Chapter 48 Ectopic Pregnancy
INTRODUCTION
If undiagnosed or untreated, ectopic pregnancy may result in rupture of the fallopian tube and massive intraperitoneal hemorrhage. In the past decade, this phenomenon accounted for approximately 9% of maternal pregnancy-related deaths in the United States.1 Because ectopic pregnancy diagnosis and treatment has moved from an inpatient to an outpatient setting, there are no clear reporting standards, and the most up-to-date incidence rates and morbidity and mortality statistics date back to the mid-1990s.1
EPIDEMIOLOGY
It is estimated that about 2% of pregnancies in the United States are ectopic pregnancies. The number of ectopic pregnancies diagnosed in the United States continues to rise, with a sixfold increase documented between 1970 and 1992.2–4 In Europe, the prevalence of ectopic pregnancy appears stable in France, Sweden, and the Netherlands, but has continued to increase in Norway.4 U.S. healthcare statistics demonstrate that of the 108,800 patients diagnosed with ectopic pregnancy in 1992, 58,000 needed hospitalization, at a cost of $1.1 billion.5 Recent trends of outpatient treatment and decreased need for hospitalization have likely led to an underreporting of the condition and a consequent underestimation in U.S. government figures.
The vast majority of ectopic pregnancies (more than 90%) occur in the tube, with 80% to 90% of these occurring in the ampullary region, 5% to 10% in the isthmic region, 5% in the fimbrial region, and about 2.4% located in the cornual (interstitial) region. The other sites include 3.2% in the ovary, 1.3% elsewhere in the abdomen, and less than 0.15% in the cervix.6–8
Etiology
Ectopic pregnancies are believed to occur primarily as a result of factors that delay or prevent the passage of the fertilized egg into the uterine cavity. Partial or complete blockage of a fallopian tube may arrest the passing embryo in the fallopian tube lumen. Even if the tube is still patent, inflammation and infection may damage the endosalpinx of the tube. Resultant abnormalities in ciliary function are believed to impede transport of the embryo through the fallopian tube.9 The most common cause of such tubal damage is believed to be clinical or subclinical infections caused by Chlamydia trachomatis or Neisseria gonorrhoeae.
It is hypothesized that in some cases embryo factors (including cytogenetic abnormalities) may lead to premature implantation in a nonendometrial site. However, recent studies that have examined the role of chromosome abnormalities in ectopic pregnancy have not supported earlier case reports of high proportions of genetically abnormal gestations at ectopic sites. Among 22 surgically excised tubal pregnancies, only one abnormal chromosomal complement was found.10 In a larger review of 62 karyotyped ectopic pregnancies, 4.8% showed chromosomal abnormalities, a figure which matched that expected for maternal and gestational age of the cases.11
Risk Factors
Multiple risk factors have been consistently shown to be associated with ectopic pregnancies (Table 48-1).12 The strongest association has been found with prior pelvic inflammatory disease (PID), a prior history of ectopic pregnancy, and previous tubal surgery (including previous tubal ligation). These conditions are believed to alter tubal integrity and thus impede the migration of the fertilized ovum to the uterus.
Weaker associations have been made between ectopic pregnancy and infertility (a possible marker in some patients for subclinical tubal infection), cigarette smoking (thought to affect tubal motility), increasing age, having more than one lifetime sexual partner, any pelvic or abdominal surgery (other than cesarean delivery), and a history of having a sexually transmitted disease. Although use of an intrauterine device (IUD) does not increase the risk of ectopic pregnancy compared to controls, women using an IUD found to have a positive pregnancy test are more likely to have an ectopic than an intrauterine pregnancy, in a manner similar to women who have had a tubal ligation.13 Unfortunately, the sensitivity of these risk factors is low, and as many as 50% of patients with proven ectopic pregnancies will have none.13
No clear association has been documented between ectopic pregnancy and oral contraceptive use, previous elective pregnancy termination or spontaneous miscarriage, or cesarean section.9,10
Improved Diagnosis
Diagnostic accuracy has improved as a result of more sensitive pregnancy tests and pelvic ultrasound. The advent of radioimmunoassay and specific antiserum to the beta-subunit of human chorionic gonadotropin (hCG) has allowed for the accurate quantification of β-hCG and the ability to closely follow its rise and fall.14 High-resolution transvaginal ultrasonography with Doppler flow imaging has led to improved visualization of adnexal masses, including ectopic pregnancies, at earlier gestational ages.
Assisted Reproductive Technologies
Assisted reproductive technologies (ART) appear to increase the risk of ectopic pregnancies as a result of both preexisting pathology and effects inherent to these techniques. Not only do many subfertile women have tubal abnormalities, but ovulation induction also results in hormonal fluctuations that are hypothesized to alter tubal motility in some women. After oocyte retrieval, in vitro fertilization (IVF), and embryo transfer, the incidence of tubal pregnancy can be as high as 4.5%.15
Heterotopic Pregnancy
IVF also appears to increase the incidence of simultaneous intrauterine and ectopic pregnancy, termed heterotopic pregnancy.16 In the late 1940s, the prevalence of heterotopic pregnancy was estimated to be 1:30,000 pregnancies.17 The prevalence is now estimated to be 1:4000 pregnancies in the general population, and as high as 1:100 pregnancies resulting from IVF.15,18,19 This dramatic increase is believed to be the result of the increased risk of multiple pregnancies and the unknown effects on tubal motility, in combination with the invasive nature of ART. The clinician must be aware that although ultrasound verification of intrauterine pregnancy dramatically decreases the chance of an ectopic pregnancy, it does not completely rule it out, especially in patients whose pregnancy has resulted from ART.
Ruptured Ectopic Pregnancy
Fallopian tube rupture secondary to ectopic pregnancy remains relatively common despite greater awareness of the disease and improved diagnostic modalities. This is because there is little or no correlation between tubal rupture time since last menstrual period, physical findings, symptoms or β-hCG level. In a large retrospective study of 700 women with ectopic pregnancies, the rupture rate was 34% and one third of patients had no symptoms prior to rupture.20 The primary risk factors for tubal rupture in this study were no prior history of ectopic pregnancy and multiparity.
Likewise, there is little relationship between the onset of ectopic pregnancy symptoms and subsequent tubal rupture. In one study with an overall rupture rate of 32%, less than one fourth of the ruptures occurred within the first 48 hours of symptom onset.21 The remaining ruptures occurred at a fairly steady rate of 2.5% per 24 hours of untreated symptoms for the next 2 weeks.
It is surprising that there is no correlation between tubal rupture and β-hCG levels. In one study, 11% of patients with ruptured ectopic pregnancies had β-hCG levels less than 100IU/L.16 Even declining β-hCG levels are not clinically reassuring, because fallopian tube rupture can occur when serial β-hCG measurements demonstrate a dropping level, as well as when the β-hCG level is very low.22,23
PRESENTATION
Symptoms
Vaginal Bleeding
Vaginal bleeding occurs in more than 50% of ectopic pregnancies and can range from scant spotting to heavy bleeding. It is hypothesized that vaginal bleeding occurs because the thickened endometrial lining is not well supported by the abnormal hormonal milieu. Theoretically, lower β-hCG production associated with most ectopic pregnancies stimulates the corpus luteum to produce an inadequate amount of progesterone. Some patients may even report passing tissue vaginally. In such cases, it is important to keep in mind that a “decidual cast” from the endometrial cavity can easily be mistaken for tissue, and only microscopically confirmed chorionic villi should be used to confirm that the pregnancy was intrauterine.17
Physical Examination
Pelvic Examination
On pelvic examination, inspection of the cervix will usually reveal a closed os, with or without bleeding. A vigorous bimanual examination in search of an adnexal mass is contraindicated if an ectopic pregnancy is suspected. In the presence of adnexal tenderness, the size and characterization of any adnexal masses is best determined by ultrasound. Bimanual pressure on a fragile ectopic pregnancy can result in rupture, converting a stable patient into a surgical emergency. Even when an ectopic pregnancy is present, an adnexal mass will be palpable in more than 10% of cases, and in one third of these cases, the mass will ultimately prove to be unrelated to the ectopic pregancy (e.g., a corpus luteum cyst).17
DIAGNOSIS
Ultrasonography
Intrauterine Pregnancy Confirmation
The confirmation of an intrauterine pregnancy requires the identification of a series of structures by vaginal ultrasound, including the gestational sac, yolk sac, and fetal pole with or without cardiac motion. The gestational sac is seen first and appears as a thick, echogenic rim surrounding a sonolucent center in an eccentric location of the endometrial stripe. This is often referred to as the double decidual sign. The yolk sac is a bright echogenic rim with a sonolucent center that can be seen by approximately 5 weeks’ gestational age. The fetal pole develops as a thickening along an edge of the yolk sac, with cardiac motion first seen around 5½ to 6 weeks after the last menstrual period, even in the case of multiple gestations. The diagnostic accuracy of transvaginal ultrasound for identifying an intrauterine pregnancy approaches 100% in gestations greater than 5½ weeks.24
Adnexal Findings
Ectopic pregnancies can often be seen in the adnexa with vaginal ultrasound. The most common finding is an inhomogeneous mass, which has been reported to be visible in approximately half of patients with ectopic pregnancies in some series.25 Less commonly, a mass with a hyperechoic ring around the gestational sac can be seen.
Human Chorionic Gonadotropin
Quantitative measurement of serum β-hCG is a very accurate method for determining gestational age in the first trimester of a normal pregnancy.26 This is extremely important in the diagnosis of ectopic pregnancy, because at the time of initial evaluation, many women will be unsure of their menstrual or conception dates; thus the exact gestational age is not known. The use of radioimmunoassay to measure serum β-hCG has greatly improved the time to obtain results as well as their accuracy.
Discriminatory Zone
An important factor when determining the viability and location of a pregnancy by vaginal ultrasound is the discriminatory zone. The discriminatory zone is defined as that level of β-hCG at which a normal singleton intrauterine pregnancy can be visualized on transvaginal ultrasonography.28 At most institutions, the discriminatory zone for a singleton pregnancy when using transvaginal ultrasonography is between 1500 and 2500mIU/mL (using the WHO Third International Standard, or International Reference Preparation).2
Serial β-hCG Determination
To distinguish a normal intrauterine pregnancy from a nonviable intrauterine or ectopic gestation, serial β-hCG determinations are performed. It is now well-established that the beta-hCG concentration rises almost linearly in the early weeks of a normally growing gestation, doubling every 1.4 to 2.1 days.27–29 Many clinicians rely on the rule of at least a 66% rise in β-hCG over 2 days based on earlier studies.27,30–33 More recent evidence suggests that the rise of β-hCG may be slower than previously reported, with 99% of all normal viable intrauterine pregnancies having an increase in β-hCG of at least 24% in 1 day and 53% in 2 days.34 Intervening when the 2-day rise in β-hCG is between 53% and 66% may result in the interruption of a viable pregnancy.
Uterine Cavity Sampling
In cases where gross products of conception (gestational sac or fetal parts) are not visible, verification of the presence of chorionic villi can be a problem, because final diagnosis with a permanent pathologic specimen takes up to 24 hours. One solution is to obtain a frozen section at the time of D&C, which has been shown to be very accurate in identifying products of conception, with almost no risk of false-positive results.35
Other techniques used to identify chorionic villi have not been found to be as sensitive. Floating the tissue obtained in saline solution will allow the trained gynecologist to identify villi in only 60% of cases where they can be identified histologically.36 The use of a stereomicroscope significantly improves the ability to identify chorionic villi, but is rarely available in common practice.37 Sampling of the uterine cavity with a pipelle biopsy instrument in an outpatient setting has been found to have relatively poor sensitivity of 30% to 63%.38,39 In the future, perhaps other forms of less invasive endometrial sampling, such as the handheld manual vacuum aspirator, will prove to have the necessary sensitivity for confirming products of conception.
Other Diagnostic Tests
Serum progesterone levels have also been used to aid in the diagnosis of ectopic pregnancy. Overall, serum progesterone levels are lower in ectopic pregnancies than in intrauterine pregnancies.40 Levels less than 5ng/mL are almost always (99.8%) associated with nonviable pregnancies, but these can be either abnormal intrauterine pregnancies (impending spontaneous abortion) or ectopic pregnancies.41 Conversely, progesterone levels of greater than 17.5ng/mL are rarely associated with ectopic pregnancies, with only 8% of ectopic pregnancies falling into this category.
Despite these strong correlations at either end of the concentration spectrum, serum progesterone levels have limited value in diagnosing ectopic pregnancies, because many patients’ values will fall between these extremes of values, where there is too much overlap to be discriminatory.42 In addition, serum progesterone levels are not readily available in many hospital laboratories on a “stat” basis, making the use of this test impractical in emergency situations.
Other laboratory tests evaluated for usefulness in the diagnosis of ectopic pregnancy include vascular endothelial growth factor (VEGF), CA-125, fetal fibronectin, and creatine kinase.43–49 Like serum progesterone, overlapping ranges of values for normal and abnormal pregnancies have prevented any of these markers from being useful in distinguishing ectopic and nonectopic gestations. Using genomics approaches, other promising serum protein markers have been identified that may ultimately prove to be discriminatory between intrauterine and ectopic pregnancies.50
Algorithm for Diagnosis
A simple diagnostic algorithm using ultrasound and serum β-hCG determinations can be helpful (Fig. 48-1).51 When a patient presents in early pregnancy with pain or uterine bleeding, the first step is transvaginal ultrasound. If a nonviable intrauterine pregnancy (e.g., impending spontaneous abortion) is visualized, standard management options are indicated based on symptomatology. Likewise, if an ectopic pregnancy is seen in the adnexa, treatment options are clear.
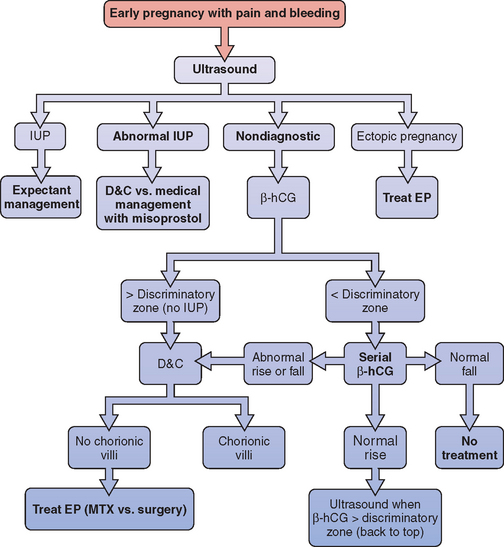
Figure 48-1 Diagnostic algorithm flow chart
(adapted from Gracia CR, Barnhart KT: Diagnosing ectopic pregnancy: A decision analysis comparing six strategies. Obstet Gynecol 97:464-470, 2001.)
In some cases where no chorionic villi are found on D&C, the clinical history is suggestive of a complete spontaneous abortion before evacuation, with heavy vaginal bleeding with passage of tissue and an open cervix. In these cases, it is appropriate to manage the patient expectantly with re-evaluation of serum β-hCG levels 12 to 24 hours after evacuation. If the β-hCG level drops sharply from preoperative levels, a complete spontaneous abortion is the most likely diagnosis, although a resolving ectopic pregnancy (sometimes referred to as a tubal abortion) is also possible. Keep in mind that 35% of women with an ectopic pregnancy are diagnosed when the β-hCG level is falling.28 If the β-hCG level plateaus or continues to rise, an ectopic pregnancy is highly likely, and immediate treatment should be instituted. This approach can also be used when the clinical suspicion of ectopic pregnancy is low, but no pathologist is available for intraoperative examination of the D&C specimen.
All patients for whom ectopic pregnancy was among the differential diagnoses should be followed with at least weekly β-hCG levels until β-hCG is no longer detectable in the serum. This may take up to several weeks, because a minimum decline in serial β-hCG concentration for a completed abortion ranges from 21% to 35% in 48 hours.52 A negative β-hCG value is the only sure way to confirm complete resolution of the ectopic pregnancy. There have been reports of tubal rupture with β-hCG levels as low as 5mIU/mL.53
Of all women with ectopic pregnancies who present with symptoms, about 50% will have β-hCG levels above the discriminatory zone and are therefore diagnosed within a single evaluation.3 The remaining 50% of women with ectopic pregnancies who seek medical attention will be found to have β-hCG levels below the discriminatory zone, and ultrasound is usually nondiagnostic. At this point in time, the sensitivity of transvaginal ultrasound for the diagnosis of intrauterine pregnancy, spontaneous miscarriage, and ectopic pregnancy has been shown to be only 25% to 33% and the predictive value is low.54
Screening Asymptomatic Patients
There may be some advantage to screening patients at high risk for ectopic pregnancy before the development of symptoms.55 Risk factors include previous history of ectopic pregnancy, tubal surgery, PID, sterilization, current IUD, and known tubal disease seen by hysterosalpingography or laparoscopy. In a study of 143 symptom-free women with these risk factors, screening was started before 7 weeks’ gestation with serial β-hCG measurements and ultrasound studies. In this particular study, 5.6% of the women were diagnosed with ectopic pregnancies. It is yet to be established that the potential benefits of this approach, including decreasing the risk of complications and patient reassurance, outweighed the drawbacks of false-positive diagnoses, increased costs, and increased emotional stress.56 For this reason, universal screening of women at increased risk for ectopic pregnancy cannot be recommended at this time.
TREATMENT
Laparotomy versus Laparoscopy
Laparotomy versus laparoscopy for the treatment of ectopic pregnancy has been compared in three prospective, randomized trials.57–59 Each concluded that the laparoscopic approach is superior to laparotomy. Laparoscopy resulted in less blood loss, less analgesia requirement, and a shorter duration of hospital stay compared to laparotomy. Laparoscopy was also found to be less costly in all three trials. Not surprisingly, a Cochrane review of the surgical treatment of ectopic pregnancy likewise concluded that laparoscopy is the treatment of choice for eligible patients.60
Exploratory Laparotomy: The Unstable Patient
Laparotomy via a pfannensteil incision will usually allow expeditious entry into the peritoneal cavity. On visualization of the pelvic structures, the site of implantation of the ectopic pregnancy should be immediately identified. A Kelly forceps (“clamp”) is then placed at the proximal portion of the fallopian tube, at the uterine cornu. This should virtually eliminate further blood loss, because most of the blood supply to the fallopian tube comes from branches of the uterine artery. A second Kelly clamp can then be placed along the mesosalpinx, meeting the end of the first clamp, so that all vessels within the mesosalpinx are occluded. Alternatively, a succession of Kelly clamps can be used to clamp the mesosalpinx as close to the tube as possible, as described by Damario and Rock.61 The entire tube and the ectopic gestation are then excised as one specimen. The pedicles are suture ligated with 2-0 or 3-0 vicryl or other synthetic absorbable suture. After assuring hemostasis, the pelvis should be evacuated of blood and clots, which can total up to several liters of blood loss (Table 48-2).
Table 48-2 Salpingectomy via Laparotomy or Laparoscopy — Surgical Steps
| Suprapubic Pfannensteil incision made for laparotomy |
| Fallopian tube elevated using Allis or Babcock clamp |
| Mesosalpinx clamped with succession of Kelly clamps or hemostats, just below the fallopian tube |
| Tube removed at site of uterine attachment close to cornua |
| Interrupted 2-0 or 3-0 delayed-absorbable suture (e.g., Vicryl) used for closure of pedicles |
| Inspection for hemostasis |
| or |
| Laparoscopic trocar ports placed (umbilical and at least 2 additional) for laparoscopic approach |
| Fallopian tube grasped and elevated distally with endo-grasper |
| Tube cauterized and then transected at cornual end, close to uterus |
Laparoscopic Approach
Salpingectomy
The surgical steps of laparoscopic salpingectomy are similar to those performed via laparotomy.56,62 Blood and clots present in the pelvis are removed with irrigation and suction so that the involved tube can be adequately visualized. The involved tube is grasped near the end where cutting will be initiated. If possible, the tube is resected starting proximally at the uterine cornu and continuing until the fimbrial end is reached. With less than ideal exposure, resection can proceed in the opposite direction.
Salpingostomy
Conservative surgical management of ectopic pregnancy can be accomplished by either salpingostomy or by segmental resection of the involved segment of fallopian tube. Segmental resection has mostly been used in patients with isthmic ectopic pregnancies because in this portion of the tube the lumen is narrower and the muscularis is thicker than in the ampullary region.63 This anatomic difference may lead to increased tubal obstruction after linear salpingostomy performed on this segment of the tube.
Bleeding from the incision edge and ectopic pregnancy site can be controlled using bipolar or, if necessary, unipolar electrosurgery. Electrosurgery should be limited as much as possible to prevent further tubal damage. Another technique to minimize bleeding is to inject the tubal mesentery under the ectopic pregnancy before making the incision with 10 mL of a dilute vasopressin solution (10U in 50 mL physiologic saline solution).61 The temporary local vasospasm induced by this method is designed to minimize the need for electrosurgery and thus limit damage to tubal mucosa during ectopic pregnancy removal.
After tissue removal and achievement of hemostasis, the tube is gently irrigated and left to close by secondary intention. The salpingostomy site closes effectively and rapidly by secondary intention with minimal risk of adhesions. Closing the salpingostomy with suture has not been shown to be beneficial during laparotomy or laparoscopy and might actually increase the risk of tissue ischemia and adhesion formation.64–66 A prospective study failed to show a difference between suturing and not suturing the salpingostomy site after laparoscopic removal of ectopic pregnancies in terms of tubal patency rates, postoperative adhesion rates, or cumulative pregnancy rates (Table 48-3).66
| Laparoscopic trocar ports placed (umbilical, suprapubic, and at least one additional in lower quadrant contralateral to ectopic site) |
| Evacuation of hemoperitoneum with suction irrigator |
| Fallopian tube grasped and kept taut with atraumatic endo-grasper |
| Laser, unipolar cautery, or edge of endosheers used to make linear salpingostomy incision along antimesenteric wall of tube |
| Hydrodissection or gentle tubal compression to extrude products of conception |
| Tissue placed in endoscopic bag and removed from abdominal cavity |
| Irrigation of tube with minimal use of cautery (micro-tip best) |
| Tube left open to heal by secondary intention |
Whether performed by laparotomy or laparoscopy, the fertility outcome after linear salpingostomy is satisfactory. After salpingostomy performed by either approach, the intrauterine pregnancy rate is approximately 60% and the recurrent ectopic pregnancy rate is 15%.14 Comparisons of reproductive outcomes following different treatment approaches are discussed here.
Persistent Ectopic Pregnancy
Although salpingostomy has a high success rate in terms of subsequent tubal patency and intrauterine pregnancy, incomplete resolution of the pregnancy, termed persistent ectopic pregnancy, occurs in 5% to 20% treated by this method.67–69 In one study, the risk of persistent ectopic pregnancy was double for patients treated with laparoscopic salpingostomy compared to patients treated with salpingostomy performed via laparotomy.14
After treating an ectopic pregnancy with salpingostomy, the β-hCG level should be checked on postoperative day 1. A decrease of less than 50% from the initial preoperative β-hCG level is associated with a relative risk of 3.51 for persistent products of conception.70 In this same series, there were no cases of persistent ectopic pregnancy when the postoperative day 1 β-hCG decreased by more than 76%. To ensure complete resolution of the ectopic pregnancy, the β-hCG level should be repeated weekly until it is no longer detectable.
Options for treatment of a persistent ectopic pregnancy include medical therapy or a second surgery. In general, if there are no signs indicating tubal rupture (which may occur even in the setting of a dropping β-hCG), medical treatment with methotrexate, using a single intramuscular dose, is preferable. Use of prophylactic methotrexate immediately after conservative surgery has been advocated by some and its use supported by one randomized trial, which showed a decrease in the rate of persistent ectopic pregnancy from 14.5% to 1.9% with single-dose methotrexate (1mg/kg IM).71 A recent decision-analysis found that prophylactic methotrexate resulted in fewer cases of tubal rupture and fewer procedures at a lower cost compared with observation alone.72 In a clinical setting where the rate of persistent ectopic pregnancy is greater than 9% with observation after surgery, the incidence of persistent ectopic pregnancy is less than 5% after prophylactic methotrexate, the probability of ectopic rupture is greater than 7.3% with persistent ectopic pregnancy, and the complication rate associated with prophylactic methotrexate is less than 18%, the use of prophylactic methotrexate optimizes the treatment. If these conditions are not met, observation alone is the better strategy. Until more data are available, following serial β-hCG levels without administering prophylactic methotrexate remains a reasonable strategy.
Medical Management with Methotrexate
Methotrexate has proven to be a safe and effective method of treating ectopic pregnancy. First introduced in 1982, it has become a common mode of treatment for appropriately selected patients. The primary advantage to medical therapy is the chance to avoid the morbidity and risks associated with surgery. In addition to its use as primary treatment for ectopic pregnancy, methotrexate is also used for the treatment of persistent ectopic pregnancy after salpingostomy, as a prophylactic method to decrease the risk of persistent ectopic pregnancy after conservative surgery, and as a primary treatment of ectopic pregnancies in unusual locations.
Mechanism of Action
Methotrexate acts to inhibit rapidly dividing cells, specifically arresting mitosis of the cytotrophoblasts of the ectopic pregnancy. It is a folic acid antagonist that inhibits the enzyme dihydrofolate reductase. Dihydrofolate reductase reduces folate to tetrahydrofolate by the addition of single carbon groups, which are subsequently transferred in the synthesis of DNA and RNA precursors. By blocking this enzyme, methotrexate leads to the depletion of cofactors required for DNA and RNA synthesis. With interruption of both DNA and RNA synthesis and impairment of the synthesis of critical proteins necessary for cell survival, methotrexate targets cells in many parts of the cell cycle.73 Methotrexate also causes the buildup of dihydrofolate polyglutamates in the cell, which both acts as a toxic substance itself and prolongs the action of methotrexate within cells.73,74
Methotrexate is predominantly cleared by the kidney and excreted in the urine, so the medication should be used with great caution and with adjusted doses in patients with renal compromise.74
Need for Definitive Diagnosis
In an attempt to expedite treatment for women with abnormal pregnancies, some clinicians have used methotrexate presumptively before making a definitive diagnosis. The most common clinical scenarios where this practice is employed are (1) in the absence of signs of a normal intrauterine pregnancy and β-hCG above the discriminatory zone, and (2) with a pleateauing β-hCG level below the discriminatory zone. Although such a strategy does avoid invasive intervention, it is not recommended. Presumed diagnosis of ectopic pregnancy has been shown to be inaccurate about 40% of the time.75 Therefore, if methotrexate were used without prior confirmation of an ectopic pregnancy, a large number of patients would receive this chemotherapeutic agent unnecessarily. In addition, methotrexate has about a 30% failure rate when used for early pregnancy termination, so it may be inadequate treatment for an abnormal intrauterine pregnancy.75 Should the pregnancy ultimately prove to be viable, the risk of congential anomalies is significant, because methotrexate is a known teratogen.
Another problem with presumptive treatment is that an incorrect diagnosis will lead to assignment of an erroneous diagnostic label to a patient, which may have implications for her future care: a patient with an ectopic pregnancy may be more readily referred to IVF for a presumed tubal factor, when one may not actually exist. In addition, the presumptive treatment of women at risk for ectopic pregnancy does not result in a reduction in cost, side effects, or time saved.76
Indications and Contraindications
To minimize the risk of tubal rupture after the initiation of medical therapy, relative contraindications for the use of methotrexate have been described.77 Although not all clinicians agree, many avoid using methotrexate in the presence of an adnexal mass greater than 3.5 cm at its greatest dimension, fetal cardiac motion visible on ultrasound, or β-hCG greater than 15,000 mIU/mL.77
Absolute contraindications to methotrexate therapy include evidence of immunodeficiency, damage to organs that metabolize methotrexate (i.e., liver and kidney), preexisting conditions that could be exacerbated by methotrexate (e.g., peptic ulcer disease, blood dyscrasias, active pulmonary disease), and breastfeeding (Table 48-4).
Table 48-4 Absolute Contraindications to Methotrexate Therapy
| Breastfeeding |
| Overt or laboratory evidence of immunodeficiency |
| Alcoholism, alcoholic liver disease, or other chronic liver disease |
| Preexisting blood dyscrasias (bone marrow hypoplasia, leukopenia, thrombocytopenia, significant anemia) |
| Known sensitivity to methotrexate |
| Active pulmonary disease |
| Peptic ulcer disease |
| Hepatic, renal, or hematologic dysfunction |
Adapted from ACOG: Medical Management of Tubal Pregnancy. Washington, DC, ACOG Practice Bulletin No. 3, 1998.
Pretreatment Evaluation
Certain baseline laboratory values should be evaluated before administering methotrexate. Patients should be screened with a complete blood count, liver function tests, and serum creatinine. A chest x-ray should be performed in women with a history of pulmonary disease due to their risk of developing interstitial pneumonitis. Patients should be excluded from medical treatment if they are found to have aspartate transaminase greater than 50 or 2 times normal; creatinine level greater than 1.3 to 1.5mg/dL, white blood cell count less than 3,000/μL, or platelet count less than 100,000/μL.78,79
Methotrexate Treatment Protocols
For the treatment of ectopic pregnancy with methotrexate, intramuscular injection is the preferred method of administration. Preliminary reports of oral administration have reported successful resolution of ectopic pregnancy, but this mode of administration is not well-studied.80
The two most common regimens employed for the treatment of ectopic pregnancy are the multidose protocol and the single-dose protocol. Under the multidose protocol, methotrexate is administered as the sodium salt at a dose of 1mg/kg per day, intramuscularly, on days 1, 3, 5, and 7 of treatment.81 Due to the relatively large overall dose, leucovorin is used to prevent cell toxicity and is given at a dose of 0.1mg/kg intramuscularly on alternating days (days 2, 4, 6, and 8). Patients receive up to four doses (1 methotrexate/1 leucovorin) until the β-hCG decreases by at least 15% on two consecutive measurements, 2 days apart. Consequently, some patients may only require one or two doses, and others need the full four-dose course. All patients need to be followed until the β-hCG is no longer detectable in the serum to confirm complete resolution of the ectopic gestation. In select cases, a second four-dose course may be given 1 week later if there is an increase or plateau in two consecutive β-hCG values; at such a point, however, most clinicians would proceed to surgical treatment.
A single-dose regimen of methotrexate was more recently introduced, with the benefit of simplified administration and less frequent need for patient follow-up.82 Under this protocol, a patient is given an intramuscular methotrexate dose of 50 mg/m2 based on the patient’s body surface area:
No leucovorin rescue is given. The single-dose regimen is somewhat of a misnomer because a second dose may be administered after 1 week if the β-hCG value does not decline by at least 15% between days 4 and 7 after initial methotrexate injection.
It has been shown that using the single-dose protocol, approximately 20% of women require more than one dose to completely resolve their ectopic gestation.83 Again, once a treatment response has been documented with serially decreasing β-hCG levels, patients are followed with surveillance β-hCG measurements until no longer detectable in serum (Tables 48-5 and 48-6).
| Treatment Day | Laboratory Tests | Intervention |
|---|---|---|
| Pretreatment | β-hCG, CBC with differential, LFTs, creatinine, type and screen |
β-hCG, β-human chorionic gonadotropin; CBC, complete blood count; LFTs, liver function tests; SAB, spontaneous abortion; MTX, methotrexate; LEU, leucovorin.
Table 48-6 Single-dose Methotrexate Protocol
| Treatment Day | Laboratory Tests | Intervention |
|---|---|---|
| Pretreatment | β-hCG, CBC with differential, LFTs, creatinine, type and screen | |
| 0 | β-hCG | MTX 50 mg/m2* IM |
| 4 | β-hCG | |
| 7 | β-hCG | MTX 50 mg/m2* IM if β-hCG <15% decrease Day 4 to Day 7 |
| Surveillance every 7 days (until β-hCG <5) | ||
β-hCG, β-human chorionic gonadotropin; CBC, complete blood count; LFTs, liver function tests; MTX, methotrexate; SAB, spontaneous abortion.
Side Effects
At methotrexate doses used to treat ectopic pregnancies, common symptoms include cramping abdominal pain, vaginal bleeding or spotting, gastrointestinal symptoms including nausea, vomiting, and indigestion, and general symptoms such as fatigue, lightheadedness, or dizziness.77,79
Major side effects are uncommon and include impaired liver function, skin sensitivity to light, stomatitis, gastritis and enteritis, temporary hair loss, bone marrow suppression, and pneumonitis.3,14,60 Hemorrhagic enteritis is manifest by nausea, vomiting, bloody diarrhea, and weight loss. Destruction of bone marrow precursors puts the patient at risk for developing thrombocytopenia, reticulocytopenia, lymphopenia, and granulocytopenia, and in the most severe cases can lead to risk of life-threatening hemorrhage and systemic infections. Rare cases of alopecia and anaphylactoid reaction have also been described.84,85
Fortunately, major side effects reported with methotrexate used to treat ectopic pregnancy are very uncommon. In one study, 2% of patients developed stomatitis and 3% had transient elevation of transaminase levels, all of which resolved spontaneously.82 The single-dose regimen appears to be associated with fewer side effects (OR=0.44; 0.31–0.63).86 However, when adjusted for initial β-hCG values, the prevalence of side effects was comparable for those who received single-dose and multidose methotrexate.
Clinical Course after Treatment
It is important for both patients and clinicians to be aware of the clinical course of ectopic pregnancies after methotrexate treatment. An increase in abdominal pain 6 to 7 days after receiving the medication is reported by one third to two thirds of patients.14,78,87 This “separation” pain is attributable to tubal distension from tubal abortion or hematoma formation. As long as other aspects of the patient’s condition remain stable, separation pain may be treated with pain medications and does not warrant surgical intervention.88
The β-hCG levels may plateau, or even rise initially, after methotrexate injection. This is believed to occur because of continued hormonal production by syncytiotrophoblastic cells, despite mitotic arrest induced by methotrexate in the cytotrophoblasts.89 Similarly, on ultrasound examination, the gestational mass may appear to enlarge, owing in part to hemorrhage distending the tubal lumen.
Efficacy of Methotrexate
Methotrexate is effective in the treatment of ectopic pregnancy. Overall, methotrexate has been shown to be more effective with absence of live embryo, smaller gestational mass (<3.5 cm), less color Doppler flow to the adnexal mass, and lower initial β-hCG level, though the absolute value for β-hCG varies among studies.87,90–92 Reviews on the subject have demonstrated that patients who are administered methotrexate therapy as single or variable dose may expect an 87% to 93% success rate, a post-treatment tubal patency rate of about 75% to 81%, with a future pregnancy rate of about 60% and a recurrent ectopic pregnancy rate of 7% to 8%.4 However, the majority of trials have been open label with no direct comparison between regimens.
A meta-analysis, including data from 1327 women treated with methotrexate for ectopic pregnancy, compared the single-dose and multidose regimens.86 The overall success rate, defined as the avoidance of surgical intervention, for the medical therapy was 89%; the success rate of the multidose therapy was 92.7% and of the single-dose was 88.1%, a statistically significant difference. The use of the single-dose regimen was associated with a significantly greater chance of failed medical management compared to the use of the multidose regimen (OR 1.71; 1.04–2.82). However, when the analysis was adjusted to account for factors independently affecting success rates such as initial β-hCG and the presence of embryonic fetal cardiac activity, it was noted that the failure rate of a single-dose regimen was even higher compared to the multidose regimen (OR 4.74, 1.77–2.62).
Methotrexate Efficacy Compared to Surgery
Several studies have compared medical management to surgical management.93,94 Multidose methotrexate therapy appears to be comparable in effectiveness to laparoscopic salpingostomy. In a multicenter, randomized, prospective trial of 100 women with laparoscopically confirmed ectopic pregnancies, 82% of those who received methotrexate were successfully treated with a single course, 4% required a second course, and 14% required surgical intervention for active bleeding or tubal rupture.93 Of the women in the salpingostomy arm, 72% were successfully treated with salpingostomy alone, 8% required conversion to salpingectomy for persistent bleeding, and 20% required adjuvant methotrexate for persistent trophoblastic tissue.
Not all studies have found that methotrexate management is equivalent to surgical management. In a smaller, randomized trial comparing single-dose methotrexate to laparoscopic salpingostomy, a 65% success rate in the methotrexate group was noted, compared to a 93% success rate in the laparoscopic salpingostomy group, calling into question the effectiveness of the single-dose regimen.95 A retrospective study from a university hospital serving an indigent population likewise compared the success of single-dose methotrexate with that of surgical treatment.96 The overall success rate for those receiving methotrexate was significantly lower than for the conservative laparoscopic surgical group (79% vs. 90%, respectively), and 11% of patients treated medically required a second methotrexate dose.
Direct Injection of Methotrexate and Other Agents
Direct injection of methotrexate into the ectopic gestational sac can be done laparoscopically, hysteroscopically, via transcervical tubal cannulation, or transvaginally under ultrasound guidance.97–100 Direct injection of methotrexate has the advantage of delivering a higher dose of medication precisely into the ectopic implantation site, reducing systemic absorption and resultant side effects. However, unlike intramuscular administration, this mode of drug delivery is invasive and requires some degree of expertise and often anesthesia.
Other Agents
Injection of other agents into the site of an ectopic pregnancy has been reported. Ultrasound-guided injection of potassium chloride has been shown to lead to cessation of cardiac activity and subsequent resorption of ectopic pregnancies.101,102 However, because potassium chloride does not affect the growth of trophoblastic tissue, this tissue may continue to proliferate, possibly leading to fallopian tube rupture.103 Laparoscopic injection of hyperosmolar glucose has also been reported to be successful in a small series of patients,104 but it is less successful than laparoscopic salpingostomy.105 Because most of these reports involve small numbers of patients, subsequent tubal function and reproductive success remain uncertain. None of these alternative methods are currently in widespread use.
Expectant Management
Prior to modern methods of early diagnosis of ectopic pregnancy and prompt interventions, many ectopic pregnancies likely spontaneously resolved with no treatment. In the 1950s, Lund106 randomized patients to expectant management versus surgical treatment and reported a 57% success rate of spontaneous resolution of the ectopic pregnancy in the nonintervention group. However, those patients whose ectopic pregnancies did not resolve on their own experienced significant symptoms and presented with hemoperitoneum and tubal rupture.
A recent review of 10 studies that prospectively examined the efficacy of expectant management included 347 patients.14 These were patients who were hemodynamically stable and whose β-hCG levels were documented to be decreasing by serial measurements. Although there was some variation between the studies in the confirmation of diagnosis, size, and location of the ectopic pregnancy, the overall success rate was 69%, with a range of 47% to 100%.
Some investigators have attempted to identify prognostic factors that predict success of expectant management.107,108 Although there appears to be increased success of spontaneous resolution with lower initial β-hCG and absence of a visible sac on ultrasound, there are still no established criteria for the inclusion and exclusion of patients. Despite close follow-up, and even in the context of declining β-hCG levels, tubal rupture may still occur.14
One study of a large cohort of women with ectopic pregnancies was able to categorize women into two groups: those who presented acutely and those who had a chronic presentation.109 Those with the chronic presentation often had a delayed diagnosis of ectopic gestation, but were also found to have a less acute clinical course with a lower chance of rupture. The authors speculated that this difference may be due to factors such as differences in the invasive nature of the trophoblast, the location of implantation, or host defenses.
Treatment of Ectopic Pregnancies in Locations Other than the Tube
Although most ectopic pregnancies are located within the fallopian tube’s ampullary, isthmic, or fimbrial portions, a small number implant in unusual sites. Of all ectopic pregnancies, about 2.4% are interstitial or cornual, 3.2% ovarian, and 1.3% abdominal, and less than 0.15% are cervical.6–8 With earlier and more accurate diagnosis of these rare ectopic pregnancies, a conservative approach to treatment in the hemodynamically stable patient is often feasible.
Cornual (Interstitial) Pregnancy
The interstitial part of the fallopian tube is the proximal portion that is embodied within the muscular wall of the uterus, measuring approximately 0.7 mm in width and 1 to 2 cm in length. Due to the surrounding myometrial layer, a gestation in this site may expand and not rupture until reaching 7 to 16 weeks in size.110 Clinically, a pregnancy implanted in this site is seen as a swelling lateral to the round ligament. Sonographic criteria suspicious for the diagnosis include an empty uterine cavity, a chorionic sac seen separately and more than 1 cm from the most lateral edge of the uterine cavity, and a thin myometrial layer surrounding the chorionic sac.111,112
Surgical Treatment
Traditional treatment of interstitial ectopic pregnancy was cornual resection via laparotomy, and this remains the approach for the unstable patient. A laparoscopic approach has been proposed in the treatment of the stable patient who does not desire medical management. Most techniques described in the literature involve injection of intramyometrial vasopressin to minimize blood loss, performance of a linear incision at the site of the ectopic pregnancy, and the use of hydrodissection to flush out the gestational products in one mass.110 Some authors advocate the closure of the cornual defect with sutures; others use electrocoagulation with closure by secondary intention.113 Successful hysteroscopic management of interstitial ectopic pregnancy has also been described.114
Medical Treatment
Studies of the use of methotrexate to treat interstitial ectopic pregnancy have yielded conflicting results. In a series of 14 patients with interstitial ectopic pregnancy, treatment with single-dose methotrexate was 100% successful, with only 1 patient requiring a second dose due to insufficient decline of β-hCG between days 4 and 7.115 In another review of 20 patients with interstitial ectopic pregnancy, treatment with methotrexate was only successful in 35%.53 In a review of 41 patients with interstitial ectopic pregnancy treated with systemic, local injection, or combined methotrexate therapy, the overall success rate was 83%, with faster resolution of β-hCG in patients treated with local injection.110 Based on these and other published reports, it appears that, in stable patients management of interstitial pregnancy with either laparoscopy or methotrexate using a multidose protocol are reasonable alternatives to laparotomy.
Ovarian Pregnancy
Ovarian ectopic pregnancies remain difficult to differentiate from tubal pregnancies before surgery because it is difficult with ultrasound to distinguish between ovarian and tubal masses. Due to ovarian vascularity, ovarian pregnancy tends to present earlier and often after having already ruptured.116 Grossly, an ovarian pregnancy can be mistaken for a bleeding corpus luteum cyst until pathologic confirmation of chorionic villi is obtained.
The approach to the treatment of ovarian ectopic pregnancy has traditionally been laparotomy with oophorectomy. Wedge resection and the laparoscopic approach have been reported in recent years.117 Medical treatment with methotrexate has also been described with success.118
Abdominal Pregnancy
Although the traditional treatment of abdominal pregnancy has been by laparotomy, early diagnosis with ultrasound can sometimes allow for more conservative management. Recent case reports have described successful treatment of abdominal ectopic pregnancies with laparoscopy, some with additional adjuvant methotrexate.119–122
Cervical Pregnancy
Before surgical management with D&C, the substantial risk of postevacuation hemorrhage can be minimized by vascular occlusion, which has been successfully reported by transvaginal ligation of the cervical branches of the uterine arteries or angiographic embolization of the uterine arteries.123 Arterial embolization in the radiology suite offers the advantage of being less invasive compared to arterial ligation.
The most common surgical approach for the treatment of cervical pregnancy is curettage, although successful hysteroscopic resection of a cervical pregnancy has also been reported.124 After removal of a cervical pregnancy, postprocedure bleeding can be controlled using a Foley balloon for tamponade, a technique that has been shown to be more effective than vaginal packing.125 In women who do not desire future fertility, hysterectomy reliably removes the cervical ectopic pregnancy and is probably the most reliable way to control procedure-related hemorrhage.
In patients desiring future fertility, medical treatment of a cervical pregnancy might lower the risk of hemorrhage compared to surgery.123,126 Medical treatments that have been described include local injection of methotrexate, prostaglandins, or hyperosmolar glucose, with or without curettage.123,126,127 Local injection of methotrexate appears to offer some advantage over systemic administration in the treatment of cervical pregnancy. Whenever medical treatment is used, the physician must be prepared to control life-threatening hemorrhage by whatever means necessary, including hysterectomy, even in women who desire future fertility.
REPRODUCTIVE OUTCOME AFTER ECTOPIC PREGNANCY
Fertility after Surgical Treatment
Recurrent Ectopic Pregnancy
The risk of a recurrent ectopic pregnancy ranges from 10% to 27%, which represents a fivefold to tenfold increase in risk compared to the general population (Table 48-7).128–132 It is not surprising that ectopic pregnancies tend to recur, because many ectopic pregnancies are the result of abnormal fallopian tube function.
Table 48-7 Recurrent Ectopic Pregnancy According to Treatment
| Study | Risk Factor | Recurrent Ectopic Pregnancy Rate |
|---|---|---|
| Butts et al.133 | History of previous ectopic pregnancy | 10–27% |
| Ego et al.134 | One previous | 13% |
| Tulandi135 | Two previous | 28% |
| After treatment of ectopic pregnancy with | ||
| Pisarska et al.4 | Methotrexate | 6–8% |
| Laparoscopic | ||
| Pisarska et al.4 | Salpingostomy | 13% |
| Barnhart et al.38 | Salpingectomy | 17% |
| Laparotomy | ||
| Barnhart et al.38 | Salpingostomy | 16% |
| Yao & Tulandi14 | Salpingectomy | 10% |
Women who have undergone salpingectomy for an ectopic pregnancy have an increased risk of developing another ectopic pregnancy in the remaining tube, due to the likely bilateral nature of the underlying process (see Table 48-7). Those who undergo tube-sparing, conservative treatment with salpingostomy or methotrexate have approximately an equal risk of recurrent ectopic pregnancy in either tube. Likewise, the risk of a recurrent ectopic pregnancy after salpingectomy (7% to 17%) appears to be no greater than after salpingostomy (8% to 16%).14,136,137
In patients with a history of ectopic pregnancy, some factors appear to increase the risk of having a recurrent ectopic pregnancy even further, including a history of any pelvic surgery (including surgery for the first ectopic pregnancy) and a previous live birth or spontaneous miscarriage.133 In this study, it was surprising that the risk of a recurrent ectopic pregnancy was not further increased by a history of infections with gonorrhea or chlamydia, pelvic inflammatory disease, cesarean delivery, or pregnancy termination. One explanation for these findings is the possibility that the occurrence of the first ectopic pregnancy is a more sensitive marker for tubal damage from a subclinical pelvic infection than either cultures or clinical history.
Subsequent Fertility
Subsequent fertility rates do not appear to be affected by whether the ectopic pregnancy is treated laparoscopically or by laparotomy. Several studies have failed to show a difference between subsequent intrauterine pregnancy rates in women treated with salpingostomy whether performed by laparoscopy (56% to 61%) or laparotomy (58% to 61%).14,57,59,138 With similar results in terms of fertility, laparoscopy (with its decreased blood loss, shorter hospital stay, and shorter postoperative recovery compared to laparotomy) has become the procedure of choice for the hemodynamically stable patient.
Although it seems logical that subsequent fertility would be higher after salpingostomy compared to salpingectomy, several studies have not demonstrated this. In one study of combined data from nine retrospective studies, subsequent intrauterine pregnancy rates were no different after salpingostomy (53%) versus salpingectomy (49%).14 Another large retrospective study found no difference in subsequent intrauterine pregnancy rates after salpingostomy (36%) versus salpingectomy (40%), as long as the remaining tube was patent.136 When the opposite tube was blocked or absent, the intrauterine pregnancy rate after salpingostomy was decreased by half (18%).136 A third study found that the intrauterine pregnancy rate after salpingostomy was almost twice that after salpingectomy; however, these differences were not statistically significant on multivariate analysis.40 A recent study found a higher cumulative pregnancy rate after salpingostomy (88%) compared to salpingectomy (66%).137
In the absence of results from a randomized study, it makes sense to perform a salpingostomy whenever possible in the presence of a relatively normal-appearing tube in patients desiring future fertility. However, if the tube is ruptured or severely damaged, or the patient does not desire future fertility, salpingectomy is often the best, and sometimes the only, surgical option (Table 48-8).
| Tubal rupture |
Fertility after Methotrexate Treatment
Reproductive outcome appears to be similar after methotrexate therapy compared to surgical therapy, and there is a suggestion that the recurrent ectopic pregnancy rate may be lower. The tubal patency rate after methotrexate treatment for ectopic pregnancy is high, ranging from 75% to 80% in a review of 40 studies of single-dose, variable-dose, and direct-injection regimens.4 In this same review, the subsequent intrauterine pregnancy rate was found to be 57% to 61%, and the ectopic pregnancy rate was 6% to 8%. In an 18-month follow-up study comparing multidose systemic methotrexate with laparoscopic salpingostomy, the cumulative spontaneous intrauterine pregnancy rate was 36% in the methotrexate-treated group and 43% in the laparoscopic salpingostomy group.137 In a small randomized trial, the overall rate of intrauterine pregnancy rate (including patients who conceived with ART) appeared higher and the ectopic pregnancy rate lower after methotrexate treatment compared to laparoscopic salpingostomy, although the study lacked the statistical power to reach definitive conclusions.138
SUMMARY
1 Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990–1992. MMWR. 1995;44:46-48.
2 Fylstra DL. Tubal pregnancy: A review of current diagnosis and treatment. Obstet Gynecol Surv. 1998;53:320-328.
3 Barnhart K, Esposito M, Coutifaris C. An update on the medical treatment of ectopic pregnancy. Obstet Gynecol Clin North Am. 2000;27:653-667.
4 Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet. 1998;351:1115-1120.
5 Washington AE, Katz P. Ectopic pregnancy in the United States: Economic consequences and payment source trends. Obstet Gynecol. 1993;81:287-292.
6 Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: A 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
7 Gun M, Mavrogiorgis M. Cervical ectopic pregnancy: A case report and literature review. Ultrasound Obstet Gynecol. 2002;19:297-301.
8 Parente JT, Ou CS, Levy J, Legatt E. Cervical pregnancy analysis: A review and report of five cases. Obstet Gynecol. 1983;62:79-82.
9 Hunter RHF. Tubal ectopic pregnancy: A patho-physiological explanation involving endometriosis. Hum Reprod. 2002;17:1688-1691.
10 Goddijn M, van der Veen F, Schuring-Blom GH, et al. Cytogenetic characteristics of ectopic pregnancy. Hum Reprod. 1996;11:2769-2771.
11 Coste J, Fernandez H, Joye N, et al. Role of chromosome abnormalities in ectopic pregnancy. Fertil Steril. 2000;74:1259-1260.
12 Ankum W, Mol B, Van de Veen F, Bossuyt P. Risk factors for ectopic pregnancy: A meta-analysis. Fertil Steril. 1996;65:1093-1099.
13 Della-Giustina D, Denny M. Ectopic pregnancy. Emerg Med Clin North Am. 2003;21:565-584.
14 Yao M, Tulandi T. Current status of surgical and nonsurgical management of ectopic pregnancy. Fertil Steril. 1997;67:421-433.
15 Maymon R, Shulman A. Controversies and problems in the current management of tubal pregnancy. Hum Reprod Update. 1996;2:541-551.
16 DeVoe RW, Pratt JH. Simultaneous intrauterine and extrauterine pregnancy. Am J Obstet Gynecol. 1948;56:1119.
17 Dart RD, Kaplan B, Vavaklis K. Predictive value of history and physical examination in patients with suspected ectopic pregnancy. Ann Emerg Med. 1999;33:283-290.
18 Richards SR, Stempel LS, Carlton BD. Heterotopic pregnancy. Reappraisal of incidence. Am J Obstet Gynecol. 1982;142:928-930.
19 Goldman GA, Fisch B, Ovadia J, Tadir Y. Heterotopic pregnancy after reproductive technologies. Obstet Gynecol Surv. 1992;47:217-221.
20 Saxon D, Falcone T, Mascha EJ, et al. A study of ruptured tubal ectopic pregnancy. Obstet Gynecol. 1997;90:46-49.
21 Bickell NA, Bodian C, Anderson RM, Kase N. Time and the risk of ruptured tubal pregnancy. Obstet Gynecol. 2004;104:789-794.
22 Tulandi T, Hemmings R, Khalifa F. Rupture of ectopic pregnancy in women with low and declining serum β-human chorionic gonadotropin concentrations. Fertil Steril. 1991;56:786-787.
23 Irvine LM, Padwick ML. Serial serum hCG measurements in a patient with an ectopic pregnancy: A case for caution. Hum Reprod. 2000;15:1646-1647.
24 Goldstein SR, Snyder JR, Watson C, Danon M. Very early pregnancy detection with endovaginal ultrasound. Obstet Gynecol. 1988;72:200-204.
25 Condous G, Okaro E, Khalid A, et al. The accuracy of transvaginal ultrasonography for the diagnosis of ectopic pregnancy prior to surgery. Hum Reprod. 2005;20:1404-1409.
26 Barnhart K, Mennuti MT, Benjamin I, et al. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol. 1994;l84:1010-1015.
27 Kadar N, Caldwell BV, Romero R. A method of screening for ectopic pregnancy and its indications. Obstet Gynecol. 1981;52:162-166.
28 Kadar N, Romero R. Observations on the log human chorionic gonadotropin-time relationship in early pregnancy and its practical implications. Am J Obstet Gynecol. 1987;157:73-78.
29 Romero R, Kadar N, Copel JA, et al. The value of serial human chorionic gonadotropin testing as a diagnostic tool in ectopic pregnancy. Am J Obstet Gynecol. 1986;155:392-394.
30 Pittaway DE, Wentz AC. Evaluation of early pregnancy by serial chorionic gonadotropin determinations: A comparison of methods by receiver operating characteristic curve analysis. Fertil Steril. 1985;43:529-533.
31 Kadar N, Freedman M, Zacher M. Further observation on the doubling time of hCG in early asymptomatic pregnancy. Fertil Steril. 1990;54:783-787.
32 Fritz M, Guo S. Doubling time of hCG in early normal pregnancy: Relationship to hCG concentration and gestational age. Fertil Steril. 1987;47:584-589.
33 Lenton EA, Neal LM, Sulaiman R. Plasma concentrations of human chorionic gonadotropin from the time of implantation until the second week of pregnancy. Fertil Steril. 1982;37:773-778.
34 Barnhart K, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early intrauterine pregnancy: hCG curves redefined. Obstet Gynecol. 2004;104:50-55.
35 Spandorfer SD, Menzin AW, Barnhart KT, et al. Gynecology: Efficacy of frozen-section evaluation of uterine curettings in the diagnosis of ectopic pregnancy. Am J Obstet Gynecol. 1996;175:603-605.
36 Lindahl B, Ahlgren M. Identification of chorionic villi in abortion specimens. Obstet Gynecol. 1986;67:79-81.
37 Kristiansen JD, Clausen I, Nielsen MN, Thomsen SG. Stereomicroscopic demonstration of chorionic villi: Differentiation between miscarriage and ectopic pregnancy. BJOG. 1993;100:839-841.
38 Barnhart KT, Gracia CR, Reindl B, Wheeler JE. Usefulness of Pipelle endometrial biopsy in the diagnosis of women at risk for ectopic pregnancy. Am J Obstet Gynecol. 2003;188:906-909.
39 Ries A, Singson P, Bidus M, Barnes JG. Use of the endometrial Pipelle in the diagnosis of early abnormal gestations. Fertil Steril. 2000;74:593-595.
40 Mol BW, Lijmer JG, Ankum WM, et al. The accuracy of single serum progesterone measurement in the diagnosis of ectopic pregnancy: A meta-analysis. Hum Reprod. 1998;13:3220-3227.
41 McCord ML, Muran D, Buster J, et al. Single serum progesterone as a screen for ectopic pregnancy: Exchanging specificity and sensitivity to obtain optimal test performance. Fertil Steril. 1996;66:513-516.
42 Buckley RG, King KJ, Riffernburgh RH, et al. Serum progesterone testing to predict ectopic pregnancy in symptomatic first-trimester patients. Ann Emerg Med. 2000;36:95-100.
43 Ness RB, McLaughlin MT, Heine RP, et al. Fetal fibronectin as a marker to discriminate between ectopic and intrauterine pregnancies. Am J Obstet Gynecol. 1998;179:697-702.
44 Vitoratos N, Gregoriou O, Papadias C, et al. Clinical value of creatinine kinase in the diagnosis of ectopic pregnancy. Gynecol Obstet Invest. 1998;46:80-83.
45 Daniel Y, Geva E, Lerner-Geva L, et al. Levels of vascular endothelial growth factor are elevated in patients with ectopic pregnancy: Is this a novel marker? Fertil Steril. 1999;72:1013-1017.
46 Predanic M. Differentiating tubal abortion from viable ectopic pregnancy with serum CA-125 and beta-human chorionic gonadotropin determinations. Fertil Steril. 2000;73:522-525.
47 Saha PK, Gupta I, Ganguly NK. Evaluation of serum creatine kinase as a diagnostic marker for tubal pregnancy. Austral NZ J Obstet Gynecol. 1999;39:366-367.
48 Felemban A, Sammour A, Tulandi T. Serum vascular endothelial growth factor as a possible marker for early ectopic pregnancy. Hum Reprod. 2002;17:490-492.
49 Fasouliotis SJ, Spandorfer SD, Witkin SS, et al. Maternal serum vascular endothelial growth factor levels in early ectopic and intrauterine pregnancies after in vitro fertilization treatment. Fertil Steril. 2004;82:309-313.
50 Gerton GL, Fan XJ, Chittams J, et al. A serum proteomics approach to the diagnosis of ectopic pregnancy. Ann NY Acad Sci. 2004;1022:306-312.
51 Gracia CR, Barnhart KT. Diagnosing ectopic pregnancy: A decision analysis comparing six strategies. Obstet Gynecol. 2001;97:464-470.
52 Barnhart K, Sammel MD, Chung K, et al. Decline of serum hCG and spontaneous complete abortion: Defining the normal curve. Obstet Gynecol. 2004;104:975-981.
53 Barnhart K, Spandorfer S, Coutifaris C. Medical treatment of interstitial pregnancy: A report of three unsuccessful cases. J Reprod Med. 1997;42:521-524.
54 Barnhart KT, Simhan H, Kamelle S. Diagnostic accuracy of ultrasound above and below the beta-hCG discriminatory zone. Obstet Gynecol. 1999;94:583-587.
55 Mol BW, Hajenius PJ, Ankum WM, et al. Screening for ectopic pregnancy in symptom-free women at increased risk. Obstet Gynecol. 1997;89:704-707.
56 Mol BW, van der Veen F, Bossuyt PM. Symptom-free women at increased risk of ectopic pregnancy: Should we screen? Acta Obstet Gynecol Scand. 2002;81:661-672.
57 Vermesh M, Silva PD, Rosen GF, et al. Management of unruptured ectopic gestation by linear salpingostomy: A prospective, randomized clinical trial of laparoscopy versus laparotomy. Obstet Gynecol. 1989;73:400-404.
58 Lundorff P, Thorburn J, Lindblom B. Fertility outcome after conservative surgical treatment of ectopic pregnancy evaluated in a randomized trial. Fertil Steril. 1992;57:998-1002.
59 Murphy AA, Nager CW, Wujek JJ, et al. Operative laparoscopy versus laparotomy for the management of ectopic pregnancy: A prospective trial. Fertil Steril. 1992;57:1180-1185.
60 Hajenius PJ, Mol BWJ, Bossuyt PMM, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 3, 2003. CD000324.
61 Damario MA, Rock JA. Ectopic pregnancy. In: Rock JA, Jones HW, editors. TeLinde’s Operative Gynecology. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2003:507-536.
62 Ory SJ. Ectopic pregnancy. In: Adamson GD, Martin DC, editors. Endoscopic Management of Gynecologic Disease. Philadelphia: Lippincott-Raven Publishers; 1996:97-108.
63 Balasch J, Barri PN. Treatment of ectopic pregnancy: The new gynaecological dilemma. Hum Reprod. 1994;9:547-548.
64 McComb P, Gomel V. Linear ampullary salpingotomy heals better by secondary versus primary closure. Fertil Steril. 1984;41:454.
65 Tulandi T, Guralnick M. Treatment of tubal ectopic pregnancy by salpingotomy with or without tubal suturing and salpingectomy. Fertil Steril. 1991;56:374-375.
66 Fujishita A, Masuzaki H, Newaz Khan K, et al. Laparoscopic salpingotomy for tubal pregnancy: Comparison of linear salpingotomy with and without suturing. Hum Reprod. 2004;19:1195-1200.
67 DiMarchi JM, Kosasa TS, Kobara TX, Hace RW. Persistent ectopic pregnancy. Obstet Gynecol. 1987;70:555-560.
68 Vermesh M, Silva PD, Sauer MV, et al. Persistent tubal ectopic gestation: Patterns of circulation of beta-human chorionic gonadotropin and progesterone, and management options. Fertil Steril. 1988;50:584-590.
69 Seifer DB, Gutman JN, Grant WD, et al. Comparison of persistent ectopic pregnancy after laparoscopic salpingostomy versus salpingostomy at laparotomy for ectopic pregnancy. Obstet Gynecol. 1993;81:378-382.
70 Spandorfer SD, Sawin SW, Benjamin I, Barnhart KT. Postoperative day 1 serum human chorionic gonadotropin level as a predictor of persistent ectopic pregnancy after conservative surgical management. Fertil Steril. 1997;68:430-434.
71 Graczykowski JW, Mishell DR. Methotrexate prophylaxis of persistent ectopic pregnancy after conservative treatment by salpingostomy. Obstet Gynecol. 1997;89:118-122.
72 Gracia CR, Brown HA, Barnhart KT. Prophylactic methotrexate after linear salpingostomy: A decision analysis. Fertil Steril. 2001;76:1191-1195.
73 Barnhart K, Coutifaris C, Esposito M. The pharmacology of methotrexate. Expert Opin Pharm. 2001;2:409-417.
74 Calabresi P, Chabner B. Antineoplastic agents. In: Gilman A, Goodman LS, Goodman A, editors. The Pharmacologic Basis of Therapeutics. 8th ed. New York: Macmillan; 2005:1275-1276.
75 Barnhart K, Katz I, Hummel A, Gracia CG. Presumed diagnosis of ectopic pregnancy. Obstet Gynecol. 2002;100:505-510.
76 Ailawadi M, Lorch SA, Barnhart KT. Cost effectiveness of presumptively medically treating women at risk for ectopic pregnancy compared to first performing a D&C. Fertil Steril. 2005;83:376-382.
77 American College of Obstetricians and Gynecologists: Medical Management of Tubal Pregnancy. Washington, DC, ACOG Practice Bulletin no. 3, 1998.
78 Glock JL, Johnson JV, Brumsted JR. Efficacy and safety of single-dose systemic methotrexate in the treatment of ectopic pregnancy. Fertil Steril. 1994;62:716-721.
79 Stovall TG, Ling FW. Single dose methotrexate: An expanded clinical trial. Am J Obstet Gynecol. 1993;168:1759-1765.
80 Lipscomb GH, Meyer NL, Flynn DE, et al. Oral methotrexate for treatment of ectopic pregnancy. Am J Obstet Gynecol. 2002;186:1192-1195.
81 Stovall TG, Ling FW, Buster JE. Outpatient chemotherapy of unruptured ectopic pregnancy. Fertil Steril. 1989;51:435-438.
82 Stovall TG, Ling FW, Gray LA. Single-dose methotrexate for treatment of ectopic pregnancy. Obstet Gynecol. 1991;77:754-757.
83 Lipscomb GH, Bran D, McCord ML, et al. Analysis of 315 ectopic pregnancies treated with single-dose methotrexate. Am J Obstet Gynecol. 1998;178:1354-1358.
84 Trout S, Kemmann E. Reversible alopecia after single-dose methotrexate treatment in a patient with ectopic pregnancy. Fertil Steril. 1995;64:866-867.
85 Straka M, Zeringue E, Goldman M. A rare drug reaction to methotrexate after treatment for ectopic pregnancy. Obstet Gynecol. 2004;103:1047-1048.
86 Barnhart KT, Gosman G, Ashby R, Sammel M. The medical management of ectopic pregnancy: A meta-analysis comparing “single dose” and “multidose” regimens. Obstet Gynecol. 2003;101:778-784.
87 Lipscomb GH, McCord ML, Stovall TG, et al. Predictors of success of methotrexate treatment in women with tubal ectopic pregnancies. NEJM. 2000;341:1974-1978.
88 Lipscomb GH, Puckett KJ, Bran D, Ling FW. Management of separation pain after single-dose methotrexate therapy for ectopic pregnancy. Obstet Gynecol. 1999;93:590-593.
89 Thompson GR, O’Shea RT, Harding A. Beta-HCG levels after conservative treatment of ectopic pregnancy: Is a plateau normal? Aust NZ J Obstet Gynecol. 1994;34:96-99.
90 Elito J, Reichmann AP, Uchiyama MN, Camano L. Predictive score for the systemic treatment of unruptured ectopic pregnancy with a single dose of methotrexate. Int J Gynecol Obstet. 1999;67:75-79.
91 Tawfiq A, Agameya A-F, Claman P. Predictors of treatment failure for ectopic pregnancy treated with single-dose methotrexate. Fertil Steril. 2000;74:877-880.
92 Potter MB, Lepine LA, Jamieson DJ. Predictors of success with methotrexate treatment of tubal ectopic pregnancy at Grady Memorial Hospital. Am J Obstet Gynecol. 2003;188:1192-1194.
93 Hajenius PJ, Engelsbel S, Mol BWJ, et al. Randomized trial of systemic methotrexate versus laparoscopic salpingostomy in tubal pregnancy. Lancet. 1997;350:774-779.
94 Saraj AJ, Wilcox JG, Najmabadi S, et al. Resolution of hormonal markers of ectopic gestation: A randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol. 1998;92:989-994.
95 Sowter MC, Farquhar CM, Petrie KJ, Gudex G. A randomized trial comparing single dose systemic methotrexate and laparoscopic surgery for the treatment of unruptured tubal pregnancy. BJOG. 2001;108:192-203.
96 Lewis-Bliehall C, Rogers RC, Kammerer-Doak DN, et al. Medical vs. surgical treatment of ectopic pregnancy. J Reprod Med. 2001;46:983-988.
97 Feichtinger W, Kemeter P. Conservative treatment of ectopic pregnancy by transvaginal aspiration under sonographic control and injection of methotrexate. Lancet. 1987;1:381-382.
98 Pansky M, Bukovsky I, Golan A. Local methotrexate injection: A nonsurgical treatment of ectopic pregnancy. Am J Obstet Gynecol. 1989;161:393-396.
99 Risquez F, Mathieson J, Pariente D, et al. Diagnosis and treatment of ectopic pregnancy by retrograde selective salpingography and intraluminal methotrexate injection. Hum Reprod. 1990;5:759-762.
100 Goldenberg M, Bider D, Oelsner G, et al. Treatment of interstitial pregnancy with methotrexate via hysteroscopy. Fertil Steril. 1992;58:1234-1236.
101 Robertson DE, Smith W, Moye MA, et al. Reduction of ectopic pregnancy by injection under ultrasound control. Lancet. 1987;1:974-975.
102 Timor-Tritsch I, Baxi L, Peisner DB. Transvaginal salpingocentesis: A new technique for treating ectopic pregnancy. Am J Obstet Gynecol. 1989;160:459-460.
103 Pansky M, Golan A, Bukovsky I, Caspi E. Nonsurgical management of tubal pregnancy: Necessity in view of the changing clinical appearance. Am J Obstet Gynecol. 1991;164:888-895.
104 Yeko TR, Mayer JC, Parsons AK, Maroulis GB. A prospective series of unruptured ectopic pregnancies treated by tubal injection with hyperosmolar glucose. Obstet Gynecol. 1995;85:265-268.
105 Laatikainen L, Tuomivara L, Kaar K. Comparison of local injection of hyperosmolar glucose solution with salpingostomy for the conservative treatment of tubal pregnancy. Fertil Steril. 1993;60:80-84.
106 Lund JJ. Early ectopic pregnancy. J Obstet Gynaecol Br Empire. 1955;62:70-75.
107 Trio D, Strobelt N, Picciolo C, et al. Prognostic factors for successful expectant management of ectopic pregnancy. Fertil Steril. 1995;63:469-472.
108 Korhonen J, Stenman UH, Ylostalo P. Serum chorionic gonadotropin dynamics during spontaneous resolution of ectopic pregnancy. Fertil Steril. 1994;61:632-636.
109 Barnhart KT, Rinaudo P, Hummel A, et al. Acute and chronic presentation of ectopic pregnancy may be two clinical entities. Fertil Steril. 2003;80:1344-1351.
110 Lau S, Tulandi T. Conservative medical and surgical management of interstitial ectopic pregnancy. Fertil Steril. 1999;72:207-215.
111 Jafri SZ, Loginsky SJ, Bouffard JA, Selis JE. Sonographic detection of interstitial pregnancy. J Clin Ultrasound. 1987;15:253-257.
112 Timor-Tritsch IE, Monteagudo A, Matera C, Veit CR. Sonographic evolution of cornual pregnancies treated without surgery. Obstet Gynecol. 1992;79:1044-1049.
113 Grobman WA, Milad MP. Conservative laparoscopic management of a large cornual ectopic pregnancy. Hum Reprod. 1998;13:2002-2004.
114 Sanz LE, Verosko J. Hysteroscopic management of cornual ectopic pregnancy. Obstet Gynecol. 2002;99:941-944.
115 Rodriguez L, Takacs P, Kang L. Single-dose methotrexate for the management of interstitial ectopic pregnancy. Intl J Gynecol Obstet. 2004;84:271-272.
116 Gaudoin MR, Coulter KL, Robins AM, et al. Is the incidence of ovarian ectopic pregnancy increasing? Eur J Obstet Gynecol. 1996;70:141-143.
117 Hage PS, Arnouk IF, Zarou DM, et al. Laparoscopic management of ovarian ectopic pregnancy. J Amican Assoc Gynecol Laparosc. 1994;1:283-285.
118 Chelmow D, Gates E, Penzias AS. Laparoscopic diagnosis and methotrexate treatment of an ovarian pregnancy: A case report. Fertil Steril. 1994;62:879-881.
119 Ginath S, Malinger G, Golan A, et al. Successful laparoscopic treatment of a ruptured primary abdominal pregnancy. Fertil Steril. 2000;74:601-602.
120 Mitra A, LeQuire MH. Minimally invasive management of 14.5-week abdominal pregnancy without laparotomy. J Ultrasound Med. 2003;22:709-714.
121 Gerli S, Rossetti D, Baiocchi G, et al. Early ultrasonographic diagnosis and laparoscopic treatment of abdominal pregnancy. Eur J Obstet Gynecol. 2004;113:103-105.
122 Rahaman J, Berkowitz R, Mitty H, et al. Minimally invasive management of an advanced abdominal pregnancy. Obstet Gynecol. 2004;103:1064-1068.
123 Yao M, Tulandi T. Surgical and medical management of tubal and non-tubal ectopic pregnancies. Curr Opin Obstet Gynecol. 1998;10:371-374.
124 Ash S, Farrell S. Hysteroscopic resection of a cervical ectopic pregnancy. Fertil Steril. 1996;66:842-844.
125 Ushakov FB, Elchalal U, Aceman PJ, Schenker JG. Cervical pregnancy: Past and future. Obstet Gynecol Surv. 1996;52:45-59.
126 Mitra AG, Harris-Owens M. Conservative medical management of advanced cervical ectopic pregnancies. Obstet Gynecol Surv. 2000;55:385-389.
127 Cepni I, Ocal P, Erkan S, Erzik B. Conservative treatment of cervical ectopic pregnancy with transvaginal ultrasound-guided aspiration and single-dose methotrexate. Fertil Steril. 2004;81:1130-1132.
128 Hallatt J. Repeat ectopic pregnancy: A study of 123 consecutive cases. Am J Obstet Gynecol. 1975;45:542-546.
129 Schoen JA, Nowak RJ. Repeat ectopic pregnancy: A 16-year clinical survey. Obstet Gynecol. 1975;45:542-546.
130 Paavonen J, Varjonen-Toivonen M, Komulainen M, Heinonen PK. Diagnosis and management of tubal pregnancy: Effect on fertility outcome. Int J Gynaecol Obstet. 1985;23:129-133.
131 Sandevei R, Bergsjo P, Ulstein M, Steier JA. Repeat ectopic pregnancy: A 20-year hospital survey. Acta Obstet Gynecol Scand. 1987;6:635-640.
132 Skjeldestad FE, Hadgu A, Eriksson N. Epidemiology of repeat ectopic pregnancy: A population-based prospective cohort study. Obstet Gynecol. 1998;91:129-135.
133 Butts S, Sammel M, Hummel A, et al. Risk factors and clinical features of recurrent ectopic pregnancy: A case control study. Fertil Steril. 2003;80:1340-1344.
134 Ego A, Subtil D, Cosson M, et al. Survival analysis of fertility after ectopic pregnancy. Fertil Steril. 2001;75:560-566.
135 Tulandi T. Reproductive performance of women after two tubal ectopic pregnancies. Fertil Steril. 1998;50:164-166.
136 Korell M, Albrich W, Hepp H. Fertility after organ-reserving surgery of ectopic pregnancy: Results of a multicenter study. Fertil Steril. 1997;68:220-223.
137 Bangsgaard N, Lund CO, Ottesen B, Nilas L. Improved fertility following conservative surgical treatment of ectopic pregnancy. BJOG. 2003;110:765-770.
138 Vermesh M, Presser SC. Reproductive outcome after linear salpingostomy for ectopic pregnancy: A prospective 3-year follow-up. Fertil Steril. 1992;57:682-684.
139 Dias Pereira G, Hajenius PJ, Mol BWJ, et al. Fertility outcome after systemic methotrexate and laparoscopic salpingostomy for tubal pregnancy. Lancet. 1999;353:724-725.
140 Fernandez H, Yves Vincent SCA, Pauthier S, et al. Randomized trial of conservative laparoscopic treatment and methotrexate administration in ectopic pregnancy and subsequent fertility. Hum Reprod. 1998;13:3239-3242.