CHAPTER 31 Ectopic Ossification About the Elbow
INTRODUCTION
Development of pathologic formation about the elbow takes on several forms including heterotopic ossification, myositis ossificans, and periarticular calcification. Heterotopic ossification refers to the formation of mature lamellar bone in nonosseous tissue. Heterotopic ossification and ectopic bone are terms that are used interchangeably for this type of bone formation. Myositis ossificans refers to abnormal formation of bone in inflammatory muscle.1 Myositis ossificans also consists of mature lamellar bone and is distinguished from ectopic bone only by its location.
Calcification about the elbow is distinctly different from ossification. Calcific deposits typically consist of calcium pyrophosphate and do not contain mature bone. These are usually amorphous globular deposits and have no trabecular structure. Calcific deposits may occur in tendon, synovium, capsular tissue, and ligaments. Calcium deposits are most commonly seen in ligamentous tissue after elbow trauma.10,12,51 Elbow motion is rarely affected to any significant degree.
ETIOLOGY
TRAUMATIC CONDITIONS THAT MAY LEAD TO ECTOPIC BONE FORMATION
Thompson and Garcia84 documented the incidence of ectopic bone formation in a series of more than 1200 traumatic elbow conditions: 3% in simple dislocations, 20% in elbow dislocations associated with radial head fractures, and 16% in elbow dislocations associated with other fractures. Although the definition of what constitutes ectopic bone is not clear, this report does serve as a worthwhile benchmark of the relative incidence among these three injury types.
Elbow Dislocations
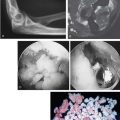
Patients with simple dislocations without fracture are less likely to develop ectopic bone formation than are those with associated fractures.72,73 Linscheid and Wheeler51 noted an incidence of some radiographic density in about 30% in a series of 110 elbow dislocations. True ectopic bone formed in the anterior capsule in five patients (4.5%), and most had calcification in the collateral ligaments below the medial and lateral epicondyles (Fig. 31-1). Josefsson and colleagues42 found true ectopic bone formation in only 1 of 52 elbow dislocations (1.6%). Periarticular calcification, however, was noted in 38 patients (76%). Eleven of the 12 patients in whom there was no evidence of periarticular calcification had sustained their dislocations before the age of 16 years.
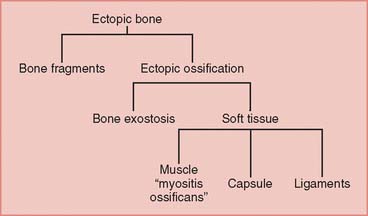
FIGURE 31-1 Distribution of possible radiographic densities about the elbow after trauma.
(Redrawn from Broberg, M. A., and Morrey, B. F.: Results of treatment of fracture-dislocations of the elbow. Clin. Orthop. Rel. Res. 216:111, 1987.)
The incidence of ectopic bone formation increases about fivefold when an elbow dislocation is associated with a radial head fracture.84 McLaughlin55 listed several principles to reduce the formation of ectopic bone: (1) excision of the radial head within 24 hours of injury, (2) complete removal of the radial head with all the fracture fragments, (3) avoidance of the formation of bone dust within the operative bed at the time of radial head excision, (4) careful hemostasis, and (5) avoidance of hematoma formation. There is little scientific evidence to support these recommendations, but they seem reasonable.
Radial Head Fractures
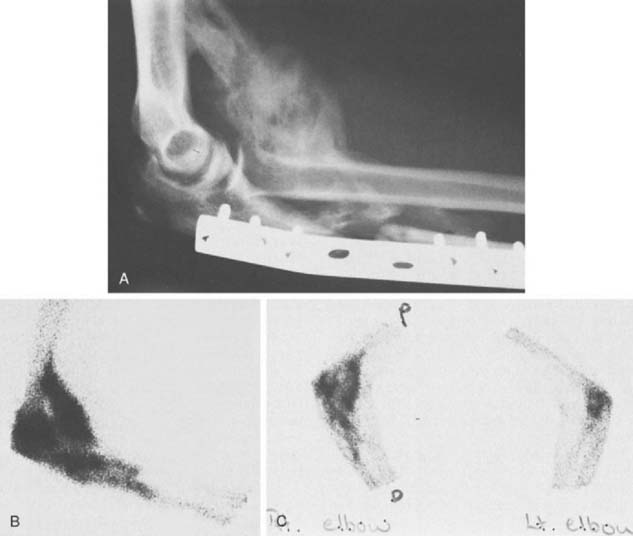
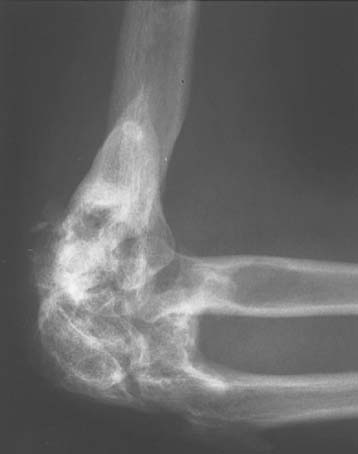
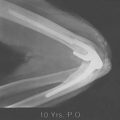
Ectopic bone formation may also occur following isolated fractures of the radial head. Patients with Mason type III comminuted fractures are probably at the greatest risk.52 In a study of 60 cases, Mikic and Vukadinovic56 noted ossification about the elbow in 32 (56%). In the 18 patients with more extensive amounts of bone, restriction of elbow motion was seen in all. In 31 (52%) of these patients, there was significant regrowth of bone at the level of resection of the radius, as noted by others.48,81 We have observed a rather consistent pattern of ectopic bone emanating from the resected radius in this clinical circumstance (Fig. 31-2).
Timing of Surgical Treatment with Elbow Trauma
We have observed that patients with open injuries undergoing several surgical procedures performed during the first 7 to 14 days of injury are at particular risk (Fig. 31-3). In McLaughlin’s series of radial head fractures,55 12% who underwent excision within 24 hours had ectopic bone formation, compared with 38% who had delayed excision.
In a study of 14 patients with fracture-dislocations of the elbow treated by either partial or complete resection of the radial head, Broberg and Morrey8 reported an incidence of significant ectopic bone formation of only 9%. The single patient with significant heterotopic ossification had undergone excision of the radial head 8 days after injury, and the results of treatment were graded as fair with continued elbow pain.
The issue of delay before definitive surgery for fracture dislocation was initially emphasized by McLaughlin.55 This opinion has been recently strengthened by the work of Ilahi and colleagues.41 Among 71 consecutive elbow injuries of various types, none developed significant ectopic bone when operated on within 48 hours. On the other hand, 8 of 24 (33%) did enounter ectopic bone formation if treatment was delayed more than 48 hours.
Post-Traumatic Radioulnar Synostosis
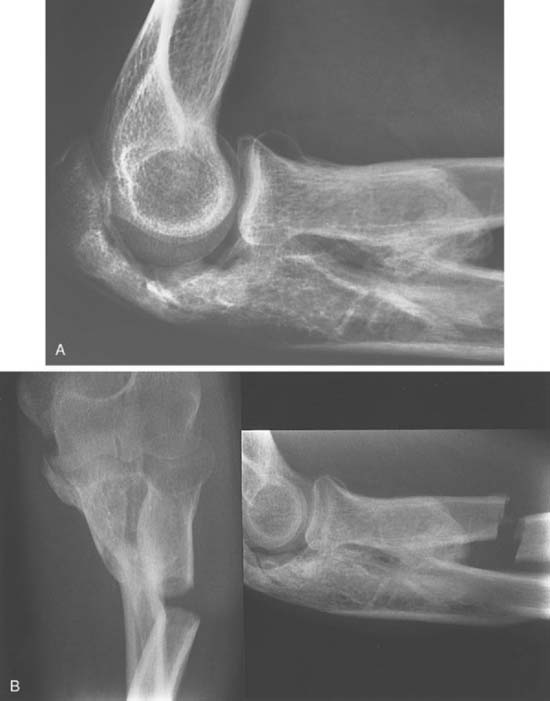
Cross-union from ectopic bone may be caused by fractures,5,11,23,65,75,85 or soft tissue injury7,60,70 of the forearm. When an associated elbow injury has occurred, the synostosis may be extensive (Fig. 31-4).
Vince and Miller86 classified synostoses of the forearm by location: type I, distal third; type II, middle third; and type III, proximal third. Two of three patients with proximal synostoses who were treated surgically had unsatisfactory results because of recurrence of the synostosis. Failla and associates22 reported a series of 20 patients from the Mayo Clinic who underwent excision, with only 7 patients achieving good or excellent results and 13 patients achieving fair or poor results. The timing of synostosis excision appeared to be important, with no good or excellent results in patients operated on less than 12 months after injury or more than 3 years after injury. We use the radiographic appearance of discrete margins and mature trabeculation as the basis of when to resect the lesion. Bone scans and serum enzymes are of little value in deciding when to resect the lesion, and hence, we do not obtain these studies.
Jupiter and Ring43 subclassified type III forearm synostosis into three types based on the location of theectopic bone. Type III A ectopic ossification is located at or distal to the bicipital tuberosity, Type III B involves the radial head and/or proximal ulnar joint, and Type III C refers to a proximal radial ulnar synostosis contiguous with ectopic bone extending across the elbow to the distal humerus. Eighteen patients underwent operative excision of post-traumatic proximal radioulnar synostosis. Recurrence was seen in only one patient. The 17 patients who did not experience a recurrence regained an average of 139 degrees of forearm rotation. No significant relationship between postoperative forearm rotation and synostosis size, the use of interpositional fat, or the concomitant use of a hinged elbow distractor was identified.43
The surgical treatment of proximal radioulnar synostosis has traditionally been viewed with pessimism. This view has been based on very little published data. The importance of forearm rotation to patient function is well established, and recent experience demonstrates that surgical excision is warranted and is likely to be successful.43
An alternative surgical approach to improve forearm rotation in this difficult clinical setting is proximal radial resection as described by Kamenini and Morrey44 (Fig. 31-5). Indications include (1) extensive synostosis not amenable to a safe and discrete resection, (2) articular surface involvement, and (3) associated anatomic deformity. A Kocher approach is recommended to explore the synostosis and the radius distal to the synostosis. One centimeter of bone is excised from the radius, and the remaining exposed bone is covered with bone wax. Postoperative arc of forearm rotation averages 98 degrees. Reankylosis occurred at the site of resection in one patient.
CLASSIFICATION
There are several discrete clinical circumstances in which ectopic bone develops around the elbow: (1) trauma, usually fracture; (2) closed head or spinal cord injury; (3) burn injury to the extremity; and (4) genetic conditions (Box 31-1). Recently, the process has also been reported to occur after adult respiratory distress syndrome37 and after orthotopic liver transplant.62
BOX 31-1 Patients at Risk for Developing Ectopic Bone About the Elbow
Radiographically, ectopic bone is seldom seen before 3 weeks after injury or surgery, but it usually can be detected if one looks for it critically. The soft density on the radiograph can be visualized under a bright light. The extent of ectopic bone formation usually is evident by 12 weeks (Fig. 31-6). Bressler and colleagues9 studied the maturation process of ectopic bone in 25 CT scans. Persistent, unossified, low-density soft tissue areas were detected adjacent to mineralized areas up to 16 years after injury. In adults, after the maturation process is completed, the bone usually does not resorb. Resorption may occur in children younger than 16 years. Ectopic ossification may be classified by its anatomic formation site or its functional affect on elbow motion and forearm rotation.
ANATOMIC CLASSIFICATION
Ectopic bone may develop in essentially any tissue about the elbow. Common anatomic location of the abnormal bone varies with etiology. In post-traumatic situations, posterolateral elbow is most often involved. A bone bridge often develops between the lateral condyle of the humerus and the posterolateral olecranon. Bone may also fill the olecranon fossa. A second common location in traumatic cases involve the anterolateral compartment of the elbow. Anterior bone may extend from the distal humerus to the radius and ulna at the level of the bicipital tuberosity. The coronoid is frequently enlarged and blocks elbow flexion because the coronoid fossa cannot accept the volume increase.87
Neurogenic ectopic ossification most commonly develops anteriorly in the flexor muscles or posteriorly in the extensors. Ossification tends to occur with the muscle and follows a single plane. This contrasts to post-traumatic situations in which multiple planes are frequently involved.6,87
FUNCTIONAL CLASSIFICATION
Hastings and Graham38 have developed the classification based on functional range of motion of the elbow and forearm rotation. Class I ectopic bone refers to the radiographic appearance of abnormal bone without any functional limitations. This should be documented because it signifies a patient’s tendency toward heterotopic bone formation. Prophylactic treatment may be warranted in these patients.
INCIDENCE OF ECTOPIC BONE FORMATION
ECTOPIC BONE FORMATION FOLLOWING CENTRAL NERVOUS SYSTEM INJURY
First described during the First World War,16 ectopic bone formation may occur after central nervous system (CNS) injury to the brain or spinal cord.29,72 Garland and others29,35,40 extensively studied the orthopedic problems encountered by patients with both traumatic brain injury and spinal cord injury (see Chapter 72).
Traumatic Brain Injury without Elbow Trauma
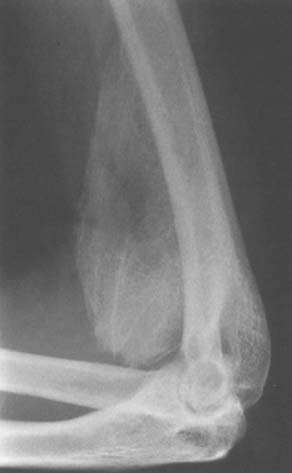
In a review of 496 patients with traumatic brain injury, Garland and colleagues32,33 found an incidence of periarticular bone formation in 100 joints in 57 patients (11%). Patients with spastic quadriparesis have the highest incidence of ectopic bone formation. The hip was the most common location, followed by the shoulder, elbow, and knee. Ectopic bone formation developed in 4% of the elbows. Anterior bone formation was deep to the biceps and brachialis muscles and anterior to the joint capsule, occasionally involving the entire brachialis muscle (Fig. 31-7). The posterior ectopic bone was beneath the triceps tendon in close association to the posterior capsule. In Garland’s series,32 the ectopic bone was anterior in six and posterior in 17, and eight of the elbows were completely ankylosed preoperatively. Posterolateral ectopic bone formation is the most common site of occurrence.30
A major determinant of potential successful surgical treatment is the residual neurologic deficit. Twenty-three patients underwent resection of ectopic bone about the elbow. In general, resection should be delayed until at least 18 months after CNS injury to allow maximum functional recovery. The results of excision correlate with the neurologic classification.32 Complications include soft tissue infections and neurologic injury. Recurrence is closely associated with spasticity.30
Traumatic Brain Injury with Associated Elbow Trauma
The incidence of heterotopic ossification about the elbow dramatically increases in patients with fracture-dislocations of the elbow and concomitant head injury. In one study,46 ectopic bone developed about the elbow in greater than 90% of patients with this injury complex. Routine prophylaxis in this patient population is logical, although studies demonstrate its efficacy.
ECTOPIC BONE FORMATION IN BURN PATIENTS
Significant ectopic bone formation in burn patients is a rare occurrence. Evans20,21 listed the following risk factors: (1) the percentage area, (2) the location of the burn, (3) the length of bed confinement, (4) osteoporosis, (5) superimposed trauma, and (6) genetic predisposition. The elbow, shoulder, and hip are the most commonly involved.20,21 In a study of more than 5000 cases at the U.S. Army Institute of Surgical Research, the incidence of ectopic bone formation was only 1.2%, with 82.5% of those cases involving the elbow. This is similar to the recent report of a 1.2% incidence after 1478 burns, of which the elbow is the most commonly involved joint.67 Although several studies have reported an incidence of ectopic bone formation varying from 2 to 35 percent,20,21,63,76,82 the incidence of significant ectopic bone formation at the elbow that requires treatment is probably about 1%.21
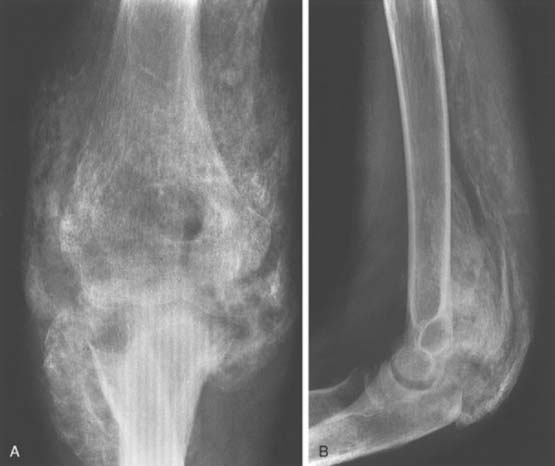
The distribution of the ectopic bone about the elbow in the burn patient is posterior and medial along the medial border of the triceps and anterior in the plane of the brachialis from the anterior surface of the humerus to the coronoid (Fig. 31-8).21
Evans21 and others19 have noted that prevention of ectopic bone in the burn patient is best accomplished by reducing the period of bed confinement and the period of postburn hypermetabolic state through the use of early wound excision and grafting. If ectopic bone formation does occur, passive stretching of the joint should be avoided. Active range of motion exercises of the joint within a pain-free arc may continue.15
Results of excision of heterotopic ossification about the elbow in children with burns was recently reported.36 All patients experienced an improvement in their arc of motion (average increase of 57 degrees), and all patients were able to reach the face and perineum after their operative procedure. No recurrent heterotopic bone was noted.
Selection criteria for excision include (1) decreased range of motion sufficient to cause functional limitations, (2) maturation of new bone confirmed on the radiograph, (3) no evidence of acute inflammation, and (4) complete healing of the skin in the area of the ectopic bone. After excision, most patients have functional range of motion arcs and recurrence is uncommon.15,39 The most common complication is ulnar nerve irritation.18
Technical factors include anterior transposition of the ulnar nerve, excision of the ectopic bone and collateral ligaments if they are ossified, and excision of the radial head when forearm rotation is limited.20 In the senior author’s (Bernard F. Morrey, MD) experience, however, radial humeral involvement very rarely occurs. If the anterior aspect of the elbow is significantly scarred by third-degree burns, excision of the scarred areas with release of the contractures and excision of the ectopic bone is recommended.17 In these complex situations, plastic surgical consultation and assistance should be considered.
FIBRODYSPLASIA OSSIFICANS PROGRESSIVA
Genetic conditions may lead to ectopic ossification about the elbow. Fibrodysplasia ossificans progressiva is a rare genetic disorder characterized by progressive soft tissue ossification. The etiology is a defect in the induction of enchondral osteogenesis.45 More than half of patients with this disorder experience ectopic bone formation about the elbow between the third and fourth decades of life. Family history and a history of prior injury causing exuberant heterotopic ossification suggests this diagnosis. Connor and Evans13 and others74 have recommended against surgical treatment. Massive amounts of ectopic bone form after surgical intervention and result in predictably poor outcomes. Avoidable factors for the precipitation of ectopic bone in these patients include local trauma, careless venipuncture, intramuscular injections, biopsy of the lumps, and operations to excise heterotopic bone.
CLINICAL PRESENTATION
Ectopic ossification about the elbow typically begins to develop approximately 2 weeks following the inciting event. Patients will often experience swelling, warmth, and pain.64 Motion loss may also begin to be evident at this time. Early presentation may be confused with cellulitis, deep infection, reflex sympathetic dystrophy, and thrombophlebitis. Infection must be considered and ruled out, especially in patients who have presented with an open injury.
DIAGNOSTIC STUDIES
LABORATORY STUDIES
Several laboratory variables have been studied in an effort to identify high-risk patients. An increased incidence of the human leukocyte antigen B18 in a group of patients with central nervous system injury and paraosteoarthropathy has been reported.31,40,50,57,78
Unfortunately, the predictive value of serum alkaline phosphatase determinations has not been consistent and the utility of the test in clinical practice is limited.2,28,49,58,64 The test lacks specificity, and, in most cases, the ideal time to initiate prophylactic treatment may have passed. Serum alkaline phosphatase levels are not predictive of bone maturation and are no longer used to determine surgical timing.
RADIOGRAPHIC EVALUATIONS
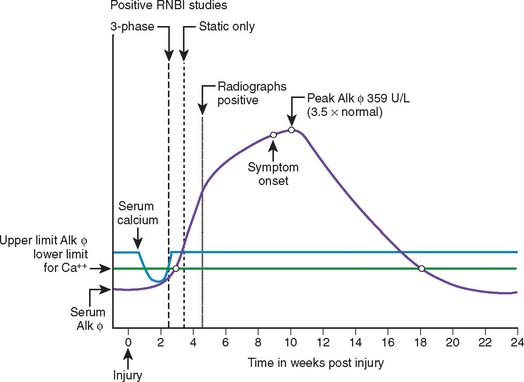
The technetium bone scan is markedly positive during the formation of ectopic bone and usually remains positive for a prolonged time (Fig. 31-9).27
The temporal relation between positive technetium bone scans, serum alkaline phosphatase determinations, radiographs, and the clinical presentation of ectopic bone formation was well illustrated by Orzel and Rudd64 in a study of 50 patients with a variety of injuries (spinal cord trauma, traumatic brain injury, extremity trauma, and so forth). Both the technetium bone scan and serum alkaline phosphatase determinations were abnormal before either the clinical onset or radiographic detection of ectopic bone formation (see Fig. 31-6).
Bone scans and magnetic resonance imaging (MRI) scans are not routinely needed in the evaluation of the stiff elbow. Pittinger68 believed that ectopic ossificationshould not be excised before 12 months after onset. This was based on the belief that excision of active ectopic bone would lead to recurrence. However, more recent studies dispute this claim. Jupiter43 and McAuliffe53 have shown that active ectopic ossification about the elbow can be excised without an increase in recurrence.
GENERAL PRINCIPLES OF TREATMENT
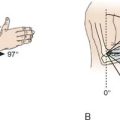
Not all ectopic bone formation about the elbow requires surgical treatment. Ectopic ossification that does not produce symptoms or interfere with the functional arc of elbow rotation should be treated without surgery. The goal of treatment is not, and should not be, normal motion. The main indication for surgical management is ectopic bone producing an arc of elbow motion that is less than functional and limits the patient’s activities of daily living or interferes with occupational or recreational pursuits. Morrey et al61 has shown that a functional arc of motion about the elbow includes a flexion-extension arc of 30 to 130 degrees and forearm rotation of 50 degrees of pronation and supination. Ninety percent of activities of daily living can be performed within this motion arc.
General principles include (1) excision of all motion-limiting heterotopic bone; (2) avoiding articular cartilage damage; (3) atraumatic tissue handling; (4) careful hemostasis; (5) suction drainage of the wound; (6) avoiding the creation and deposit of bone dust in the joint by the use of osteotomes rather than saws; (7) meticulous lavage; (8) avoiding neurologic injury; and (9) early postoperative range of motion. Careful preoperative planning is crucial to identify areas to be resected and the appropriate and safe surgical approach to access all involved heterotopic bone.
Timing of Surgical Treatment
The appropriate timing of surgical intervention remains controversial; however, the trend is toward earlier surgical excision of function-limiting ectopic ossification about the elbow. As previously discussed, recent articles have demonstrated no evidence of increased recurrence rates when early excision is performed.38,43,53 Various methods of postoperative prophylaxis were used in this study (including no prophylaxis). Long surgical delays are not without risks. Severe limitation of elbow rotation is extremely disabling and persistent contracture may lead to articular cartilage destruction. Many surgeons now consider a 3- to 4-month interval adequate and proceed with surgical excision at that time. The senior author still prefers to wait at least 6 months.
Indications to Interpositional Arthroplasty and Total Elbow Arthroplasty
Concomitant interpositional arthroplasty is indicated in a younger patient with coexisting articular surface damage representing greater than 50% of the joint surface.59 This same scenario in an older patient or more sedentary patient, is probably best managed with total elbow replacement. The age cutoff for these procedures is controversial.66
ULNAR NEUROPATHY IN ASSOCIATION WITH ECTOPIC BONE FORMATION
Late ulnar neuropathy may occur as a result of compression in the cubital tunnel from ectopic bone. Sometimes when completely encased in bone, it is further at risk if elbow motion increases while it remains tethered or compressed by the ectopic ossification. Although ulnar neuropathy from ectopic bone is most often found in the brain-injured adult,32,34,47,89 it occurs after burns88 and trauma as well. In a 5-year period, 2.5% of the adult brain-injured population in one study developed late ulnar neuropathy.47 Fourteen percent had a history of trauma, and 86% were found to have idiopathic heterotopic ossification associated with spasticity. Treatment consisted of ulnar nerve transposition anteriorly, with 85% of the patients having complete recovery. We completely free the nerve from the ectopic bone for a distance of 3 cm with preservation of function. Simple subcutaneous translocation of the nerve is adequate. If completely surrounded by bone, I usually release the nerve, remove the ectopic bone and replace it in its bed, or translocate the nerve if it is stretched by motion.
SURGICAL TECHNIQUES
Surgical Approach
For the posterolateral resection, the triceps mechanism is retracted medially without disturbing its insertion, and the ectopic bone is exposed subperiosteally. The central bridge of ectopic bone is resected initially. The elbow is then flexed, and attachments of the ectopic bone to the humerus and olecranon are removed. Anterior capsule release is not necessary. Varying amounts of the olecranon are excised to reduce olecranon impingement. The posterior bar is the easiest to resect and, in our experience, has an excellent prognosis.
POSTOPERATIVE MANAGEMENT
If the process involves muscle fibers, the surgical field is treated with 700 cGy radiation. Otherwise, 75 mg indomethacin is prescribed 3 weeks before and 8 weeks after surgery. The elbow is managed with continuous motion and splints as described in Chapters 10 and 11. When motion goals are not met, manipulation under anesthesia is performed 6 weeks after surgery.
The use of postoperative radiation remains controversial. Gaur et al36 found no recurrence in children with burns as the etiology. Jupiter and Ring43 found no recurrence in 17 patients in whom post-traumatic radioulnar synostoses were excised. No prophylaxis was used in either patient group. However, Jupiter and Ring43 later reported on surgical treatment of complete anklyosis about the elbow and found recurrences in one third of the trauma cohort and one patient in the burn cohort. The authors now use prophylactic radiation routinely. Prospective randomized studies are needed to further define the use of this modality.
ADJUVANT TREATMENT TO PREVENT ECTOPIC BONE FORMATION
Several authors have shown that indomethacin is an effective agent54,71,77 that significantly reduces the formation of ectopic bone about the hip. The recommended dosage is 75 mg daily for 6 weeks after surgery.54,71,77 We typically begin the treatment 1 to 2 weeks before the surgery but have no scientific basis for this practice.
Oral diphosphonates have been used,24,79,83 and experimental data have shown this class of drug to delay the mineralization of osteoid.69 Unfortunately, when these drugs are discontinued, the osteoid may mineralize.77,80
Low-dose external beam irradiation has been shown to be an effective method of preventing ectopic bone formation about the hip after total hip arthroplasty3,4,14,49 and following acetabular fractures.6 Recent studies noted effective control of ectopic bone with only 700 cGy in a single dose.3,4 External beam irradiation should be delivered to the high-risk patient within 24 hours and no later than 72 hours after surgery. Delay of radiation treatments beyond 72 hours significantly reduces its effectiveness. Potential or theoretical problems of low-dose irradiation include wound healing problems and nonunion. The risk of postirradiation sarcoma is extremely rare. In the past 10 years, we have treated more than 100 patients with low-dose irradiation for control of ectopic bone formation about the elbow and hip, and we have never detected delayed wound healing that was attributable to the low-dose irradiation. When necessary, bone graft fracture sites may be shielded.25 In our experience, there have been no nonunions directly attributable to the low-dose irradiation. There are more than 130 postirradiation sarcomas in the Mayo Clinic files, and none has been caused by the use of low-dose irradiation for the prevention of ectopic bone.26 We have found no instance of sarcoma to have developed after doses of 3000 cGy or less.
1 Ackerman L.V. Extraosseous localized nonneoplastic bone and cartilage formation (so-called myositis ossificans). J. Bone Joint Surg. 1958;40A:279.
2 Andersen P.K., Pedersen P., Kristensen S.S., Schmidt S.A., Pedersen N.W. Serum alkaline phosphatase as an indicator of heterotopic bone formation following total hip arthroplasty. Clin. Orthop. Rel. Res. 1988;234:102.
3 Ayers D.C., Evarts C.M., Parkinson J.R. The prevention of heterotopic ossification in high-risk patients by low-dose radiation after total hip arthroplasty. J. Bone Joint Surg. 1986;68A:1423.
4 Ayers D.C., Pellegrini V.D., Evarts C.M. Prevention of heterotopic ossification in high-risk patients by radiation therapy. Clin. Orthop. Rel. Res. 1991;263:87.
5 Benjamin A.. Injuries of the forearm. Wilson J.N., editor. Watson-Jones Fractures and Joint Injuries, 6th ed., vol. 2. New York: Churchill Livingstone, 1982;650.
6 Bosse M.J., Poka A., Reinert C.M., Ellwanger F., Slawson R., McDevitt E.R. Heterotopic ossification as a complication of acetabular fracture. J. Bone Joint Surg. 1988;70A:1231.
7 Botting T.D.J. Posttraumatic radioulnar cross union. J. Trauma. 1970;10:16.
8 Broberg M.A., Morrey B.F. Results of delayed excision of the radial head after fracture. J. Bone Joint Surg. 1986;68A:669.
9 Bressler E.L., Marn C.S., Gore R.M., Hendrix R.W. Evaluation of ectopic bone by CT. Am. J. Roentgenol. 1987;148:931.
10 Broberg M.A., Morrey B.F. Results of treatment of fracture-dislocations of the elbow. Clin. Orthop. 1987;216:109.
11 Bunnell S. Surgery of the Hand, 2nd ed., Philadelphia: J. B. Lippincott; 1948:591.
12 Buxton J.D. Ossification in the ligaments of the elbow joint. J. Bone Joint Surg. 1938;20:709.
13 Connor J.M., Evans D.A.P. Fibrodysplasia ossificans progressiva: the clinical features and natural history of. 34 patients. J. Bone Joint Surg.. 1982;64B:76.
14 Coventry M.B., Scanlon P.W. The use of radiation to discourage ectopic bone. J. Bone Joint Surg. 1981;63A:201.
15 Crawford C.M., Varghese G., Mani M., Neff J.R. Heterotopic ossification: are range-of-motion exercises contraindicated? J. Burn Care Rehabil. 1986;7:323.
16 Dejerine A., Ceiller M.A. Paraosteoarthropathies of paraplegic patients by spinal cord lesion. Clin. Orthop. Rel. Res. 1991;263:3.
17 Dias D.A. Heterotopic para-articular ossification of the elbow with soft tissue contracture in burns. Burns. 1983;9:128.
18 Djurickovic S., Meek R.N., Snelling C.F., Broekhuyse H.M., Blachut P.A., O’Brien P.J., Boyle J.C. Range of motion and complications after post burn heterotopic bone excision about the elbow. J. Trauma. 1996;41:825.
19 Elledge E.S., Smith A.A., McManus W.F., Pruitt B.A. Heterotopic bone formation in burned patients. J. Trauma. 1988;28:684.
20 Evans E.B. Orthopaedic measures in the treatment of severe burns. J. Bone Joint Surg. 1966;48A:643.
21 Evans E.B. Heterotopic bone formation in thermal burns. Clin. Orthop. Rel. Res. 1991;263:94.
22 Failla J.M., Amadio P.C., Morrey B.F. Posttraumatic proximal radioulnar synostosis: Results of surgical treatment. J. Bone Joint Surg. 1989;71A:1206.
23 Fielding J.W. Radioulnar crossed union following displacement of the proximal radial epiphysis: A case report. J. Bone Joint Surg. 1964;46A:1277.
24 Finerman G.A.M., Krengel W.F., Lowell J.D. Role of diphosphonate (EHDP) in the prevention of heterotopic ossification after total hip arthroplasty: A preliminary report. In: The Hip: Proceedings of the Fifth Open Scientific Meeting of the Hip Society. St. Louis: C. V. Mosby; 1977:222.
25 Frassica F.J., Coventry M.B. Ectopic bone following total hip arthroplasty. In: Morrey B., editor. Joint Replacement Arthroplasty. New York: Churchill Livingstone; 1991:867.
26 Frassica F.J., Sim F.H., Frassica D.A., Wold L.E. Survival and management considerations in postradiation osteosarcoma and Paget’s osteosarcoma. Clin. Orthop. Rel. Res. 1991;263:200.
27 Freed J.H., Hahn H., Meneter R., Dillon T. The use of the three phase bone scan in the early diagnosis of heterotopic ossification (HO) and in the evaluation of didronel therapy. Paraplegia. 1982;20:208.
28 Furman R., Nicholas J.J., Jovoff L. Elevation of the serum alkaline phosphatase coincident with ectopic bone formation in paraplegic patients. J. Bone Joint Surg. 1970;52A:1131.
29 Garland D.E. A clinical perspective on common forms of acquired heterotopic ossification. Clin. Orthop. Rel. Res. 1991;263:13.
30 Garland D.E. Surgical approaches for resection of heterotopic ossification in traumatic brain-injured adults. Clin. Orthop. Rel. Res. 1991;263:59.
31 Garland D.E., Alday B., Venos K.G. Heterotopic ossification and HLA antigens. Arch. Phys. Med. Rehabil. 1984;65:531.
32 Garland D.E., Blum C., Waters R.L. Periarticular heterotopic ossification in head injured adults: Incidence and location. J. Bone Joint Surg. 1980;62A:1143.
33 Garland D.E., Hanscom D.A., Keenan M.A., Smith C., Moore T. Resection of heterotopic ossification in the adult with head trauma. J. Bone Joint Surg. 1985;67A:1261.
34 Garland D.E., O’Halloren R.M. Fractures and dislocations about the elbow in the head injured adult. Clin. Orthop. Rel. Res. 1982;168:38.
35 Garland D.E., Orwin J.F. Resection of heterotopic ossification in patients with spinal cord injuries. Clin. Orthop. Rel. Res. 1989;242:169.
36 Gaur A., Sinclair M., Caruso E., Peretti G., Zaleske D. Hetertopic ossification around the elbow following burns in children: Results after excision. J. Bone Joint Surg. 2003;85A:1538.
37 Goodman T.A., Merkel P.A., Perlmutter G., Doyle M.K., Krange S.M., Polisson R.P. Heterotopic ossification in the setting of neuromuscular blockade. Arthritis Rheum. 1997;40:1619.
38 Hastings H., Graham T.J. The classification and treatment of heterotopic ossification about the elbow and forearm. Hand Clinic. 1994;102:417-437.
39 Hoffer M.M., Brody G., Ferlic F. Excision of heterotopic ossification about the elbows in patients with thermal injury. J. Trauma. 1978;18:667.
40 Hunter T., Dubo H.I.C., Hildahl C.R., Smith N.J., Schroeder M.L. Histocompatibility antigens in patients with spinal cord injury or cerebral damage complicated by heterotopic ossifications. Rheum. Rehabil. 1980;19:97.
41 Ilahi O.A., Strausser D.W., Gabel G.T. Post-traumatic heterotopic ossification about the elbow. Orthopedics. 1998;21:265.
42 Josefsson P.O., Johnell O., Gentz C.F. Long-term sequelae of simple dislocation of the elbow. J. Bone Joint Surg. 1984;66A:927.
43 Jupiter J.B., Ring D. Operative treatment of posttraumatic proximal radioulnar synostosis. J. Bone Joint Surg. 1998;80A:248.
44 Kamineni S., Maritz N.G., Morrey B.F. Proximal radial resection for posttraumatic radial synostosis: A new technique to improve forearm rotation. J. Bone Joint Surg. 2002;84A:745.
45 Kaplan F.S., Tabas J.A., Gannon F.H., Finkel G., Hahn G.V., Zasloff M.A. The histopathology of fibrodysplasia ossifican progressive. An enchondral process. J. Bone Joint Surg. 1993;75A:220.
46 Keenan M.A.E., Haider T. The formation of heterotopic ossification after traumatic brain injury: A biopsy study with ultrastructural analysis. J. Head Trauma Rehabil. 1996;11:8-22.
47 Keenan M.A., Kauffman D.L., Garland D.E., Smith C. Late ulnar neuropathy in the brain-injured adult. J. Hand Surg. 1988;13A:120.
48 King B.B. Resection of the radial head and neck: An end result of thirteen cases. J. Bone Joint Surg. 1939;21:839.
49 Klein L., Van Den Noort S., Dejak J.J. Sequential studies of urinary hydroxyproline and serum alkaline phosphatase in acute paraplegia. Med. Serv. J. Can. 1966;22:524.
50 Larson J.M., Michalski J.P., Collacott E.A., Eltorai D., McCombs C.C., Madorsky J.B. Increased prevalence of HLAB27 in patients with ectopic ossification following traumatic spinal cord injury. Rheum. Rehabil. 1981;20:193.
51 Linscheid R.L., Wheeler D.K. Elbow dislocations. J. A. M. A. 1965;194:1171.
52 Mason M.L. Some observations on fractures of the head of the radius with a review of 100 cases. Br. J. Surg. 1954;42:123.
53 McAuliffe J.A., Woitson A. Early excision of heterotopic ossification about the elbow followed by radiotherapy. J. Bone Joint Surg. 1997;79A:749-755.
54 McLaren A.C. Prophylaxis with indomethacin for heterotopic bone after open reduction of fractures of the acetabulum. J. Bone Joint Surg. 1990;72A:245.
55 McLaughlin H.L. Some fractures with a time limit. Surg. Clin. North Am. 1955;35:553.
56 Mikic Z.D., Vukadinovic S.M. Late results in fractures of the radial head treated by excision. Clin. Orthop. Rel. Res. 1983;181:220.
57 Minaire P., Betuel H., Girard R., Pilonchery G. Neurologic injuries, paraosteoarthropathies, and human leukocyte antigens. Arch. Phys. Med. Rehabil. 1980;61:214.
58 Mollan R.A.B. Serum alkaline phosphatase in heterotopic para-articular ossification after total hip replacement. J. Bone Joint Surg. 1979;61B:423.
59 Morrey B.F. Posttraumatic stiffness: Distraction arthroplasty. Orthopedics. 1992;15:863-869.
60 Morrey B.F., Askew L.J., An K.N., Dobyns J.H. Rupture of the distal tendon of the biceps brachii: A biomechanical study. J. Bone Joint Surg. 1985;67A:418.
61 Morrey B.F., Askew L.J., Chao E.Y. A biomedical study of functional elbow motion. J. Bone Joint Surg. 1981;63A:872-877.
62 Munin M.C., Balu G., Sotereanos D.G. Elbow complications after organ transplantation. Case reports. Am. J. Phys. Med. Rehab. 1995;74:672.
63 Munster A.M., Bruck H.M., Johns L.A., von Prince K., Kikman E.M., Remig R.L. Heterotopic calcification following burns: A prospective study. J. Trauma. 1973;12:1071.
64 Orzel J.A., Rudd T.G. Heterotopic bone formation: Clinical, laboratory, and imaging correlation. J. Nucl. Med. 1985;26:125.
65 Newman J.H. Displaced radial neck fractures in children. Injury. 1977;9:114.
66 Peden, J. P: Total elbow arthroplasty for the management of the ankylosed or fused elbow. J. Bone Joint Surg. [Br.] (Accepted for publication, 2008)
67 Peterson S.L., Mani M.M., Crawford C.M., Neff J.R., Hiebert J.M. Post burn heterotopic ossification: insights for management decision making. J. Trauma. 1989;29:365.
68 Pittinger D.E. Hetertopic ossification. Orthop. Rev.. 1991;20:33.
69 Plasmans C.M.T., Kuypers E.I.M., Sloof T.J.J.H. The effect of ethane-1-hydroxy-1, l diphosphonic acid (EHDP) on matrix-induced ectopic bone formation. Clin. Orthop. Rel. Res.. 1978;132:233.
70 Razemon J.P., Decoulx J., Leclair H.P. Les synostoses radiocubitales posttraumatiques de l’adulte. Acta Orthop. Belgica. 1965;31:5.
71 Ritter M.A., Sieber J.M. Prophylactic indomethacin for the prevention of heterotopic bone formation following total hip arthroplasty. Clin. Orthop. Rel. Res.. 1985;196:217.
72 Roberts P.H. Heterotopic ossification complicating paralysis of intracranial origin. J. Bone Joint Surg.. 1968;50B:70.
73 Roberts P.H. Dislocation of the elbow. Br. J. Surg.. 1969;56:806.
74 Rogers J.G., Geho W.B. Fibrodysplasia ossificans progressiva: a survey of forty-two cases. J. Bone Joint Surg.. 1979;61A:909.
75 Russell T.A.. Malunited fractures. Crenshaw A.H., editor. Campbell’s Operative Orthopaedics, 7th ed., vol. 3. St. Louis: C. V. Mosby, 1987;2041.
76 Schiele H.P., Hubbard R.B., Bruck H.M. Radiographic changes in burns of the upper extremity. Radiology. 1972;104:13.
77 Schmidt S.A., Kjaersgaard-Andersen P., Pedersen N.W., Kristensen S.S., Pedersen P., Neilson J.B. The use of indomethacin to prevent the formation of heterotopic bone after total hip arthroplasty. J. Bone Joint Surg. 1988;70A:834.
78 Seignalet J., Moulin M., Pelissier J., Romain M., Bouffeard-Vercelli M., Lapinski H., Roquefeuil B. HLA and neurogenic paraosteoarthropathies. Tissue Antigens. 1983;21:268.
79 Stover S.L., Niemann K.M., Miller J.M. Disodium etidronate in the prevention of postoperative recurrence of heterotopic ossification in spinal cord injury patients. J. Bone Joint Surg. 1976;58A:683.
80 Stover S.L., Niemann K.M., Tullos J.R. Experience with surgical resection of heterotopic bone in spinal cord injury patients. Clin. Orthop Rel. Res. 1991;263:71.
81 Sutro C.J. Regrowth of bone at the proximal end of the radius following resection in this region. J. Bone Joint Surg. 1935;17:867.
82 Tepperman P.S., Hilbert L., Peters W.J., Pritzker K.P.H. Heterotopic ossification in burns. J. Burn Care Rehabil. 1984;5:283.
83 Thomas B.J., Amstutz H.C. Results of the administration of diphosphonate for the prevention of heterotopic ossification after total hip arthroplasty. J. Bone Joint Surg. 1985;67A:400.
84 Thompson H.C., Garcia A. Myositis ossificans: Aftermath of elbow injuries. Clin. Orthop. Rel. Res. 1967;50:130.
85 Tooms R.E.. Complications of treatment of injuries to the forearm. Epps C.H., editor. Complications in Orthopaedic Surgery, 2nd ed., vol. 1. Philadelphia: J. B. Lippincott, 1986;325.
86 Vince K.G., Miller J.E. Cross-union complicating fracture of the forearm. Part 1. Adults. J. Bone Joint Surg.. 1987;69A:640.
87 Viola R., Hastings H. Treatment of ectopic ossification about the elbow. CORR. 2000;370:65-86.
88 Vorenkamp S.E., Nelson T.L. Ulnar nerve entrapment due to heterotopic bone formation after a severe burn. J. Hand Surg. 1987;12A:378.
89 Wainapel S.F., Rao P.U., Schepsis A.A. Ulnar nerve compression by heterotopic ossification in a head-injured patient. Arch. Phys. Med. Rehabil. 1985;66:512.