Emergency Cardiac Pacing
Emergency Transvenous Cardiac Pacing
The transvenous method of endocardial pacing is commonly used and is both safe and effective. In skilled hands, the semifloating transvenous catheter is successfully placed under electrocardiographic (ECG) guidance in 80% of patients.1 The technique can be performed in less than 20 minutes in 72% of patients and in less than 5 minutes in 30%. However, in some instances, anatomic, logistic, and hemodynamic impediments can prohibit successful pacing by even the most skilled clinician. As with other medical procedures, it should not be performed without a thorough understanding of its indications, contraindications, and complications.2
Background
The ability of muscle to be artificially depolarized was recognized as early as the 18th century. Initial efforts focused on the transcutaneous approach (see later in this section). Over the succeeding years several scattered experiments were reported, and in 1951 Callaghan and Bigelow first used the transvenous approach to stimulate asystolic hearts in hypothermic dogs.3
Furman and Robinson demonstrated the transvenous endocardial approach in humans in 1958.4 They treated two patients with complete heart block and Stokes-Adams seizures, thus reconfirming that low-voltage pacing could completely control myocardial depolarization. The catheter remained in the second patient for 96 days without complication. Other early clinical studies also demonstrated the utility of transvenous pacing.5 Fluoroscopic guidance was used for placement of the pacing catheter in all these studies.
In 1964 Vogel and coworkers demonstrated the use of a flexible catheter passed without fluoroscopic guidance for intracardiac electrocardiography.6 One year later, Kimball and Killip used this technique to insert endocardial pacemakers at the bedside.7 They noted technical problems in 20% of their patients, including intermittent capture, difficulty passing the catheter, and catheter knotting. During the same year, Harris and colleagues confirmed the ease and speed with which this procedure could be accomplished.8
Before 1965 all intracardiac pacing was done asynchronously, which meant that the pacing catheter could cause electrical stimulation during any phase of the cardiac cycle. Asynchronous pacing frequently resulted in the pacemaker firing during the vulnerable period of an intrinsic depolarization; this occasionally caused ventricular tachycardia or fibrillation. In 1967 a demand pacemaker generator that sensed intrinsic depolarizations and inhibited the pacemaker for a predetermined period was used successfully by Zuckerman and associates in six patients.9 Since then there has been steady progress in the design and functionality of pacemakers. Table 15-1 summarizes the four-letter code that is used to describe modern pacemakers (there is a fifth letter for combined pacemaker-cardioverter/defibrillators). The most commonly used emergency transvenous pacemaker is represented by the code VVI: the ventricle is paced, the ventricle is sensed, and when a native impulse is sensed, the pacemaker is inhibited. Dual-chamber pacing (DDD or DDDR) is the preferred methodology for permanent pacing but is rarely used on an emergency basis because of the increased complexity of the procedure.
Rosenberg and coworkers introduced an improved pacing catheter known as the Elecath semifloating pacing wire.1 The Elecath was stiffer than the Flexon steel wire electrode that was in prevailing use. Rosenberg and coworkers1 achieved pacing in 72% of their patients with an average procedure time of 18 minutes. They also noted that 30% of their patients were paced in 5 minutes or less. In 1970, Swan and Ganz introduced the technique of heart catheterization with a flow-directed balloon-tipped catheter.10 Schnitzler and colleagues successfully used this method for placement of a right ventricular pacemaker in 15 of 17 patients.11
In 1981 Lang and associates compared bedside use of the flow-directed balloon-tipped catheter with insertion of a semirigid electrode catheter in 111 perfusing patients.12 These researchers found a significantly shorter insertion time (6 minutes 45 seconds versus 13 minutes 30 seconds), a lower incidence of serious arrhythmias (1.5% versus 20.4%), and a lower incidence of catheter displacement (13.4% versus 32%) with the balloon-tipped catheter. They concluded that the balloon-tipped catheter was the method of choice for temporary transvenous pacing (Table 15-2).
TABLE 15-2
| DATE | INVESTIGATOR | EVENT |
| 1700 | Early investigators | First restimulation studies |
| 1951 | Callaghan and Bigelow | First transvenous approach in dogs |
| 1952 | Zoll | Transcutaneous cardiac stimulator |
| 1958 | Falkmann and Walkins | Implanted pacing wires after surgery |
| 1959 | Furman and Robinson | First transvenous pacer in humans |
| 1964 | Vogel et al. | Flexible electrocardiographic catheter without fluoroscopy |
| 1965 | Kimball and Killip | First bedside transvenous pacing |
| 1966 | Goetz et al. | Demand pacemaker developed |
| 1967 | Zuckerman et al. | Use of a demand pacemaker clinically |
| 1969 | Rosenberg et al. | Semifloating pacing catheter |
| 1973 | Schnitzler et al. | Balloon-tipped pacers |
Birkhahn and coworkers retrospectively compared the experience of emergency physicians with that of cardiologists in placing transvenous pacemakers under ECG guidance.13 They reported a 13% risk for major complications in both groups of specialists. They concluded that pacemaker placement by emergency physicians under ECG guidance without fluoroscopy had success and complication rates that were comparable to those of their cardiology colleagues.
Indications
In general, the indications can be grouped into those that cause either tachycardias or bradycardias (see Review Box 15-1). Transcutaneous cardiac pacing (TCP) has become the mainstay of emergency cardiac pacing and is often used pending placement of a transvenous catheter or to determine whether potentially terminal bradyasystolic rhythms will respond to pacing.
Bradycardias
Sinus Node Dysfunction: Sinus node dysfunction may be manifested as sinus arrest, tachybrady (sick sinus) syndrome, or sinus bradycardia. Although symptomatic sinus node dysfunction is a common indication for elective permanent pacing, it is seldom cause for emergency pacemaker insertion.
Seventeen percent of patients with acute myocardial infarction (AMI) will experience sinus bradycardia.14 It occurs more frequently with inferior than with anterior infarction and has a relatively good prognosis when accompanied by a hemodynamically tolerable escape rhythm. However, sinus bradycardia is not a benign rhythm in this situation; it has a mortality rate of 2% with inferior infarction and 9% with anterior infarction.15 Sinus node dysfunction frequently responds to medical therapy but requires prompt pacing if such therapy fails.
Asystolic Arrest: Transvenous pacing in an asystolic or bradyasystolic patient has little value and is not recommended.16 In a study of 13 patients who had suffered cardiac arrest, capture of the myocardium was noted in 4 patients, but there were no survivors.17 Transvenous pacing alone may also not be effective for post-countershock pulseless bradyarrhythmias.18 This failure of pacing has likewise been demonstrated with transcutaneous pacemakers, thus suggesting that failure of effective pacing is primarily related to the state of the myocardial tissue.17 Cardiac pacing may be used as a “last-ditch” effort in bradyasystolic patients but is rarely successful and is not considered standard practice. Early pacing is essential when done for this purpose if success is to be achieved19 (see later in this section). Most importantly, given the continued emphasis on the importance of maximizing chest compressions during cardiopulmonary resuscitation (CPR), interrupting CPR to institute emergency pacing is not recommended.20
AV Block: AV block is the classic indication for pacemaker therapy. In symptomatic patients without myocardial infarction (MI) and in asymptomatic patients with a ventricular rate lower than 40 beats/min, pacemaker therapy is indicated.21
In patients with AMI, 15% to 19% progress to heart block: first-degree block develops in approximately 8%, second-degree block in 5%, and third-degree block in 6%.22 First-degree block progresses to second- or third-degree block 33% of the time, and second-degree block progresses to third-degree block about one third of the time.23
Trauma: Pacing is not a standard intervention in traumatic cardiac arrest, but in selected cases it may be considered. Several rhythm and conduction disturbances have been documented in patients with nonpenetrating chest trauma. In these patients, traumatic injury to the specialized conduction system may predispose to life-threatening dysrhythmias and blocks that can be treated by cardiac pacing.24
Hypovolemia and hypotension can cause ischemia of conduction tissue and cardiac dysfunction.25 Marked bradyarrhythmias that persist even after vigorous volume replacement may rarely respond to cardiac pacing in patients with such trauma.26
Bundle Branch Block and Ischemia
Bundle branch block occurring in AMI is associated with a higher mortality rate and a greater incidence of third-degree heart block than is uncomplicated infarction. Atkins and colleagues noted that 18% of patients with MI had bundle branch block.27 Of these patients, complete heart block developed in 43% who had right bundle branch block (RBBB) and left axis deviation, in 17% who had left bundle branch block (LBBB), in 19% who had left anterior hemiblock, and in 6% who had no conduction block. The investigators concluded that RBBB with left axis deviation should be paced prophylactically.
A study by Hindman and associates confirmed the natural history of bundle branch block during MI.28 In their study the presence or absence of first-degree AV block, the type of bundle branch block, and the age of the block (new versus old) were used to determine the relative risk for progression to type II second-degree or third-degree block (Table 15-3).
TABLE 15-3
Influence of Different Variables on the Risk for High-Degree AVB in Patients with BBB during MI
| PATIENTS | PROGRESSING TO HIGH-DEGREE AVB (%) |
| Infarct location | |
| Anterior | 25 |
| Indeterminate | 12 |
| Inferior or posterior | 20 |
| PR interval | |
| >0.20 sec | 25 |
| ≤0.20 sec | 19 |
| Type of BBB | |
| LBBB | 13 |
| RBBB | 14 |
| RBBB + LAFB | 27 |
| RBBB + LPFB | 29 |
| ABBB | 44 |
| Onset of BBB | |
| Definitely old | 13 |
| Possibly new | 25 |
| Probably new | 26 |
| Definitely new | 23 |
From American Heart Association from Hindman MC, Wagner GS, JaRo M, et al. The clinical significance of bundle branch block complicating acute myocardial infarction. 2. Indications of temporary and permanent pacemaker insertion. Circulation. 1978;58:690.
Because of the increased risk, consider pacing for the following conduction blocks: new-onset LBBB, RBBB with left axis deviation or other bifascicular block, and alternating bundle-branch block.28 Though controversial, one authority recommends prophylactic pacing for all new bundle branch blocks when MI is evident.29
Whether to place a transvenous pacemaker prophylactically in patients with LBBB before insertion of a flow-directed pulmonary artery catheter (PAC) remains controversial. Some researchers strongly advocate this procedure because of the risk for transient RBBB and life-threatening complete heart block associated with PAC placement.30 One study noted that this risk is low in patients with previous LBBB but continued to recommend temporary catheter placement for all cases of new LBBB.31 One solution to this problem is to place a transcutaneous pacemaker before catheterization as an emergency measure should heart block develop. In these cases a temporary transvenous pacemaker can be placed in a semi-elective manner when needed.32 In any event, the trend toward decreased PAC use, particularly outside the critical care setting, makes it unlikely that this will be an issue in the ED.33
Tachycardias
Hemodynamically compromising tachycardias are usually treated by medical means or electrical cardioversion. Since 1980 there has been increasing interest in pacing therapy for symptomatic tachycardias. Supraventricular dysrhythmias, with the exception of atrial fibrillation, respond well to atrial pacing. By “overdrive” pacing the atria at rates 10 to 20 beats/min faster than the underlying rhythm, the atria become entrained, and when the rate is slowed, the rhythm frequently returns to normal sinus. A similar procedure is done for ventricular dysrhythmias.34 Overdrive pacing is especially useful for arrhythmias with recurrent prolonged QT intervals such as those seen with quinidine toxicity or torsades de pointes.35 Though an attractive thought, there is no reported experience with these techniques in the ED. Transvenous pacing is also useful in patients with digitalis-induced dysrhythmias, in whom direct current cardioversion may be dangerous, or in patients in whom there is further concern about myocardial depression with drugs.36
Cardiac Pacing for Drug-Induced Dysrhythmias
Severe bradycardia and heart block often accompany overdose of digitalis preparations, β-adrenergic blockers, and calcium channel blockers. Although intuitively attractive, cardiac pacing is not generally effective for serious toxin-induced bradycardias, even though there have been case reports of success.37–40 In β-blocker overdose, pacing may increase the heart rate but rarely benefits blood pressure or cardiac output. Worsening of blood pressure may occur as a result of loss of atrial contractions with ventricular pacing. Likewise, calcium channel blocker overdose and digitalis-induced bradycardia and heart block rarely benefit from cardiac pacing. Pharmacologic interventions, such as digoxin-specific Fab, glucagon, calcium, inotropic medications, and vasopressors, remain the mainstay in the treatment of drug-induced dysrhythmias. Given the lack of success of pacing, possible downsides, and the greater effectiveness of specific antidotes, it is not standard to routinely attempt transvenous cardiac pacing in the setting of drug overdose. However, as a last resort, cardiac pacing can be supported.41
Contraindications
The presence of a prosthetic tricuspid valve is generally considered to be an absolute contraindication to transvenous cardiac pacing.42 Also, severe hypothermia will occasionally result in ventricular fibrillation when pacing is attempted. Because ventricular fibrillation under these conditions is difficult to convert, caution is advised when considering pacing severely hypothermic and bradycardiac patients. Rapid and careful rewarming is often recommended first, followed by pacing if the patient’s condition does not improve.
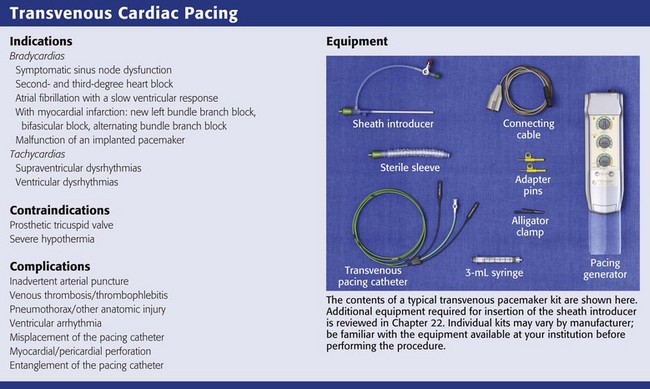
Equipment
Several items are required to insert a transvenous pacemaker adequately. Like most special procedures, a prearranged tray is convenient. The usual components required to insert a transvenous cardiac pacemaker are depicted in Review Box 15-1.
Pacing Generator
Temporary pacing generators are battery operated, and thus it is always good practice to install a fresh battery whenever pacing is anticipated. An example of a pacing generator is shown in Figure 15-1.
Pacing Catheters and Electrodes
Several sizes and brands of pacing catheters are available. In general, most range from 3 to 5 Fr in size and are approximately 100 cm in length. Lines are marked along the catheter surface at approximately 10-cm intervals and can be used to estimate catheter position during insertion. Pacing catheters differ with respect to their stiffness, electrode configurations, floating characteristics, and other qualities. For emergency pacing, the semifloating bipolar electrode catheter with a balloon tip is used most frequently (Fig. 15-2). The balloon holds approximately 1.5 mL of air, and the air injection port has a locking lever to secure balloon expansion. Before insertion, the balloon is checked for leakage of air by inflating and immersing it in sterile water. The presence of an air leak is noted by a stream of bubbles rising to the surface of the water. An inflated balloon helps the catheter “float” into the heart, even in low-flow states, but is obviously not advantageous in the cardiac arrest situation.
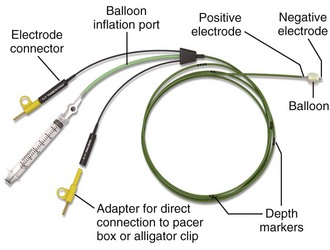
Figure 15-2 Balloon-tipped pacing catheter.
ECG Machine
The ECG machine should be placed in a manner that allows easy visibility of the rhythm during insertion. One method is to place the machine near the level of the patient’s midthorax facing the operator, on either side of the patient as logistics and operator preference allow (Fig. 15-3). Note that the operator stands at the head of the patient during passage of the catheter through the internal jugular or subclavian vein and at the midabdomen for insertion through the femoral or brachiocephalic vein. Newer patient monitors may be equipped with suitable ECG connections to allow their use in place of a stand-alone ECG machine. Because these patients will already be attached to a monitor, it may prove convenient to use the same piece of equipment to assist in insertion of the pacemaker.
Introducer Sheath
An introducer set or sheath is required for venous access (see Chapter 22). Some pacing catheters are prepackaged with the appropriate equipment, whereas others require a separate set. The introducer set is used to enhance passage of the pacing catheter through the skin, subcutaneous tissue, and vessel wall. The sheath must be larger than the pacing catheter to allow it to pass. The size of the pacing catheter refers to its outside diameter, whereas the size of the introducer refers to its inside diameter. Thus, a 5-Fr pacing catheter will fit through a 5-Fr introducer. Introducer sheaths are available with a perforated elastic seal covering the opening through which the pacing catheter is passed (pacer port). The seal allows the catheter to be manipulated while preventing blood from escaping or air from entering the vein. A side port allows the sheath to be used for central venous access. A makeshift sheath can be fashioned with an appropriately sized intravenous (IV) catheter. For a 3-Fr balloon-tipped catheter, a 14-gauge 1.5- to 2-inch IV catheter is suitable. A 4-Fr balloon-tipped catheter will also fit through a 14-gauge catheter or needle. However, without a seal over the hub, blood will leak from the end of the IV catheter.
Procedure
A checklist for the preparation and initial setup of a pacing generator is shown in Box 15-1. It may be useful to have a copy of this checklist or a similar list stored with the pacemaker to have on hand in emergency situations.
Site Selection
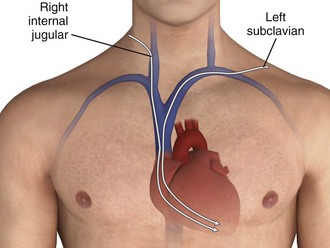
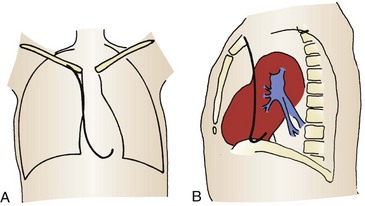
The four venous channels that provide easy access to the right ventricle are the brachial, subclavian, femoral, and internal jugular veins (Table 15-4). The route selected is often one of personal or institutional preference. The right internal jugular and left subclavian veins have the straightest anatomic pathway to the right ventricle and are generally preferred for temporary transvenous pacing (Fig. 15-4). In some centers a particular site is preferred for permanent transvenous pacemaker placement, and if possible, this site should be avoided for temporary placement.
TABLE 15-4
Advantages and Disadvantages of Pacemaker Placement Sites
| VENOUS CHANNELS | ADVANTAGES | DISADVANTAGES |
| Brachial | Very safe route Vessel easily accessible, either by cutdown or a percutaneous approach Compressible |
Often requires a cutdown Easily displaced and poor patient mobility Not reusable if a cutdown technique is performed The catheter is more difficult to advance than in central or larger vessels |
| Subclavian | Direct access to the right side of the heart (especially via the left subclavian) Rapid insertion time Good patient mobility |
Pneumothorax and other intrathoracic trauma are possible Noncompressible |
| Femoral | Direct access to the right side of the heart Rapid insertion time Compressible |
Increased incidence of thrombophlebitis Can be dislodged by leg movement Poor patient mobility Infection |
| Internal jugular | Direct access to the right side of the heart (especially via the right internal jugular) Rapid insertion time Compressible |
Possible carotid artery puncture Dislodgment with movement of the head Thrombophlebitis |
The subclavian vein can be accessed through both an infraclavicular and a supraclavicular approach; the infraclavicular approach is most commonly reported for all temporary transvenous pacemaker insertions. This route is preferred because of its easy accessibility, close proximity to the heart, and ease in catheter maintenance and stability. The supraclavicular approach has been described in the literature for several years and has gained popularity among some clinicians.43,44
The left subclavian vein is preferred because of the less acute angle traversed than with the right-sided approach, but either side may be used.43,44
During CPR, use of the right internal jugular vein and the left subclavian vein for pacemaker insertion has been demonstrated to result in the highest rates of proper placement in the right ventricle.45 The right internal jugular vein is the more direct route of the two and may be the most appropriate site.
Femoral veins, like neck veins, are compressible and easily catheterized. Problems include easy dislodgment, infection, and increased risk for thrombophlebitis.46,47
Brachial vein catheterization is easy to perform but results in a high incidence of infection and vessel thrombosis.48 In addition, the catheter is easily dislodged with arm motion. This approach is seldom used in the emergency setting.
Skin Preparation and Venous Access
With the guidewire in place, pass a dilator and introducer sheath together over the guidewire, as is done in the Seldinger technique. Remove the dilator and guidewire and pass the pacing catheter through the introducer sheath (Fig. 15-5, step 1). One key additional step to help preserve sterility while manipulating the pacing catheter is to attach an extensible sleeve on the end of the introducer before inserting the pacing catheter (see Fig. 15-2, steps 1 and 6). In this way the pacing catheter can be advanced and withdrawn multiple times without fear of contamination.
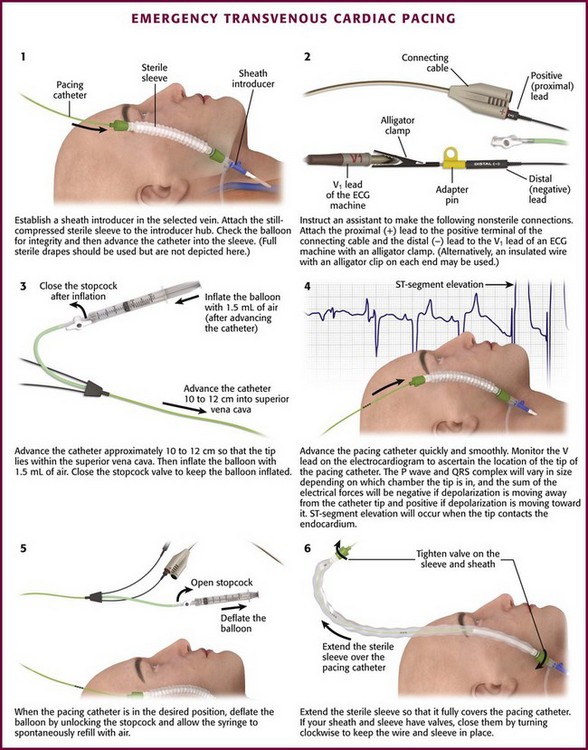
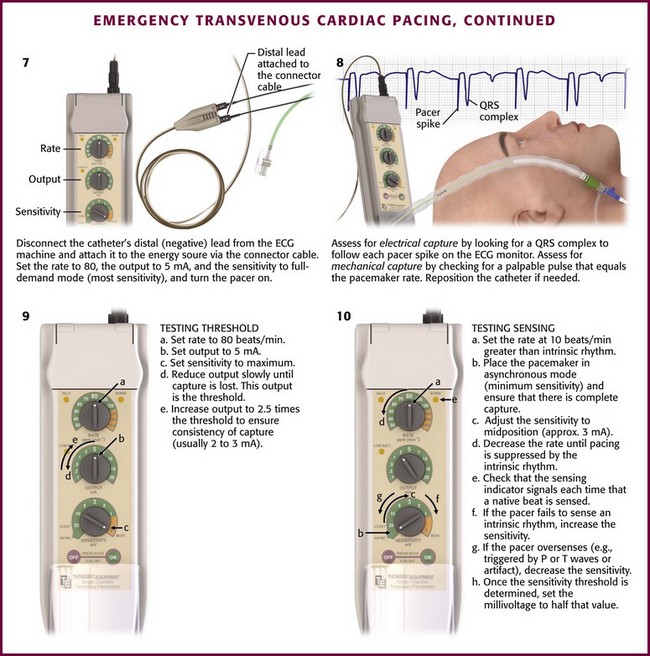
Figure 15-5 Emergency transvenous cardiac pacing. Note that by attaching the pacing catheter to the electrocardiographic (ECG) machine (step 2) and observing the V1 ECG tracing, the negative lead now becomes an exploring lead that tells the operator the position of the tip of the pacing catheter in the body (see Fig. 15-6).
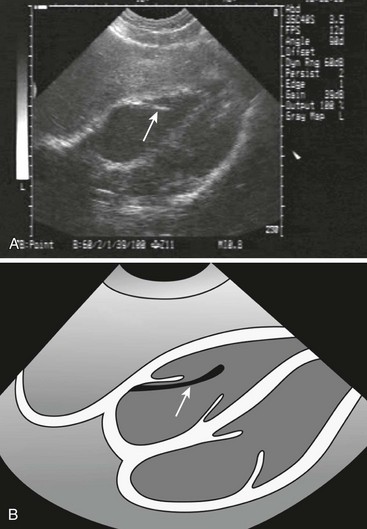
Bedside ultrasound (US) can be useful as an aid in securing central venous access, and its use in the setting of emergency transvenous pacing has been reported.49,50
Pacemaker Placement
ECG Guidance: Connect the patient to the limb leads of an ECG machine, and turn the indicator to record the chest (V) lead.
With newer ECG machines, the pacemaker may be attached to any of the V leads (usually V1 or V5) that are displayed during rhythm monitoring. The distal terminal of the pacing catheter (the cathode or lead marked “negative”, “−.” or “distal”) must be connected to the V lead of the ECG machine by a male-to-male connector or by an insulated wire with an alligator clip on each end. Some prepackaged kits contain an alligator clamp that can be connected to the lead with an adapter pin (see Fig. 15-5, step 2). The pacing catheter is thus an exploring electrode that creates a unipolar electrode for intracardiac ECG recording. The ECG tracing recorded from the electrode tip localizes the position of the tip of the pacing electrode. Because the tracing on the ECG machine may be slightly delayed, advancement of the catheter after initial insertion must be evaluated carefully. If a balloon-tipped catheter is used, inflate the balloon with air after the catheter enters the superior vena cava (≈10 to 12 cm for a subclavian or internal jugular insertion) (see Fig. 15-5, step 3). The inflation port should be locked and the syringe left attached.
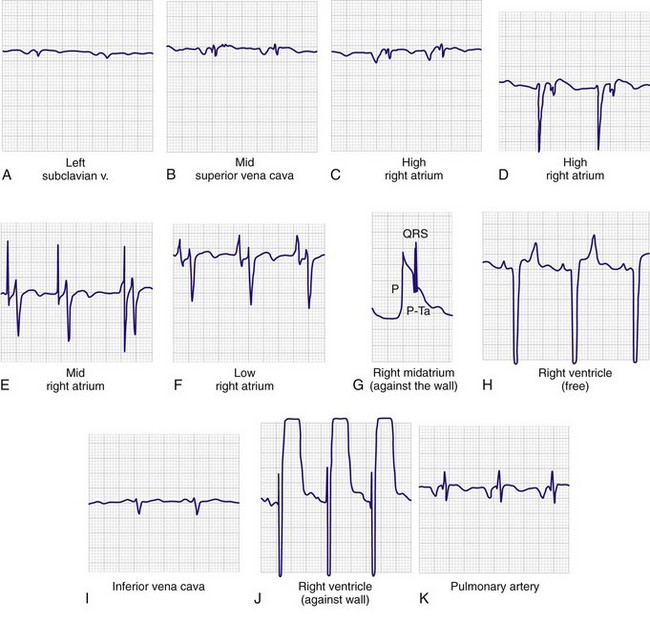
Advance the pacing catheter both quickly and smoothly. Monitor the V lead, and observe the P wave and QRS complex to ascertain the location of the tip of the pacing catheter (see Fig. 15-5, step 4). Use of electrocardiography to guide placement of a pacing catheter is based on two concepts. First, the complex will vary in size depending on which chamber is entered. For example, when the tip of the pacing catheter is in the atrium, one will see large P waves, often larger than the corresponding QRS complex. Second, the sum of the electrical forces will be negative if the depolarization is moving away from the catheter tip and positive if the depolarization is moving toward the catheter tip. Therefore, if the tip of the catheter is above the atrium, both the P wave and QRS complex will be negative (i.e., the electrical forces of a normally beating heart will be moving away from the catheter tip). As the tip progresses inferiorly in the atrium, the P wave will become isoelectric (biphasic) and will eventually become positive as the wave of atrial depolarization advances toward the tip of the catheter. The ECG tracing resembles an aVR lead initially when in the left subclavian vein (Fig. 15-6A) or the midportion of the superior vena cava (see Fig. 15-6B). At the high right atrial level, both the P wave and QRS complex are negative. The P wave is larger than the QRS complex and is deeply inverted (see Fig. 15-6C and D). As the center of the atrium is approached, the P wave becomes larger and biphasic (see Fig. 15–6E). As the catheter approaches the lower atrium (see Fig. 15-6F), the P wave becomes smaller and upright. The QRS complex is fairly normal. When striking the right atrial wall, an injury pattern with a P-Ta segment is seen (Fig. 15-6G). As the electrode passes through the tricuspid valve, the P wave becomes smaller and the QRS complex becomes larger (see Fig. 15-6H). Placement in the inferior vena cava may be recognized by a change in the morphology of the P wave and a decrease in the amplitude of both the P wave and the QRS complex (see Fig. 15-6I).
Once the pacing catheter is in the desired position, deflate the balloon by unlocking the port, observing that the syringe refills with air spontaneously, and then removing the syringe (Fig. 15-5, step 5). One should avoid drawing back on the syringe because this may rupture the balloon. If the syringe does not refill spontaneously, the operator should suspect that the balloon might be ruptured. The balloon should not be inflated and the pacing catheter should be withdrawn and the balloon checked for leaks. If a leak is found, a new pacing catheter should be used.
After successful passage of the catheter into the right ventricle, advance the tip until contact is made with the endocardial wall. When this occurs, the QRS segment will show ST-segment elevation (see Fig. 15-6J). Ideally, the tip of the catheter should be lodged in the trabeculae at the apex of the right ventricle; however, pacing may also be successful if the catheter is in various other positions within the ventricle or outflow tract.
If the pacer enters the pulmonary artery outflow tract, the P wave again becomes negative, and the QRS amplitude diminishes (see Fig. 15-6K). If the catheter is in the pulmonary artery, withdraw the pacing catheter into the right ventricle and readvance it. Sometimes a clockwise or counterclockwise twist of the catheter will redirect its path in a more favorable direction. If catheter-induced ectopy develops, withdraw the catheter slightly until the ectopy stops, and then readvance it. Occasionally, an antidysrhythmic drug such as lidocaine may be needed to desensitize the myocardium. Once ventricular endocardial contact is made, disconnect the catheter from the ECG machine and connect the distal lead to the negative terminal on the pacing generator (see Fig. 15-5, step 7). Set the pacing generator at a rate of 80 beats/min or 10 beats/min faster than the underlying ventricular rhythm, whichever is higher. Select the full-demand mode with an output of about 5 mA. The pacing generator is then turned on.
Assess the patient for electrical and mechanical capture. Electrical capture will be manifested on the ECG monitor as a pacer spike followed by a QRS complex (see Fig. 15-5, step 8). If a pacer spike is seen but no QRS follows, capture is not occurring. Mechanical capture means that a pacer spike with its corresponding QRS triggers a myocardial contraction. This can be assessed by checking that a palpable pulse is present and equal to the rate set on the pacemaker. If complete capture does not occur or if it is intermittent, the pacer will need to be repositioned. When proper capture occurs, assess the pacer for optimal positioning. This is done by testing the thresholds for pacing and sensing and by physical examination, electrocardiography, and chest radiography.
Catheter Placement in Low-Flow States: If cardiac output is too low to “float” a pacing catheter or if the patient is in extremis, there may not be enough time to advance a pacing catheter via the previously described techniques. Such a situation would be asystole or complete heart block with malignant ventricular escape rhythms (although one can make a case for TCP in such conditions). In such emergency situations, connect the pacing catheter to the energy source, turn the output to the maximum amperage, and select the asynchronous mode. Advance the catheter blindly in the hope that it will enter the right ventricle and pacing will be accomplished. Rotate, advance, withdraw, or otherwise manipulate the pacing catheter according to clinical response. The right internal jugular approach is the most practical access route in this situation.
US Guidance: As bedside US has become more widely available in the ED, new uses have been discovered. One promising technique involves using US to assist in the placement of emergency transvenous pacing catheters.51,52 US may also help demonstrate whether mechanical capture has been achieved. The advantages of US over fluoroscopy are its safety and ready availability. Further experience will be necessary to confirm its utility (see Ultrasound Box).
Testing Threshold
To determine the threshold, set the pacing generator to maximum sensitivity (full-demand mode) at 5-mA output and a rate of approximately 80 beats/min (or at least 10 beats/min greater than the patient’s intrinsic rate) (see Fig. 15-5, step 9). Reduce the current (output) slowly until capture is lost. This current is the threshold. Carry out this maneuver two or three times to ensure that this value is consistent. Increase the amperage to 2.5 times the threshold to ensure consistency of capture (usually between 2 and 3 mA).
Testing Sensing
Test the sensing function in patients who have underlying rhythms. Set the rate at about 10 beats/min greater than the endogenous rhythm, place the pacemaker in asynchronous mode (minimum sensitivity, which is the maximum setting on the sensitivity voltage control), and ensure that there is complete capture (see Fig. 15-5, step 10). Then adjust the sensitivity control to its midposition or approximately 3 mV, and gradually decrease the rate until pacing is suppressed by the patient’s intrinsic rhythm. The sensing indicator on the pacing generator should signal each time that a native beat is sensed and should be in synchrony with each QRS complex on the ECG monitor. If the pacer fails to sense the intrinsic rhythm, increase the sensitivity (decrease the millivolts) until the pacer is suppressed. Conversely, if the sensing indicator is triggered by P or T waves or by artifact, decrease the sensitivity until only the QRS complex is sensed. Once the sensitivity threshold is determined, set the millivoltage to about half that value.
Securing and Final Assessment
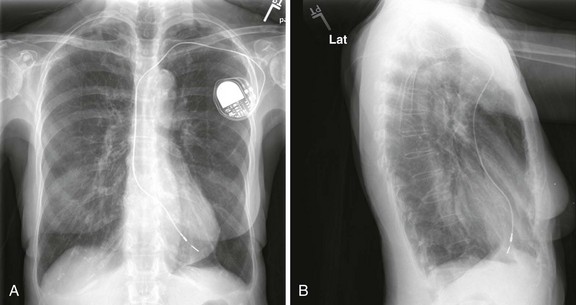
After the pacemaker’s position has been tested for electrical accuracy, it must be secured in place. If a sealed introducer sheath was used, the hub should be fixed firmly to the skin with suture (e.g., 4-0 nylon or silk). A fastening suture should be sewn to the skin and the hub tied securely in place. If a plain introducer was used, withdraw it to prevent leakage and suture the catheter in place. In either case, coil the excess pacing catheter and secure it in a sterile manner underneath a large sterile dressing. Assess pacemaker function again, and take a chest film to ensure proper positioning. Ideal positioning of the pacing catheter is at the apex of the right ventricle (Fig. 15-7).
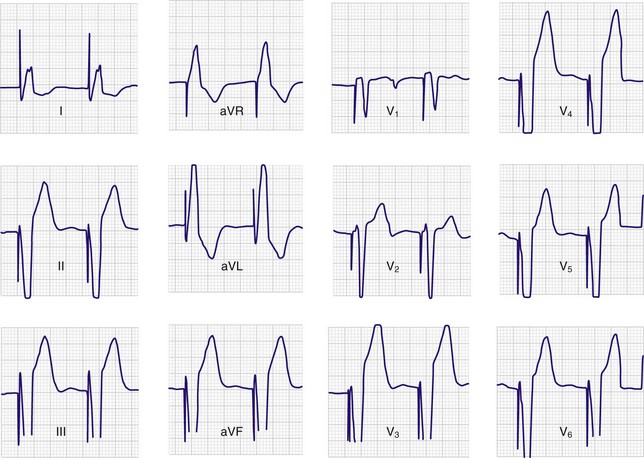
A 12-lead ECG tracing should be obtained after placement of the transvenous pacemaker. If the catheter is within the right ventricle, a left bundle branch pattern with left axis deviation should be evident in paced beats (Fig. 15-8). If an RBBB pattern is noted, coronary sinus placement or left ventricular pacing secondary to septal penetration should be suspected.
With a properly functioning ventricular pacemaker, large cannon waves may be noted on inspection of the venous pulsations at the neck. When the pacemaker achieves ventricular capture, there may be times when the atria contract against a closed tricuspid valve and a cannon wave results. On auscultation of the heart a slight murmur may be evident secondary to tricuspid insufficiency from the catheter interfering with the tricuspid valve apparatus.53 Following each pacemaker impulse a clicking sound may be heard during expiration that is believed to represent either intercostal or diaphragmatic muscular contractions caused by the pacemaker.54 Note that this can also be a sign of cardiac perforation.55 On auscultation of the second heart sound, paradoxical splitting may be noted. This represents a delay in closure of the aortic valve because of delayed left ventricular depolarization.
Complications
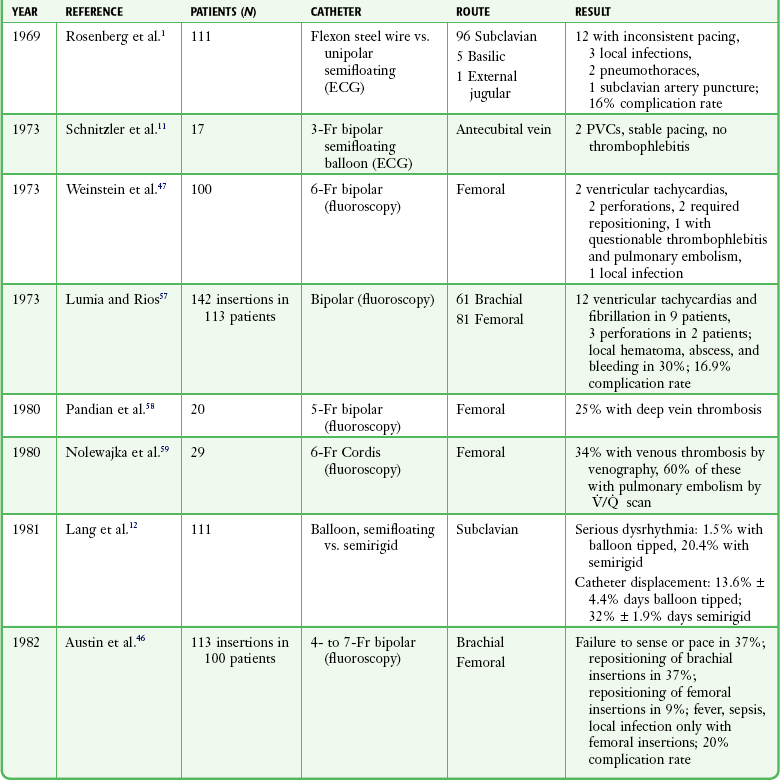
The complications associated with emergency transvenous cardiac pacing are numerous and represent a compendium of those related to central venous catheterization,13,56 those related to right-sided heart catheterization, and those unique to the pacing catheter itself (Table 15-5).
TABLE 15-5
Complications of Transvenous Cardiac Pacing
ECG, electrocardiogram; PVC, premature ventricular contraction;  , ventilation-perfusion.
, ventilation-perfusion.
Problems Related to Central Venous Catheterization
Inadvertent arterial puncture is a well-known complication of the percutaneous approach to the venous system.60 This problem is usually recognized quickly because of the rapid return of arterial blood. Firm compression over the puncture site will almost always result in hemostasis in 5 minutes or less.
Venous thrombosis and thrombophlebitis are also potential problems with central venous catheterization. Thrombophlebitis, which occurs early after insertion, is an uncommon complication. Thrombosis of the innominate vein is also a rare problem, with pulmonary embolism being an even more uncommon event.61 Femoral vein thrombosis, however, appears to be a much more common event associated with femoral vein catheterization.46,59 Studies using noninvasive techniques have shown a 37% incidence of femoral vein thrombosis, with 55% of these patients having evidence of pulmonary embolism on ventilation-perfusion scans.59 At least one study suggests that the use of low-dose enoxaparin is safe and effective in reducing pacemaker-related femoral vein thrombosis.62
Pneumothorax is consistently a problem with the various approaches to the veins at the base of the neck. The decision to place a chest tube in patients with this complication depends on the extent of the air leak and the clinical status of the patient. In addition, laceration of the subclavian vein with hemothorax,63 laceration of the thoracic duct with chylothorax, air embolism, wound infections, pneumomediastinum, hydromediastinum, hemomediastinum,64 phrenic nerve injury,65 and fracture of the guidewire with embolization66,67 are all potential complications.35,63
Complications of Right-Sided Heart Catheterization
A frequent complication of the pacing catheter is dysrhythmia, with premature ventricular contractions being a common occurrence. One study noted a 1.5% incidence of serious dysrhythmias with a balloon-tipped catheter inserted under ECG guidance as opposed to a 32% incidence with insertion of a semirigid catheter under fluoroscopic guidance, thus suggesting that the balloon catheter was the preferred type of catheter.12 Another study noted a 6% incidence of ventricular tachycardia during insertion.46 An ischemic heart is more prone than a nonischemic heart to dysrhythmias.68 Therapy for catheter-induced ectopy during insertion involves repositioning the catheter in the ventricle. This usually stops the ectopy; however, if after repeated attempts it is found that the catheter cannot be passed without ectopy, myocardial suppressant therapy may be used to desensitize the myocardium.
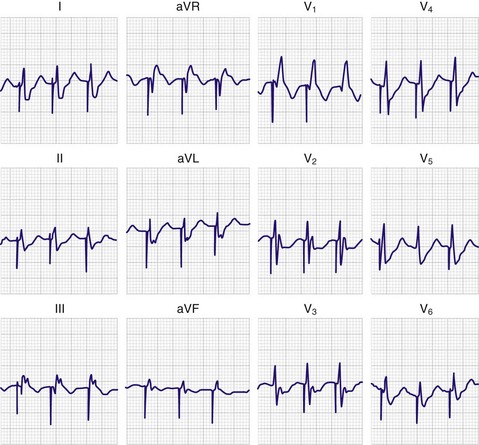
Misplacement of the pacing catheter has been well studied. Passage of the catheter into the pulmonary artery can be diagnosed electrocardiographically by observing the return of an inverted P wave and a decrease in the voltage of the QRS complex. Misplacement in the coronary sinus may occur and should be suspected in patients in whom a paced RBBB pattern on the electrocardiogram is seen with right ventricular pacing (Fig. 15-9). Rarely, an RBBB pattern can be seen with a normal right ventricular position; therefore, all RBBB patterns do not represent coronary sinus pacing.69 Further evidence of coronary sinus location can be obtained by viewing the lateral chest film. Normally, the tip of the catheter should point anteriorly toward the apex of the heart; however, with placement in the coronary sinus, the tip of the catheter is displaced posteriorly and several centimeters away from the sternum (Fig. 15-10). Other potential forms of misplacement include left ventricular pacing through an atrial septal defect or a ventricular septal defect, septal puncture, extraluminal insertion, and arterial insertion.70
Perforation of the ventricle is a well-described complication that can result in loss of capture,71 hemopericardium, and tamponade.72,73 Reported symptoms and signs of this problem include chest pain, pericardial friction rub, and diaphragmatic or chest wall muscular pacing.74 At least one case of a postpericardiotomy-like syndrome and two cases of endocardial friction rub have been reported without perforation.75,76
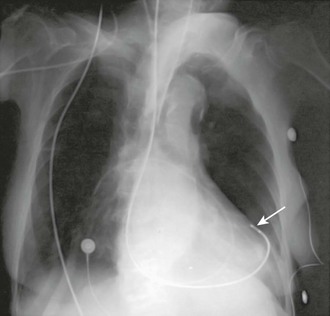
Pericardial perforation is suggested radiographically when the pacing catheter is outside or abuts the cardiac silhouette and is not in proper position within the right ventricular cavity (Fig. 15-11).77 ECG clues include a change in the QRS complex and T-wave axis or failure to properly sense. In suspected cases, a two-dimensional echocardiogram usually demonstrates the catheter’s extracardiac position. Simply pulling the catheter back and repositioning it in the right ventricle can usually treat uncomplicated perforation.
During insertion of a temporary pacing catheter when a nonfunctioning permanent catheter is in place, there is a small risk of entanglement or knotting.78 This potential also exists with other central lines and PACs. Even without the presence of other lines, the pacing catheter can become knotted.79 Frequently, these lines can be untangled under fluoroscopy with the use of specialized catheters.
Local and systemic infection,48 balloon rupture, pulmonary infarction,80 phrenic nerve pacing,81 and rupture of the chordae tendineae are also potential complications.
Complications of the Pacing Electrode
Mechanical failures include displacement, fracture of the catheter, and loose leads. Displacement can result in intermittent or complete loss of capture or improper sensing, malignant dysrhythmias, diaphragmatic pacing, or perforation. Displacement should be suspected with changes in amplitude, with changes of greater than 90 degrees in vector, or with a change in threshold.82 Frequently, catheter fractures may be detected by careful review of the chest film or may be suspected because of a change in the sensing threshold. As with displacement, catheter fractures may result in intermittent or complete loss of capture.
Organic causes of pacemaker failure result in changes in the threshold or sensing function.83 Progressive inflammation, fibrosis, and thrombosis may result in more than doubling of the original threshold.84 However, this process takes several weeks and is of no concern in the setting of pacemaker placement in the ED.
Electrical problems with pacing in the past have included failure of the pacemaker generator, dysrhythmias, and outside interference. Modern devices are extremely reliable and resistant to outside interference. Although ventricular tachycardia and ventricular fibrillation have been reported to result from pacemakers, these dysrhythmias are rare. Therefore, patients with such dysrhythmias should be evaluated for a non–pacemaker-induced cause.85 Defibrillation and cardioversion are safe in patients who have temporary pacemakers.
Emergency Transcutaneous Cardiac Pacing
In 1872, Duchenne de Boulogne reported successful resuscitation of a child by attaching one electrode to a limb while a second electrode was rhythmically touched to the precordium of the thorax.86 Successful overdrive pacing of the human heart with a precordial electrode was reported by VonZiemssen in 1882.87
In 1952, Zoll introduced the first practical means of TCP. Using a ground electrode attached to the skin and a subcutaneous needle electrode over the precordium, he reported successful resuscitation of two patients in ventricular standstill.88 One patient was paced for 5 days and subsequently discharged from the hospital. Zoll later introduced a machine that delivered impulses lasting 2 msec through metal paddles 3 cm in diameter pressed firmly against the anterior chest wall. This device was the first commercial transcutaneous cardiac pacemaker. During the 1950s, Zoll and coworkers and Leatham and colleagues demonstrated the effectiveness of TCP in patients with bradycardia and asystole.89–92 Leatham and colleagues used larger electrodes (4 × 6 cm) and a longer pulse duration (20 msec) to successfully pace two patients with bradydysrhythmias.92
Until the late 1950s, TCP was the only clinically accepted method of cardiac pacing. The original technique involving bare metal electrodes had adverse effects, including local tissue burns, muscle contraction, and severe pain.88,92 With the development of the first implantable pacemakers from 1958 through 1960 and the improvement in transvenous electrodes during the early 1960s, TCP was rapidly discarded.93
Refinements in electrode size and pulse characteristics led to the reintroduction of TCP into clinical practice.94,95 Increasing the pulse duration from 2 to 20 msec or longer was found to decrease the current output required for cardiac capture.96,97 Longer impulse durations also make induction of ventricular fibrillation less likely.96 Electrodes with a larger surface area (80 to 100 cm2) decrease the current density at the underlying skin and therefore decrease pain and the possibility of tissue burns.94
Indications and Contraindications
General indications for cardiac pacing were discussed earlier. TCP is the fastest and easiest method of emergency pacing. This technique is useful for initial stabilization of patients in the ED who require emergency pacing while arrangements or decisions about transvenous pacemaker insertion are being made. The equipment is readily mastered, the procedure is fast, and it is minimally invasive.95,98 Refinements in equipment have made TCP the emergency pacing procedure of choice. TCP is also widely used in the prehospital environment, as well as in the hospital in the cardiac catheterization laboratory, operating room, and intensive care units and on general medical floors.99–101 The technique may be preferable to transvenous pacing in patients who have received thrombolytic agents or other anticoagulants. No central venous puncture, with the attendant risk of hemorrhage, is required. Limited experience suggests that TCP may also be useful in the treatment of refractory tachydysrhythmias by overdrive pacing.102–107 Although small pediatric electrodes for TCP have been developed, experience with pediatric TCP has been limited.108–110
TCP is indicated for the treatment of hemodynamically significant bradydysrhythmias that have not responded to medical therapy. Hemodynamically significant implies hypotension, anginal chest pain, pulmonary edema, or evidence of decreased cerebral perfusion. This technique is temporary and is indicated for short intervals as a bridge until transvenous pacing can be initiated or the underlying cause of the bradydysrhythmia (e.g., hyperkalemia,98 drug overdose) can be reversed.111 Though generally unsuccessful, TCP may be attempted for the treatment of asystolic cardiac arrest. In this setting the technique is efficacious only if used early after the onset of arrest (usually within 10 minutes).112,113 TCP is not indicated for the treatment of prolonged arrest victims with a final morbid rhythm of asystole.109,114–116
Delay from the onset of arrest to the initiation of pacing is a major problem that limits the usefulness of TCP in prehospital care. Hedges and associates reported that the everyday availability of pacing increased the number of patients who underwent pacing within 10 minutes of hemodynamic decompensation and increased long-term patient survival as well.113 Prehospital pacing may be most useful in the treatment of patients with a hemodynamically significant bradycardia who have not yet progressed to cardiac arrest (e.g., heart block in the setting of AMI) or in patients who arrest after the arrival of prehospital providers.112,113
In conscious patients with hemodynamically stable bradycardia, TCP may not be necessary. It is reasonable to attach electrodes to such patients and to leave the pacemaker in standby mode against the possibility of hemodynamic deterioration while further efforts at treatment of the patient’s underlying disorder are being made. This approach has been used successfully in patients with new heart block in the setting of cardiac ischemia.117 Generally, when a transvenous pacemaker becomes available, it is preferred because of better patient tolerance.
Equipment
Since their reintroduction, transcutaneous pacemakers have undergone rapid evolution and are now standard equipment in most EDs, as well as in other hospital settings and the prehospital environment. The pacemakers introduced in the early 1980s tended to be asynchronous devices with a limited selection of rate and output parameters. Units introduced more recently have demand mode pacing and more output options and are often combined with a defibrillator in a single unit. Combined defibrillator-pacers offer advantages in cost, ease, and rapidity of use when compared with stand-alone devices. An example of a combined unit is shown in Review Box 15-2.
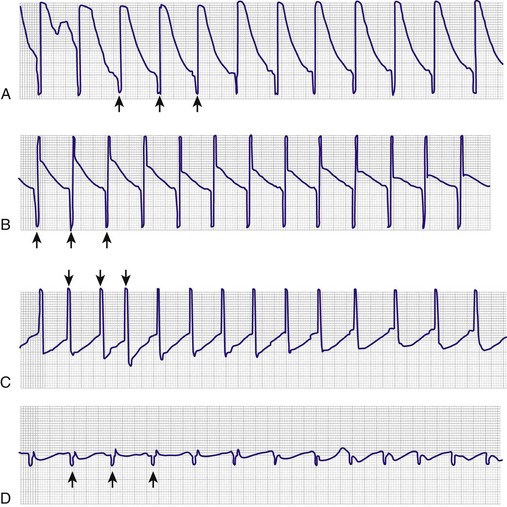
All transcutaneous pacemakers have similar basic features. Most allow operation in either a fixed rate (asynchronous) or a demand mode (VVI). Most allow rate selection in a range from 30 to 200 beats/min. Current output is usually adjustable from 0 to 200 mA. If an ECG monitor is not an integral part of the unit, an output adapter to a separate monitor is required to “blank” the large electrical spike from the pacemaker impulse and allow interpretation of the much smaller ECG complex. Without blanking protection, the standard ECG machine is swamped by the pacemaker spike and uninterpretable. This could be disastrous because the large pacing artifacts can mask treatable ventricular fibrillation (Fig. 15-12). Pulse durations on available units vary from 20 to 40 msec and are not adjustable by the operator.
Technique
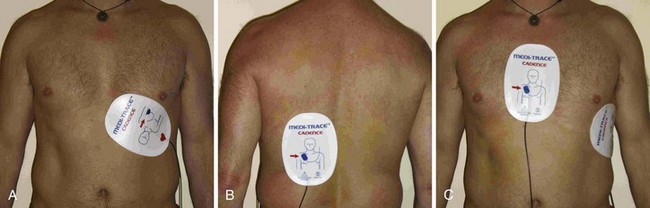
The pacing electrodes are self-adhesive. Position them as shown in Figure 15-13. Take care to avoid placing the electrodes over an implanted pacemaker or defibrillator. Remove any transdermal drug delivery patches if they are in the way. Remove excessive hair if time permits. Place the anterior electrode (cathode or negative electrode) as close as possible to the point of maximal impulse on the left anterior chest wall. Place the second electrode directly posterior to the anterior electrode (Fig.15-13A and B). The posterior electrode serves as the ground. An alternative arrangement for the pacing electrodes is shown in Figure 15-13C. On females, place the electrode beneath the breast and against the chest wall. Because data regarding optimum electrode placement are scarce, selection can be based on the clinician’s preference and the patient’s habitus.118,119 Nonetheless, suboptimal capture as a result of poor electrode placement may be rectified with a small change in electrode position. Although the polarity of the electrodes does not appear to be important for defibrillation, at least one study has indicated that it may important be for pacing.120 The electrodes are labeled by the manufacturer to indicate which should be placed over the precordium, and it is prudent to observe this recommendation. ECG electrodes (if used) are placed on the chest wall or limbs, or both, as required and connected to the instrument cable. Some clinicians prophylactically apply pacing electrodes to all critically ill patients with bradycardia to facilitate immediate TCP should decompensation occur.
There is little risk for electrical injury to health care providers during TCP. The power delivered during each impulse is less than 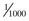 of that delivered during defibrillation.121 Chest compressions (CPR) can be administered directly over the insulated electrodes while pacing.122 Inadvertent contact with the active pacing surface results in only a mild shock.
of that delivered during defibrillation.121 Chest compressions (CPR) can be administered directly over the insulated electrodes while pacing.122 Inadvertent contact with the active pacing surface results in only a mild shock.
Pacing Bradycardiac Rhythms
To initiate TCP, apply the pacing electrodes and activate the device (Fig. 15-14, steps 1 and 2). Slowly increase the output from minimal settings until capture is achieved. (Fig. 15-14, step 4) Rate and current (output) selections are adjustable. Generally, a heart rate of 60 to 70 beats/min will maintain adequate blood pressure (by blood pressure cuff or arterial catheter) and cerebral perfusion.

Figure 15-14 Emergency transcutaneous cardiac pacing.
Assess electrical capture by monitoring the ECG tracing on the filtered monitor of the pacing unit (see Fig. 15-14, steps 5 and 6). Assess mechanical capture by palpating the pulse as for transvenous pacing. Because of muscular contractions triggered by the pacer, carotid pulses may be difficult to assess, so palpating the femoral pulse may be easier. Additionally, bedside US may prove useful in determining ventricular capture.123,124 Ideally, continue pacing at an output level just above the threshold of initial electrical capture to minimize discomfort. One study involving 16 normal male volunteers who were paced without sedation noted cardiac capture at a mean current of 54 mA (range, 42 to 60 mA).125 Most subjects could tolerate pacing at their capture threshold; only one subject required discontinuation of pacing at 60 mA because of intolerable pain. Heller and coworkers compared subjective pain perception and capture thresholds in 10 volunteers paced with five different transcutaneous pacers.126 Capture rates (40% to 80%), thresholds (66.5 to 104 mA), and subjective discomfort varied from pacemaker to pacemaker.
Failure to capture with TCP may be related to electrode placement or patient size. Patients with barrel-shaped chests and large amounts of intrathoracic air conduct electricity poorly and may prove refractory to capture. In one study, the scarring associated with thoracotomy was found to nearly double the pacing threshold.127 A large pericardial effusion or tamponade will also increase the output required for capture.128 Failure to electrically capture with a transcutaneous device in these settings is an indication to consider immediate placement of a transvenous pacer.
Patients who are conscious or who regain consciousness during TCP will experience discomfort because of muscle contraction.117,125,126 Analgesia with incremental doses of an opioid agent, sedation with a benzodiazepine compound, or both, will make this discomfort tolerable until transvenous pacing can be instituted.
Overdrive Pacing
Overdrive pacing of ventricular tachycardia or paroxysmal supraventricular tachycardia is performed in patients who are stable enough to tolerate the brief delay associated with the preparation needed for this technique.102–107 Few data exist on the efficacy or use of this procedure in the ED. Sedate the patient as explained earlier, place pacing and monitoring electrode pads in the standard positions as detailed earlier, and initiate brief trains (6 to 10 beats) of asynchronous pacing. Set the pacer rate at approximately 20 to 60 pulses/min greater than the dysrhythmia rate.129 Generally, an impulse rate of 200 pulses/min is used for ventricular tachycardias (the rate is usually 150 to 180 beats/min), and a rate of 240 to 280 pulses/min is used for paroxysmal supraventricular tachycardias (the rate is commonly 200 to 250 beats/min).
Complications
A rare complication of TCP is induction of ventricular fibrillation. Studies of fibrillation thresholds using large precordial electrodes have shown that the longer impulse durations used in modern devices seem to decrease the chance of inducing ventricular fibrillation with TCP. Nonetheless, asynchronous TCP for tachydysrhythmias has been associated with acceleration in rhythm and the development of ventricular fibrillation.105
Studies looking at prolonged TCP in humans have not been extensive. Zoll and colleagues reported 25 humans paced for up to 108 hours with impulses of 20-msec duration.91 Pacer-induced dysrhythmias did not occur. Leatham and colleagues paced one patient for 68 hours with impulses 20 msec in duration.92 The patient died 2 days after pacing was discontinued. Pathologic examination revealed no evidence of pacer-induced myocardial damage. Madsen and colleagues paced 10 healthy volunteers at threshold for 30 minutes and found no enzyme or echocardiographic abnormalities.130 TCP appears unlikely to produce cardiac injury with short-term use in the ED.
Soft tissue discomfort with the potential for injury may still occur with current transcutaneous pacemakers. Most patients are able to tolerate the discomfort, especially after sedation and analgesia. Nonetheless, prolonged use may still induce local cutaneous injury, particularly in pediatric patients because of the use of smaller electrodes.131,132 Patients who cannot tolerate TCP or who will need long-term pacing are candidates for transvenous pacing.
References
1. Rosenberg, AS, Grossman, JI, Escher, DJW, et al. Bedside transvenous cardiac pacing. Am Heart J. 1969;77:697.
2. Francis, GS, Williams, SV, Achord, JL, et al. Clinical competence in the insertion of a temporary transvenous ventricular pacemaker. Circulation. 1994;89:1913.
3. Callaghan, JC, Bigelow, WG. Electrical artificial pacemaker for standstill of heart. Ann Surg. 1951;134:8.
4. Furman, S, Robinson, G. The use of intracardiac pacemaker in the correction of total heart block. Surg Forum. 1958;9:245.
5. Furman, S. The early history of cardiac pacing. Pacing Clin Electrophysiol. 2003;26:2023.
6. Vogel, JHK, Tabari, K, Averill, KH, et al. A simple technique for identifying P-waves in complex arrhythmias. Am Heart J. 1964;67:158.
7. Kimball, JT, Killip, T. A simple bedside method for transvenous intracardiac pacing. Am Heart J. 1965;70:35.
8. Harris, CW, Hurlburt, JC, Floyd, WL, et al. Percutaneous technique for cardiac pacing with a platinum-tipped electrode catheter. Am J Cardiol. 1965;15:48.
9. Zuckerman, W, Zaroff, L, Berkovits, BV, et al. Clinical experience with a new implantable demand pacemaker. Am J Cardiol. 1967;20:232.
10. Swan, HJ, Ganz, W. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 1970;283:447.
11. Schnitzler, RN, Caracta, AR, Damato, AN. Floating catheter for temporary transvenous ventricular pacing. Am J Cardiol. 1973;31:351.
12. Lang, R, David, D, Herman, HO, et al. The use of the balloon-tipped floating catheter in temporary transvenous cardiac pacing. Pacing Clin Electrophysiol. 1981;4:491.
13. Birkhahn, RH, Gaeta, TJ, Tloczkowski, J, et al. Emergency medicine–trained physicians are proficient in the insertion of transvenous pacemakers. Ann Emerg Med. 2004;43:469.
14. Julian, DG, Valentine, DA, Miller, GG. Disturbances of rate, rhythm, and conduction in acute myocardial infarction: a prospective study of 100 consecutive unselected patients with the aid of electrocardiographic monitoring. Am J Med. 1965;37:915.
15. Baba, N. Experimental cardiac ischemia, observation of the sinoatrial and atrioventricular node. Lab Invest. 1970;23:168.
16. Link, MS, Atkins, DL, Passman, RS, et al. 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 6: electrical therapies—automated external defibrillators, defibrillation, cardioversion, and pacing. Circulation. 2010;122:S706–S719.
17. Hazard, PB. Transvenous cardiac pacing in cardiopulmonary resuscitation. Crit Care Med. 1981;9:666.
18. Niemann, JT, Adomian, GE, Garner, D, et al. Endocardial and transcutaneous cardiac pacing, calcium chloride, and epinephrine in postcountershock asystole and bradycardias. Crit Care Med. 1985;13:699.
19. Syverund, SA, Dalsey, WC, Hedges, JR. Transcutaneous and transvenous cardiac pacing for early bradyasystolic cardiac arrest. Ann Emerg Med. 1986;15:121.
20. Field, JM, Hazinski, MF, Sayre, MR, et al. 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 1: executive summary. Circulation. 2010;122:S640–S656.
21. Conklin, EF, Giannelli, S, Nealon, TF. Four hundred consecutive patients with permanent transvenous pacemakers. J Thorac Cardiovasc Surg. 1975;69:1.
22. Simon, AB, Steinke, WE, Curry, JJ. Atrioventricular block in acute myocardial infarction. Chest. 1972;62:156.
23. Resuekov, L, Lipp, H. Pacemaking in acute myocardial infarction. Prog Cardiovasc Dis. 1972;14:475.
24. Lazaros, GA, Ralli, DG, Moundaki, VS, et al. Delayed development of complete heart block after a blunt chest trauma. Injury. 2004;35:1300.
25. White, BC, Hoehner, PJ, Petinga, TJ, et al. HIS electrocardiographic characterization of terminal arrhythmias of hemorrhagic shock in dogs. JACEP. 1979;8:298.
26. Millikan, JS, Moore, EE, Dunn, EL, et al. Temporary cardiac pacing in traumatic arrest victims. Ann Emerg Med. 1980;9:591.
27. Atkins, JM, Leshin, SJ, Blumquist, G, et al. Ventricular conduction blocks and sudden death in acute myocardial infarction. N Engl J Med. 1978;288:281.
28. Hindman, MC, Wagner, GS, JaRo, M. The clinical significance of bundle-branch block complicating acute myocardial infarction. 1. Clinical characteristics, hospital mortality, and one year follow-up. Circulation. 1978;58:679.
29. Escher, DJ. The use of cardiac pacemakers. In: Braunwald E, ed. Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia: Saunders; 1980:749.
30. Thompson, JR, Dolton, BC, Lapis, DG, et al. Right bundle-branch block in complete heart block caused by Swan Ganz catheter. Anesthesiology. 1979;51:359.
31. Morris, D, Mulvihill, D, Lew, WYW. Risk of developing complete heart block during bedside pulmonary artery catheterization in patients with left bundle-branch block. Arch Intern Med. 1987;147:2005.
32. Buran, MJ. Transcutaneous pacing as an alternative to prophylactic transvenous pacemaker insertion. Crit Care Med. 1987;15:623.
33. Bessman, ES. Invasive monitoring, pacing techniques, and automatic and implantable defibrillators. In: Tintinalli JR, Kelen GB, Stapczynski JS, eds. Emergency Medicine: A Comprehensive Study Guide. 6th ed. New York: McGraw-Hill; 2003:132.
34. DeSanctis, RW, Kastor, JA. Rapid intracardiac pacing for treatment of recurrent ventricular arrhythmias in the absence of heart block. Am Heart J. 1968;76:168.
35. Goldberger, E. Temporary cardiac pacing. In: Goldberger E, Wheet MW, Jr., eds. Treatment of Cardiac Emergencies. 3rd ed. St. Louis: Mosby; 1982:233.
36. Weiner, I. Pacing techniques in the treatment of tachycardias. Ann Intern Med. 1980;93:326.
37. Rothenhausler, HB, Hoberl, C, Ehrentrout, S, et al. Suicide attempt by pure citalopram overdose causing long-lasting severe sinus bradycardia, hypotension and syncopes: successful therapy with a temporary pacemaker. Pharmacopsychiatry. 2000;33:150.
38. Punukollu, G, Gowda, RM, Khan, IA, et al. Delayed presentation of calcium channel antagonist overdose. Am J Ther. 2000;10:132.
39. Miller, MA, Crystal, CS, Helphenstine, J, et al. Successful resuscitation of hypermagnesaemic asystolic cardiac arrest with the use of early transvenous cardiac pacemaker: a case report. Emerg Med J. 2006;23:e22.
40. Suleyman, T, Tevfik, P, Abdulkadir, G, et al. Complete atrioventricular block and ventricular tachyarrhythmia associated with donepezil. Emerg Med J. 2006;23:641.
41. ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care, Part 10.2: toxicology in ECC. Circulation. 2005;112:IV126.
42. Davidson, NC, Mond, HG. Ventricular pacing in the presence of tricuspid valve disease. Pacing Clin Electrophysiol. 2002;25:129.
43. Dronen, S, Thompson, B, Nowak, R, et al. Subclavian vein catheterization during cardiopulmonary resuscitation. JAMA. 1982;247:3227.
44. Laczika, K, Thalhammer, F, Locker, G, et al. Safe and efficient emergency transvenous ventricular pacing via the right supraclavicular route. Anesth Analg. 2000;90:784–789.
45. Syverund, SA, Dalsey, WC, Hedges, JR. Radiographic assessment of transvenous pacemaker placement during CPR. Ann Emerg Med. 1986;15:131.
46. Austin, JL, Preis, LK, Crampton, RS, et al. Analysis of pacemaker malfunction and complications of temporary pacing in the coronary care unit. Am J Cardiol. 1982;44:301.
47. Weinstein, J, Gnoj, J, Mazzara, JT, et al. Temporary transvenous pacing via the percutaneous femoral approach: a prospective study of 100 cases. Am Heart J. 1973;85:695.
48. Furman, S. Pacemaker emergencies. Med Clin North Am. 1979;63:113.
49. Fyke, FE, III. Doppler guided extrathoracic introducer insertion. Pacing Clin Electrophysiol. 1995;18:1017–1021.
50. Nash, A, Burrell, CJ, Ring, NJ, et al. Evaluation of an ultrasonically guided venipuncture technique for the placement of permanent pacing electrodes. Pacing Clin Electrophysiol. 1998;21:452–455.
51. Macedo, W, Jr., Sturman, K, Kim, JM, et al. Ultrasonic guidance of transvenous pacemaker insertion in the emergency department: a report of three cases. J Emerg Med. 1999;17:491–496.
52. Aguilera, PA, Durham, BA, Riley, DA. Emergency transvenous cardiac pacing placement using ultrasound guidance. Ann Emerg Med. 2000;36:224–227.
53. Nachnani, GH, Gooch, AS, Hsu, I. Systolic murmurs induced by pacemaker catheters. Arch Intern Med. 1969;24:202.
54. Kluge, WF. Pacemaker sound and its origin. Am J Cardiol. 1970;25:362.
55. Kramer, DH, Moss, AJ, Shah, PM. Mechanisms and significance of pacemaker-induced extracardiac sound. Am J Cardiol. 1970;25:367.
56. Hynes, JK, Holmes, DR, Harrison, CE. Five-year experience with temporary pacemaker therapy in the coronary care unit. Mayo Clin Proc. 1983;58:122.
57. Lumia, FJ, Rios, JC. Temporary transvenous pacemaker therapy: an analysis of complications. Chest. 1973;64:604.
58. Pandian, NG, Kosowsky, BD, Gurewich, V. Transfemoral temporary pacing and deep venous thrombosis. Am Heart J. 1980;100:847.
59. Nolewajka, AT, Goddard, MD, Brown, TG. Temporary transvenous pacing and femoral vein thrombosis. Am J Cardiol. 1980;45:459.
60. Herbst, CA. Indications, management, and complications of percutaneous subclavian catheters. Arch Surg. 1978;113:1421.
61. Sethi, GK, Bhayana, JN, Scott, SM, et al. Innominate venous thrombosis: a rare complication of transvenous pacemaker electrodes. Am Heart J. 1974;87:770.
62. Zibaeenezhad, MJ, Atabati, E, Aghasadeghi, K. Prevention of deep vein thrombosis associated with temporary transvenous cardiac pacemakers by enoxaparin. Iranian Cardiovasc Res J. 2009;3:153.
63. Johnson, CL, Jazarchick, J, Lynn, HB. Subclavian venipuncture, preventable complications. Mayo Clin Proc. 1970;45:719.
64. Arbitman, M, Kart, BH. Hydromediastinum after aberrant central venous catheter placement. Crit Care Med. 1979;7:27.
65. Drachler, DH, Koepte, GH, Wey, JG. Phrenic nerve injury from subclavian vein catheterization. JAMA. 1976;236:2880.
66. Cope, C. Intravascular breakage of Seldinger spring guide wires. JAMA. 1962;180:1061.
67. Schwartz, AJ, Harrow, JC, Jobes, DR, et al. Guide wires—a caution. Crit Care Med. 1981;9:347.
68. Mehra, R, Furman, S, Crump, J. Vulnerability of the mildly ischemic ventricle to cathodal, anodal, and bipolar stimulation. Circ Res. 1977;41:159.
69. Abernathy, WS, Crevey, BJ. Right bundle-branch block during transvenous ventricular pacing. Am Heart J. 1975;90:774.
70. Campo, I, Garfield, GJ, Escher, DJW, et al. Complications of pacing by pervenous subclavian semifloating electrodes including extraluminal insertions. Am J Cardiol. 1970;26:627.
71. Danielson, GK, Shabetai, R, Bryant, LR. Failure of endocardial pacemaker due to myocardial perforation. J Thorac Cardiovasc Surg. 1967;54:42.
72. Goswani, M, Gould, L, Gompiecht, RF, et al. Perforation of the heart by flexible transvenous pacemaker. JAMA. 1971;216:2013.
73. Kalloor, GJ. Cardiac tamponade. Report of a case after insertion of transvenous endocardial electrode. Am Heart J. 1974;88:88.
74. Jorgensen, EO, Lyngborg, K, Wennevold, A. Unusual sign of perforation of a pacemaker catheter. Am Heart J. 1967;74:732.
75. Kaye, D, Frankl, W, Arditi, LI. Probable postcardiotomy syndrome following implantation of a transvenous pacemaker: report of the first case. Am Heart J. 1975;90:627.
76. Glassman, RD, Noble, RJ, Tavel, ME, et al. Pacemaker-induced endocardial friction rub. Am J Cardiol. 1977;40:811.
77. Tarver, RB, Gillespie, KR. The misplaced tube. Emerg Med. 1988;20:97.
78. Boal, BH, Keller, BD, Ascheim, RS, et al. Complication of intracardiac electrical pacing—knotting together of temporary and permanent electrodes. N Engl J Med. 1969;280:650.
79. Johansson, L, Malmstrom, G, Uggla, LG. Intracardiac knotting of the catheter in heart catheterization. J Thorac Surg. 1954;27:605.
80. Foote, GA, Schabel, SI, Hodges, M. Pulmonary complications of the flow-directed balloon-tipped catheter. N Engl J Med. 1974;290:927.
81. Escher, DJ, Furman, S, Solomon, N, et al. Transvenous pacing of the phrenic nerve. Am Heart J. 1977;72:283.
82. Preston, TA. Electrocardiographic diagnosis of pacemaker catheter displacement. Am Heart J. 1973;854:445.
83. Smith, ND. Pacemaker dysfunction. In: Greenberg MI, Roberts JR, eds. Emergency Medicine: A Clinical Approach to Challenging Problems. Philadelphia: Davis; 1982:355.
84. Preston, TA, Fletcher, RD, Lucchesi, BR, et al. Changes in myocardial threshold, physiologic, and pharmacologic factors in patients with implanted pacemakers. Am Heart J. 1967;74:235.
85. Leung, FW, Oill, PA. Ticket of admission: unexplained syncopal attacks in patients with cardiac pacemaker. Ann Emerg Med. 1980;9:527.
86. Duchenne de Boulogne. De l’Electrisation Localise et Son Application a la Pathologique et a la Therapeutique. Paris: Bailliere; 1872.
87. VonZiemssen, H, Studienüber die Bewegungsvorgänge am menschlichen Herzen, sowieüber die mechanische und elektrische Erregbarkeit des Herzens und des Nervus, 1882.
88. Zoll, PM. Resuscitation of the heart in ventricular standstill by external electrical stimulation. N Engl J Med. 1952;247:768.
89. Zoll, PM, Linenthal, AJ, Norman, LR, et al. Treatment of unexpected cardiac arrest by external electric stimulation of the heart. N Engl J Med. 1956;254:541.
90. Zoll, PM, Linenthal, AJ, Norman, LR. Treatment of Stokes-Adams disease by external stimulation of the heart. Circulation. 1954;9:482.
91. Zoll, PM, Linenthal, AJ, Norman, LR, et al. External electric stimulation of the heart in cardiac arrest. Arch Intern Med. 1955;96:639.
92. Leatham, A, Cook, P, Davis, JG. External electric stimulator for treatment of ventricular standstill. Lancet. 1956;2:1185.
93. Chardack, WM, Gage, AA, Greatbatch, W. A transistorized self-contained, implantable pacemaker for the long-term correction of complete heart block. Surgery. 1960;48:643.
94. Zoll, PM. External noninvasive electric stimulation of the heart. Crit Care Med. 1981;9:393.
95. Dalsey, WC, Syverud, SA, Trott, A. Transcutaneous cardiac pacing. J Emerg Med. 1984;1:201.
96. Jones, M, Geddes, LA. Strength duration curves for cardiac pacemaking and ventricular fibrillation. Cardiovasc Res Bull. 1977;15:101.
97. Varghese, PJ, Bren, G, Ross, A. Electrophysiology of external cardiac pacing: a comparative study with endocardial pacing. Circulation. 1982;66:349.
98. Clinton, JE, Zoll, PM, Zoll, R, et al. External noninvasive cardiac pacing. J Emerg Med. 1985;2:155.
99. Berliner, D, Okun, M, Peters, RW, et al. Transcutaneous pacing in the operating room. JAMA. 1985;254:84.
100. Johnson, DQ, Vukov, LF, Farnell, MB. External transcutaneous pacemakers in air medical transport services [abstract]. Aeromed J. 1987;2:23.
101. Noe, R, Cockrell, W, Moses, HW, et al. Transcutaneous pacemaker use in a large hospital. Pacing Clin Electrophysiol. 1986;9:101.
102. Rosenthal, ME, Stamato, NJ, Marchlinski, FE, et al. Noninvasive cardiac pacing for termination of sustained, uniform ventricular tachycardia. Am J Cardiol. 1986;58:561.
103. Sharkey, SW, Chaffee, V, Kapsner, S. Prophylactic external pacing during conversion of atrial tachyarrhythmias. Am J Cardiol. 1985;55:1632.
104. Altamura, G, Bianconi, L, Boccadamo, R, et al. Treatment of ventricular and supraventricular tachyarrhythmias by transcutaneous cardiac pacing. Pacing Clin Electrophysiol. 1989;12:331.
105. Grubb, BP, Temsey-Armos, P, Hahn, H, et al. The use of external, noninvasive pacing for the termination of ventricular tachycardia in the emergency department setting. Ann Emerg Med. 1992;21:174.
106. Grubb, BP, Samoil, D, Temsey-Armos, P, et al. The use of external, noninvasive pacing for the termination of supraventricular tachycardia in the emergency department setting. Ann Emerg Med. 1993;22:714.
107. Monraba, R, Sala, C. Percutaneous overdrive pacing in the out-of-hospital treatment of torsades de pointes. Ann Emerg Med. 1999;33:356.
108. Beland, MJ, Hesslein, PS, Finlay, CD, et al. Noninvasive transcutaneous cardiac pacing in children. Pacing Clin Electrophysiol. 1987;10:1262.
109. Quan, L, Graves, JR, Kinder, DR, et al. Transcutaneous cardiac pacing in the treatment of out-of-hospital pediatric cardiac arrests. Ann Emerg Med. 1992;21:905.
110. Hatlestad, D. The benefits of electricity: transcutaneous pacing in EMS. Emerg Med Serv. 2002;31:38.
111. Cummins, RO, Haulman, J, Quan, L, et al. Near-fatal yew berry intoxication treated with external cardiac pacing and digoxin specific FAB antibody fragments. Ann Emerg Med. 1990;19:38.
112. Barthell, E, Troiano, P, Olson, D, et al. Prehospital external cardiac pacing: a prospective, randomized, controlled clinical trial. Ann Emerg Med. 1988;17:1221.
113. Hedges, JR, Feero, S, Shultz, B, et al. Prehospital transcutaneous cardiac pacing for symptomatic bradycardia. Pacing Clin Electrophysiol. 1991;14:1473.
114. Eitel, DR, Guzzardi, LJ, Stein, SE, et al. Noninvasive transcutaneous cardiac pacing in prehospital cardiac arrest. Ann Emerg Med. 1987;16:531.
115. Cummins, RO, Graves, JR, Larsen, MP, et al. Out-of-hospital transcutaneous pacing by emergency medical technicians in patients with asystolic cardiac pacing. N Engl J Med. 1993;328:1377.
116. Hedges, JR, Syverud, SA, Dalsey, WC, et al. Prehospital trial of emergent transcutaneous pacing. Circulation. 1987;76:1337.
117. Zoll, PM, Zoll, RH, Falk, RH, et al. External non-invasive temporary cardiac pacing: clinical trials. Circulation. 1985;71:937.
118. Deakin, CD, Nolan, JP, European Resuscitation Council. European Resuscitation Council guidelines for resuscitation 2005. Section 3. Electrical therapies: automated external defibrillators, defibrillation, cardioversion and pacing. Resuscitation. 2005;67(suppl 1):S25.
119. Panescu, D, Webster, JG, Tompkins, WJ, et al. Optimisation of transcutaneous cardiac pacing by three-dimensional finite element modelling of the human thorax. Med Biol Eng Comput. 1995;33:769.
120. Falk, RH, Ngai, ST. External cardiac pacing: influence of electrode placement on pacing threshold. Crit Care Med. 1986;14:931.
121. Syverud, SA, Dalsey, WC, Hedges, JR. Transcutaneous cardiac pacing [letter]. Ann Emerg Med. 1984;13:982.
122. Dalsey, WC, Syverud, SA, Hedges, JR. Emergency department use of transcutaneous cardiac pacing for cardiac arrests. Crit Care Med. 1985;13:399.
123. Ettin, D, Cook, T. Using ultrasound to determine external pacer capture. J Emerg Med. 1999;17:1007–1009.
124. Holger, JS, Lamon, RP, Minnigan, HJ, et al. Use of ultrasound to determine ventricular capture in transcutaneous pacing. Am J Emerg Med. 2003;21:227.
125. Falk, RH, Zoll, PM, Zoll, RH. Safety and efficacy of noninvasive cardiac pacing: a preliminary report. N Engl J Med. 1983;309:1166.
126. Heller, MB, Kaplan, RM, Peterson, J, et al. Comparison of performance of five transcutaneous pacing devices [abstract]. Ann Emerg Med. 1987;16:493.
127. Kemnitz, J, Winter, J, Vester, EG, et al. Transcutaneous cardiac pacing in patients with implantable cardioverter defibrillators and epicardial patch electrodes. Anesthesiology. 1992;77:258.
128. Hedges, JR, Syverud, SA, Dalsey, WC, et al. Threshold enzymatic and pathologic changes associated with prolonged transcutaneous pacing in a chronic heart block model. J Emerg Med. 1989;7:1.
129. Fisher, JD, Matos, JA, Kim, SC. Anti-tachycardia pacing and stimulation. In: Josephson ME, Wellens HJJ, eds. Tachycardias: Mechanisms, Diagnosis, and Treatments. Philadelphia: Lea & Febiger; 1984:413–425.
130. Madsen, JK, Pedersen, F, Grande, P, et al. Normal myocardial enzymes and normal echocardiographic findings during noninvasive transcutaneous pacing. Pacing Clin Electrophysiol. 1988;11:1188.
131. Pride, HB, McKinley, DF. Third-degree burns from the use of an external cardiac pacing device. Crit Care Med. 1990;18:572.
132. Haas, NA, Kulasekaran, K, Camphausen, C. Beneficial hemodynamic response of transthoracic cardiac pacing in a 2 kg preterm neonate. Intensive Care Med. 2005;31:877.