Endobronchial ultrasound
(CONSULTANT-LEVEL EXAMINATION)
Overview
Convex probe endobronchial ultrasound-guided transbronchial needle aspiration is used for diagnosing (and staging) intrathoracic lymphadenopathy in lung cancer,1 other intrathoracic tumors,2 and lymphadenopathy from extrathoracic malignancies.3 The aspiration yield for benign causes of intrathoracic lymphadenopathy is less than with cancer, mostly because of lack of tissue architecture in cytology specimens, but sarcoidosis, and bacterial, mycobacterial, and fungal infections can be diagnosed.4–6 High-frequency endobronchial ultrasound (EBUS), using a radial scanning probe, is used to diagnose peripheral pulmonary nodules7 and to define airway wall structures in tracheal stenosis, tracheobronchomalacia, excessive dynamic airway collapse, and tumor invasion.8–10
Extraluminal vascular structures (superior vena cava, azygos vein, main pulmonary artery and its branches, ascending and descending aorta, aortic arch, pulmonary veins, and left atrium) are visualized using convex probe EBUS (see Figure 25-5 in the imaging case box). Bronchoscopists can assess volume status, pericardial fluid, or the presence of pulmonary embolism.11,12 Thoughtful resource use for intensive care unit (ICU) admissions13 warrants that EBUS should be considered in critically ill patients with suspected lung, mediastinal, cardiovascular, and hemodynamic disorders. This chapter illustrates potential applications of EBUS and EBUS–transbronchial needle aspiration (TBNA) in patients admitted to the ICU or undergoing general anesthesia. Moreover, we suggest algorithms built on the strengths of EBUS diagnostic abilities, its minimally invasive nature, and its proven safety profile for the noncritically ill patient setting (based on our own institutional experience).
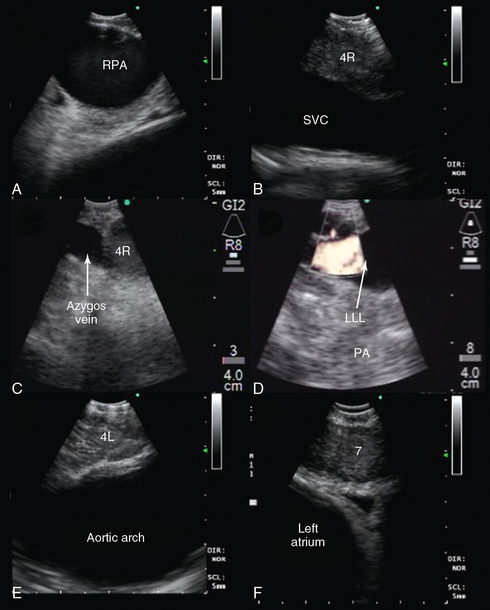
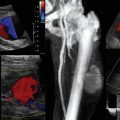
Figure 25-5 Convex probe endobronchial ultrasound (EBUS) visualization of mediastinal vascular structure. After introduction of the bronchoscope via the endotracheal tube (ETT), the right lower lobe bronchus is approached. The transducer is turned 90 degrees to the lateral wall to visualize the right lower lobe pulmonary artery (PA). Pulling back the bronchoscope, the take-off of the middle lobe PA is examined. With the transducer oriented anteriorly, the right pulmonary artery (RPA) is visualized crossing the right main bronchus (A), and the right upper lobe PA take-off is seen if the transducer is oriented anterolaterally near the main carina. After turning the bronchoscope medially, the pulmonary trunk is inspected. Above the RPA, with the transducer oriented anterolateral and pulled back, the superior vena cava (SVC) is examined (B). Continuing to turn the bronchoscope laterally, the azygos vein is visualized (C). The investigation of the left side also starts in the lower lobe bronchus after turning the bronchoscope to the lateral wall to visualize the left lower lobe (LLL) PA (D) and, after pulling back the bronchoscope and orienting it anteromedial, the left upper lobe PA. The left PA is visualized behind the lateral wall of the left main bronchus up to the left lower paratracheal region; continuing to pull back and orient the transducer laterally, the aortic arch is encountered (E). Subcarinal region with pulmonary veins and left atrium is visualized either from the left or right main bronchi when the transducer is oriented medially (F).
Intrathoracic lymphadenopathy
Many patients admitted to the ICU demonstrate enlarged intrathoracic lymph nodes on computed tomography (CT) scans performed for a variety of reasons. Sampling lymph nodes could assist in management. For example, patients with hypoxemic respiratory failure and parenchymal or interstitial infiltrates may have associated lymphadenopathy of variable significance. Although a patient with pneumonia likely has reactive lymphadenopathy, needle aspiration could reveal the actual organism. Patients with sarcoidosis and anthracosis have characteristic findings on EBUS-TBNA specimens (granulomatous reaction and anthracotic histiocytes, respectively).4,14
EBUS-TBNA can make the diagnosis of primary lung cancer or extrathoracic malignancy, metastatic to mediastinal, and hilar lymph nodes. It allows staging or restaging of patients with known or suspected malignancy.1–3 This information is used to redefine level of care, ICU priority scores, and guide treatment. A patient with cancer on mechanical ventilation, for example, may be at risk for transbronchial or transthoracic lung biopsies. EBUS-TBNA can make a diagnosis of progressive disease, infections, or granulomatoid reaction (Figure 25-1).
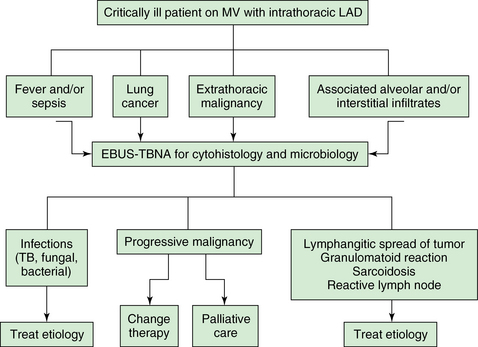
Figure 25-1 Proposed algorithm for using (convex probe) endobronchial ultrasound (EBUS) in critically ill patients with intrathoracic lymphadenopathy. A No. 8.5 to 9 endotracheal tube (ETT) is used to facilitate passage of the EBUS bronchoscope (outer diameter [OD], 6.9 mm) and prevent auto–peak end-expiratory pressure (PEEP). LAD, Lymphadenopathy; MV, mechanical ventilation; TB, tuberculosis; TBNA, transbronchial needle aspiration.
Extrinsic central airway obstruction, pulmonary artery compression, and superior vena cava syndrome
Extrinsic compression is often amenable to interventional procedures, such as airway stent insertion,15 so that patients with respiratory failure on mechanical ventilation may be weaned from ventilators.16 Bronchoscopy and EBUS-TBNA are performed for the dual purpose of diagnosing the central airway obstruction (CAO) (Figure 25-2) and making a cytohistologic diagnosis by sampling impinging masses. Doppler mode is useful to assess the vascularity of the mass and suggests tumor engorgement with potential worsening airway obstruction in the supine position or hypervolemic state. A rapid diagnosis allows prompt referral for stent insertion and systemic therapy. A patient with a large mediastinal cyst could even be drained17 rather than be referred for open surgery.
Figure 25-2 Potential role of endobronchial ultrasound (EBUS) in critically ill patients with mediastinal masses (arrows). A, Large pretracheal mass compressing the lower trachea and main carina. B, Near-complete obstruction in the lower trachea and proximal mainstem bronchi because of extrinsic compression and excessive dynamic airway collapse. C, Large (3 cm in anteroposterior diameter), heterogeneous mass confirmed to be small cell lung cancer (SCLC) on rapid on-site cytology examination (ROSE). D, Proposed use of EBUS and white light bronchoscopy (WLB) in patients with mediastinal masses requiring mechanical ventilation. CTX, Chemotherapy; EBRT, external beam radiotherapy; MV, mechanical ventilation; PA, pulmonary artery; PAH, pulmonary arterial hypertension; SVC, superior vena cava; TBNA, transbronchial needle aspiration; *Tracheal wall invasion by a primary lung tumor defines a T4 and thus at least stage IIIB lung cancer.
** Surgical treatment is considered for cases of large mediastinal cysts compressing the airway and not treatable by other minimally invasive techniques. For lung cancer, surgery is considered if the tumor is deemed resectable after complete staging AND if the patient is operable. ***In general, CTX and EBRT are offered once patient’s functional status improves after liberation from mechanical ventilation.
Expiratory central airway collapse, tracheal stenosis, and airway wall tumor invasion
Expiratory central airway collapse (ECAC) is a reported cause of hypercarbic respiratory failure as well as inability to wean from mechanical ventilation. Diagnosis can be made with white light bronchoscopy (WLB), but assessment of airway wall structures requires visualization in cross section (Figure 25-3). A high-frequency (20 MHz) radial probe, used through a bronchoscope with a working channel of 2.8 mm, allows identification of hypoechoic and hyperechoic layers of the airway wall and identifies cartilage abnormalities in diffuse malacia, excessive dynamic airway collapse (EDAC), and tracheal stenosis.8–10 Cartilage integrity in ECAC, its fracture in posttracheostomy or postintubation stenosis, its invasion by extraluminal tumor, and precise identification of cross-sectional extent of hypertrophic stenotic tissues in tracheal stenosis can impact management. For instance, a patient with ECAC-related respiratory failure might have excessive dynamic airway collapse on WLB but no evidence of cartilage abnormality. This should prompt a search for causes of obstructive ventilatory impairment resulting from small airway disease/parenchymal changes.18 On the other hand, a patient with diffuse malacia, from relapsing polychondritis, for example,10 may warrant stent placement to restore airway patency (see Figure 25-3). A patient with cartilage invasion by primary lung tumor might warrant systemic chemoradiotherapy, whereas in tracheal stenosis, if the cartilage is destroyed, the risk of recurrence is high after laser-assisted or balloon dilation. EBUS findings might accelerate appropriate referrals in each of these instances (see Figure 25-3).
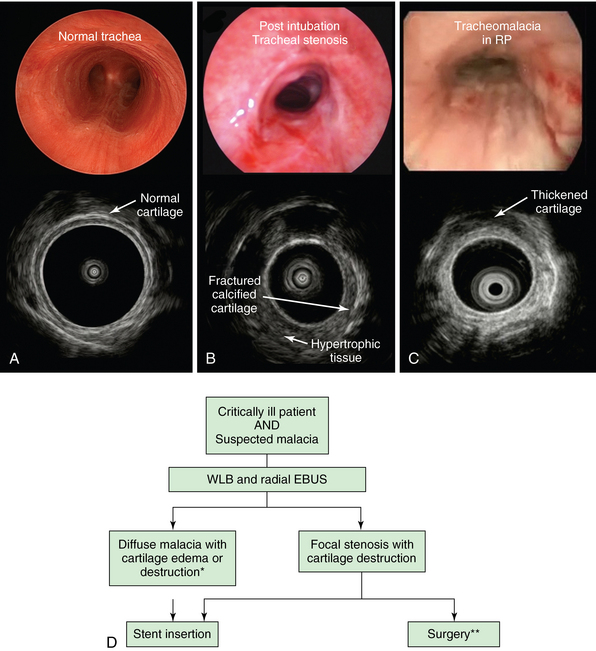
Figure 25-3 Potential role of high-frequency endobronchial ultrasound (EBUS) (radial probe) in critically ill patients with suspected malacia. A, Normal tracheal lumen on white light bronchoscopy (WLB) and on EBUS; the sonographic image (bottom) showed a thin, intact cartilaginous wall. B, WLB and EBUS in posttracheostomy tracheal stenosis; although WLB shows circumferential airway lumen narrowing, only ultrasonographic imaging reveals extent of hypertrophic stenotic tissues and disruption of cartilage. C, WLB shows diffuse circumferential tracheomalacia and mucosal edema in a patient with relapsing polychondritis (RP); EBUS shows cartilage thickening. D, Proposed algorithm for use of radial EBUS in conjunction with WLB in patients with suspected malacia. In diffuse malacia resulting from cartilage abnormality, stent insertion may be warranted. *Surgery for diffuse expiratory central airway collapse (ECAC) is offered to patients with cartilage weakness and crescent-type malacia, but not to those with diffuse cartilage collapse as seen in RP. In patients with tracheal stenosis, laser-assisted mechanical dilation has a high recurrence rate if cartilage is disrupted. These patients benefit from surgical referral for sleeve resection.
**Nonsurgical candidates may benefit from restoring airway patency with indwelling airway stents.
Peripheral pulmonary nodules
In critically ill patients, peripheral pulmonary nodules may be caused by primary or metastatic malignancy, or fungal (i.e., coccidioidomycosis, invasive pulmonary aspergillosis, cryptococcus, histoplasmosis), mycobacterial (i.e., tuberculosis), or bacterial septic emboli. They are also seen in patients admitted to the ICU with diagnoses of autoimmune disease, granulomatosis with polyangiitis, or rheumatoid arthritis. If tissue diagnosis is needed, patients on mechanical ventilation are at high risk for pneumothorax (PTX) from transbronchial lung biopsy or CT-guided fine-needle aspiration (FNA). Furthermore, there are dangers transporting critically ill patients outside the ICU. Radial probe EBUS has a diagnostic yield of 50% to 80%, depending on the clinician’s ability to visualize the nodule, the size of the nodule, presence of air bronchus sign on CT scan, the nodule’s distance from the hilum, and the position of the probe in relation to the nodule.19 Compared with CT-guided FNA, lower rates of PTX and bleeding are described.7
Pulmonary embolism
Central pulmonary embolism (PE) (defined as thrombus in the pulmonary trunk or a main or a lobar PA) is diagnosed using contrast-enhanced chest computed tomography (chest CT angiogram). Acute PE with hemodynamic instability, contrast allergy, or severe renal dysfunction may occur and often preclude intravenous contrast administration. Bedside transthoracic echocardiography (TTE) has low sensitivity for confirming PE, although it might reveal right ventricular dysfunction. Pulmonary angiograms or ventilation-perfusion scans require transport to radiology, which is risky in unstable patients. Because the majority of patients who succumb from PE die within 2 hours from the onset of symptoms (and those who do survive may succumb from recurrent PE), a firm diagnosis is warranted. Visualization of pulmonary arteries by using convex probe EBUS is possible because depth of penetration is approximately 5 cm. Vessels run adjacent to the central airways and there is no interposition of ventilated lung parenchyma between airways and vessels.12 In a prospective, multicenter pilot study of 32 patients with CT angiogram-confirmed, hemodynamically stable PE, CT angiogram documented 101 PEs, of which 97 (96%) were also detected by EBUS.12 EBUS missed only 4 emboli (1 in a middle lobe and 3 in a left upper lobe artery). There were no EBUS-related complications. The entire main pulmonary trunk, main PAs, the lower lobe arteries, and at least 10 mm of the upper lobe and middle lobe arteries can be examined in just a few minutes (Figure 25-4A and B, see Figure 25-1D). Whether EBUS might worsen hemodynamic instability because of increased intrathoracic pressures during bronchoscopy is unknown. A large endotracheal tube (No. 8.5-9) should probably be used to avoid expiratory flow limitation, which might lead to intrinsic peak end-expiratory pressure (PEEP) and worsening hypotension. Future research in hemodynamically unstable patients may be hampered by the fact that diagnostic bronchoscopy is generally contraindicated in patients with ongoing cardiac ischemia or severe refractory hypoxemia, both of which can occur in the setting of acute massive PE.
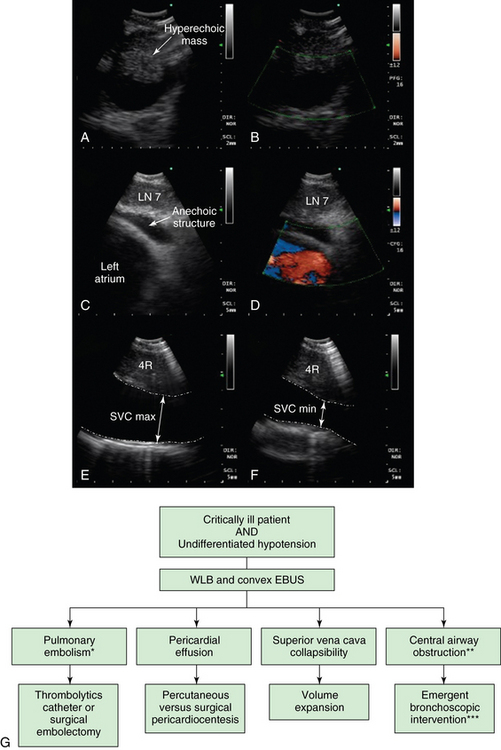
Figure 25-4 Potential role of convex probe endobronchial (EBUS) in undifferentiated hypotension. (Images A and B, C and D, and E and F represent different patients.) A, Hyperechogenic mass inside a tubular anechoic structure resembling EBUS description of a thrombus inside a pulmonary artery. B, In this case, however, the structure imaged was a subcarinal node, and Doppler mode showed that the anechoic signal was not a vessel. EBUS–transbronchial needle aspiration (TBNA) showed recurrent squamous cell carcinoma. C, Anechoic layer surrounding the heart in a patient undergoing EBUS-TBNA for subcarinal lymphadenopathy (LAD) (lymph node [LN] 7). D, Doppler mode confirms presence of fluid around the left atrium (fluid is Doppler negative, heart is Doppler positive). E, Superior vena cava (SVC) maximum diameter measured during EBUS and during expiratory phase (patient on positive pressure ventilation during EBUS-TBNA). F, SVC minimum diameter during inspiration is measured and the SVC collapsibility index calculated. G, Diagram illustrates potential role of convex probe EBUS with white light bronchoscopy (WLB) for diagnosis of undifferentiated hypotension. Pulmonary embolism (PE), pericardial effusions, and volume status may be assessed when computed tomography (CT) angiogram, transthoracic echocardiogram (TTE), or transesophageal echocardiogram (TEE) is nondiagnostic or not feasible. *PE may be missed in lobar and segmental airways. **Central airway obstruction (CAO) can cause hypotension through ball-valve mechanism, leading to auto–peak end-expiratory pressure (PEEP) and increased intrathoracic pressure. ***The type of intervention depends on the nature of obstruction (e.g., dilation and stent for extrinsic compression, laser- or electrosurgery-assisted debulking for exophytic obstruction, foreign bodies, mucous plugs, or blood clots removal).
Pericardial effusion
EBUS allows visualization of the middle mediastinum and images pericardial effusion around the left atrium (see Figure 25-4). Although most pericardial disorders are diagnosed by TTE or transesophageal echocardiography (TEE), in case of acute hemodynamic instability during general anesthesia or in the ICU, EBUS could be useful because it allows assessment of pulmonary vessels, SVC, and portions of the pericardium. If TTE is suboptimal (obesity, chest deformity, hyperinflation while on positive-pressure ventilation, emphysema, subcutaneous edema or emphysema, lack of patient cooperation, inability to rotate the patient for optimal scanning, presence of surgical dressings) and TEE is not readily available, EBUS can be used to visualize posterior cardiac structures in great detail. A patient with hypotension in whom tamponade is suspected might thus warrant EBUS when TTE is nondiagnostic and/or not readily available. We have seen patients in whom pericardial effusion around the left atrium is clearly identified (see Figure 25-4C and D). In theory, when tamponade physiology affects the left atrium (LA), the respiratory variation in LA chamber size and diastolic collapse could be detected. The single scanning plane and limited chamber visualization, however, limit the benefit of EBUS in patients with pericardial disease. Relevant to patients in the operating room under general anesthesia or in the ICU on positive-pressure ventilation is that a reversed sequence of intrathoracic pressure occurs during the respiratory cycle. Intrathoracic pressure is higher during inspiration, and therefore venous return is smaller. Further experience is needed to better understand anatomic structures in scanning planes before EBUS can be recommended for cardiac assessment.
Volume status
The assessment of fluid responsiveness is relevant to managing patients with acute hypotension. If patients on mechanical ventilation have no significant respiratory variation in the inferior vena cava (IVC) or SVC diameter, it is unlikely that cardiac output will increase with volume expansion, which could actually be harmful. Whereas the diameter of the IVC at the junction with right atrium is measured using the subcostal view on TTE, the SVC diameter measurement is usually performed by TEE.20 Measurement of IVC diameter is less predictive than SVC diameter because the former is unreliable in patients with high intraabdominal pressures, whereas the latter directly reflects the relationship between central blood volume and intrathoracic pressures. Maximal and minimal SVC diameter (measured at the top of the right atrium) are observed during respiration.20 Studies using TEE demonstrate that the collapsibility index of the SVC, defined as [(maximum diameter on expiration − minimum diameter on inspiration)/(maximum diameter on expiration)], is highly predictive of fluid responsiveness. Results show that in septic patients on mechanical ventilation, an SVC collapsibility index above 36% predicted an increase in cardiac index after volume expansion of more than 11%, (specificity 100% and sensitivity 90%).20 We observed that cyclic increase in intrathoracic pressure can induce partial or complete collapse of the vessel seen by EBUS, when the SVC is visualized with the scope in the lower trachea/proximal right main bronchus and oriented anterolaterally toward the right (see Figure 25-4E and F). Using the signal from the ventilator, airway pressures are displayed on the screen of the ultrasound monitor, allowing accurate timing of SVC cyclic changes during the respiratory cycle. Interpretation should be evaluated in the context of the patient’s clinical condition, radiographic findings, degree of hypoxemia, urine output, and fluid balance (see Figure 25-4G).
Pearls and highlights
• Critically ill patients with intrathoracic lymphadenopathy may warrant EBUS-TBNA for unclear sources of fever, sepsis, initial diagnosis, and recurrence or progression of thoracic or extrathoracic malignancy.
• Combination WLB and EBUS-TBNA can differentiate and diagnose types of CAO.
• Radial probe EBUS reveals cartilage abnormalities in stenosis and malacia and may impact treatment decisions.
• Convex probe EBUS reveals SVC collapsibility and may be helpful for assessing intravascular volume status.
• Convex probe EBUS allows visualization of the pulmonary trunk, main and lobar branches, and sections of the pericardium, potentially helping diagnose pulmonary embolism and pericardial effusion.
References
1. Yasufuku, K, Pierre, A, Darling, G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg. 2011; 142(6):1393–1400.
2. Colt, HG, Davoudi, M, Murgu, S. Scientific evidence and principles for the use of endobronchial ultrasound and transbronchial needle aspiration. Expert Rev Med Devices. 2011; 8(4):493–513.
3. Navani, N, Nankivell, M, Woolhouse, I, et al, Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of intrathoracic lymphadenopathy in patients with extrathoracic malignancy: a multicenter study. J Thorac Oncol. 2011;6(9):1505–1509.
4. Agarwal, R, Srinivasan, A, Aggarwal, AN, et al, Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: a systematic review and meta-analysis. Respir Med. 2012;106(6):883–892.
5. Navani, N, Molyneaux, PL, Breen, RA, et al, Utility of endobronchial ultrasound-guided transbronchial needle aspiration in patients with tuberculous intrathoracic lymphadenopathy: a multicentre study. Thorax. 2011;66(10):889–893.
6. Fujikura, Y, Kouzaki, Y, Ohta, S, et al. A case of Nocardia asteroids infection in a patient with HIV/AIDS diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). Intern Med. 2012; 51(11):1413–1417.
7. Steinfort, DP, Khor, YH, Manser, RL, et al, Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J. 2011;37(4):902–910.
8. Murgu, SD, Colt, HG, Mukai, D, et al. Multimodal imaging guidance for laser ablation in tracheal stenosis. Laryngoscope. 2010; 120(9):1840–1846.
9. Murgu, S, Kurimoto, N, Colt, H. Endobronchial ultrasound morphology of expiratory central airway collapse. Respirology. 2008; 13(2):315–319.
10. Kurimoto, N, Murayama, M, Yoshioka, S, et al. Assessment of usefulness of endobronchial ultrasonography in determination of depth of tracheobronchial tumor invasion. Chest. 1999; 115(6):1500–1506.
11. Park, JS, Chung, JH, Jheon, S, et al. EBUS-TBNA in the differential diagnosis of pulmonary artery sarcoma and thromboembolism. Eur Respir J. 2011; 38(6):1480–1482.
12. Aumiller, J, Herth, FJ, Krasnik, M, et al, Endobronchial ultrasound for detecting central pulmonary emboli: a pilot study. Respiration. 2009;77(3):298–302.
13. Guidelines for intensive care unit admission, discharge and triage. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 1999; 27(3):633–638.
14. Gounant, V, Ninane, V, Janson, X, et al. Release of metal particles from needles used for transbronchial needle aspiration. Chest. 2011; 139(1):138–143.
15. Freitag, L. Airway stents. In: Strausz J, Bolliger CT, eds. Interventional pulmonology. Sheffield, UK: European Respiratory Society; 2010:190–217.
16. Murgu, S, Langer, S, Colt, H. Bronchoscopic intervention obviates the need for continued mechanical ventilation in patients with airway obstruction and respiratory failure from inoperable non-small-cell lung cancer. Respiration. 2012; 84(1):55–61.
17. Nakajima, T, Yasufuku, K, Shibuya, K, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the treatment of central airway stenosis caused by a mediastinal cyst. Eur J Cardiothorac Surg. 2007; 32(3):538–540.
18. Baram, D, Smaldone, G, Tracheal collapse versus tracheobronchomalacia: normal function versus disease. Am J Respir Crit Care Med. 2006;174(6):724. [author reply 724-725].
19. Kurimoto, N, Miyazawa, T, Okimasa, S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest. 2004; 126(3):959–965.
20. Vieillard-Baron, A, Chergui, K, Rabiller, A, et al. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med. 2004; 30(9):1734–1739.