Drying
Michael E. Aulton
Chapter contents
Fundamental properties and interrelationships
Moisture content of wet solids
Dryers in the pharmaceutical industry
Types of pharmaceutical dryers
Convective drying of wet solids
Conductive drying of wet solids
Radiation drying of wet solids
Dryers for solutions and suspensions
Stages of the freeze-drying process
Solute migration during drying
Consequences of solute migration
Influence of formulation factors on solute migration
Influence of process factors on solute migration
Key points
• The selection of the best drying method for a product is a key decision.
• The phenomenon of solute migration should be minimized during drying processes.
Introduction
Drying is an important operation in primary pharmaceutical manufacture (i.e. the synthesis of active pharmaceutical ingredients or excipients) since it is usually the last stage of manufacturing before packaging. It is important that the residual moisture, say from the final crystallization step, is rendered low enough to prevent product deterioration during storage and ensure free-flowing properties during use. It is equally important (and probably encountered more frequently) in secondary (dosage form) manufacture following the common operation of wet granulation (see Chapter 28) during the preparation of granules prior to tablet compaction. Hence, stability (see Chapter 48 and 49), flow properties (see Chapter 12) and compactability (see Chapter 30) are all influenced by residual moisture.
This chapter is concerned with drying to the ‘dry’ solid state, starting with either a wet solid or a solution or suspension. The former is usually achieved by exposing the wet solid to moving, relatively dry air (elevated temperatures to accelerate the process are common). The latter is possible with equipment such as the spray dryer (see later in this chapter) that is capable of producing a dry product from a solution or suspension in one operation.
Most pharmaceutical materials are not completely free from moisture (i.e. they are not ‘bone dry’) but contain some residual water, the amount of which may vary with the temperature and humidity of the ambient air to which they are exposed. This is discussed in more detail in this chapter.
For the purpose of this chapter, drying is defined as the removal of all or most of the liquid associated with a wet pharmaceutical product. All drying processes of relevance to pharmaceutical manufacturing involve evaporation or sublimation of the liquid phase and the removal of the subsequent vapour. The process must provide the latent heat for these processes without a significant temperature rise. Naturally the latter will enhance the potential of thermal degradation of the product. In the majority of cases the ‘liquid’ will be water but also more volatile organic solvents, such as isopropanol, may need to be removed in a drying process. The physical principles of aqueous or organic solvent drying are similar, regardless of the nature of the liquid, though volatile solvents are normally recovered by condensation rather than being vented into the atmosphere. This is for environmental and economic reasons. Also, the toxicity and flammability of organic solvents pose additional safety and process considerations.
Drying of wet solids
Fundamental properties and interrelationships
An understanding of this operation requires some preliminary explanation of the following important terms. To avoid confusion and repetition, these terms will be defined and explained in the context of water (the most commonly used pharmaceutical solvent) but the explanations and concepts are equally applicable to other relevant liquids (e.g. ethanol, isopropanol, etc.).
Moisture content of wet solids
The moisture content of a wet solid is expressed as kg of moisture associated with 1 kg of the moisture-free or ‘bone-dry’ solid. Thus, a moisture content of 0.4 means that 0.4 kg of water is present per kg of the ‘bone-dry’ solid that will remain after complete drying. It is sometimes calculated as percentage moisture content; thus this example would be quoted as 40% moisture content.
Total moisture content
This is the total amount of liquid associated with a wet solid. Some of this water can be easily removed by the simple evaporative processes employed by most pharmaceutical dryers and some cannot. The amount of easily removable water (unbound water) is known as the free moisture content and the moisture content of the water that is more difficult to remove in practice (bound water) is the equilibrium moisture content. Thus, the total moisture content of a solid is equal to its free moisture content plus its equilibrium moisture content.
Unbound water.
The unbound water associated with a wet solid exists as a liquid and it exerts its full vapour pressure. It can be removed readily by evaporation. During a drying process this unbound water is readily lost but the resulting solid will not be completely free from water molecules as it remains in contact with atmospheric air that inevitably contains dissolved water. Consequently the resulting solid is often known as air dry.
Equilibrium moisture content
As mentioned above, evaporative drying processes will not remove all the possible moisture present in a wet product because the drying solid equilibrates with the moisture that is naturally present in air. The moisture content of a solid under steady-state ambient conditions is termed the equilibrium moisture content. Its value will change with the temperature and humidity of the air, and with the nature of the solid (see later in this chapter).
Bound water.
Part of the moisture present in a wet solid may be adsorbed on surfaces of the solid or be absorbed within its structure to such an extent that it is prevented from developing its full vapour pressure and therefore from being easily removed by evaporation. Such moisture is described as bound water and is more difficult to remove than unbound water. Adsorbed water is attached to the surface of the solid as individual water molecules which may form a mono- (or bi-) layer on the solid surface. Absorbed bound water exists as a liquid but is trapped in capillaries within the solid by surface tension.
Moisture content of air
An added complication to the drying process is that the drying air also contains moisture. Many pharmaceutical plants have air-conditioning systems to reduce the humidity of the incoming process air, but removing water from air is a very expensive process and therefore not all the water will be removed. The moisture content of air is expressed as kg of water per kg of ‘bone-dry’ (water-free) dry air.
The moisture content of air is not altered by changes in its temperature alone, only by changes in the amount of moisture taken up by the air. The moisture content of air should be carefully distinguished from the relative humidity.
Relative humidity (RH) of air
Ambient air is a simple solution of water in a mixture of gases and as such follows the rules of most solutions – such as increased water solubility with increasing temperature, a maximum solubility at a particular temperature (saturation) and precipitation of the solute on cooling (condensation, rain!). Incidentally, this is exactly why rain is sometimes called precipitation.
At a given temperature, air is capable of ‘taking up’ (i.e. dissolving) water vapour until it is saturated (at 100% RH). Lower relative humidities can be quantified in terms of percentage relative humidity, which is given by:
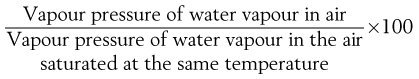 (29.1)
(29.1)
This is approximately equal to the percentage saturation, which is:
 (29.2)
(29.2)
Percentage saturation is the more fundamental measure but the expression ‘relative humidity’ is most commonly used. The two differ only very slightly in practice and only because water vapour does not behave exactly like an ideal gas.
These relationships show that the relative humidity of air is dependent not only on the amount of moisture in the air but also on its temperature. This is because the amount of water required to saturate air is itself dependent on temperature. As mentioned before, in ambient air, water is in solution in the air gases and in this case its solubility increases with increasing temperature. If the temperature of the air is raised whilst its moisture content remains constant, the air will theoretically be capable of taking up more moisture and therefore its relative humidity falls. A re-examination of Equations 29.1 and 29.2 will show this.
It is important to understand the difference between moisture content and relative humidity of air. This is important in many contexts (powder properties, granulation, drying, compaction, storage conditions) but these terms are often confused.
An additional complication to be taken into account is that during a drying process both the temperature and moisture content of the drying air (and therefore its relative humidity) could change significantly. This arises from two separate factors:
Relationship between equilibrium moisture content, relative humidity and the nature of the solid
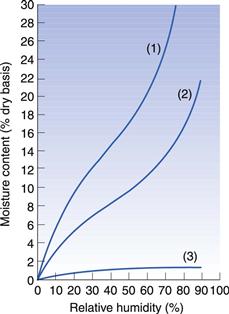
The equilibrium moisture content of a solid exposed to moist air varies with the relative humidity and with the nature of the solid, as shown in some typical plots (Fig. 29.1). If we assume that the atmospheric conditions are of the order of 20 °C and 70–75% relative humidity, a mineral such as kaolin will contain about 1% bound moisture, while a starch-based product may have as much as 30% or more.
Loss of water from wet solids
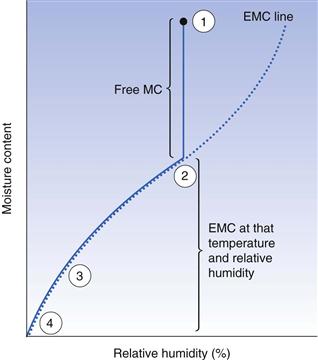
As explained above, unbound water is easily lost by evaporation until the equilibrium moisture content of the solid is reached. This is shown in Figure 29.2. Once the solid reaches its equilibrium moisture content, extending the time of drying will not change the moisture content since an equilibrium situation has been reached. The only way to reduce the moisture content of the solid shown in Figure 29.2 is to reduce the relative humidity of the ambient air. This can be achieved on a large scale with an air-conditioning system. On a laboratory scale, desiccators are used. Silica gel (a common laboratory and packaging desiccant) does not directly take water from a solid; instead it acts by removing the water from the air, thereby reducing its relative humidity to around 5–10%. This in turn causes the equilibrium to move along the drying curve in Figure 29.2 to the left, thus reducing the moisture content of the solids. Phosphorus pentoxide works in an identical manner but it has an even greater affinity for the water in the storage air.
If dried materials are exposed to humid ambient conditions they will quickly regain moisture from the atmosphere since this relationship is an equilibrium. Figure 29.1 shows this. Thus it is unnecessary to ‘overdry’ a product and there is no advantage in drying to a moisture content lower than that which the material will have under the normal conditions of use.
If low residual moisture content is necessary due to a hydrolytic instability in the material, the dried product must be efficiently sealed during or immediately after the drying process to prevent ingress of moisture. It also worth noting that some solid pharmaceutical materials perform better when they contain a small amount of residual water. Powders will flow better; the flow of very dry powders is inhibited by static charge. Tablet granules have superior compaction properties with a small amount (1–2 %) of residual moisture.
Types of drying method
Choice of drying method
When considering how to dry a material, the following points should be considered:
• heat sensitivity of the material being dried
• physical characteristics of the material
The general principles for efficient drying can be summarized as:
Dryers in the pharmaceutical industry
The types and variety of drying equipment have reduced in recent years as pharmaceutical companies strive for standardization and globalization of manufacturing. The types of dryers that have proved the most successful have become commonplace and less efficient (or more damaging) drying processes have largely disappeared. An additional trend is the manufacture of ‘mini’ versions of manufacturing equipment to be used in formulation and process development. Previously, very distinctly different dryers were sometimes used in laboratories but this resulted in numerous problems during scale-up. The use of the miniaturized production equipment (processing just a few hundred grams) will minimize later problems during the scaling up to manufacturing batches (typically a few hundred kilograms).
Types of pharmaceutical dryers
A variety of pharmaceutical dryers are still used and it is convenient to categorize these according to the heat transfer method that they employ, i.e. convection, conduction or radiation.
Convective drying of wet solids
Dynamic convective dryers
Fluidized-bed dryer
An excellent method of obtaining good contact between the warm drying air and wet particles is found in the fluidized-bed dryer. The general principles of the technique of fluidization will be summarized before discussing its application to drying.
Consider the situation in which particulate matter is contained in a vessel, the base of which is perforated, enabling a fluid to pass through the bed of solids from below. The fluid can be liquid or gas, but air will be assumed for the purposes of this description, as it is directly relevant to the drying process.
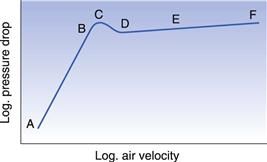
If the air velocity through the bed is increased gradually and the pressure drop through the bed is measured, a graph of the operation shows several distinct regions, as indicated in Figure 29.3. At first, when the air velocity is low, in the region A to B, flow takes place between the particles without causing disturbance, but as the velocity is increased a point is reached, C, when the pressure drop has attained a value where the frictional drag on the particle is equal to the force of gravity on the particle. Rearrangement of the particles occurs to offer least resistance, D, and eventually they are suspended in the air and can move. The pressure drop through the bed decreases slightly because of the greater porosity at D. Further increase in the air velocity causes the particles to separate and move freely and the bed is fully fluidized, region D to E. Any additional increase in velocity separates the particles further, that is, the bed expands, without appreciable change in the pressure drop. In the region E to F, fluidization is irregular, much of the air flowing through in bubbles; the term boiling bed is used to describe this. At a very high air flow rate, F, the air velocity is sufficient to entrain the solid particles and transport them out of the top of the bed in a process known as pneumatic transport.
The important factor is that fluidization produces conditions of great turbulence, the particles mixing with good contact between air and particles. Hence, if hot air is used, the turbulent conditions lead to high heat and mass transfer rates, the fluidized-bed technique therefore offers a means of rapid drying.
The fluidized-bed dryer was developed to make use of this process of fluidization to improve the efficiency of heat transfer and vapour removal compared with the older static tray dryers that it replaced. A reason for this is the more efficient transfer of the required latent heat of evaporation from the air to the drying solid. The rate of heat transfer (dH/dt) in convective drying may be written as:
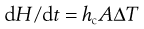 (29.3)
(29.3)
where hc is the heat transfer coefficient for convective heat transfer, A is the surface area available for heat transfer and ΔT is the difference in temperature between the drying air and the solid to be dried. The heat transfer coefficient is high in a fluidized bed as the vigorous motion of the particles reduces the thickness of the boundary layer. Also, the process fluidizes individual powder particles or granules and thus the surface area available for drying is maximized to the total surface area of the powder bed. An equivalent to Equation 29.3 shows that the rate of mass transfer (in this case vapour removal in the opposite direction) is similarly improved.
Heat and mass transfer are therefore relatively efficient and the process, even for a large manufacturing batch, takes between about 20 and 40 minutes.
The arrangement of a typical fluidized-bed dryer is shown in Figure 29.4. Sizes are available with capacities ranging from about 400 g to 1200 kg. These are now manufactured so that dryers throughout the range have similar geometric and hydrodynamic features to aid manufacturing scale-up from laboratory experiments.
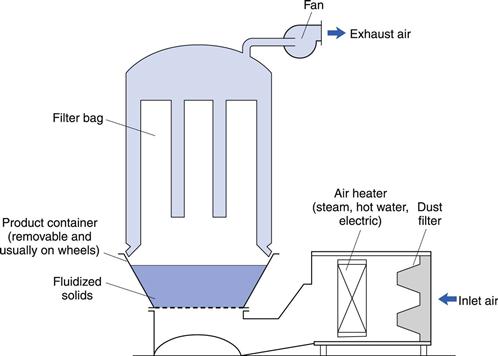
Fig. 29.4 Fluidized bed dryer.
Advantages of fluidized-bed drying
Disadvantages of fluidized-bed drying
Conductive drying of wet solids
In this process, the wet solid is in thermal contact with a hot surface and the bulk of heat transfer occurs by conduction.
Vacuum oven
This equipment is a good example of a conduction dryer, though it is not used as extensively as it was formerly. The vacuum oven (Fig. 29.5) consists of a jacketed vessel sufficiently strong in construction to withstand a vacuum within the oven and possibly steam pressure in the jacket. In addition, the supports for the shelves form part of the jacket, giving a larger area for conduction heat transfer. The oven should be closed by a door that can be locked tightly to give an airtight seal. The oven is connected through a condenser and liquid receiver to a vacuum pump, although if the liquid to be removed is water and the pump is of the ejector type that can handle water vapour, the pump can be connected directly to the oven.
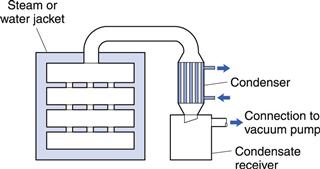
Fig. 29.5 Vacuum oven.
Operating pressure can be as low as 0.03–0.06 bar, at which water boils at 25–35 °C. Some ovens may be large (for example, about 1.5 m3 and with 20 shelves).
The main advantage of a vacuum oven is that drying takes place at a low temperature, and since there is little air present, there is minimal risk of oxidation. The temperature of the drying solid can rise to the steam or heating water temperature at the end of the drying but this is not usually harmful.
Vacuum ovens are rarely used nowadays for production but are still worthy of mention as they may be the only method available to dry particularly thermolabile or oxygen-sensitive materials. Additionally, small-scale vacuum ovens are frequently found in development laboratories where they are commonly used for the drying of small development samples, particularly when the heat stability of the drug or formulation is uncertain.
Radiation drying of wet solids
Radiant heat transmission
Heat transmission by radiation differs from heat transfer by conduction or convection in that no transfer medium (solid, liquid or gaseous) needs be present. Heat energy in the form of radiation can cross empty space or travel through the atmosphere virtually without loss. If it falls on a body capable of absorbing it, then it appears as heat although a proportion may be reflected or transmitted.
Use of microwave radiation
Microwave radiation in the wavelength range 10 mm to 1 m has been found to be an efficient heating and drying method. Microwave dryers are used in the pharmaceutical industry.
Generation and action of microwaves
Microwaves are produced by an electronic device known as a magnetron. Microwave energy can be reflected down a rectangular duct (termed a waveguide) or simply beamed through a transparent polypropylene window into the drying chamber. To avoid interference with radio and television, it is permitted to operate only at certain frequencies which are normally 960 and 2450 MHz.
The penetration of microwaves into the wet product is so good that heat is generated uniformly within the solid.
When microwaves fall on substances of suitable electronic structure (i.e. small polar molecules, such as water), the electrons in the molecule attempt to resonate in sympathy with the radiation. The resulting molecular ‘friction’ generates heat. The large molecules of the solids do not resonate as well as, say, water molecules, so further heating may be avoided once the water is removed. This is indicated clearly by the ‘loss factors’ listed in Table 29.1. The loss factor is the ratio of the microwave energy absorbed by individual molecules to the microwave energy provided. Thus, the higher the value, the greater is the absorption of microwave energy. Table 29.1 lists these values for some common solvents and excipients. Clearly, the absorption of the microwave energy is far greater for small polar molecules than larger and less polar molecules.
Table 29.1
Microwave energy loss factors for some pharmaceutical solvents and excipients
| Material | Loss factor |
| Methanol | 13.6 |
| Ethanol | 8.6 |
| Water | 6.1 |
| Isopropanol | 2.9 |
| Acetone | 1.25 |
| Maize starch | 0.41 |
| Magnesium carbonate | 0.08 |
| Lactose | 0.02 |
Microwave dryers for granulates
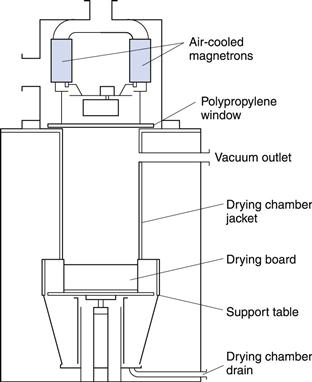
Figure 29.6 is a sketch of a microwave dryer used for drying granules. It is designed to operate under a slight vacuum. That, in itself, is not essential for the use of microwaves but the air flow through the chamber facilitates the continuous removal of evaporated solvent. The radiation is generated by multiple magnetrons each producing 0.75 kW at 2450 MHz. The radiation passes through the polypropylene window into the drying chamber where it is absorbed by the liquid in the wet granules contained on a tray. The heat generated in the mass drives off the moisture.
The evolved vapour is drawn away in the air flow as it is formed. When drying is nearly complete, the radiation field intensity within the chamber will rise since the dry solids do not absorb as readily as water. This rise is detected and the magnetrons are progressively turned down automatically to give an accurate control of the final moisture content and minimize the danger of overheating.
Advantages of microwave drying
The following advantages are claimed for microwave drying:
Disadvantages of microwave drying
Dryers for solutions and suspensions
The objective of these dryers is to generate a large surface area in the liquid for heat and mass transfer and to provide an effective means of collecting the dry solid. The most useful type disperses the liquid as a spray of small droplets – the spray dryer.
Spray dryer
The spray dryer provides a large surface area for heat and mass transfer by atomizing the liquid into small droplets. These are sprayed into a stream of circulating hot air, so that each droplet dries to an individual solid particle. Thus, particle formation and drying occur in the one process.
There are many forms of spray dryer (e.g. GEA Niro) and Figure 29.7 shows a typical design in which the drying chamber resembles a cyclone. This ensures good circulation of air, facilitates heat and mass transfer and encourages the separation of dried particles from the moving air by the centrifugal action.
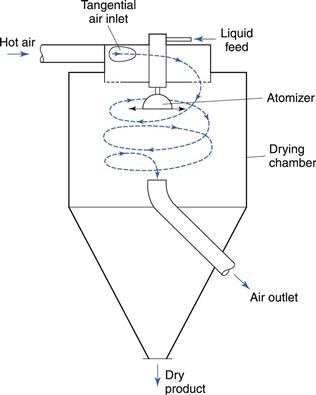
Fig. 29.7 Spray dryer.
Atomizer
The character of the particles is controlled by the droplet size, so the type of atomizer is important. Early simple jet atomizers tended to block easily as a result of rapid evaporation and deposition of solid on the nozzle. This caused the flow rate to vary and this had a detrimental effect on the uniformity and predictability of droplet size.
This was resolved by the use of rotary types of atomizer, one form of which is shown in Figure 29.8. Liquid is fed on to the disc that is rotated at high speed (10 000–30 000 revolutions per minute). A film is formed and spreads from the small disc to a larger, inverted hemispherical bowl, becoming thinner, and eventually being dispersed from the edge in a fine spray of uniform droplet size. In addition, the rotary atomizer has the advantage of being equally effective with either solutions or suspensions of solids and it can operate efficiently at various feed rates.
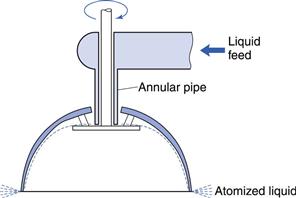
Fig. 29.8 Rotary atomizer.
Modern two-fluid nozzles have overcome the problems of earlier jet atomizers and have proved successful. In this context, the two fluids are the liquid product and the atomizing air. Two-fluid nozzles can handle feed rates between 30 and 80 litres per hour. They can be positioned either to spray their droplets co-currently into the air stream from the top of the dryer, or to spray from the base of the dryer in what is known as ‘fountain mode’. The two-fluid nozzles produce a narrow droplet size range with the mean size being affected by nozzle position. In general, a nozzle used co-currently will produce smaller droplets than one used in fountain mode. Rotary atomizers produce droplets in the mid-range. To illustrate this, data from one experiment performed under similar conditions showed that the mean droplet size from a co-current two-fluid nozzle was 2 µm, from a rotary atomizer was 50 µm and from a two-fluid nozzle used in fountain mode was 100 µm.
Drying chamber
The air enters the chamber tangentially and rotates the drying droplets around the chamber to increase their residence time and therefore time for drying. For pharmaceutical purposes, it is usual to filter the air and to heat it indirectly by means of a heat exchanger. Dust carried over in the air outlet stream may be recovered by a cyclone separator or filter bag. It is also possible to recycle up to 60% of the exhaust air to the air inlet of the dryer. This greatly improves the efficiency of the process.
A range of sizes of spray dryer is available, from a laboratory model with a volume of 100–200 mL capable of producing just a few grams of experimental material from aqueous or organic solution, through a pilot-scale model with a chamber diameter of 800 mm and a height of 3 m capable of evaporating 7 kg of water per hour, to a production-scale model that could have a chamber diameter of 3.5 m and be 6 m high (or even larger) with an evaporative capacity of about 50–100 kg water per hour. Larger spray dryers, with a capacity of up to 4000 kg/h, are used in other industries, notably in food production. Typically, modern spray dryers have a 60° cone at the base of the hollow cylinder.
Product
Spray-dried products are easily recognizable, being uniform in their appearance. The particles have a characteristic shape, in the form of hollow spheres sometimes with a small hole. This arises from the drying process, since the droplet enters the hot air stream and dries on the outside to form an outer crust with liquid still in the centre. This liquid then vaporizes and the internal vapour escapes by blowing a hole in the sphere. Figure 29.9 shows the mechanism of formation of the spherical product.
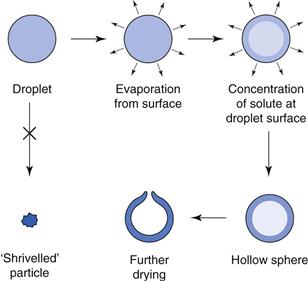
Fig. 29.9 Formation of product in spray drying.
Intelligent computer control is available to control process parameters as these, in turn, can affect particle size, bulk density, moisture content, dissolution rate and dispersibility of the resulting product.
Advantages of the spray-drying process
Disadvantages of the spray-drying process
Uses of spray-drying
The spray dryer can be used for drying almost any substance, in solution or in suspension. It is most useful for thermolabile materials, particularly if handled continuously and in reasonably large quantities.
Examples of both soluble and insoluble substances that are spray dried include citric acid, sodium phosphate, gelatin, starch, barium sulfate and calcium phosphate. The process is also used for some powdered antibiotic formulations where the spray-dried powder is packaged and distributed. This is then reconstituted as a syrup at the time of dispensing. The dry product is spray dried from a formulation containing all the necessary ingredients, including colours and flavours. Since only water is removed in the spray-drying process, these products must be reconstituted with pure water only.
Spray drying is also capable of producing spherical particles in the respirable range of 1–7 µm that are necessary for the delivery of drugs from dry powder inhalers (see Chapter 37).
It is possible to operate spray dryers aseptically using heated filtered air to dry products such as serum hydrolysate. Also, some spray dryers operate in a closed circuit mode with an inert gas to minimize oxidation of the product. Volatile solvents can be recovered from such systems.
Pharmaceutical spray drying has been reviewed by Wendel & Çelik (1997) and the reader is referred to this article if additional information is required.
Fluidized spray dryer
A development of the spray dryer is the fluidized spray dryer (Niro). This has a small fluidized bed mounted in the base of the cone at the point where the product is collected. The moving air created in the fluidized bed overcomes any cohesion of spray-dried particles after they fall into the collection chamber. This allows spheres with a higher moisture content to be handled and also ones made from stickier and more cohesive substances than were previously possible to process.
Freeze drying
Freeze drying is a process used to dry extremely heat-sensitive materials. It can allow the drying, without excessive damage, of proteins, blood products and even microorganisms which retain a small but significant viability.
In this process, the initial liquid solution or suspension is frozen, the pressure above the frozen state is reduced and the water removed by sublimation. Thus an overall liquid-to-vapour transition takes place, as with all the previous dryers discussed, but all three states of matter are involved: liquid to solid, then solid to vapour.
The theory and practice of freeze drying are based on an understanding and application of the phase diagram for the water system.
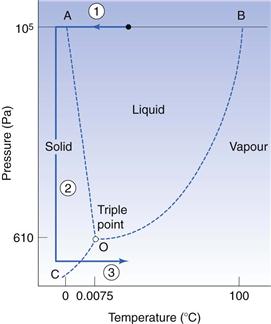
The phase diagram for water
The phase diagram for the water system (Fig. 29.10) consists of three separate areas. Each area represents a single phase of water – vapour, liquid or solid. Two phases can coexist along a line under the conditions of temperature and pressure defined by any point on the line. The point O is the one unique point where all three phases can coexist and is known as the triple point. Its coordinates for pure water are a pressure of 610 Pa (as a comparison, atmospheric pressure is approximately 105 Pa) and a temperature of 0.0075 °C.
The lines on the phase diagram represent the interphase equilibrium lines which show:
• the boiling point of water as it is lowered by reduction of the external pressure above the water (BO in Fig. 29.10)
• the reduction of the vapour pressure exerted by ice as the temperature is reduced (CO).
On heating ice at atmospheric pressure, it will melt when the temperature rises to 0 °C, i.e. at this temperature the ice will change to liquid water. Continued heating at atmospheric pressure will raise the temperature of the water to 100 °C. If heating is continued, the liquid water will be converted into water vapour at 100 °C.
If, however, solid ice is maintained at a pressure below the triple point then on heating, the ice will sublime and pass directly to water vapour without passing through the liquid phase. This sublimation, and therefore drying, will only occur at a temperature below that of the triple point. Thus it will only happen if the pressure is prevented from rising above the triple point pressure during the process. To ensure that this is so, the vapour evolved must be removed as fast as it is formed.
Application of the phase diagram of water to freeze drying
The process of freeze drying is superimposed on the phase diagram for water in Figure 29.10. In its basic form freeze drying comprises three steps:
2. reducing the atmospheric pressure above the ice to below that of the triple point of the product
3. adding heat to the system to raise the temperature to the sublimation curve (CO in Fig. 29.10) to provide the latent heat of sublimation.
These are discussed in detail below.
Stages of the freeze-drying process
Freezing stage
The liquid material is frozen before the application of a vacuum to avoid frothing. The depression of the freezing point caused by the presence of dissolved solutes means that the solution must be cooled to well below the normal freezing temperature for pure water and it is usual to work in the range –10 to –30 °C, typically below –18 °C. The presence of dissolved solutes will shift the pure-water phase diagram. Since the subsequent stage of sublimation is slow, several methods are used at this stage to produce a large frozen surface to speed up that later stage.
Shell freezing.
This is employed for fairly large volumes such as blood products. The bottles are rotated slowly and almost horizontally in a refrigerated bath. The liquid freezes in a thin shell around the inner circumference of the bottle. Freezing is slow and large ice crystals form, which is a drawback of this method as they may damage blood cells and reduce the viability of microbial cultures.
In vertical spin freezing, the bottles are spun individually in a vertical position so that centrifugal force forms a circumferential layer of solution which is cooled by a blast of cold air. The solution supercools and freezes rapidly with the formation of small ice crystals.
Centrifugal evaporative freezing.
This is a similar method where the solution is spun in small containers within a centrifuge. This prevents foaming when a vacuum is applied. The vacuum causes boiling at room temperature and this removes so much latent heat that the solution cools quickly and ‘snap’ freezes. About 20% of the water is removed prior to freeze drying and there is no need for separate refrigeration. Ampoules are usually frozen in this way, a number being spun in an angled position (approximately 30° to the horizontal) in a special centrifuge head so that the liquid is thrown outwards and freezes as a wedge with a larger surface area.
Vacuum application stage
The containers and the frozen material must be connected to a source of vacuum sufficient to drop the pressure below the triple point and remove the large volumes of low-pressure vapour formed during drying. Again, an excess vacuum is normal in practice to ensure that the product in question is below the triple point of the formulation.
Commonly a number of bottles or vials are attached to individual outlets of a manifold which is connected to vacuum.
Sublimation stage
Heat of sublimation must be supplied. It may be thought that, as the process takes place at a low temperature, the additional heat needed to sublime the ice will be small. In fact, the latent heat of sublimation of ice is 2900 kJ kg–1, appreciably larger than the latent heat of evaporation of water at atmospheric pressure. This heat must be supplied for the process to take place.
Sublimation can only occur at the frozen surface. It is a slow process (approximately 1 mm thickness of ice per hour) and is surface area dependent. This step must therefore be considered at the freezing stage (as discussed above). The procedures discussed earlier not only increase the surface area, but also reduce the thickness of ice to be sublimated.
Primary drying.
Under those conditions the ice sublimes, leaving a porous solid which still contains about 0.5% moisture after primary drying. Further reduction can be effected by secondary drying. During the primary drying, the latent heat of sublimation must be provided and the vapour removed.
Heat transfer.
Heat transfer is critical; insufficient heat input prolongs the process, which is already slow, and excess heat will cause melting.
Small ampoules may be left on the centrifuge head or may be placed on a manifold; in either case heat from the atmosphere is sufficient. Large volumes, bottles of blood for example, are placed in individually heated cylinders or are connected to a manifold when heat can be taken from the surrounding environment that must be replaced by some form of input heat.
In all cases, heat transfer must be controlled since only about 5 W m–2 K–1 is needed and overheating will lead to melting. It is important to appreciate that, although a significant amount of heat is required, there should be no significant increase in temperature; the added heat should be sufficient to provide the latent heat of sublimation only and little sensible heat.
Vapour removal.
The vapour formed must be removed continually to prevent the pressure within the container rising above the triple point pressure and thus preventing sublimation. To reduce the pressure sufficiently, it is necessary to use efficient vacuum pumps, usually two-stage rotary pumps on the small scale and ejector pumps on the large scale. On the small scale, vapour is absorbed by a desiccant such as phosphorus pentoxide, or is cooled in a small condenser with solid carbon dioxide. Mechanically refrigerated condensers are used on the large scale.
For vapour flow to occur, the vapour pressure at the condenser must be less than that at the frozen surface and a low condenser temperature is necessary. On a large scale, vapour is commonly removed by pumping, but the pumps must be of large capacity and must not be affected by moisture. The extent of the necessary pumping capacity will be realized from the fact that, under the pressure conditions used during primary drying, 1 g of ice will form 1000 litres of water vapour. Ejector pumps are most satisfactory for this purpose.
Rate of drying.
The rate of drying in freeze drying is very slow. The drying rate curve illustrated in Figure 29.11 shows a similar shape to a normal drying curve, the drying being at constant rate during most of the time.
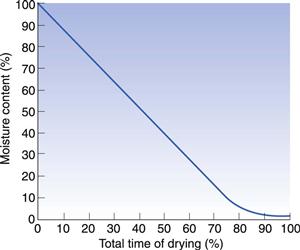
Fig. 29.11 Sublimation drying: rate of drying curve.
Computer control enables the drying cycle to be monitored. There is an optimum vapour pressure for a maximum sublimation rate, and the heat input and other variables are adjusted to maintain this value. Continuous freeze drying is possible in modern equipment where the vacuum chamber is fitted with a belt conveyor and vacuum locks. Despite these advances, the overall drying rate is still slow.
Secondary drying
The removal of the final amounts of residual moisture at the end of primary drying is performed by raising the temperature of the solid to as high as 50 or 60 °C. A high temperature is permissible for many materials because the small amount of moisture remaining at the end of primary drying is not sufficient to cause spoilage.
Packaging
Attention must be paid to packaging freeze-dried products to ensure protection from moisture during storage. Containers should be closed without contacting the atmosphere, if possible, and ampoules, for example, are sealed on the manifold while still under vacuum. Otherwise, the closing must be carried out under controlled atmospheric conditions. The dry material often needs to be sterile.
Advantages of freeze drying
Freeze drying, as a result of the character of the process, has certain special advantages:
Disadvantages of freeze drying
There are two main disadvantages of freeze drying:
Uses of freeze drying
The method is used for those products which could not be dried by any other heat method. These include biological products; for example, some antibiotics, blood products, vaccines (such as BCG, yellow fever, smallpox), enzyme preparations (such as hyaluronidase) and microbiological cultures. The latter enables specific microbiological species and strains to be stored for long periods with viability of about 10% on reconstitution.
Solute migration during drying
Solute migration is the phenomenon that can occur during drying which results from the movement of a solution within a wet system. The solvent moves towards the surface of a solid (from where it evaporates), taking any dissolved solute with it. Many drugs and binding agents are soluble in granulating fluid and during the drying of granulates these solutes can move towards the surface of the drying bed or granule and be deposited there when the solvent evaporates. Solute migration during drying can lead to localized variability in the concentration of soluble drugs and excipients within the dried product.
Migration associated with drying granules can be of two types: intergranular migration (between granules) and intragranular migration (within individual granules).
Intergranular migration
Intergranular migration, where the solutes move from granule to granule, may result in gross maldistribution of active drug. It can occur during the drying of static beds of granules (e.g. tray drying) since the solvent and accompanying solute(s) move from granule to granule towards the top surface of the bed where evaporation takes place. When the granules are compressed, the tablets may have a deficiency or an excess of drug. For example, experimentation found that only 12% of tablets made from a tray-dried warfarin granulate were within the USP limits for drug content.
Intragranular migration
Drying methods based on fluidization and vacuum tumbling keep the granules separate during drying and so prevent the intergranular migration that may occur in fixed beds. However, intragranular migration, where the solutes move towards the periphery of each granule, may take place.
Consequences of solute migration
Solute migration of either type can result in a number of problems and occasional benefits.
Loss of active drug
The periphery of each granule may become enriched, with the interior suffering depletion. This will be of no consequence unless the enriched outer layer is abraded and lost, as may happen during fluidized-bed drying when the fine drug-rich dust will be eluted in the air and carried to the filter bag or lost. The granules suffer a net loss of drug and as a result will be below specification with respect to quantity of active ingredient.
Mottling of coloured tablets
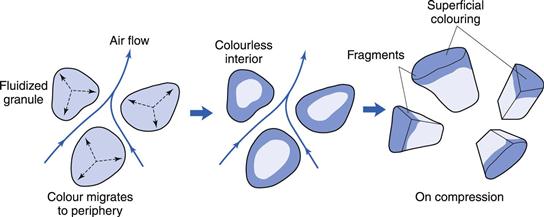
Coloured tablets can be made by adding soluble colour during wet granulation. Intragranular migration of the colour may give rise to dry granules with a highly coloured outer zone and a colourless interior (Fig. 29.12). During compaction granule fracture takes place and the colourless interior is exposed. The eye then sees the coloured fragments against a colourless background and the tablets appear mottled.
Migration may be reduced by using the insoluble aluminium ‘lake’ of the colouring material (in which the soluble dye is adsorbed strongly onto insoluble alumina particles) in preference to the soluble dye itself. Studies have indicated that the production of small granules, which do not fracture so readily, are preferable to larger ones if mottling is troublesome.
Migration of soluble binders
Intragranular migration may deposit a soluble binder at the periphery of the granules and so confer a ‘hoop stress’ resistance, making the granules harder and more resistant to abrasion. It has been shown that this migration can aid the bonding process during tablet compaction as a result of binder–binder (rather than drug–drug or drug–excipient) contact and is therefore sometimes beneficial.
Many other factors such as granule formulation, drying method and moisture content have been shown to affect solute migration.
Influence of formulation factors on solute migration
Nature of substrate
The principles governing solute migration are similar to those of thin-layer chromatography. Thus, if the granule substrate has an affinity for the solute then migration will be impeded. Luckily, many of the common tablet excipients possess this affinity. The presence of absorbent materials, such as starch and microcrystalline cellulose, will minimize tablet solute migration.
The use of water-insoluble aluminium lakes (pigments) reduces mottling compared with water-soluble dyes. This effect has also been seen with film-coat colours (Chapter 32).
Viscosity of granulating fluid
The popular granulating fluids are solutions of polymers whose viscosity is appreciably greater than water alone. This viscosity impedes the movement of moisture by increasing the fluid friction. Increasing the concentration and therefore the viscosity of PVP solution has been shown to slow the migration of drugs in fixed beds of wet granules. Solution of methylcellulose with comparable viscosities gave similar migration rates showing that the effect is due to viscosity alone and not to any specific action of either of the binders.
Influence of process factors on solute migration
Drying method
Intergranular migration in fixed beds of granules will occur whenever a particular method of drying creates a temperature gradient. This results in greater evaporation from the hotter zones.
In slow convective drying (e.g. during static tray drying), the maximum concentration of migrated solute will normally occur in the surface of the drying bed since the process of drying is slow enough to maintain a capillary flow of solvent/solute to the surface over a long period of time.
Drying by microwave radiation results in uniform heating that in turn minimizes solute migration.
Drying methods which keep the granules in motion will abolish the problem of intergranular migration, but intragranular migration can still occur. This is marked in fluidized granules.
Initial moisture content
The initial moisture content of the granule will also influence the extent of migration. The greater the moisture content, the greater will be the moisture movement before the pendular state is reached, at which migration cannot continue as there is no longer a continuous layer of mobile liquid water within the wet solid (see Fig. 29.2).
Some practical means of minimizing solute migration
It may be useful to list the measures that can be taken to minimize migration:
Reference
1. Wendel S, Çelik M. An overview of spray-drying applications. Pharmaceutical Technology. 1997;10:124–144.
Bibliography
1. Banker GS, Rhodes CT. Modern Pharmaceutics. 4th edn NY, USA: Marcel Dekker; 2002.
2. Broadhead J, Rouan SK, Hau I, Rhodes CT. The effect of process and formulation variables on the properties of spray-dried beta-galactosidase. Journal of Pharmacy and Pharmacology. 1994;46(6):458–467.
3. am Ende DJ. The freeze drying process. Chap. 41 in: am In: Ende DJ, ed. Chemical Engineering in the Pharmaceutical Industry: R&D to Manufacture. New Jersey, USA: John Wiley & Sons (in conjunction with AIChE); 2010.
4. Franks F, Auffret T. Freeze Drying of Pharmaceuticals and Biopharmaceuticals: Principles and Practice. London: RSC Publishing; 2008.
5. Masters K. Spray Drying Handbook. 5th edn Longman Scientific and Technical, Harlow, Essex 1991.
6. Travers DN. Problems with solute migration. Manufacturing Chemist and Aerosol News. 1983;53(3):67–71.
7. Troy DB, ed. Remington: The Science and Practice of Pharmacy. 21st edn Maryland: Lippincott, Williams and Wilkins; 2006.
8. Tsotsas E, Mujumbar AS. Modern Drying Technology. vol. 3 Germany: Wiley-VCH; 2011.
9. Wan LS, Heng PW, Chia CG. Preparation of coated particles using a spray drying process with an aqueous system. International Journal of Pharmaceutics. 1991;77:183–191.
10. Zoglie, M.A., Carstensen, J.T. (1981) Drying. In: Leiberman, H.A., Lachman, L. Pharmaceutical Dosage Forms: Tablets. Marcel Dekker, New York.