Emergency Ultrasound
Perspective and Definitions
Emergency ultrasound (US) is the simultaneous performance and interpretation of a focused sonographic examination at the bedside of the patient for the diagnosis, resuscitation, monitoring, guidance, and treatment of emergency medical conditions.1,2
Emergency US is an emergency clinician–performed study.1,3 Although the operator is typically an emergency physician, he or she may be an emergency physician assistant, nurse practitioner, or trained emergency nurse or paramedic—all under the supervision of a trained, credentialed emergency physician.4–6 Emergency physicians who have advanced training in US imaging are also known as emergency sonologists.6 Emergency US is typically performed in the emergency department (ED) but may also be performed in other areas of the hospital, emergency stand-alone centers, out-of-hospital mobile transport (such as ambulances and helicopters), disaster scenes, military engagements, international rescue work, and remote settings (such as space, sea, or land centers with limited or no medical access).7–13
With emergency US, the physician or clinician (1) performs, (2) simultaneously interprets the examination, and (3) integrates the results in the clinical scenario.1,5 This is unlike other specialties, in which a trained technologist performs the examination and a physician interprets or “reads” the images.5,14
Emergency US examinations are focused examinations of an area of the body, organ system, or physiologic pattern or goal-directed investigations of an emergency symptom or sign. Not all pathologic processes will be detected by such examinations, usually because of the interference of acoustic entities that reduce transmission and reflection of sound and the lack of an appropriate window to the tissue of choice. Other limitations include experience, skill, and confidence of the performing physician.15 The main risk management issue associated with emergency US is not using the modality when it is available and appropriate.16
History
A half-century of scientific work in many countries produced the initial medical uses of US in the 1950s by physicians interested in its advantages over traditional imaging, including noninvasiveness, lack of ionizing radiation, and superior resolution.14 In the 1980s emergency physicians started to use US as a way of ruling out emergent “silent” diagnoses, such as ectopic pregnancy, intraperitoneal hemorrhage, hemopericardium, cholelithiasis, renal colic, and aortic aneurysm. A model curriculum was created by the Society for Academic Emergency Medicine (SAEM) that initiated formal guidance for emergency US programs.17,18 Because of resistance from traditional imaging specialties, such as radiology and cardiology, the American Medical Association House of Delegates, led by the American College of Emergency Physicians (ACEP), passed a resolution that hospital credentialing committees follow specialty-specific guidelines for US-based credentialing.19
Specialty-specific guidelines, created in 2001 and updated in 2008 by the ACEP and endorsed by the SAEM in 2010, specify emergency US scope of practice, primary applications, training pathways, number of procedures required for training before credentialing, quality assurance and documentation guidance, and training course outlines.1 Today, more than 95% of emergency medicine residencies teach US, and approximately 30 to 60% of community hospitals have instituted bedside US performed by emergency physicians.20,21
Since 2008, emergency US has been recognized by outside organizations, including the American Institute of Ultrasound in Medicine, with implementation of the focused assessment with sonography for trauma (FAST) examination, and the American Society of Echocardiography, with application of focused cardiac US (FOCUS) for shock, effusion, chest pain, and cardiac arrest.22,23 Several other organizations have recognized emergency or point-of-care US, including the World Interactive Network Focused on Critical Ultrasound internationally and the Society of Medical Ultrasound in Medical Education for medical education.
US programs typically require some investment in education and hardware, but reimbursement for both professional and hospital fees is adequate to meet return on investment within years, depending on number and types of US, payer mix, and documentation.24 More EDs have recently invested in digital-based archiving and storage systems. The more traditional solution is the picture archiving and communication system. A new, more comprehensive solution seems to be the web-based US management systems, which provide archiving, documentation, quality assurance, and credential solutions to the emergency medicine and point-of-care market.
Training, Credentialing, and Accreditation
Emergency US is one of the competency assessments required of emergency medicine residents by the Residency Review Committee for Emergency Medicine, with defined requirements as set by national organizations.25–27 For emergency physicians in practice, initial training often takes place through nationally defined hands-on continuing medical education courses followed by a period of proctoring or supervision.1,28 Credentialing at the hospital level is based on specialty-specific national guidelines. Recent educational advances, such as simulation, task trainers, and web-based training, enhance US training.29–32 US is not defined as advanced imaging by the Centers for Medicare and Medicaid Services or the 2008 Medicare Improvements for Patients and Providers Act.33
Medical student emergency US education has also been defined by national organizations and may be incorporated in more extensive undergraduate US programs.1 US fellowships have become the most prevalent postgraduate emergency medicine fellowship.34 See the website of the American College of Emergency Physicians (http://www.acep.org/Clinical–Practice-Management/Ultrasound/; accessed April 12, 2013) for helpful references for program development and education.
Applied Ultrasound Physics and Instrumentation
By definition, US is sound with frequencies higher than 20,000 Hz. US is kinetic energy, a longitudinal, mechanical, and directional wave, so all users of this technology should observe the ALARA (as low as reasonably achievable) principle by performing procedures only when they are needed and limiting the time of sonographic investigation.35
Other US terms and artifacts commonly used are listed in Box 196-1.
Equipment and Safety
US biosafety, including use of the ALARA principle, appropriate Doppler technology, and appropriate microbiologic disinfection of the probe, has been reinforced by recent articles.36 The ALARA principle suggests that the least amount of energy be used to produce satisfactory imaging. Doppler modes should be minimized over sensitive tissue, including early gestation, germinal tissue, and mucosal or neural tissue. The sonographer should be familiar with the thermal index (TI), which should be kept below 2.0, and the mechanical index (MI), which should be kept below 1.9. Surface probes should be cleaned at the bedside with mechanical removal of the gels and debris and then disinfected with an appropriate spray or wipe. Endocavitary probes will need more prolonged and substantial cleaning. Safety also includes the use of appropriate barriers over transducers, including condoms, gloves, or commercial barriers over endocavitary probes and sterile transducer covers for sterile procedures.37 For nonsterile procedures, barriers can include translucent sterile transdermal barriers.
Applications and Categorization
In 1994 the SAEM categorized US applications broadly into abdominal, cardiac, obstetric-gynecologic, and special applications.17 In 2001 the ACEP developed guidelines that categorized applications more specifically to include trauma, pregnancy, abdominal aortic aneurysm, cardiac (for pericardial effusion and cardiac activity), biliary, renal, and procedural guidance.38 In 2008 the ACEP added deep venous thrombosis (DVT), thoracic studies for pneumothorax and pleural effusion, soft tissue and musculoskeletal studies, and ocular examination as core appplications.1 Advanced applications at that time included testicular, adnexal, bowel, advanced echocardiography, transesophageal echocardiography, transcranial Doppler, and contrast studies. Therapeutic applications of transcranial Doppler are being studied by emergency physicians.39 Grouping of these US applications into categories of resuscitative, diagnostic, symptom or sign based, procedural guidance, and monitoring and therapeutic helps describe the relationship between the uses of US in emergency medicine (Table 196-1).
The most common uses of emergency US in decreasing order are trauma, cardiac (cardiac arrest and pericardial effusion), abdominal aortic aneurysm, pelvic, biliary, procedural, renal, and DVT.21 Among recent emergency medicine residency graduates, US use was high (98%), with those in academic centers reporting highest use.
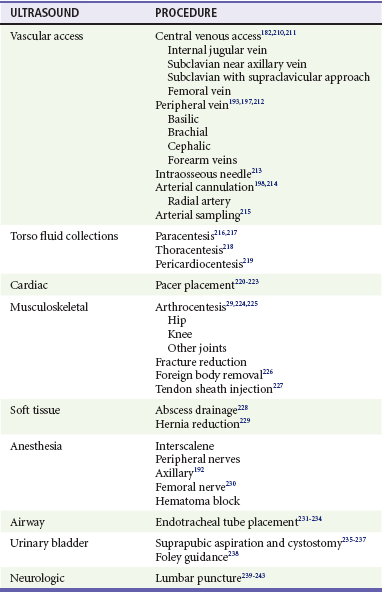
US guidance can assist many percutaneous procedures, such as vascular access, torso cavities evacuation (e.g., thoracentesis, paracentesis, and pericardiocentesis), arthrocentesis, lumbar puncture, abscess drainage, nerve blocks, and others.3 Emergency US is used in clinical pathways or algorithms to identify or to exclude emergency medical conditions rapidly.40,41
Trauma Ultrasound
The trauma US examination is also called the focused assessment with sonography for trauma (FAST) examination.1,22,42 The trauma examination originally focused on the peritoneal space, as an evaluation for hemoperitoneum, with use of the right flank, left flank, and pelvic windows for detection of dependent peritoneal fluid and pleural effusion.43 Newer versions include the EFAST (extended FAST) for the evaluation of potential pneumothorax and FASTER for evaluation of the extremities.40,44–46
The FAST examination is based on the premise that a treating physician can sample known sonographic windows within minutes to evaluate the peritoneal, pericardial, and pleural spaces for fluid and abnormal fluid and air. Within the peritoneum, fluid circulates throughout the abdomen and pelvis and settles in dependent spaces.47,48 The volume of fluid required for a positive US study depends on the site of injury and site of sonographic detection, but 250 mL or more is generally visible, and nearly 600 mL of fluid is necessary to cause a positive flank stripe when fluid is infused from the pelvis.49
Pericardial fluid is contained within the parietal and visceral pericardium. Once a certain volume is reached, the pressure in the pericardial space increases exponentially, causing pericardial tamponade.50 In general, 50 mL is required to cause hemodynamic compromise in a patient without prior pericardial inflammation. However, tears in the pericardium that communicate with the pleural space or even the peritoneum can cause false-negative examination findings.
Pleural fluid can be detected by US, but this depends on the position of the body.51 Pneumothorax detection is based on the absence of the normal sonographic sliding of the visceral and parietal pleurae, although there are other signs (see thoracic section).
The torso trauma US examination (often called the EFAST) can detect pathologic free intraperitoneal fluid (typically hemoperitoneum, uroperitoneum, bile, or ascites), hemopericardium, hemothorax, or pneumothorax.45,46 Typically, fluid in the peritoneum, pericardium, and pleural cavity is anechoic, but it can have echogenicity with clotting, depending on the age of the clot or bowel contents. Compared with other fluid-filled structures in the abdomen and pelvis, peritoneal free fluid generally has sharp edges and an irregular shape, whereas most visceral or vascular structures have intrinsically smooth oval contours and less abrupt edge detail.
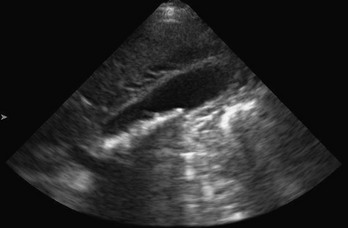
The FAST technique uses a low- to middle-frequency probe (2-5 MHz) to evaluate dependent peritoneal spaces, pleural spaces, and the pericardium. Within the peritoneum, dependent spaces include the following, grouped by tissue window: the right flank, which evaluates the hepatorenal space (also called Morison’s pouch; Fig. 196-1), the right subphrenic space, and the right costophrenic angle; the left flank, which evaluates the perisplenic space between the diaphragm and spleen, the splenorenal space, the perirenal space, and the left subphrenic space; and the suprapubic view, which is performed by placement of the transducer superior to the pubic bone with a full bladder to visualize the pouch of Douglas in women and the retrovesical space in men. Extra views include the paracolic gutters inferior from the flank views and medial from the iliac spine to visualize free fluid surrounding the bowel.52
For pleural fluid, the superiorly angled flank views allow visualization of the costophrenic angles. The evaluation for pneumothorax uses either a low-frequency probe at a shallow depth or, more typically, a high-frequency probe for better resolution, placed at the anterior chest in the second intercostal space for anterior pneumothorax and at the axilla for large pneumothoraces and pleural effusion. Diaphragm injuries have been described with dislocation of typical organ patterns.53
The sensitivity of the FAST examination ranges from 60 to 99%, and its specificity ranges from 80 to 99%.54–57 The large range of sensitivity is related to the comparative “gold standard” used for each study, with clinical endpoints suggesting higher accuracy. The FAST does not capture every peritoneal injury, although the need for intervention in undetected injuries has not been studied.58 Randomized, controlled trials show lower morbidity, time to operating room, and charges for patients with US use during resuscitation, and a positive US study increases the odds ratio for laparotomy.59,60
Patients with traumatic pericardial effusions are taken to the operating room sooner and have lower mortality rates with US.44 However, the mediastinal injuries more common in blunt trauma have not been assessed without transesophageal echocardiography.50,61 US can diagnose pneumothorax with excellent sensitivity and specificity compared with plain chest radiography and variable accuracy compared with computed tomography scanning.62,63
There are certain populations of patients in whom FAST evaluation has limited benefit. With penetrating chest injuries, the FAST examination seems to be efficacious for both pericardial and nearby peritoneal injuries; but in patients with purely anterior penetrating trauma to the abdomen, the sensitivity of trauma US is poor because bowel injuries can occur without significant hemoperitoneum.58,59,64–66
The accuracy of the FAST examination may be lower in children than in adults, and many pediatric injuries can be observed or treated nonoperatively with angiography.67 In obstetric patients, the few observational studies that have been performed have shown reasonable sensitivity and specificity, although abruption and fetal viability may necessitate an earlier operative course.68
With pelvic fractures, the sensitivity ranges from 20 to 80%.69–72 More important, the detection of free fluid in an unstable patient with a pelvic fracture may be due to uroperitoneum from bladder injury rather than hemoperitoneum from vascular injury, clouding the decision for laparotomy versus pelvic embolization.64 In addition, retroperitoneal injuries to the genitourinary tract are not assessed with four-quadrant FAST examination.
Pelvic Ultrasound
Pelvic US by emergency physicians was initiated to address one of the epidemics of the modern era—ectopic pregnancy.73–77 Typically, pelvic US is used to confirm intrauterine pregnancy, which indirectly excludes ectopic pregnancy in most patients.78,79 A recent meta-analysis revealed a pooled sensitivity of 99.3% to rule out ectopic pregnancy when an intrauterine pregnancy was detected. Another use during pregnancy is the detection of fetal viability, incomplete abortion, ectopic pregnancy, and molar pregnancy. Pelvic US is used in nonpregnancy states to detect tubo-ovarian abscesses, masses, and hemoperitoneum in the hemodynamically unstable patient.80
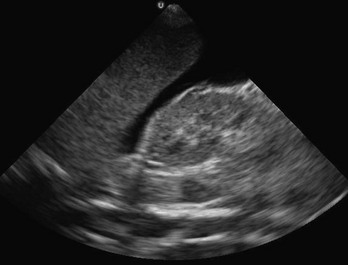
Indications for sonographic evaluation of the first-trimester pregnant patient include symptoms or signs that suggest an ectopic pregnancy, molar pregnancy, or fetal demise and dating of the pregnancy. Intrauterine pregnancy is confirmed with a gestational sac with a yolk sac or fetal pole within the fundus of the uterus (Fig. 196-2).78 An embryonic or fetal demise has US findings of a fetal pole measuring more than 5 mm without fetal heart rate or a gestational sac of more than 20 mm in diameter without a fetal pole. A molar pregnancy appears as an echogenic, cystic uterus with disorganized echoes and is associated with high β-human chorionic gonadotropin concentrations without the sonographic finding of intrauterine pregnancy. Findings of an ectopic pregnancy include a chorionic ring or gestational sac with evidence of a yolk sac or fetal pole outside the uterus or in an abnormal location in the uterus, such as the cornu or cervix.73 Excluding the groups of intrauterine pregnancy, embryonic demise, molar pregnancy, and ectopic pregnancy, there is a class called indeterminate, which may account for 20% of pregnant patients presenting to the ED in the first and early second trimesters. Heterotopic pregnancies can occur at a rate of 1/5000 and be detected by the same techniques and definitions.81
Emergency physician–performed pelvic US has reduced the morbidity of ectopic pregnancy by shortening the time to diagnosis and operating room treatment and has resulted in greater initial detection of abnormal pregnancy, reduced ED patient throughput times, and increased patient satisfaction with care.74,76,82–85 The detection of live intrauterine pregnancy predicts a rate of 91% continuation past 20 weeks, except for those with vaginal bleeding, who drop to a rate of 86%.86 Further continuation of the pregnancy to term occurs in 77% of patients.87 Emergency physicians can date pregnancies accurately by pelvic US with typical fetal biometry.88
Emergency physicians are adept at identification of ectopic pregnancies by both transabdominal and endovaginal techniques.85,89–93 In addition, molar pregnancies and some malformation, such as conjoint twins, have been described.94,95 The gynecologic differential diagnosis of significant free fluid in a pregnant woman, in addition to ectopic pregnancy, should include uterine rupture and ruptured corpus luteum pregnancy.96 Pelvic US in nonpregnancy states has shown good accuracy for tubo-ovarian abscess and improved decision-making for female patients with right lower abdominal pain.97,98
Cardiac Ultrasound
The use of cardiac US by emergency physicians is presentation and symptom specific and focused in nature. Indications include cardiac arrest, possible pericardial effusion, trauma, chest pain, hypotension, and procedural guidance.1,3,23,99 Cardiac US is often included in algorithms and protocols for certain symptoms or signs, such as dyspnea or hypotension.
Cardiac US is performed through the transthoracic and transabdominal windows with use of small curvilinear or phased array transducers. Typical views include the subcostal four-chamber and long-axis views, parasternal long-axis view, parasternal short-axis view, and apical four-chamber view. Although most emergency physicians are comfortable with the subcostal view from the FAST examination, the parasternal long-axis view is a good alternative and good window for left ventricular assessment.100,101 In addition, the apical four-chamber view provides excellent comparison of the right and left ventricles in terms of size and function. The long-axis subcostal view shows some of the right side of the heart but highlights the IVC, including size and respiratory variation.
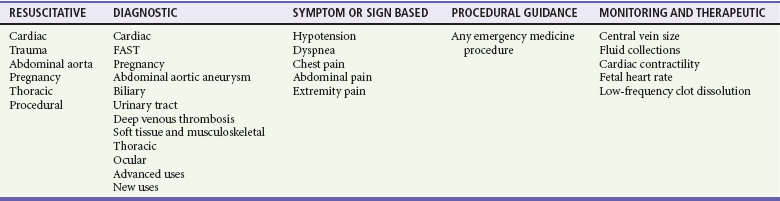
Cardiac US performed by emergency physicians shows good accuracy in detection of pericardial effusion (Fig. 196-3), assessment of left ventricular function, and evaluation of patients with undifferentiated shock. In addition, aspects of cardiac US are being used for the assessment of intravascular volume status and cardiac output and in the evaluation of dyspnea.
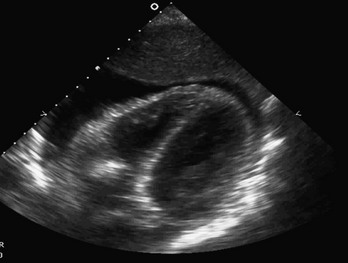
Figure 196-3 Pericardial effusion in subcostal view.
In cardiac arrest, US can detect ventricular motion in both asystole and pulseless electrical activity (PEA).102,103 In asystole, two studies have shown poor prognosis in the absence of sonographic ventricular activity.84 Cardiac US can detect pseudoasystole by revealing ventricular fibrillation or coordinated cardiac contractions without a palpable pulse.85,104 Use of cardiac US in PEA and near-PEA states can be diagnostic for pericardial effusion.82 Cardiac US can detect ventricular capture when the patient is paced, transcutaneously or by a transvenous pacer. US can also identify pneumothorax, another treatable cause of cardiac arrest.103 Cardiac US is feasible during pulse checks and prehospital life support, as advanced cardiac life support guidelines suggest minimizing non–cardiopulmonary resuscitation intervals.105–108
Emergency cardiac US is diagnostic for both medical and traumatic pericardial effusions.109,110 In patients with dyspnea, pericardial effusion is detected with excellent accuracy.111 In trauma, use of cardiac US reduces time to operative intervention and reduces mortality in patients with penetrating cardiac injury.50 Detection of effusions in blunt trauma has also been described. Patients with cardiac US performed by emergency physicians for pericardial effusion have significantly reduced hospital length of stay and charges.112
Cardiac US is used for detection of pericardial effusions, chamber enlargement, and global activity in chest pain syndromes.113 In chest pain, US has been studied for the evaluation of pericardial tamponade, pulmonary embolus, cardiogenic shock, aortic dissection, pneumothorax, and bony chest wall fracture.114–116
US protocols have been developed to evaluate undifferentiated hypotension.41,117–119 Cardiac US windows with the addition of abdominal views can assess for effusion, global ventricular activity, ventricular chamber size, IVC size and respiratory change, peritoneal fluid, and abdominal aortic aneurysm to narrow the differential diagnosis. Sepsis is the most common diagnosis for patients with hyperdynamic ventricular activity.120
Central pressures can be estimated by examination of the IVC size and collapsibility.121,122 Studies show that this technique has good accuracy in assessment of blood loss, hypovolemia, and congestive heart failure.123,124 Correlation studies have shown moderate agreement between central venous pressure and IVC measurements, with actual cardiac qualitative assessment as good as or better than the IVC measurements.125–129 The internal jugular vein measurement may correlate with central venous pressure,130 and esophageal Doppler examination may also correlate with hemodynamic measurements.131
Abdominal Vascular Ultrasound
Emergency physicians use abdominal US to detect abdominal aortic aneurysm, another silent disease in patients with flank, abdominal, or back pain, and to evaluate unexplained hypotension in the older patient.132,133 The use of US to detect aortic dissection and occlusion is described as well.134–136
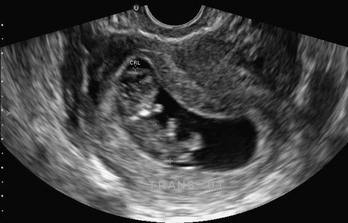
The technique involves imaging of the aorta from the subxiphoid space to the umbilicus to evaluate for dilation above a diameter of 3 cm (Fig. 196-4). For the detection of infrarenal aneurysms, the technique should visualize the aorta from the diaphragm to the aortic bifurcation. Fusiform aneurysms are more common, but detection of saccular aneurysms requires both the transverse and longitudinal planes.137 If an aneurysm is found, a peritoneal view of Morison’s pouch is performed to detect intraperitoneal fluid. Proximal iliac arteries may be followed if it is technically feasible or time allows.
Emergency physician use of US for abdominal aortic aneurysm detection has shown good accuracy compared with other imaging modalities and laparotomy.133,138–140 It also has management implications in older patients with lower back pain, in whom the elimination of the presence of abdominal aortic aneurysm can lead to other management decisions. Screening for abdominal aortic aneurysm in the ED with US has been shown to be effective.141,142
Endovascular repair versus the more traditional open repair has become more popular.143,144 The detection of leaks (often called endoleaks) from the stent would require Doppler imaging and may require further imaging with computed tomography or magnetic resonance imaging.144
Aortic dissection may be detected by a combination of abdominal and cardiac scanning.136 A linear echogenic flap, anywhere across the lumen of the aorta, is suggestive of dissection, sometimes with the detection of different flows on either side of the flap by color Doppler scanning. The cardiac US examination can show unexplained pericardial effusion; a dilated aortic root; aortic insufficiency; and a linear echogenic flap seen in the ascending aorta, aortic arch (through a suprasternal notch), or descending aorta.
Aortic occlusion is another condition that may be detected by US.145 Echogenic thrombus may fill the aorta and disrupt perfusion below the diaphragm with resulting pain, pallor, numbness, and reduction or absence of distal pulses. Clear identification of the borders and lack of Doppler flow in the abdominal aorta are characteristic of the syndrome.
Biliary Ultrasound
Biliary US to detect gallstones and associated cholecystitis was one of the early applications of US in emergency medicine.146–148 The sonographic technique involves a modified biplanar approach to the right upper quadrant. A complete evaluation includes visualization of the common bile duct. The diagnosis of cholelithiasis is made after identification of echogenic foci within the gallbladder lumen with shadowing (Fig. 196-5). Other image patterns include stones that will not shadow, sludge, and the wall echo sign of a gallbladder full of gallstones. Signs of cholecystitis include a dilated gallbladder, increased gallbladder wall thickness, sonographic Murphy’s sign, and pericholecystic fluid. A nonmobile stone in the gallbladder neck is highly suggestive of eventual cholecystitis.149 A common bile duct larger than 6 mm in people younger than 60 years and smaller than 10 mm in elders may indicate choledocholithiasis. Biliary US is fast and accurate, with a sensitivity of 87 to 94% and a specificity of 82 to 96% in detection of gallstones, comparable to radiology ultrasonography.150–153 Biliary US has replaced nuclear medicine testing.148,154
Urinary Tract Ultrasound
Renal US includes biplanar views of the kidneys with emphasis on dilation of the calyceal system and pelvis of the respective kidney (Fig. 196-6). In addition, visualization of the bladder can diagnose secondary hydronephrosis from an obstructed bladder stone and may demonstrate nonobstructive bladder jets through the use of Doppler US. The windows for the two kidneys are similar to those of the trauma flank series, with the exception that the patient may be rolled on the opposite side so that the transducer may be placed more posteriorly on the back if necessary. The bladder view is performed suprapubically, and calculations of volume may be made with on-machine calculators or by formulas.
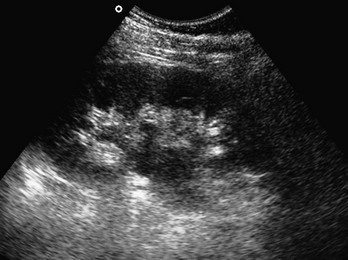
Figure 196-6 Hydronephrosis in renal pelvis and calyces.
Renal US has a sensitivity of 83% and a specificity of 92% in detection of hydronephrosis (see Fig. 196-6).155,156 Genitourinary tract obstruction protocols have great sensitivity in elimination of renal obstruction from a differential diagnosis.157 Bladder US is useful for detection of a full bladder and the presence of a Foley catheter and for guidance during suprapubic aspiration or Foley placement.119–124,158–160
Deep Venous Thrombosis Ultrasound
The swollen extremity often requires sonographic imaging to assess for DVT. Emergency physicians commonly use compression US to rule out DVT.125–128,161–165 The two-level compression technique involves visualization of the compressibility of the common femoral and popliteal veins. The three-level technique adds the junction of the superficial femoral and deep femoral veins (Fig. 196-7). Use of upper extremity veins is less common because Doppler US is needed for the subclavian vein, which is not compressible under the clavicle.166
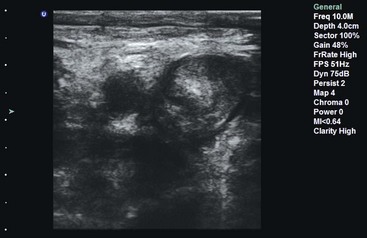
Figure 196-7 Deep venous thrombosis with layering clot in popliteal vein seen on compression ultrasound examination.
The accuracy of this technique ranges from 70 to 99%, depending on operator experience.164,167–169 Hospital charges and time in the ED are reduced for patients who have bedside venous US performed to rule out DVT.170–172
Bernardi also investigated a whole-leg strategy versus a 2-point strategy in combination with a D-dimer test, which required 1-week follow-up with an abnormal level.173 Both strategies were equivalent in detection of symptomatic DVT at 3-month follow-up. Depending on the skill of the sonographer, patient characteristics, and availability of follow-up, ED US may guide lower extremity DVT institutional protocols.
Thoracic and Tracheal Ultrasound
Thoracic applications include the detection of pleural effusion, pneumothorax, pneumonia, and interstitial edema.174,175 The technique of thoracic US uses a low-frequency probe to survey for pleural effusions and a high-frequency probe to detect pleural lines and related artifacts. Pleural fluid appears as an anechoic collection above the diaphragm (Fig. 196-8). In addition, a collapsed lung may be visualized as an echogenic floating structure. Normally, the parietal and visceral pleural lines slide against each other, and lack of sliding is the finding most consistent with pneumothorax (on M-mode, called the seashore sign). Additional findings of pneumothorax include the presence of horizontal artifacts called A-lines (on M-mode, called the stratosphere sign) and the absence of B-lines (vertical white lines from reverberation of air with fluid) at the parietal-visceral pleural line (Fig. 196-9).176–179 Confounding factors include adhesions of the pleura, chronic obstructive pulmonary disease, prior pneumothorax, and mainstem bronchus intubation. A lung point sign is the edge of the pneumothorax where the lung is still adherent to the parietal pleura, and part of the image shows no sliding until the lung moves into the interspace with respirations.177 The accuracy of US for detection of pneumothorax (sensitivity 100%, specificity 98%) is better than that of plain chest radiography in the acute setting, but there may be reduced accuracy after 24 hours.180
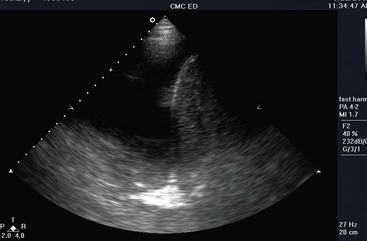
Figure 196-8 Large pleural effusion.
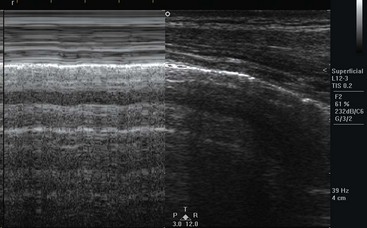
Figure 196-9 M-mode image of normal lung sliding.
Severe pneumonia is visualized as echogenic “liver-like” echogenicity as the lung accumulates fluid with consolidation.181 Peripneumonic collections are common and may indicate inflammation. The dynamic air bronchogram, hyperechoic areas within bronchi that move with respiration, usually within “heparinized” lung, has been described as another sign of alveolar consolidation.182
Pulmonary edema is indicated by the presence of comet tails (B-lines), which are reverberation artifacts that reflect from the parietal pleural interface into the lung. Normally found in dependent areas of the lung, the widespread distribution of these artifacts in an apical lung may indicate increased lung congestion.183–185
Dynamic tracheal US for the confirmation of endotracheal intubation has relatively good sensitivity and specificity.186–188 The dynamic movement of the endotracheal tube creates a flutter through the recognized shadow of the airway, but static US seems to lack accuracy.189,190 This technique may have a role in patients in cardiac arrest or with equivocal end-tidal carbon dioxide levels, and confirmation is significantly faster than with chest radiography in children.190
Ocular Ultrasound
The eye is an excellent acoustic window, and short-duration gray scale US over a closed eyelid can visualize both the anterior and the posterior chamber well.191,192 The globe, pupillary size, and extraocular movements can be examined even with facial swelling.193,194 Ocular US has been described for intraocular disease, such as retinal detachment (Fig. 196-10), retinal hemorrhage, vitreal hemorrhage, intraocular foreign body, dislocated lens, and retro-orbital hemorrhage.191,192,195,196 The sensitivity of ocular US for retinal detachment by emergency physicians is 100%, and the specificity is 83 to 92%.197,198 In addition, the optic nerve sheath diameter can be measured behind the eye, reflecting intracranial pressure.199–202 The sensitivity of such measurements ranges from 83% in children to 100% in adults.199,203
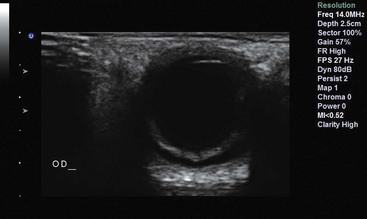
Figure 196-10 Retinal detachment.
Soft Tissue Ultrasound
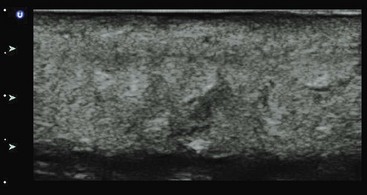
Except in the hand and foot, US in soft tissue is straightforward because of the lack of air and bone in the skin and subcutaneous tissue. Soft tissue US is used to differentiate cellulitis from abscess, to detect foreign bodies and hernias, and to evaluate other soft tissue pathologic processes, including masses, pseudoaneurysms, and glands.204–208 A high-frequency linear transducer moves from normal skin to the abnormal area. Cellulitis or edema will cause an echogenic pattern with cobblestoning between fat lobules (Fig. 196-11). Abscesses are irregular hypoechoic to anechoic collections within the subcutaneous layer but may connect with the surface (Fig. 196-12). US is diagnostic in soft tissue disease for cellulitis, abscess, and necrotizing fasciitis.150–153,209,210 In the ABSCESS study, clinical examination had a sensitivity of 86% and specificity of 70%, whereas US had a sensitivity of 98% and specificity of 88%.205 In other studies, use of US changed management for half of adult patients and 22% of children with clinical cellulitis.206,211 One study showed that incision and drainage is superior to US-guided aspiration in a cohort of patients with soft tissue infection in regard to resolution at 1 week (80% vs. 26%, respectively).212
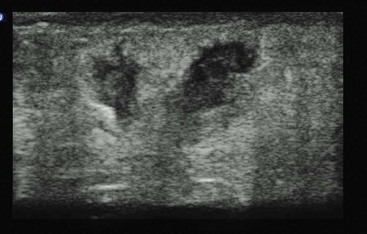
Figure 196-12 Sonographic subcutaneous abscess.
US can differentiate peritonsillar abscess from cellulitis and can be used for guidance of peritonsillar aspiration.213,214 It can also detect early Ludwig’s angina with abscess, dental abscess, epiglottitis, glandular abnormalities, and pseudoaneurysm in soft tissues and fascia of the face.207,208,215–217 US has also been used in various body areas, including the penis and scrotum, for signs of disruption or infection.218,219
Detection of foreign bodies is characterized by variable echogenicity in the tissue with shadowing beneath the foreign body. Metal foreign bodies, such as bullets and intrauterine devices, have characteristically high-reflective echogenicity and ring-down artifacts. Bedside US has variable accuracy for detection of foreign bodies in simulated cases but better accuracy in true clinical settings.220–222 Use of a water bath may aid in the detection of superficial foreign bodies.223
Musculoskeletal Ultrasound
US has been particularly useful for joint effusion and muscle, tendon, and bone injury. US is excellent for detection of fluid in joints, confirmation of effusions, and guidance of drainage procedures.29,160,161,224–230 Joint effusions typically are anechoic or echogenic, depending on type and age (Fig. 196-13). Arthrocentesis of both the adult and pediatric hip, in addition to other joints, has been reported with good success.229,231,232
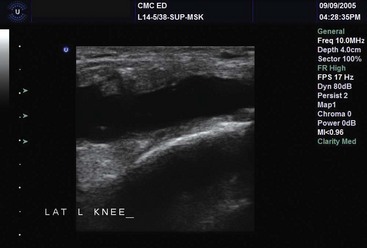
Figure 196-13 Knee joint effusion.
Diagnosis of ligamentous injuries and muscle avulsion and hemorrhage has also been reported.224,233 Muscle and tendon abnormalities are detected by anechoic and heterogeneous abnormalities (Fig. 196-14). Detection is often assisted by movement of the limb. Tendons and their anisotropic longitudinal fibrils can be visualized near joints, with pathologic tears appearing as a discontinuity of the tendon. Muscles are hypoechoic with echogenic borders. Tears and hemorrhage are interruptions of this normal pattern.224,234,235 Water bath techniques provide a better acoustic window for shallow tissue, such as fingers.236
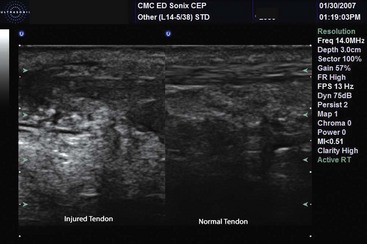
Figure 196-14 Achilles tendon disruption.
The characteristic reflection of bone with immediate shadowing is used to identify normal bone and its contour. Fractures are seen as a defect in the bony cortex.46,224,237–240 The image of the cortex can be useful for guidance of fracture reduction.241–243 Comparison with the contralateral side may also be helpful. Confirmation can be done by US imaging before and after reduction.244
US is accurate in the detection of fractures, joint effusions, bursitis, cysts, and hematomas.140,229,231,245–247 Prospective evaluation of finger fractures demonstrates good accuracy228 (Fig. 196-15). Other fractures studied include femur, sternal, rib, and forearm fractures.224,237–240
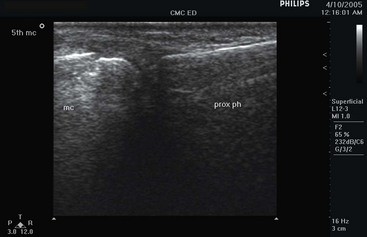
Figure 196-15 Metacarpal fracture.
Transcranial Doppler Ultrasound
Transcranial Doppler US has been used for detection of abnormal flow patterns in the brain and for detection and treatment of middle cerebral artery strokes. An advanced procedure, typically using a phased array transducer over a bone or orbital window in the skull, color Doppler and spectral Doppler scanning is performed at different levels of intracranial vessels for detection of abnormal patterns.39
Testicular Ultrasound
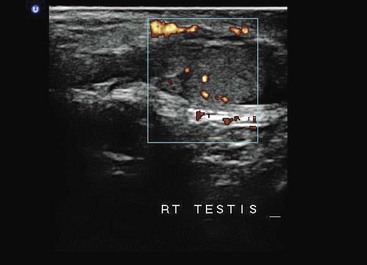
Testicular scanning is an advanced US application that requires visualization of the normal and symptomatic testicle with a high-frequency probe. The testicular structures, including the normal homogeneity of the testicle, are evaluated, and Doppler scanning is performed for evaluation of normal venous and arterial flow (Fig. 196-16). Absent or decreased flow on the affected side indicates possible torsion, whereas increased flow indicates epididymitis. Experienced emergency physicians accurately diagnosed testicular pain with a sensitivity of 95% and specificity of 94% in a study of 36 patients, which included 3 patients with torsion.248
Abdominal Bowel Ultrasound
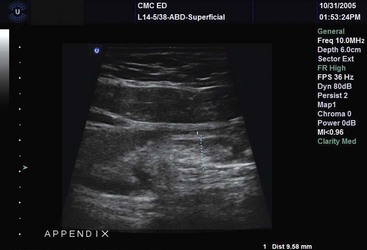
The use of bedside US for evaluation of appendicitis, diverticulitis, and other bowel disease is an area of increasing interest. There has been variable experience with the use of graded compression US to detect appendicitis (>6 mm), with reported sensitivity of 67% and specificity of 92% (Fig. 196-17).249 Whereas there is considerable effort looking at the use of US first and then computed tomography for appendicitis, the accuracy of emergency physician–performed US for appendicitis has been equivocal.249–251 In women, endovaginal US can be used to rule out adnexal disease.98
The use of US for diverticulitis, intussusception, volvulus, and small bowel obstruction has been reported.252–254 Thickening of the bowel, dilated bowel with fluid-filled loops, and changes in vascularity suggest disease. Tracing of the bowel to normality may also show cutoff points.
Hernias, described in the soft tissue section, may also be detected as masses in the ventral, inguinal, paramedian, femoral, and scrotal areas.255 Free air, with comet tails and artifacts in areas that are usually normal, such as between the liver and diaphragm, may also be detected in patients with suspected perforated viscus.256
Pediatric Emergency Ultrasound
The use of US for children in the ED has two paths, one that mirrors the applications that have been used for adults and a second exploring the more specific pediatric abdominal applications.257 The use for patient management and accuracy can vary per application.
The FAST examination for evaluation of abdominal trauma in children is specific (98%), but the sensitivity is only 20%, making it difficult to use without further imaging or other testing.67 In addition, specific pediatric abdominal applications that are common in the radiology suite have begun to be explored. Case reports and case series of intussusception, appendicitis, and pyloric stenosis have been reported.251,252,258–260
Ultrasound for Procedural Guidance
In 2001, in response to the Institute of Medicine report To Err Is Human, the Agency for Healthcare Research and Quality (AHRQ) sanctioned a report on actions that may improve patient safety.261,262 The report contains a recommendation for US guidance for internal jugular central line insertion. Since then, the use of US for procedural guidance has expanded for many emergency procedures, especially vascular access, and is advocated for error reduction.263
Procedural guidance can be static or dynamic.264 Static guidance suggests that US has been placed over the anatomic area, and the area is marked while angle and distance information is noted. Dynamic guidance describes procedures performed with real-time US visualization of the needle entering the anatomic area on the monitor as well as on the patient.
There are two common axis approaches to vein cannulation: transverse, in which the vein appears as a circular structure on the screen; and longitudinal, in which the vein appears as a tubular structure along the width of the screen. In the transverse approach (also known as out of plane), the probe’s long axis is centered transverse (90 degrees) to the long axis of the anatomic area, guiding the needle to bisect the probe at its center, which gives centering and depth information (Fig. 196-18). In the longitudinal axis (also known as in-plane), the probe is placed along the long axis of the anatomic area, and the needle is introduced in the long axis of the probe, giving depth and trajectory information (Fig. 196-19). Whereas the short-axis approach is more popular with novices, the long-axis approach may be safer because of the visualization of the needle tip during the entire dynamic procedure.150 Finally, an oblique axis has been described, essentially approaching the vessels at 45 degrees but visualizing the target vessels and surrounding structures.265
The most studied and most advocated use of US procedural guidance is for central line insertion.266–269 Both the AHRQ and the National Institute for Health and Clinical Excellence have recommended US guidance for central line insertion.262,270 US guidance for internal jugular central line insertion can be recommended as a safe and best practice.271
US permits the physician to assess the internal jugular for overlap with the carotid artery, vessel diameter, and presence of luminal clot or vessel obliteration.186 Internal jugular central line insertion has been studied in the ED, intensive care unit, and radiology suite. In the ED, US-guided internal jugular cannulation is associated with decreased time to flashback and improved success in the difficult-stick patient, improved overall success rate and first attempt and overall rates, reduced time to insertion, and reduced complication rate.268,272,273 The Valsalva maneuver and Trendelenburg position may assist in increasing the size of the cross-sectional area.274
US does not prevent posterior vein wall penetration as reported in case reports and simulated models.275,276 Adverse events (hematoma, arterial cannulation, pneumothorax, and unsuccessful placement) can occur in up to 20% of patients having US-guided internal jugular catheterization.277–279
Femoral vein insertion has been studied in cardiac arrest patients, and improved cannulation rate and reduced complications have been reported.280 Several maneuvers can optimize the success rate of femoral vein cannulation, including the reverse Trendelenburg position and placement of the pressure on the iliac vein proximal to the femoral vein.188,281,282
US does not contribute to procedural success in subclavian vein cannulation because of the lack of a convenient window for the subclavian vein under the clavicle. Laterally, toward the junction of the axillary vein and subclavian veins, the vein can be visualized in the chest wall.283,284 Although it may be cannulated there, the challenge is to insert the catheter through the pliable soft tissue of the anterior-superior chest.191,284,285 The supraclavicular fossa is a window for central line insertion, and the subclavian and brachiocephalic veins may be imaged at this site.192,286
US-guided central access decreases complications and attempts but did not improve success rates in pediatric critical care patients.287
Emergency physicians are able to use US to insert peripheral intravenous catheters in patients with difficult access with high success rates.193–195,288,289 Only one randomized study showed no differences in number of attempts, time to successful catheterization, or patient satisfaction.290 Veins that are large in width (0.4 cm) or at moderate depth (0.3-1.5 cm) have a higher success rate.291 Nurses and ED technicians have also been taught to use US for peripheral venous guidance.292–294
The use of one operator versus two operators has not been shown to increase success rates.295 US can facilitate peripheral vascular access by the Seldinger technique.296 Marking of the skin in its long axis while using the short axis has not increased success rates.297 Pediatric US-guided intravenous catheter placement in difficult-access pediatric patients has been shown to be more successful than traditional techniques.298,299 Arterial access, including radial artery aspiration and cannulation, is more successful with US guidance.300,301 US has confirmed intravascular location with a combination of B-mode and Doppler mode, in which the needle, guidewire, and catheter can be located by US.302–304
Sonographic confirmation of intraosseous placement has also been described with detection of Doppler flow outside the bone and inside the marrow.305,306 Numerous other procedures using US guidance have been described in emergency practice (Table 196-2).
Torso Drainage Procedures
Paracentesis, including US-guided diagnostic peritoneal lavage, has been described. Static or dynamic guidance may assist this procedure by identifying the deepest location of ascitic fluid and obstructing bowel loops (Fig. 196-20). Thoracentesis is assisted by definition of the diaphragm as the most inferior anatomic landmark to avoid solid organ injury. In the left thorax, potential cardiac laceration may be avoided by the use of US. Routine use of sonography has been associated with a reduced rate of pneumothorax and tube thoracostomy.307 Pericardiocentesis also can be facilitated by location of the best window (subcostal, apical, or parasternal long) and needle guidance into the pericardial space, lessening the likelihood of ventricular puncture.
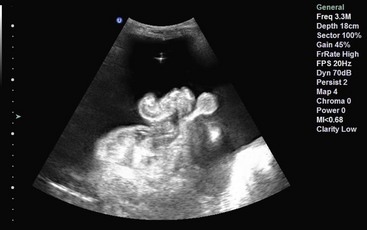
Figure 196-20 Needle entering peritoneum during paracentesis.
Soft Tissue and Musculoskeletal Procedures
Arthrocentesis and joint injection may be assisted by US to detect effusion, to identify joint landmarks, and to confirm the needle tip in the joint (Fig. 196-21). Use of US reduces the number of attempts and increases the overall success rate in non-knee joints but not in knee joints.226,231,308 Sonographic needle guidance also improves performance and outcomes in intra-articular joint injections.309
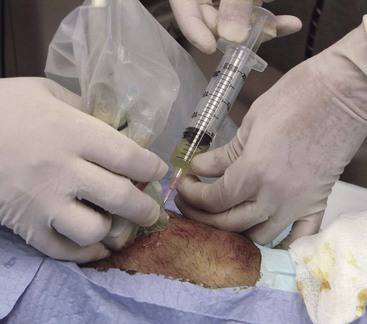
Figure 196-21 Needle entering joint space during arthrocentesis.
US may assist hematoma aspiration and blocks by directing the needle into the site.310 US-guided fracture reduction, especially in pediatric fractures, has been described with good results.
Nerve Blocks
US-guided nerve blocks confirm needle placement around the nerve and allow anesthetic to be delivered at sites around the nerve, avoiding fascicular deposition (Fig. 196-22). Multiple US-guided nerve blocks have been described in both the emergency medicine and anesthesia literature, and it is clear that most percutaneous nerve blocks can be performed with assistance of US. US-guided femoral nerve blocks produce a more complete blockade than the landmark or fascial pop techniques.311 In the setting of knee trauma, femoral nerve blocks appear to reduce pain more effectively than intravenous analgesics.312,313
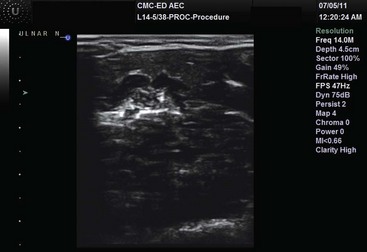
Figure 196-22 Sonographic ulnar nerve block.
Abdominal Procedures
In addition to paracentesis, described earlier, US-guided hernia reduction, suprapubic cystostomy, and gastrostomy tube placement have been described in the literature. Hernia reduction may be assisted with gentle, constant pressure over the hernia with the probe in the direction of opening through the peritoneum with resultant confirmation that the hernia has been reduced. Suprapubic aspiration and cystostomy are assisted by the detection of a full bladder with secondary guidance of the needle into the bladder for aspiration or removal. US has been touted in a pilot study to guide gastrostomy tube replacement with confirmation.314
Lumbar Puncture
Lumbar puncture can be assisted by US by the identification of the spinal processes for sonographic landmarks for placement of the needle in both the transverse and longitudinal plane, even in large patients.315,316 In addition, foraminal location can be identified, allowing a paramedian approach to lumbar puncture.
Out-of-Hospital Ultrasound: Disasters and Remote Settings
US has been used in the out-of-hospital, military battlefield, disaster, and international medicine settings. The use of US has been described in European and other settings by physicians in the out-of-hospital arena, including ground, helicopter, air, and space,9,317–323 and by paramedics for FAST and abdominal aorta examinations.324
The military forced development of small compact US machines and has been an extensive user of US in combat situations, including the frontline, combat support hospital, and tertiary hospital settings.9,11,325 US has been used in the Armenian earthquake of 1988, the Haitian earthquake of 2010, and other multiple casualty situations.326–328
US interpretation may be enhanced by satellite and telesonography systems that allow remote guidance and interpretation.329 In remote and international low-resource settings, US has become the imaging test of choice for emergency care.330,331
Integration Into Emergency Medicine Practice
US has been integrated into emergency medicine practice because of the need for effective, noninvasive, nonpainful, portable imaging techniques.14 US, whether it is introduced in medical school, residency, or clinical practice, is a skill that requires constant attention with hands-on practice and interpretation.5,332 Emergency US, the performance and interpretation of ultrasonographic examination at the bedside in the emergency setting, has enhanced many aspects of emergency care, making diagnosis and treatment more rapid, efficient, safe, and accurate.
References
1. American College of Emergency Physicians. Emergency ultrasound guidelines. Ann Emerg Med. 2009;53:550–570.
2. Durston, WE, Carl, ML, Guerra, W, Eaton, A, Ackerson, LM. Ultrasound availability in the evaluation of ectopic pregnancy in the ED: Comparison of quality and cost-effectiveness with different approaches. Am J Emerg Med. 2000;18:408–417.
3. American College of Emergency Physicians. Emergency Ultrasound Imaging Criteria Compendium. www.acep.org/WorkArea/downloadasset.aspx?id=32886, 2008.
4. Daymude M, Mehta S, Gruppo L, McManus J: Developing an emergency physician assistant formal ultrasound education program. 3rd World Congress on Ultrasound in Emergency and Critical Care Medicine; Paris, France; May 2007.
5. Cook, T, Hunt, P, Hoppman, R. Emergency medicine leads the way for training medical students in clinician-based ultrasound: A radical paradigm shift in patient imaging. Acad Emerg Med. 2007;14:558–561.
6. American College of Emergency Physicians, Section of Emergency Ultrasound. Emergency Ultrasound Fellowship Guideline—An Information Paper. www.acep.org/webportal/PracticeResources/issues/ultra/ultrasoundfellowshipguide.htm, 2006.
7. Lapostolle, F, et al. Usefulness of hand-held ultrasound devices in out-of-hospital diagnosis performed by emergency physicians. Am J Emerg Med. 2006;24:237–242.
8. Bahner, D, Yeager, S, Werman, H. Ultrasound image quality in the out-of-hospital environment. Acad Emerg Med. 2001;8:568.
9. Brooks, A, Price, V, Simms, M. FAST on operational military deployment. Emerg Med J. 2005;22:263–265.
10. Heegaard, W, et al. Ultrasound for the air medical clinician. Air Med J. 2004;23:20–23.
11. Roberts, J, McManus, J, Harrison, B. Use of ultrasonography to avoid an unnecessary procedure in the prehospital combat environment: A case report. Prehosp Emerg Care. 2006;10:502–506.
12. Zafren, K. How useful is on-mountain sonography? Wilderness Environ Med. 2001;12:230–231.
13. Blaivas, M, Lyon, M, Brannam, L. Emergency ultrasonography utility in a rural emergency department with limited radiology services. Ann Emerg Med. 2003;42:S88.
14. Kendall, JL, Hoffenberg, SR, Smith, S. History of emergency and critical care ultrasound: The evolution of a new imaging paradigm. Crit Care Med. 2007;35:S126–S130.
15. Davis, DP, Campbell, CJ, Poste, JC, Ma, G. The association between operator confidence and accuracy of ultrasonography performed by novice emergency physicians. J Emerg Med. 2005;29:259–264.
16. Blaivas, M, Pawl, R. Analysis of lawsuits filed against emergency physicians for point-of-care emergency ultrasound examination performance and interpretation over a 20-year period. Am J Emerg Med. 2012;30:338–341.
17. Mateer, J, et al. Model curriculum for physician training in emergency ultrasonography. Ann Emerg Med. 1994;23:95–102.
18. Bahner, D, Quick, G, Huff, S, Pepera, M, Fox, C, Ultrasound research design at the United States SAEM National Conference (1999-2003). September 2003.
19. American Medical Association House of Delegates. H-230.960. Privileging for Ultrasound Imaging. www.ama-assn.org/apps/pf_online/pf_online?f_n=resultLink&doc=policyfiles/HOD/H-230.960.HTM&s_t=Ultrasound&catg=AMA/HOD&&nth=1&&st_p=0&nth=2&, 2001.
20. Moore, C, Gregg, S, Lambert, M. Performance, training, quality assurance, and reimbursement of emergency physician–performed ultrasonography at Academic Medical Centers. J Ultrasound Med. 2004;23:459–466.
21. Moore, C, Molina, A, Lin, H. Ultrasonography in community emergency departments in the United States: Access to ultrasonography performed by consultants and status of emergency physician–performed ultrasonography. Ann Emerg Med. 2006;47:147–153.
22. Bahner, D, et al. AIUM practice guideline for the performance of the focused assessment with sonography for trauma (FAST) examination. J Ultrasound Med. 2008;27:313–318.
23. Labovitz, AJ, et al. Focused cardiac ultrasound in the emergent setting: A consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23:1225–1230.
24. Soremekun, OA, Noble, VE, Liteplo, AS, Brown, DF, Zane, RD. Financial impact of emergency department ultrasound. Acad Emerg Med. 2009;16:674–680.
25. Accreditation Council for Graduate Medical Education, Residency Review Committee. Emergency Medicine Guideline: Guidelines for Procedures and Resuscitations. www.acgme.org/acWebsite/RRC_110/110_guidelines.asp#res, 2006.
26. Perina, DG, et al. The 2011 model of the clinical practice of emergency medicine. Acad Emerg Med. 2012;19:e19–40.
27. Heller, M, et al. Residency training in emergency ultrasound: Fulfilling the mandate. Acad Emerg Med. 2002;9:835–839.
28. Mandavia, DP, Aragona, J, Chan, L, Chan, D, Henderson, SO. Ultrasound training for emergency physicians. Acad Emerg Med. 2000;7:1009–1014.
29. Girzadas, DV, Jr., et al. Hybrid simulation combining a high fidelity scenario with a pelvic ultrasound task trainer enhances the training and evaluation of endovaginal ultrasound skills. Acad Emerg Med. 2009;16:429–435.
30. Platz, E, Liteplo, A, Hurwitz, S, Hwang, J. Are live instructors replaceable? Computer vs. classroom lectures for EFAST training. J Emerg Med. 2011;40:534–538.
31. Platz, E, et al. Comparison of Web- versus classroom-based basic ultrasonographic and EFAST training in 2 European hospitals. Ann Emerg Med. 2010;56:660–667.
32. Chenkin, J, Lee, S, Huynh, T, Bandiera, G. Procedures can be learned on the Web: A randomized study of ultrasound-guided vascular access training. Acad Emerg Med. 2008;15:949–954.
33. Medicare Improvements for Patients and Providers Act (MIPPA) H. R. 6331 (110th). frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=110_cong.
34. Emergency Ultrasound Fellowships. www.eusfellowships.com.
35. Kremkau FW, ed. Diagnostic Ultrasound: Principles and Instruments, 4th ed, Philadelphia: WB Saunders, 1993.
36. Frazee, BW, Fahimi, J, Lambert, L, Nagdev, A. Emergency department ultrasonographic probe contamination and experimental model of probe disinfection. Ann Emerg Med. 2011;58:56–63.
37. American Institute of Ultrasound in Medicine. Guidelines for Cleaning and Preparing Endocavitary Ultrasound Transducers Between Patients. www.aium.org/publications/statements.aspx, 2003.
38. American College of Emergency Physicians. ACEP emergency ultrasound guidelines—2001. Ann Emerg Med. 2001;38:470–481.
39. Shafe, M, Blaivas, M, Hooker, E, Straus, L. Noninvasive intracranial cerebral flow velocity evaluation in the emergency department by emergency physicians. Acad Emerg Med. 2004;11:774–777.
40. Use of ultrasound imaging by emergency physicians. Ann Emerg Med. 1997;30:364–365.
41. Jones, AE, Tayal, VS, Sullivan, DM, Kline, JA. Randomized controlled trial of immediate vs. delayed goal-directed ultrasound to identify the etiology of nontraumatic hypotension in emergency department patients. Crit Care Med. 2004;32:1703–1708.
42. Scalea, TM, et al. Results from an international consensus conference. J Trauma. 1999;46:466–472.
43. Rozycki, GS, et al. A Prospective study of surgeon-performed ultrasound as the primary adjuvant modality for injured patient assessment. J Trauma. 1995;39:492–500.
44. Hoffmann, B. Sonoguide. www.sonoguide.com.
45. Kirkpatrick, AW, et al. Hand-held sonography for detecting post-traumatic pneumothoraces: The Extended Focused Assessment with Sonography for Trauma (EFAST). J Trauma. 2004;57:288–295.
46. Dulchavsky, SA, et al. Advanced ultrasonic diagnosis of extremity trauma: The FASTER examination. J Trauma. 2002;53:28–32.
47. Bode, PJ, van Vugt, AB. Ultrasound in the diagnosis of injury. Injury. 1996;27:379–383.
48. Boulanger, BR, et al. A prospective study of emergent abdominal sonography after blunt trauma. J Trauma. 1996;39:325–330.
49. Branney, SW, et al. Quantitative sensitivity off ultrasound in detecting free intraperitoneal fluid. J Trauma. 1995;39:375–380.
50. Plummer, D, Brunnette, D, Asinger, R, Ruiz, E. Emergency department echocardiography improves outcome in penetrating cardiac injury. Ann Emerg Med. 1992;21:709–712.
51. Abboud, P, Kendall, J. Can emergency department ultrasound be used reliably for detecting hemothorax after blunt traumatic injury? Acad Emerg Med. 2001;8:579.
52. Ma, OJ, Kefer, MP, Mateer, JR, Thoma, B. Evaluation of hemoperitoneum using a single- vs multiple-view ultrasonographic examination. Acad Emerg Med. 1995;2:581–586.
53. Gangahar, R, Doshi, D. FAST scan in the diagnosis of acute diaphragmatic rupture. Am J Emerg Med. 2010;28:e1–3.
54. Nordenholz, KE, Rubin, MA, Gularte, GG, Liang, HK. Ultrasound in the evaluation and management of blunt abdominal trauma. Ann Emerg Med. 1997;29:357–366.
55. Soyuncu, S, Cete, Y, Bozan, H, Kartal, M, Akyol, A. Accuracy of physical and ultrasonographic examinations by emergency physicians for the early diagnosis of intraabdominal haemorrhage in blunt abdominal trauma. Injury. 2007;38:564–569.
56. Dolich, M, et al. 2576 Ultrasounds for blunt abdominal trauma. J Trauma. 2001;50:108–112.
57. Gaarder, C, et al. Ultrasound performed by radiologists—confirming the truth about FAST in trauma. J Trauma. 2009;67:323–327.
58. Bauwens, SD, Rademacher, SJ, Mutze, S, Ekkernkamp, A, Porzsolt, F. Emergency ultrasound-based algorithms for diagnosing blunt abdominal trauma [review]. Cochrane Collaboration. 2006;2:1–19.
59. Melniker, LA, et al. Randomized controlled clinical trial of point-of-care, limited ultrasonography for trauma in the emergency department: The First Sonography Outcomes Assessment Program trial. Ann Emerg Med. 2006;48:227–235.
60. Moylan, M, et al. Association between a positive ED FAST examination and therapeutic laparotomy in normotensive blunt trauma patients. J Emerg Med. 2007;33:265–271.
61. Schiavone, WA, et al. The use of echocardiography in the emergency management of nonpenetrating traumatic cardiac rupture. Ann Emerg Med. 1991;20:1248–1250.
62. Abboud, PA, Kendall, J. Emergency department ultrasound for hemothorax after blunt traumatic injury. J Emerg Med. 2003;25:181–184.
63. Duggal, S, Blaivas, M, Brannam, L, Lyon, M. A prospective comparison of supine chest x-ray and bedside ultrasound for diagnosis of traumatic pneumothorax. Acad Emerg Med. 2004;11:579.
64. Tayal, VS, Beatty, MB, Marx, JA, Tomaszewski, CA, Thomason, MH. FAST (focused assessment with sonography in trauma) accurate for cardiac and intraperitoneal injury in penetrating anterior chest trauma. J Ultrasound Med. 2004;23:467–472.
65. Rochester, AL, Tayal, VS, Marx, JA, Thomason, MH. The accuracy of trauma ultrasound in penetrating trauma to the anterior abdomen. Acad Emerg Med. 2006;13:S142.
66. Udobi, KF, Rodriquez, A, Chiu, WC, Scalea, TM. Role of ultrasonography in penetrating abdominal trauma: A prospective clinical study. J Trauma. 2001;50:475–479.
67. Fox, JC, et al. Test characteristics of focused assessment of sonography for trauma for clinically significant abdominal free fluid in pediatric blunt abdominal trauma. Acad Emerg Med. 2011;18:477–482.
68. Ma, OJ, Mateer, JR, DeBehnke, DJ. Use of ultrasonography for the evaluation of pregnant trauma patients. J Trauma. 1996;40:665–668.
69. Tayal, VS, et al. Accuracy of trauma ultrasound in major pelvic injury. J Trauma. 2006;61:1453–1457.
70. Friese, R, Malekzadeh, S, Shafi, S, Gentilello, L, Starr, A. Abdominal ultrasound is an unreliable modality for the detection of hemoperitoneum in patients with pelvic fracture. J Trauma. 2007;63:97–102.
71. Jones, AE, Mason, PE, Tayal, VS, Gibbs, MA. Sonographic intraperitoneal fluid in patients with pelvic fracture: Two cases of traumatic intraperitoneal bladder rupture. J Emerg Med. 2003;25:373–377.
72. Hoffman, L, Pierce, D, Puumala, S. Clinical predictors of injuries not identified by focused abdominal sonogram for trauma (FAST) examinations. J Emerg Med. 2009;36:271–279.
73. Mateer, JR, Aiman, EJ, Brown, MH, Olson, DW. Ultrasonographic examination by emergency physicians of patients at risk for ectopic pregnancy. Acad Emerg Med. 1995;2:867–873.
74. Mateer, JR, et al. Outcome analysis of a protocol including bedside endovaginal sonography in patients at risk for ectopic pregnancy. Ann Emerg Med. 1996;27:283–289.
75. Dart, R. Role of pelvic ultrasonography in evaluation of symptomatic first-trimester pregnancy. Ann Emerg Med. 1999;33:310–320.
76. Durham, B, Lane, B, Burbridge, L, Balasubramaniam, S. Pelvic ultrasound performed by emergency physicians for the detection of ectopic pregnancy in complicated first-trimester pregnancies. Ann Emerg Med. 1997;29:338–347.
77. Schlager, D, Lazzarreschi, G, Whitten, D, Sanders, AB. A prospective study of ultrasonography in the ED by emergency physicians. Am J Emerg Med. 1994;12:185–189.
78. Tayal, VS, Cohen, H, Norton, J. Outcome of patients with indeterminate first-trimester emergency department pelvic ultrasound. Acad Emerg Med. 2004;11:526.
79. Stein, JC, et al. Emergency physician ultrasonography for evaluating patients at risk for ectopic pregnancy: A meta-analysis. Ann Emerg Med. 2010;56:674–683.
80. Rodgerson, JD, et al. Emergency department right upper quadrant ultrasound reduces time to diagnosis and treatment of ruptured ectopic pregnancies. Acad Emerg Med. 1999;6:540–541.
81. Press, G, Martinez, A. Heterotopic pregnancy diagnosed by emergency ultrasound. J Emerg Med. 2007;33:25–27.
82. Shih, CH. Effect of emergency physician–performed pelvic sonography on length of stay in the emergency department. Ann Emerg Med. 1997;29:348–352.
83. Burgher, S, Tandy, T, Dawdy, M. Transvaginal ultrasonography by emergency physicians decreases patient time in the emergency department. Acad Emerg Med. 1998;5:802–807.
84. Blaivas, M, Bell, G. Benefit from emergency physician identified ectopic pregnancy using bedside ultrasound. Acad Emerg Med. 2000;7:500.
85. Adhikari, S, Blaivas, M, Lyon, M. Diagnosis and management of ectopic pregnancy using bedside transvaginal ultrasonography in the ED: A 2-year experience. Am J Emerg Med. 2007;25:591–596.
86. Juliano, M, Dabulis, S, Heffner, A. Characteristics of women with fetal loss in symptomatic first trimester pregnancies with documented fetal cardiac activity. Ann Emerg Med. 2008;52:143–147.
87. Choi, H, Blaivas, M. Gestational outcome in patients with first-trimester pregnancy complications and ultrasound-confirmed live intrauterine pregnancy. Acad Emerg Med. 2000;7:200–203.
88. Shah, S, et al. Accuracy of emergency physicians using ultrasound to determine gestational age in pregnant women. Am J Emerg Med. 2010;28:834–838.
89. Sergel, MJ, Greenberg, DT. Live twin ectopic pregnancy with advanced gestation. J Emerg Med. 2009;37:77–78.
90. Nicks, BA, Fitch, MT, Manthey, DE. A case of intrauterine molar pregnancy with coexistent ectopic pregnancy. J Emerg Med. 2009;36:246–249.
91. Kouliev, T, Cervenka, K. Emergency ultrasound in cervical ectopic pregnancy. J Emerg Med. 2010;38:55–56.
92. Gaber-Patel, K, Smith, MD. Thirteen-week cornual ectopic pregnancy. Am J Emerg Med. 2009;27:900.e1–900.e2.
93. Corrigan, KJ, Kowalzyk, DR. Ectopic ovarian pregnancy in a second-trimester patient. Am J Emerg Med. 2007;25:1085.e3–1085.e4.
94. Saul, T, Sonson, J. Molar pregnancy. J Emerg Med. 2011;40:e39–e40.
95. Amin, S, Weekes, A. Live conjoined twins: A rare first trimester diagnosis during emergency department sonography. J Emerg Med. 2010;39:e105–e108.
96. Dabulis, S, McGuirk, T. An unusual case of hemoperitoneum: Uterine rupture at 9 weeks gestational age. J Emerg Med. 2007;33:285–287.
97. Adhikari, S, Blaivas, M, Lyon, M. Role of bedside transvaginal ultrasonography in the diagnosis of tubo-ovarian abscess in the emergency department. J Emerg Med. 2008;34:429–433.
98. Tayal, V, et al. ED endovaginal pelvic ultrasound in nonpregnant women with right lower quadrant pain. Am J Emerg Med. 2008;26:81–85.
99. Quick, G. Ultrasound: Where will the puck be? ACEPs The Connection. 2000:1–7.
100. Mark, D, et al. Directed bedside transthoracic echocardiography: Preferred cardiac window for left ventricular ejection fraction estimation in critically ill patients. Am J Emerg Med. 2007;25:284–900.
101. Levinson, MM, Boniface, KS, Adler, JD, Miller, K. Emergency physician performance of single parasternal long-axis view to visually estimate left ventricular ejection fraction. Acad Emerg Med. 2005;12(Suppl 1):48-b.
102. Tayal, VS, Kline, JA. Emergent echocardiographic detection of pericardial effusion in patients with hemodynamic collapse. Acad Emerg Med. 1999;6:540.
103. Hernandez, C, et al. C.A.U.S.E.: Cardiac arrest ultra-sound exam—a better approach to managing patients in primary non-arrhythmogenic cardiac arrest. Resuscitation. 2008;76:198–206.
104. Amaya, SC, Langsam, A. Ultrasound detection of ventricular fibrillation disguised as asystole. Ann Emerg Med. 1999;33:344–346.
105. Tsung, JW, Blaivas, M. Feasibility of correlating the pulse check with focused point-of-care echocardiography during pediatric cardiac arrest: A case series. Resuscitation. 2008;77:264–269.
106. Breitkreutz, R, et al. Focused echocardiographic evaluation in life support and peri-resuscitation of emergency patients: A prospective trial. Resuscitation. 2010;81:1527–1533.
107. Volpicelli, G. Usefulness of emergency ultrasound in nontraumatic cardiac arrest. Am J Emerg Med. 2011;29:216–223.
108. Price, S, et al. Peri-resuscitation echocardiography: Training the novice practitioner. Resuscitation. 2010;81:1534–1539.
109. Mandavia, D, Hoffner, R, Mahaney, K, Henderson, S. Bedside echocardiography by emergency physicians. Ann Emerg Med. 2001;38:377–382.
110. Nagdev, A, Stone, MB. Point-of-care ultrasound evaluation of pericardial effusions: Does this patient have cardiac tamponade? Resuscitation. 2011;82:671–673.
111. Blaivas, M. Incidence of pericardial effusion in patients presenting to the emergency department with unexplained dyspnea. Acad Emerg Med. 2001;8:1143–1146.
112. Sierzenski, PR, O’Connor, RE. Emergency physician echocardiography reduces cost of hospitalization for patients with pericardial effusion. Acad Emerg Med. 2005;12(Suppl 1):77–b.
113. Lainscak, M, Pernat, A. Importance of bedside echocardiography for detection of unsuspected isolated right ventricular infarction as a cause of cardiovascular collapse. Am J Emerg Med. 2007;25:110–114.
114. Mandavia, DP, Mallon, WK. Using bedside ultrasonography to evaluate trauma patients. J Crit Illness. 2000;15:387–394.
115. Fojtik, JP, Handly, N, Dean, AJ, Costantino, TG. The prevalence of pericardial effusion in patients presenting to two urban emergency departments. Ann Emerg Med. 2002;40:S58.
116. Bova, C, et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med. 2003;21:180–183.
117. Rose, J, Bair, A, Mandavia, D, Kinser, D. The UHP ultrasound protocol: A novel ultrasound approach to the empiric evaluation of the undifferenttiated hypotensive patient. Am J Emerg Med. 2001;19:299–302.
118. Bahner, DP. Trinity: A hypotensive ultrasound protocol. J Diagn Med Sonogr. 2002;18:193–198.
119. Moore, C, et al. Determination of left ventricular function by emergency physician echocardiography of hypotensive patients. Acad Emerg Med. 2002;9:186–193.
120. Jones, A, Craddock, P, Tayal, V, Kline, J, Diagnostic accuracy of left ventricular function for identifying sepsis among emergency department patients with non-traumatic undifferentiated hypotension. Shock. 2005;(24):513–517.
121. Chen, L, Kim, Y, Santucci, K. Use of ultrasound measurement of the inferior vena cava diameter as an objective tool in the assessment of children with clinical dehydration. Acad Emerg Med. 2007;14:841–845.
122. Lyon, M, Blaivas, M, Brannam, L. Sonographic measurement of the inferior vena cava as a marker of blood loss. Am J Emerg Med. 2005;23:45–50.
123. Blehar, DJ, Dickman, E, Gaspari, R. Identification of congestive heart failure via respiratory variation of inferior vena cava diameter. Am J Emerg Med. 2009;27:71–75.
124. Wang, HK, et al. Cardiac ultrasound helps for differentiating the causes of acute dyspnea with available B-type natriuretic peptide tests. Am J Emerg Med. 2010;28:987–993.
125. Carr, BG, et al. Intensivist bedside ultrasound (INBU) for volume assessment in the intensive care unit: A pilot study. J Trauma. 2007;63:495–500.
126. Ferrada, P, Murthi, S, Anand, RJ, Bochicchio, GV, Scalea, T. Transthoracic focused rapid echocardiographic examination: Real-time evaluation of fluid status in critically ill trauma patients. J Trauma. 2011;70:56–62.
127. Nagdev, AD, Merchant, RC, Tirado-Gonzalez, A, Sisson, CA, Murphy, MC. Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55:290–295.
128. Schefold, JC, et al. Inferior vena cava diameter correlates with invasive hemodynamic measures in mechanically ventilated intensive care unit patients with sepsis. J Emerg Med. 2010;38:632–637.
129. Lamia, B, et al. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33:1125–1132.
130. Donahue, SP, Wood, JP, Patel, BM, Quinn, JV. Correlation of sonographic measurements of the internal jugular vein with central venous pressure. Am J Emerg Med. 2009;27:851–855.
131. Jain, S, et al. Noninvasive Doppler ultrasonography for assessing cardiac function: Can it replace the Swan-Ganz catheter? Am J Surg. 2008;196:961–967.
132. Tayal, VS, Graf, CD, Gibbs, MA. Prospective study of accuracy and outcome of emergency ultrasound for abdominal aortic aneurysm over two years. Acad Emerg Med. 2003;10:867–871.
133. Costantino, TG, Bruno, EC, Handly, N, Dean, AJ. Accuracy of emergency medicine ultrasound in the evaluation of abdominal aortic aneurysm. J Emerg Med. 2005;29:455–460.
134. Sherman, S, Cosby, K. Emergency physician ultrasonography—aortic dissection. J Emerg Med. 2004;26:217–218.
135. Sierzenski, P, Dickman, E, Leech, S, Gukhook, J, Bollinger, M. Emergency physician ultrasound decreases physician times to diagnosis, beta-blocker therapy, and operative repair in patients with acute aortic dissection. Acad Emerg Med. 2004;11:580–581.
136. Fojtik, J, Costantino, T, Dean, A. The diagnosis of aortic dissection by emergency medicine ultrasound. J Emerg Med. 2007;32:191–196.
137. Ernst, CB. Abdominal aortic aneurysm. N Engl J Med. 1999;328:1167–1172.
138. Kuhn, M, Bonnin, RL, Davey, MJ, Rowland, JL, Langlois, SL. Emergency department ultrasound scanning for abdominal aortic aneurysm: Accessible, accurate, and advantageous. Ann Emerg Med. 2000;36:219–223.
139. Knaut, AL, Kendall, JL, Patten, R, Ray, C. Ultrasonographic measurement of aortic diameter by emergency physicians approximates results obtained by computed tomography. J Emerg Med. 2005;28:119–126.
140. Dent, B, Kendall, RJ, Boyle, AA, Atkinson, PR. Emergency ultrasound of the abdominal aorta by UK emergency physicians: A prospective cohort study. Emerg Med J. 2007;24:547–549.
141. Moore, CL, Holliday, RS, Hwang, JQ, Osborne, MR. Screening for abdominal aortic aneurysm in asymptomatic at-risk patients using emergency ultrasound. Am J Emerg Med. 2008;26:883–887.
142. Melanson, S, et al. Can an emergency department ultrasound screening program identify abdominal aortic aneurysm in asymptomatic geriatric patients? Ann Emerg Med. 2000;36:S21.
143. Schermerhorn, ML, et al. Defining perioperative mortality after open and endovascular aortic aneurysm repair in the US Medicare population. J Am Coll Surg. 2011;212:349–355.
144. Monge, M, Eskandari, MK. Strategies for ruptured abdominal aortic aneurysms. J Vasc Interv Radiol. 2008;19(Suppl):S44–S50.
145. Roxas, R, Gallegos, L, Bailitz, J. Rapid detection of aortic occlusion with emergency ultrasonography. Ann Emerg Med. 2011;58:21–23.
146. Durston, W, et al. Comparison of quality and cost-effectiveness in the evaluation of symptomatic cholelithiasis with different approaches to ultrasound availability in the ED. Am J Emerg Med. 2001;19:260–269.
147. Rosen, CL, et al. Gallbladder ultrasonography: Agreement between emergency physicians and radiologists. Acad Emerg Med. 1998;5:417.
148. Blaivas, M, Harwood, RA, Lambert, MJ. Decreasing length of stay with emergency department gallbladder ultrasonography. Acad Emerg Med. 1999;6:541.
149. Nelson, M, Raio, C, Gerardo, C, Theodoro, D, Chun, P. Can emergency physicians utilize the S. I. N. phenomenon (stone impacted in gallbladder neck) to accurately diagnose cholecystitis? Ann Emerg Med. 2005;46:80.
150. Summers, SM, et al. A prospective evaluation of emergency department bedside ultrasonography for the detection of acute cholecystitis. Ann Emerg Med. 2010;56:114–122.
151. Miller, AH, Delaney, KA, Brockman, CR, Pepe, PE. ED ultrasound in hepatobiliary disease. J Emerg Med. 2006;30:69–74.
152. Jang, TB, Ruggeri, W, Dyne, P, Kaji, AH. The learning curve of resident physicians using emergency ultrasonography for cholelithiasis and cholecystitis. Acad Emerg Med. 2010;17:1247–1252.
153. Durston, W, et al. Comparison of quality and cost-effectiveness in the evaluation of symptomatic cholelithiasis with different approaches to ultrasound availability in the ED. Am J Emerg Med. 2001;19:260–269.
154. Blaivas, M, Adhikari, S. Diagnostic utility of cholescintigraphy in emergency department patients with suspected acute cholecystitis: Comparison with bedside RUQ ultrasonography. J Emerg Med. 2007;33:47–52.
155. Gaspari, RJ, Horst, K. Emergency ultrasound and urinalysis in the evaluation of flank pain. Acad Emerg Med. 2005;12:1180–1184.
156. Kartal, M, Eray, O, Erdogru, T, Yilmaz, S. Prospective validation of a current algorithm including bedside US performed by emergency physicians for patients with acute flank pain suspected for renal colic. Emerg Med J. 2006;23:341–344.
157. Henderson, SO, et al. Bedside emergency department ultrasonography plus radiography of the kidneys, ureters, and bladder vs intravenous pyelography in the evaluation of suspected ureteral colic. Acad Emerg Med. 1998;8:666–671.
158. McCans, KW, Baumann, B. Use of ultrasound bladder volume determinations decreases the number of unsuccessful catheterization attempts in pediatric emergency department patients. Acad Emerg Med. 2004;11:446.
159. Baumann, B, et al. Volumetric bladder ultrasound performed by trained nurses increases catheterization success in pediatric patients. Am J Emerg Med. 2008;26:18–23.
160. Chen, L, Hsiao, A, Moore, C, Santucci, K. Utility of bedside bladder ultrasound prior to urethral catheterization in infants. Acad Emerg Med. 2004;11:598.
161. Blaivas, M, Lambert, MJ, Harwood, RC, Wood, JP, Konicki, JP. Lower extremity Doppler for deep venous thrombosis—can emergency physicians be accurate and fast? Acad Emerg Med. 2000;6:120–126.
162. Frazee, BW, Snooey, ER, Levitt, A. Emergency department compression ultrasound to diagnose proximal deep vein thrombosis. J Emerg Med. 2001;20:107–111.
163. Jacoby, J, et al. Can emergency medicine residents detect acute deep venous thrombosis with a limited, two-site ultrasound examination? J Emerg Med. 2006;32:197–200.
164. Kline, JA, et al. Emergency clinician–performed compression ultrasonography for deep venous thrombosis of the lower extremity. Ann Emerg Med. 2008;52:437–445.
165. Jang, T, Docherty, M, Aubin, C, Polites, G. Resident-performed compression ultrasonography for the detection of proximal deep vein thrombosis: Fast and accurate. Acad Emerg Med. 2004;4:319–322.
166. Leech, SJ, Gukhool, JA, Dickman, E, Sierzenski, PR. Emergency physician–performed ultrasound accurately identifies upper extremity deep venous thrombosis. Acad Emerg Med. 2004;11:585–586.
167. Magazzini, S, et al. Duplex ultrasound in the emergency department for the diagnostic management of clinically suspected deep vein thrombosis. Acad Emerg Med. 2007;14:216–220.
168. Crisp, JG, Lovato, LM, Jang, TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56:601–610.
169. Burnside, PR, Brown, MD, Kline, JA. Systematic review of emergency physician–performed ultrasonography for lower-extremity deep vein thrombosis. Acad Emerg Med. 2008;15:493–498.
170. Morrison, D, Lyon, M, Blaivas, M. Emergency physicians decrease hospital charges by performing bedside ultrasound for deep venous thrombosis. Acad Emerg Med. 2005;12(Suppl 1):79.
171. Theodoro, DL, Blaivas, M, Duggal, D, Snyder, G, Lucas, M. Emergency physician–performed lower extremity Doppler results in significant time savings. Acad Emerg Med. 2002;9:541.
172. Farahmand, S, Farnia, M, Shahriaran, S, Khashayar, P. The accuracy of limited B-mode compression technique in diagnosing deep venous thrombosis in lower extremities. Am J Emerg Med. 2011;29:687–690.
173. Bernardi, E, et al. Serial 2-point ultrasonography plus D-dimer vs whole-leg color-coded Doppler ultrasonography for diagnosing suspected symptomatic deep vein thrombosis: A randomized controlled trial. JAMA. 2008;300:1653–1659.
174. Reissig, A, Copetti, R, Kroegel, C. Current role of emergency ultrasound of the chest. Crit Care Med. 2011;39:839–845.
175. Lichtenstein, D, Meziere, G, Biderman, P, Gepner, A. The comet-tail artifact: An ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25:383–388.
176. Lichtenstein, DA, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33:1231–1238.
177. Soldati, G, et al. The ultrasonographic deep sulcus sign in traumatic pneumothorax. Ultrasound Med Biol. 2006;32:1157–1163.
178. Blaivas, M, Lyon, M, Duggal, S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med. 2005;12:844–849.
179. Simon, BC, Paolinetti, L. Two cases where bedside ultrasound was able to distinguish pulmonary bleb from pneumothorax. J Emerg Med. 2005;29:201–205.
180. Dente, C, et al. The accuracy of thoracic ultrasound for detection of pneumothorax is not sustained over time: A preliminary study. J Trauma. 2007;62:1384–1389.
181. Parlamento, S, Copetti, R, Di Bartolomeo, S. Evaluation of lung ultrasound for the diagnosis of pneumonia in the ED. Am J Emerg Med. 2009;27:379–384.
182. Gillman, LM, Panebianco, N, Alkadi, A, Blaivas, M, Kirkpatrick, AW. The dynamic sonographic air bronchogram: A simple and immediate bedside diagnosis of alveolar consolidation in severe respiratory failure. J Trauma. 2011;70:760.
183. Picano, E, et al. Ultrasound lung comets: A clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356–363.
184. Fagenholz, P, et al. Chest ultrasonography for the diagnosis and monitoring of high-altitude pulmonary edema. Chest. 2007;131:1013–1018.
185. Liteplo, AS, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): Sonographic B-lines and N-terminal pro-brain–type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med. 2009;16:201–210.
186. Weaver, B, Blaivas, M, Lyon, M. Confirmation of endotracheal tube placement after intubation using the sliding lung sign. Acad Emerg Med. 2005;12:91–92.
187. Milling, T, et al. Transtracheal 2-D ultrasound for identification of esophageal intubation. J Emerg Med. 2007;32:409–414.
188. Muslu, B, et al. Use of sonography for rapid identification of esophageal and tracheal intubations in adult patients. J Ultrasound Med. 2011;30:671–676.
189. Ma, G, et al. The sensitivity and specificity of transcricothyroid ultrasonography to confirm endotracheal tube placement in a cadaver model. J Emerg Med. 2007;20:1–3.
190. Galicinao, J, Bush, A, Godambe, S. Use of bedside ultrasonography for endotracheal tube placement in pediatric patients: A feasibility study. Pediatrics. 2007;120:1297–1303.
191. Blaivas, M. Bedside emergency department ultrasonography in the evaluation of ocular pathology. Acad Emerg Med. 2000;7:947–950.
192. Blaivas, M, Theodoro, DL, Sierzenski, PR. A study of bedside ocular ultrasonography in the emergency department. Acad Emerg Med. 2002;9:791–799.
193. Chiao, L, et al. Ocular examination for trauma; clinical ultrasound aboard the International Space Station. J Trauma. 2005;58:885–889.
194. Harries, A, Shah, S, Teismann, N, Price, D, Nagdev, A. Ultrasound assessment of extraocular movements and pupillary light reflex in ocular trauma. Am J Emerg Med. 2010;28:956–959.
195. Shiver, S, Lyon, M, Blaivas, M. Detection of ocular foreign bodies using compact ultrasound. Acad Emerg Med. 2005;12(Suppl 1):74.
196. McIlrath, ST, Blaivas, M, Lyon, M. Diagnosis of periorbital gas on ocular ultrasound after facial trauma. Am J Emerg Med. 2005;23:517–520.
197. Yoonessi, R, Hussain, A, Jang, TB. Bedside ocular ultrasound for the detection of retinal detachment in the emergency department. Acad Emerg Med. 2010;17:913–917.
198. Shinar, Z, Chan, L, Orlinsky, M. Use of ocular ultrasound for the evaluation of retinal detachment. J Emerg Med. 2011;40:53–57.
199. Tayal, V, et al. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007;49:508–514.
200. Jang, T, Aubin, C. The use of serial ocular ultrasonography in the care of patients with head injury. Ann Emerg Med. 2005;45:336–337.
201. Tsung, JW, Blaivas, M, Cooper, A, Levick, NR. A rapid noninvasive method of detecting elevated intracranial pressure using bedside ocular ultrasound: Application to 3 cases of head trauma in the pediatric emergency department. Pediatr Emerg Care. 2005;21:94–98.
202. Kimberly, H, Shah, S, Marill, K, Noble, V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15:201–204.
203. Le, A, et al. Bedside sonographic measurement of optic nerve sheath diameter as a predictor of increased intracranial pressure in children. Ann Emerg Med. 2009;53:785–791.
204. Page-Wills, C, Simon, BC, Christy, D, Levitt, MA. Utility of ultrasonography on emergency department management of suspected cutaneous abscess. Acad Emerg Med. 2000;7:493.
205. Squire, B, Fox, J, Zlidenny, A, Barajas, G. ABSCESS: Applied bedside sonography for convenient evaluation of superficial soft tissue infections. Ann Emerg Med. 2004;44:S62.
206. Tayal, VS, Hasan, N, Norton, HJ, Tomaszewski, CA. The effect of soft-tissue ultrasound on the management of cellulitis in the emergency department. Acad Emerg Med. 2006;13:384–388.
207. Charlton, N, Cook, T. Clinician-based ultrasound facilitates the evaluation of lateral neck mass in the ED. Am J Emerg Med. 2008;26:386.e3–386.e6.
208. Jenq, KY, Panebianco, NL, Lee, PA, Chen, EH, Dean, AJ. Diagnosis of a facial artery pseudoaneurysm using emergency bedside ultrasound. J Emerg Med. 2010;38:642–644.
209. Morrison, D, Blaivas, M, Lyon, M. Emergency diagnosis of Fournier’s gangrene with bedside ultrasound. Am J Emerg Med. 2005;23:544–547.
210. Yen, ZS, Wang, HP, Ma, HM, Chen, SC, Chen, WJ. Ultrasonographic screening of clinically-suspected necrotizing fasciitis. Acad Emerg Med. 2002;9:1448–1451.
211. Sivitz, AB, Lam, SH, Ramirez-Schrempp, D, Valente, JH, Nagdev, AD. Effect of bedside ultrasound on management of pediatric soft-tissue infection. J Emerg Med. 2010;39:637–643.
212. Gaspari, RJ, Resop, D, Mendoza, M, Kang, T, Blehar, D. A randomized controlled trial of incision and drainage versus ultrasonographically guided needle aspiration for skin abscesses and the effect of methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2011;57:483–491.
213. Blaivas, M, Theodoro, D, Duggal, S. Ultrasound-guided drainage of peritonsillar abscess by the emergency physician. Am J Emerg Med. 2003;21:155–158.
214. Lyon, M, Glisson, P, Blaivas, M. Bilateral peritonsillar abscess diagnosed on the basis of intraoral sonography. J Ultrasound Med. 2003;22:993–996.
215. Gaspari, R. Bedside ultrasound of the soft tissue of the face: A case of early Ludwig’s angina. J Emerg Med. 2006;31:287–291.
216. Adhikari, S, Blaivas, M, Lander, L. Comparison of bedside ultrasound and Panorex radiography in the diagnosis of a dental abscess in the ED. Am J Emerg Med. 2011;29:790–795.
217. Hung, TY, et al. Bedside ultrasonography as a safe and effective tool to diagnose acute epiglottitis. Am J Emerg Med. 2011;29:359.e1–359.e3.
218. Nomura, JT, Sierzenski, PR. Ultrasound diagnosis of penile fracture. J Emerg Med. 2010;38:362–365.
219. Mahler, SA, Manthey, DE. Diagnosis of a preputial cavity abscess with bedside ultrasound in the emergency department. J Emerg Med. 2008;35:273–276.
220. Jacobson, JA, Powell, A, Craig, JG, Bouffard, JA, van Holsbeeck, MT. Wooden foreign bodies in soft tissue: Detection at US. Radiology. 1998;206:45–48.
221. Turkcuer, I, et al. Do we really need plain and soft-tissue radiographies to detect radiolucent foreign bodies in the ED? Am J Emerg Med. 2006;24:763–768.
222. Schlager, D, Sanders, AB, Wiggins, D, Boren, W. Ultrasound for the detection of foreign bodies. Ann Emerg Med. 1991;20:189–191.
223. Raio, CC, et al. Do patients who have had negative focused emergency department lower extremity ultrasounds to rule out deep venous thrombosis receive their necessary repeat ultrasounds in 5-7 days? Acad Emerg Med. 2006;13:S116–a.
224. Valley, VT. Clinical applications of musculoskeletal ultrasound scanning in an emergency department setting. Ann Emerg Med. 2001;38:S99.
225. Valley, VT, Stahmer, SA. Targeted musculoarticular sonography in the detection of joint effusions. Acad Emerg Med. 2001;8:361–367.
226. Roy, S, Dewitz, A, Paul, I. Ultrasound-assisted ankle arthrocentesis. Am J Emerg Med. 1999;17:300–301.
227. Tayal, V, Pariyadath, M, Norton, J. Randomized controlled trial of ultrasound-guided peripheral non-knee arthrocentesis in the emergency department. Acad Emerg Med. 2006;13:S122-b–123S-b.
228. Tayal, V, Pariyadath, M, Norton, J. Prospective use of ultrasound imaging to detect bony hand injuries in adults. J Ultrasound Med. 2007;26:1143–1148.
229. Tsung, JW, Blaivas, M. Emergency department diagnosis of pediatric hip effusion and guided arthrocentesis using point-of-care ultrasound. J Emerg Med. 2008;35:393–399.
230. LaRocco, BG, Ziupko, G, Sierzenski, P. Ultrasound diagnosis of quadriceps tendon rupture. J Emerg Med. 2008;35:293–295.
231. Freeman, K, Dewitz, A, Baker, W. Ultrasound guided hip arthrocentesis in the ED. Am J Emerg Med. 2007;2(5):80–86.
232. Vieira, RL, Levy, JA. Bedside ultrasonography to identify hip effusions in pediatric patients. Ann Emerg Med. 2010;55:284–289.
233. Valley, VS, Shermer, CD. Use of musculoskeletal ultrasonography in the diagnosis of pes anserine tendinitis: A case report. J Emerg Med. 2001;20:43–45.
234. Sisson, C, Nagdev, A, Tirado, A, Murphy, M, Suner, S. Ultrasound diagnosis of traumatic partial triceps tendon tear in the emergency department. J Emerg Med. 2011;40:436–438.
235. Nagdev, A, Murphy, M, Sisson, C. Bedside ultrasound for the detection of diabetic myonecrosis. Am J Emerg Med. 2008;26:969.e3–969.e4.
236. Sierzenski, PR, Leech, SJ, Gukhool, J, Blaivas, M. ED ultrasound evaluation of the index flexor tendon: A comparison of water-bath evaluation technique (WET) versus direct contact ultrasound. Acad Emerg Med. 2003;10:573.
237. Marshburn, TH, et al. Goal-directed ultrasound in the detection of long-bone fractures. J Trauma. 2004;57:329–332.
238. Chan, SS. Emergency bedside ultrasound for the diagnosis of rib fractures. Am J Emerg Med. 2009;27:617–620.
239. Kirkpatrick, AW, et al. Rapid diagnosis of an ulnar fracture with portable hand-held ultrasound. Mil Med. 2003;168:312–313.
240. O’Malley, P, Tayal, VS. Use of emergency musculoskeletal sonography in diagnosis of an open fracture of the hand. J Ultrasound Med. 2007;26:679–682.
241. Durston, W, Swartzentruber, R. Ultrasound guided reduction of pediatric forearm fractures in the ED. Am J Emerg Med. 2000;18:72–77.
242. Chinnock, B, Khaletskiy, A, Kuo, K, Hendey, GW. Ultrasound-guided reduction of distal radius fractures. J Emerg Med. 2011;40:308–312.
243. Ang, SH, Lee, SW, Lam, KY. Ultrasound-guided reduction of distal radius fractures. Am J Emerg Med. 2010;28:1002–1008.
244. Halberg, MJ, Sweeney, TW, Owens, WB. Bedside ultrasound for verification of shoulder reduction. Am J Emerg Med. 2009;27:134.e5–134.e6.
245. Carl, ML, Durston, WE. Survey of staff opinions on ultrasound by emergency physicians. Am J Emerg Med. 2000;18:342.
246. Costantino, TG, Roemer, B, Leber, EH. Septic arthritis and bursitis: Emergency ultrasound can facilitate diagnosis. J Emerg Med. 2007;32:295–297.
247. Shiver, SA, Blaivas, M. Acute lower extremity pain in an adult patient secondary to bilateral popliteal cysts. J Emerg Med. 2008;34:315–318.
248. Blaivas, M, Sierzenski, P, Lambert, M. Emergency evaluation of patients presenting with acute scrotum using bedside ultrasonography. Acad Emerg Med. 2001;8:90–93.
249. Fox, JC, et al. Retrospective analysis of emergency department ultrasound for acute appendicitis. Cal J Emerg Med. 2007;8:41–45.
250. Fox, JC, Solley, M, Zlidenny, A, Anderson, C. Bedside ultrasound for appendicitis. Acad Emerg Med. 2005;12(Suppl 1):76.
251. Halm, BM, Eakin, PJ, Franke, AA. Diagnosis of appendicitis by a pediatric emergency medicine attending using point-of-care ultrasound: A case report. Hawaii Med J. 2010;69:208–211.
252. Halm, BM, Boychuk, RB, Franke, AA. Diagnosis of intussusception using point-of-care ultrasound in the pediatric ED: A case report. Am J Emerg Med. 2011;29:354.e1–354.e3.
253. Baker, JB, Mandavia, D, Swadron, SP. Diagnosis of diverticulitis by bedside ultrasound in the emergency department. J Emerg Med. 2006;30:327–329.
254. Chin, LW, Hsu, CK, Wang, HP. Ultrasonographic diagnosis of elderly midgut volvulus in the ED. Am J Emerg Med. 2006;24:900–902.
255. Chin, L-W, Chou, M-C, Wang, H-P, Bell, W. Ultrasonography diagnosis of occult obturator hernia presenting as intestinal obstruction in ED [letter]. Am J Emerg Med. 2005;23:237–239.
256. Moriwaki, Y, et al. Ultrasonography for the diagnosis of intraperitoneal free air in chest-abdominal-pelvic blunt trauma and critical acute abdominal pain. Arch Surg. 2009;144:137–141.
257. Levy, J, Noble, V. Bedside ultrasound for pediatric emergency medicine. Pediatrics. 2008;121:e1404–1412.
258. Tsung, JW, Raio, CC, Ramirez-Schrempp, D, Blaivas, M. Point-of-care ultrasound diagnosis of pediatric cholecystitis in the ED. Am J Emerg Med. 2010;28:338–342.
259. Malcom, GE, 3rd., Raio, CC, Del Rios, M, Blaivas, M, Tsung, JW. Feasibility of emergency physician diagnosis of hypertrophic pyloric stenosis using point-of-care ultrasound: A multi-center case series. J Emerg Med. 2009;37:283–286.
260. Rozycki, GS, et al. The role of ultrasound in patients with possible penetrating cardiac wounds: A prospective multicenter study. J Trauma. 1999;46:543–552.
261. Arger, PH, Schultz, SM, Sehgal, CM, Cary, TW, Aronchick, J. Teaching medical students diagnostic sonography. J Ultrasound Med. 2005;24:1365–1369.
262. Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Rockville, Md:Agency for Healthcare Research and Quality; July 2001. www.ahrq.gov/clinic/ptsafety/summary.htm.
263. Blackstock, U, Stone, MB. Emergency ultrasonography and error reduction. Ann Emerg Med. 2009;54:53–55.
264. Milling, T, et al. Use of real-time, single-operator dynamic ultrasound guidance for internal jugular central venous cannulation: A randomized, controlled clinical trial. Ann Emerg Med. 2005;46:26.
265. Phelan, M, Hagerty, D. The oblique view: An alternative approach for ultrasound-guided central line placement. J Emerg Med. 2009;37:403–408.
266. Milling, TJ, et al. Use of ultrasonography to identify infants for whom urinary catheterization will be unsuccessful because of insufficient urine volume: Validation of the urinary bladder index. Ann Emerg Med. 2005;45:510–513.
267. Milling, TJ, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: The Third Sonography Outcomes Assessment Program (SOAP-3) trial. Crit Care Med. 2005;33:1764–1769.
268. Leung, J, Duffy, M, Finckh, A. Real-time ultrasonographically-guided internal jugular vein catheterization in the emergency department increases success rates and reduces complications: A randomized, prospective study. Ann Emerg Med. 2006;48:540–547.
269. Hudson, PA, Rose, JS. Real-time ultrasound guided internal jugular vein catheterization in the emergency department. Am J Emerg Med. 1997;15:79–82.
270. National Institute for Clinical Excellence. Central venous catheters—ultrasound locating devices: Guidance. http://guidance.nice.org.uk/TA49/Guidance/pdf/English, 2002.
271. Verghese, S, et al. Ultrasound-guided internal jugular venous cannulation in infants: A prospective comparison with the traditional palpation method. Anesthesiology. 1999;91:71–77.
272. Karakitsos, D, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: A prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10:R162.
273. Balls, A, et al. Ultrasound guidance for central venous catheter placement: Results from the Central Line Emergency Access Registry Database. Am J Emerg Med. 2010;28:561–567.
274. Bellazzini, MA, Rankin, PM, Gangnon, RE, Bjoernsen, LP. Ultrasound validation of maneuvers to increase internal jugular vein cross-sectional area and decrease compressibility. Am J Emerg Med. 2009;27:454–459.
275. Blaivas, M, Adhikari, S. An unseen danger: Frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med. 2009;37:2345–2349.
276. Moon, CH, et al. Incidence of posterior vessel wall puncture during ultrasound-guided vessel cannulation in a simulated model. Acad Emerg Med. 2010;17:1138–1141.
277. Theodoro, D, Krauss, M, Kollef, M, Evanoff, B. Risk factors for acute adverse events during ultrasound-guided central venous cannulation in the emergency department. Acad Emerg Med. 2010;17:1055–1061.
278. Stone, MB, Hern, HG. Inadvertent carotid artery cannulation during ultrasound guided central venous catheterization. Ann Emerg Med. 2007;49:720.
279. Chiles, K, Nagdev, A. Accidental carotid artery cannulation detected by bedside ultrasound. West J Emerg Med. 2011;12:100–101.
280. Hilty, WM, Hudson, PA, Levitt, MA, Hall, JB. Real-time ultrasound-guided femoral vein catheterization during cardiopulmonary resuscitation. Ann Emerg Med. 1997;29:331–337.
281. Rippey, JC, Pascu, O, Jacobs, I. Abdominal compression effectively increases the size of the common femoral vein, as measured by ultrasonography. Ann Emerg Med. 2008;52:446–452.
282. Werner, SL, Jones, RA, Emerman, CL. Effect of hip abduction and external rotation on femoral vein exposure for possible cannulation. J Emerg Med. 2008;35:73–75.
283. Bentley, SK, Seethala, R, Weingart, SD. Ultrasound-guided axillary vein approach to the subclavian vein for central venous access. Ann Emerg Med. 2008;52:475.
284. Sharma, A, Bodenham, AR, Mallick, A. Ultrasound-guided infraclavicular axillary vein cannulation for central venous access. Br J Anaesth. 2004;93:188–192.
285. Fragou, M, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: A prospective randomized study. Crit Care Med. 2011;39:1607–1612.
286. Mallin, M, Louis, H, Madsen, T. A novel technique for ultrasound-guided supraclavicular subclavian cannulation. Am J Emerg Med. 2010;28:966–969.
287. Froehlich, CD, et al. Ultrasound-guided central venous catheter placement decreases complications and decreases placement attempts compared with the landmark technique in patients in a pediatric intensive care unit. Crit Care Med. 2009;37:1090–1096.
288. Stone, MB, Moon, C, Sutijono, D, Blaivas, M. Needle tip visualization during ultrasound-guided vascular access: Short-axis vs long-axis approach. Am J Emerg Med. 2010;28:343–347.
289. Mills, CN, Liebmann, O, Stone, MB, Frazee, BW. Ultrasonographically guided insertion of a 15-cm catheter into the deep brachial or basilic vein in patients with difficult intravenous access. Ann Emerg Med. 2007;50:68–72.
290. Stein, J, George, B, River, G, Hebig, A, McDermott, D. Ultrasonographically guided peripheral intravenous cannulation in emergency department patients with difficult intravenous access: A randomized trial. Ann Emerg Med. 2009;54:33–40.
291. Witting, MD, Schenkel, SM, Lawner, BJ, Euerle, BD. Effects of vein width and depth on ultrasound-guided peripheral intravenous success rates. J Emerg Med. 2010;39:70–75.
292. Brannam, L, Blaivas, M, Lyon, M, Flake, M. Emergency nurses’ utilization of ultrasound guidance for placement of peripheral intravenous lines in difficult -access patients. Acad Emerg Med. 2004;11:1361–1363.
293. Chinnock, B, Thornton, S, Hendey, GW. Predictors of success in nurse-performed ultrasound-guided cannulation. J Emerg Med. 2007;33:401–405.
294. Schoenfeld, E, Boniface, K, Shokoohi, H. ED technicians can successfully place ultrasound-guided intravenous catheters in patients with poor vascular access. Am J Emerg Med. 2011;29:496–501.
295. Rose, JS, Norbutas, CM. A randomized controlled trial comparing one-operator versus two-operator technique in ultrasound-guided basilic vein cannulation. J Emerg Med. 2008;35:431–435.
296. Mahler, SA, et al. Short- vs long-axis approach to ultrasound-guided peripheral intravenous access: A prospective randomized study. Am J Emerg Med. 2011;29:1194–1197.
297. Resnick, JR, Cydulka, RK, Donato, J, Jones, RA, Werner, SL. Success of ultrasound-guided peripheral intravenous access with skin marking. Acad Emerg Med. 2008;15:723–730.
298. Doniger, SJ, Ishimine, P, Fox, JC, Kanegaye, JT. Randomized controlled trial of ultrasound-guided peripheral intravenous catheter placement versus traditional techniques in difficult-access pediatric patients. Pediatr Emerg Care. 2009;25:154–159.
299. Schnadower, D, Lin, S, Perera, P, Smerling, A, Dayan, P. A pilot study of ultrasound analysis before pediatric peripheral vein cannulation attempt. Acad Emerg Med. 2007;14:483–485.
300. Schwemmer, U, et al. Ultrasound-guided arterial cannulation in infants improves success rate. Eur J Anaesthesiol. 2006;23:476–480.
301. Sandhu, NS, Patel, B. Use of ultrasonography as a rescue technique for failed radial artery cannulation. J Clin Anesth. 2006;18:138–141.
302. Lanza, C, Russo, M, Fabrizzi, G. Central venous cannulation: Are routine chest radiographs necessary after B-mode and colour Doppler sonography check? Pediatr Radiol. 2006;36:1252–1256.
303. Stone, MB, Nagdev, A, Murphy, MC, Sisson, CA. Ultrasound detection of guidewire position during central venous catheterization. Am J Emerg Med. 2010;28:82–84.
304. Stone, MB. Identification and correction of guide wire malposition during internal jugular cannulation with ultrasound. CJEM. 2007;9:131–132.
305. Tsung, JW, Blaivas, M, Stone, MB. Feasibility of point-of-care colour Doppler ultrasound confirmation of intraosseous needle placement during resuscitation. Resuscitation. 2009;80:665–668.
306. Stone, M, Teismann, N, Wang, R. Ultrasonographic confirmation of intraosseous needle placement in an adult unembalmed cadaver model. Ann Emerg Med. 2007;49:515–519.
307. Barnes, TW, et al. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33:442–446.
308. Tayal, V, Pariyadath, M, Norton, J. Randomized controlled trial of ultrasound-guided peripheral non-knee arthrocentesis in the emergency department. Acad Emerg Med. 2006;13:S197.
309. Sibbitt, WL, Jr., et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36:1892–1902.
310. Crystal, CS, Miller, MA, Young, SE. Ultrasound guided hematoma block: A novel use of ultrasound in the traumatized patient. J Trauma. 2007;62:532–533.
311. Reid, N, Stella, J, Ryan, M, Ragg, M. Use of ultrasound to facilitate accurate femoral nerve block in the emergency department. Emerg Med Australas. 2009;21:124–130.
312. Barker, R, et al. Femoral nerve blockade administered preclinically for pain relief in severe knee trauma is more feasible and effective than intravenous metamizole: A randomized controlled trial. J Trauma. 2008;64:1535–1538.
313. Stone, M, Price, D, Wang, R. Ultrasound-guided supraclavicular block for the treatment of upper extremity fractures, dislocations, and abscesses in the ED. Am J Emerg Med. 2007;25:472–475.
314. Wu, TS, Leech, SJ, Rosenberg, M, Huggins, C, Papa, L. Ultrasound can accurately guide gastrostomy tube replacement and confirm proper tube placement at the bedside. J Emerg Med. 2009;36:280–284.
315. Ferre, RM, Sweeney, TW. Emergency physicians can easily obtain ultrasound images of anatomical landmarks relevant to lumbar puncture. Am J Emerg Med. 2007;25:291–296.
316. Nomura, J, et al. A randomized controlled trial of ultrasound-assisted lumbar puncture. J Ultrasound Med. 2007;26:1341–1348.
317. Nelson, BP, Melnick, ER, Li, J. Portable ultrasound for remote environments, Part I: Feasibility of field deployment. J Emerg Med. 2011;40:190–197.
318. Nelson, BP, Melnick, ER, Li, J. Portable ultrasound for remote environments, Part II: Current indications. J Emerg Med. 2011;40:313–321.
319. Fincke, E, et al. Evaluation of shoulder integrity in space: First report of musculoskeletal US on the International Space Station. Radiology. 2005;234:319–322.
320. Boniface, K, Smith, R, Breit, A. FAST exam by prehospital providers under real time remote physician guidance. J Ultrasound Med. 2008;27:S87.
321. Heegard, WG, et al. Ultrasound in helicopter EMS. Acad Emerg Med. 2003;10:449.
322. Melanson, S, McCarthy, J, Kostenbader, J, Greissinger, P, Heller, M. Aeromedical FAST by nonphysicians with a miniature ultrasound unit. Ann Emerg Med. 2000;36:S21.
323. Busch, M. Portable ultrasound in pre-hospital emergencies: A feasibility study. Acta Anaesthesiol Scand. 2006;50:754–758.
324. Heegaard, W, et al. Prehospital ultrasound by paramedics: Results of field trial. Acad Emerg Med. 2010;17:624–630.
325. Lyons, R, Stamilio, D, McReynolds, T, Endrizzi, J. Diagnosis and treatment of a ruptured ectopic pregnancy in a combat support hospital during operation Iraqi Freedom: Case report and critique of a field-ready sonographic device. Mil Med. 2004;169:681–683.
326. Sarkisian, AE, et al. Sonographic screening of mass casualties for abdominal and renal injuries following the 1988 Armenian earthquake. J Trauma. 1991;31:247–250.
327. Sustic, A, et al. Ultrasonography in the evaluation of hemoperitoneum in war casualities. Mil Med. 1999;164:600–602.
328. Shah, S, et al. Impact of portable ultrasound in trauma care after the Haitian earthquake of 2010. Am J Emerg Med. 2010;28:970–971.
329. Dyer, D, et al. The clinical and technical evaluation of a remote telementored telesonography system during the acute resuscitation and transfer of the injured patient. J Trauma. 2008;65:1209–1216.
330. Shah, S, et al. Development of an ultrasound training curriculum in a limited resource international setting: Successes and challenges of ultrasound training in rural Rwanda. Int J Emerg Med. 2008;1:193–196.
331. Adler, D, Mgalula, K, Price, D, Taylor, O. Introduction of a portable ultrasound unit into the health services of the Lugufu refugee camp, Kigoma District, Tanzania. Int J Emerg Med. 2008;1:261–266.
332. Jang, T, Naunheim, R, Sineff, S, Aubin, C. Operator confidence correlates with more accurate abdominal ultrasounds by emergency medicine residents. J Emerg Med. 2007;33:175–179.