Chapter 89 Embolization of Tumors
Brain, Head, Neck, and Spine
Hypervascular neoplasms of the central nervous system (CNS) can be formidable surgical challenges associated with significant morbidity and mortality. Vascular tumors can result in excessive intraoperative blood loss, prompting termination of the surgery before achieving its goals. Multiple reports have suggested preoperative embolization can reduce intraoperative blood loss, the need for transfusions, operative time, and the length of hospitalization.1–4 Embolization also may reduce mass effect and alleviate pain.5 Furthermore, preoperative embolization can facilitate a more complete surgical extirpation by clarifying the surgical field, enhancing tumor boundaries, and shrinking the tumor.
In most cases, preoperative embolization of arterial pedicles in various vascular CNS tumors is technically feasible, regardless of the tumor’s origin and location (Table 89-1). The goals of tumor embolization are sacrificing the feeding vessels and obliterating the tumor capillary bed to the greatest extent possible. These goals must be balanced against the risks of embolization, which include occlusion of en passage vessels, pulmonary emboli, retained microcatheters, and compression of eloquent neural tissues by expanding intratumoral edema or hemorrhage.
TABLE 89-1 Barrow Neurologic Institute Series of 169 Embolized CNS Tumors (1995-2009)
| Tumors | No. |
|---|---|
| Meningiomas | |
| Olfactory groove | 2 |
| Convexity | 10 |
| Skull base | 1 |
| Parasagittal | 3 |
| Frontal | 5 |
| Sphenoid | 3 |
| Paraganglioma | |
| Glomus jugulare | 17 |
| Glomus tympanicum | 1 |
| Carotid body tumors | 7 |
| Hemangioblastoma | 12 |
| Juvenile Nasal Angiofibroma | 25 |
| Hemangiopericytoma | 3 |
| Others | |
| Plasmacytoma (spinal) | 1 |
| Aneurysmal bone cyst (spinal) | 2 |
| Thyroid carcinoma met (spinal) | 1 |
| Hemangioma (skull base) | 1 |
| Hemangioma (facial) | 2 |
| Hemangioma (spinal) | 4 |
| Renal carcinoma met (spinal) | 12 |
| Renal carcinoma met (cranial) | 1 |
| Giant cell tumor | 3 |
| Vestibular schwannoma | 1 |
| Jugular foramen nerve sheath tumor | 1 |
| Synovial cell sarcoma (spinal) | 1 |
| Osteogenic sarcoma (spinal) | 1 |
| Schwannoma (spinal) | 2 |
| Pharyngeal carcinoma | 2 |
| Chordoma (spinal) | 1 |
| Nasal polyp | 1 |
| Thyroid carcinoma | 1 |
| Melanoma (spinal) | 1 |
| Dural cavernous malformation | 1 |
| Total | 129 |
Tumors of the Head
Meningiomas
Meningiomas originate from arachnoid cap cells and can be hypervascular. They are slightly more common in females than in males and account for 13% to 18% of all intracranial tumors.6 Typically, they are benign, with the potential for a surgical cure with complete resection. Recurrence rates are inversely proportional to the extent of surgical resection. Angiography and preoperative embolization of intracranial meningiomas are common practices used to improve the chances obtaining complete resection and a cure. The resection of many large meningiomas has been aborted due to heavy intraoperative blood loss, a complication that can be mitigated with judicious use of preoperative embolization. Angiography can also assist surgical planning by delineating the vascular supply to the tumor, the encasement and patency of vascular structures (arteries or dural venous sinuses), the degree of displacement of neuronal elements, and the site of dural attachment.
The vascular supply of posterior fossa meningiomas is usually from the posterior meningeal artery, MMA, or accessory meningeal artery. Classically, tentorial meningiomas receive arterial feeders from the tentorial branch of the meningohypophyseal trunk (MHT), but they can be supplied by the infratentorial trunk, MMA, or accessory meningeal artery. Petroclival lesions are often supplied by the MMA (frequently from the petrosal, petrosquamosal, or occipital branches), transmastoid branches of the posterior auricular or occipital arteries, anterior inferior cerebellar artery (AICA, via the subarcuate branch), or neuromeningeal branch of the ascending pharyngeal artery.
Outcomes
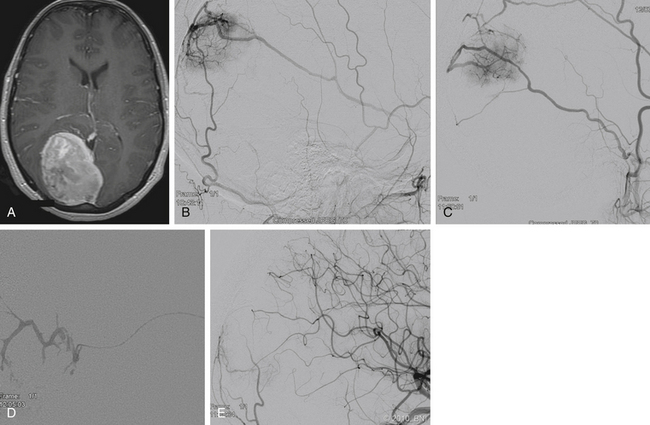
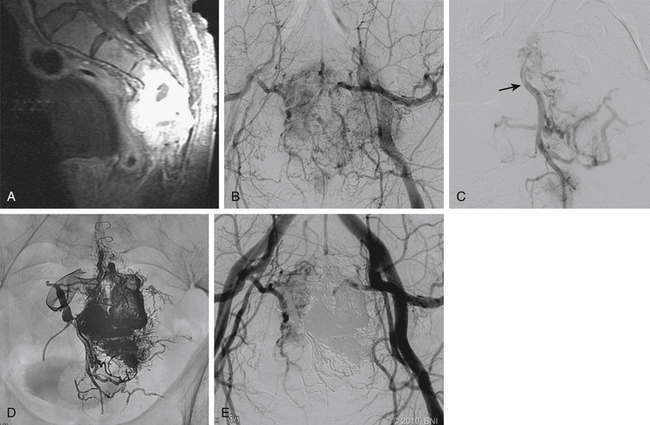
Many meningiomas do not require preoperative embolization because they often can be easily devascularized at surgery. At our institution, we recommend preoperative embolization of giant meningiomas, meningiomas involving the middle cranial fossa or skull base, falcine or parasagittal meningiomas, or meningiomas of the pineal region (Fig. 89-1). During surgery, the vascular supply to skull base meningiomas is frequently obscured until a substantial portion of the tumor has been excised, emphasizing the beneficial utility of embolization in these cases. In patients who are poor surgical candidates, embolization may be offered as a palliative measure to slow tumor growth.
Large, hypervascular skull base tumors whose vascular supply is not readily accessible via surgery should be evaluated for preoperative embolization. Embolization of deep-feeding arteries such as the inferolateral trunk and MHT can be technically challenging due to their small caliber and acute angle of origin. Technological advances in microcatheters and microguidewires have facilitated superselective catheterization of these blood vessels and expanded the range of treatable intracranial lesions through embolization. Even so, the potential for reflux of embolic material into the ICA remains a serious concern, and these lesions continue to present major challenges for even the most experienced neurointerventionalists. Abdel Kerim et al. described a technique of inflating a balloon in the MHT distal to the exit of a tumoral feeding artery to improve the penetration of Onyx (ev3 Endovascular, Irvine, CA) into the feeding vessel.7 Several authors have successfully embolized middle fossa tumors with deep-feeding arteries with good surgical and radiographic outcomes.8–10 The inferolateral trunk occasionally has collaterals with the ophthalmic artery via the deep recurrent ophthalmic artery. During embolization of the inferolateral trunk, extreme caution is warranted to minimize the risk of potential blindness.
Embolization of pial or ophthalmic branches is usually considered too perilous to undertake. However, Kaji et al. reported two cases in which distal cortical ICA branches were successfully embolized with Gelfoam (Pharmacia & Upjohn Company LLC, Peapack, NJ) before surgery.11 Based on their experience, these authors recommend that embolization of pial or cortical vessels only be undertaken if the following conditions are met: (1) The tumor is supplied exclusively by the ICA. (2) The tumor is located in a noneloquent portion of the brain. (3) The patient has a negative sodium amytal test. (4) Superselective catheterization is performed with the catheter directly abutting the tumor capsule. (5) Particulate, rather than glue-based, embolisate is used.
Pineal region meningiomas are rare, accounting for 0.3% of intracranial meningiomas and 6% to 8% of pineal region tumors.12 These uncommon lesions can draw their blood supply from a variety of sources, including meningeal branches of the ECA, the tentorial artery, medial or lateral posterior choroidal branches, branches of the superior vermian or superior cerebellar artery (SCA), meningeal branches of the posterior inferior cerebellar artery (PICA), or VAs. Sagoh et al. reported successful embolization of a pineal region meningioma with estrogen alcohol and polyvinyl alcohol (PVA) via the bilateral MMAs.13
Optic nerve meningiomas are seldom amenable to endovascular treatment because of the shared blood supply between the tumor and the optic nerve. Terada et al. concluded that if the microcatheter can be positioned distal to the origin of the central retinal artery, embolization is possible. However, the risk of causing blindness is high if reflux occurs into the central retinal artery.14 In many cases, aggressive embolization of optic nerve meningiomas is neither beneficial nor advisable.
Indications for Embolization
In general, the primary rationale for the embolization of meningiomas is to reduce blood flow to the tumor, thereby facilitating a more complete surgical resection. Several studies have attempted to compare the risks associated with preoperative embolization for meningioma resection with its benefits. Bendszus et al. concluded that intraoperative blood loss was reduced only in patients who underwent complete tumor embolization as defined by absence of tumor blush on angiography.15
We performed a retrospective study to determine the risk-to-benefit profile of preoperative embolization of meningiomas. In the study, 33 patients underwent preoperative embolization followed by surgical resection. These patients were compared to an appropriately matched group of 193 nonembolized meningiomas that were extirpated. Preoperative embolization significantly reduced intraoperative blood loss and the need for transfusion. The operative time, total cost, length of stay, and rates of complication were similar in both groups. Other authors have found similar findings.16,17
Complications
The overall risk associated with endovascular embolization of meningiomas is low.18,19 Major complications include stroke, blindness, intratumoral edema, or hemorrhage. Migration of embolic material via reflux or an unappreciated EC-to-IC anastomosis is the most common cause of major morbidity associated with embolization. The neurointerventionalist must have a working knowledge of the highly variable anatomy of EC-to-IC anastomoses and must constantly remain vigilant for the possibility of proximal reflux. Cataclysmic intratumoral swelling follows embolization, particularly if performed with particle embolisates, and can require emergent resection. Our practice is to resect meningiomas the day after embolization. Tumor swelling can sometimes be mitigated by the administration of corticosteroids.
Minor complications occur in as many as 30% of patients and include facial pain, trismus, or both.19 These side effects can be managed symptomatically with corticosteroids or analgesics and are usually self-limited. Rare complications such as cranial nerve damage (thought to be related to occlusion of the vaso vasorum of the cranial nerves), subarachnoid hemorrhage, or retinal embolus have been reported.20,21 Scalp necrosis is a rare but serious complication occasionally associated with embolization of ECA vessels. Several authors recommend preserving the STA as a donor vessel for a free tissue transfer in the event of massive scalp necrosis.22,23
Paragangliomas
Certain familial patterns or association with genetic syndromes (multiple endocrine neoplasia II, neurofibromatosis 1, von Hippel-Lindau (VHL) disease, familial paraganglioma, or Carney triad) have been associated with the diagnosis of paragangliomas. Multiple paragangliomas have been found in 22% and 87% of sporadic and familial paragangliomas, respectively.24,25 Indium-111 octreotide, a radioisotope somatostatin analogue, has been used as a labeling tracer to selectively identify multiple or metastatic paragangliomas.26
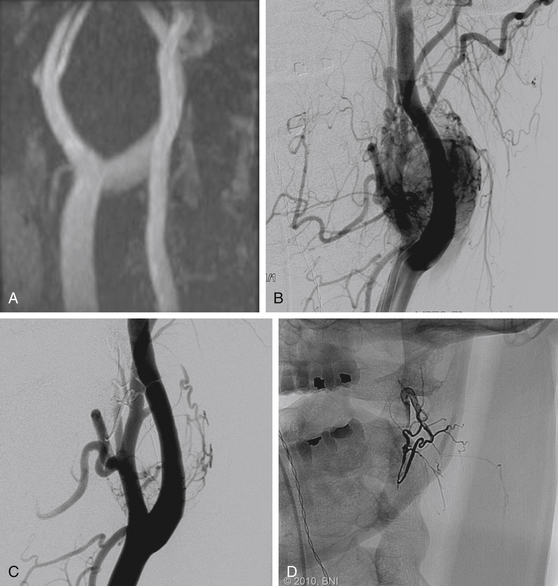
The most common presentation associated with carotid body tumors is a painless, slowly enlarging neck mass. These tumors can cause lower cranial nerve dysfunction (i.e., hoarseness, stridor, or hypoglossal palsy) due to local mass effect, but they rarely grow larger than 4 cm. The diagnosis of carotid body tumors can be confused with glomus vagale. The latter lesions typically arise from paraganglionic tissue rests within the nodose ganglion and are found immediately rostral to the carotid bifurcation. The angiographic appearance of carotid body tumors and glomus vagale tumors differs in that carotid body tumors characteristically splay the ICA and ECA (Fig. 89-2), whereas vagal paragangliomas tend to displace the carotid arteries anteriorly and medially.27
Angiography of these lesions must identify the intracranial and extracranial supply to the tumor, as well as the involvement of the dural venous sinus system. The patency of both transverse and sigmoid sinuses must be evaluated to determine whether sacrifice of the involved sinus is feasible without causing venous hypertension and infarction. The blood supply to a carotid body tumor is typically derived from proximal ECA branches or is derived directly from the bifurcation. The blood supply to tympanojugular tumors is almost uniformly derived from the ascending pharyngeal artery.28 Glomus tympanicum tumors usually receive blood supply from the inferior tympanic branch of the ascending pharyngeal artery, while branches of the neuromeningeal trunk supply the hypoglossal canal and jugular fossa lesions. These lesions tend to be small and rarely require preoperative embolization. Glomus tumors within the temporal bone are often fed by branches of the petrous (via the vidian artery) or cavernous (clival branch of the MHT) segments of the ICA.
Glomus jugulare tumors, particularly those that extended into the intracranial compartment, require preoperative embolization.29 These lesions frequently are multicompartmentalized, with a separate arterial supply to each compartment. To achieve complete embolization of a glomus jugulare tumor, the neurointerventionalist must selectively catheterize and embolize each arterial feeding vessel. In general, the ascending pharyngeal artery supplies the inferomedial compartment, while the stylomastoid branch of the occipital or posterior auricular artery contributes to the posterolateral compartment. The anterior compartment tends to be supplied by branches of the internal maxillary artery or the caroticotympanic artery. Branches of the MMA typically feed the superior compartment. If sacrifice of the jugular vein or sigmoid sinus will be necessary, the intracranial venous outflow system should be evaluated during angiography.
Superselective catheterization of the arterial pedicles is crucial for evaluating the angioarchitecture of the tumor and for identifying EC-to-IC anastomoses. Many such superselective microcatheterizations may be required to opacify or embolize the entire tumor. Tumors with substantial supply from the ICA or significant encasement of the ICA may not be amenable to surgical resection and can be evaluated for possible vessel sacrifice with balloon test occlusion.
Embolization reduces operative time and intraoperative blood loss.30,31 In the hands of an experienced neurointerventionalist, the risk of embolization for carotid body tumors is acceptably low, although the yield is probably too low to justify embolization of lesions smaller than 2 cm. Due to local soft-tissue inflammatory response, surgery within 48 hours of embolization is strongly recommended.
Complications
Most severe complications associated with embolization of head and neck paragangliomas are related to inadvertent migration of embolisate into the intracranial circulation, either through reflux or through the rich and highly variable EC-to-IC anastomotic network. Embolization of glomus jugulare tumors can cause lower cranial nerve palsies, presumably from embolization of the vaso vasorum supplying these nerves. Facial nerve palsies and even herniation syndromes have also been reported as rare complications of glomus jugulare tumor embolization.32,33 Temporary facial nerve paresis is common after embolization because the facial nerve often receives its blood supply from the stylomastoid artery and the petrosal branches of the MMA or accessory meningeal artery. Recovery of facial nerve paresis is more common when PVA is used as the embolic agent because the vessels tend to recanalize. Provocative testing should be undertaken before glue embolization because this embolisate is relatively permanent and may lead to irreversible deficits.
Hemangioblastomas
Hemangioblastomas are benign, hypervascular neoplasms primarily found in the cerebellum or spinal cord. They account for 1% to 2% of craniospinal tumors and occur most commonly within the cerebellar hemispheres, followed by the vermis, cerebellopontine angle, or brain stem.6 Most hemangioblastomas are sporadic, but 20% are associated with VHL disease. The disease has an autosomal dominant inheritance pattern with incomplete penetrance. Multiple hemangioblastomas are common in patients with VHL disease.
Operative morbidity is high because of uncontrollable bleeding, so naturally these lesions have been targeted for preoperative embolization.34 The blood supply to cerebellar hemangioblastomas is typically from PICA, but AICA or SCA branches can also contribute. Pontomedullary lesions often derive their blood supply from SCA branches, while cervicomedullary lesions are supplied by branches of the VA or anterior spinal artery. Superficial lesions can draw blood supply from dural branches of the VA (i.e., posterior meningeal artery). Due to the highly vascular nature of these lesions, the caliber of the feeding artery can exceed that of the basilar artery.
The risk associated with embolization of hemangioblastomas is high because the feeding arteries are often pial vessels. Suboptimal penetration of embolisate into the tumor nidus offers little in terms of reducing operative blood loss, particularly in posterior fossa lesions. The patient is thereby exposed to the risk of the embolization procedure without incurring any benefit.35 Embolization of posterior fossa hemangioblastomas has been associated with complication rates as high as 50%, although some studies have shown preoperative embolization to be a helpful adjunct to resection.36,37 Some authors postulate that postembolization hemorrhage is related to venous outflow obstruction.38,39
Hemangiopericytomas
Hemangiopericytomas are highly vascular lesions, and intraoperative hemorrhage can be significant. Hemorrhage is the most common cause for subtotal resection or operative morbidity. Embolization substantially reduces intraoperative bleeding and facilitates resection.40–42 However, embolization can be technically difficult because these tumors tend to parasitize cortical vessels. Ethanol and direct surgical puncture have been successfully employed in the past, although we have had success with Onyx, n-BCA, and PVA. Postembolization swelling is common with these lesions; therefore, resection within 48 hours of embolization is recommended.
Juvenile Nasal Angiofibromas
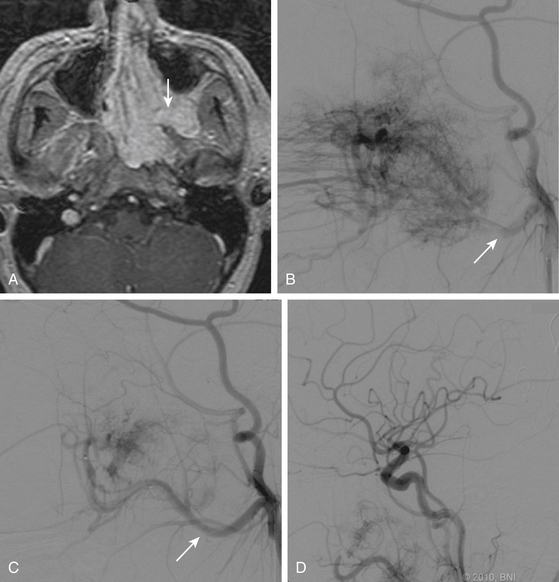
Juvenile nasal angiofibromas (JNAs) are benign, extremely vascular, nonencapsulated neoplasms consisting of vascular and connective tissue. They usually arise from the superior posterior margin of the sphenopalatine foramen. JNAs are the most common benign tumor of the nasopharynx and account for 0.05% to 0.5% of all head and neck tumors.43 These tumors almost exclusively affect adolescent boys; the mean age at diagnosis is 14 years. JNAs rarely metastasize but display locally malignant behavior and exhibit high rates of recurrence after subtotal resection.44 Approximately 30% of JNAs manifest with intracranial extension.45 The most common presenting symptoms are epistaxis and prolonged nasal obstruction.
The unique appearance of JNAs on CT and MRI eliminates the need for biopsy, which can result in uncontrollable bleeding. Angiography demonstrates multiple tortuous feeding vessels followed by a dense, homogeneous blush in the capillary phase (Fig. 89-3). Prominent draining veins are apparent immediately in the early venous phase. JNAs are typically supplied by branches of the internal maxillary artery, with contributions from the ascending pharyngeal artery in as many as 33% of cases.46 Bilateral carotid angiography is mandatory in all cases, particularly if there is intracranial extension, because these tumors can recruit blood supply from the ophthalmic artery, contralateral internal maxillary artery, and branches of either ICA.
Surgical resection is regarded as the primary treatment modality.47 JNAs are highly vascular, which can lead to significant intraoperative blood loss, increased morbidity, and incomplete resection. Preoperative embolization is an important adjunct to help reduce intraoperative blood loss and to facilitate surgical extirpation.48–50 For embolization to be effective, the small distal vessels within the tumor must be obstructed. Failure of the embolisate to penetrate the tumor parenchyma results in inadequate devascularization and may lead to an unsatisfactory reduction in intraoperative blood loss.
Older case series recommend against embolization of JNAs, citing ineffective reduction of intraoperative blood loss or excessive risks of the procedure.51,52 However, our experience has been positive. From 1995 to 2009, we successfully and safely embolized 30 JNAs without complications and with improvement in intraoperative blood loss based on the subjective opinion of the operators. We have successfully used PVA, n-BCA, and more recently, Onyx. Gay et al. reported an unusual case of postoperative palatal necrosis and oronasal fistula after a staged embolization followed by transpalatal resection of a JNA.53 The authors believe that this complication was potentiated by the embolization but maintain that preoperative embolization is still warranted for these tumors.
After embolization of JNAs, the most common complications include fever and local pain. Fever is thought to arise from tissue ischemia and should not delay surgical resection. Bradycardia occasionally follows embolization of the internal maxillary artery or ascending pharyngeal artery. Intracranial embolization can arise as a result of unrecognized EC-to-IC collaterals or direct reflux of embolisate around the tip of the microcatheter.
Miscellaneous Lesions
The treatment of choice is surgical excision. Preoperative embolization can be used for tumor devascularization or as the sole treatment modality in surgically inaccessible lesions.54–56 Koci et al. described a patient treated with embolization as monotherapy for an aneurysmal bone cyst that regressed (characterized by involution of the soft-tissue component, sclerosis, and ossification) for 2 years.57 However, a recurrence at 4 years led the authors to conclude that these lesions require continued surveillance. Successful preoperative embolization of large aneurysmal bone cysts of the skull base has also been reported.58
Embolization of metastatic disease to the skull has been reported in hepatocellular carcinoma and thyroid cancer (follicular and papillary histologic subtypes). These lesions tend to be supplied by branches of the ECA. Embolization can be used as a presurgical adjunct or as a palliative measure for pain control.59 Lin et al. described the successful embolization of metastatic osteosarcoma to the orbit in a single patient.60
Bingaman et al. described presurgical embolization of an intracranial and extracranial mesenchymal chondrosarcoma of the right frontal region.61 The lesion was supplied by branches of the ECA on both sides, and embolization was performed through the ipsilateral MMA. The authors emphasized the importance of gross total resection, as well as close surveillance of this potentially malignant tumor.
Avellino et al. described the embolization of a recurrent Masson’s vegetant intravascular hemangioendothelioma involving the cerebellopontine angle and middle cranial fossa.62 Despite attempted embolization followed by resection on two separate occasions, the lesion recurred. The tumor ultimately derived blood supply from the AICA, MHT, and bilateral occipital arteries. Embolization of the left occipital artery was initially successful, but angiography at the time of the recurrence demonstrated substantial contribution from the ECA and left MHT. The patient tolerated balloon test occlusion and ultimately underwent sacrifice of the left ICA.
Spinal Tumors
Presurgical embolization of vascular tumors has become common in many centers. Surgeons’ experiences have been used to infer a definitive decrease in intraoperative blood loss associated with spinal tumors embolized before surgery. However, conflicting results regarding the benefits of preoperative embolization of vascular spinal tumors have also been reported. Multiple reports have suggested that preoperative embolization reduces intraoperative blood loss. Guzman et al. described their experience with preoperative embolization of vertebral metastases in 24 patients.63 Two patients did not undergo embolization because the feeding pedicle branched from the artery of Adamkiewicz. The estimated operative blood loss was 5500 ml in those two patients compared to 1900 ml in the remaining patients whose tumors were successfully embolized.63 Berkefeld et al. reported a median intraoperative blood loss of 4350 and 1800 ml in surgical cases of nonembolized and embolized spinal tumors using PVA particles, respectively.64 In contrast, Jackson et al. found no significant reduction in intraoperative blood loss between embolized and nonembolized spinal tumors.65 Empirically, however, they believed that embolization facilitated resection of renal cell carcinomas. King et al. also reported no significant differences in intraoperative blood loss between embolized and nonembolized spinal tumors.66 However, they had to abort surgical resection due to excessive blood loss in three patients whose tumors were not embolized.
Primary Spinal Tumors
Paragangliomas
As neuroendocrine tumors, paragangliomas most often arise in the intradural extramedullary compartment and in the lumbosacral segment when found in the spine. Patients usually develop symptoms consistent with cauda equina or spinal cord compression. Intracranial hypertension has been reported to be associated with paragangliomas.67 On MRI, paragangliomas can be difficult to distinguish from other cauda equina tumors (i.e., ependymomas). Angiographically, paragangliomas are densely vascular with possible intratumoral arteriovenous shunting. Serpiginous vessels surrounding the tumor blush, which are often visible on angiograms, correspond to dilated veins.68
Hemangioblastomas
The microsurgical resection of spinal hemangioblastomas can be challenging, especially large tumors extending beyond one spinal segment or in the lower spinal territory. The rate of surgical complications associated with hemangioblastomas in the CNS exceeds 15%.69,70 Several investigators have described their successful experiences in embolizing spinal hemangioblastomas.37,71 At our institution, we are rarely requested to perform preoperative embolization for spinal hemangioblastomas.
Giant Cell Tumors
Although usually considered benign tumors, 5% to 10% of giant cell tumors may undergo malignant degeneration and assume a more aggressive course.72 These tumors have a predilection for the thoracolumbar and sacral regions. They can remain relatively asymptomatic until they grow to a significant size. Sacral giant cell tumors are difficult to manage, and their recurrence rate after initial therapy is higher than that of any other location. Treatment for these tumors includes surgical, radiation, and endovascular options.
Surgical management may be effective in treating some sacral giant cell tumors. Large space-occupying sacral giant cell tumors are seldom amenable to surgical resection without causing pelvic instability or neurologic dysfunctions. In a literature review of 159 patients with 166 lesions, Leggon et al. reported no recurrences after en bloc resection with a wide margin (0 of 8 patients).73 The rate of local recurrence was 51% (25 of 49 lesions), 51% (18 of 31 lesions), and 49% (35 of 71 lesions) in patients treated with radiation, surgery with intralesional margins, and surgery with intralesional margins and radiation, respectively. The mortality rate attributable to the disease was 23%. When en bloc extirpation is infeasible, alternative forms of management should be considered with the understanding that the recurrence rate is relatively high.
By minimizing intraoperative blood loss, preoperative embolization can be a valuable adjuvant therapy for improving surgical safety, particularly for intralesional resections. In a number of small series, the results of embolization have been promising as the primary treatment modality. Lackman et al. treated four of five patients with sacral giant cell tumors exclusively with embolization and reported that symptoms resolved, tumor growth was arrested, and there were no recurrences.74 Hosalkar et al. also reported their experiences in nine patients with sacral giant cell tumors treated with serial embolization as the primary modality.5 No tumor progression was noted in seven of the nine patients with a mean follow-up of almost 9 years. All of Hosalkar et al.’s patients experienced significant pain relief.
Hemangiomas
Spinal hemangiomas are benign aggregates of hamartomatous proliferations of vascular tissue within endothelium-lined spaces that are found in about 11% of autopsy specimens.75 Occasionally, hemangiomas can cause local pain or neurologic deficits related to nerve root compression. On CT, they have a polka-dot appearance with punctate sclerotic foci representing thickened vertical trabeculae. Bulging of the posterior cortex and paravertebral soft-tissue extension may be associated with aggressive hemangiomas.
Symptomatic spinal hemangiomas have been treated with a variety of modalities. For pain control, vertebroplasty, kyphoplasty, ethanol injection, radiotherapy, and transarterial embolization have been used with success.76–80 For aggressive hemangiomas requiring surgical management, preoperative embolization can play a significant adjunctive role. Hurley et al. successfully used Onyx to embolize vertebral hemangiomas preoperatively in two cases, with a reported operative blood loss of 1500 and 100 ml.81 Acosta et al. also found a significant reduction in blood loss after presurgical embolization of vertebral hemangiomas in their series.76
Aneurysmal Bone Cysts
Preoperative embolization can play an important role before surgical resection or as a primary modality of treatment in selected nonsurgical cases.82 Embolization of aneurysmal bone cysts causes involution of soft-tissue components, sclerosis, and ossification. However, these radiographic findings may not appear for months or years. A few investigators have reported diffuse involution and ossification after embolization of these lesions and claimed that surgical intervention is unnecessary.57,83 However, continued surveillance is recommended because foci can reappear, and cystic changes have been observed as long as 2 years after embolization.57
Metastatic Spinal Tumors
Metastatic vascular spinal tumors represent the largest group of spinal lesions that are often embolized before surgical resection. Hypervascular metastatic spinal tumors usually originate from renal cell carcinomas, sarcomas, thyroid cancer, and neuroendocrine tumors. Several retrospective studies reported a mean or median intraoperative blood loss that ranged between 1540 and 4300 ml after embolization of spinal tumors of various origins.64,84,85
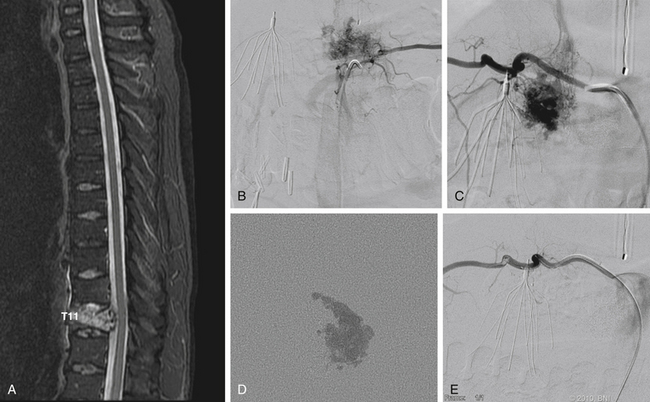
Renal cell carcinomas represent the largest group of vertebral metastases that are treated with preoperative embolization (Fig. 89-4). Radical surgery is increasingly being used in the management of spinal metastatic renal cell carcinomas. Alternative methods of treatment, including chemotherapy and radiotherapy, offer limited benefit. The hypervascular nature of these tumors can result in life-threatening intraoperative blood loss.
Numerous investigators have suggested that preoperative embolization can facilitate radical extirpation of renal cell carcinoma in toto with a concomitant decrease in intraoperative blood loss.3,64,84,86 Manke et al. reported a median intraoperative blood loss of 1500 ml in 19 embolized vertebral renal cell carcinomas and 5000 ml in 11 nonembolized renal cell carcinomas.87 Even in patients with a partially embolized renal cell carcinoma, intraoperative blood loss was reduced compared to the control group. However, many other studies lacked control groups undergoing various degrees of embolization by different operators. Rehak et al. noted a greater-than-average amount of blood loss in their group undergoing embolization of renal cell carcinomas (4750 ml) compared to their nonembolized patients (1786 ml).88 However, the groups differed in many respects, including the size of the tumor, the extent of metastases, and the complexity of the surgery. Tumors in the embolized group were twice as large as tumors in nonembolized group. An anterior approach was used twice as often in the embolized group as in the nonembolized group.
In selected cases, embolization offers palliative treatment of metastatic spinal lesions. Kuether et al. reported successful transarterial embolization of a T5 vertebral renal cell carcinoma metastasis in a patient with neurologic deficits.89 They noted a dramatic reduction in spinal cord compression after embolization, and the patient improved neurologically. Four months later, her neurologic state deteriorated, and she was found to have a new sacral mass. The sacral lesion was embolized, and the patient again improved after treatment. Smit et al. reported their experience with the embolization of four follicular thyroid carcinoma metastases to the spine in patients with neurologic deficits. After the procedure, the neurologic symptoms of all of the patients improved.90 Other investigators have reported significant pain reduction in patients who underwent embolization of spinal metastases.64,84
Technical Nuances
Craniospinal Embolization
An over-the-wire or flow-directed microcatheter is used to perform superselective catheterization and angiography of the feeding pedicles. Meticulous evaluation of the superselective angiography for characteristics similar to those described earlier is needed before the next stage of intervention.
Direct Tumor Embolization
In carotid body tumors and selected JNAs, tumor embolization has been described by direct tumoral puncture with Onyx injection.91,92 After angiography is performed in the appropriate common carotid artery, a 20-gauge spinal needle is inserted percutaneously into the lateral aspect of the neck. The needle is advanced under fluoroscopic road map guidance until arterial blood return is observed. Inadvertent puncture of the carotid artery must be avoided. Intratumoral angiography is performed through the spinal needle to confirm its location within the tumor. Potential dangerous anastomoses are also identified. Embolization then proceeds through the spinal needle. Postprocedural cerebral angiography is performed to rule out embolic events.
Types of Embolisates
The ideal embolisate is one that can penetrate deeply into the capillary bed while providing sufficient control over its injection to avoid occlusion of the normal arterial or venous vasculature. Other characteristics of an ideal embolisate include radiopacity for visualization, ease of surgical handling, and nontoxicity. Given that the treatment goal is surgical extirpation of the tumor, long-term durability of the embolisate is a less important consideration. Embolic materials can be divided into three major categories: solid occlusive devices, particles, and liquid embolic agents.
Particles
Embospheres
Embospheres (BioSphere Medical, Rockland, MA) are clear, radiolucent, acrylic microspheres composed of trisacryl gelatin microspheres. Unlike PVA, Embospheres have a uniform shape and size. Embospheres are compressible and may be associated with less clumping and clogging of microcatheters than PVA. They do not degrade and generate only a moderate inflammatory response.93 Microspheres have been described to penetrate more distally than PVA during embolization and to result in significantly less operative blood loss.94
Liquid Embolic Agents
Alcohol
Alcohol is a potent sclerotic, devascularizing agent. It causes anoxic cellular damage and fibrinoid necrosis of the intimal lining.95 Because of its low viscosity, alcohol can penetrate more deeply than other agents. However, the use of alcohol is associated with a high risk of cranial nerve deficits or normal tissue infarction.96,97 Intraoperative intratumoral injections of ethanol have been performed with success. Lonser et al.98 described their technique of injecting ethanol intratumorally in three spinal epidural lesions and one posterior fossa hemangioblastoma. They used a 28-gauge needle to inject alcohol into the tumors until the lesions blanched visibly. Although ethanol is a powerful embolisate, it must be used judiciously and with great caution to prevent infarction of normal tissue.
EVOH Copolymer–DMSO Solvent (Onyx)
Unlike n-BCA, Onyx is a cohesive polymer. Although Onyx does not adhere to the endothelium, it can adhere to the microcatheter, making withdrawal of the catheter precarious. The DMSO solvent prevents polymerization of the Onyx. When Onyx is injected and contacts the aqueous solution, the DMSO diffuses away rapidly. The copolymer precipitates into a soft, spongy solid. The toxicity of DMSO in humans is well established. DMSO is angiotoxic, inducing vasospasm, angionecrosis, arterial thrombosis, and vascular rupture. However, these detrimental qualities are directly related to the volume of DMSO infused and its length of contact with the endothelium. Limiting the rate of DMSO infusion (less than 0.25 ml every 90 seconds) eliminates these undesirable side effects. An inadequate mixture of the solution before infusion can lead to sedimentation of the tantalum powder, producing suboptimal opacification and visualization.
The advantages of Onyx are that it can penetrate much deeper than other agents and that countless capillary networks can be occluded with a single pedicle injection. Unlike n-BCA, Onyx permits discontinuous injections on the order of minutes and allows continuous angiographic analysis of the angioarchitecture of the lesion. In particular, small collaterals not visualized on the initial angiogram may become apparent with progressive infusion of Onyx (Fig. 89-5). The ability to stop the infusion and analyze the progress of the Onyx can minimize complications from its unwanted diffusion into potentially dangerous anastomoses or collaterals. The ability of Onyx to penetrate enables progressive filling and deeper casting of the fine capillary network, and it can migrate in a retrograde fashion into arterial feeders from other vessels through a single pedicle. Venous filling can be stopped temporarily without the threat of unintended migration of Onyx into draining veins. These advantages allow exceptional control of Onyx flow and its progression to enable a large amount to be delivered via a single injection.
Another advantage of Onyx is the ease with which it can be manipulated during surgery. The black, embolized vessels are easy to see, divide, and cauterize. The microneurosurgeons in the study by Akin et al. reported that Onyx-embolized vessels have better handling characteristics than vessels that were embolized by n-BCA.99 This finding was reported despite the endovascular interventionalist’s belief that the n-BCA provided better angiographic embolization than Onyx in the same rete mirabile model of the swine.
Complications
Presurgical tumor embolization can cause significant morbidity and mortality. The most common complications from tumor embolization are fever and localized pain.100 Other potential devastating complications include unintentional embolic events involving the craniospinal axis or pulmonary circulations, intratumoral hemorrhage, and cranial nerve injuries.
Embolization can injure cranial nerves by disrupting the vaso vasorum to the nerves. For example, the neuromeningeal branch of the ascending pharyngeal artery frequently supplies the spinal accessory and hypoglossal nerves. Provocative testing can be used to determine whether the potential embolized vessel supplies a cranial nerve. The rates of false positives and false negatives associated with provocative testing are high, and reflux of lidocaine intracranially can cause seizures. These limitations prevent its widespread use as a standard procedure. Careful patient selection and meticulous techniques are essential tools for successful preoperative endovascular embolization of tumors.
Adler J.R., Upton J., Wallman J., Winston K.R. Management and prevention of necrosis of the scalp after embolization and surgery for meningioma. Surg Neurol. 1986 April;25(4):357-360.
Akin E.D., Perkins E., Ross I.B. Surgical handling characteristics of an ethylene–vinyl alcohol copolymer compared with n-butyl cyanoacrylate used for embolization of vessels in an arteriovenous malformation resection model in swine. J Neurosurg. 2003 February;98(2):366-370.
Alen J.F., Lobato R.D., Gomez P.A., et al. Intracranial hemangiopericytoma: study of 12 cases. Acta Neurochir (Wien). 2001;143(6):575-586.
Bendszus M., Rao G., Burger R., et al. Is there a benefit of preoperative meningioma embolization? Neurosurgery. 2000 December;47(6):1306-1311.
Berkefeld J., Scale D., Kirchner J., et al. Hypervascular spinal tumors: influence of the embolization technique on perioperative hemorrhage. AJNR Am J Neuroradiol. 1999 May;20(5):757-763.
Breslau J., Eskridge J.M. Preoperative embolization of spinal tumors. J Vasc Interv Radiol. 1995 November;6(6):871-875.
Cornelius J.F., Saint-Maurice J.P., Bresson D., et al. Hemorrhage after particle embolization of hemangioblastomas: comparison of outcomes in spinal and cerebellar lesions. J Neurosurg. 2007 June;106(6):994-998.
Dean B.L., Flom R.A., Wallace R.C., et al. Efficacy of endovascular treatment of meningiomas: evaluation with matched samples. AJNR Am J Neuroradiol. 1994 October;15(9):1675-1680.
Deshmukh V.R., Fiorella D.J., McDougall C.G., et al. Preoperative embolization of central nervous system tumors. Neurosurg Clin N Am. 2005 April;16(2):411-432. xi
Elhammady M.S., Farhat H., Ziayee H., ziz-Sultan M.A. Direct percutaneous embolization of a carotid body tumor with Onyx. J Neurosurg. 2009 January;110(1):124-127.
Elhammady M.S., Wolfe S.Q., Ashour R., et al. Safety and efficacy of vascular tumor embolization using Onyx: is angiographic devascularization sufficient? J Neurosurg. 2009 August 21.
Eskridge J.M., McAuliffe W., Harris B., et al. Preoperative endovascular embolization of craniospinal hemangioblastomas. AJNR Am J Neuroradiol. 1996 March;17(3):525-531.
Gellad F.E., Sadato N., Numaguchi Y., Levine A.M. Vascular metastatic lesions of the spine: preoperative embolization. Radiology. 1990 September;176(3):683-686.
Gruber A., Bavinzski G., Killer M., Richling B. Preoperative embolization of hypervascular skull base tumors. Minim Invasive Neurosurg. 2000 June;43(2):62-71.
Hosalkar H.S., Jones K.J., King J.J., Lackman R.D. Serial arterial embolization for large sacral giant-cell tumors: mid- to long-term results. Spine (Phila Pa 1976). 2007 May 1;32(10):1107-1115.
Jacobsson M., Petruson B., Svendsen P., Berthelsen B. Juvenile nasopharyngeal angiofibroma. A report of eighteen cases. Acta Otolaryngol. 1988 January;105(1-2):132-139.
Kaji T., Hama Y., Iwasaki Y., et al. Preoperative embolization of meningiomas with pial supply: successful treatment of two cases. Surg Neurol. 1999 September;52(3):270-273.
Lackman R.D., Khoury L.D., Esmail A., Donthineni-Rao R. The treatment of sacral giant-cell tumours by serial arterial embolisation. J Bone Joint Surg Br. 2002 August;84(6):873-877.
Liapis C.D., Evangelidakis E.L., Papavassiliou V.G., et al. Role of malignancy and preoperative embolization in the management of carotid body tumors. World J Surg. 2000 December;24(12):1526-1530.
Liu J.K., Brockmeyer D.L., Dailey A.T., Schmidt M.H. Surgical management of aneurysmal bone cysts of the spine. Neurosurg Focus. 2003 November 15;15(5):E4.
Murakami R., Baba Y., Furusawa M., et al. Short communication: the value of embolization therapy in painful osseous metastases from hepatocellular carcinomas; comparative study with radiation therapy. Br J Radiol. 1996 November;69(827):1042-1044.
Sundaresan N., Choi I.S., Hughes J.E., et al. Treatment of spinal metastases from kidney cancer by presurgical embolization and resection. J Neurosurg. 1990 October;73(4):548-554.
Tampieri D., Leblanc R., TerBrugge K. Preoperative embolization of brain and spinal hemangioblastomas. Neurosurgery. 1993 September;33(3):502-505.
Wang S.J., Wang M.B., Barauskas T.M., Calcaterra T.C. Surgical management of carotid body tumors. Otolaryngol Head Neck Surg. 2000 September;123(3):202-206.
White J.B., Link M.J. Endovascular embolization of paragangliomas: a safe adjuvant to treatment. J Vasc Interv Neurol. 2008;1(2):37-41.
1. Dean B.L., Flom R.A., Wallace R.C., et al. Efficacy of endovascular treatment of meningiomas: evaluation with matched samples. AJNR Am J Neuroradiol. 1994 October;15(9):1675-1680.
2. Deshmukh V.R., Fiorella D.J., McDougall C.G., et al. Preoperative embolization of central nervous system tumors. Neurosurg Clin N Am. 2005 April;16(2):411-432. xi
3. Gellad F.E., Sadato N., Numaguchi Y., Levine A.M. Vascular metastatic lesions of the spine: preoperative embolization. Radiology. 1990 September;176(3):683-686.
4. Gruber A., Bavinzski G., Killer M., Richling B. Preoperative embolization of hypervascular skull base tumors. Minim Invasive Neurosurg. 2000 June;43(2):62-71.
5. Hosalkar H.S., Jones K.J., King J.J., Lackman R.D. Serial arterial embolization for large sacral giant-cell tumors: mid- to long-term results. Spine (Phila Pa 1976). 2007 May 1;32(10):1107-1115.
6. Russell D.S., Rubenstein L.J.. Tumors and Hematomas of the blood vessel, In: Russell DS, Rubinstein LJ (eds). Pathology of the Nervous System, Baltimore, Williams and Wilkins, 1977 :116-127
7. Abdel KerimA, Bonneville F., Jean B., et al. Balloon-assisted embolization of skull base meningioma with liquid embolic agent. J Neurosurg. 2010 January;112(1):70-72.
8. Halbach V.V., Higashida R.T., Hieshima G.B., Hardin C.W. Embolization of branches arising from the cavernous portion of the internal carotid artery. AJNR Am J Neuroradiol. 1989 January;10(1):143-150.
9. Hirohata M., Abe T., Morimitsu H., et al. Preoperative selective internal carotid artery dural branch embolisation for petroclival meningiomas. Neuroradiology. 2003 September;45(9):656-660.
10. Robinson D.H., Song J.K., Eskridge J.M. Embolization of meningohypophyseal and inferolateral branches of the cavernous internal carotid artery. AJNR Am J Neuroradiol. 1999 June;20(6):1061-1067.
11. Kaji T., Hama Y., Iwasaki Y., et al. Preoperative embolization of meningiomas with pial supply: successful treatment of two cases. Surg Neurol. 1999 September;52(3):270-273.
12. Fukushima S., Terasaki M., Shigemori M. Chordoid meningioma arising in the pineal region: a case report. Brain Tumor Pathol. 2008;25(2):91-95.
13. Sagoh M., Onozuka S., Murakami H., Hirose Y. Successful removal of meningioma of the pineal region after embolization. Neurol Med Chir (Tokyo). 1997 November;37(11):852-855.
14. Terada T., Kinoshita Y., Yokote H., et al. Preoperative embolization of meningiomas fed by ophthalmic branch arteries. Surg Neurol. 1996 February;45(2):161-166.
15. Bendszus M., Rao G., Burger R., et al. Is there a benefit of preoperative meningioma embolization? Neurosurgery. 2000 December;47(6):1306-1311.
16. Gruber A., Killer M., Mazal P., et al. Preoperative embolization of intracranial meningiomas: a 17-years single center experience. Minim Invasive Neurosurg. 2000 March;43(1):18-29.
17. Hieshima G.B., Everhart F.R., Mehringer C.M., et al. Preoperative embolization of meningiomas. Surg Neurol. 1980 August;14(2):119-127.
18. Ahuja A.K., Gibbons K.J., Hopkins L.N. Endovascular techniques to treat brain tumors. In: Ahuja A.K., Gibbons K.J., Hopkins L.N. Youmans Neurological Surgery. Philadelphia: Saunders; 1996:2826-2840.
19. Manelfe C., Lasjaunias P., Ruscalleda J. Preoperative embolization of intracranial meningiomas. AJNR Am J Neuroradiol. 1986 September;7(5):963-972.
20. Hayashi T., Shojima K., Utsunomiya H., et al. Subarachnoid hemorrhage after preoperative embolization of a cystic meningioma. Surg Neurol. 1987 March;27(3):295-300.
21. Probst E.N., Grzyska U., Westphal M., Zeumer H. Preoperative embolization of intracranial meningiomas with a fibrin glue preparation. AJNR Am J Neuroradiol. 1999 October;20(9):1695-1702.
22. Adler J.R., Upton J., Wallman J., Winston K.R. Management and prevention of necrosis of the scalp after embolization and surgery for meningioma. Surg Neurol. 1986 April;25(4):357-360.
23. Chan R.C., Thompson G.B. Ischemic necrosis of the scalp after preoperative embolization of meningeal tumors. Neurosurgery. 1984 July;15(1):76-81.
24. Grufferman S., Gillman M.W., Pasternak L.R., et al. Familial carotid body tumors: case report and epidemiologic review. Cancer. 1980 November 1;46(9):2116-2122.
25. Netterville J.L., Jackson C.G., Miller F.R., et al. Vagal paraganglioma: a review of 46 patients treated during a 20-year period. Arch Otolaryngol Head Neck Surg. 1998 October;124(10):1133-1140.
26. Hammond S.L., Greco D.L., Lambert A.T., et al. Indium In-111 pentetreotide scintigraphy: application to carotid body tumors. J Vasc Surg. 1997 May;25(5):905-908.
27. White J.B., Link M.J. Endovascular embolization of paragangliomas: a safe adjuvant to treatment. J Vasc Interv Neurol. 2008;1(2):37-41.
28. Moret J., Lasjaunias P. Vascular architecture of tympanojugular glomus tumors. New York, Paris, Barcelona: Masson; 1986.
29. George B. Jugulare foramen paragangliomas. Acta Neurochir (Wien). 1992;118(1-2):20-26.
30. Liapis C.D., Evangelidakis E.L., Papavassiliou V.G., et al. Role of malignancy and preoperative embolization in the management of carotid body tumors. World J Surg. 2000 December;24(12):1526-1530.
31. Wang S.J., Wang M.B., Barauskas T.M., Calcaterra T.C. Surgical management of carotid body tumors. Otolaryngol Head Neck Surg. 2000 September;123(3):202-206.
32. Marangos N., Schumacher M. Facial palsy after glomus jugulare tumour embolization. J Laryngol Otol. 1999 March;113(3):268-270.
33. Pandya S.K., Nagpal R.D., Desai A.P., Purohit A.V. Death following external carotid artery embolization for a functioning glomus jugulare chemodectoma. Case report. J Neurosurg. 1978 June;48(6):1030-1034.
34. Djindjian M. Successful removal of a brainstem hemangioblastoma. Surg Neurol. 1986 January;25(1):97-100.
35. Takeuchi S., Tanaka R., Fujii Y., et al. Surgical treatment of hemangioblastomas with presurgical endovascular embolization. Neurol Med Chir (Tokyo). 2001 May;41(5):246-251.
36. Standard S.C., Ahuja A., Livingston K., et al. Endovascular embolization and surgical excision for the treatment of cerebellar and brain stem hemangioblastomas. Surg Neurol. 1994 May;41(5):405-410.
37. Tampieri D., Leblanc R., TerBrugge K. Preoperative embolization of brain and spinal hemangioblastomas. Neurosurgery. 1993 September;33(3):502-505.
38. Cornelius J.F., Saint-Maurice J.P., Bresson D., et al. Hemorrhage after particle embolization of hemangioblastomas: comparison of outcomes in spinal and cerebellar lesions. J Neurosurg. 2007 June;106(6):994-998.
39. Montano N., Doglietto F., Pedicelli A., et al. Embolization of hemangioblastomas. J Neurosurg. 2008 May;108(5):1063-1064.
40. Alen J.F., Lobato R.D., Gomez P.A., et al. Intracranial hemangiopericytoma: study of 12 cases. Acta Neurochir (Wien). 2001;143(6):575-586.
41. Fountas K.N., Kapsalaki E., Kassam M., et al. Management of intracranial meningeal hemangiopericytomas: outcome and experience. Neurosurg Rev. 2006 April;29(2):145-153.
42. Kaye A.H., Laws E.R. Brain Tumors. Tokyo: Livingstone; 1995.
43. Sivanadan R., Willard E.F. Benign and malignant tumors of the nasopharynx. In: Cummings C.W., Haughey B.H., Thomas J.R., Harker L.A. Cummings Otolaryngology: head and Neck Surgery: Pathology & Genetics Head and Neck Tumors. Philadelphia: Mosby; 2005:1669-1684.
44. McCombe A., Lund V.J., Howard D.J. Recurrence in juvenile angiofibroma. Rhinology. 1990 June;28(2):97-102.
45. Harwood A.R., Cummings B.J., Fitzpatrick P.J. Radiotherapy for unusual tumors of the head and neck. J Otolaryngol. 1984 December;13(6):391-394.
46. Roberson G.H., Price A.C., Davis J.M., Gulati A. Therapeutic embolization of juvenile angiofibroma. AJR Am J Roentgenol. 1979 October;133(4):657-663.
47. Siniluoto T.M., Luotonen J.P., Tikkakoski T.A., et al. Value of pre-operative embolization in surgery for nasopharyngeal angiofibroma. J Laryngol Otol. 1993 June;107(6):514-521.
48. Davis K.R. Embolization of epistaxis and juvenile nasopharyngeal angiofibromas. AJR Am J Roentgenol. 1987 January;148(1):209-218.
49. Jacobsson M., Petruson B., Svendsen P., Berthelsen B. Juvenile nasopharyngeal angiofibroma. A report of eighteen cases. Acta Otolaryngol. 1988 January;105(1-2):132-139.
50. Steinberger S.J., Wetmore R.F. Current management of juvenile nasopharyngeal angiofibroma. Trans Pa Acad Ophthalmol Otolaryngol. 1984;37(1):65-70.
51. Duvall A.J.III, Moreano A.E. Juvenile nasopharyngeal angiofibroma: diagnosis and treatment. Otolaryngol Head Neck Surg. 1987 December;97(6):534-540.
52. Piquet J.J., Chevalier D. Surgical treatment of angiofibromas of the nasopharynx—34 cases. Rhinology. 1989 September;27(3):149-154.
53. Gay I., Elidan J., Gordon R. Oronasal fistula—a possible complication of preoperative embolization in the management of juvenile nasopharyngeal angiofibroma. J Laryngol Otol. 1983 July;97(7):651-656.
54. DeRosa G.P., Graziano G.P., Scott J. Arterial embolization of aneurysmal bone cyst of the lumbar spine. A report of two cases. J Bone Joint Surg Am. 1990 June;72(5):777-780.
55. Dysart S.H., Swengel R.M., van Dam B.E. Aneurysmal bone cyst of a thoracic vertebra. Treatment by selective arterial embolization and excision. Spine (Phila Pa 1976). 1992 July;17(7):846-848.
56. Misasi N., Sadile F. Selective arterial embolization in orthopaedic pathology. Analysis of long-term results. Chir Organi Mov. 1991 October;76(4):311-316.
57. Koci T.M., Mehringer C.M., Yamagata N., Chiang F. Aneurysmal bone cyst of the thoracic spine: evolution after particulate embolization. AJNR Am J Neuroradiol. 1995 April;16(Suppl 4):857-860.
58. Sheikh B.Y., Kanaan I., Alwatban J., et al. Aneurysmal bone cyst involving the skull base: report of three cases. Skull Base Surg. 1999;9(2):145-148.
59. Murakami R., Baba Y., Furusawa M., et al. Short communication: the value of embolization therapy in painful osseous metastases from hepatocellular carcinomas; comparative study with radiation therapy. Br J Radiol. 1996 November;69(827):1042-1044.
60. Lin P.Y., Chen W.M., Hsieh Y.L., et al. Orbital metastatic osteosarcoma. J Chin Med Assoc. 2005 June;68(6):286-289.
61. Bingaman K.D., Alleyne C.H.Jr, Olson J.J. Intracranial extraskeletal mesenchymal chondrosarcoma: case report. Neurosurgery. 2000 January;46(1):207-211.
62. Avellino A.M., Grant G.A., Harris A.B., et al. Recurrent intracranial Masson’s vegetant intravascular hemangioendothelioma. Case report and review of the literature. J Neurosurg. 1999 August;91(2):308-312.
63. Guzman R., Dubach-Schwizer S., Heini P., et al. Preoperative transarterial embolization of vertebral metastases. Eur Spine J. 2005 April;14(3):263-268.
64. Berkefeld J., Scale D., Kirchner J., et al. Hypervascular spinal tumors: influence of the embolization technique on perioperative hemorrhage. AJNR Am J Neuroradiol. 1999 May;20(5):757-763.
65. Jackson R.J., Loh S.C., Gokaslan Z.L. Metastatic renal cell carcinoma of the spine: surgical treatment and results. J Neurosurg. 2001 January;94(Suppl 1):18-24.
66. King G.J., Kostuik J.P., McBroom R.J., Richardson W. Surgical management of metastatic renal carcinoma of the spine. Spine (Phila Pa 1976). 1991 March;16(3):265-271.
67. Sankhla S., Khan G.M. Cauda equina paraganglioma presenting with intracranial hypertension: case report and review of the literature. Neurol India. 2004 June;52(2):243-244.
68. Osborn A.G.. Diagnostic Neuroradiology, St. Louis, Mosby 1994:pp 876-918
69. Conway J.E., Chou D., Clatterbuck R.E., et al. Hemangioblastomas of the central nervous system in von Hippel-Lindau syndrome and sporadic disease. Neurosurgery. 2001 January;48(1):55-62.
70. Wang C., Zhang J., Liu A., Sun B. Surgical management of medullary hemangioblastoma. Report of 47 cases. Surg Neurol. 2001 October;56(4):218-226.
71. Eskridge J.M., McAuliffe W., Harris B., et al. Preoperative endovascular embolization of craniospinal hemangioblastomas. AJNR Am J Neuroradiol. 1996 March;17(3):525-531.
72. Bell G.R. Surgical treatment of spinal tumors. Clin Orthop Relat Res. 1997 February;335:54-63.
73. Leggon R.E., Zlotecki R., Reith J., Scarborough M.T. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res. 2004 June;423:196-207.
74. Lackman R.D., Khoury L.D., Esmail A., Donthineni-Rao R. The treatment of sacral giant-cell tumours by serial arterial embolisation. J Bone Joint Surg Br. 2002 August;84(6):873-877.
75. Doppman J.L., Oldfield E.H., Heiss J.D. Symptomatic vertebral hemangiomas: treatment by means of direct intralesional injection of ethanol. Radiology. 2000 February;214(2):341-348.
76. Acosta F.L.Jr, Sanai N., Chi J.H., et al. Comprehensive management of symptomatic and aggressive vertebral hemangiomas. Neurosurg Clin N Am. 2008 January;19(1):17-29.
77. Faria S.L., Schlupp W.R., Chiminazzo H.Jr. Radiotherapy in the treatment of vertebral hemangiomas. Int J Radiat Oncol Biol Phys. 1985 February;11(2):387-390.
78. Munk P.L., Marotta T.R. Intralesional injection of absolute alcohol into vertebral hemangiomas: a new treatment option? AJNR Am J Neuroradiol. 1999 June;20(6):959-960.
79. Peh W.C., Gilula L.A. Percutaneous vertebroplasty: indications, contraindications, and technique. Br J Radiol. 2003 January;76(901):69-75.
80. Zapalowicz K., Skora P., Myslinski R., et al. Balloon kyphoplasty for painful C-7 vertebral hemangioma. J Neurosurg Spine. 2008 May;8(5):458-461.
81. Hurley M.C., Gross B.A., Surdell D., et al. Preoperative Onyx embolization of aggressive vertebral hemangiomas. AJNR Am J Neuroradiol. 2008 June;29(6):1095-1097.
82. Liu J.K., Brockmeyer D.L., Dailey A.T., Schmidt M.H. Surgical management of aneurysmal bone cysts of the spine. Neurosurg Focus. 2003 November 15;15(5):E4.
83. De C.R., Biagini R., Boriani S., et al. Selective arterial embolization in the treatment of aneurysmal bone cyst and angioma of bone. Skeletal Radiol. 1992;21(8):523-527.
84. Breslau J., Eskridge J.M. Preoperative embolization of spinal tumors. J Vasc Interv Radiol. 1995 November;6(6):871-875.
85. Sundaresan N., Choi I.S., Hughes J.E., et al. Treatment of spinal metastases from kidney cancer by presurgical embolization and resection. J Neurosurg. 1990 October;73(4):548-554.
86. Prabhu V.C., Bilsky M.H., Jambhekar K., et al. Results of preoperative embolization for metastatic spinal neoplasms. J Neurosurg. 2003 March;98(Suppl 2):156-164.
87. Manke C., Bretschneider T., Lenhart M., et al. Spinal metastases from renal cell carcinoma: effect of preoperative particle embolization on intraoperative blood loss. AJNR Am J Neuroradiol. 2001 May;22(5):997-1003.
88. Rehak S., Krajina A., Ungermann L., et al. The role of embolization in radical surgery of renal cell carcinoma spinal metastases. Acta Neurochir (Wien). 2008 November;150(11):1177-1181.
89. Kuether T.A., Nesbit G.M., Barnwell S.L. Embolization as treatment for spinal cord compression from renal cell carcinoma: case report. Neurosurgery. 1996 December;39(6):1260-1262.
90. Smit J.W., Vielvoye G.J., Goslings B.M. Embolization for vertebral metastases of follicular thyroid carcinoma. J Clin Endocrinol Metab. 2000 March;85(3):989-994.
91. Elhammady M.S., Farhat H., Ziayee H., ziz-Sultan M.A. Direct percutaneous embolization of a carotid body tumor with Onyx. J Neurosurg. 2009 January;110(1):124-127.
92. Elhammady M.S., Wolfe S.Q., Ashour R., et al. Safety and efficacy of vascular tumor embolization using Onyx: is angiographic devascularization sufficient? J Neurosurg. 2009 August 21.
93. Beaujeux R., Laurent A., Wassef M., et al. Trisacryl gelatin microspheres for therapeutic embolization, II: preliminary clinical evaluation in tumors and arteriovenous malformations. AJNR Am J Neuroradiol. 1996 March;17(3):541-548.
94. Bendszus M., Klein R., Burger R., et al. Efficacy of trisacryl gelatin microspheres versus polyvinyl alcohol particles in the preoperative embolization of meningiomas. AJNR Am J Neuroradiol. 2000 February;21(2):255-261.
95. Ellman B.A., Green C.E., Eigenbrodt E., et al. Renal infarction with absolute ethanol. Invest Radiol. 1980 July;15(4):318-322.
96. Jungreis C.A. Skull-base tumors: ethanol embolization of the cavernous carotid artery. Radiology. 1991 December;181(3):741-743.
97. Latshaw R.F., Pearlman R.L., Schaitkin B.M., et al. Intraarterial ethanol as a long-term occlusive agent in renal, hepatic, and gastrosplenic arteries of pigs. Cardiovasc Intervent Radiol. 1985;8(1):24-30.
98. Lonser R.R., Heiss J.D., Oldfield E.H. Tumor devascularization by intratumoral ethanol injection during surgery. Technical note. J Neurosurg. 1998 May;88(5):923-924.
99. Akin E.D., Perkins E., Ross I.B. Surgical handling characteristics of an ethylene–vinyl alcohol copolymer compared with n-butyl cyanoacrylate used for embolization of vessels in an arteriovenous malformation resection model in swine. J Neurosurg. 2003 February;98(2):366-370.
100. Head, neck, and brain tumor embolization. AJNR Am J Neuroradiol. 2001 September;22(Suppl 8):S14-S15.