CHAPTER 51 Elbow Arthroplasty: Historical Perspective and Emerging Concepts
INTRODUCTION
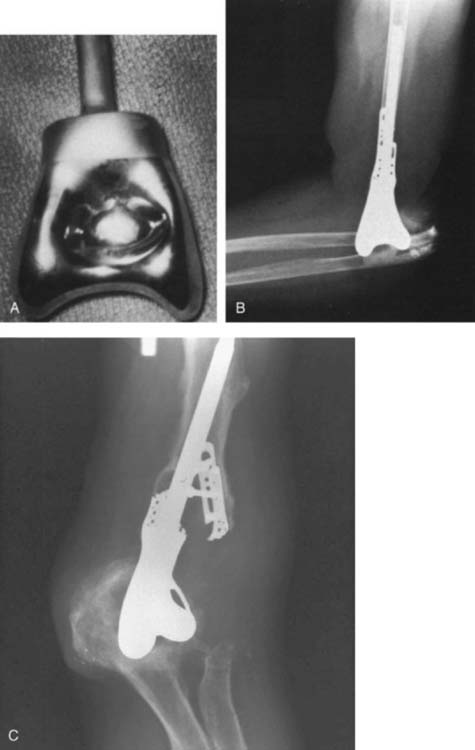
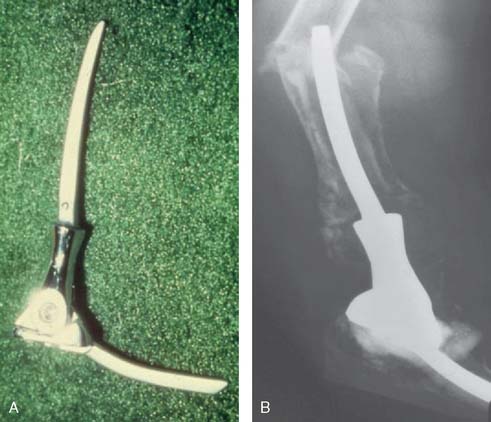
The first “modern” total elbow arthroplasty using bone cement was performed by Dee in 1972. However, in the late 1940s and early 1950s, documentation of replacement of the elbow by means of hemiarthroplasties as well as custom distal humerus or proximal ulna articular bearing materials exists. All of these efforts suffered from poor fixation and lack of understanding of the joint kinematics. In 1952, Venable48 and in 1965 Barr and Eaton3 designed endoprostheses for the distal humerus, usually for reconstructing traumatic deficiencies (Fig. 51-1). There were also efforts made to create a custom ulnohumeral joint when the elbow was unstable and fusion was the only alternative.11,28,29 These early elbow designs exhibited various articular and fixation concepts (Fig. 51-2). However, without bone cement, these prostheses usually became loose and unstable (see Fig. 51-1).
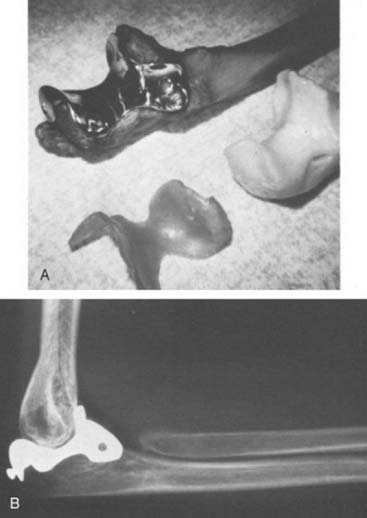
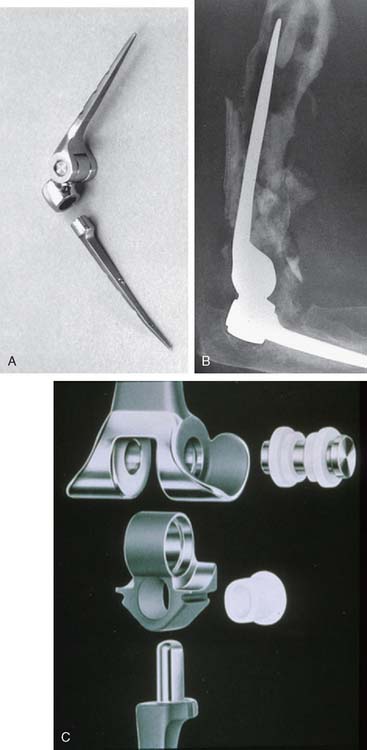
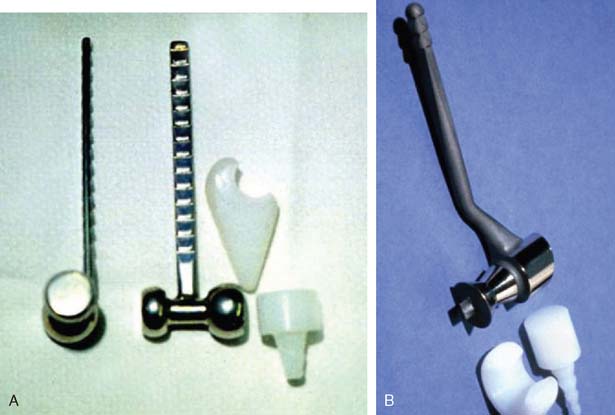
In the 1970s, the concept of resurfacing emerged. These designs were intended for more limited pathology. Mayo was actively involved with early efforts to design both hemi and articulated devices (Fig. 51-3).23 The so-called “saddle” was a true resurfacing of the olecranon designed at Mayo in the later 1960s, and was successful in some instances38 (Fig. 51-4). Street and Stevens47 are credited with the first use of a convex trochlea and capitellum replacement of the distal humerus (Fig. 51-5). The goal of these implants was a hemiarthroplasty replacement that required less bone resection and, it was hoped, preservation of the collateral ligaments. Materials such as nylon, acrylic, stainless steel, and even vulcanized rubber were used in these early prostheses. Although some were successful in relieving pain, most had limited motion, were often unstable without adequate ligamentous support and loosened over time. A few long-term successes were reported; but in general, joint function was limited and the clinical experience consisted of only a few patients.
The era of total elbow joint prosthetic replacement followed the limited success with these custom-designed and resurfacing hemiarthroplasty. We consider the “modern” era of prosthetic replacement to have begun in the early 1970s with the design of Dee, followed by a number of hinged elbow arthroplasties7,9,19,21,39,46 (Fig. 51-6). This and other prostheses almost uniformly provided pain relief and a reasonable range of elbow motion (20 degrees of extension, to 110 to 120 degrees of flexion).
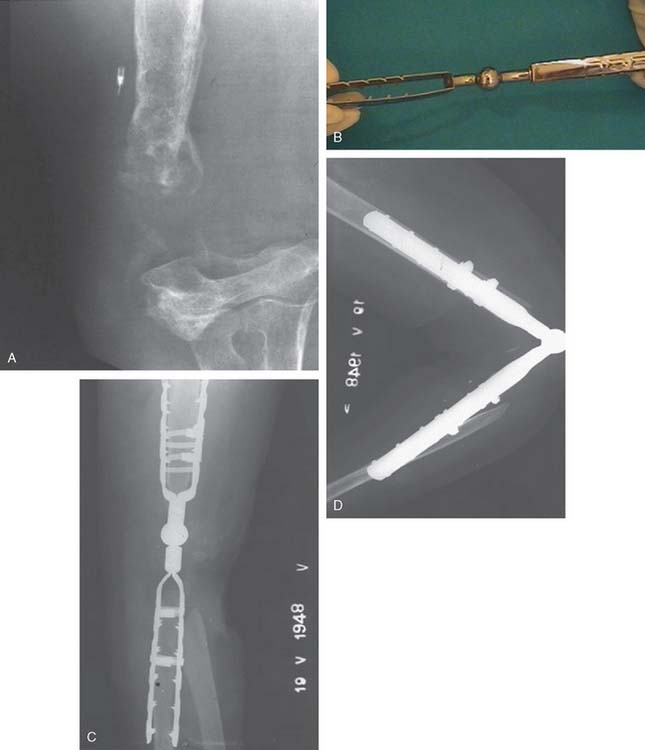
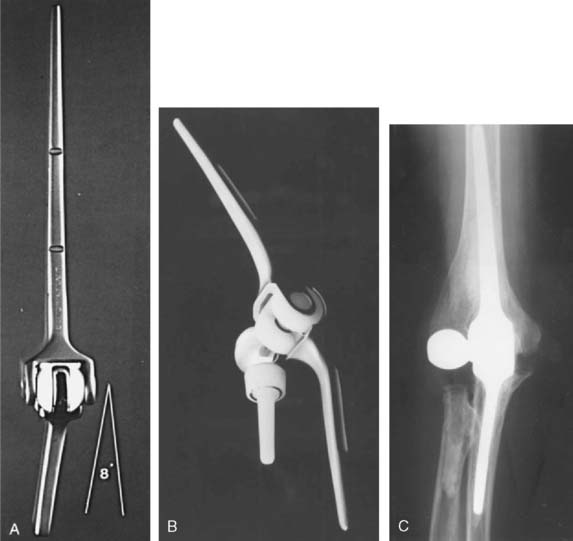
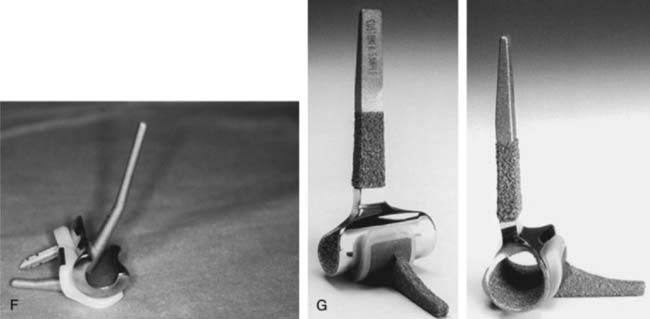
The important features of the Dee prosthesis is that it was the first implant to use cement fixation. However, the lack of understanding the complex anatomy and biomechanics resulted in a particularly high early failure rate that progressed to virtually 100% failure with time. The disadvantage of rigid fixation and predictable failure was recognized worldwide. Some devices were modified to address the initial design flaws such as the GSB from Zurich (Fig. 51-7). Roland Pritchard was one of the first in this country to recognize the value of a “loose hinge” and a polyethylene bushing (Fig. 51-8). Others sought the desirability of stability and flexibility with a “snap-fit” articulation. The design by Volz also incorporated a radial head to better distribute the forces crossing the joint49 (see Fig. 51-8). Unfortunately these design concepts suffered from deficiencies of technique, soft tissue balance and bushing wear. A less constrained articulation was also attained by a snap fit with some systems (see Fig. 51-8). Finally, Coonrad recognized the problem with a medial/lateral articulation and a collared polyethylene bushing was introduced in 1970 (Fig. 51-9).
Unfortunately, complication rates proved very high from loosening, component fracture, and infection leading to a rate of reoperation of 22% to 30%. Hence, the whole concept was reassessed.
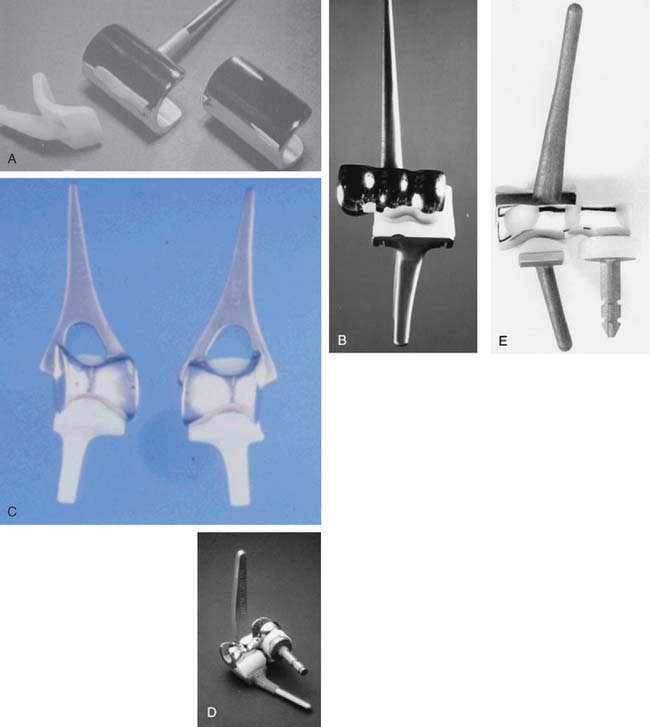
Loosening at the bone-cement interfaces was the predicated price paid for rigid-constraint prostheses.7,10,17 Early recognition of this problem prompted interest in and the development of more prostheses that were not mechanically coupled. These so-called resurfacing or uncoupled designs14,16,25,46 (Fig. 51-10) are best termed unlinked implants and prompted various efforts to restore the anatomic contour. The earliest designs also tried, unsuccessfully, to avoid the use of a stemmed implant. These implants intended to lessen stresses on the bone-cement interface, allowing the soft tissue capsule and ligaments to transmit forces and decrease the risk of mechanical loosening. Over the years, these theoretical advantages have proved to be effective, however, only to the extent that the unlinked device is also less constrained at the articulation. We now know that it is the articular constraint, not the linkage per se, that influences loosening.
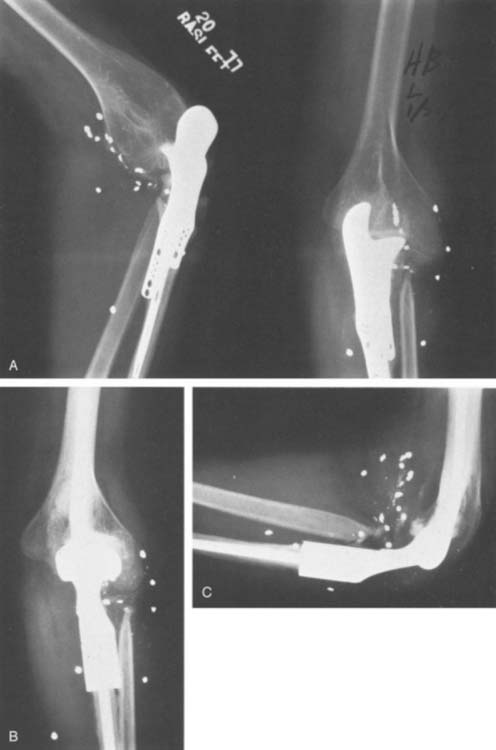
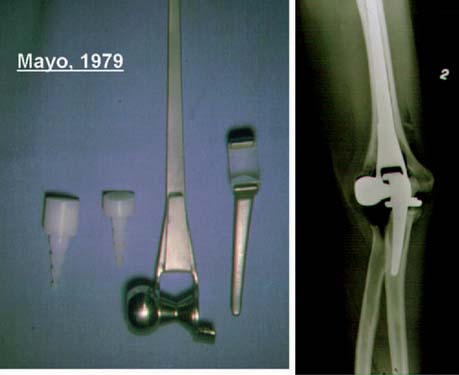
Our clinical experience with the more modern designs at the Mayo Clinic began in 1971 with the original Coonrad hinged prosthesis. This was composed of humeral and ulna components separated by a high-density polyethylene bushing (see Fig. 51-9).35 Our experience with this design was flawed by poor stem fixation (Fig. 51-11). With a more sophisticated understanding of biomechanics and after studying our own and the clinical experience of others, a three-piece, semiconstrained, flanged device was designed in 1978 (Fig. 51-12). Morrey inserted two of these devices, and the second one dislocated. The design was abandoned, but the value of the flange concept was believed to be sound and it was incorporated into the Coonrad implant.
In 1981, we36 reported the results of 80 Mayo and Coonrad prostheses in 72 patients. The results were good in 60%, fair in 16%, and poor in 24%.36 Revisions were frequent, however, and were related to the early deficiencies of surgical technique, poor understanding of essential joint design features, and poor knowledge of elbow biomechanics. The complication rate was 55%, including loosening, infection, triceps rupture, ulnar neuropathy, and medial or lateral condyle fracture. Nevertheless, pain relief was excellent and functional motion (24 to 129 degrees) encouraged our group to the further use and development of total elbow joint replacement.
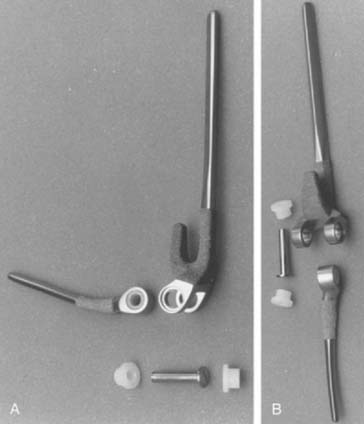
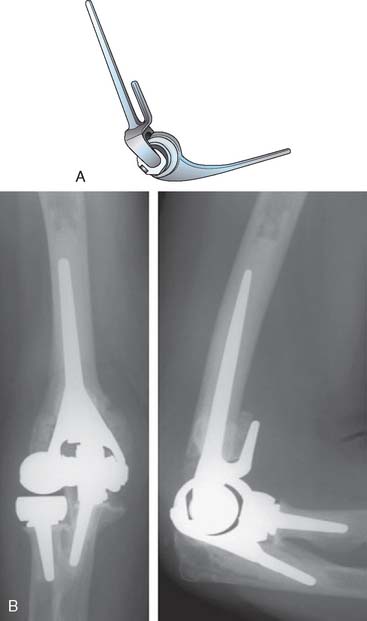
Two major developments in the redesign of the Coonrad prosthesis, along with an improved surgical approach and cementing techniques, led to the continued pursuit of the ideal total elbow joint arthroplasty. Under the direction of Dr. Richard Bryan, a study group of Mayo investigators examined the issue of prosthetic loosening and implant failure. It was concluded that the Mayo snap-fit relatively unconstrained implant was potentially too unstable and the stem design caused a rotational torque to the humeral articulation. Biomechanical studies suggested that humeral loosening, in particular, might be prevented by better force transfer across what is, in effect, a “weight-bearing” joint. The addition of the anterior flange significantly reduced the problems of the “windshield wiper” effect of stem loosening at the humerus (Fig. 51-13). Better intramedullary cavity bone preparation plus cementing techniques provided more secure fixation of both proximal ulna and distal humerus. As a consequence of these changes, implantation of the Mayo-modified Coonrad prosthesis, the Coonrad II, became the procedure of choice at our institution for the majority of patients requiring total elbow arthroplasty from 1978 to 1981. We applied this design not only in advanced rheumatoid disease but also in osteoarthritis, post-traumatic arthritis, distal humerus nonunion, and supracondylar fractures, as noted earlier. The final design change was the addition of the anterior flange in 1981 and altering the surface preparation of the proximal ulna, and distal humerus, to provide further improvement in prosthetic fixation and overall stability. This is termed the Coonrad-Morrey and, with some modifications, is still in use today.
CURRENT DIRECTION
As discussed subsequently, especially in Chapter 52, innovation and reassessment continues. The advantages of unlinked devices is being readdressed by considering a radial head to enhance stability. The flange is recognized to stabilize the bone cement interface (Fig. 51-14). Challenges of technique are being recognized by designing more sophisticated instrumentation. The more anatomic designs have also prompted the implantation of the humeral component as a “hemireplacement” when the proximal radial and ulnar articulations are intact (see Chapter 52B). Finally, the ability to convert an unlinked to a linked articulation real time during surgery is also now available in some designs (Acclaim, Lattitude) (see Fig. 51-14).
Complications
Complications related to total elbow replacement have also decreased (see Chapter 61) and overall, a safe, functional, and long-term satisfactory outcome to the multiple types of arthritic deformity may be anticipated. Yet, the history of total elbow arthroplasty still has not been fully told because better materials, biomechanical analysis of forces, prosthetic designs, ligament reconstructions, and protection against infection are developed by interested clinical and basic researchers.37,42 The story, to date, has been an interesting and challenging effort of combining anatomy, mechanics, and surgical experience to produce options for total elbow replacement with progress continued to be made.
CHOICE OF JOINT IMPLANT REPLACEMENT
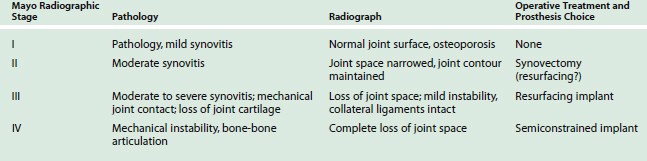
Today, given the options available, the choice of a joint implant for elbow reconstructive surgery is usually based on three factors (Table 51-1): (1) the extent and the etiology of the disease process (post-traumatic, degenerative, or rheumatoid process),22,30 (2) the specific needs of the patient, and (3) the experience or preference of the surgeon.30 The disease process is influenced most significantly by the degree of pain, the amount of joint instability, and the limitation of motion that affects the elbow (Table 51-2). Relief of pain is clearly the primary goal of implant arthroplasty.6,10,12,14 In general, with all of the current elbow prostheses, relief of pain can and should be anticipated in 90% to 95% of all patients. The difference between the unlinked and the linked implants is related to the spectrum of pathology that can be successfully addressed by the design. The potential for instability is greater with the unlinked implant,14,16,40 and hence, limits soft tissue release.4,9,30–33,35,39 The complica tions related to neuropathy (ulnar), surgical approach (triceps weakness), and infection are not dissimilar between the design concepts (see Chapters 61 and 63).
| Unlinked | Linked |
|---|---|
In the past, elbow surgeons might select different implants based on the primary pathology and potential soft tissue supports requiring treatment. Hence, unlinked implants might be selected for both juvenile- and adult-onset rheumatoid disease, provided that collateral ligament stability was reasonably present or could be obtained by implant insertion.1,8,14,15,25,43,45,50 In primary and post-traumatic arthritis, linked implants are generally preferred because there is more clinical experience with their use than with unlinked implants.33 In post-traumatic arthritis cases with bone loss,24 in particular, either the original or, less commonly, a custom-designed semiconstrained implants, loose-hinged articulated implants of Coonrad/Morrey type provide the best option, if not the only solution. In reality, today a surgeon will commonly select a single specific design and employ it for all indications, based on data, preference, or both.
Improved motion following elbow arthroplasty is important because after relief of pain, what the patient needs most is the ability to flex the elbow to touch the face and the head and to extend the elbow forward to reach and grasp objects. Unlike the case with the hip or the knee, improved motion at the elbow may be a primary indication for joint replacement. Motion is particularly relevant in patients with concomitant shoulder and wrist disease. Current studies suggest that 30 to 130 degrees will provide a functional arc of motion.34 Unlinked implants13,25,46 reportedly have less extension, 25 to 40 degrees, possibly as a consequence of longer postoperative immobilization to prevent instability. Semiconstrained linked implants average 20 to 130 degrees of motion.18,21,31,36 It is for this reason that a semiconstrained, loose-hinge implant is preferred when the goal is improved motion and stability for the elbow. The unlinked arthroplasty might be preferred by some in the younger patient in whom pain relief is desired and collateral ligaments and joint stability are close to normal, with motion being a secondary consideration.
In addition to motion, strength is a relevant consideration.5,10 However, improvement of strength alone is not an indication for elbow replacement. The absence of strength in the triceps and biceps, in itself, is a contraindication to elbow arthroplasty unless muscle transfer (see contraindications) can restore this function. Improvement in strength prompted the development of the triceps-sparing approach of Bryan/Morrey. This has resulted in fewer difficulties with the function and loss of elbow extension strength.
Finally, with improved technique and stem designs, reliable fixation is reflected by a lower loosening rate. This, in turn, has resulted in more frequent demonstration of bushing wear, which has been shown to be associated with longevity and correcting longstanding deformity.26
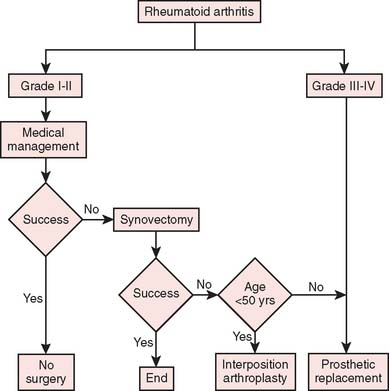
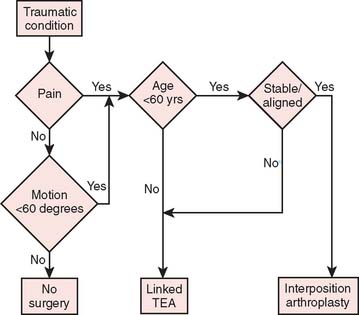
Our current “logic” for the management of rheu-matoid arthritis and for post-traumatic conditions including joint replacement is demonstrated in the treatment algorithms depicted in Figures 51-15 and 51-16, respectively.
1 Ackerman G., Jupiter J.B. Nonunion of fractures of the distal end of the humerus. J. Bone Joint Surg. 1988;70A:75.
2 Allieu Y., Meyer zu Reckendorf G., Daude O. Long-term results of unconstrained Roper-Tuke total hip arthroplasty in patients with rheumatoid arthritis. J. Shoulder Elbow Surg. 1998;7:560.
3 Barr J.S., Eaton R.G. Elbow reconstruction with a new prosthesis to replace the distal end of the humerus: A case report. J. Bone Joint Surg. 1965;47A:1408.
4 Bell S., Gschwend N., Steiger U. Arthroplasty of the elbow. Experience with the mark III GSB prosthesis. Aust. N.Z. J. Surg. 1986;56:823.
5 Bryan R.S., Morrey B.F. Extensive posterior exposure of the elbow: Triceps sparing approach. Clin. Orthop. 1982;166:199.
6 Connor P.M., Morrey B.F. Total elbow arthroplasty in patients who have juvenile rheumatoid arthritis. J. Bone Joint Surg. 1998;80A:678.
7 Coonrad R.W. History of total elbow arthroplasty. In: Inglis A.E., editor. Upper Extremity Joint Replacement (Symposium on Total Joint Replacement of the Upper Extremity, 1979. St. Louis: C. V. Mosby Co., 1982.
8 Davis R.F., Weiland A.J., Hungerford D.S., Moore J.R., Volenec-Dowling S. Nonconstrained total elbow arthroplasty. Clin. Orthop. 1982;171:156.
9 Dee R. Total replacement arthroplasty of the elbow for rheumatoid arthritis. J. Bone Joint Surg. 1972;54B:88.
10 Dobyns J.H., Bryan R.S., Linscheid R.L., Peterson L.F.A. The special problems of total elbow arthroplasty. Geriatrics. 1976;31:57.
11 Engelbrecht E., Bucholz H.W., Rottger J., Siegal A. Total elbow replacement with a hinge and a nonblocked system. In: Joint Replacement of the Upper Limb. London: Mechanical Engineering Publications; 1978.
12 Ewald F.C. Total elbow replacement. Orthop. Clin. North Am. 1975;6:685.
13 Ewald F.C. Nonconstrained metal to plastic total elbow arthroplasty. In: Inglis A.E., editor. Symposium on Total Joint Replacement of the Upper Extremity. St. Louis: C.V. Mosby Co.; 1982:141.
14 Ewald F.C., Scheinberg R.D., Poss R., Thomas W.H., Scott R.D., Sledge C.B. Capitellocondylar total elbow arthroplasty: Two- to five-year follow-up in rheumatoid arthritis. J. Bone Joint Surg. 1980;62A:1259.
15 Ewald F.C., Simmons E.D., Sullivan J.A., Thomas W.H., Scott R.D., Poss R., Thornhill T.S., Sledge C.B. Capitellocondylar total elbow replacement in rheumatoid arthritis: Long-term results. J. Bone Joint Surg. 1993;75:498.
16 Friedman R.J., Lee D.E., Ewald F.C. Noncons-trained total elbow arthroplasty. J. Arthroplasty. 1989;4:31.
17 Garrett J.C., Ewald F.C., Thomas W.H., Sledge C.B. Loosening associated with GSB hinge total elbow replacement in patients with rheumatoid arthritis. Clin. Orthop. Rel. Res. 1977;127:170.
18 Gill D.R., Morrey B.F. The Coonrad-Morrey total elbow arthroplasty in patients who have rheumatoid arthritis: A. 10 to 15 year follow-up study. J. Bone Joint Surg. 1998;80A:1327.
19 Gschwend N., Loehr J., Ivosevic-Radovanovic D., Scheler H. Semiconstrained elbow prostheses with special reference to the GBS III prosthesis. Clin. Orthop. 1988;232:104.
20 Gschwend N., Simmen B.R., Matejovsky Z. Late complications in elbow arthroplasty. J. Shoulder Elbow Surg. 1996;5(2 Pt 1):86.
21 Inglis A.E., Pellicci P.M. Total elbow replacement. J. Bone Joint Surg. 1980;62A:1252.
22 Inglis A.E., Ranawat C.S., Straub L.R. Synovectomy and débridement of the elbow in rheumatoid arthritis. J. Bone Joint Surg. 1971;53A:652.
23 Johnson E.W.Jr., Schlein A.P. Vitallium prosthesis for the olecranon and proximal part of the ulna: Case report with thirteen-year follow-up. J. Bone Joint Surg. 1970;52A:721.
24 King G.J., Itoi E., Niebur G.L., Morrey B.F., An K.N. Kinematics and stability of the Norway elbow. Acta Orthop. Scand. 1993;64:657.
25 Kudo H., Iwano K., Watanabe S. Total replacement of the rheumatoid elbow with a hingeless prosthesis. J. Bone Joint Surg. 1980;62A:277.
26 Lee B.P., Adams R.A., Morrey B.F. Polyethylene wear after total elbow arthroplasty. J. Bone Joint Surg. 2005;87A:1080.
27 London J.T. Endoprosthetic prosthetic replacement of the elbow. In: Morrey B.F., editor. The Elbow and Its Disorders. Philadelphia: W. B. Saunders; 1985:540.
28 MacAusland W.R. Replacement of the distal end of the humerus with a prosthesis: Report of four cases. West. J. Surg. 1954;65:557.
29 Mellen R.H., Phalen G.S. Arthroplasty of the elbow by replacement of the distal end of the humerus with an acrylic prosthesis. J. Bone Joint Surg. 1947;29:348.
30 Morrey B.F. Elbow replacement arthroplasty: Indications and patient selection. In: Morrey B.F., editor. Joint Replacement Arthroplasty. New York: Churchill Livingstone; 1991:275.
31 Morrey B.F., Adams R.A. Semiconstrained total elbow arthroplasty for rheumatoid arthritis. J. Bone Joint Surg. 1992;74A:479.
32 Morrey B.F., Adams R.A. Semiconstrained elbow replacement for distal humeral nonunion. J. Bone Joint Surg. 1995;77B:67.
33 Morrey B.F., Adams R.A., Bryan R.S. Total replacement for post-traumatic arthritis of the elbow. J. Bone Joint Surg. 1991;73B:607.
34 Morrey B.F., Askew L.J., An K.N., Chao E.Y. A biomechanical study of normal functional elbow motion. J. Bone Joint Surg. 1981;63A:87.
35 Morrey B.F., Bryan R.S. Total joint arthroplasty: The elbow. Mayo Clin. Proc. 1979;54:507.
36 Morrey B.F., Bryan R.S., Dobyns J.H., Linscheid R.L. Total elbow arthroplasty: A five-year experience at the Mayo Clinic. J. Bone Joint Surg. 1981;63A:1050.
37 O’Driscoll S.W., Tanaka S., An K.N., Morrey B.F. The semiconstrained total elbow replacement: A biomechanical analysis. J. Bone Joint Surg. 1992;74B:297.
38 Peterson L.F.A., James J.A. Surgery of the rheumatoid elbow. Orthop. Clin. North Am. 1971;2:667.
39 Pritchard R.W. Long-term follow-up study: Semiconstrained elbow prosthesis. Orthopedics. 1981;4:151.
40 Pritchard R.W. Anatomic surface elbow arthroplasty: A preliminary report. Clin. Orthop. 1983;179:223.
41 Risung, F.: The Norway elbow prosthesis system: Six years experience. Presented at the 1993 European Rheumatoid Arthritis Surgery Society, Oslo, Norway.
42 Risung F. The Norway elbow replacement: Design, technique and results after nine years. J. Bone Joint Surg. 1997;79B:394.
43 Ruth J.T., Welde A.H. Capitellocondylar total elbow arthroplasty. J. Bone Joint Surg. 1992;74A:95.
44 Schlein A.P. Semiconstrained total elbow arthroplasty. Clin. Orthop. Relat. Res. 1976;121:222.
45 Schwyzer H.K., Simmen B.F., Gschwend N. Infekt nach Schulter-und Ellbogenarthroplastik. Diagnostik Therapie. Orthopade. 1995;24:367.
46 Souter W.A. Arthroplasty of the elbow: With particular reference to metallic hinge arthroplasty in rheumatoid patients. Orthop. Clin. North Am. 1973;4:395.
47 Street D.M., Stevens P.S. A humeral replacement prosthesis for the elbow: Results in ten elbows. J. Bone Joint Surg. 1974;56A:1147.
48 Venable C.S. An elbow and an elbow prosthesis: Case of complete loss of the lower third of the humerus. Am. J. Surg. 1952;83:271.
49 Volz R.G. Development and clinical analysis of a new semiconstrained total elbow prosthesis. In: Inglis A.E., editor. Upper Extremity Joint Replacement Symposium on Total Elbow Joint Replacement of the Upper Extremity, 1979. St. Louis: C. V. Mosby Co., 1982.
50 Weiland A.J., Weirs A.P.C., Wells R.P., Moore J.R. Capitellocondylar total elbow replacement. J. Bone Joint Surg. 1989;71A:217.