Chapter 52 Drugs to Treat Parasitic Infections
| Abbreviations | |
|---|---|
| AIDS | Acquired immunodeficiency syndrome |
| CNS | Central nervous system |
| DNA | Deoxyribonucleic acid |
| G6PD | Glucose-6-phosphate dehydrogenase |
| GI | Gastrointestinal |
| IM | Intramuscular |
| IV | Intravenous |
Therapeutic Overview
CLASSIFICATION OF MAJOR PARASITIC GROUPS
There are two major groups of parasites: multicellular helminths (worms) and single-celled protozoa.
| Therapeutic Overview |
|---|
| Prevention Strategies |
| Control disease vectors or reduce contact with them |
| Improve hygiene and sanitation |
| Vaccine development |
| Drugs |
| Transmission of Protozoal Infection |
| Malaria—mosquitoes |
| Leishmaniasis—sand flies |
| African trypanosomiasis—tsetse flies |
| Chagas’ disease—reduviid bugs |
| Amebiasis—food, water |
| Giardiasis—food, water |
| Toxoplasmosis—cats, undercooked meats |
Helminths have sophisticated organ systems and many have complex life cycles. Clinical manifestations of helminthic diseases are usually proportionate to the worm burden. Infections with light worm burdens are often asymptomatic, whereas heavy worm burdens can result in life-threatening disease. Exceptions occur when one or more helminths gain access to a critical organ such as the brain or an eye, or when an adult worm migrates into and obstructs the common bile duct, such as with A. lumbricoides. Helminths have finite life spans. Infestations resolve over time, unless there is autoinfection, as in the case of Strongyloides stercoralis or Hymenolepis nana, or the parasite has an extremely long life span, as in the case of Clonorchis sinensis. Eosinophilia is common when helminths migrate through tissue but may be absent after intestinal helminths have reached maturity in the bowel lumen.
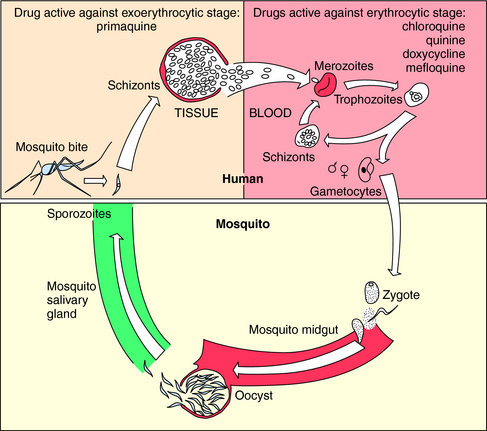
The vectors by which parasites spread are varied. Enteric pathogens are spread in fecally contaminated food and water, T. vaginalis is spread by intimate personal contact, whereas Plasmodium species, which cause malaria, are transmitted by anopheline mosquitoes, whose life cycle is depicted in Figure 52-1. Sporozoites are inoculated into the host when an infected female attempts to take a blood meal. The sporozoites travel to the liver through the circulation, invade hepatocytes, and develop within liver cells in 1 to 3 weeks. The erythrocytic stage, which is the only symptomatic stage, begins when merozoites are released from the liver and invade red blood cells. Plasmodium vivax and Plasmodium ovale, in contrast, can persist for months in the liver as hypnozoites before completing development and initiating symptomatic malaria.
Mechanisms of Action
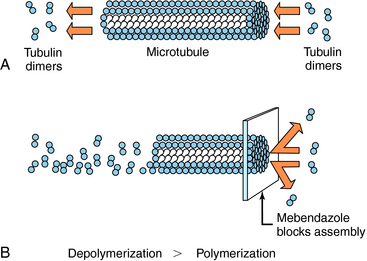
Albendazole sulfoxide, the primary metabolite of albendazole, and mebendazole bind to β-tubulin in susceptible nematodes and inhibit microtubule assembly, leading to disruption of microtubules and selective and irreversible inhibition of glucose uptake (Fig. 52-2). This results in depletion of glycogen stores, reduced formation of adenosine triphosphate, disruption of metabolic pathways, and ultimately parasitic death. Serum glucose concentrations are not affected in the human host.
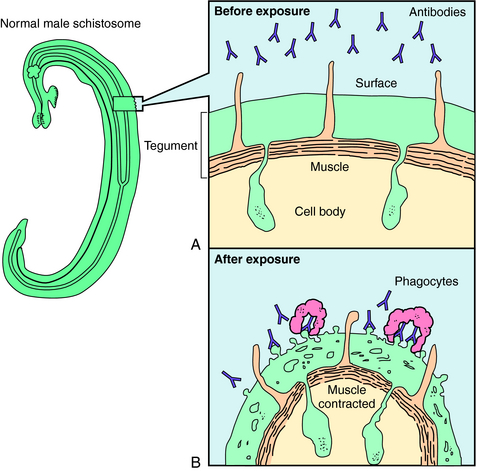
Praziquantel is a heterocyclic pyrazine-isoquinoline derivative. It is rapidly taken up by tapeworms and flukes, but its precise mechanism of action is not known. Studies of the tapeworm Hymenolepis diminuta indicate that praziquantel releases Ca++ from endogenous stores, resulting in contraction and subsequent expulsion of the worm from the GI tract. In the schistosomes, praziquantel damages the tegument, causing intense vacuolation, exposure of sequestered schistosomal antigens, and increased permeability to Ca++, causing tetanic contraction and paralysis. Adult schistosomes are then swept back through the portal circulation to the liver, where they are destroyed by phagocytes. Figure 52-3 depicts the marked alterations in the schistosomal surface after drug exposure.
A summary of the observed effects and possible mechanisms of action of the major antihelminthic drugs is in Table 52-1.
TABLE 52–1 Observed Effects and Possible Mechanisms of Action of the Major Anthelmintic Drugs
| Drug | Observed Effects on Helminths | Possible Mechanism of Action |
|---|---|---|
| Albendazole | Inhibition of glucose transport; depletion of glycogen stores, inhibition of fumarate reductase | Binding to β-tubulin, prevents microtubule polymerization |
| Mebendazole | Inhibition of glucose transport; depletion of glycogen stores | Binding to β-tubulin |
| Pyrantel pamoate | Muscles depolarize, increased spike wave activity, spastic paralysis | Depolarizing neuromuscular blockade |
| Diethylcarbamazine | Hyperpolarization and paralysis of worm’s musculature; exposure of antigens, leading to antibody binding and attack by phagocytes | Hyperpolarization and neuromuscular blockade |
| Ivermectin | Alters chloride currents, resulting in death of microfilariae | Altered chloride channel function |
| Praziquantel | Depolarization of muscles, increased intracellular Ca++, displacement of schistosomes to the human liver, exposure of surface antigens, binding by antibody and phagocytes, tegument disruption | Uncertain |
| Niclosamide | Uncouples phosphorylation; may inhibit anaerobic metabolism | Uncertain |
Metronidazole has a broad spectrum of activity against anaerobic bacteria and protozoa. It is activated when reduced by ferredoxins or their equivalents in protozoa or bacteria. The resultant products react with deoxyribonucleic acid (DNA) and other intracellular parasite constituents, causing damage and death. Tinidazole has a similar mechanism of action. Paromomycin, an aminoglycoside antibiotic (see Chapter 47), inhibits protein synthesis. Iodoquinol acts against E. histolytica cysts and, to a lesser extent, trophozoites by an unknown mechanism. Diloxanide furoate is directly amebicidal, and little is known about its mechanism of action also. Nitazoxanide has a broad spectrum of activity against protozoa and helminths and is approved for giardiasis and cryptosporidiosis in children. The mechanism involves inhibition of electron transport reactions essential to metabolism of anaerobic organisms. Furazolidone interferes with several bacterial enzyme systems, but its mechanism of action against G. lamblia is uncertain.
Atovaquone has activity against Plasmodium spp., Babesia spp., P. jiroveci, and T. gondii. It selectively inhibits electron transport, resulting in collapse of the mitochondrial membrane potential. It also inhibits pyrimidine biosynthesis, which is obligatorily coupled to electron transport in Plasmodium spp. Pyrimethamine binds to and irreversibly inhibits dihydrofolate reductase. It is approximately 1000-fold more active against plasmodium dihydrofolate reductase-thymidylate synthetase than against human dihydrofolate reductase. Pyrimethamine is often used with one of the sulfonamides to inhibit sequential steps in folate metabolism. Trimethoprim inhibits the dihydrofolate reductase of many bacteria and some protozoa and is frequently administered with sulfamethoxazole (see Chapter 48). Proguanil is metabolized to an active cyclic triazine metabolite that selectively inhibits plasmodium dihydrofolate reductase-thymidylate synthetase. Nifurtimox undergoes partial reduction followed by auto-oxidation, forming superoxide anion, hydrogen peroxide, and hydroxyl radicals that damage cell membranes and DNA. Eflornithine is an irreversible inhibitor of ornithine decarboxylase, the enzyme that catalyzes the rate-limiting step in polyamine synthesis. Although polyamines are essential for growth and differentiation of all cells, eflornithine has clinical activity only against T. brucei gambiense.
A number of the antibiotics discussed in previous chapters have activity against some protozoa. Tetracycline, doxycycline, and clindamycin inhibit protein synthesis, and sulfonamides inhibit dihydropteroate synthetase and para-aminobenzoic acid binding to it. Amphotericin B is thought to act on leishmania as it does on susceptible fungi, by disrupting membranes (see Chapter 50). In addition, liposomal and lipid-associated amphotericin is selectively targeted to macrophages, the cells in which this parasite resides.
A summary of the possible mechanisms of action of the major antiprotozoal drugs is found in Table 52-2.
TABLE 52–2 Possible Mechanisms of Action of Major Antiprotozoal Drugs
| Drug | Possible Mechanism of Action |
|---|---|
| Metronidazole | Activated when reduced by ferredoxins, reacts with DNA and other parasite constituents |
| Paromomycin | Inhibits protein synthesis |
| Nitazoxanide | Inhibition of electron transfer reactions essential to the metabolism of anaerobic organisms |
| Chloroquine | Concentrated in hemoglobin-containing digestive vesicles, inhibits heme polymerase |
| Quinine | Concentrated in food vacuoles, probably inhibits heme polymerase |
| Mefloquine | Concentrated in food vacuoles, may form toxic complexes with heme |
| Pyrimethamine | Inhibits dihydrofolate reductase |
| Sulfonamides | Inhibit binding of p-aminobenzoic acid to dihydropteroate synthetase |
| Nifurtimox | Forms reactive O2 species that damage cell membranes and DNA |
| Eflornithine | Irreversibly inhibits ornithine decarboxylase, inhibits polyamine synthesis |
| Melarsoprol | Reacts with sulfhydryl groups, inhibits proteins |
Pharmacokinetics
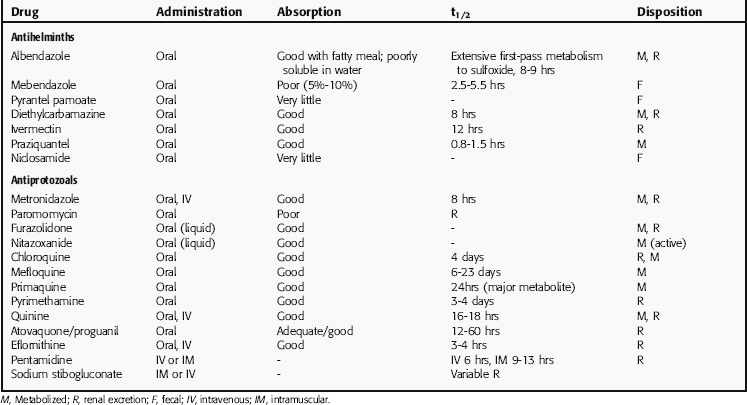
Pharmacokinetic parameters for selected antiparasitic drugs are listed in Table 52-3.
Chloroquine phosphate is well absorbed when taken orally, with peak serum concentrations reached in 3.5 hours. It is eliminated slowly after treatment is terminated. Approximately 50% of the drug is excreted unchanged in the urine, and the rest is metabolized in the liver.
Atovaquone/proguanil combinations have pharmacokinetics as described for the individual drugs.
Doxycycline, tetracycline, ciprofloxacin, and sulfonamides are well absorbed when taken orally. Their pharmacokinetics are discussed in detail in Chapters 47 and 48. Amphotericin B is discussed in Chapter 50.
Relationship of Mechanisms of Action to Clinical Response
Praziquantel is active against all Schistosoma species and all but one of the human flukes, F. hepatica, for which treatment with triclabendazole or bithionol is effective. Both praziquantel and niclosamide are effective against adult T. saginata, T. solium, D. latum, and H. nana in the human GI tract. Praziquantel is preferable for the treatment of T. solium because it is active against larvae and adults and may prevent internal autoinfection, a theoretical possibility after niclosamide treatment. In the case of H. nana, praziquantel is effective as a single dose, because it kills cysticerci in the wall of the intestine and kills adult worms, whereas niclosamide kills only adult worms and must be administered for 7 days.
The recommendations for prophylaxis and treatment of malaria are reviewed regularly and are available from the Centers for Disease Control and Prevention (www.cdc.gov/travel) and in The Medical Letter on Drugs and Therapeutics (www.medicalletter.org).
Treatment of Other Protozoal Diseases
Treatment of symptomatic toxoplasmosis consists of pyrimethamine and a short-acting sulfonamide, such as sulfadiazine. Leucovorin (discussed in Chapter 54) is administered concurrently to prevent bone marrow suppression. The combination of pyrimethamine and clindamycin is also effective and frequently used in patients with AIDS. The macrolide spiramycin has been used to treat women infected with T. gondii during pregnancy.
The drug of choice for P. jerovici is trimethoprim-sulfamethoxazole. There is, however, a very high incidence of adverse reactions to sulfonamides in patients with AIDS, and it is often necessary to use alternatives, such as pentamidine isethionate, trimethoprim plus dapsone, atovaquone, or primaquine plus clindamycin. Prophylactic administration of trimethoprim-sulfamethoxazole, dapsone, atovaquone, or pentamidine aerosol is effective in reducing recurrences and preventing disease in AIDS patients with low CD4+ counts.
Pharmacovigilance: Side Effects, Clinical Problems, and Toxicity
Quinine has the poorest therapeutic index among antimalarial drugs. Common side effects include tinnitus, decreased hearing, headache, dysphoria, nausea, vomiting, and mild visual disturbances. Quinine therapy has been associated with severe hypoglycemia in persons with heavy P. falciparum infections as a result of the parasite’s use of glucose and the quinine-mediated release of insulin from the pancreas, which responds to intravenous administration of glucose. Rare complications include allergic skin rashes, pruritus, agranulocytosis, hepatitis, and massive hemolysis in patients with P. falciparum malaria, which has been termed “blackwater fever.” Quinine causes respiratory paralysis in persons with myasthenia gravis, stimulates uterine contractions, and may produce abortion but has been used successfully to treat serious cases of malaria during pregnancy. Quinidine gluconate, the stereoisomer of quinine, is also a Class 1A antiarrhythmic drug (see Chapter 22). It decreases ventricular ectopy, affects cardiac conduction, and prolongs the QTc interval. Life-threatening dysrhythmias can occur but are rare. Hypotension may result if the drug is administered too rapidly.
Sodium stibogluconate and meglumine antimonate have frequent side effects, but they usually do not prevent completion of therapy. Chemical pancreatitis is common, and recipients also frequently experience myalgias, arthralgias, fatigue, and nausea. Nonspecific ST-T wave changes are observed on the electrocardiogram. Untoward effects are more common in persons with renal failure. Side effects of antibiotics and amphotericin B are discussed in Chapter 46 through 48 and 50.
| Albendazole and Mebendazole | GI discomfort, alopecia, bone marrow suppression, hepatic injury |
| Diethylcarbamazine | Central and GI effects, Mazzotti reaction |
| Ivermectin | Inflammatory reactions |
| Praziquantel | GI side effects, allergic reactions caused by release of helminthic antigens |
| Metronidazole | GI side effects, neurotoxicity, alcohol intolerance |
| Paromomycin | GI side effects, ototoxicity, renal toxicity |
| Chloroquine | Headache, nausea, vomiting, blurred vision, retinal damage |
| Primaquine | GI side effects, hemolysis in people with glucose-6-phosphate deficiency |
| Quinine | Tinnitus, decreased hearing, headache, dysphoria, GI side effects, visual disturbances |
| Mefloquine | Neuropsychiatric reactions |
| Pyrimethamine | Rare blood dyscrasias, rash, vomiting, seizures, shock |
New Horizons
The emergence of multidrug-resistant P. falciparum has stimulated the search for new forms of treatment and prophylaxis for malaria. Among the exciting new compounds are the artemisinin derivatives, which include artesunate. These were identified in studies of quinghaosu, the Chinese herbal treatment for malaria derived from the wormwood plant Artemisia annua. Artemisinin and other artemisinin relatives are endoperoxide-containing compounds. In the presence of intraparasitic iron, these drugs may be converted into free radicals and other intermediates that alkylate specific malaria proteins. Artesunate and its derivatives have been used successfully to treat acute P. falciparum malaria in areas where mefloquine- and quinine-resistant strains are endemic. In many instances artemisinin derivatives are used concurrently with mefloquine or another antimalarial drug. Currently available artemisinin derivatives are not useful prophylactically because of their short half-lives and concern about neurotoxicity with long-term use. Other anti-malarials are being studied in combinations to determine their additive or synergistic effects and whether they may be useful against drug-resistant isolates.
| Antihelminths | Antimalarials |
| Albendazole (Albenza) | Atovaquone and proguanil (Malarone) |
| Bithionol (Bitin) | Chloroquine (Aralen) |
| Diethylcarbamazine (Hetrazan) | Doxycycline (Vibramycin) |
| Ivermectin (Stromectol) | Mefloquine (Lariam) |
| Mebendazole (Vermox) | Primaquine |
| Niclosamide (Yomesan) | Quinine |
| Praziquantel (Biltricide) | Quinidine |
| Proguanil (Paludrine) | |
| Pyrantel pamoate (Antiminth) | |
| Pyrimethamine (Daraprim) | |
| Antiprotozoals | Antikinetoplastides |
| Clindamycin (Cleocin) | Amphotericin B (lipid associated) |
| Diloxanide furoate (Furamide) | Melarsoprol (Arsobal) |
| Furazolidone (Furoxone) | Pentamidine isethionate (Pentam 300) |
| Iodoquinol (Yodoxin) | Trimethoprim-Sulfamethoxazole (Bactrim, Septra) |
| Metronidazole (Flagyl) | Stibocluconate sodium (Pentostam) |
| Nitazoxanide (Alinia) | |
| Pentamidine isethionate (Pentam 300) | |
| Paromomycin (Humatin) | |
| Pyrimethamine (Daraprim) | |
| Spiramycin (Rovamycine) | |
| Tinidazole (Tindamax) | |
| Trimethoprim-Sulfamethoxazole (Bactrim, Septra) |
Griffith et al. 2007 Griffith KS, Lewis LS, Mali S, Parise ME. Treatment of malaria in the United States. JAMA. 2007;297:2264-2277.
Anonymous. Drugs for parasitic infections. Med Lett. 2007;49(Suppl):1-15.
Pearson RD. Antiparasitic drugs. In Mandell GL, Bennett JE, Dolin R, editors: Principles and practice of infectious diseases, ed 5, Philadelphia: Churchill Livingstone, 2005.
Centers for et al. 2007 Centers for Disease Control and Prevention. Traveler’s Health: 2007, US Department of Health and Human Resources, Public Health Service. wwwn.cdc.gov/travel.