred to as morules. Intercellular bridges and keratin deposits may be seen. The nuclei of the squamous cells are uniform, and lack prominent nucleoli (Figure 18.35). Mitotic figures are rare. In poorly differentiated tumors, the squamous cells show grade 2 or 3 nuclear atypia and are not confined to the lumens of glands. Occasionally, the squamous cells have a spindle morphology, simulating a sarcoma, and may invade the myometrium or vascular spaces. Keratin pearls are often found. Care must be taken in distinguishing between squamous differentiation and solid foci of adenocarcinoma. The undifferentiated epithelial component should be considered glandular unless intercellular bridges are seen or the cells show ample eosinophilic cytoplasm with well-defined borders (Table 18.5).68
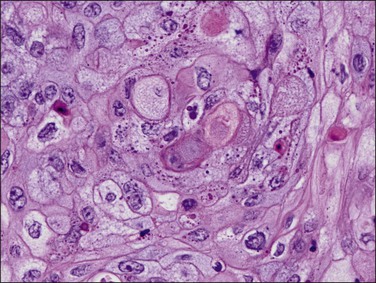
Figure 18.35 Endometrial adenocarcinoma, endometrioid type, with squamous change. High-power view of squamous component.
Foreign-body-type granulomas may form in the peritoneum in response to the keratin component of endometrioid carcinomas with squamous differentiation. These lesions do not seem to affect the prognosis adversely in the absence of viable-appearing tumor cells. The granulomas probably result from transtubal spread of exfoliated necrotic tumor cells.
Mucinous carcinoma in pure form is an uncommon variant of endometrial carcinoma with cells containing prominent intracytoplasmic mucin, resembling the mucinous carcinoma of the endocervix. Mucinous carcinoma constitutes 1–9% of all endometrial carcinomas.69,70 Patients range in age from 47 to 89 years and their clinical features are similar to those of patients with endometrioid carcinoma. Most patients present with stage I disease.
More commonly mucinous endometrial adenocarcinomas arise in conjunction with an endometrioid component and develop in endometrial polyps in approximately one-fourth of cases. A higher frequency of mucinous adenocarcinomas has been reported in patients receiving tamoxifen and synthetic progestogens71 suggesting that there might be a different histogenetic mechanism for this tumor, namely progestogens encouraging mucinous metaplasia.72 The tumors lack distinctive macroscopic features.
Microscopically, the tumors show a glandular architectural pattern or a villoglandular configuration (Figure 18.36). The epithelial cells lining the glands and papillae are tall with basal nuclei, prominent intracytoplasmic mucin, and minimal stratification (Figure 18.37). Nuclear atypia is mild to moderate, and mitotic activity is not prominent. Mucicarmine, periodic acid–Schiff (PAS) and Alcian blue staining can highlight the mucin, but this is rarely necessary for diagnosis. Sometimes, mucinous differentiation is associated with squamous differentiation. Most mucinous carcinomas are well differentiated (grade 1) but grade 2 and grade 3 tumors are occasionally described.69 More likely than not, poorly differentiated tumors lose their ability to produce mucin. Lymph node metastases may be extremely well differentiated.
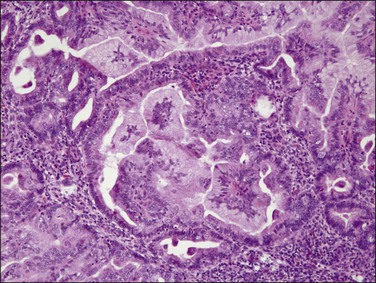
Figure 18.36 Endometrial adenocarcinoma, endometrioid type, with mucinous differentiation. An area of mucinous carcinoma is present in the center.
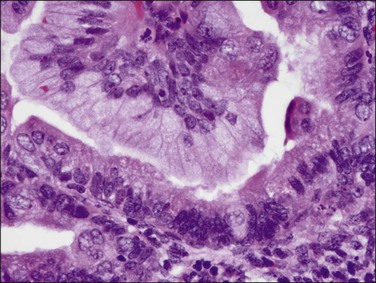
Figure 18.37 Endometrial adenocarcinoma, endometrioid type, with mucinous differentiation. Detail of columnar cells with cytoplasmic mucin.
Sometimes, complex filiform papillary fragments of mucinous endometrial adenocarcinoma are encountered in biopsy specimens (Figure 18.38). An extremely bland cytology, rare mitoses, and tendency to break apart into small fragments make these tumors extremely difficult to recognize, as they resemble endocervical epithelium. The abundance of mucinous epithelium, delicate supportive stroma, and alternating mucinous and non-mucinous differentiation often coexisting with a microglandular cribriform component (Figure 18.39)73 are characteristic of carcinoma.74 At hysterectomy, a non-exophytic tumor component may be found, and this may or may not retain the extensive mucinous differentiation of the surface papillary tumor (Figure 18.40). Minor quantities of mucinous elements in an otherwise typical endometrioid adenocarcinoma do not warrant a diagnosis of mucinous adenocarcinoma. Tumors showing typical endometrioid carcinoma with less than 50% of a mucinous component are best designated as endometrioid carcinomas with mucinous differentiation.
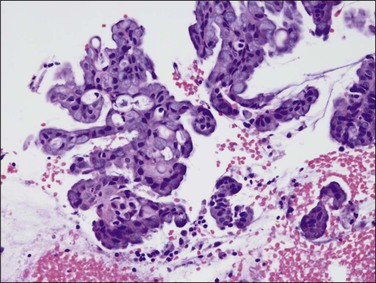
Figure 18.38 Endometrial adenocarcinoma, endometrioid type, with mucinous differentiation. Exophytic fronds of branching mucinous epithelium contain micro cribriform structures.
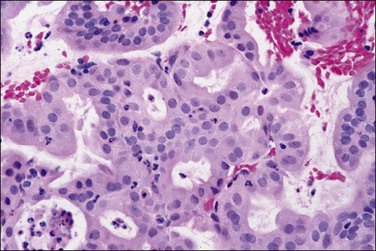
Figure 18.39 Microglandular cribriform architecture in an endometrial adenocarcinoma, endometrioid type, with mucinous differentiation.
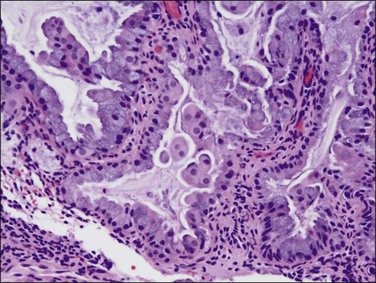
Figure 18.40 Endometrial adenocarcinoma, endometrioid type, with mucinous differentiation. Non-exophytic component of the same tumor as in Figure 18.39 showing a greater degree of endometrioid differentiation and scattered intracytoplasmic mucin.
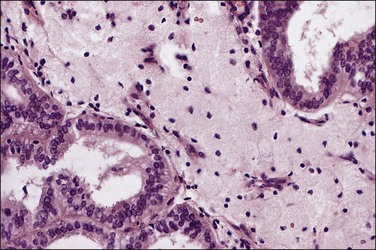
Secretory carcinoma is an uncommon variant of endometrioid adenocarcinoma composed of well-differentiated glands resembling those of early or midsecretory endometrium (Figure 18.41).75 It accounts for only 1–2% of endometrial carcinomas. The most common changes are subnuclear and/or supranuclear vacuolation in unstratified columnar cells usually exhibiting grade 1 nuclei. The secretory appearance may be focal or diffuse, and not infrequently is admixed with endometrioid carcinoma.
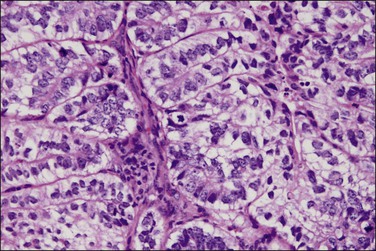
Figure 18.41 Endometrial adenocarcinoma, endometrioid type, with secretory differentiation. This tumor resembles a day 17 secretory endometrium with characteristic and prominent subnuclear vacuoles.
There are two types of secretory carcinoma: those in which the secretory change is induced by circulating progestins, and those in which secretory differentiation is an intrinsic feature of the neoplasm independent of the background hormonal state. The presence or absence of progestin-induced stromal change, and the menopausal status of the patient, distinguishes both types.76
Secretory adenocarcinomas are associated with a good prognosis.68 Treatment is the same as that for endometrioid carcinoma of the same stage and grade.
Ciliated carcinoma is rare.77 Ciliated cells are uncommon in adenocarcinomas of the endometrium but occasionally individual ciliated cells can be found with diligent searching. More rarely, extensive ciliated differentiation is present throughout (Figure 18.42). Only if at least 75% of the tumor cells are ciliated should the tumor be termed a ‘ciliated cell carcinoma.’78 Some well-differentiated ciliated tumors may be difficult to distinguish from premalignant EIN/AH lesions. Thus the diagnosis of ciliated cell carcinoma should be made with caution. In some cases only the presence of myometrial or lymphatic invasion establishes the diagnosis. Ciliated carcinoma has an association with exogenous estrogen treatment. The tumors are well differentiated and have a good prognosis.78
Villoglandular carcinomas are characterized by long, slender, villous papillae with thin fibrovascular cores usually admixed with typical glandular endometrioid carcinoma (Figure 18.43).79 The cytologic features are the same as those of typical endometrioid carcinoma (Figure 18.44). Myometrial invasion usually is superficial. Clinical outcomes are based on grade, and are comparable to those seen for other types of endometrioid adenocarcinomas.
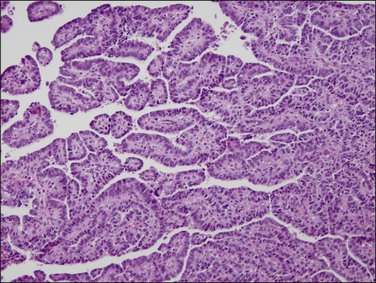
Figure 18.43 Villoglandular architecture in an endometrioid carcinoma, which can be distinguished from its serous counterpart by its simple to pseudostratified rather than exfoliating epithelium.
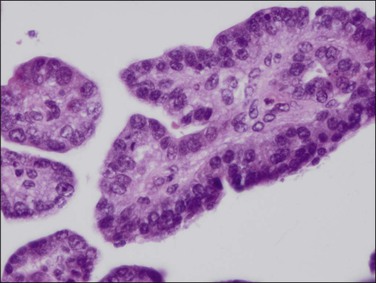
Figure 18.44 Detail of villoglandular endometrioid carcinoma with moderate degree of cytologic atypia. Same case as Figure 18.43.
Endometrioid adenocarcinomas with sertoliform differentiation80 are tumors with areas composed of glands resembling sex cord–stromal tumors. The glands are in the form of closely packed tubules or trabeculae with basally oriented nuclei and clear to fibrillary cytoplasm (Figure 18.45). The non-sertoliform areas of the tumors consist of typical endometrioid adenocarcinoma.
Differential Diagnosis
Well-differentiated endometrioid adenocarcinoma may be difficult to distinguish from EIN/AH. This problem has been discussed in detail in Chapter 17. EIN consists of individual lumen-bearing glands grouped together, whereas a cribriform pattern, solid growth, meandering interconnected lumens, or villoglandular architecture (Figure 18.12) generally are diagnostic of adenocarcinoma.
Myoinvasion clearly favors a diagnosis of carcinoma, but as biopsy and curettage devices rarely sample the myometrial wall it is a criterion only available in hysterectomy specimens and therefore only of use in establishing the final diagnosis. Several groups have systematically examined endometrial biopsy material in women with and without myoinvasive adenocarcinoma to discover features associated with myoinvasion. Variation in nuclear size (anisokaryosis, measured as standard deviation of nuclear diameter) emerged as the single variable most associated with deep myoinvasion. This finding confirms the earlier observation that the presence of extreme nuclear pleomorphism worsens clinical outcome relative to that expected by architectural features alone.58
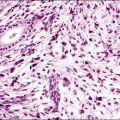
Well-differentiated endometrioid adenocarcinoma with myometrial invasion may be difficult to distinguish from atypical polypoid adenomyoma, particularly in curettings (Figure 18.46). This is an obviously important distinction since most atypical polypoid adenomyomas are treated conservatively. Postmenopausal age of the patient and marked nuclear atypia favor a myoinvasive adenocarcinoma, because atypical polypoid adenomyoma usually shows no more than mild to moderate nuclear atypia. In contrast to the elongated fibers of the normal myometrium, the stromal component of atypical polypoid adenomyoma grows in short interlacing fascicles. Furthermore, in curettings or biopsy from an atypical polypoid adenomyoma, there are usually also fragments of normal background endometrium; in contrast, on a biopsy of an endometrioid adenocarcinoma, it would be most unusual to obtain only fragments of myoinvasive neoplasm without free tumor fragments. Immunohistochemistry is helpful in distinguishing between atypical polypoid adenomyoma and a myoinvasive endometrioid adenocarcinoma; whereas the stromal component of atypical polypoid adenomyoma is CD10 negative, the myoinvasive glands of endometrioid adenocarcinoma are typically surrounded by CD10 immunoreactive stromal cells.81
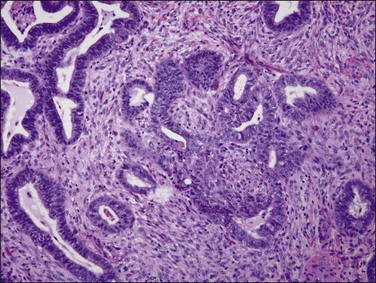
Figure 18.46 Atypical polypoid adenomyoma. Neoplastic glands with squamous morules are offset by a densely muscular stroma.
Although atypical polypoid adenomyoma has an excellent prognosis, there is a risk of recurrence (around 45%) if curettage or polypectomy is undertaken. There is also a small, but definite, risk of transition to endometrioid adenocarcinoma, which has been estimated at 8.8% in one meta-analysis.82
Metaplastic changes as described in Chapter 16 may be seen in benign, premalignant (EIN/AH), or carcinoma states.83 Hormonally induced metaplasias, such as tubal metaplasia resulting from estrogen stimulation, have a distinctive topography in which metaplastic glands are diffusely interspersed among non-metaplastic glands. This differs from the expanding geographic foci of premalignant and malignant neoplastic processes in which the metaplastic component itself may be cohesive and geographic. Even if metaplastic changes are frequently associated with cancer, the benign cytologic features, architectural pattern, and lack of invasion help to make the correct diagnosis of hormonally induced metaplasias.
Poorly differentiated carcinomas composed of extensive solid tumor may show spindled cells and give a ‘pseudosarcomatous’ appearance. Just the presence of recognizable glands does not mean the tumor is biphasic and, in such cases, a diagnosis of carcinosarcoma would be unjustified. Conversely, it is also apparent that the mesenchymal and epithelial elements of carcinosarcomas may both show reactivity with cytokeratins and vimentin.84 Thus, cytokeratin reactivity in the stromal element does not necessarily mean that a carcinomatous component is present in a sarcoma and, in some cases, a distinction is impossible to make.
The interpretation of menstrual endometrium or endometrium with glandular and stromal breakdown in curettings may be difficult, especially in distinguishing it from adenocarcinoma. The absence of stroma between the glandular elements may give the impression of confluent glands or in some cases even of solid epithelial growth. The piled up epithelium may lose nuclear polarity and acquire prominent nucleoli. However, the coarse chromatin and nuclear pleomorphism of malignancy are lacking and a genuine cribriform pattern and invasion are never present. Mitoses are also lacking. Furthermore, the glands of menstrual endometrium regularly exhibit some residual secretory change and a careful search will identify typical areas of stromal fragmentation as opposed to areas of coagulative necrosis (see Chapter 14).
The distinction between endometrial adenocarcinoma and endocervical adenocarcinoma may be difficult, if not impossible, to make in curettings. Fractional curettage assists in delimiting the site of involvement, but surface extension along the lower uterine segment may occur. Even though most endometrial carcinomas are of endometrioid type and most endocervical carcinomas are of mucinous type, endometrioid carcinomas may arise in the cervix and mucinous adenocarcinomas not uncommonly arise in the endometrium. The stroma of the tumor may also help; in cervical adenocarcinomas it is typically fibrous whereas endometrial carcinomas usually contain very little stroma. An immunostain panel of three markers can be used in the differential diagnosis. ER and vimentin are more likely to be expressed in endometrial than cervical adenocarcinomas, whereas cervical adenocarcinomas more frequently show strong immunoreactivity for p16.84,85 In endometrial adenocarcinomas p16 immunostaining is weak or patchy.86 Reactivity for integrated human papillomavirus (HPV) in DNA isolated from tumor tissue strongly supports a cervical origin, as it is present in 80–90% of cervical, and essentially no endometrial, adenocarcinomas.87 Tissue processing and HPV polymerase chain reaction testing for this purpose are not generally available, however.
Behavior and Treatment
Endometrioid adenocarcinoma and its variants present early as postmenopausal bleeding, which is both obvious and alarming. Eighty percent of patients present with clinical stage 1 disease, although some patients are found to have more advanced disease at surgery. Five year survival rates are currently 96% for stage 1 disease, 67% for stage 2, and 23% for stage 3.1 The behavior of the endometrioid histologic variants is the same, grade for grade, as that of typical endometrioid adenocarcinoma.88
The standard treatment is surgical, consisting of hysterectomy with bilateral salpingo-oophorectomy, with or without adjuvant radiotherapy. Practices vary regionally regarding pelvic and para-aortic lymph node dissection. It is doubtful whether lymphadenectomy has any added benefit in the patient whose primary lesion is well differentiated and superficially invasive.89,90 Lymph node dissections are commonly carried out, however, if frozen section of the hysterectomy specimen demonstrates deep myometrial invasion, cervical involvement, or a non-endometrioid histology. Local delivery of high-dose progestins by hormone-impregnated intrauterine devices may be effective in controlling well-differentiated local disease with a low rate of systemic complications.91 Systemic treatment with progestins may be beneficial but, as the tumors that respond are those with ER and PRs that are of early stage and low grade with a favorable outcome anyway, practical use of these agents is limited.92 Chemotherapy and adjuvant radiotherapy are used for advanced and recurrent disease.93,94
Appearances Following Radiation
Curetted specimens may be received following radiation therapy for endometrial carcinoma. Currently, however, this is an infrequent event since radiotherapy is predominantly given postoperatively. The tumor’s histologic response to radiation varies and sometimes no changes can be detected. Changes may be seen in both neoplastic and non-neoplastic epithelial cells. Most prominent among these are nuclear enlargement, pleomorphism, and hyperchromasia, often resulting in markedly bizarre forms (Figure 18.47). As these alterations are seen in both benign and malignant cells, identifying residual carcinoma can be difficult.
Serous carcinoma
Serous carcinoma is an aggressive form of endometrial cancer exhibiting a predominantly papillary architecture composed of exfoliative bulbous hobnail-like cells with marked nuclear atypia.5,95 It is the uniformly severe nuclear atypia that distinguishes serous carcinoma from other less aggressive papillary endometrial carcinomas. Therefore, the term ‘serous carcinoma’ is preferred to ‘papillary’ serous carcinoma.
Clinical Features
The tumor accounts for 1–10% of all endometrial cancers. It is lower in population-based studies but higher in reports from gynecologic oncology centers.95 The patients are generally about 4–10 years older than women with endometrioid carcinomas, rarely have received exogenous estrogen therapy, and lack previous or concurrent EIN or AH.96 Most women are parous (90%). Few are obese (10%) or have diabetes.97 Typically they have normal serum estrogen levels.98
Gross Features
Serous carcinomas generally have the same gross features as endometrioid carcinomas, i.e., exophytic and papillary; however, many tumors appear bulky and necrotic.8 The uterus is more frequently atrophic as compared with endometrioid carcinomas. Not infrequently the tumor is confined to an endometrial polyp.
Microscopic Features
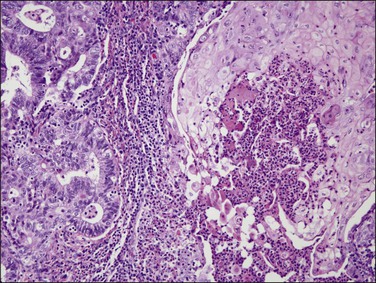
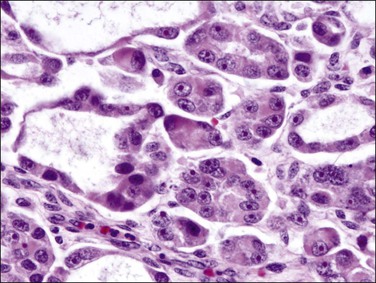
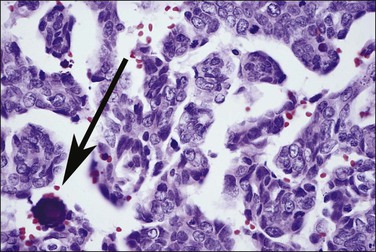
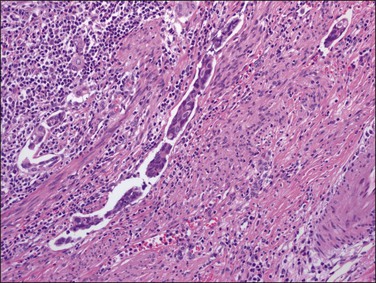
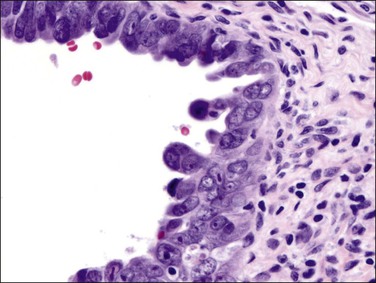
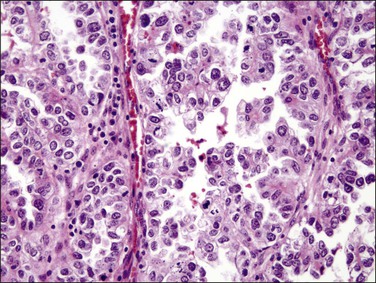
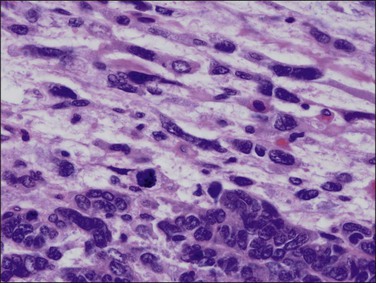
Histologically, serous carcinomas closely resemble the more common ovarian high-grade serous carcinomas (see Chapter 25). They typically have complex, branching papillae with broad, thick fibrovascular cores, but occasionally thin to delicate cores (Figure 18.48). The papillae are covered by a stratified epithelium with a prominent and very characteristic tufting or budding pattern (Figure 18.49), with many groups of detached cells lying free between the papillae (Figure 18.50). This pattern notwithstanding, serous carcinoma may also show a glandular or solid pattern. Unlike endometrioid adenocarcinoma, the glandular structures are irregularly shaped and often lined by polygonal rather than columnar cells.95 The tumor cells are often hobnail and contain abundant granular eosinophilic or clear cytoplasm. The nuclei are usually high grade, with marked pleomorphism and large macronucleoli along with occasional bizarre and hyperchromatic giant nuclei. Mitotic figures are numerous and abnormal mitoses are easily identified. Psammoma bodies are found in about 25% of cases (Figure 18.51).99 A high percentage of cases exhibit striking lymphovascular invasion (Figure 18.52) and deep myometrial invasion.5 The invasive component can show contiguous downgrowth of papillary processes, or solid masses or glands. Serous endometrial carcinomas also have a higher incidence of cervical and lower uterine segment involvement.100 The uninvolved endometrium adjacent to serous carcinoma is atrophic in 76% of the cases and hyperplastic in only 5%.115
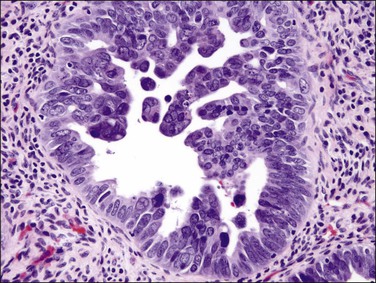
Figure 18.49 Endometrial carcinoma, serous type. High-grade nuclei with exfoliation in a ‘hobnail’ pattern.
Serous carcinoma of the endometrium may occur in a pure form or associated with other types of endometrial carcinoma, such as endometrioid or clear cell adenocarcinoma.8,101
Mixed tumors where at least 25% of the tumor has features of serous differentiation behave as serous cancers and should be treated as such.95 There is less data and thus some controversy regarding how to manage patients with lesser quantities of admixed serous cancer. In these cases, a clear indication of the amount of serous component present should be mentioned in the report. Unlike its ovarian counterpart, serous carcinoma of the endometrium is not graded but considered high grade by definition.102
Immunohistochemistry
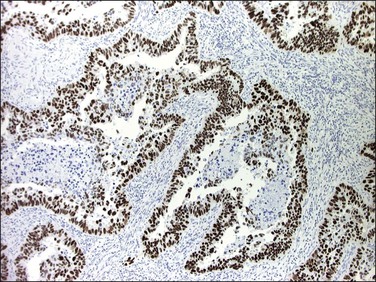
The molecular changes of p53 mutation in 80–90% of serous carcinomas is reflected in p53 immunohistochemistry, which typically shows a diffuse and intense nuclear staining pattern involving almost all tumor cells (Figure 18.53).8,103 Less frequently, p53 frameshift mutations lead to a truncated protein that is not detected by the antibodies, creating a ‘null phenotype’ that can be recognized compared to the low level of intermittent staining seen in adjacent normal tissues that serve as an internal positive control for the stain.103 Ki-67 immunohistochemistry shows a high labeling index (about 40%) in serous carcinoma and ERs and PRs are absent or only weakly expressed in most tumors.104 The PTEN gene is intact, and expressed in normal levels in most serous carcinomas.105 Strong and diffuse p16 immunoreaction occurs in serous carcinomas without implying HPV infection. Like endometrioid adenocarcinoma, serous carcinoma usually expresses epithelial membrane antigen (EMA), CK7, CA125, Ber EP4, B72.3, and vimentin. WT1 expression occurs infrequently (20% of cases).106,107
Serous Endometrial Intraepithelial Carcinoma (EIC)
In over 90% of cases of serous carcinoma, the surface epithelium adjacent to the carcinoma is replaced by one or several layers of markedly atypical cells lying in the vicinity of atrophic endometrial glands (Figure 18.54). This lesion, which has been designated serous EIC, can extend into the underlying glands without apparent stromal invasion. However, the cells of EIC resemble the cells of the adjacent invasive serous tumor and, like them, show strong nuclear immunoreactivity for p53 (Figure 18.55), high Ki-67 index, and loss of ERs and PRs.8 Serous EIC may also be observed in association with clear cell adenocarcinoma.104 Cases of isolated serous EIC without evidence of invasive serous carcinoma are rare, and often confined to endometrial polyps.108 Extensive serous EIC may cover most of the surface and the glands of the endometrium and, thus, hardly be distinguished from minimally invasive serous carcinoma.
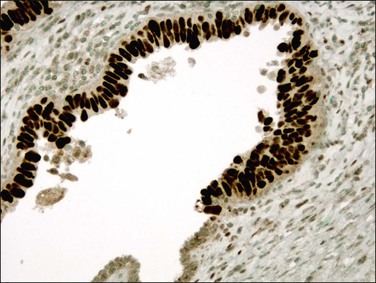
Figure 18.55 Serous EIC. Lesional cells are highlighted on the surface and within overtaken glands by p53 immunohistochemistry.
Even in the absence of local uterine myoinvasion, serous EIC has been associated with metastasis in the ovary, peritoneum, or omentum, probably resulting from implantation of exfoliated tumor cells, which pass through the fallopian tube.109 Thus, serous EIC cannot be considered a precursor lesion but rather an intraepithelial stage of carcinoma capable of spreading to distant sites.
Differential Diagnosis
Serous carcinomas with predominant glandular pattern lacking papillary features can be misinterpreted as high-grade endometrioid carcinoma. However, grade 3 endometrioid carcinoma is almost always solid, not glandular. On the other hand, discohesive solid areas of poorly differentiated endometrioid tumor may develop cleft-like separations that mimic the architecture of serous carcinoma. In these cases, however, the nuclear features help in distinguishing both tumors. The glands and cleft-like fractures in serous carcinoma are lined by cells with high-grade nuclei, often hobnail shaped, whereas the glands in endometrioid carcinoma are lined by columnar cells with grade 1 or 2 nuclei (Figure 18.56). Serous carcinomas almost always (90–95%) have an abnormal p53 immunoreaction, so if this is normal it is unlikely to be serous. Positive p53 staining is not, however, specific for serous cancers, as 40% of poorly differentiated endometrioid carcinomas are p53 abnormal.110
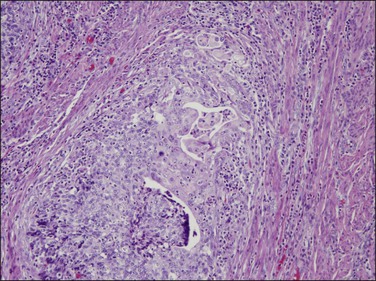
Figure 18.56 Area of invasive poorly differentiated endometrioid carcinoma with separating clefts that resemble serous adenocarcinoma.
Serous carcinoma must be distinguished from its endometrioid counterpart with villoglandular architecture.79 Architecturally, villoglandular adenocarcinoma exhibits long, convoluted glands usually covered by flat columnar epithelium with pseudostratified nuclei (Figure 18.44), whereas serous carcinoma shows plump papillary excrescences with tufting (Figure 18.49). Nuclear atypia is only mild to moderate in villoglandular endometrioid carcinoma compared to the marked nuclear atypia in serous carcinoma.
Aside from mixed carcinomas, overlap of cellular features exists between serous and clear cell carcinomas.95 In particular, hobnail-shaped cells, which are characteristic of clear cell carcinoma, may also occur in serous carcinoma but they are usually larger. In contrast to clear cell carcinomas, serous carcinomas lack a tubulocystic or a solid pattern as well as stromal hyalinization and lack a considerable amount of cells with clear cytoplasm. Compared to clear cell carcinomas, serous carcinomas may also show substantial nuclear polymorphism and frequent mitoses. p53 mutation is infrequent (<10%111) in clear cell carcinomas compared to (>90%) serous carcinomas.112
An artifact that can be confused with serous carcinoma is caused by intracavitary infusion of hypertonic mannitol or electrolyte solutions during uterine hysteroscopy. These nonphysiologic electrolyte solutions can cause exposed epithelia to exfoliate in a hobnail-like manner (Figure 18.57). Hyperchromatic nuclei appear bland and condensed, rather than enlarged with coarse chromatin. Widespread distribution throughout the specimen, along with minimal to absent pleomorphism, is a clue to its benign character.
Behavior and Treatment
The most striking feature of endometrial serous carcinoma is its aggressive behavior leading to a poor overall prognosis. The 5 and 10 year actuarial survival rates for all stages are 36% and 18%, respectively.113 The 5 year survival for pathologic stage I carcinoma is 40%.114
This poor outcome particularly relates to frequent extrauterine disease at the time of diagnosis; 72% of patients whose disease preoperatively appears confined to the uterus already have extrauterine disease found at operation.97 Even minimal myometrial invasion is frequently associated with widespread disease.115 Early clinical studies have shown poor outcome for stage I cases, but this may be due in part to incomplete staging procedure.116 Cancer death may even occur in stage IA disease.117 Unlike endometrioid carcinomas, grade and depth of myometrial invasion of serous carcinomas are not significant predictors for peritoneal spread of disease. Several studies have demonstrated that serous carcinomas that have not invaded the myometrium can be associated with extrauterine disease or recur after the initial treatment.104 Most likely, this reflects spread via tubal reflux and subsequent peritoneal carcinomatosis,118 which is unusual for other forms of endometrial cancer. There is no evidence that endometrial serous carcinoma is a component of a multifocal disease process arising independently in the endometrium, and pelvic and abdominal serosa. On the other hand, spread of serous carcinoma between these sites, including tube and endometrium, has been documented.119 Not surprisingly, a disproportionately large fraction of patients with relapsed endometrial carcinomas have serous carcinoma. Whereas serous carcinomas constitute less than 10% of endometrial carcinomas, they account for about 50% of treatment failures.5
Lymphovascular space invasion, often seen within the myometrium, cervix, parametrium, fallopian tubes, and ovaries, is associated with a high frequency of extrauterine disease. Metastasis to the para-aortic lymph nodes is found in 13% of serous carcinomas confined to the endometrium.104 Similarly, 6 of 10 serous carcinomas discovered in endometrial polyps with little or no myometrial invasions recurred, and four patients died of disease.101
First-line treatment for women with endometrial serous carcinoma is surgery including total abdominal hysterectomy with bilateral salpingo-oophorectomy along with omentectomy and careful surgical staging, including peritoneal cytology and pelvic and para-aortic lymph node sampling. Because this tumor is aggressive, some form of adjunctive therapy is desirable for all tumors except those that qualify as minimal uterine serous carcinoma. In a series of 21 cases with pure serous EIC or minimal serous carcinoma (less than 1 cm of intraendometrial carcinoma) all 14 patients whose tumors lacked myometrial or vascular invasion and who had no evidence of extrauterine disease survived without adjuvant treatment.118 Patients with early stage disease have a survival advantage, underlining the need for precise staging procedures.120
Clear Cell Adenocarcinoma
Clinical Features
Clear cell adenocarcinomas account for 1–6% of endometrial carcinomas and occur at an older age (mean age 65–69 years) than endometrioid adenocarcinoma.121 The women generally are less often obese, less often have diabetes mellitus, and less frequently have taken hormone replacement therapy.122 Like all other forms of endometrial cancer, bleeding is the most common initial clinical manifestation.
Microscopic Features
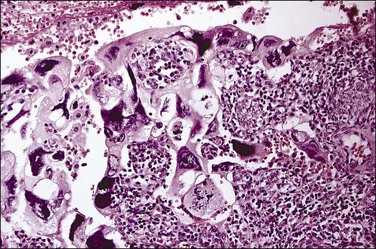
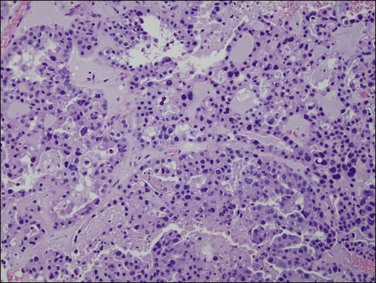
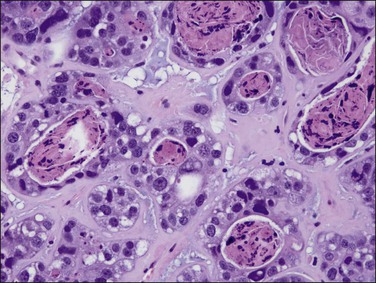
As in other locations along the female genital tract, endometrial clear cell carcinoma may show papillary (Figures 18.58 and 18.59), solid, and tubulocystic (Figure 18.60) patterns. Most clear cell adenocarcinomas have a mixture of at least two of these patterns. The epithelial cells lining the cysts in the tubulocystic areas have discharged their glycogen and have scanty cytoplasm; thus, they show enlarged and pleomorphic nuclei that appear to protrude into the lumens, the hobnail cells (Figure 18.61). Cystic spaces are often lined by flattened cells. However, the most striking feature of the tumor is the clear cytoplasm in many cells (although not necessarily the majority). Nothing in the cytoplasm stains with hematoxylin and eosin (H&E) for the glycogen is leached out during fixation and processing. Mucin, if present, is found only in the lumens of the glands and not in the cell cytoplasm. The stroma may be dense and hyalinized, particularly in tubulocystic areas. The hyalinized stroma is a disorganized type of basement membrane material secreted by tumor cells, which replaces a ‘mucoid’ material that may persist in some areas (Figure 18.60).123 Nuclear atypia is often marked and the tumors show a high nuclear grade. Nucleoli are prominent and the frequency of mitotic figures variable. PAS-positive, diastase-resistant intracellular and extracellular hyaline bodies are found in approximately two-thirds of clear cell carcinomas. Clear cell carcinomas are not graded, as all are considered high grade by definition.102
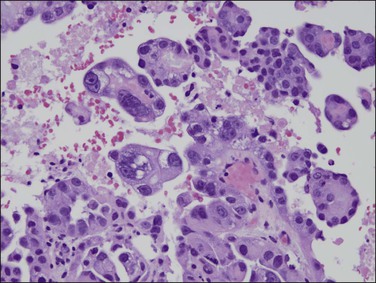
Figure 18.58 Endometrial adenocarcinoma, clear cell type. Papillary pattern with partly hyalinized cores.
Immunohistochemistry
Like endometrioid and serous carcinomas, most clear cell carcinomas express pan-cytokeratins, EMA, CK7, BerEP4, B72.3, CA125, and vimentin, while they are negative for CK20 and WT1. Clear cell carcinomas are typically ER/PR negative and show p53, p16, and Ki-67 expression, which are intermediate between endometrioid and serous carcinomas.
Rare cases associated with the HNPCC syndrome lack expression of MSH-2 and MSH-6.
Differential Diagnosis
Yolk sac tumors, particularly with clear cytology, are rare in the uterus and exceedingly rare in the endometrium.124 In contrast to clear cell carcinoma, which typically occurs in postmenopause, the patients are young, in the third or fourth decade. As Schiller–Duval bodies are seen in only a 20% minority of yolk sac tumors, immunoreaction for α-fetoprotein (AFP) or glypican-3 as well elevated serum AFP are helpful diagnostic tools.
Clear cell adenocarcinomas may at times be exceedingly difficult to distinguish from Arias-Stella change in the endometrium (Figure 18.62), but the distinction is critical as therapy differs significantly. While both show bulbous nuclear enlargement, pleomorphism, and hyperchromasia, there is less pleomorphism within flat areas of epithelium in Arias-Stella. Nuclear chromatin is often smudged in Arias-Stella change, whereas the chromatin material in at least some areas in clear cell adenocarcinoma should be crisp. Other signs are often useful. Arias-Stella change is often associated with decidua and in women who are usually young, whereas clear cell adenocarcinoma may show solid areas composed of clear cells and the patients are invariably past menopause.
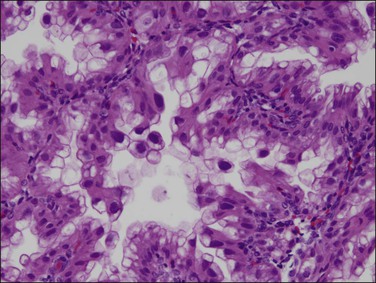
Figure 18.62 Focus of Arias-Stella phenomenon where the cells have pleomorphic, enlarged, and hyperchromatic nuclei that protrude into the glandular lumen, resulting in a ‘hobnail’ appearance. Non-hobnail cells are less pleomorphic. At times, this change is difficult to distinguish from clear cell adenocarcinoma. Mitoses are extremely rare if not absent.
Behavior and Treatment
Clear cell carcinomas are high-grade, invasive tumors that tend to present at an advanced stage. Like serous carcinomas, clear cell carcinomas are more often associated with deep myometrial invasion, lymphovascular space invasion, and pelvic lymph node metastasis than ordinary endometrioid carcinomas. Thus, the prognosis for women with endometrial clear cell adenocarcinoma is poorer than for endometrioid carcinoma, with 5 and 10 year disease-free survival rates reported as 43–68% and 39% respectively.115,125 Two-thirds of patients suffer relapses outside the pelvis. Pathologic stage and age are the two most important prognostic factors.125 Stage I tumors, particularly if confined to an endometrial polyp, are associated with better prognosis.115 Although treatment is primarily surgical, because of the poor prognosis and high nuclear grade of the tumors, adjuvant therapy is often administered.
Mixed Types of Carcinoma
Although arbitrarily chosen, 10% of a minor component is an amount that can readily be recognized and thus a practical and commonly applied guideline for designation as a ‘mixed carcinoma.’ If a diagnosis of mixed carcinoma is made, the components present should always be specified along with their approximate volume percentage. Mixed endometrioid and serous carcinomas containing at least 25% of a serous component behave as pure serous carcinomas.95 However, it is unclear how the presence of 10–25% of tumor elements of an unfavorable histologic type affects prognosis.
Carcinosarcoma
Uterine carcinosarcomas are epithelial malignancies with a malignant mesenchymal component that may include homologous or heterologous sarcomatous elements (Figures 18.63 and 18.64). Long known as malignant mixed müllerian tumors (MMMT) or sometimes as malignant mixed mesodermal tumors, they were renamed carcinosarcomas by the WHO in 2003 to reflect current understanding that they are primarily epithelial tumors that have developed a mesenchymal component mimicking epithelial to mesenchymal transition.126–128 Evidence suggests that carcinosarcomas share some molecular and epidemiologic risk factors with endometrioid-type endometrial carcinoma, including PTEN mutation,129 microsatellite instability,130 obesity, use of exogenous hormones, and nulliparity.131 Carcinosarcomas, however, may demonstrate an expression profile independent of other forms of endometrial cancer,132 and have a worse prognosis than other high-grade endometrial carcinomas.133 Many studies have attempted to document a prognostic effect of the extent or type of heterologous elements, but there is no consistent conclusion.134 One recent study suggested that the effect is stage dependent, with the presence of heterologous elements as an adverse indicator in stage 1 disease.135 Carcinosarcoma is sufficiently distinctive that it might be considered a separate entity of its own rather than a subset within the type 2 class.
Other Types of Endometrial Carcinoma
Undifferentiated Carcinoma
This term, while semantically incorrect, is applied, by common usage, to those neoplasms that are demonstrably epithelial in nature but not otherwise differentiated. Less than 2% of endometrial tumors are classified as undifferentiated carcinomas.88,136 Some of these are of large cell type and may show some attempt at gland formation. These are therefore probably the most anaplastic examples of the tumors described above. The most common tumor considered in the differential diagnosis is grade 3 endometrioid adenocarcinoma with only focal glandular differentiation.
Undifferentiated endometrial carcinomas may contain foci of moderately or well-differentiated endometrioid adenocarcinoma. These tumors have been named ‘de-differentiated endometrial carcinoma.’137 In these neoplasms, the glandular or differentiated components are superficial, whereas the undifferentiated areas are deeper in the endomyometrium and clearly separated the differentiated tumor.
Neuroendocrine carcinomas are classified separately, even though no difference in survival has been shown between large cell and small cell types.136 In addition to a characteristic neuroendocrine histology, positive staining for a neuroendocrine marker (chromogranin, CD56, synaptophysin) should be seen in 10% or more of the tumor.
Small cell carcinomas (Figure 18.65), both with138 and without139 positive neuroendocrine markers, have been reported, some associated with paraneoplastic syndromes.140 The prognosis is poor.
Squamous Cell Carcinoma
Pure squamous cell carcinoma of the endometrium is rare (Figure 18.66). Earlier reports included a high proportion of women who had endometritis and pyometra but this has been less apparent for the last four decades.141 Many cases are associated with benign squamous metaplasia of the endometrium (ichthyosis uteri; Figure 18.67), a condition encountered only in older women, often in association with intrauterine infection. The diagnosis should be made only if there is no evidence of coexistent adenocarcinoma and if careful examination of the cervix excludes a primary tumor in that organ. Where squamous cell carcinoma is present synchronously in both the uterine body and cervix, the assumption has been that the tumor originated in the cervix and spread upward. More recent views suggest that sometimes the opposite may be true.142
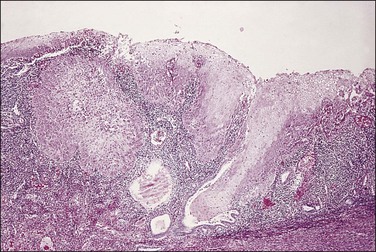
Figure 18.66 Squamous cell carcinoma, arising in a field of squamous metaplasia (‘ichthyosis uteri’).
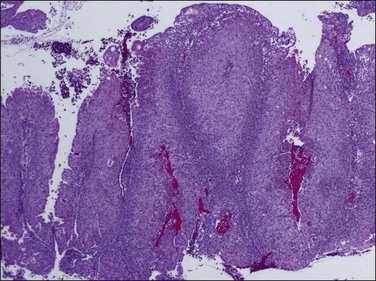
Figure 18.67 Ichthyosis uteri, extensive squamous metaplasia. Most of the endometrium, with the exception of a few basal glandular elements, is replaced by stratified squamous epithelium.
The prognosis of squamous cell carcinoma of the endometrium is exceedingly poor,68 with 26% of reported cases surviving only a median of 9 months after diagnosis.141 Treatment is primarily by surgery. Adjuvant radiotherapy does not improve survival. The addition of cisplatin-based chemotherapy to postoperative radiotherapy may prolong survival in some patients.143
Signet-ring cell carcinoma,144 transitional cell carcinoma,145 glassy cell carcinoma,146 mucinous adenocarcinoma of intestinal type,73,147 and lymphoepithelioma-like carcinoma148 have also been reported.
Another rare variant is endometrial adenocarcinoma with trophoblastic differentiation.149 These tumors are poorly differentiated endometrial adenocarcinomas containing syncytiotrophoblast-like giant cells that are reactive for the β-subunit of human chorionic gonadotropin (hCG), some patients also having raised levels of serum β-hCG.149 The behavior of these tumors is aggressive.
Synchronous Endometrial and Ovarian Carcinoma
A perplexing problem encountered all too often by the practicing pathologist is to determine whether a tumor involving the endometrium and one or both ovaries has arisen in one organ and metastasized to the other(s) or whether the tumors are independent synchronous primaries.150 This distinction can be very difficult when the histologies are similar but not exact, or if the tumor in the second organ occurs at a subsequent time. This distinction, however, is of great clinical importance, due to the significantly better prognosis of patients with synchronous primary tumors.151
Several histologic features help distinguish primary from metastatic tumors in the endometrium and ovaries.152 The presence of a precancerous lesion is strong evidence of in situ genesis. If the cancer is endometrioid, this would include EIN/AH. Serous carcinomas do not have such a definitive counterpart for this purpose, because the presumed precursor lesion (serous EIC) is closely mimicked by secondary surface spread outward from a main tumor mass. Potentially precancerous processes in the ovary, such as endometriosis or a pre-existing benign or borderline tumor of similar histologic type, suggest de novo development of the cancer in the ovary. Disparate histologic types (but not grade) of synchronous endometrial and ovarian tumors are also good evidence of independent primaries. On the other hand, similar histology cannot be taken as evidence of metastasis from one organ to the other. About 15–25% of ovarian tumors with endometrioid histology are associated with a histologically similar lesion in the endometrium. These lesions are usually regarded as well-differentiated independent neoplasms due to their high survival rates.
While many molecular genetic tests are useful, most lack the specificity needed to distinguish primary tumors from metastases. Conservation of highly specific molecular genetic changes, such as unique point mutations, in tumors occurring at different sites supports metastasis of a single primary tumor.153 There are, however, two fundamental barriers in applying this in practice: (1) the most commonly performed tests (immunohistochemistry) examine nonspecific protein endpoints without characterizing more specific underlying genetic changes and (2) independent primary tumors may share genetic alterations that are common for a particular histotype. An excellent example is p53 immunohistochemistry of synchronous ovarian and endometrial serous carcinomas. Irrespective of origin, most of these will abnormally accumulate p53 protein in their nuclei. DNA sequencing to characterize underlying mutations as shared or different is required to resolve them as metastatic (unicentric) versus independent (multicentric) carcinomas. Such specialized, and expensive, sequence confirmation of mutations that serve as clonal ‘markers’ is not routine practice in most pathology departments today. Furthermore, there is the additional complication that metastases may acquire additional changes resulting from tumor progression, thereby diverging from the primary tumor. It must be emphasized, however, that the results of the molecular genetic analyses should always be interpreted in the light of the clinicopathologic findings.
Tumors Metastatic to the Endometrium
The metastatic tumor in the endometrium frequently has a characteristic appearance that enables the primary tumor to be identified. For instance, lobular carcinoma from the breast often presents a characteristic ‘single-cell’ pattern. Metastatic signet-ring cell carcinomas of the stomach or colon are equally characteristic. More often, however, the metastatic tumor cells have no diagnostic features and are diffusely distributed through the stroma (Figure 18.68).
Prognostic Factors in Endometrial Carcinoma
Stage and Depth of Myometrial Invasion
A critical determinant in the outcome of a woman with endometrial carcinoma is the stage of the tumor at the time of diagnosis.154 This is true for all tumor types. The staging system most widely used is that of FIGO as revised in 2009,57 and since adopted by the International Union Against Cancer (UICC).155
Several significant changes were made in the 2009 revision (Table 18.6) including:
Table 18.6
Surgical Staging of Carcinoma of the Corpus Uteri (FIGO 2009)60
| Stage I | Tumor confined to the corpus uteri |
| IA | No or less than half myometrial invasion |
| IB | More than half myometrial invasion |
| Stage II | Tumor invades cervical stroma, but does not extend beyond the uterus |
| Stage III | Local and/or regional spread of the tumor |
| IIIA | Tumor invades the serosa of the corpus uteri and/or adnexaa |
| IIIB | Vaginal and/or parametrial involvementa |
| IIIC | Metastases to pelvic and/or para-aortic lymph nodesa |
| IIIC1 | • Positive pelvic nodes |
| IIIC2 | • Positive para-aortic lymph nodes with or without positive pelvic lymph nodes |
| Stage IV | Tumor invades bladder and/or bowel mucosa, and/or distant metastases |
| IVA | Tumor invasion of bladder and/or bowel mucosa |
| IVB | Distant metastases, including intra-abdominal metastases and/or inguinal lymph nodes |
aPositive cytology should be reported separately in stage II tumors, without changing the stage.
• Stage I disease confined to the corpus. Three tiers of myoinvasion were collapsed to two (1A, 1B), across a 50% myoinvasion threshold.
• Stage II disease extension to cervix uteri. Cervical involvement is considered present only when tumor invades into the cervical stroma. Noninvasive intraglandular/surface extension along the cervical canal is not a scored feature.
• Stage III local and/or regional spread. Positive peritoneal cytologies are no longer part of the staging system, but should be reported separately. Uterine serosal involvement is stage IIIA. Vaginal and/or parametrial extension is stage IIIB. Lymph node involvement (stage IIIC) is stratified by positive pelvic nodes (IIIC1) and positive para-aortic nodes with or without positive pelvic nodes (stage IIIC2).
Precise histologic assessment of myometrial depth of invasion and cervical stromal involvement is crucial for the staging of early disease in FIGO 2009.
Stage IA tumors are those confined to the uterine corpus with up to 50% depth of myoinvasion. This has improved intraoperative assessments, by increasing accuracy (87%) of assessment across a 50% myoinvasion threshold, compared to that (77%) for any invasive disease.156 Patients with more than 50% myometrial thickness invasion, those with stage 1B disease, are at increased risk for extrauterine metastases, including pelvic and para-aortic lymph node metastases. These patients often require more aggressive surgical staging,157 which may include pelvic and para-aortic lymphadenectomy as well as postoperative adjuvant therapy.
Physical displacement of fragmented tumor, either during the surgical procedure or within the pathology laboratory during processing, should not be misinterpreted as tumor spread and a reason to upstage the patient. Detached fragments are commonly squeezed into the fallopian tube lumen or adhere to uterine serosa. Evidence of invasion, encasement of the tumor in reactive exudates, or modeling against the adjacent tissue is all evidence of tumor spread rather than displacement. The introduction of robotic laparoscopic hysterectomy has created new artifacts.158,159 During these procedures a rigid manipulator is inserted through the cervical os and a high-pressure balloon inflated against the endometrial surface to stabilize the device. The resultant forces can cause deep myometrial fractures perpendicular to the endometrial surface (Figure 18.69), through which extruded tumor fragments enter the myometrium and vessels (Figure 18.70), or fallopian tube.
Figure 18.69 Operative myometrial fracture by a robotic manipulator with avulsion of tumor fragments into the artifactual cleft. This well-differentiated carcinoma had no true myoinvasion and was confined to the endometrium.
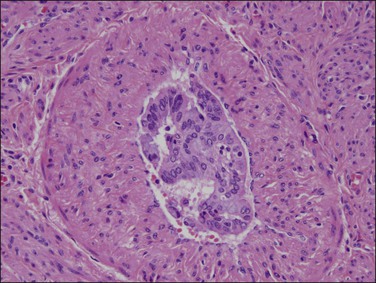
Figure 18.70 Artifactual intravascular extrusion of tumor shards from the myometrial fractures in the same case as in Figure 18.69. The epithelium is torn but not degenerated, and there is no thrombus or adhesion to the endothelial lining.
Lymphovascular Invasion
Vascular and lymphatic channel invasion are seen in approximately one-fifth of endometrial carcinomas and the finding correlates significantly with depth of myometrial invasion and histologic grade.117,160 Patients with lymph node metastasis have a significantly higher incidence of vascular invasion than those without. The presence of vascular invasion has been shown to be an independent indicator for increased risk of recurrence160 and diminished survival in patients with clinical stage I endometrial adenocarcinoma.136 Women with stage I endometrial adenocarcinoma who have vascular invasion are candidates for adjuvant therapy.56
Age
The patient’s age at the time of diagnosis is often a critical factor in determining the prognosis of a patient with endometrial carcinoma.136 Generally, endometrial carcinoma in younger women, particularly before menopause, is associated with a 5 year survival approaching 100%. One study found a 5 year survival of 96% for women aged 40–49 years compared with only 53% for women of 70–79 years.166 Increasing age is associated with a higher grade and stage of endometrioid tumors, and likelihood of a non-endometrioid type tumor, but this only accounts for part of the difference in survival. An additional factor may be that a relative lack of immunocompetence may be more prevalent in older patients.
Steroid Hormone Receptors
Essentially all endometrioid carcinomas are reactive for ERs, whereas PR levels depend on the histologic grade of the tumor; well-differentiated carcinomas generally have higher PR concentrations than poorly differentiated tumors.110,164 There is some evidence that immunohistochemical determination of PRs may identify an independent predictor of clinical course in patients with endometrioid carcinoma, and receptor studies may prove to be of some value to help determine which patients might or might not be suitable for hormonal therapy.162 A multitude of recently described ER and PR subtypes has increased the complexity of possible hormonal responses beyond a simple one-hormone, one-receptor model. Relevance of receptor subtypes to disease course and management is not yet completely understood, and is a subject of ongoing investigation.
The Spread of Endometrial Carcinoma
An alternate route of tumor dissemination is by exfoliated carcinoma cells passing retrograde through the fallopian tube and gaining access to the peritoneal cavity. Intra-abdominal spread of serous carcinomas may occur without any myoinvasion at the primary endometrial site.165 The ovary is involved in 10–15% of advanced cases of endometrial carcinoma. When the ovary contains an appreciable amount of tumor of endometrial pattern, the possibility of a coexistent but separate primary ovarian tumor of endometrioid type must be borne in mind.
Disease distribution at the time of death by disease is similar for endometrioid and non-endometrioid tumor types, with 32% and 40%, respectively, having non-pelvic abdominal disease (liver in 35% of affected cases) and 56% and 51%, respectively, having disease outside the abdomen.165
References
1. Ries, L, Eisner, AG, Kosary, MP, et al. SEER cancer statistics review, 1975–2002. Bethesda, MD: National Cancer Institute; 1975.
2. Jemal, A, Siegel, R, Xu, J, Ward, E. Cancer statistics, 2010. CA Cancer J Clin. 2010; 60:277–300.
3. Sherman, ME, Carreon, JD, Lacey, JV, Jr., Devesa, SS. Impact of hysterectomy on endometrial carcinoma rates in the United States. J Natl Cancer Inst. 2005; 97:1700–1702.
4. Merrill, RM. Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monit. 2008; 14:CR24–CR31.
5. Hendrickson, M, Martinez, A, Ross, J, et al. Uterine papillary serous carcinoma, a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982; 6:93–108.
6. Bokhman, J. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983; 15:10–17.
7. Lax, SF, Kurman, RJ. A dualistic model for endometrial carcinogenesis based on immunohistochemical and molecular genetic analyses. Verh Dtsch Ges Pathol. 1997; 81:228–232.
8. Sherman, ME, Bur, ME, Kurman, RJ. p53 in endometrial cancer and its putative precursors: Evidence for diverse pathways of tumorigenesis. Hum Pathol. 1995; 26:1268–1274.
9. Moreno-Bueno, G, Sanchez-Estevez, C, Cassia, R, et al. Differential gene expression profile in endometrioid and nonendometrioid endometrial carcinoma: STK15 is frequently overexpressed and amplified in nonendometrioid carcinomas. Cancer Res. 2003; 63:5697–5702.
10. Monte, NM, Webster, KA, Neuberg, D, et al. Joint loss of PAX2 and PTEN expression in endometrial precancers and cancer. Cancer Res. 2010; 70:6225–6232.
11. Matias-Guiu, X, Prat, J. Molecular pathology of endometrial carcinoma. Histopathology. 2013; 62:111–123.
12. Cheung, LW, Hennessy, BT, Li, J, et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011; 1:170–185.
13. Matias-Guiu, X, Prat, J. Molecular pathology of endometrial cancer. In: Cheng L, Eble JN, eds. Molecular surgical pathology. New York: Springer, 2013.
14. Brinton, LA, Hoover, RN. Estrogen replacement therapy and endometrial cancer risk: unresolved issues. The Endometrial Cancer Collaborative Group. Obstet Gynecol. 1993; 81:265–271.
15. Grady, D, Gebretsadik, T, Kerlikowske, K, et al. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995; 85:304–313.
16. Pike, MC, Peters, RK, Cozen, W, et al. Estrogen-progestin replacement therapy and endometrial cancer. J Natl Cancer Inst. 1997; 89:1110–1116.
17. Calle, EE, Rodriguez, C, Walker-Thurmond, K, Thun, MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003; 348:1625–1638.
18. Reeves, GK, Pirie, K, Beral, V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007; 335:1134.
19. Lindemann, K, Vatten, LJ, Ellstrom-Engh, M, Eskild, A. Body mass, diabetes and smoking, and endometrial cancer risk: a follow-up study. Br J Cancer. 2008; 98:1582–1585.
20. Adams, TD, Stroup, AM, Gress, RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring). 2009; 17:796–802.
21. Ward, KK, Shah, NR, Saenz, CC, et al. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol Oncol. 2012; 126:176–179.
22. Schmandt, RE, Iglesias, DA, Co, NN, Lu, KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol. 2011; 205:518–525.
23. Cust, AE, Kaaks, R, Friedenreich, C, et al. Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J Clin Endocrinol Metab. 2007; 92:255–263.
24. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004; 19:41–47.
25. Ehrmann, DA. Polycystic ovary syndrome. N Engl J Med. 2005; 352:1223–1236.
26. Park, JC, Lim, SY, Jang, TK, et al. Endometrial histology and predictable clinical factors for endometrial disease in women with polycystic ovary syndrome. Clin Exp Reprod Med. 2011; 38:42–46.
27. Chittenden, BG, Fullerton, G, Maheshwari, A, Bhattacharya, S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online. 2009; 19:398–405.
28. Malmstrom, H, Hogberg, T, Risberg, B, Simonsen, E. Granulosa cell tumors of the ovary: prognostic factors and outcome. Gynecol Oncol. 1994; 52:50–55.
29. Watson, P, Vasen, HFA, Mecklin, JP, et al. The risk of endometrial cancer in hereditary nonpolyposis colorectal cancer. Am J Med. 1994; 96:516–520.
30. Bandera, CA, Boyd, J. The molecular genetics of endometrial carcinoma. Prog Clin Biol Res. 1997; 396:185–203.
31. Renkonen-Sinisalo, L, Butzow, R, Leminen, A, et al. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int J Cancer. 2007; 120:831–834.
32. Sutter, C, Dullenbach-Hellweg, G, Schmidt, D, et al. Molecular analysis of endometrial hyperplasia in HNPCC-suspicious patients may predict progression to endometrial carcinoma. Am J Pathol. 2004; 23:18–25.
33. Hutter, P, Couturier, A, Membrez, V, et al. Excess of hMLH1 germline mutations in Swiss families with hereditary non-polyposis colorectal cancer. Int J Cancer. 1998; 78:680–684.
34. Peel, DJ, Ziogas, A, Fox, EA, et al. Characterization of hereditary nonpolyposis colorectal cancer families from a population-based series of cases. J Natl Cancer Inst. 2000; 92:1517–1522.
35. Zhou, XP, Kuismanen, S, Nystrom-Lahti, M, et al. Distinct PTEN mutational spectra in hereditary non-polyposis colon cancer syndrome-related endometrial carcinomas compared to sporadic microsatellite unstable tumors. Hum Mol Genet. 2002; 11:445–450.
36. Kwon, JS, Scott, JL, Gilks, CB, et al. Testing women with endometrial cancer to detect Lynch syndrome. J Clin Oncol. 2011; 29:2247–2252.
37. Levine, DA, Lin, O, Barakat, RR, et al. Risk of endometrial carcinoma associated with BRCA mutation. Gynecol Oncol. 2001; 80:395–398.
38. Beiner, ME, Finch, A, Rosen, B, et al. The risk of endometrial cancer in women with BRCA1 and BRCA2 mutations. A prospective study. Gynecol Oncol. 2007; 104:7–10.
39. Cohen, I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol. 2004; 94:256–266.
40. Bland, AE, Calingaert, B, Secord, AA, et al. Relationship between tamoxifen use and high risk endometrial cancer histologic types. Gynecol Oncol. 2009; 112:150–154.
41. Hoogendoorn, WE, Hollema, H, van Boven, HH, et al. Prognosis of uterine corpus cancer after tamoxifen treatment for breast cancer. Breast Cancer Res Treat. 2008; 112:99–108.
42. Chin, J, Konje, JC, Hickey, M. Levonorgestrel intrauterine system for endometrial protection in women with breast cancer on adjuvant tamoxifen. Cochrane Database Syst Rev. 2009.
43. McPherson, CP, Sellers, TA, Potter, JD, et al. Reproductive factors and risk of endometrial cancer. The Iowa Women’s Health Study. Am J Epidemiol. 1996; 143:1195–1202.
44. Schlesselman, JJ. Risk of endometrial cancer in relation to use of combined oral contraceptives. A practitioner’s guide to meta-analysis. Hum Reprod. 1997; 12:1851–1863.
45. Escobedo, LG, Lee, NC, Peterson, HB, Wingo, PA. Infertility-associated endometrial cancer risk may be limited to specific subgroups of infertile women. Obstet Gynecol. 1991; 77:124–128.
46. La Vecchia, C, Franceschi, S, Decarli, A, et al. Risk factors for endometrial cancer at different ages. J Natl Cancer Inst. 1984; 73:667–671.
47. Brinton, LA, Berman, ML, Mortel, R, et al. Reproductive, menstrual, and medical risk factors for endometrial cancer: Results from a case-control study. Am J Obstet Gynecol. 1992; 167:1317–1325.
48. Al-Zoughool, M, Dossus, L, Kaaks, R, et al. Risk of endometrial cancer in relationship to cigarette smoking: results from the EPIC study. Int J Cancer. 2007; 121:2741–2747.
49. Austin, H, Drews, C, Partridge, EE. A case-control study of endometrial cancer in relation to cigarette smoking, serum estrogen levels, and alcohol use. Am J Obstet Gynecol. 1993; 169:1086–1091.
50. Daniell, HW. More advanced-stage tumors among smokers with endometrial cancer. Am J Clin Pathol. 1993; 100:439–443.
51. Mutter, GL, Baak, JPA, Crum, CP, et al. Endometrial precancer diagnosis by histopathology, clonal analysis, and computerized morphometry. J Pathol. 2000; 90:462–469.
52. Schneller, JA, Nicastri, AD. Intrauterine pregnancy coincident with endometrial carcinoma: a case study and review of the literature. Gynecol Oncol. 1994; 54:87–90.
53. Horwitz, RI, Feinstein, AR, Horwitz, SM, Robboy, SJ. Necropsy diagnosis of endometrial cancer and detection-bias in case/control studies. Lancet. 1981; 2:66–68.
54. Dijkhuizen, FP, Mol, BW, Brolmann, HA, Heintz, AP. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer. 2000; 89:1765–1772.
55. Mariani, A, Webb, MJ, Keeney, GL, et al. Surgical stage I endometrial cancer: predictors of distant failure and death. Gynecol Oncol. 2002; 87:274–280.
56. Connelly, PJ, Alberhasky, RC, Christopherson, WM. Carcinoma of the endometrium. III. Analysis of 865 cases of adenocarcinoma and adenoacanthoma. Obstet Gynecol. 1982; 59:569–575.
57. Creasman, W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009; 105:109.
58. Zaino, RJ, Kurman, RJ, Diana, KL, Morrow, CP. The utility of the revised International Federation of Gynecology and Obstetrics histologic grading of endometrial adenocarcinoma using a defined nuclear grading system: a Gynecologic Oncology Group study. Cancer. 1995; 75:81–86.
59. Silverberg, SG. Problems in the differential diagnosis of endometrial hyperplasia and carcinoma. Mod Pathol. 2000; 13:309–327.
60. Mutch, DG. The new FIGO staging system for cancers of the vulva, cervix, endometrium and sarcomas. Gynecol Oncol. 2009; 15:325–328.
61. Creasman, WT, Odicino, F, Maisonneuve, P, et al. Carcinoma of the corpus uteri. Int J Gyneol Obstet. 2003; 83:79–118.
62. Hall, JB, Young, RH, Nelson, JH, Jr. The prognostic significance of adenomyosis in endometrial carcinoma. Gynecol Oncol. 1984; 17:32–40.
63. Quick, CM, May, T, Horowitz, NS, Nucci, MR. Low-grade, low-stage endometrioid endometrial adenocarcinoma: a clinicopathologic analysis of 324 cases focusing on frequency and pattern of myoinvasion. Int J Gynecol Pathol. 2012; 31:337–343.
64. Stewart, CJ, Little, L. Immunophenotypic features of MELF pattern invasion in endometrial adenocarcinoma: evidence for epithelial-mesenchymal transition. Histopathology. 2009; 55:91–101.
65. Schorge, JO, Hossein, SM, Hynan, L, Ashfaq, R. ThinPrep detection of cervical and endometrial adenocarcinoma: a retrospective cohort study. Cancer. 2002; 96:338–343.
66. Maksem, JA. Performance characteristics of the Indiana University Medical Center endometrial sampler (Tao Brush) in an outpatient office setting, first year’s outcomes: recognizing histological patterns in cytology preparations of endometrial brushings. Diagn Cytopathol. 2000; 22:186–195.
67. Kinde, I, Bettegowda, C, Wang, Y, et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med. 2013; 5:167ra4.
68. Silverberg, S, Kurman, R. Endometrial carcinoma. Tumors of the uterine corpus and gestational trophoblastic disease. Washington, DC: Armed Forces Institute of Pathology; 1991.
69. Melhem, MF, Tobon, H. Mucinous adenocarcinoma of the endometrium: a clinico-pathological review of 18 cases. Am J Pathol. 1987; 6:347–355.
70. Ross, JC, Eifel, PJ, Cox, RS, et al. Primary mucinous adenocarcinoma of the endometrium. A clinicopathologic and histochemical study. Am J Surg Pathol. 1983; 7:715–729.
71. Dallenbach-Hellweg, G, Hahn, U. Mucinous and clear cell adenocarcinomas of the endometrium in patients receiving antiestrogens (tamoxifen) and gestagens. Int J Gynecol Pathol. 1995; 14:7–15.
72. Cheng, W, Liu, J, Yoshida, H, et al. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med. 2005; 11:531–537.
73. Zaloudek, C, Hayashi, GM, Ryan, IP, et al. Microglandular adenocarcinoma of the endometrium: a form of mucinous adenocarcinoma that may be confused with microglandular hyperplasia of the cervix. Am J Pathol. 1997; 16:52–59.
74. Nucci, M, Crum, CP, Prasad, C, Mutter, GL. Mucinous endometrial epithelial proliferations: a morphologic spectrum of changes with diverse clinical significance. Mod Path. 2000; 12:1137–1142.
75. Clement, PB, Young, RH. Endometrioid carcinoma of the uterine corpus: a review of its pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2002; 9:145–184.
76. Parra-Herran, CE, Monte, NM, Mutter, GL. Endometrial intraepithelial neoplasia with secretory differentiation: diagnostic features and underlying mechanisms. Mod Pathol. 2013.
77. Lax, SF, Pizer, ES, Ronnett, BM, Kurman, RJ. Comparison of estrogen and progesterone receptor, Ki-67, and p53 immunoreactivity in uterine endometrioid carcinoma and endometrioid carcinoma with squamous, mucinous, secretory, and ciliated cell differentiation. Hum Pathol. 1998; 29:924–931.
78. Hendrickson, MR, Kempson, RL. Ciliated carcinoma–a variant of endometrial adenocarcinoma: a report of 10 cases. Int J Gynecol Pathol. 1983; 2:1–12.
79. Zaino, RJ, Kurman, RJ, Brunetto, VL, et al. Villoglandular adenocarcinoma of the endometrium: a clinicopathologic study of 61 cases: a gynecologic oncology group study. Am J Surg Pathol. 1998; 22:1379–1385.
80. Eichhorn, JH, Young, RH, Clement, PB. Sertoliform endometrial adenocarcinoma: a study of four cases. Am J Pathol. 1996; 15:119–126.
81. Ohishi, Y, Kaku, T, Kobayashi, H, et al. CD10 immunostaining distinguishes atypical polypoid adenomyofibroma (atypical polypoid adenomyoma) from endometrial carcinoma invading the myometrium. Hum Pathol. 2008; 39:1446–1453.
82. Heatley, MK. Atypical polypoid adenomyoma: a systematic review of the English literature. Histopathology. 2006; 48:609–610.
83. Carlson, JW, Mutter, GL. Endometrial intraepithelial neoplasia is associated with polyps and frequently has metaplastic change. Histopathology. 2008; 53:325–332.
84. McCluggage, WG. Recent advances in immunohistochemistry in gynaecological pathology. Histopathology. 2002; 40:309–326.
85. Alkushi, A, Irving, J, Hsu, F, et al. Immunoprofile of cervical and endometrial adenocarcinomas using a tissue microarray. Virchows Arch. 2003; 442:271–277.
86. Ansari-Lari, MA, Staebler, A, Zaino, RJ, et al. Distinction of endocervical and endometrial adenocarcinomas—immunohistochemical p16 expression correlated with human papillomavirus (HPV) DNA detection. Am J Surg Pathol. 2004; 28:160–167.
87. Moreira, MA, Longato-Filho, A, Taromaru, E, et al. Investigation of human papillomavirus by hybrid capture II in cervical carcinomas including 113 adenocarcinomas and related lesions. Int J Gynecol Cancer. 2006; 16:586–590.
88. Sherman, ME, Silverberg, SG. Advances in endometrial pathology. Clin Lab Med. 1995; 15:517–543.
89. Dowdy, SC, Borah, BJ, Bakkum-Gamez, JN, et al. Prospective assessment of survival, morbidity, and cost associated with lymphadenectomy in low-risk endometrial cancer. Gynecol Oncol. 2012; 127:5–10.
90. Kitchener, H, Swart, AM, Qian, Q, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009; 373:125–136.
91. Montz, FJ, Bristow, RE, Bovicelli, A, et al. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol. 2002; 186:651–657.
92. Randall, TC, Kurman, RJ. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol Surv. 1997; 90:434–440.
93. Shaeffer, DT, Randall, ME. Adjuvant radiotherapy in endometrial carcinoma. Oncologist. 2005; 10:623–631.
94. Vaidya, AP, Littell, R, Krasner, C, Duska, LR. Treatment of uterine papillary serous carcinoma with platinum-based chemotherapy and paclitaxel. Int J Gynecol Cancer. 2006; 16(Suppl 1):267–272.
95. Sherman, ME, Bitterman, P, Rosenshein, NB, et al. Uterine serous carcinoma. A morphologically diverse neoplasm with unifying clinicopathologic features. Am J Surg Pathol. 1992; 16:600–610.
96. Hendrickson, MR, Longacre, TA, Kempson, RL. Uterine papillary serous carcinoma revisited. Gynecol Oncol. 1994; 54:261–263.
97. Goff, BA, Kato, D, Schmidt, RA, et al. Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol Oncol. 1994; 54:264–268.
98. Sherman, ME, Sturgeon, S, Brinton, LA, et al. Risk factors and hormone levels in patients with serous and endometrioid uterine carcinomas. Mod Pathol. 1997; 10:963–968.
99. Demopoulos, RI, Genega, E, Vamvakas, E, et al. Papillary carcinoma of the endometrium: morphometric predictors of survival. Am J Pathol. 1996; 15:110–118.
100. Longacre, TA, Chung, MH, Jensen, DN, Hendrickson, MR. Proposed criteria for the diagnosis of well-differentiated endometrial carcinoma. A diagnostic test for myoinvasion. Am J Surg Pathol. 1995; 19:371–406.
101. Silva, EG, Jenkins, R. Serous carcinoma in endometrial polyps. Mod Pathol. 1990; 3:120–128.
102. Tavassoli, FA, Stratton, MR. Tumors of the breast and female genital organs. Lyons, France: IARC Press; 2003.
103. Lax, SF, Kendall, B, Tashiro, H, et al. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer. 2000; 88:814–824.
104. Lax, SF, Pizer, ES, Ronnett, BM, Kurman, RJ. Clear cell carcinoma of the endometrium is characterized by a distinctive profile of p53, Ki-67, estrogen, and progesterone receptor expression. Hum Pathol. 1998; 29:551–558.
105. Darvishian, F, Hummer, AJ, Thaler, HT, et al. Serous endometrial cancers that mimic endometrioid adenocarcinomas: a clinicopathologic and immunohistochemical study of a group of problematic cases. Am J Surg Pathol. 2004; 28:1568–1578.
106. Acs, G, Pasha, T, Zhang, PJ. WT1 is differentially expressed in serous, endometrioid, clear cell, and mucinous carcinomas of the peritoneum, fallopian tube, ovary, and endometrium. Int J Gynecol Pathol. 2004; 23:110–118.
107. Goldstein, NS, Uzieblo, A. WT1 immunoreactivity in uterine papillary serous carcinomas is different from ovarian serous carcinomas. Am J Clin Pathol. 2002; 117:541–545.
108. Zheng, W, Khurana, R, Farahmand, S, et al. p53 immunostaining as a significant adjunct diagnostic method for uterine surface carcinoma: precursor of uterine papillary serous carcinoma. Am J Surg Pathol. 1998; 22:1463–1473.
109. Baergen, RN, Warren, CD, Isacson, C, Ellenson, LH. Early uterine serous carcinoma: clonal origin of extrauterine disease. Am J Pathol. 2001; 20:214–219.
110. Lax, SF. Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Arch. 2004; 444:213–223.
111. Okuda, T, Otsuka, J, Sekizawa, A, et al. p53 mutations and overexpression affect prognosis of ovarian endometrioid cancer but not clear cell cancer. Gynecol Oncol. 2003; 88:318–325.
112. An, HJ, Logani, S, Isacson, C, Ellenson, LH. Molecular characterization of uterine clear cell carcinoma. Mod Pathol. 2004; 17:530–537.
113. Abeler, VM, Kjorstad, KE. Serous papillary carcinoma of the endometrium: a histopathological study of 22 cases. Gynecol Oncol. 1990; 39:266–271.
114. Carcangiu, ML, Chambers, JT. Uterine papillary serous carcinoma: a study on 108 cases with emphasis on the prognostic significance of associated endometrioid carcinoma, absence of invasion, and concomitant ovarian carcinoma. Gynecol Oncol. 1992; 47:298–305.
115. Carcangiu, ML, Chambers, JT. Early pathologic stage clear cell carcinoma and uterine papillary serous carcinoma of the endometrium: comparison of clinicopathologic features and survival. Int J Gynecol Pathol. 1995; 14:30–38.
116. Gitsch, G, Friedlander, ML, Wain, GV, Hacker, NF. Uterine papillary serous carcinoma: a clinical study. Cancer. 1995; 75:2239–2243.
117. Carcangiu, ML, Tan, LK, Chambers, JT. Stage IA uterine serous carcinoma: a study of 13 cases. Am J Surg Pathol. 1997; 21:1507–1514.
118. Snyder, MJ, Bentley, R, Robboy, SJ. Transtubal spread of serous adenocarcinoma of the endometrium: an underrecognized mechanism of metastasis. Int J Gynecol Pathol. 2006; 25:155–160.
119. Jarboe, EA, Miron, A, Carlson, JW, et al. Coexisting intraepithelial serous carcinomas of the endometrium and fallopian tube: frequency and potential significance. Int J Gynecol Pathol. 2009; 28:308–315.
120. Kato, DT, Ferry, JA, Goodman, A, et al. Uterine papillary serous carcinoma (UPSC): a clinicopathologic study of 30 cases. Gynecol Oncol. 1995; 59:384–389.
121. Abeler, VM, Kjorstad, KE. Clear cell carcinoma of the endometrium: a histopathological and clinical study of 97 cases. Gynecol Oncol. 1991; 40:207–217.
122. Kanbour-Shakir, A, Tobon, H. Primary clear cell carcinoma of the endometrium: a clinicopathologic study of 20 cases. Int J Gynecol Pathol. 1991; 10:67–78.
123. Kato, N, Takeda, J, Fukase, M, Motoyama, T. Hyalinized stroma in clear cell carcinoma of the ovary: how is it formed? Hum Pathol. 2012; 43:2041–2046.
124. Spatz, A, Bouron, D, Pautier, P, et al. Primary yolk sac tumor of the endometrium: a case report and review of the literature. Gynecol Oncol. 1998; 70:285–288.
125. Abeler, VM, Vergote, IB, Kjorstad, KE, Trope, CG. Clear cell carcinoma of the endometrium. Prognosis and metastatic pattern. Cancer. 1996; 78:1740–1747.
126. Fujii, H, Yoshida, M, Gong, ZX, et al. Frequent genetic heterogeneity in the clonal evolution of gynecological carcinosarcoma and its influence on phenotypic diversity. Cancer Res. 2000; 60:114–120.
127. Silverberg, SG, Mutter, GL, Kurman, RJ, et al. Tumors of the uterine corpus: epithelial tumors and related lesions. In: Tavassoli FA, Stratton MR, eds. WHO classification of tumors: pathology and genetics of tumors of the breast and female genital organs. Lyons, France: IARC Press; 2003:221–232.
128. Castilla, MA, Moreno-Bueno, G, Romero-Perez, L, et al. Micro-RNA signature of the epithelial-mesenchymal transition in endometrial carcinosarcoma. J Pathol. 2011; 223:72–80.
129. Amant, F, de la, Rey M, Dorfling, CM, et al. PTEN mutations in uterine sarcomas. Gynecol Oncol. 2002; 85:165–169.
130. Taylor, NP, Zighelboim, I, Huettner, PC, et al. DNA mismatch repair and TP53 defects are early events in uterine carcinosarcoma tumorigenesis. Mod Pathol. 2006; 19:1333–1338.
131. Inthasorn, P, Carter, J, Valmadre, S, et al. Analysis of clinicopathologic factors in malignant mixed Mullerian tumors of the uterine corpus. Int J Gynecol Cancer. 2002; 12:348–353.
132. Maxwell, GL, Chandramouli, GV, Dainty, L, et al. Microarray analysis of endometrial carcinomas and mixed mullerian tumors reveals distinct gene expression profiles associated with different histologic types of uterine cancer. Clin Cancer Res. 2005; 11:4056–4066.
133. Vaidya, AP, Horowitz, NS, Oliva, E, et al. Uterine malignant mixed mullerian tumors should not be included in studies of endometrial carcinoma. Gynecol Oncol. 2006; 103:684–687.
134. Lopez-Garcia, MA, Palacios, J. Pathologic and molecular features of uterine carcinosarcomas. Semin Diagn Pathol. 2010; 27:274–286.
135. Ferguson, SE, Tornos, C, Hummer, A, et al. Prognostic features of surgical stage I uterine carcinosarcoma. Am J Surg Pathol. 2007; 31:1653–1661.
136. Abeler, VM, Kjorstad, KE. Endometrial adenocarcinoma in Norway. A study of a total population. Cancer. 1991; 67:3093–3103.
137. Silva, EG, Deavers, MT, Bodurka, DC, Malpica, A. Association of low-grade endometrioid carcinoma of the uterus and ovary with undifferentiated carcinoma: a new type of dedifferentiated carcinoma? Int J Gynecol Pathol. 2006; 25:52–58.
138. van Hoeven, KH, Hudock, JA, Woodruff, JM, Suhrland, MJ. Small cell neuroendocrine carcinoma of the endometrium. Am J Pathol. 1995; 14:21–29.
139. Huntsman, DG, Clement, PB, Gilks, CB, Scully, RE. Small-cell carcinoma of the endometrium. A clinicopathological study of sixteen cases. Am J Surg Pathol. 1994; 18:364–375.
140. Campo, E, Brunier, MN, Merino, MJ. Small cell carcinoma of the endometrium with associated ocular paraneoplastic syndrome. Cancer. 1992; 69:2283–2288.
141. Kennedy, AS, Demars, LR, Flannagan, LM, Varia, MA. Primary squamous cell carcinoma of the endometrium: a first report of adjuvant chemoradiation. Gynecol Oncol. 1995; 59:117–123.
142. Dalrymple, JC, Russell, P. Squamous endometrial neoplasia-are Fluhmann’s postulates still relevant? Int J Gynecol Cancer. 1995; 5:421–425.
143. Sorosky, JI, Kaminski, PF, Kreider, J, et al. Endometrial squamous cell carcinoma following whole pelvic radiation therapy: response to carboplatin. Gynecol Oncol. 1995; 57:426–429.
144. Mooney, EE, Robboy, SJ, Hammond, CB, et al. Signet-ring cell carcinoma of the endometrium: a primary tumor masquerading as a metastasis. Am J Pathol. 1997; 16:169–172.
145. Fukunaga, M, Ushigome, S. Transitional cell carcinoma of the endometrium. Histopathology. 1998; 32:284–286.
146. Hachisuga, T, Sugimori, H, Kaku, T, et al. Glassy cell carcinoma of the endometrium. Gynecol Oncol. 1990; 36:134–138.
147. McCluggage, WG, Roberts, N, Bharucha, H. Enteric differentiation in endometrial adenocarcinomas: a mucin histochemical study. Int J Gynecol Pathol. 1995; 14:250–254.
148. Vargas, MP, Merino, MJ. Lymphoepitheliomalike carcinoma: an unusual variant of endometrial cancer. A report of two cases. Am J Pathol. 1998; 17:272–276.
149. Bradley, CS, Benjamin, I, Wheeler, JE, Rubin, SC. Endometrial adenocarcinoma with trophoblastic differentiation. Gynecol Oncol. 1998; 69:74–77.
150. Zaino, R, Whitney, C, Brady, MF, et al. Simultaneously detected endometrial and ovarian carcinomas—a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study. Gynecol Oncol. 2001; 83:355–362.
151. Liu, Y, Li, J, Jin, H, et al. Clinicopathological characteristics of patients with synchronous primary endometrial and ovarian cancers: a review of 43 cases. Oncol Lett. 2013; 5:267–270.
152. Robboy, SJ, Datto, MB. Synchronous endometrial and ovarian tumors: metastatic disease or independent primaries? Hum Pathol. 2005; 36:597–599.
153. Irving, JA, Catasus, L, Gallardo, A, et al. Synchronous endometrioid carcinomas of the uterine corpus and ovary: alterations in the beta-catenin (CTNNB1) pathway are associated with independent primary tumors and favorable prognosis. Hum Pathol. 2005; 36:605–619.
154. Kato, T, Watari, H, Endo, D, et al. New revised FIGO 2008 staging system for endometrial cancer produces better discrimination in survival compared with the 1988 staging system. J Surg Oncol. 2012; 106:938–941.
155. Ugaki, H, Kimura, T, Miyatake, T, et al. Intraoperative frozen section assessment of myometrial invasion and histology of endometrial cancer using the revised FIGO staging system. Int J Gynecol Cancer. 2011; 21:1180–1184.
156. Ali, A, Black, D, Soslow, RA. Difficulties in assessing the depth of myometrial invasion in endometrial carcinoma. Am J Pathol. 2007; 26:115–123.
157. Inoue, Y, Obata, K, Abe, K, et al. The prognostic significance of vascular invasion by endometrial carcinoma. Cancer. 1996; 78:1447–1451.
158. Krizova, A, Clarke, BA, Bernardini, MQ, et al. Histologic artifacts in abdominal, vaginal, laparoscopic, and robotic hysterectomy specimens: a blinded, retrospective review. Am J Surg Pathol. 2011; 35:115–126.
159. Delair, D, Soslow, RA, Gardner, GJ, et al. Tumoral displacement into fallopian tubes in patients undergoing robotically assisted hysterectomy for newly diagnosed endometrial cancer. Int J Gynecol Pathol. 2013; 32:188–192.
160. Gal, D, Recio, FO, Zamurovic, D, Tancer, ML. Lymphvascular space involvement—a prognostic indicator in endometrial adenocarcinoma. Gynecol Oncol. 1991; 42:142–145.
161. Satyaswaroop, PG, Mortel, R. Sex steroid receptors in endometrial carcinoma. Gynecol Oncol. 1993; 50:278–280.
162. Fukuda, K, Mori, M, Uchiyama, M, et al. Prognostic significance of progesterone receptor immunohistochemistry in endometrial carcinoma. Gynecol Oncol. 1998; 69:220–225.
163. Kerner, H, Sabo, E, Friedman, M, et al. An immunohistochemical study of estrogen and progesterone receptors in adenocarcinoma of the endometrium and in the adjacent mucosa. Int J Gynecol Cancer. 1995; 5:275–281.
164. Matias-Guiu, X, Catasus, L, Bussaglia, E, et al. Molecular pathology of endometrial hyperplasia and carcinoma. Hum Pathol. 2001; 32:569–577.
165. Barlin, JN, Wysham, WZ, Ferda, AM, et al. Location of disease in patients who die from endometrial cancer: a study of 414 patients from a single institution. Int J Gynecol Cancer. 2012; 22:1527–1531.
166. Connelly, PJ, Alberhasky, RC, Christopherson, WM. Carcinoma of the endometrium. III. Analysis of 865 cases of adenocarcinoma and adenoacanthoma. Obstet Gynecol. 1982; 59:569–575.
167. Ambros, RA, Sherman, ME, Zahn, CM, et al. Endometrial intraepithelial carcinoma: a distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol. 1995; 26:1260–1267.
168. Berchuck, A, Boyd, J. Molecular basis of endometrial cancer. Cancer. 1995; 76(Suppl):2034–2040.
169. Mutter, GL. PTEN, a protean tumor suppressor. Am J Pathol. 2001; 158:1895–1898.
170. Kounelis, S, Kapranos, N, Kouri, E, et al. Immunohistochemical profile of endometrial adenocarcinoma: a study of 61 cases and review of the literature. Mod Pathol. 2000; 13:379–388.
171. Faquin, WC, Fitzgerald, JT, Lin, MC, et al. Sporadic microsatellite instability is specific to neoplastic and preneoplastic endometrial tissues. Am J Clin Pathol. 2000; 113:576–582.
172. Esteller, M, Levine, R, Baylin, SB, et al. MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene. 1998; 17:2413–2417.