Chapter 89 Drug Toxicity of the Posterior Segment
A variety of systemic medications can generate retinal toxicity. Fortunately, in the majority of cases the loss of visual function is minimal or reversible following discontinuation of the inciting drug. Nevertheless, permanent or progressive visual loss may occur in some instances. We present those medications known to produce a well-described anomaly and have omitted others that have not been definitively proven to cause retinal abnormalities. The medications are grouped according to the type of retinal toxicity they produce, summarized in Box 89.1.
Box 89.1
Patterns of retinal toxicity
Disruption of the retina and retinal pigment epithelium
Phenothiazines
Thioridazine
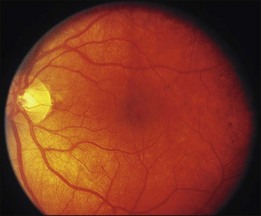
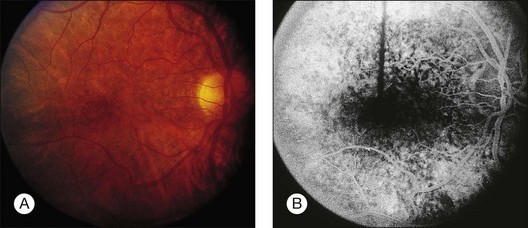
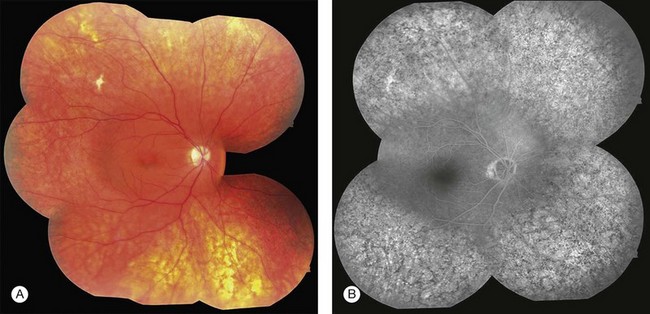
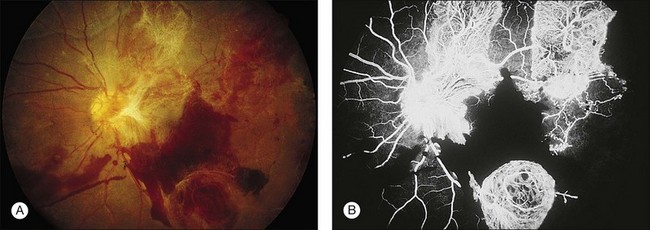
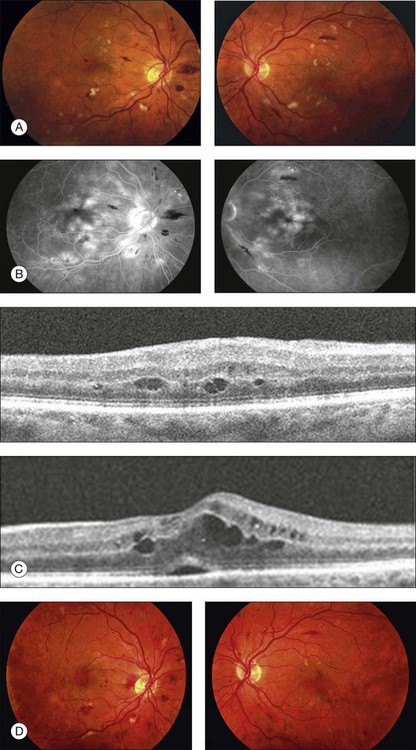
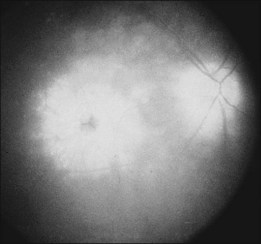
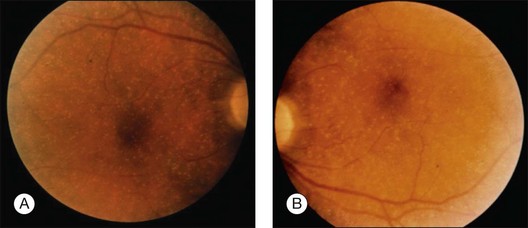
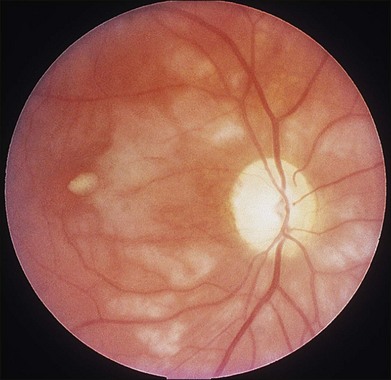
Blurred vision, dyschromatopsia (reddish or brownish discoloration of vision), and nyctalopia characterize acute toxicity with thioridazine.1 In the earliest stages the fundus appearance may be normal or display only mild granular pigment stippling (Fig. 89.1). An intermediate stage is characterized by circumscribed nummular areas of retinal pigment epithelial (RPE) loss from the posterior pole to the midperiphery2 (Fig. 89.2A). Fluorescein angiography (FA) reveals disruption of the choriocapillaris in these zones of pigment rarefaction (Fig. 89.2B). In late stages of thioridazine toxicity, widespread areas of depigmentation alternating with hyperpigmented plaques, vascular attenuation, and optic atrophy are seen3 (Fig. 89.3).
Retinal toxicity from thioridazine is dependent more on the total daily dose than on the cumulative amount of drug received.4 With higher daily doses, toxicity can occur rapidly, even within the first two weeks of therapy.5 Toxicity is rare at dosages less than 800 mg/day. Nonetheless, a few cases have been reported with lower doses given over several years.6–10 As a result, many now suggest that any patient taking thioridazine, regardless of the daily dose, should be monitored for the development of visual symptoms or fundus changes.
In the initial stages of toxicity, visual field testing can reveal mild constriction, paracentral scotomas, or ring scotomas. Electroretinography (ERG) is either normal or shows decreased oscillatory potentials. In the later stages, both the rod and cone functions of the ERG, as well as electrooculography (EOG), are markedly abnormal.11 If the drug is stopped early, ERG testing often improves over the first year.12 Histologic studies demonstrate that atrophy and disorganization of photoreceptor outer segments occurs primarily, with a secondary loss of the RPE and choriocapillaris.3
The early fundus changes associated with thioridazine often progress despite discontinuation of therapy.2 It is unclear whether this degeneration represents continued toxicity of the drug or a delayed expansion of chorioretinal scarring to areas of subclinical, preexisting damage.12 Visual function, in contrast to fundus appearance, usually improves over the first year after a toxic reaction, but there has been one report of severe progressive decline in vision after cessation of the drug.13
The mechanism of thioridazine-mediated toxicity remains unknown. Many phenothiazines bind melanin granules of the RPE and uveal tissue, but not all commonly instigate retinal toxicity.14–16 The compound NP-207 (piperidyl-chlorophenothiazine hydrochloride) has a remarkably similar chemical structure to thioridazine, including the same piperidyl side chain. NP-207 was never marketed because of the pronounced pigmentary retinopathy that developed during early clinical trials.17 This piperidyl side chain is not present in other phenothiazines such as chlorpromazine, which exhibit much less retinal toxicity. Experimental studies demonstrate that phenothiazines both alter enzyme kinetics and inhibit oxidative phosphorylation with subsequent abnormalities in rhodopsin synthesis.18–20 Other studies postulate that phenothiazine toxicity is due to the drug’s effect on the dopamine receptors in the retina.21 Further study is necessary to determine whether these observed effects are involved in the pathogenesis of thioridazine toxicity.
Chlorpromazine
Chlorpromazine is a piperazine similar to thioridazine but lacks the piperidyl side chain mentioned above. The compound binds strongly to melanin and can cause hyperpigmentation in the skin, conjunctiva, cornea, lens, and retina22–28 (Fig. 89.4). Other ocular effects include oculogyric crisis, miosis, and blurred vision caused by paralysis of accommodation. Usual doses range from 40 to 75 mg/day, but dosages up to 800 mg/day are not uncommon.
Retinal toxicity from chlorpromazine is rare. When massive doses are given (e.g., 2400 mg/day for 12 months), pigmentary changes may occur in the retina with attenuation of retinal vessels and optic nerve pallor25 (Fig. 89.5). Similar to thioridazine, the development and extent of toxicity are more closely related to daily dosage than total amount of drug taken.
Chloroquine derivatives
Chloroquine
Chloroquine was first used as an antimalarial drug in World War II. Currently it is prescribed for treatment of amebiasis, rheumatoid arthritis, and systemic lupus erythematosus in countries primarily outside the United States, and for prophylaxis against malaria. Retinal toxicity with degeneration of the RPE and neurosensory retina as a result of long-term daily use of chloroquine has been well described.29–35 However, most cases of retinopathy have developed when a higher than currently recommended (3 mg/kg/day using lean body weight) dose was used.36A daily dose exceeding 250 mg with a total cumulative dose between 100 and 300 g is customarily needed to produce toxicity.37 One study showed a 19% incidence of chloroquine retinopathy in patients taking a mean daily dose of 329 mg.38 Conversely, with strict adherence to a low dose per diem, the incidence of retinal abnormalities is minimal even when cumulative doses reach over 1000 g.39
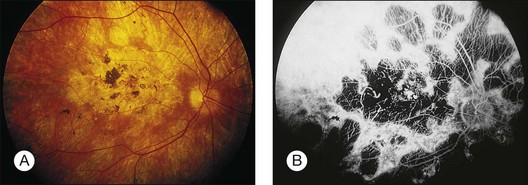
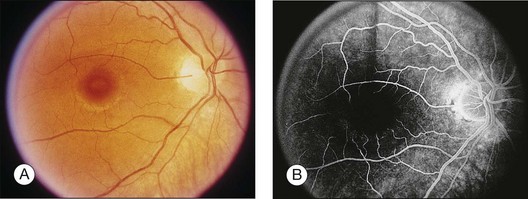
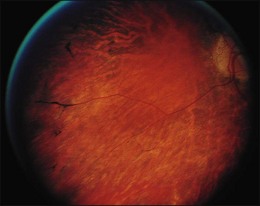
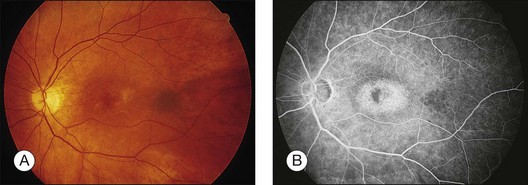
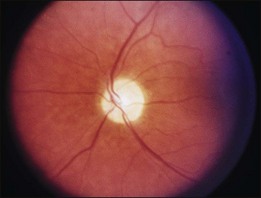
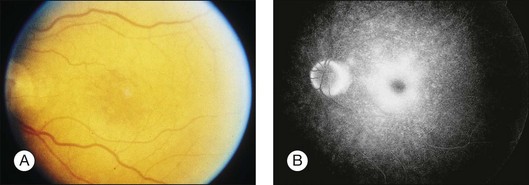
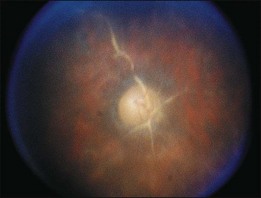
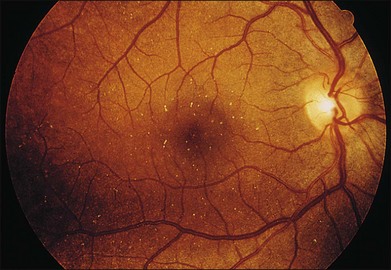
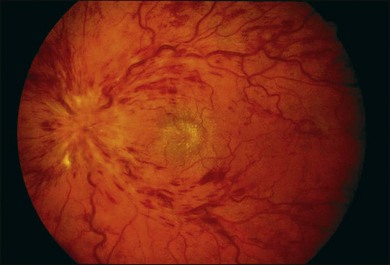
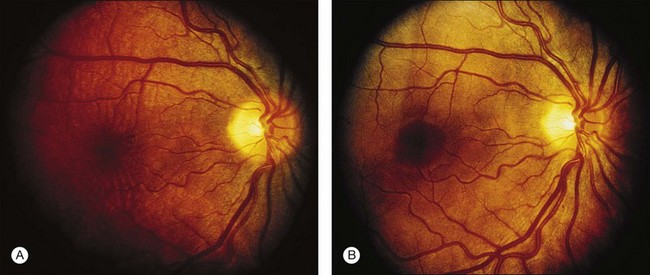
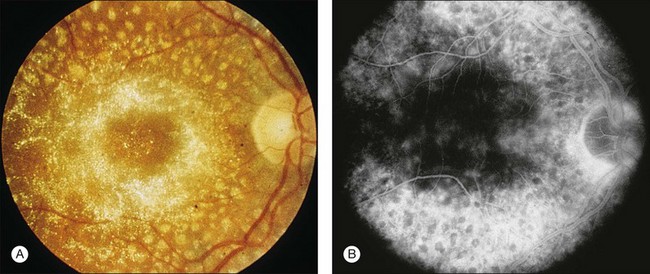
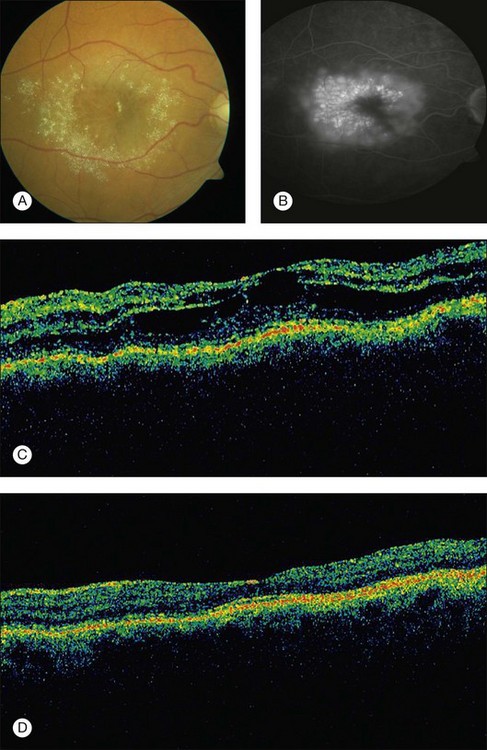
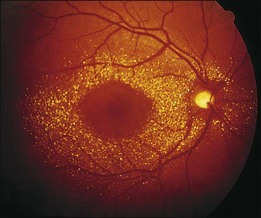
A paracentral scotoma may be the earliest manifestation of retinal toxicity and can precede the development of any ophthalmoscopic or ERG abnormality.40 Subtle macular pigment stippling with a loss of the foveal light reflex (Fig. 89.6) usually appears on fundus examination before the development of a classic bull’s-eye maculopathy, in which a ring of depigmentation surrounded by an area of hyperpigmentation is seen centered on the fovea (Fig. 89.7). Visual acuity decreases when the RPE abnormalities involve the center of the fovea. The peripheral retina can display pigment mottling, which may, in severe cases, develop into the appearance of primary tapetoretinal degeneration with narrowed retinal vessels, optic disc pallor, and eventual blindness (Fig. 89.8).
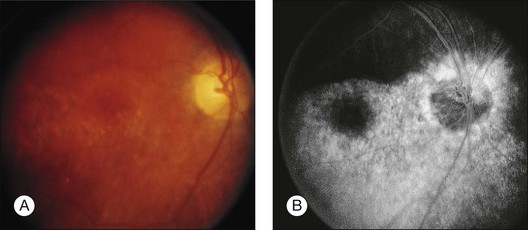
Fig. 89.7 Advanced chloroquine toxicity. Later photograph (A) and fluorescein angiogram (B) from the patient in Fig. 89.6 show marked progression with advanced widespread pigmentary changes.
(Reproduced with permission from Mieler WF. Focal points. American Academy of Ophthalmology, December 1997.)
After the cessation of chloroquine treatment, early subtle macular changes can revert to normal. Although far advanced cases may progress despite discontinuation of the drug, most patients remain stable with long-term follow-up.41,42 Chloroquine, however, is very slowly excreted from the body. It has been detected in the plasma, red blood cells, and urine of patients 5 years after their last known ingestion.43 This prolonged presence may account for the rare cases of delayed onset of chloroquine retinopathy seen up to 7 years or longer after discontinuation.44,45
Fluorescein angiography can be helpful in the early demonstration of pigment abnormalities in the macula (see Figs 89.6, 89.7). There is minimal evidence of damage to the choriocapillaris on FA in the areas of pigment disturbance. The ERG and EOG may be abnormal early, although the EOG is sometimes supernormal initially and is not as helpful diagnostically.46 Histopathologic sections demonstrate loss of RPE pigmentation with an accumulation of pigment-laden cells in the outer retinal layers with damage and reduction of photoreceptors.47 Electron microscopic studies reveal more widespread damage to the retina, especially the ganglion cell layer.48 The retinal nerve fiber layer thickness has been shown to be significantly decreased compared to normal in patients on chloroquine therapy and is correlated to the daily dose taken.49 Fundus autofluorescence (FAF) and optical coherence tomography (OCT) findings suggest that the ganglion cell layer is affected by toxicity initially, especially surrounding the retinal vasculature.50
Like the phenothiazines, chloroquine is bound by melanin and concentrated in the RPE and uveal tissues.51 It appears that chloroquine toxicity may be mediated by disruption of lysosomal function in the RPE and neural retina and by inhibition of critical enzymes and interference with their metabolic function.44,52,53
With the availability of hydroxychloroquine, a less toxic but similar medication, use of chloroquine has steadily waned. Screening for toxicity has been more fully evaluated recently for hydroxychloroquine (see below), and similar testing is likely appropriate for chloroquine as well. Color vision can be abnormal in early toxicity and use of the Standard Pseudoisochromatic Plates Part 2 (SPP-2) or the American Optical Hardy Rand Rittler (AO-HRR) color vision plates provides adequate sensitivity and specificity for detection of abnormalities.54,55 Multifocal ERG testing may be abnormal in early toxicity even when other tests such as visual field and full-field ERG are normal.56,57
Hydroxychloroquine
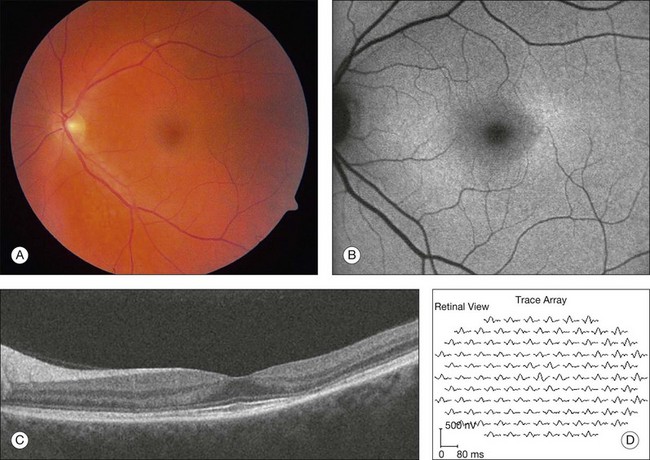
Given the incidence of toxicity with chloroquine, most rheumatologists prefer hydroxychloroquine for the treatment of rheumatoid arthritis and systemic lupus erythematosus. Although it can produce a retinopathy identical to chloroquine, its occurrence is much less common.58–61 Toxicity involving decreased visual acuity, paracentral scotoma, and a bull’s-eye maculopathy has been documented62–67 (Figs 89.9, 89.10). Many of these patients received above the recommended daily dosage of 6.5 mg/kg/day, but the classic fundus findings have been reported at lower doses as well.62,64,67–69 Screening for toxicity becomes more important the longer the patient has been taking the drug as toxicity approaches 1% after 5–7 years of therapy and/or a cumulative dose of 1000 g.70–72
Several authors have questioned the utility of screening given the low yield, high cost, and the difficulty in diagnosing the condition early enough to prevent damage.73–76 Nevertheless, if retinal and functional changes are detected early, severe visual impairment can be averted.69,77
The revised American Academy of Ophthalmology guidelines for screening include a baseline examination performed at the commencement of therapy.78 Screening exams during the first five years of therapy can be performed during routine ophthalmic examination (interval to be determined by the age of the patient and the presence or absence of retinal or macular disease). Earlier recommendations emphasized dosing by weight. As most patients are given 400 mg/day of hydroxychloroquine, this dose is acceptable for all except for those with short stature (generally 5 feet 2 inches or less in height). These patients should be given a dose based on their ideal body weight, otherwise overdosage may occur.79 Furthermore, the dosage may need to be altered if the patient has renal or liver dysfunction.
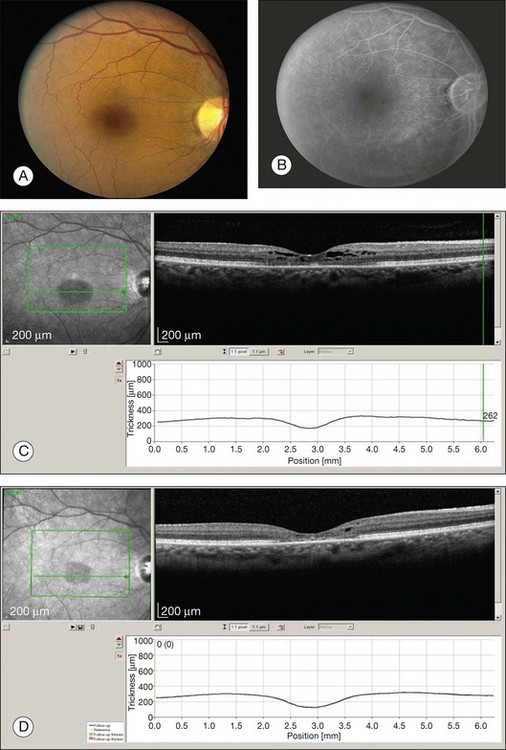
After five years of therapy, screening should be performed at least annually.71 Current guidelines are centered around tests found to detect early toxicity often prior to any appreciable fundus findings. Patients should have a Humphrey 10–2 automated visual field test with a white test object and in addition should have one of three objective tests at each screening: multifocal electroretinogram (mfERG),70,80–84 spectral domain OCT,85–87 and/or FAF88 (Fig. 89.11). Any abnormalities of the pattern deviation on the Humphrey 10–2 visual fields should be taken seriously and the test repeated to confirm its reproducibility. In most situations, and since SD-OCT testing is so readily available, SD-OCT should also be obtained. While abnormalities on FAF are generally associated with concerns for active disease, the test has not yet been shown to be reliably predictable as a screening tool for future toxicity.
As noted in the preceding paragraph, it is imperative to discuss the risk of toxicity with patients and the rationale for screening (to detect, but not necessarily prevent visual loss).78 If ocular toxicity occurs,63,65,66,89 and is recognized at an early stage, efforts should be made to communicate this directly to the prescribing physician so that alternative treatment options can be discussed with the patient. In almost all cases, cessation of the drug should be suggested.
Quinine sulfate
Quinine sulfate was first used for the treatment of malaria in World War II, but it currently is prescribed for the management of nocturnal muscle cramps or “restless leg syndrome”. The recommended daily dose is less than 2 g. Signs of systemic toxicity occur with doses greater than 4 g, and the fatal oral dose is 8 g. Ocular toxicity with quinine develops after an overdose, either by accidental ingestion or by attempted abortion or suicide. Rarely, chronic ingestion at low levels can result in ocular toxicity as well.90 With an overdose, a syndrome known as cinchonism is rapidly produced, consisting of nausea, vomiting, headache, tremor, and sometimes hypotension and loss of consciousness. When patients awake they often are completely blind and have dilated, unreactive pupils.91 In the acute stages of toxicity, fundus examination reveals mild venous dilation with minimal retinal edema and normal arterial caliber. The FA displays minimal abnormalities. ERG testing shows an acute slowing of the a-wave with increased depth, loss of oscillatory potentials, and a decreased b-wave.92 EOG and visual-evoked potential (VEP) testing are also abnormal.
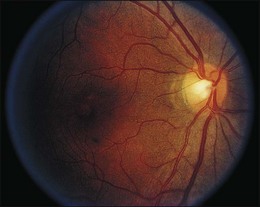
Over the next few days visual acuity returns, but the patient is left with a small central island of vision. There is a progressive attenuation of the retinal arterioles with the development of optic disc pallor over the next few weeks to months and iris depigmentation can occur93 (Fig. 89.12). Early investigators believed the mechanism of quinine toxicity to be vascular in origin. This was based primarily on the fundus appearance several weeks after ingestion, which showed marked arteriolar attenuation and optic disc pallor.91,94 More recent experimental and clinical studies have demonstrated minimal involvement of the retinal vasculature in the early stages of quinine toxicity.91,94,95 Furthermore, ERG and histologic studies show that the site of toxicity is likely the retinal ganglion, bipolar, and photoreceptor cells.91,95 The exact mechanism of quinine toxicity is unidentified, but some have suggested that it may act as an acetylcholine antagonist and disrupt cholinergic transmission in the retina.96
Clofazimine
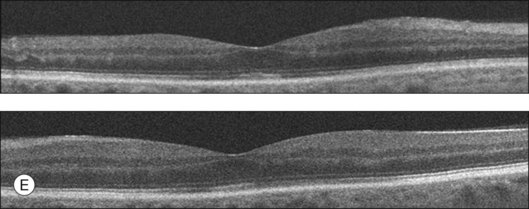
Clofazimine is a red phenazine dye that has been used to treat dapsone-resistant leprosy, psoriasis, pyoderma gangrenosum, discoid lupus, and more recently, Mycobacterium avium-complex infections in AIDS patients. With treatment over several months, clofazimine crystals may accumulate in the cornea. Two cases of bull’s-eye maculopathy with pigmentary retinopathy (Fig. 89.13) have been reported in AIDS patients with doses of 200 to 300 mg/day (total dose, 40–48 g).97,98 Visual acuity was mildly affected, with reduced scotopic, photopic, and flicker ERG amplitudes. Cessation of treatment may result in the clearance of the corneal deposits but does not appear to affect the retinopathy.
Dideoxyinosine (DDI)
A midperipheral pigmentary retinopathy has been noted in three children with AIDS receiving high-dose therapy with the antiviral 2’, 3’-dideoxyinosine.99 The cases were associated with ERG and EOG changes. The retinal toxicity stabilized after discontinuation of the medication. Several cases have now been described involving adult patients as well. A very similar midperipheral pattern of RPE abnormality has been noted (Fig. 89.14).
Deferoxamine
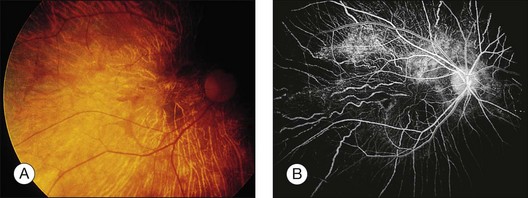
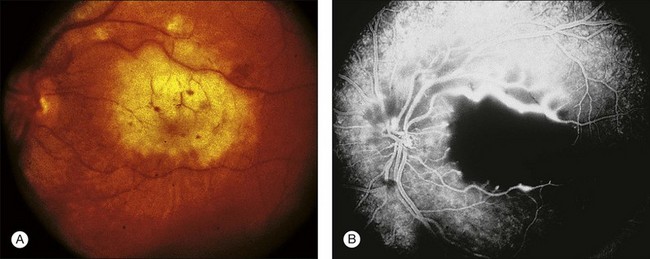
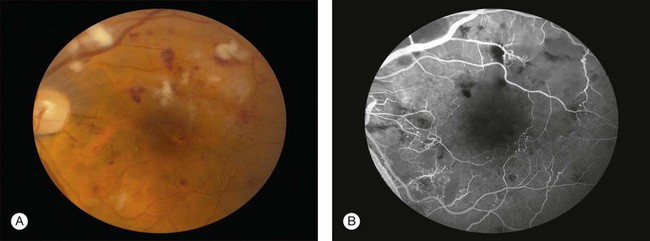
Intravenous (IV) and subcutaneous (SQ) administration of deferoxamine has been used to treat patients who require repeated blood transfusions and subsequently develop complications of iron overload. High-dose IV and SQ therapy has produced visual loss, nyctalopia, peripheral and central field loss, and reduced ERG amplitudes and EOG ratios.100,101 The fundus examination can be normal initially, or there may be a faint graying of the macula.102 Pigmentary changes in the macula and periphery develop within a few weeks and are particularly highlighted by fluorescein angiography103 (Fig. 89.15). Return of visual function occurs with cessation of therapy. Deferoxamine chelates many metals other than iron, and it is possible that the mechanism of toxicity may involve the removal of copper from the RPE.100 Histopathologic changes occur primarily in the RPE and include loss of microvilli from the apical surface, patchy depigmentation, vacuolation of the cytoplasm, swelling and calcification of mitochondria, and disorganization of the plasma membrane.104
Corticosteroid preparations
The vehicles of several common corticosteroid preparations have been shown to cause retinal necrosis when inadvertently injected into the eye105,106 (Fig. 89.16). The corticosteroids themselves probably have a minimal toxic effect on the retina.107 Celestone Soluspan, with its vehicle benzalkonium chloride, and Depo-Medrol, with myristyl gamma-picolinium chloride, caused the most extensive retinal damage in an experimental study comparing several depot steroids.108 If one of these agents is inadvertently injected, immediate surgical removal should be instituted.
Cisplatin and BCNU (carmustine)
Cisplatin and BCNU are used for the treatment of malignant gliomas and metastatic breast cancer. Three different types of retinal toxicity have been reported with these agents. One type of change consists of a pigmentary retinopathy of the macula with markedly decreased visual acuity and frequently abnormal electrophysiologic testing. This pigmentary change has been reported after administration of combined intra-arterial cisplatin and BCNU and with cisplatin alone for malignant glioma.109,110 These findings probably are the result of platinum toxicity of the retina. Severe bilateral visual loss was reported after intravenous cisplatin in a patient that received four times the intended dose for treatment of lymphoma.111 Later histology showed a splitting of the outer plexiform layer.
A second type of retinopathy has been described and consists of cotton-wool spots, intraretinal hemorrhages, macular exudate, and optic neuropathy with disc swelling. This was reported in the setting of high-dose chemotherapy with cisplatin, cyclophosphamide, carmustine, and autologous bone-marrow transplantation for metastatic breast cancer.112 The third type of change involves a vascular retinopathy or optic neuropathy, which can include arterial occlusion, vasculitis, and papillitis. This has been seen in approximately 65% of patients receiving intra-arterial BCNU alone or combined with cisplatin for malignant glioma.110 These fundus changes are associated with a profound visual loss that begins about 6 weeks after the start of therapy. Other ocular effects may include orbital pain, chemosis, secondary glaucoma, internal ophthalmoplegia, and cavernous sinus syndrome. Injection of medication above the ophthalmic artery can still result in toxicity.113 The visual loss usually is progressive, and no treatment is known.
Miscellaneous agents
Overdose of potassium iodate, an iodized salt used for iodine supplementation in areas endemic for goiter, has been shown to cause profound visual loss and extensive fundus pigmentary abnormalities.114 Fluorescein angiography reveals RPE window defects and ERG and VEP testing shows marked impairment of retinal function. Visual acuity may improve slowly over several months. There have been two cases of pigmentary retinopathy with diminished ERG amplitudes reported after administration of denileuken diftitox.115
Vascular damage
Talc
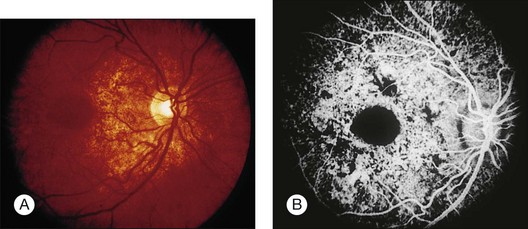
A characteristic retinopathy consisting of small, white, glistening crystals concentrated in the end arterioles of the posterior pole has been described in intravenous (IV) drug abusers116–118 (Fig. 89.17). These addicts crush oral medications such as methylphenidate hydrochloride (Ritalin) or methadone HCl and then create an aqueous suspension by adding water and heating the mixture. The solution is subsequently drawn up into a syringe, with occasional attempts at filtering the mixture with cotton fibers, gauze, or cigarette filters. These oral medications contain talc (hydrous magnesium silicate) as inert filler material; after IV administration, talc particles embolize to the pulmonary vasculature, where the larger particles are trapped. After repeated injections over months to years, collateral vasculature develops, allowing the particles to enter the systemic circulation and embolize to other organs, including the eye. Even before shunt development, particles smaller than 7 µm can traverse the pulmonary capillary bed and enter the retinal circulation.119
Once a large number of talc particles lodge in the small arterioles of the retinal vasculature, a characteristic picture of an ischemic retinopathy begins to develop. Capillary nonperfusion, microaneurysm formation, cotton-wool spots, and venous loops can all be seen.120 In severe cases optic disc and peripheral neovascularization and vitreous hemorrhage can develop121,122 (Fig. 89.18). An experimental model of talc retinopathy in monkeys has demonstrated with light and electron microscopic techniques that the vascular abnormalities induced are very similar to other ischemic retinopathies seen in humans, such as sickle cell and hypertensive retinopathy.123–125
Oral contraceptives
Oral contraceptives have been implicated in some cases of central retinal vein occlusion (CRV), retinal and cilioretinal artery obstruction, and retinal edema occurring in young women.126–132 The synthetic estrogen and progesterone contained in contraceptive pills are thought to adversely effect coagulation factors and induce a hypercoagulable state leading to thromboembolic complications. Most of the studies reporting ocular complications are from the 1960s and 1970s, when the estrogen concentrations used in “the pill” were much higher (Fig. 89.19). Some recent prospective studies have failed to show an increased incidence of ocular complications with the drug, though one large study showed an increase in “retinal vascular findings”.133–135
Aminoglycoside antibiotics
Retinal toxicity from aminoglycoside antibiotics has been reported after inadvertent intraocular injection of massive doses, intravitreal injection for bacterial endophthalmitis, prophylactic intravitreal injection after pars plana vitrectomy, prophylactic subconjunctival injections after routine ocular surgery, and with the use of small amounts in the infusion fluid during cataract extraction.136–139 Gentamicin is the most toxic antibiotic in the aminoglycoside family, followed by tobramycin and amikacin.140 Massive doses result in early superficial and intraretinal hemorrhages, retinal edema, cotton-wool patches, arteriolar narrowing, and venous beading139 (Fig. 89.20). Fluorescein angiography reveals severe vascular nonperfusion in the acute stages. Visual loss is profound, and late rubeosis iridis, neovascular glaucoma, pigmentary retinopathy, and optic atrophy are common. Intravitreal injection of smaller doses thought to be safe for the eye (100–400 µg) can still cause toxicity with less severe fundus changes.137–139 The major preservatives found in injectable gentamicin (methylparaben, propylparaben, sodium bisulfite, and edetate disodium) likely play an additive role in its ocular toxicity.
A number of factors appear to affect the extent of toxicity observed with similar doses of these medications. Peyman found that retinal toxicity could be enhanced with an intravitreal injection directed at the posterior pole with the bevel of the needle pointed toward the retina, and Zachary and Forster demonstrated that an increased rate of injection during intraocular administration could also increase the retinal toxicity observed.141,142 One investigator stated that eyes that have undergone a previous pars plana vitrectomy are at greater risk for gentamicin toxicity, but an experimental model has shown no difference between eyes that had cataract extraction alone compared with those that underwent lensectomy and vitrectomy.143,144 Finally, increased ocular pigmentation protects the rabbit retina from aminoglycoside toxicity and may explain some of the wide variability seen with intraocular exposure in humans.145,146
Although clinical aminoglycoside toxicity appears to affect the retinal vasculature primarily, pathologic studies have revealed that gentamicin in small doses causes the formation of abnormal lamellar lysosomal inclusions in the RPE, and larger doses cause increasing amounts of retinal necrosis, first of the outer then inner segments.147–150 Histologically, vessel closure appears to result from granulocytic plugging.
Prevention of aminoglycoside toxicity can be accomplished by abandoning the use of these medications as routine prophylaxis following intraocular surgery, eliminating them from intraocular infusion fluids used in vitrectomy and cataract surgery, and using alternative medications for the treatment of bacterial endophthalmitis. Animal studies have demonstrated that thinned sclera alone without perforation can result in markedly elevated intraocular gentamicin levels after subconjunctival injection.151 If inadvertent intraocular injection does occur, immediate pars plana vitrectomy with posterior segment lavage should be performed.152,153 Since there is some evidence that gravity plays a role in the predilection of gentamicin-induced toxicity for the macula, the patient should be placed upright as soon as possible after surgery.154
Interferon
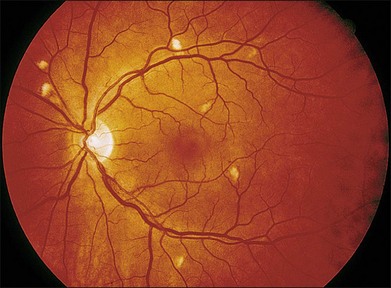
Interferon-α is used to treat Kaposi’s sarcoma, hemangiomas of infancy, chronic hepatitis C, melanoma, renal cell carcinoma and in chemotherapy protocols for leukemia, lymphoma, and hemangiomatosis. Interferon therapy has been associated with the development of multiple cotton-wool spots associated with retinal hemorrhages155–157 (Fig. 89.21). Optic disc edema, branch arterial and venous occlusion, central retinal venous obstruction, anterior ischemic optic neuropathy and CME have been reported with the more severe findings observed in patients receiving high dose therapy.158–162 Visual acuity usually is not affected if the fundus findings are limited to cotton-wool spots and intraretinal hemorrhage. Changes are noted within the first 4–8 weeks of therapy and are seen more frequently in diabetic and hypertensive patients163 (Fig. 89.22).
Intravitreal injection of interferon-α-2b is well tolerated in the rabbit eye up to dosages of 1 million units; 2 million units causes a vitreous haze and intraretinal hemorrhages.164 Interferon toxicity may be caused by an increase in immune complex deposition and activated complement C5a with leukocyte infiltration. EOG testing may become abnormal in early toxicity.165
Miscellaneous agents
Ergot alkaloids in higher than recommended doses have been reported to cause retinal vasoconstriction,166,167 and over-the-counter phenylpropanolamine used in appetite suppressants and decongestants has been implicated in one case of central retinal vein occlusion.168 Gemcitabine is a chemotherapeutic agent used for non-small-cell lung carcinoma, breast, ovarian and pancreatic cancers. It has been associated with one case of Purtscher-like retinopathy169 (Fig. 89.23).
Cystoid macular edema
Epinephrine
The use of epinephrine compounds in glaucoma has decreased with the advent of newer, more efficacious agents. Topical epinephrine can cause macular edema in aphakic eyes, indistinguishable clinically and angiographically from postoperative aphakic cystoid macular edema (CME). In the largest controlled study, 28% of aphakic eyes treated with epinephrine and 13% of untreated aphakic eyes had macular edema, a difference that was statistically significant.170 Most cases of CME resolve with cessation of epinephrine usage. This medication should be avoided in the treatment of the glaucomatous aphakic and pseudophakic eyes.
Nicotinic acid
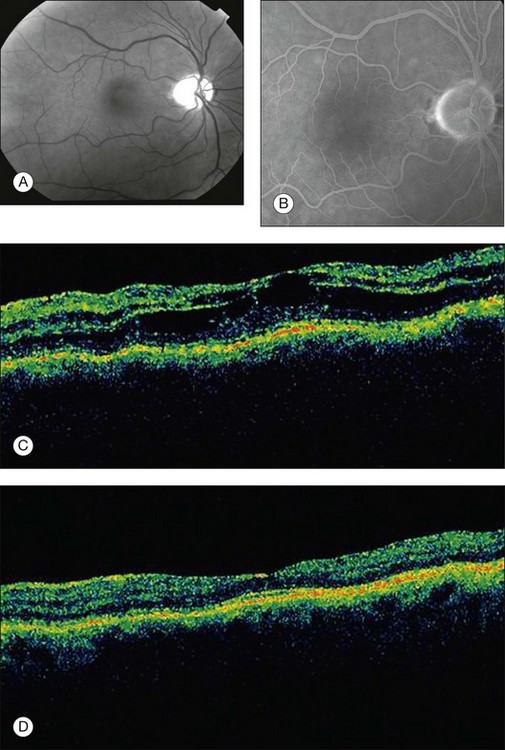
High doses of niacin have been used to reduce serum lipid and cholesterol levels. Better-tolerated HMG-CoA reductase inhibitor agents have curtailed their utilization, though there has been a recent resurgence in their use in both monotherapy and combination therapy with statins. At doses greater than 1.5 g/day, a minority of patients will report blurred central vision, sometimes associated with a paracentral scotoma or metamorphopsia.171 Fluorescein angiography fails to demonstrate vascular leakage despite the typical clinical appearance of CME172,173 (Fig. 89.24). This has led to speculation of a direct toxic effect on Müller cells, resulting in intracellular edema.174 Optical coherence tomography reveals cystoid spaces in the inner nuclear and outer plexiform layers.175,176 With cessation of treatment, the CME resolves, and vision generally returns to normal. Given the rarity of this condition, only patients who are taking high-dose niacin and who have visual symptoms should be evaluated.
Latanoprost
Latanoprost is a prostaglandin analogue that is used for the control of a variety of forms of glaucoma. Although initial human and animal studies did not show an association between latanoprost and CME, recent case reports and studies have documented that approximately 2–5% of susceptible patients with glaucoma may develop CME and anterior uveitis, which resolves after discontinuation of the drug177–185 (Fig. 89.25). This may be caused by the preservative used in the drug formulation.186 Patients with CME who are taking latanoprost should undergo a trial off the medication before initiating further therapy for the edema. High-risk CME patients, such as those with a history of recent surgery or uveitis, should be managed with other agents.
Paclitaxel/docetaxel
Paclitaxel and docetaxel are similar antimicrotubule agents that are used for treatment of breast, lung, and prostate cancer. They have both been associated with angiographically negative CME (Fig. 89.26). The edema appears to respond to treatment with topical or systemic acetazolamide, and/or intravitreal anti-VEGF therapy.187,188
Retinal folds
Sulfa antibiotics, acetazolamide, chlorthalidone, disothiazide, ethoxyzolamide, hydrochlorothiazide, metronidazole, sulphonamide, topiramate, triamterene
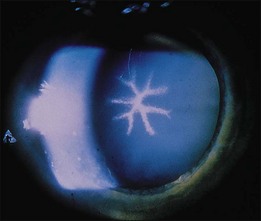
Several medications, most with a structure similar to sulfanilamide, as those above, can cause a syndrome of transient acute myopia and anterior chamber shallowing. This is thought to occur as a result of ciliary body swelling, choroidal effusion, or both, or with swelling of the lens itself with subsequent forward rotation of the lens–iris diaphragm.189–198 Retinal folds in the macula are seen in young patients with this syndrome, but FA does not reveal retinal leakage (Fig. 89.27). The folds presumably develop as a result of vitreous traction on the macula that is caused by the forward shift of the lens and iris.
Crystalline retinopathy
Tamoxifen
Tamoxifen is an antiestrogen agent used in the treatment of estrogen-receptor-positive tumors such as advanced breast carcinoma (and in some estrogen-receptor-negative tumors such as hepatocellular carcinoma) and as adjuvant therapy after surgical resection of early disease. Retinal toxicity consisting of decreased visual acuity and color vision with white intraretinal crystalline deposits, macular edema, and punctate retinal pigmentary changes can occur.199 The intraretinal deposits appear to reside in the inner retina and are most numerous in paramacular areas (Fig. 89.28). Early reports involved patients who had received high doses (60–100 mg/day, total dosage >100 g) of the drug over 1 year.200 More recent studies have demonstrated that chronic low-dose administration (10–20 mg/day) with as little as 7.7 g total, also can cause ocular toxicity.201–205 Even asymptomatic patients may exhibit intraretinal crystalline formation.206 Visual function and edema improve after discontinuation of the drug, but the refractile deposits remain.
There has been a recent upturn in cases of tamoxifen-induced retinal crystals, as patients with aggressive glioblastoma are now being treated with 100–200 mg of tamoxifen on a daily basis (Fig. 89.29). Concurrent cystoid macular edema may be treated with intravitreal anti-VEGF therapy.
Fluorescein angiography demonstrates late focal staining in the macula consistent with CME. Decreased photopic and scotopic a- and b-wave amplitude is noted on ERG testing.207 Light microscopy reveals lesions confined to the nerve fiber and inner plexiform layers, which stain positive for glycosaminoglycans. Small (3–10 µm) intracellular and large (30–35 µm) extracellular lesions within axons are noted on electron microscopy.208 The lesions appear to represent products of axonal degeneration similar to corpora amylacea. Experimentally, tamoxifen inhibits glutamate uptake by RPE cells.209 OCT findings in lower-dose therapy interestingly do not show an increase in macular edema, but rather a foveal cyst with disruption of the photoreceptor line, while high-dose therapy can show CME.210–212
Decreased vision with bilateral optic disc swelling and retinal hemorrhages has been reported in a patient just 3 weeks after commencement of therapy with tamoxifen. These findings resolved completely after the drug was stopped.213 It is unclear whether the findings in this patient were related to the more commonly seen toxic effects. Optic neuropathy with tamoxifen has been reported as well.214 With current low-dose therapy (10–20 mg/day), retinal lesions are rare, and routine examination of asymptomatic patients is not indicated.206,215 If a patient taking tamoxifen is noted to have intraretinal crystals, FA should be performed, primarily to rule out juxtafoveal telangiectasis, which can have similar-appearing lesions.216 With confirmed evidence of toxicity causing a visual disturbance, the medication should be stopped. For persistent macular edema, which has been noted after prolonged high-dose therapy after cessation of the drug, antivascular endothelial growth factor injection may be beneficial.217
Canthaxanthine
Canthaxanthine is a naturally occurring carotenoid. It is used as a food-coloring agent, for skin pigmentation in the treatment of vitiligo, and for the treatment of photosensitivity disorders such as erythropoietic protoporphyria, psoriasis, and photosensitive eczema. It also has been used over-the-counter in high doses as an oral tanning agent. Many reports have described a characteristic ring-shaped deposition of yellow-orange crystals in the superficial retina with high doses (usually a total dose greater than 19 g over 2 years)218–220 (Fig. 89.30). The crystals appear more prominently in eyes with preexisting retinal disease and with concurrent use of beta-carotene.218,221
Patients usually are asymptomatic, and FA usually is normal. There have been published reports of both normal and abnormal ERG, EOG, dark adaptation, and static threshold perimetry.222–225 Although only clinically evident in the macula, the lipid-soluble crystals are found pathologically in the entire inner retina and ciliary body.226 The crystals are, as would be expected, larger and more numerous surrounding the fovea. Canthaxanthine crystals are localized to the spongy degeneration of the inner neuropil and are associated with atrophy of the Müller cells. Interestingly, a single case report showed OCT localization of crystals to the outer plexiform layer.227 An experimental model of canthaxanthine-induced retinopathy also has demonstrated RPE cell vacuolization and disruption of phagolysosomes.228
With discontinuation of treatment, deposits may slowly clear over many years.229,230 This slow reversal correlates with the detection of high plasma levels of canthaxanthine many months after discontinuation of the drug. Rarely, a fundus picture identical to canthaxanthine maculopathy can be seen in patients who have no known history of extradietary canthaxanthine.231 A high dietary intake concurrent with preexisting retinal disease is thought to partially explain this phenomenon.
Methoxyflurane
Methoxyflurane is an inhalational anesthetic, which, if used for extended periods, especially in patients with renal insufficiency, causes irreversible renal failure as a result of deposition of calcium oxalate crystals in the kidney. These crystals are also deposited elsewhere throughout the body. Fundus examination of these patients reveals numerous yellow-white punctate lesions in the posterior pole and periarterially232,233 (Fig. 89.31). The deposits are located histologically in both the RPE and inner retina.234,235
Miscellaneous agents
A single case of crystalline retinopathy following 19 years of nitrofurantoin (Macrodantin) use has been reported.236 Three cases of rapid visual loss with fludarabine phosphate, a nucleoside analog, during treatment for SLE or metastatic melanoma were reported, with two of the cases exhibiting deep yellow retinal flecks.237 Three patients receiving long-term ritonavir therapy as part of a highly active antiretroviral therapy regimen were reported to have a retinal pigment epitheliopathy, parafoveal telangiectasis, and intraretinal crystal deposits.238
Uveitis
Rifabutin
Rifabutin is a semisynthetic rifamycin antibiotic that is used for the treatment and prevention of disseminated Mycobacterium avium-complex (MAC) infection in patients with and without AIDS.239–247 A small percentage of patients treated with higher doses of rifabutin (>450 mg/day) for systemic MAC infection, or lower doses (300 mg/day) for prophylaxis against MAC, can develop uveitis.248 The uveitis usually is bilateral and can be severe enough to cause a hypopyon that simulates infectious endophthalmitis.249 It can occur from 2 weeks to 14 months after initiation of the drug.247 Concomitant use of clarithromycin and/or fluconazole (or itraconazole), especially when lower doses of rifabutin are used, greatly increases the chance of a uveitic episode.250 Both systemic fluconazole and clarithromycin elevate rifabutin levels by inhibiting metabolism of the drug via the hepatic microsomal cytochrome P-450.245 Although most cases have reported mainly an anterior uveitis and corneal endothelial deposits, posterior vitritis and retinal vasculitis have been described as well.239,251
Rifabutin-associated uveitis can be treated successfully with topical corticosteroids or by decreasing or discontinuing the medication. Long-term use may result in ERG abnormalities.252 Patients without systemic MAC infection who are taking rifabutin for prophylaxis and also are taking fluconazole or clarithromycin should be warned about the potential for uveitis and counseled as to its signs and symptoms.
Cidofovir
Cidofovir, also known as HPMPC, is a nucleotide analogue that inhibits viral DNA polymerase and is used for the treatment of cytomegalovirus (CMV) retinitis.253–262 Cidofovir therapy, with both intravenous and intravitreous (20 µg) routes of administration, has been associated with an anterior uveitis, hypotony, and visual loss.263 These complications can be treated and sometimes prevented with the use of topical corticosteroids, cycloplegics, and oral probenecid. Cidofovir has been shown experimentally and clinically to cause a direct toxic effect to the ciliary body, with a resulting iritis and intraocular pressure decrease.253,261 Although a 10 µg intravitreous dose had fewer side effects, it is also much less effective against CMV retinitis.262 Investigations continue to try to determine the optimal dose and route of administration of cidofovir.
Miscellaneous
Cardiac glycosides
Cardiac glycosides such as digoxin are used in the treatment of chronic heart failure and as antiarrhythmic agents. Although these drugs do not cause a characteristic fundus abnormality, ocular symptoms including blurred vision, scintillating scotomas, and xanthopsia (yellowing of vision) are common.264,265 These changes probably are caused by direct toxicity to the photoreceptors. The visual symptoms are reversible with discontinuation of the drug.
Methanol
Methanol occasionally is ingested by alcoholics. Visual blurring and field deficits are seen within 18 hours. Early fundus findings include optic nerve hyperemia and retinal edema, and late findings include optic atrophy8,266–273 (Fig. 89.32). OCT findings have shown the optic nerve head swelling with adjacent edema of the retinal nerve fiber layer.274 Optic nerve toxicity is mediated by formic acid, a breakdown product of methanol, which directly affects the inner retina and optic nerve. The degree of systemic acidosis correlates well with the extent of visual dysfunction. Early hemodialysis is effective in removing methanol from the body, but if visual recovery is not evident by 6 days, it often remains permanently decreased.
Vigabatrin
Vigabatrin is used for treatment of epilepsy, and has been associated with optic atrophy and visual field defects.275,276
Sildenafil, tadalafil, vardenafil
Sildenafil, tadalafil, and vardenafil are a class of drugs that are potent inhibitors of phosophodiesterase-5 (PDE-5) and are used for the treatment of erectile dysfunction. Sildenafil also blocks PDE-6, though with only about 1/10 of its effect on PDE-5. PDE-6 is a key enzyme in the phototransduction cascade, and sildenafil modifies this cascade in photoreceptor outer segments causing a rise in cyclic guanosine monophosphate (cGMP). Commonly, patients notice a bluish dyschromotopsia (dose dependent) 1–2 hours after ingestion and this has been associated with a significant transient depression in the ERG in at least one study, though others have shown minimal to no effect on the ERG.277–286 There appears to be no appreciable effect to slight increase in choroidal circulation with use of sildenafil on the ocular circulation and no adverse effects on those with early age-related macular degeneration.287–292 There have been reported cases of nonarteritic ischemic optic neuropathy, central serous retinopathy, and cilioretinal artery obstruction with use of sildenafil and tadalafil.293–305
1 Weekley RD, Potts AM, Reboton J, et al. Pigmentary retinopathy in patients receiving high doses of a new phenothiazine. Arch Ophthalmol. 1960;64:65–76.
2 Meredith TA, Aaberg TM, Willerson D. Progressive chorioretinopathy after receiving thioridazine. Arch Ophthalmol. 1978;96:1172–1176.
3 Miller FS, III., Bunt-Milam AH, Kalina RE. Clinical-ultrastructural study of thioridazine retinopathy. Ophthalmology. 1982;89:1478–1488.
4 Connell MM, Poley BJ, McFarlane JR. Chorioretinopathy associated with thioridazine therapy. Arch Ophthalmol. 1964;71:816–821.
5 Hagopian V, Stratton DB, Busick RD. Five cases of pigmentary retinopathy associated with thioridazine administration. Am J Psychiatr. 1966;123:97–100.
6 Hamilton D. Thioridazine retinopathy within the upper dosage limit [letter]. Psychosomatics. 1985;26:823–824.
7 Heshe J, Engelstoft FH, Kirk L. Retinal injury developing under thioridazine treatment. Nord Psykiat T. 1961;15:442–447.
8 Lam RW, Remick RA. Pigmentary retinopathy associated with low-dose thioridazine treatment. Can Med Assoc J. 1985;132:737.
9 Neves MS, Jordan K, Dragt H. Extensive chorioretinopathy associated with very low dose thioridazine [letter]. Eye. 1990;4:767–770.
10 Tekell JI, Silva JA, Maas JA, et al. Thioridazine-induced retinopathy. Am J Psychiatry. 1996;153:1234–1235.
11 Miyata M, Imai H, Ishikawa S, et al. Changes in human electroretinography associated with thioridazine administration. Ophthalmologica. 1980;181:175–180.
12 Marmor MF. Is thioridazine retinopathy progressive? Relationship of pigmentary changes to visual function. Br J Ophthalmol. 1990;74:739–742.
13 Chaudhry TA, Shamsi FA, Weitzman ML. Progressive severe visual loss after long-term withdrawal from thioridazine treatment. Eur J Ophthalmol. 2006;16:651–653.
14 Potts AM. Further studies concerning accumulation of polycyclic compounds on uveal melanin. Invest Ophthalmol Vis Sci. 1964;3:399–404.
15 Potts AM. The concentration of phenothiazines in the eye of experimental animals. Invest Ophthalmol Vis Sci. 1962;1:522–530.
16 Potts AM. The reaction of uveal pigment in vitro with polycyclic compounds. Invest Ophthalmol Vis Sci. 1964;3:405–416.
17 Kinross-Wright JT. Clinical trial of a new phenothiazine compound NP-207. Psychiatr Res Rep Am Psychiatr Assoc. 1956;4:89–94.
18 Bonting SL, Caravaggio LL, Canady MR. Studies on sodium potassium–activated adenosine triphosphatase. X. Occurrence in retinal rods and relation to rhodopsin. Exp Eye Res. 1964;3:47–56.
19 Cerletti A, Meier-Ruge W. Toxicological studies on phenothiazine-induced retinopathy. Excerpts Medica International Congress Series. 1968;145:170–188.
20 Muirhead JF. Drug effects on retinal oxidation: retinal alcohol: NAD+ oxidoreductase. Invest Ophthalmol Vis Sci. 1967;6:635–641.
21 Fornaro P, Calabria G, Corallo G, et al. Pathogenesis of degenerative retinopathies induced by thioridazine and other antipsychotics: a dopamine hypothesis. Doc Ophthalmol. 2002;105:41–49.
22 DeLong SL, Poley BJ, McFarlane JR. Ocular changes associated with long-term chlorpromazine therapy. Arch Ophthalmol. 1965;73:611–617.
23 Mathalone MBR. Eye and skin changes in psychiatric patients treated with chlorpromazine. Br J Ophthalmol. 1967;51:86–93.
24 Oshika T. Ocular adverse effects of neuropsychiatric agents: incidence and management. Drug Safety. 1995;12:256–263.
25 Siddal JR. The ocular toxic findings with prolonged and high dosage chlorpromazine intake. Arch Ophthalmol. 1965;74:460–464.
26 Wolf ME, Richer S, Berk MA, et al. Cutaneous and ocular changes associated with the use of chlorpromazine. Int J Clin Pharmacol Ther Toxicol. 1993;31:365–367.
27 Webber SK, Domniz Y, Sutton GL, et al. Corneal deposition after high-dose chlorpromazine hydrochloride therapy. Cornea. 2001;20:217–219.
28 Razeghinejad MR, Nowroozzadeh MH, Zamani M, et al. In vivo observations of chlorpromazine ocular deposits in a pt on long-term chlorpromazine therapy. Clin Exp Ophthalmol. 2008;36:560–563.
29 Cambiaggi A. Unusual ocular lesions in a case of systemic lupus erythematosus. Arch Ophthalmol. 1957;57:451–453.
30 Henkind P, Rothfield NF. Ocular abnormalities in patients treated with synthetic antimalarial drugs. N Engl J Med. 1963;269:433–439.
31 Hobbs HE, Edeadie SP, Sommervile F. Ocular lesions after treatment with chloroquine. Br J Ophthalmol. 1961;45:284–297.
32 Hobbs HE, Sorsby A, Freedman A. Retinopathy following chloroquine therapy. Lancet. 1959;2:478–480.
33 Marks JS. Chloroquine retinopathy: is there a safe daily dose? Ann Rheum Dis. 1982;41:52–58.
34 Nylander U. Ocular damage in chloroquine therapy. Acta Ophthalmol. 1967;92:5–71.
35 Okun E, Gouras P, Bernstein H, et al. Chloroquine retinopathy. Arch Ophthalmol. 1963;69:59–71.
36 Ochsendorf FR, Runne U. Chloroquine: consideration of maximum daily dose (3.5 mg/kg ideal weight) prevents retinopathy. Dermatology. 1996;192:382–383.
37 Tobin DR, Krohel GB, Rynes RL. Hydroxychloroquine: seven-year experience. Arch Ophthalmol. 1982;100:81–83.
38 Finbloom DS, Silver K, Newsome DA, et al. Comparison of hydroxychloroquine and chloroquine use and the development of retinal toxicity. J Rheumatol. 1985;12:692–694.
39 Mackenzie AH, Scherbel AL. A decade of chloroquine maintenance therapy: rate of administration governs incidence of retinotoxicity. Arthritis Rheum. 1968;11:496.
40 Hart WM, Burde RM, Johnston GP, et al. Static perimetry in chloroquine retinopathy: perifoveal patterns of visual field depression. Arch Ophthalmol. 1984;102:377–380.
41 Brinkley JR, Dubois EL, Ryan SJ. Long-term course of chloroquine retinopathy after cessation of medication. Am J Ophthalmol. 1979;88:1–11.
42 Carr RE, Henkind P, Rothfield N, et al. Ocular toxicity of antimalarial drugs. Am J Ophthalmol. 1968;66:738.
43 Rubin M, Bernstein HN, Zvaifler NJ. Studies on the pharmacology of chloroquine. Arch Ophthalmol. 1963;70:80–87.
44 Ehrenfeld M, Nesher R, Merin S. Delayed-onset chloroquine retinopathy. Br J Ophthalmol. 1986;70:281–283.
45 Sassani JW, Brucker AJ, Cobbs W, et al. Progressive chloroquine retinopathy. Ann Ophthalmol. 1983;15:19–22.
46 Heckenlively JR, Matin D, Levy J. Chloroquine retinopathy. Am J Ophthalmol. 1980;89:150.
47 Wetterholm DH, Winter FC. Histopathology of chloroquine retinal toxicity. Arch Ophthalmol. 1964;71:82–87.
48 Ramsey MS, Fine BS. Chloroquine toxicity in the human eye: histopathologic observation by electron microscopy. Am J Ophthalmol. 1972;73:229–235.
49 Bonanomi MT, Dantas NC, Medeiros FA. Retinal nerve fiber layer thickness measurements in patients using chloroquine. Clin Experiment Ophthalmol. 2006;34:130–136.
50 Kellner U, Kellner S, Weinitz S. Chloroquine retinopathy: lipofuscin- and melanin-related fundus autofluorescence, optical coherence tomography and multifocal electroretinography. Doc Ophthalmol. 2008;116:119–127.
51 Bernstein H, Zvaifler N, Rubin M, et al. The ocular deposition of chloroquine. Invest Ophthalmol. 1963;2:384–392.
52 Mahon GJ, Anderson HR, Gardiner TA, et al. Chloroquine causes lysosomal dysfunction in neural retina and implications for retinopathy. Curr Eye Res. 2003;28:277–284.
53 Ivanina TA, Zueva MY, Lebedeva MM, et al. Ultrastructural alterations in rat and cat retina and pigment epithelium induced by chloroquine. Graefes Arch Clin Exp Ophthalmol. 1983;22:32–38.
54 Vu BL, Easterbrook M, Hovis JK. Detection of color vision defects in chloroquine retinopathy. Ophthalmol. 1999;106:1799–1803.
55 Neubauer AS, Samari-Kermani K, Schaller U, et al. Detecting chloroquine retinopathy: electro-oculogram versus colour vision. Br J Ophthalmol. 2002;87:902–908.
56 Kellner U, Kraus H, Forester MH. Multifocal ERG in chloroquine retinopathy: regional variance of retinal dysfunction. Graefes Arch Clin Exp Ophthalmol. 2000;238:94–97.
57 Tzekov R. Ocular toxicity due to chloroquine and hydroxychloroquine. Doc Ophthalmol. 2005;110:111–120.
58 Grierson DJ. Hydroxychloroquine and visual screening in a rheumatology outpatient clinic. Ann Rheum Dis. 1997;56:188–190.
59 Levy GD, Munz SJ, Paschal J, et al. Incidence of hydroxychloroquine retinopathy in 1207 patients in a large multicenter outpatient practice. Arthritis Rheum. 1997;40:1482–1486.
60 Rynes RI. Ophthalmologic considerations in using antimalarials in the United States. Lupus. 1996;5:73–74.
61 Coyle JT. Hydroxychloroquine retinopathy. Ophthalmol. 2001;108:243–244.
62 Falcone PM, Paolini L, Lou PL. Hydroxychloroquine toxicity despite normal dose therapy. Ann Ophthalmol. 1993;25:385–388.
63 Johnson MW, Vine AK. Hydroxychloroquine therapy in massive total doses without retinal toxicity. Am J Ophthalmol. 1987;104:139–144.
64 Mavrikakis M, Papazoglou S, Sfikakis PP, et al. Retinal toxicity in long-term hydroxychloroquine treatment. Ann Rheum Dis. 1996;55:187–189.
65 Shearer RV, Dubois EL. Ocular changes induced by long-term hydroxychloroquine therapy. Am J Ophthalmol. 1967;64:245–252.
66 Weiner A, Sandberg MA, Gaudio AR, et al. Hydroxychloroquine retinopathy. Am J Ophthalmol. 1991;112:528–534.
67 Weiser A, Sandberg MA, Gaadio AR, et al. Hydroxychloroquine retinopathy. Am J Ophthalmol. 1991;121:582–584.
68 Bienfang D, Coblyn JS, Liang MH, et al. Hydroxychloroquine retinopathy despite regular ophthalmologic evaluaton: A consecutive case series. J Rheumatol. 2000;27:2703–2706.
69 Browning DJ. Hydroxychloroquine and chloroquine retinopathy: screening for drug toxicity. Am J Ophthalmol. 2002;133:649–656.
70 Lyons JS, Severns ML. Detection of early hydroxychloroquine retinal toxicity enhanced by ring ratio analysis of multifocal electroretinography. Am J Ophthalmol. 2007;143:801–809.
71 Mavrikakis I, Sfikakis PP, Mavrikakis E, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmol. 2003;110:1321–1326.
72 Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res. 2010;62:775–784.
73 Easterbrook M, Bernstein H. Ophthalmic monitoring of patients taking antimalarials: preferred practice patterns. J Rheumatol. 1997;24:1390–1392.
74 Morsman CDG, Livesey SJ, Richards IM, et al. Screening for hydroxychloroquine retinal toxicity: is it necessary? Eye. 1990;4:572–576.
75 Shipley M, Silman A. Should patients on hydroxychloroquine have their eyes examined regularly? Br J Rheumatol. 1997;30:514–515.
76 Silman A, Shipley M. Ophthalmological monitoring for hydroxy-chloroquine toxicity: a scientific review of available data. Br J Rheumatol. 1997;36:599–601.
77 Easterbrook M. Hydroxychloroquine retinopathy. Ophthalmol. 2001;108:2158–2159.
78 Marmor MF, Kellner U, Lai TYY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmol. 2011;118:415–422.
79 Michaelides M, Stover N, Francis P, et al. Retinal toxicity associated with hydroxychloroquine and chloroquine. Arch Ophthalmol. 2011;129:30–39.
80 Maturi RK, Yu M, Weleber RG. Multifocal electroretinographic evaluation of long-term hydroxychloroquine users. Arch Ophthalmol. 2003;122:973–981.
81 Penrose PF, Tzekov RT, Sutter EE, et al. Multifocal electroretinography evaluation for early detection of retinal dysfunction in patients taking hydroxychloroquine. Retina. 2003;23:503–512.
82 Moschos MN, Moschos MM, Apostolopoulos M, et al. Assessing hydroxychloroquine toxicity by the multifocal ERG. Doc Ophthalmol. 2004;108:47–53.
83 Lai TY, Chan WM, Li H, et al. Multifocal electroretinographic changes in patients receiving hydroxychloroquine therapy. Am J Ophthalmol. 2005;140:794–807.
84 Lai TY, Ngai JW, Chan WM, et al. Visual field and multifocal electroretinography and their correlations in patients on hydroxychloroquine therapy. Doc Ophthalmol. 2006;112:177–187.
85 Rodriguez-Padrilla JA, Hedges TR, 3rd., Monson B, et al. High-speed ultra-high resolution optical coherence tomography findings in hydroxychloroquine retinopathy. Arch Ophthalmol. 2007;125:775–780.
86 Stepien KE, Han DP, Schell J, et al. Spectral-domain optical coherence tomography and adaptive optics may detect hydroxychloroquine retinal toxicity before symptomatic visual loss. Trans Am Ophthalmol Soc. 2009;107:28–33.
87 Pasadhika S, Fishman GA, Choi D, et al. Selective thinning of the perifoveal inner retina as an early sign of hydroxychloroquine retinal toxicity. Eye. 2010;24:75–762.
88 Kellner U, Renner AB, Tillack H. Fundus autofluorescence and mfERG for the early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest Ophthalmol Vis Sci. 2006;47:3531–3538.
89 Maturi RK, Folk JC, Nichols B, et al. Hydroxychloroquine retinopathy. Arch Ophthalmol. 1999;117:1262–1263.
90 Horgan SE, Williams RW. Chronic retinal toxicity due to quinine in Indian tonic water. Eye. 1995;9:637–663.
91 Brinton GS, Nortona EWD, Zahn JR, et al. Ocular quinine toxicity. Am J Ophthalmol. 1980;90:403–410.
92 Lochhead J, Movaffaghy A, Falsini B, et al. The effect of quinine on the electroretinogram of children with pediatric cerebral malaria. J Infect Dis. 2003;187:1342–1345.
93 Traill A, Patmaraj R, Zamir E. Quinine iris toxicity. Arch Ophthalmol. 2007;125:430.
94 Bacon P, Spalton DJ, Smith SE. Blindness from quinine toxicity. Br J Ophthalmol. 1988;72:219–224.
95 Buchanan TAS, Lyness RW, Collins AD, et al. An experimental study of quinine blindness. Eye. 1987;1:522–524.
96 Canning CR, Hague S. Ocular quinine toxicity. Br J Ophthalmol. 1988;72:23–26.
97 Craythorn JM, Swartz M, Creel DJ. Clofazimine-induced bull’s-eye retinopathy. Retina. 1986;6:50–52.
98 Cunningham CA, Friedberg DN, Carr RE. Clofazimine-induced generalized retinal degeneration. Retina. 1990;10:131–134.
99 Whitcup SM, Butler KM, Caruso R, et al. Retinal toxicity in human immunodeficiency virus-infected children treated with 2’,3’-dideoxyinosine. Am J Ophthalmol. 1992;113:1–7.
100 Davies SC, Hungerford JL, Arden GB, et al. Ocular toxicity of high-dose intravenous desferrioxamine. Lancet. 1983;2:181–184.
101 Mehta AM, Engstrom RE, Kreiger AE. Deferoxamine-associated retinopathy after subcutaneous injection. Am J Ophthalmol. 1994;118:260–262.
102 Gass JDM. Stereoscopic atlas of macular diseases: diagnosis and treatment, 4th ed. St Louis: Mosby; 1997.
103 Haimovici R, D’Amico DJ, Gragoudas ES, et al. Deferoxamine Retinopathy Study Group: The expanded clinical spectrum of deferoxamine retinopathy. Ophthalmol. 2002;109:164–171.
104 Rahl AHS, Hungerford JL, Ahmed AI. Ocular toxicity of desferrioxamine: light microscopic histochemical and ultrastructural findings. Br J Ophthalmol. 1986;70:373–381.
105 Hida T, Chandler D, Arena JE, et al. Experimental and clinical observations of the intraocular toxicity of commercial corticosteroid preparations. Am J Ophthalmol. 1986;101:190–195.
106 Pendergast SD, Eliott D, Machemer R. Retinal toxic effects following inadvertent intraocular injection of celestone soluspan. Arch Ophthalmol. 1995;113:1230–1231.
107 McCuen BW, II., Bessler M, Tano Y, et al. The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol. 1981;91:785–788.
108 Piccolino FC, Pandolfo A, Polizzi A, et al. Retinal toxicity from accidental intraocular injection of depomedrol. Retina. 2002;22:117–119.
109 Kupersmith MJ, Seiple WH, Holopigian K, et al. Maculopathy caused by intra-arterially administered cisplatin and intravenously administered carmustine. Am J Ophthalmol. 1992;113:435–438.
110 Miller DF, Bay JW, Lederman RJ, et al. Ocular and orbital toxicity following intracarotid injection of BCNU (carmustine) and cisplatinum for malignant gliomas. Ophthalmology. 1985;92:402–406.
111 Katz BJ, Ward JH, Digre KB, et al. Persistent severe visual and electroretinographic abnormalities after intravenous Cisplatin therapy. J Neruoophthalmol. 2003;23:132–135.
112 Khawly JA, Rubin P, Petros W, et al. Retinopathy and optic neuropathy in bone marrow transplantation for breast cancer. Ophthalmology. 1996;103:87–95.
113 Margo CE, Murtagh FR. Ocular and orbital toxicity after intra-carotid cisplatin therapy. Am J Ophthalmol. 1993;116:508–509.
114 Singalavaniga A, Ruangvaravate N, Dulayajinda D. Potassium iodate toxic retinopathy: a report of five cases. Retina. 2000;20:378–383.
115 Ruddle JB, Harper CA, Honemann D, et al. A denileukin diftitox (Ontak) associated retinopathy? Br J Ophthalmol. 2006;90:1070–1071.
116 AtLee WE. Talc and cornstarch emboli in eyes of drug users. JAMA. 1972;219:49.
117 Murphy SB, Jackson WB, Pare JAP. Talc retinopathy. Can J Ophthalmol. 1978;13:152–156.
118 Tse DT, Ober RR. Talc retinopathy. Am J Ophthalmol. 1980;90:624–640.
119 Schatz H, Drake M. Self-injected retinal emboli. Ophthalmology. 1979;86:468.
120 Friberg TR, Gragoudas ES, Regan CDJ. Talc emboli and macular ischemia in intravenous drug abuse. Arch Ophthalmol. 1979;97:1089.
121 Brucker AJ. Disk and peripheral retinal neovascularization secondary to talc and cornstarch emboli. Am J Ophthalmol. 1979;88:864.
122 Kresca LJ, Goldberg MF, Jampol LM. Talc emboli and retinal neovascularization in a drug abuser. Am J Ophthalmol. 1979;87:334.
123 Jampol LM, Setogawa T, Rednam KRV, et al. Talc retinopathy in primates: a model of ischemic retinopathy. I. Clinical studies. Arch Ophthalmol. 1981;99:1273–1280.
124 Kaga N, Tso MOM, Jampol LM, et al. Talc retinopathy in primates: a model of ischemic retinopathy. II. A histopathologic study. Arch Ophthalmol. 1982;100:1644–1648.
125 Kaga N, Tso MOM, Jampol LM. Talc retinopathy in primates: a model of ischemic retinopathy. III. An electron microscopic study. Arch Ophthalmol. 1982;100:1649–1657.
126 Gombos GM, Moreno DH, Bedrossian PB. Retinal vascular occlusion induced by oral contraceptives. Ann Ophthalmol. 1975;7:215–217.
127 Goren GB. Retinal edema secondary to oral contraceptives. Am J Ophthalmol. 1967;64:447–449.
128 Lyle TK, Wybar K. Retinal vasculitis. Br J Ophthalmol. 1961;45:778–788.
129 Perry HD, Mallen FJ. Cilioretinal artery occlusion associated with oral contraceptives. Am J Ophthalmol. 1977;84:56–58.
130 Stowe GC, Jakov AN, Albert DM. Central retinal vascular occlusion associated with oral contraceptives. Am J Ophthalmol. 1978;86:798–801.
131 Varga M. Recent experiences on the ophthalmological complications of oral contraceptives. Ann Ophthalmol. 1976;8:925–934.
132 Walsh FB, Clark DB, Thompson RS, et al. Oral contraceptives and neuro-ophthalmologic interest. Arch Ophthalmol. 1965;74:628–640.
133 Garg SK, Chase P, Marshall G, et al. Oral contraceptives and renal and retinal complications in young women with insulin-dependent diabetes mellitus. JAMA. 1994;271:1099–1102.
134 Petersson GJ, Fraunfelder FT, Meyer SM. Oral contraceptives. Ophthalmology. 1981;88:368–371.
135 Vessey MP, Hannaford P, Mant J, et al. Oral contraception and eye disease: findings in two large cohort studies. Br J Ophthalmol. 1998;82:538–542.
136 Balian JV. Accidental intraocular tobramycin injection: a case report. Ophthalmic Surg. 1983;14:353–354.
137 Campochiaro PA, Conway BP. Aminoglycoside toxicity – a survey of retinal specialists: implications for ocular use. Arch Ophthalmol. 1991;109:946–950.
138 Campochiaro PA, Lim JI. Aminoglycoside toxicity in the treatment of endophthalmitis. Arch Ophthalmol. 1994;112:48–53.
139 McDonald HR, Schatz H, Allen AW, et al. Retinal toxicity secondary to intraocular gentamicin. Ophthalmology. 1986;93:871–877.
140 D’Amico DJ, Caspers-Velu L, Libert J, et al. Comparative toxicity of intravitreal aminoglycoside antibiotics. Am J Ophthalmol. 1985;100:264–275.
141 Peyman GA, Vastine DW, Crouch ER, et al. Clinical use of intravitreal antibiotics to treat bacterial endophthalmitis. Trans Am Acad Ophthalmol Otolaryngol. 1974;78:862–875.
142 Zachary IG, Forster RK. Experimental intravitreal gentamicin. Am J Ophthalmol. 1976;82:604–611.
143 Rosenbaum JD, Krumholz DM, Metz DM. Gentamicin retinal toxicity after cataract surgery in an eye that underwent vitrectomy. Ophthalmic Surg Laser. 1997;28:236–238.
144 Talamo JH, D’Amico DJ, Hanninen LA, et al. The influence of aphakia and vitrectomy on experimental retinal toxicity of aminoglycoside antibiotics. Am J Ophthalmol. 1985;100:840–847.
145 Kane A, Barza M, Baum J. Intravitreal injection of gentamicin in rabbits: effect of inflammation and pigmentation on half-life and ocular distribution. Invest Ophthalmol Vis Sci. 1981;20:593–597.
146 Zemel E, Loewenstein A, Lei B, et al. Ocular pigmentation protects the rabbit retina from gentamicin-induced toxicity. Invest Ophthalmol Vis Sci. 1995;36:1875–1884.
147 Brown GC, Eagle RC, Shakin EP, et al. Retinal toxicity of intravitreal gentamicin. Arch Ophthalmol. 1990;108:1740–1744.
148 D’Amico DJ, Libert J, Kenyon KR, et al. Retinal toxicity of intravitreal gentamicin: an electron microscopic study. Invest Ophthalmol Vis Sci. 1984;25:564–572.
149 Hines J, Vinores SA, Campochiaro PA. Evolution of morphologic changes after intravitreous injection of gentamicin. Curr Eye Res. 1993;12:521–529.
150 Conway BP, Tabatabay CA, Campochiaro PA, et al. Gentamicin toxicity in the primate retina. Arch Ophthalmol. 1989;107:107–112.
151 Loewenstein A, Zemel E, Vered Y, et al. Retinal toxicity of gentamicin after subconjunctival injection performed adjacent to thinned sclera. Ophthalmol. 2001;108:759–764.
152 Chu TG, Ferreira M, Ober RR. Immediate pars plana vitrectomy in the management of inadvertent intracameral injection of gentamicin: a rabbit experimental model. Retina. 1994;14:59–64.
153 Burgansky Z, Rock T, Bartov E. Inadvertent intravitreal gentamicin injection. Eur J Ophthalmol. 2002;12:138–140.
154 Lim JI, Anderson CT, Hutchinson A, et al. The role of gravity in gentamicin-induced toxic effects in a rabbit model. Arch Ophthalmol. 1994;112:1363–1367.
155 Guyer DR, Tiedeman J, Yannuzzi LA, et al. Interferon-associated retinopathy. Arch Ophthalmol. 1993;111:350–356.
156 Kawano T, Shegehira M, Uto H, et al. Retinal complications during interferon therapy for chronic hepatitis C. Am J Gastroenterol. 1996;91:309–313.
157 Schulman JA, Liang C, Kooragayala LM, et al. Posterior segment complications in patients with hepatitis C treated with interferon and ribavirin. Ophthalmol. 2003;110:437–442.
158 Kiratli H, Irkee M. Presumed interferon-associated bilateral macular arterial branch obstruction. Eye. 2000;14:920–922.
159 Tokai R, Ikeda T, Miyaura T, et al. Interferon-associated retinopathy and cystoid macular edema. Arch Ophthalmol. 2001;119:1077–1079.
160 Hejny C, Sternberg P, Lawson DH, et al. Retinopathy associated with high-dose interferon alfa-2b therapy. Am J Ophthalmol. 2001;131:782–787.
161 Fraunfelder FW, Fraunfelder FT. Interferon alfa-associated anterior ischemic optic neuropathy. Ophthalmology. 2011;118:408–411.
162 Rubio JE, Charles S. Interferon-associated combined branch retinal artery and central retinal vein obstruction. Retina. 2003;23:546–548.
163 Wilson RL, Ross RD, Wilson LM, et al. Interferon-associated retinopathy in a young, insulin-dependent diabetic patient. Retina. 2000;20:413–415.
164 Kertes PJ, Britton WA, Addison DJ, et al. Toxicity of intravitreal interferon alpha-2b in the rabbit. Can J Ophthalmol. 1995;30:355–359.
165 Crochet M, Ingster-Moati I, Even G, et al. Retinography caused by interferon alpha associated ribavirin therapy and the importance of the electro-oculogram: a case report. J Fr Ophthalmol. 2004;27:257–262.
166 Gupta DR, Strobos RJ. Bilateral papillitis associated with Cafergot therapy. Neurology. 1972;22:793.
167 Mindel JS, Rubenstein AE, Franklin B. Ocular ergotamine tartrate toxicity during treatment of Vacor-induced orthostatic hypotension. Am J Ophthalmol. 1981;92:492–496.
168 Gilmer G, Swartz M, Teske M, et al. Over-the-counter phenylpropanolamine: a possible cause of central retinal vein occlusion. Arch Ophthalmol. 1986;104:642.
169 Banach MJ, Williams GA. Purtscher retinopathy and necrotizing vasculitis with gemcitabine therapy. Arch Ophthalmol. 2000;118:726–727.
170 Thomas JV, Gragoudas ES, Blair NP, et al. Correlation of epinephrine use and macular edema in aphakic glaucomatous eyes. Arch Ophthalmol. 1978;96:625–628.
171 Fraunfelder FW, Fraunfelder FT, Illingworth DR. Adverse ocular effects associated with niacin therapy. Br J Ophthalmol. 1995;79:54–56.
172 Gass JDM. Nicotinic acid maculopathy. Am J Ophthalmol. 1973;76:500–510.
173 Millay RH, Klein ML, Illingworth DR. Niacin maculopathy. Ophthalmology. 1988;95:930–936.
174 Jampol LM. Niacin maculopathy. Ophthalmology. 1988;95:1704–1705.
175 Spirn MJ, Warren FA, Guyer DR, et al. Optical coherence tomography findings in nicotinic acid maculopathy. Am J Ophthalmol. 2003;135:913–914.
176 Dajani HM, Lauer AK. Optical coherence tomography findings in niacin maculopathy. Can J Ophthalmol. 2006;41:197–200.
177 Hoyng PFJ, Rulo AH, Greve EL, et al. Fluorescein angiographic evaluation of the effect of latanoprost treatment on blood–retinal barrier integrity: a review of studies conducted on pseudophakic glaucoma patients and on phakic and aphakic monkeys. Surv Ophthalmol. 1997;41(Suppl 2):83–88.
178 Moroi S, Gottfredsdottir MS, Johnson MW, et al. Anterior uveitis and cystoid macular edema associated with latanoprost. Am Acad Ophthalmol Abstracts. 172, 1997.
179 Rowe JA, Hattenhauer MG, Herman DC. Adverse side effects associated with latanoprost. Am J Ophthalmol. 1997;124:683–685.
180 Warwar RE, Bullock JD, Ballal D. Cystoid macular edema and anterior uveitis associated with latanoprost use. Ophthalmology. 1998;105:263–268.
181 Halpern DL, Pasquale LR. Cystoid macular edema in aphakia and pseudophakia after use of prostaglandin analogs. Semin Ophthalmol. 2002;17:181–186.
182 Wand M, Gaudio AR, Shields MB. Latanoprost and cystoid macular edema in high-risk aphakic or pseudophakic eyes. J Cataract Refract Surg. 2001;27:1397–1401.
183 Furuichi M, Chiba T, Abe K, et al. Cystioid macular edema associated with topical latanaprost in glaucomatous eyes with a normally functioning blood–ocular barrier. J Glaucoma. 2001;10:233–236.
184 Lima MC, Paranhos A, Jr., Salim S, et al. Visually significant cystoid macular edema in pseudophakic and aphakic patients with glaucoma receiving latanaprost. J Glaucoma. 2000;9:317–321.
185 Schumer RA, Camras CB, Mandahl AK. Latanoprost and cystoid macular edema: is there a causal relation? Curr Opin Ophthalmol. 2000;11:94–100.
186 Miyake K, Ibaraki N. Prostaglandins and cystoid macular edema. Surv Ophthalmol. 2002;47(Suppl1):S203–S218.
187 Joshi MM, Garretson B. Paclitaxel retinopathy. Arch Ophthalmol. 2007;125:709–710.
188 Telender DG, Sarraf D. Cystoid macular edema with Docetaxel chemotherapy and the fluid retention syndrome. Semin Ophthalmol. 2007;22:151–153.
189 Grinbaum A, Ashkenazi I, Avni I, et al. Transient myopia following metronidazole treatment for trichomonas vaginalis. JAMA. 1992;267:511–512.
190 Ryan EH, Jampol LM. Drug-induced acute transient myopia with retinal folds. Retina. 1986;6:220–223.
191 Soylev MF, Green RL, Feldon SE. Choroidal effusion as a mechanism for transient myopia induced by hydrochlorothiazide and triamterene. Am J Ophthalmol. 1995;120:395–397.
192 Sen HA, O’Halloran HS, Lee WB. Case reports and small case series: topiramate-induced acute myopia and retinal striae. Arch Ophthalmol. 2001;119:775–777.
193 Rhee DJ, Goldberg MJ, Parrish RK. Bilateral angle-closure glaucoma and ciliary body swelling from topiramate. Arch Ophthalmol. 2001;119:1721–1723.
194 Sankar PS, Pasquale LR, Grosskreutz CL. Uveal effusion and secondary angle-closure glaucoma associated with topiramate use. Arch Ophthalmol. 2001;119:1210–1211.
195 Medeiros FA, Zhang XY, Bernd AS, et al. Angle-closure glaucoma associated with ciliary body detachment in patients using topiramate. Arch Ophthalmol. 2003;121:282–285.
196 Fraunfelder FW, Fraunfelder FT, Keates EU. Topiramate-associated acute, bilateral, secondary angle-closure glaucoma. Ophthalmol. 2004;111:109–111.
197 Craig JE, Ong TJ, Louis DL, et al. Mechanism of topiramate-induced acute-onset myopia and angle closure glaucoma. Am J Ophthalmol. 2004;137:193–195.
198 Mahesh G, Giridhar A, Saikumar SJ, et al. Drug-induced acute myopia following chlorthalidone treatment. Indian J Ophthalmol. 2007;55:386–388.
199 Alwitry A, Gardner I. Tamoxifen maculopathy. Arch Ophthalmol. 2002;120:1402.
200 Kaiser-Kupfer MI, Kupfer C, Rodrigues MM. Tamoxifen retinopathy. Cancer Treat Rep. 1978;62:315–320.
201 Chang T, Gonder JR, Ventresca MR. Low-dose tamoxifen retinopathy. Can J Ophthalmol. 1992;27:148–149.
202 Griffiths MFP. Tamoxifen retinopathy at low dosage. Am J Ophthalmol. 1987;104:185–186.
203 Pavlidis NA, Petris C, Briassoulis E, et al. Clear evidence that long-term, low-dose tamoxifen treatment can induce ocular toxicity. Cancer. 1992;69:2961–2964.
204 Noureddin BN, Seoud M, Bashshur Z, et al. Ocular toxicity in low-dose tamoxifen: a prospective study. Eye. 1999;13:729–733.
205 Yanyali AC, Freund KB, Sorenson JA, et al. Tamoxifen retinopathy in a male patient. Am J Ophthalmol. 2001;131:386–387.
206 Heier JS, Dragoo RA, Enzenauer RW, et al. Screening for ocular toxicity in asymptomatic patients treated with tamoxifen. Am J Ophthalmol. 1994;117:772–775.
207 McKeown CA, Swartz M, Blom J, et al. Tamoxifen retinopathy. Br J Ophthalmol. 1981;65:177–179.
208 Kaiser-Kupfer MI, Kupfer C, Rodrigues MM. Tamoxifen retinopathy: a clinicopathologic report. Ophthalmology. 1981;88:89–93.
209 Maenpaa H, Mannerstrom M, Toimela T, et al. Glutamate uptake is inhibited by tamoxifen and toremifene in cultured retinal pigment epithelial cells. Pharmacol Toxicol. 2002;91:116–122.
210 Gualino V, Cohen SY, Delyfer MN, et al. Optical coherence tomography findings in tamoxifen retinopathy. Am J Ophthalmol. 2005;140:757–768.
211 Park SS, Zawadzki RJ, Truong SN, et al. Microcystoid maculopathy associated with tamoxifen use diagnoses by high-resolution fourier-domain optical coherence tomography. Retin Cases Brief Rep. 2009;3:33–35.
212 Bourla DH, Sarraf D, Schwartz SD. Peripheral retinopathy and maculopathy in high-dose tamoxifen therapy. Am J Ophthalmol. 2007;144:126–128.
213 Ashford AR, Donev I, Tiwari RP, et al. Reversible ocular toxicity related to tamoxifen therapy. Cancer. 1988;61:33–35.
214 Colley SM, Elston JS. Tamoxifen optic neuropathy. Clin Exp Ophthalmol. 2004;32:105–106.
215 Nayfield SG, Gorin MB. Tamoxifen-associated eye disease: a review. J Clin Oncol. 1996;14:1018–1026.
216 Kalina RE, Wells CG. Screening for ocular toxicity in asymptomatic patients with tamoxifen [letter]. Am J Ophthalmol. 1995;119:112–113.
217 Bourla DH, Gonzales CR, Mango CW, et al. Intravitreous vascular endothelial growth factor (VEGF) inhibitor therapy for tamoxifen induced macular edema. Semin Ophthalmol. 2007;22:87–88.
218 Chang TS, Aylward W, Clarkson JG, et al. Asymmetric canthaxanthine retinopathy. Am J Ophthalmol. 1995;119:801–802.
219 Lonn LI. Canthaxanthine retinopathy. Arch Ophthalmol. 1987;105:1590–1591.
220 Espaillat A, Aiello LP, Arrigg PG, et al. Canthaxanthine retinopathy. Arch Ophthalmol. 1999;117:412–413.
221 Cortin P, Boudreault G, Rousseau AP, et al. La retinopathie a la canthaxanthine. II. Facteurs predisposants. Can J Ophthalmol. 1984;19:215–219.
222 Boudreault G, Cortin P, Corriveau LA, et al. La retinopathie a la canthaxanthine. I. Etude clinique de 51 consommateurs. Can J Ophthalmol. 1983;18:325–328.
223 Harnois C, Cortin P, Samson J, et al. Static perimetry in canthaxanthine maculopathy. Arch Ophthalmol. 1988;106:58–60.
224 Metge P, Mandirac-Bonnefoy C, Bellaube P. Thesaurismose retinienne à la canthaxanthine. Bull Mem Soc Fr Ophtalmol. 1984;95:547–549.
225 Weber U, Goerz G, Hennekes R. Carotenoid retinopathie. I. Morphologische und funktionell befunde. Klin Monatsbl Augenheilkd. 1985;186:351–354.
226 Daicker B, Schiedt K, Adnet JJ, et al. Canthaxanthin retinopathy: an investigation by light and electron microscopy and physiochemical analysis. Graefes Arch Clin Exp Ophthalmol. 1987;225:189–197.
227 Chan A, Duker JS. Ultrahigh-resolution optical coherence tomography of canthaxanthin retinal crystals. Ophthalmic Surg Lasers Imaging. 2006;37:138–139.
228 Scallon LJ, Burke JM, Mieler WF, et al. Canthaxanthine-induced retinal pigment epithelial changes in the cat. Curr Eye Res. 1988;7:687–693.
229 Harnois C, Samson J, Malenfant M, et al. Canthaxanthine retinopathy: anatomic and functional reversibility. Arch Ophthalmol. 1989;107:538–540.
230 Leyon H, Ros A, Nyberg S, et al. Reversibility of canthaxanthin deposits within the retina. Acta Ophthalmol. 1990;68:607–611.
231 Oosterhuis JA, Remky H, Nijman NM, et al. Canthaxanthine-retinopathie ohne canthaxanthine-einnahime. Klin Monatsbl Augenheilkd. 1989;194:110–116.
232 Bullock JD, Albert DM. Fleck retina: appearance secondary to oxalate crystals from methoxyflurane anesthesia. Arch Ophthalmol. 1975;93:26–31.
233 Novak MA, Roth AS, Levine MR. Calcium oxalate retinopathy associated with methoxyflurane abuse. Retina. 1988;8:230–236.
234 Albert DM, Bullock JD, Lahav M, et al. Flecked retina secondary to oxalate crystals from methoxyflurane anesthesia: clinical and experimental studies. Trans Am Acad Ophthalmol Otolaryngol. 1975;79:817–826.
235 Wells CG, Johnson RJ, Qingli L, et al. Retinal oxalosis: a clinicopathological report. Arch Ophthalmol. 1989;107:1638–1643.
236 Ibanez HE, Williams DF, Boniuk I. Crystalline retinopathy associated with long-term nitrofurantoin therapy. Arch Ophthalmol. 1994;112:304–305.
237 Bishop R, Ding X, Heller C, et al. Rapid vision loss associated with fludarabine administration. Retina. 2010;30:1272–1277.
238 Roe RH, Jumper JM, Gualino V, et al. Retinal pigment epitheliopathy, macular telangiectasis, intraretinal crystal deposits in HIV-positive patients receiving ritonavir. Retina. 2011;31:559–565.
239 Arevalo JF, Russack V, Freeman WR. New ophthalmic manifestations of presumed rifabutin-related uveitis. Ophthalmic Surg Laser. 1997;28:321–324.
240 Becker K, Schimkat M, Jablonowski H, et al. Anterior uveitis associated with rifabutin medication in AIDS patients. Infection. 1996;24:36–38.
241 Jacobs DS, Piliero PJ, Kuperwaser MG, et al. Acute uveitis associated with rifabutin use in patients with human immunodeficiency virus infection. Am J Ophthalmol. 1994;118:716–722.
242 Karbassi M, Nikou S. Acute uveitis in patients with acquired immunodeficiency syndrome receiving prophylactic rifabutin. Arch Ophthalmol. 1995;113:699–701.
243 Kelleher P, Helbert M, Sweeney J, et al. Uveitis associated with rifabutin and macrolide therapy for Mycobacterium avium-intracellulare infection in AIDS patients. Genitourin Med. 1996;72:419–421.
244 Nichols CW. Mycobacterium avium complex infection, rifabutin, uveitis: is there a connection? Clin Infect Dis. 1996;22:43–49.
245 Rifai A, Peyman GA, Daun M, et al. Rifabutin-associated uveitis during prophylaxis for Mycobacterium avium complex infection. Arch Ophthalmol. 1995;113:707.
246 Saran BR, Maguire AM, Nichols C, et al. Hypopyon uveitis in patients with acquired immunodeficiency syndrome: treatment for systemic Mycobacterium avium complex infection with rifabutin. Arch Ophthalmol. 1994;112:1159–1165.
247 Tseng AL, Walmsley SL. Rifabutin-associated uveitis. Ann Pharmacother. 1995;29:1149–1155.
248 Bhagat N, Read RW, Rao NA, et al. Rifabutin-associated hypopyon uveitis in human immunodeficiency virus-negative immunocompetent individuals. Ophthalmol. 2001;108:750–752.
249 Khan MA, Singh J, Dhillon B. Rifabutin-induced uveitis with inflammatory vitreous infiltrate. Eye. 2000;14:344–346.
250 Saha N, Bansal S, Bishop F, et al. Bilateral hypopyon and vitritis associated with rifabutin therapy in an immunocompetent patient taking itraconazole. Eye. 2009;23(6):1481.
251 Chaknis MJ, Brooks SE, Mitchell KT, et al. Inflammatory opacities of the vitreous in rifabutin-associated uveitis. Am J Ophthalmol. 1996;122:580–582.
252 Ponjavic V, Granse L, Bengtsson Stigmar E, et al. Retinal dysfunction and anterior sefment depostits in a patient treated with rifabutin. Acta Ophthalmol Scand. 2002;80:553–556.
253 Banker AS, Arevalo JF, Munguia D, et al. Intraocular pressure and aqueous humor dynamics in patients with AIDS treated with intravitreal cidofovir (HPMPC) for cytomegalovirus retinitis. Am J Ophthalmol. 1997;124:168–180.
254 Davis JL, Taskintuna I, Freeman WR, et al. Iritis and hypotony after treatment with intravenous cidofovir for cytomegalovirus retinitis. Arch Ophthalmol. 1997;115:733–737.
255 Friedberg DN. Hypotony and visual loss with intravenous cidofovir treatment of cytomegalovirus retinitis. Arch Ophthalmol. 1997;115:801–802.
256 Jabs DA. Cidofovir. Arch Ophthalmol. 1997;115:785–786.
257 Kirsch LS, Arevalo JF, De Clercq E, et al. Phase I/II study of intra-vitreal cidofovir for the treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1995;119:466–476.
258 Kirsch LS, Arevalo JF, de la Paz EC. Intravitreal cidofovir (HPMPC) treatment of cytomegalovirus retinitis in patients with acquired immune deficiency syndrome. Ophthalmology. 1995;102:533–543.
259 Lea AP, Bryson HM. Cidofovir. Drugs. 1996;52:225–230.
260 Rahal FM, Arevalo JF, Munguia D, et al. Intravitreal cidofovir for the maintenance treatment of cytomegalovirus retinitis. Ophthalmology. 1996;103:1078–1083.
261 Taskintuna I, Banker AS, Rao NA, et al. An animal model for cidofovir (HPMPC) toxicity: intraocular pressure and histopathologic effects. Exp Eye Res. 1997;64:795–806.
262 Taskintuna I, Rahhal FM, Arevalo F, et al. Low-dose intravitreal cidofovir (HPMPC) therapy of cytomegalovirus retinitis in patients with acquired immune deficiency syndrome. Ophthalmology. 1997;104:1049–1057.
263 Wang L, Damji KF, Chialant D, et al. Hypotony after intravenous cidofovir therapy for the treatment of cytomegalovirus retinitis. Can J Ophthalmol. 2002;37:419–422.
264 Blair JR, Mieler WF. Retinal toxicity associated with commonly encountered systemic agents. Int Ophthalmol Clin. 1995;35:137–156.
265 Weleber RG, Shults WT. Digoxin retinal toxicity: clinical and electrophysiologic evaluation of a cone dysfunction syndrome. Arch Ophthalmol. 1981;99:1568–1572.
266 Baumbach GL, Cancilla PA, Martin-Amat G, et al. Methyl alcohol poisoning. IV. Alterations of the morphological findings of the retina and optic nerve. Arch Ophthalmol. 1977;95:1859–1865.
267 Eells JT, Makar AB, Noker PE, et al. Methanol poisoning and formate oxidation in nitrous oxide–treated rats. J Pharmacol Exp Ther. 1981;217:57–61.
268 Gilger AP, Potts AM. Studies on the visual toxicity of methanol: the role of acidosis in experimental methanol poisoning. Am J Ophthalmol. 1955;39:63–85.
269 Hayreh MS, Hayreh SS, Baumbach GL, et al. Methyl alcohol poisoning. III. Ocular toxicity. Arch Ophthalmol. 1977;95:1851–1858.
270 Ingemansson SO. Clinical observations on ten cases of methanol poisoning. Acta Ophthalmol. 1984;62:15–24.
271 Martin-Amat G, McMartin KE, Hayreh SS, et al. Methanol poisoning: ocular toxicity produced by formate. Toxicol Appl Pharmacol. 1978;45:201–208.
272 Martin-Amat G, Tephly TR, McMartin KE, et al. Methyl alcohol poisoning. II. Development of a model for ocular toxicity in methyl alcohol poisoning using the rhesus monkey. Arch Ophthalmol. 1977;95:1847–1850.
273 Treichel JL, Murray TG, Lewandowski MF, et al. Retinal toxicity in methanol poisoning. Retina. 2004;24:309–312.
274 Fujihara M, Kikuchi M, Kurimoto Y. Methanol-induced retinal toxicity patient examined by optical coherence tomography. Jpn J Ophthalmol. 2006;50:239–241.
275 Frisen L, Malmgren K. Characterization of vigabatrin-associated optic atrophy. Acta Opthalmol Scand. 2003;81:466–473.
276 Malmgren K, Ben-Menachem E, Frisen L. Vigabatrin visual toxicity: evolution and dose dependence. Epilepsia. 2001;42:609–615.
277 Vobig MA, Klotz T, Staak M, et al. Retinal side-effects of sildenafil [letter]. Lancet. 1999;353:375.
278 Vobig MA. Retinal side-effects of sildenafil [letter]. Lancet. 1999;353:9162.
279 Zrenner E. No cause for alarm over retinal side-effects of sildenafil. Lancet. 1999;353:340–341.
280 Marmor MF, Kessler R. Sildenafil (Viagra) and ophthalmology. Surv Ophthalmol. 1999;44:153–162.
281 Marmor MF. Sildenafil (Viagra) and ophthalmology [Editorial]. Arch Ophthalmol. 1999;117:518–519.
282 Luu JK, Chappelow AV, McCulley TJ, et al. Acute effects of sildenafil on the electroretinogram and multifocal electroretinogram. Am J Ophthalmol. 2001;132:388–394.
283 Jagle H, Jagle C, Serey L, et al. Visual short-term effects of Viagra: double-blind study in healthy young subjects. Am J Ophthalmol. 2004;137:842–849.
284 Jagle H, Jagle C, Serey L, et al. Dose-dependency and time-course of electrophysiologic short-term effects of Viagra: a case study. Doc Ophthalmol. 2005;110:247–254.
285 Zoumalan CI, Zamanian RT, Doyle RL, et al. ERG evaluation of daily, high-dose sildenafil usage. Doc Ophthalmol. 2008;118:225–231.
286 Cordell WH, Maturi RK, Costigan TM, et al. ERG testing during chronic PDE5 inhibitor administration (ERG-PDEFi) consortium. Arch Ophthalmol. 2009;127:367–373.
287 Grunwald JE, Siu KK, Jacob SS, et al. Effect of sildenafil (Viagra) on the ocular circulation. Am J Ophthalmol. 2001;131:751–755.
288 Grunwald JE, Metelitsina T, Grunwald L. Effect of sildenafil citrate (Viagra) on retinal blood vessel diameter. Am J Ophthalmol. 2002;133:809–812.
289 McCulley TJ, Luu JK, Marmor MF, et al. Effects of sildenafil citrate on choroidal congestion. Ophthalmologica. 2002;216:455–458.
290 Metelitsina TI, Grunwald JE, DuPont JC, et al. Effect of Viagra on the foveolar choroidal circulation of AMD patients. Exp Eye Res. 2005;81:159–164.
291 Birch DG, Toler SM, Swanson WH, et al. A double-blind placebo-controlled evaluation of the acute effects of sildenafil citrate (Viagra) on visual function in subjects with early-stage age-related macular degeneration. Am J Ophthalmol. 2002;133:665–672.
292 Harris A, Kagemann L, Ehrlich R, et al. The effect of sildenafil on ocular blood flow. Br J Ophthalmol. 2008;92:469–473.
293 Tripathi A, O’Donnell NP. Branch retinal artery occlusion: Another complication of sildenafil [letter]. Br J Ophthalmol. 2000;84:928.
294 Pomeranz HD, Smith KH, Hart WM, Jr., et al. Sildenafil-associated nonarteritic anterior ischemic optic neuropathy. Ophthalmol. 2002;109:584–587.
295 Allibhai AZ, Gale JS, Sheidow TS. Central serous chorioretinopathy in a patient taking sildenafil citrate. Ophthalmic Surg Lasers Imaging. 2004;35:165–167.
296 Akash R, Hrishikesh D, Amith P, et al. Case report: association of combined nonarteritic anterior ischemic optic neuropathy (NAION) and obstruction of cilioretinal artery with overdose of Viagra. J Ocul Pharmacol Ther. 2005;21:315–317.
297 Pomeranz HD, Bhavsar AR. Nonarteritic ischemic optic neuropathy developing soon after use of sildenafil (Viagra): a case report of seven new cases. J Neuroophthalmol. 2005;25:9–13.
298 Quiram P, Dumars S, Parwar B, et al. Viagra-associated serous macular detachment. Graefes Arch Clin Exp Ophthalmol. 2005;243:339–344.
299 Escaravage GK, Wright JD, Givre SJ. Tadalafil associated with anterior ischemic optic neuropathy. Arch Ophthalmol. 2005;123:399–400.
300 Bollinger K, Lee MS. Recurrent visual field defect and ischemic optic neuropathy associated with tadalafil rechallenge. Arch Ophthalmol. 2005;123:400–401.
301 Peter NM, Singh MV, Fox PD. Tadalafil-associated anterior ischaemic optic neuropathy. Eye. 2005;19:715–717.
302 Hayreh SS. Erectile dysfunction drugs and non-arteritic anterior ischemic optic neuropathy: is there a cause and effect relationship? J Neuroophthalmol. 2005;25:295–298.
303 Gedik S, Yilmaz G, Akova YA. Sildenafil-associated consecutive nonarteritic anterior ischaemic optic neuropathy, cilioretinal artery occlusion, central retinal vein occlusion in a haemodialysis patient. Eye. 2007;21:129–130.
304 Carter JE. Anterior ischemic optic neuropathy and stroke with use of PDE-5 inhibitors for erective dysfunction: cause or coincidence? J Neurol Sci. 2007;262:89–92.
305 Fraunfelder FW, Fraunfelder FT. Central serous chorioretinopathy associated with sildenafil. Retina. 2008;28:606–609.