Chapter 38 Drug Delivery
A brief history of the field of drug delivery
The most common form of administration of a medication is the pill. There is evidence of the use of pills at least as early as ancient Egypt in the Ebers Papyrus (1500 bc). They consist, generally, of a medication mixed with a number of excipients or additives such as sugars and starches to protect and stabilize the formulation.1 The basic concept is that one swallows the pill, it dissolves in the stomach, and the medication is absorbed in the intestines to the circulation. This approach works well for many drugs, but not for those which can break down in the stomach. Insulin is usually presented as a case in point which must be injected because it cannot survive the environment of the stomach. Insulin is injected into the fatty tissue2 and is then absorbed into the systemic circulation.
Systemic administration of medications by either route can be problematic for certain tissues, particularly those with strong barrier characteristics or those which are poorly vascularized. Early versions of local delivery systems to the eye involve ointments which were described in ancient Mesopotamia.3 Pilocarpine drops have been used for at least 100 years to lower intraocular pressure (IOP) and treat glaucoma.4
Drug delivery systems are a broad area that includes targeted systems as well as systems that provide controlled release of the drug. One of the earliest targeted systems was the liposome, which has been shown to build up in tumors due to the enhanced permeation effect.5 Liposomes were discovered in the 1960s and the first US Food and Drug Administration (FDA) approval came with Doxil, the liposomal-encapsulated formulation of doxorubicin, an anticancer agent in the 1990s.6 More recent variants on targeted systems have focused on adding molecules on the outside of liposomes or other nanoparticles that can allow them to target the tissue of interest.7 Targeted systems may allow the systemic delivery of a molecule that is released in the ocular tissues.8 However, in and of themselves, targeted systems do not provide sustained delivery of drugs. They can be engineered to do so, but if one looks at the half-life of doxorubicin, it is still on the order of hours as opposed to days or weeks. This does not mean that targeting cannot be combined with sustained delivery, but they are two components to consider.
Sustained delivery is better termed controlled delivery. The fundamental difference is that controlled delivery is dictated by the device and not the environment around the device.9 This is fundamental to the drug delivery. One does not want to administer a device to a patient and have different patients get the drug over different time courses, particularly in an unexpected way. One of the first controlled delivery systems was actually in the eye, the Ocusert system to deliver pilocarpine for glaucoma. The Ocusert system is a reservoir system that relies on the transport of the drug through a membrane. Membranes are effective for small molecules (less than 600 Da),10 but are not suitable for delivering larger molecules such as antibodies, growth factors, and proteins more broadly.
The watershed in the drug delivery world occurred in 1976. Robert Langer and Judah Folkman showed that large molecules could be delivered over days and weeks from polymer matrices, and they showed that the release profile could be tailored based on the matrix.11 The concept was not warmly received and there were huge questions about the mechanism and plausibility of the approach,12 but the drug delivery field has grown from being nonexistent in the USA in the 1980s to 11.5 billion dollars in 1996,13 to an estimated 85 billion dollars in 2010.14 The economic impact is a reflection of a number of factors, including the ability to provide new patent protection for off-patent medications, but it also reflects the impact on human health.
Drug delivery
Formulating sustained-delivery systems
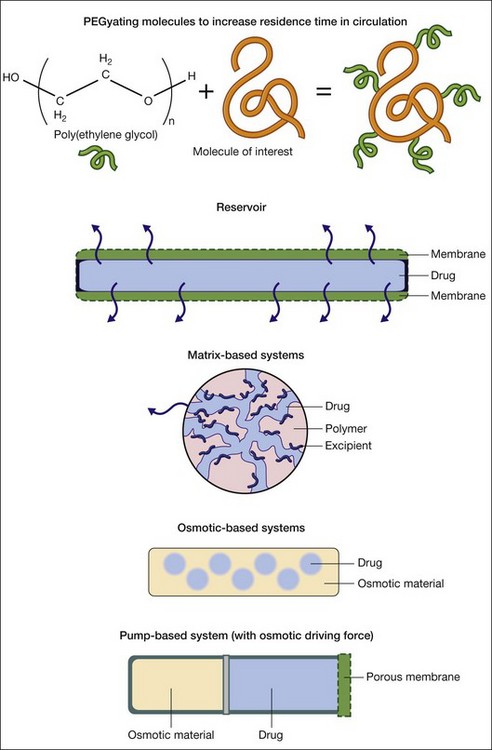
There are five broad approaches for the sustained delivery of drugs (Fig. 38.1). The first approach involves attaching molecules to the drug to increase its residence time. Poly(ethylene gycol) or PEG is the workhorse of this approach. PEGylating or attaching PEG molecules can increase the circulation time of a drug in the bloodstream by creating a highly hydrated volume around the drug.15 The second broad approach is to encapsulate the drug of interest in a reservoir system that includes a membrane that allows the drug to diffuse through the device and into the surrounding tissue. This approach lies at the basis of drug delivery systems, including the Ocusert implant delivering pilocarpine.16 Diffusion-based systems work for limited, typically small, molecules with molecular weights less than 600 Da.10 There are great benefits to the membrane-based systems, such as the ability to deliver many drugs over very long timescales, but there are risks, including the rupture of the membrane, leading to spontaneous delivery of the entire reservoir.9
The third approach involves mixing the drug with polymers which entrap the drug in a matrix. The drug typically phase separates on the micro- or nanoscale from the polymer, leading to veins of the drug in the polymer matrix. The tortuosity of the veins plays a tremendous role in the release of the drug. With nondegradable polymers, the drug delivery profile is dictated by diffusion.17 With degradable polymers, the drug delivery profile is dictated by a combination of diffusion and degradation events that occur over time.17 The challenges in the matrix-based approaches involve achieving good mixing of the drug in the matrix to form tortuous pathways as well as promoting interactions between the drug and matrix. The closer the two associate with each other, the more likely one will achieve very long (weeks to months to years) release. Thinking about this interaction becomes part of the process of formulating drug delivery systems.
The fourth approach involves osmotic systems. In these systems the diffusion of water into the matrix leads to swelling and drives the release of the drug via diffusion. Typically, osmotic systems lead to relatively fast release of drugs with a significant burst effect. Investigators are developing asymmetric membranes to control the delivery in a more uniform manner over time,18 but osmotic systems have not been used extensively other than as components to drive pump technologies which do have robust, long-term delivery.
In thinking about which approach makes the most sense in formulating a drug delivery system, one needs to consider the following: the size, nature, and stability of the drug, the length of time one wants to deliver the drug, and the amount of the drug one wants to deliver over time. Regarding size, small molecules (<600 Da) can diffuse through polymers such as silicone.10 Larger molecules, macromolecules such as proteins, are not able to diffuse through polymers on a useful timescale for most applications.11 If one is hoping to deliver larger molecules, one will need to consider the methods outside of the membrane approach.
The nature of the drug refers to its charge and solubility. Most of the popular biodegradable polymers are extremely hydrophobic. If the drug of interest is very hydrophilic, it can be challenging to encapsulate the hydrophilic drug in the hydrophobic polymer matrix. One of the classic methods to overcome this challenge is to use excipients that interact with both the drug and the polymer. Fu et al. showed how excipients could greatly improve loading and delivery of glial cell line-derived neurotrophic factor (GDNF) through the use of excipients.19 Likewise, charged molecules can interact poorly in uncharged or similarly charged polymer systems. Poly(lactic-co-glycolic acid) (PLGA) can be synthesized with a carboxyl group on the end that carries a negative charge depending on the pH. This can improve the encapsulation of positively charged molecules.
Beyond the drug, one needs to consider the amount of the drug one needs to deliver and the time course. Matrix-based formulations have been shown to deliver medications for years, but they rely on relatively high polymer-to-drug ratios, which makes them more suitable for applications where one can either deliver a large volume of material (which is unlikely in the eye) or where one has a relatively potent medication. As we will see in the examples below, many of the commonly used drugs are effective at low concentrations, but it is an important consideration. Membrane and pump systems can both deliver larger amounts of drugs over time, but the volume they deliver determines the necessary reservoir size and overall device dimensions. The time course is the second critical component. Membrane and pump approaches can both lead to very constant delivery depending on the geometry of the devices.10 Careful formulation can lead to very constant delivery over time from matrix-based formulations, but they are prone to burst release due to drug adsorbed on or near the surface of the materials.10
Delivering drugs in a targeted manner
For some compounds, though, one may want either to deliver them systemically or to deliver them to a particular cell type in the eye and not to all cell types. A good example of this would be the delivery of a specific agent for a specific cell type, such as the retinal pigment epithelium (RPE).20 The most common forms of targeting have involved creating particles, typically nanoparticles, from either liposomes or other polymers that are covered in PEG arms to reduce their aggregation and promote transport, and attaching molecules to the particles that bind to receptors or molecules on the cells of interest. Antibodies,21 peptides,22 and aptamers23,24 have all been used to facilitate targeting.
The key issues to consider in designing a targeting molecule are the specificity and affinity of the targeting moiety to the molecule of interest on the cell. Poor affinity or specificity will lead to poor targeting. Once one has identified targeting molecules with appropriate affinity and specificity, one needs to insure the stability of targeting molecule in vivo. Larger molecules, such as antibodies, have the potential to be denatured or enzymatically cleaved in vivo, which has motivated the identification of smaller molecules with similar specificity and affinity such as aptamers.23,24
Targeting is primarily used to concentrate systemically administered particles in the tissue of interest. In the retina, much of the work has focused on targeting the choroidal vessels.25,26 There is evidence that, when particles are small enough, they can cross through to the retina when administered systemically,27 which opens up the possibility of delivering targeted nanoparticles to the retina via systemic administration.
Gene delivery
Gene therapy has the potential to replace or restore function in the retina as well as produce growth factors which protect the eye. It is very challenging, though, to get the genetic material both to the cells and into the genome without breakdown of the material. There have been many studies looking at the transfection efficiency of deoxyribonucleic acid (DNA) through routes of administration, including drops, subconjunctival, intravitreal, and subretinal injections, and in all cases the transfection efficiency has been low to the retina and the subsequent protein expression is typically small and over a short duration.29
To facilitate more effective delivery of DNA and subsequent protein expression, a number of vectors have been pursued. The two basic approaches involve viral and nonviral delivery of constructs to the retina. Viruses have adapted over millions of years to deliver genes to cells efficiently, and viral delivery is generally efficient. Viral delivery does raise safety concerns and there were substantial complications and deaths in early viral-based gene therapy trials.30,31 Nonviral gene delivery has sought to provide safer alternatives, but the challenge lies in getting efficient delivery and incorporation of the genetic material.
Viral systems
There are three major classes of virus that have been used for transduction: retroviruses, lentiviruses, and adenoviruses. Retroviral vectors only infect dividing cells, making them relatively unattractive for ocular applications.32 Lentiviral vectors are designed to infect nondividing cells in the G0 or G1 phase of the cell cycle.32 There are significant safety concerns with both retroviral and lentiviral vectors because they have the potential to insert into the genome. Of the three major classes, adenoviruses have drawn tremendous interest for gene therapy because they do not incorporate into the genome, which is thought to reduce their oncogenic potential, and they are extremely effective at transducing cells.32 However, adenoviruses have been found to be very immunogenic, and one of the early clinical trials using an adenovirus had to be halted when an 18-year-old died due to a systemic inflammatory response syndrome.31
Adenovirus-based vectors have continued to be pursued in the eye because of the local delivery and immune-privileged nature. GenVec has used an adenovirus to deliver the gene for pigment epithelium-derived factor (PEDF) for the treatment of neovascular AMD in a phase I trial.33 The adenovirus-based delivery system was well tolerated by patients with only transient, mild inflammation in some patients which was managed with topical medications. There was no control group in this phase I trial, but the size of the choroidal neovascular lesions was smaller in the high-dose group. Much of the follow-on research has focused on repeated administrations, longer-term expression of PEDF, and using inducible promoters to direct expression of the protein of interest.34–36
A variant on the adenovirus, first found as a contaminant of adenoviruses, the adeno-associated virus (AAV), has proven to be an effective alternative. Like adenoviruses, AAV vectors do not integrate into the genome. Furthermore, they do not elicit a strong immune response.32 One of the first major successes for gene therapy with AAV vectors has been the treatment of Leber congenital amaurosis, a severe form of retinal degeneration that leads to vision loss in early childhood.37 A replication-deficient AAV vector was used to deliver a gene for isomerohydrolase activity (AAV-hRPE65v2). The vector was injected subretinally, and vision gains were found in younger patients.
Nonviral systems
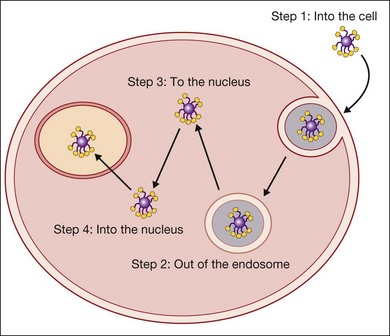
There are five basic steps that have to be achieved to transfect a cell (Fig. 38.2). The genetic material must get to the cell, into the cell, out of the endosome, to the nucleus, and into the nucleus. Viruses have evolved a complex set of tools to achieve this. For example, the adenovirus has a targeting moiety for the cell of interest and leverages the pH drop in the endosome by having the capsid undergo a conformational change which opens the endosome. It is thought to bind to dynein, a molecular motor that moves along the microtubules to travel to the nucleus.38
Nonviral systems have been designed to address one or more of these issues, and the more recent systems have been designed to address several or all of these critical steps for transfection. Liposomes were among the earliest transfection agents. Liposomes are capable of shielding DNA from enzymatic breakdown until the material gets to the cell, but their fusion with the cell membranes is not efficient.39 However, in 1987, the use of cationic liposomes was first identified as a means to facilitate high rates of transfection.40 The cationic component of the system facilitates interactions with the negatively charged cell membranes and the subsequent cell fusion which delivers the DNA into the cell. While this is a tremendous improvement over previous nonviral approaches, the efficiency is still far lower than viral systems.
Positively charged polymers like polylysine and polyethylenimine (PEI) were very early in the nonviral vector development timeline. PEI, one of the major cationic polymers, was first shown to be an effective polymer for gene delivery in 1995.41 It does two critical things. First, it is a positively charged polymer that complexes with negatively charged DNA to create positively charged nanoparticles which stick to the anionic membranes of cells. Second, it is a proton sponge, meaning that as the pH is lowered in the endosome, the amines on the polymer take up the hydrogen ions and the polymer protonates. It does this to the point that it swells and bursts the endosome. The polymer, however, has no specific method for getting the DNA to or into the nucleus. PEI tends to be most effective in dividing cells where the organization of the mitotic spindles facilitates movement of the particles to the nucleus.41,42 PEI has been used in animal models for transfection of retinal cells.43 The particles were taken up primarily by what appear to be Müller glial cells but no quantification is discussed so it is not possible to assess what sort of efficiency was achieved.
In light of the complexities of getting DNA to the nucleus, more recent research has focused on developing multicomponent systems that incorporate protection of DNA with endosomal escape mechanisms and nuclear targeting moieties.44–46 The challenges of specific transfection of retinal cells in the eye with nonviral vectors are significant, and it is likely that careful engineering of the vector will be needed for successful, long-term transfection, but the field is moving forward rapidly. It is likely to be a very different landscape in the next decade.
Cellular delivery for sustained drug delivery
Engineering cells for delivery
The attraction of using cells for the delivery of therapeutic agents is that cells can deliver a molecule for very long periods of time, potentially years. Furthermore, cells have the potential to integrate into the retina and deliver molecules in layers that may not be easily reached by standard drug delivery of gene therapy approaches. For example, it can be challenging to deliver genes to the retina and to get robust expression of the molecule of interest via intravitreal injections.47 However, there are studies showing that certain cells may be able to migrate and integrate with the retina following intravitreal administration and produce molecules such as GDNF and brain-derived neurotrophic factor (BDNF) to preserve retinal cells in a number of models of retinal degeneration.48,49 Mesenchymal stem cells,49 embryonic stem cells,48 Schwann cells,50 and fibroblasts51 have all been engineered as a means to promote neuroprotection and have all successfully been used for several months in animal models.
Engineering materials for immunological protection
While the eye is immune-privileged,52,53 the presence of disease can alter this privilege, and long-term survival of transplanted cells is not guaranteed. Since the primary purpose of the engineered cells is to deliver their molecular payload, encapsulating them to protect them from the host while allowing their molecules to reach the retina makes a great deal of sense. It also facilitates their removal should complications arise.
As noted in the section on sustained delivery systems, solid polymer membranes do not allow large molecules (> 600 Da) to diffuse through.10 Most of the growth factors noted above are significantly greater than 600 Da. The solution, then, is to engineer the materials with higher-molecular-weight cutoffs that allow the growth factors through but still block the transport of antibodies and other larger molecules.
Alginate gels and variants on alginate gels have been one of the most used materials to encapsulate cells for this purpose since the early 1980s. Alginate is an attractive material in that it does not adsorb proteins, facilitating their transport, but the molecular weight of the transported molecules can be controlled by the crosslinking density.54–56 Because alginate gels are crosslinked water-swollen gels, the encapsulated cells are able to remain hydrated and transport of nutrients and waste is maintained. Other materials, primarily synthetic hydrogels, have become popular because the permeability can be tightly controlled through crosslinking agents and density, membrane thickness, and the use of multiple layers.57,58 Kristi Anseth’s group has shown that functionalizing hydrogels with moieties that interact with cells can still maintain the long-term delivery of the protein of interest while leading to higher and longer-term cell viability, the ultimate goal of these encapsulation systems.59
Routes of delivery to the retina
Traditional routes of administration
Oral delivery
Oral delivery is the most preferred route of delivery of medications by patients. Drugs such as timolol maleate can be given orally and do have an ocular effect, namely the lowering of IOP.60 However, the presence of the blood–retinal barrier makes transport to the eye difficult and the challenges of stabilizing drugs through the gut to systemic absorption and further on to the eye are possible but very difficult. Therefore, most ocular medications have been delivered topically.
Topical delivery
A large range of medications are delivered topically either as solutions or as gels. Because a large portion of the drop or gel is cleared from the tear film quickly, topical delivery leads to significant (typically 80%) systemic absorption of the drug.61 There is also relatively limited adsorption of the drug to the ocular tissues.62 There are a number of models of pharmacokinetics of topically delivered medications, and the drug chemistry, particularly the hydrophobic versus hydrophilic nature of the drug, plays a role, but overall, there is very little drug delivered to the vitreous and the retina.63 This makes topical administration challenging for retinal diseases.
There have been clinical trials in Europe looking at delivery of nerve growth factor (NGF) for neuroprotection of the retina, and in particular, there are trials looking at topical administration of NGF for glaucoma.64 Three glaucoma patients who were losing vision received drops containing murine NGF four times daily for 3 months. There are no data from the human or animal studies regarding the concentration of NGF that reaches the back of the eye, but some patients undergoing this treatment exhibited some signs of improvements in their electroretinograms.
Injections
When oral medications and drops are not effective, injections are typically considered. Subconjunctival injections are one of the least invasive injections but there are several barriers between the subconjunctival space and the retina, although a number of molecules can diffuse in low concentrations to the retina with this approach, depending on their size and chemistry.65–67 Intravitreal injections, though more invasive, provide a means to put molecules near the retina. The vitreous is also capable of acting as a sink for many molecules, absorbing them and slowly releasing them, which can increase the residence time of these molecules in the intravitreal space.68–70 Subtenon injections have drawn more interest with the thought that the drug will be delivered over a prolonged period through this route.71–73
Novel approaches for administration
Devices
Inserts
The Ocusert system is one of the best-known and most widely studied membrane-based drug delivery systems. The device consists of two membranes of poly(ethylene-co-vinyl acetate) and a ring of the same material filled with pilocarpine to lower IOP.74 The insert was designed to deliver the drug for 7 days when placed in the cul de sac of the eye. The system worked to manage IOP, but patients found that the device could be uncomfortable especially if it twisted, and it could fall out.75 While follow-on designs sought to make the insert remain in the eye,76 a fundamental problem was that patients had to be taught how to use the insert systems and older patients did not like the device.9
The landscape for inserts may be changing. Approximately 40 million people in the USA wear contact lenses and the contact lens market is expanding.77 Modifying contact lenses makes sense in that many patients are familiar with them and their use.78 Soft contact lenses, those primarily used at this point, are hydrogels, water-soluble polymers that are crosslinked to form networks, and hydrogels are a potential material for controlled drug delivery.79 It should be noted, however, that when drug delivery researchers start looking at contact lenses or modified lenses as the solution to patient- and clinician-friendly inserts, they often forget that most patients remove their contact lenses at night. If one wants to use contact lenses as a depot for drug delivery, one either needs to account for the actual time patients wear the lenses or design a wearable long-term lens.
While hydrogels can be used as a drug delivery vehicle, water-soluble drugs, such as those likely to cross the conjunctiva most effectively, tend to elute very quickly from the highly hydrated polymer networks on the order of minutes or hours.80 Timolol maleate, a water-soluble drug for lowering IOP, has been shown to be able to be delivered from contact lenses consisting of polymers of N,N-diethylacrylamide and methacrylic acid for approximately 24 hours.81 A study of 3 patients using contact lenses delivering timolol showed the lenses controlled IOP as well as drops.82 While this is promising, timolol, like pilocarpine, does not have to reach the retina. It only needs to be in a reasonable concentration near the ciliary body to affect aqueous production.
Getting molecules into lenses or inserts that then are delivered to the retina requires crossing many physical barriers in the eye. Liposomes, surfactants, and penetrating agents may facilitate transport through these barriers,83 but a formulation that achieves this safely has yet to be demonstrated.
MEMs devices
Replenish external scleral fixated refillable device
Implanting a reservoir system in the subconjunctival space opens the door to well-controlled long-term delivery of both small and large molecules without the need for repeated intravitreal injections. The MEMs device uses electrolysis to create bubbles that push the drug out of the reservoir of the implantable device, and there is a port that facilitates loading of the drug into the system.84 Implantation of the device is similar to a glaucoma drainage device in complexity and it can be reloaded several times and has been well tolerated in initial rabbit studies.85
Ionophoresis system
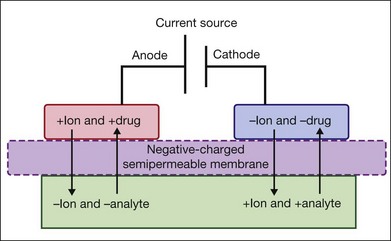
EyeGate Pharma has developed a delivery system composed of an annular scleral ionthoresis reservoir which is filled with drug (Fig. 38.3). A potential is applied between an electrode on the forehead of the patient and the device to drive the charged drug into the anterior and posterior segments of the eye over 2–4 minutes. This device is currently in clinical trials, including a study to assess the safety and efficacy of a dexamethasone phosphate iontophoresis formulated solution (EGP-437) at two different dose levels for dry eye (NCT01129856), and a trial of four iontophoretic doses of EGP-437 in patients with noninfectious anterior-segment uveitis (NCT00694135). While the system has not yet been tested for diseases of the retina, it may be an alternative to different retinal pathologies due to the good penetration in human sclera of drugs like bevacizumab.86
Implants
Vitrasert ganciclovir implant
There are several implants either in or through clinical trials which deliver drugs to the retina for long periods of time, up to several years. The Vitrasert implant was approved by the FDA in 1996 for the treatment of cytomegalovirus (CMV) retinitis.87 The implant delivers ganciclovir, an antiviral drug, from an implant of poly(vinyl alcohol) and poly(ethylene vinyl acetate), one of the early workhorses of the controlled-release field.9 The implant delivers the medication for approximately 32 weeks and has been shown to halt the progression of CMV.88,89 One of the challenges with a nondegradable implant is removal, and to deal with this, the implant is sutured to the eye wall once inserted.90 Figure 38.4 shows a comparison of the sizes of the Iluvien, Retisert, and Vitrasert implants.
Retisert fluocinolone implant
Retisert is an implant the size of a grain of rice that delivers fluocinolone acetonide intravitreally for 30 months for uveitis and was approved in 1995.91 It has also been studied in trials for diabetic retinopathy.91 The implant consists of a blend of the drug with poly(vinyl alcohol) and methylcellulose in a tablet geometry that is secured with a suture once inserted into the vitreal space through a scleral incision, similarly to the Vitrasert implant. The implant is very effective at treating uveitis, but there are side-effects, including an increase in IOP associated with the steroid delivery and formation of cataracts.92
Iluvien fluocinolone implant
The Iluvien implant is composed of the same materials and drug as the Retisert implant but is designed with a very different geometry (a narrow cylinder 3.5 × 0.37 mm) that can be injected into the intravitreal space through a 25-gauge needle.93 The implant and injector are shown in Fig. 38.5. The system delivers a lower level of fluocinolone than the Retisert implant and in clinical trials for diabetic macular edema, there have been fewer complications. In the lowest dose format (0.2 µg/day), no elevation in IOP has been seen in a phase II trial.93 Five of 17 subjects with the higher-dose (0.5 µg/day) system required IOP-lowering drops and one patient required a glaucoma drainage device.93 A total of 34% of the low-dose patients and 71% of the high-dose patients showed progression of cataracts, and 14% and 29% in each group had surgery.93 Some improvements in visual function were seen, and all patients showed reduction in foveal thickness.
Ozurdex dexamethasone implant
Ozurdex (Allergan) is a dexamethasone implant that delivers the steroid intravitreally for 6 months.94,95 The implant consists of poly(lactic-co-glycolic acid), a degradable polyester and dexamethasone. It is inserted into the intravitreal space with a single-use applicator through a 22-gauge needle. The implant and applicator are shown in Fig. 38.6. The implant is not tethered in the vitreous, like many of the other devices. Because it degrades, it does not have to be removed. It has been approved for the treatment of uveitis and macular edema caused by retinal vein occlusion, and it is associated with relatively few side-effects, with 15% of patients exhibiting increased IOP in trials and no differences in cataract surgeries between the treatment and sham groups.95
There is always a concern with implants and any delivery technology that if there is a problem, how will the implant or drug delivery system be removed? By using well-characterized drugs and well-defined delivery systems, that risk may be more limited, but careful assessment of side-effects in the clinical trials is critical. There are instances where drug delivery technologies have led to new side-effects not seen with the drug on its own due to the changes in pharmacokinetics associated with the new formulation. The classic example of this is Doxil, a PEGylated liposomal formulation of doxorubicin. The formulation is much more effective than free doxorubicin at treating cancer, but the longer circulation time due to the liposomal formulation and ability to circulate through the capillary beds of the hands and feet can lead to hand–foot syndrome.96,97
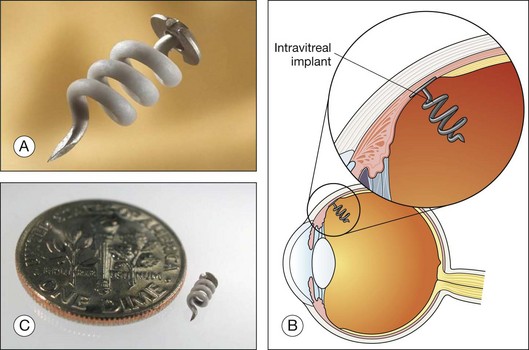
I-vation triamcinolone implant
Surmodics has developed the I-vation implant, a helical screw coated with triamcinolone acetonide that delivers the drug intravitreally for 36 months.98,99 The implant is designed to be inserted through the sclera once a hole has been made with a 25-gauge needle. The implant and a schematic are shown in Fig. 38.7. The helical design increases the surface area from which the drug is released. One of the striking features of the implant is that the drug is entirely within the coating on the helical structure and not within the bulk of the device. Like the Retisert device, long-term usage is associated with an increase in IOP and cataracts.98
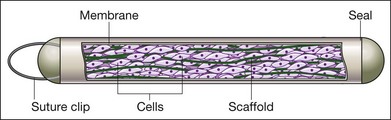
Encapsulated cell technology (ECT) ciliary neurotropic factor (CNTF) implant
CNTF can be delivered from a rice-sized implant via ECT for an estimated time of 2 years or more.100 The implant contains immortalized RPE cells engineered to deliver CNTF in an encapsulated format, and it is inserted into the vitreous cavity and sutured to the eye wall. A schematic is shown in Fig. 38.8. The implant was studied in a phase I trial for retinitis pigmentosa, it was well tolerated by patients, and some patients showed improvements in visual acuity.101 The implant (designated NT-501) was then studied in phase II trials for early- and late-stage retinitis pigmentosa. The retinal thickness was higher for those receiving the implant over a year, and Neurotech, the company developing the implant, has been given a fast-track designation by the FDA for the treatment of visual loss from retinitis pigmentosa.
Injectables
Micro- and nanoparticles
The majority of micro- and nanoparticles used in the eye are based on the degradable polyesters poly(lactic acid) (PLA) and PLGA. These polymers degrade by hydrolysis and the rate of degradation is controlled by the ratio of lactic acid to glycolic acid subunits, the molecular weight of the polymers, and, in the case of poly(l-lactic acid) (PLLA), the crystallinity of the polymer. The FDA has approved a number of devices using these materials and there is a wealth of literature looking at these materials for use in the eye.102
Micro- and nanoparticles based on PLA and PLGA have been used for many years to deliver a range of drugs from small molecules to large proteins. One of the biggest challenges associated with these polymers in spherical form is that they tend to release the drug in multiple phases (typically triphasic) beginning with a burst phase.103 However, by adding the appropriate excipients that interact with both the polymer and the drug, such as GDNF, one can achieve reduced burst and relatively constant delivery over very long times up to several months from microparticles.104 GDNF microspheres based on this approach have been used in vivo in the DBA/2 J mouse model of retinal ganglion cell (RGC) degeneration. The spheres delivering GDNF were injected intravitreally and led to significantly higher numbers of fluorogold-labeled RGCs as compared to the blank spheres or untreated animals.105 The same formulation was shown to preserve RGC and function in the pig following a model of acute ocular ischemia and found that GDNF microspheres led to the preservation of a greater number of RGCs as well as improved function over time as measured by multifocal electroretinogram.106
A number of drugs have been formulated into microspheres for intravitreal and subconjunctival administration. PLGA microparticles delivering celecoxib have been administered subconjunctivally to reduce the concentration of VEGF in the retina and vascular leakage in a diabetic rat model.107 Likewise, microparticles delivering the corticosteroid, budesonide, have been injected subconjunctivally in rats to alter VEGF expression.108 Nanoparticles delivering carboplatin for a mouse model of retinoblastoma showed a reduction in tumors.109 Nanoparticles delivering dexamethasone showed a reduction in choroidal neovascularization in a laser-induced rat model.110 Intravitreal administration of microspheres delivering BDNF showed neuroprotective effects in a rat model of retinal ischemia.111
With careful formulation, and a few predictive models,112 one can develop injectable formulations that facilitate long-term delivery (typically over several months) of the drug of interest at drug-loading efficiencies and with release kinetics that are suitable for long-term therapy for retinal diseases.
Nanoscale systems
Novagali Pharma is developing an emulsion-based delivery system called Eyeject for the sustained delivery of drugs to the intravitreal space. The system is currently in a phase I trial delivering corticosteroid prodrug for the treatment of diabetic macular edema (clinical trial identifier NCT00665106). The system involves combining the prodrug such as lipophilic ester of dexamethasone with an oil such as mineral or vegetable oil, a surfactant, and glycerol. The combination of these components leads to the formation of an emulsion that can be injected intravitreally and deliver the prodrug for 1–6 months.113
Ocular uptake of systemically delivered nanoparticles
Size plays a critical role in whether nanoparticles can be delivered systemically and be found in the retina. While there is a blood–retina barrier, in one study, a small concentration of 20 nm gold nanoparticles was found in the retina in mice following intravenous administration114; 100 nm gold nanoparticles were not found. In another study, PLA-based nanoparticles encapsulating rhodamine (100–200 nm) were not found in normal rat retinas, but a few were seen in a rat model of experimental autoimmune uveoretinitis where the blood–retinal barrier is compromised.25 Generally, few nanoparticles of any size make it to the retina. The vast majority are likely picked up by the reticuloendothelial system and cleared.115,116
Nanocomposites for topical delivery
Eye drops are far preferred by patients and clinicians to more invasive injections, and a great deal of research has focused on methods to make drops a viable system for delivery of drugs to the posterior segment of the eye. Nanocomposites typically involve liposomes or other nanoscaled particles to deliver the drug. One of the primary objectives of nanocomposite research has been to increase the residence time of the solution on the eye to facilitate transport of the drug. The charge or lack thereof on the nanoparticles plays a critical role in their behavior. Neutrally charged liposomes delivering pilocarpine were shown to lead a drop in IOP for twice as long as free drug alone, suggesting that the liposomes increased the residence of the drug following administration.117 Providing more direct evidence, rhodamine-loaded colloidal solutions of nanocapsules were administered as drops in the eye.118 Neutral particles showed greater delivery of rhodamine than negatively charged particles.
The question, though, is whether these nanocomposite systems can lead to drug delivery in the back of the eye. Hironaka et al. investigated the delivery of coumarin-6 to the retina via nanocomposites of liposomes containing coumarin-6 as eye drops in mice.119 Coumarin-6 was found in the inner plexiform layer over the first 60 minutes postadministration. The group found that there was better delivery with smaller liposomes, and coating with mucoadhesive molecules increased the delivery of coumarin-6. The mechanism for delivery was not clear in this work, but the group hypothesized that conjunctival adsorption played a role. What is also not clear from this work is how much of the dose was absorbed and how much went into the systemic circulation. The group extended this work to rabbits and monkeys with similar results.120
Pharmacokinetics in the eye
Barriers to delivery
The major barriers to delivery of molecules in the eye depending on route of administration include the conjunctiva, the sclera, the choriocapillaris, the RPE, the external limiting membrane, the internal limiting membrane, and the blood–retinal barrier. The conjunctiva is permeable to molecules up to ~26 kDa, the sclera is permeable to molecules up to 150 kDa, the choriocapillaris up to 55 kDa, the RPE up to 30 kDa, the external limiting membrane up to 70 kDa, the internal limiting membrane up to 150 kDa, and the blood–retinal barrier is not even permeable to 3 kDa dextran.121
Modeling delivery
Impact of drug chemistry
There are good models for predicting drug release from nondegradable systems.99 There are also good models for the release of drugs from degradable polymers that are highly predictive and based on a combination of diffusion and degradation behaviors.122 The one limitation of this model is that it does not consider the interaction between the polymer matrix and the drug.
This interaction is crucial for understanding release of many of the molecules of interest for the retina from polymer matrices. While size plays an important role in delivery, the drug chemistry is also critical. A good example of a place where the chemistry has a striking effect is in the delivery of NGF, BDNF, and neurotrophin-3. These molecules are from the same family of growth factors and are essentially identical in structure with minor changes in their amino acid residues123 and yet their delivery profiles from identically processed matrices can be extremely different.124–128
More models that consider the drug–polymer interactions are being developed129–131 which can be combined with the diffusion and degradation-based models to predict release of drugs from matrices. This, however, is only the first step.
One must also consider how the drugs are absorbed by the surrounding tissues in vivo. Depending on the drug chemistry, several of the tissues of the eye can absorb the drug and, potentially, prolong the delivery.132 When performing pharmacokinetic experiments, it is useful to look at not only the vitreous and aqueous concentrations, but if possible, to consider whether the lens, iris, and other tissues are acting as sinks for the drug.
Impact of depot placement
One will get a very different delivery profile of a drug to the retina if the depot is placed in the intravitreal, subconjunctival, or subtenon space. What is becoming exciting is that more and more assessments of pharmacokinetics for the major drugs in the eye are being published, leading to more data regarding the diffusivities of the drugs through the major barriers in the eye. Often, simple compartment models based on traditional diffusion can be used to predict surprisingly well the potential pharmacokinetics of a drug delivery system placed in the subconjunctival versus intravitreal space.133–136 The availability of this data increases the probability of efficiently designing delivery systems for treating the retina.
1 Royal Pharmaceutical Society of Great Britain. History of pharmacy. Available online at www.rpharms.com (cited 20 June 2011)
2 Frid A, Hirsch L, Gaspar R, et al. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36(Suppl 2):S3–18.
3 Vogel WH, Berke A. Brief history of vision and ocular medicine. Amsterdam: Kugler; 2009.
4 Remis LL, Epstein DL. Treatment of glaucoma. Annu Rev Med. 1984;35:195–205.
5 Gabizon A. Liposome circulation time and tumor targeting: implications for cancer chemotherapy. Adv Drug Deliv Rev. 1995;16:285–294.
6 Lian T, Ho RJ. Trends and developments in liposome drug delivery systems. J Pharm Sci. 2001;90:667–680.
7 Farokhzad OC, Cheng J, Teply BA, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103:6315–6320.
8 Janoria KG, Gunda S, Boddu SH, et al. Novel approaches to retinal drug delivery. Expert Opin Drug Deliv. 2007;4:371–388.
9 Langer R. Implantable controlled release systems. Pharmacol Ther. 1983;21:35–51.
10 Langer RS, Peppas NA. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials. 1981;2:201–214.
11 Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;263:797–800.
12 Langer R. Drug delivery and targeting. Nature. 1998;392S:5–10.
13 Chess R. Economics of drug delivery. Pharm Res. 1998;15:172–174.
14 Takeda Pacific. Drug delivery systems report. Fremont, CA: Takeda Pacific; 2006.
15 Harris JM, Chess RB. Effect of PEGylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221.
16 Stewart RH, Novak S. Introduction of the Ocusert ocular system to an ophthalmic practice. Ann Ophthalmol. 1978;10:325–330.
17 Langer R. New methods of drug delivery. Science. 1990;249:1527–1533.
18 Philip AK, Pathak K. Osmotic flow through asymmetric membrane: a means for controlled delivery of drugs with varying solubility. AAPS PharmSciTech. 2006;7:56.
19 Fu K, Harrell R, Zinski K, et al. A potential approach for decreasing the burst effect of protein from PLGA microspheres. J Pharm Sci. 2003;92:1582–1591.
20 Kaneko H, Dridi S, Tarallo V, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–330.
21 Rudnick SI, Adams GP. Affinity and avidity in antibody-based tumor targeting. Cancer Biother Radiopharm. 2009;24:155–161.
22 Brown KC. New approaches for cell-specific targeting: identification of cell-selective peptides from combinatorial libraries. Curr Opin Chem Biol. 2000;4:16–21.
23 Cerchia L, de Franciscis V. Targeting cancer cells with nucleic acid aptamers. Trends Biotechnol. 2010;28:517–525.
24 Levy-Nissenbaum E, Radovic-Moreno AF, Wang AZ, et al. Nanotechnology and aptamers: applications in drug delivery. Trends Biotechnol. 2008;26:442–449.
25 Sakai T, Kohno H, Ishihara T, et al. Treatment of experimental autoimmune uveoretinitis with poly(lactic acid) nanoparticles encapsulating betamethasone phosphate. Exp Eye Res. 2006;82:657–663.
26 Sun D, Nakao S, Xie F, et al. Superior sensitivity of novel molecular imaging probe: simultaneously targeting two types of endothelial injury markers. FASEB J. 2010;24:1532–1540.
27 Kim JH, Kim KW, Kim MH, et al. Intravenously administered gold nanoparticles pass through the blood–retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology. 2009;20:505101.
28 Choonara YE, Pillay V, Danckwerts MP, et al. A review of implantable intravitreal drug delivery technologies for the treatment of posterior segment eye diseases. J Pharm Sci. 2010;99:2219–2239.
29 Bloquel C, Bourges JL, Touchard E, et al. Non-viral ocular gene therapy: potential ocular therapeutic avenues. Adv Drug Deliv Rev. 2006;58:1224–1242.
30 Friedmann T. A brief history of gene therapy. Nat Genet. 1992;2:93–98.
31 Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158.
32 Heilbronn R, Weger S. Viral vectors for gene transfer: current status of gene therapeutics. Handb Exp Pharmacol. 2010;197:143–170.
33 Campochiaro PA, Nguyen QD, Shah SM, et al. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum Gene Ther. 2006;17:167–176.
34 Chen P, Hamilton M, Thomas CA, et al. Persistent expression of PEDF in the eye using high-capacity adenovectors. Mol Ther. 2008;16:1986–1994.
35 Hamilton MM, Brough DE, McVey D, et al. Repeated administration of adenovector in the eye results in efficient gene delivery. Invest Ophthalmol Vis Sci. 2006;47:299–305.
36 McVey D, Hamilton MM, Hsu C, et al. Repeat administration of proteins to the eye with a single intraocular injection of an adenovirus vector. Mol Ther. 2008;16:1444–1449.
37 Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605.
38 Chailertvanitkul VA, Pouton CW. Adenovirus: a blueprint for non-viral gene delivery. Curr Opin Biotechnol. 2010;21:627–632.
39 Chonn A, Cullis PR. Recent advances in liposomal drug-delivery systems. Curr Opin Biotechnol. 1995;6:698–708.
40 Felgner PL, Gadek TR, Holm M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417.
41 Boussif O, Lezoualc’h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–7301.
42 Horbinski C, Stachowiak MK, Higgins D, et al. Polyethyleneimine-mediated transfection of cultured postmitotic neurons from rat sympathetic ganglia and adult human retina. BMC Neurosci. 2001;2:2.
43 Gomes dos Santos AL, Bochot A, Tsapis N, et al. Oligonucleotide–polyethylenimine complexes targeting retinal cells: structural analysis and application to anti-TGFbeta-2 therapy. Pharm Res. 2006;23:770–781.
44 Won YW, Lim KS, Kim YH. Intracellular organelle-targeted non-viral gene delivery systems. J Control Release. 2011;152:99–109.
45 van Gaal EV, Oosting RS, van Eijk R, et al. DNA nuclear targeting sequences for non-viral gene delivery. Pharm Res. 2011;28:1707–1722.
46 Liechty WB, Caldorera-Moore M, Phillips MA, et al. Advanced molecular design of biopolymers for transmucosal and intracellular delivery of chemotherapeutic agents and biological therapeutics. J Control Release. 2011;30:119–127.
47 Dalkara D, Kolstad KD, Caporale N, et al. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol Ther. 2009;17:2096–2102.
48 Gregory-Evans K, Chang F, Hodges MD, et al. Ex vivo gene therapy using intravitreal injection of GDNF-secreting mouse embryonic stem cells in a rat model of retinal degeneration. Mol Vis. 2009;15:962–973.
49 Harper MM, Grozdanic SD, Blits B, et al. Transplantation of BDNF-secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes. Invest Ophthalmol Vis Sci. 2011;52:4506–4515.
50 Lawrence JM, Keegan DJ, Muir EM, et al. Transplantation of Schwann cell line clones secreting GDNF or BDNF into the retinas of dystrophic Royal College of Surgeons rats. Invest Ophthalmol Vis Sci. 2004;45:267–274.
51 Liu Y, Kim DH, Himes BT, et al. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J Neurosci. 1999;19:4370–4387.
52 Taylor AW. Ocular immune privilege. Eye (Lond). 2009;23:1885–1889.
53 Taylor AW, Kaplan HJ. Ocular immune privilege in the year 2010: ocular immune privilege and uveitis. Ocul Immunol Inflamm. 2010;18:488–492.
54 Bjerkvig R, Read TA, Vajkoczy P, et al. Cell therapy using encapsulated cells producing endostatin. Acta Neurochir Suppl. 2003;88:137–141.
55 Siebers U, Horcher A, Bretzel RG, et al. Alginate-based microcapsules for immunoprotected islet transplantation. Ann N Y Acad Sci. 1997;831:304–312.
56 Kontturi LS, Yliperttula M, Toivanen P, et al. A laboratory-scale device for the straightforward production of uniform, small sized cell microcapsules with long-term cell viability. J Control Release. 2011;152:376–381.
57 Chong SF, Lee JH, Zelikin AN, et al. Tuning the permeability of polymer hydrogel capsules: an investigation of cross-linking density, membrane thickness, and cross-linkers. Langmuir. 2011;27:1724–1730.
58 Dusseault J, Leblond FA, Robitaille R, et al. Microencapsulation of living cells in semi-permeable membranes with covalently cross-linked layers. Biomaterials. 2005;26:1515–1522.
59 Lin CC, Anseth KS. Glucagon-like peptide-1 functionalized PEG hydrogels promote survival and function of encapsulated pancreatic beta-cells. Biomacromolecules. 2009;10:2460–2467.
60 Hoyng PF, van Beek LM. Pharmacological therapy for glaucoma: a review. Drugs. 2000;59:411–434.
61 Marquis RE, Whitson JT. Management of glaucoma: Focus on pharmacological therapy. Drugs Aging. 2005;22:1–21.
62 Uusitalo H, Kahonen M, Ropo A, et al. Improved systemic safety and risk–benefit ratio of topical 0.1% timolol hydrogel compared with 0.5% timolol aqueous solution in the treatment of glaucoma. Graefes Arch Clin Exp Ophthalmol. 2006;244:1491–1496.
63 Worakul N, Robinson JR. Ocular pharmacokinetics/pharmacodynamics. Eur J Pharm Biopharmaceutics. 1997;44:71–83.
64 Lambiase A, Aloe L, Centofanti M, et al. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc Natl Acad Sci U S A. 2009. ; epub ahead of print: doi 10.1073/pnas.0906678106
65 Amrite AC, Edelhauser HF, Kompella UB. Modeling of corneal and retinal pharmacokinetics after periocular drug administration. Invest Ophthalmol Vis Sci. 2008;49:320–332.
66 Kim TW, Lindsey JD, Aihara M, et al. Intraocular distribution of 70-kDa dextran after subconjunctival injection in mice. Invest Ophthalmol Vis Sci. 2002;43:1809–1816.
67 Lee TWY, Robinson JR. Drug delivery to the posterior segment of the eye: Some insights on the penetration pathways after subconjunctival injection. J Ocular Pharmacol Ther. 2001;17:565–572.
68 Dureau P, Bonnel S, Menasche M, et al. Quantitative analysis of intravitreal injections in the rat. Curr Eye Res. 2001;22:74–77.
69 Kim H, Csaky KG, Chan CC, et al. The pharmacokinetics of rituximab following an intravitreal injection. Exp Eye Res. 2006;82:760–766.
70 Taylor SRJ, Habot-Wilner Z, Pacheco P, et al, editors. Intraocular methotrexate in the treatment of uveitis and uveitic cystoid macular edema. 112th Annual Meeting of the American Academy of Ophthalmology; 2008 Nov 08–11; Atlanta, GA.
71 Byun YS, Park YH. Complications and safety profile of posterior subtenon injection of triamcinolone acetonide. J Ocular Pharmacol Ther. 2009;25:159–162.
72 Lee SJ, Kim ES, Geroski DH, et al. Pharmacokinetics of intraocular drug delivery of Oregon green 488-labeled triamcinolone by subtenon injection using ocular fluorophotometry in rabbit eyes. Invest Ophthalmol Vis Sci. 2008;49:4506–4514.
73 Verma LK, Vivek MB, Kumar A, et al. A prospective controlled trial to evaluate the adjunctive role of posterior subtenon triamcinolone in the treatment of diffuse diabetic macular edema. J Ocul Pharmacol Ther. 2004;20:277–284.
74 Macoul KL, Pavan-Langston D. Pilocarpine ocusert system for sustained control of ocular hypertension. Arch Ophthalmol. 1975;93:587–590.
75 Pollack IP, Quigley HA, Harbin TS. Ocusert pilocarpine system – advantages and disadvantages. South Med J. 1976;69:1296–1298.
76 Saettone MF, Salminen L. Ocular inserts for topical delivery. Adv Drug Deliv Rev. 1995;16:95–106.
77 American Academy of Ophthalmology. Eye health statistics at a glance. San Francisco: American Academy of Ophthalmology; 2009.
78 White CJ, Byrne ME. Molecularly imprinted therapeutic contact lenses. Expert Opin Drug Deliv. 2010;7:765–780.
79 Hoffman A. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3–12.
80 Peppas N, Bures P, Leobandung W, et al. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharmaceutics. 2000;50:27–46.
81 Hiratani H, Alvarez-Lorenzo C. Timolol uptake and release by imprinted soft contact lenses made of N,N-diethylacrylamide and methacrylic acid. J Control Release. 2002;83:223–230.
82 Schultz CL, Poling TR, Mint JO. A medical device/drug delivery system for treatment of glaucoma. Clin Exp Optom. 2009;92:343–348.
83 Kompella UB, Kadam RS, Lee VH. Recent advances in ophthalmic drug delivery. Ther Deliv. 2010;1:435–456.
84 Saati S, Lo R, Li PY, et al. Mini drug pump for ophthalmic use. Trans Am Ophthalmol Soc. 2009;107:60–70.
85 Saati S, Lo R, Li PY, et al. Mini drug pump for ophthalmic use. Curr Eye Res. 2010;35:192–201.
86 Chopra P, Hao J, Li SK. Iontophoretic transport of charged macromolecules across human sclera. Int J Pharm. 2010;388:107–113.
87 First eye implant approved. AIDS Alert. 1996;11:59.
88 Chiron Vision files FDA application to market intraocular implant for CMV retinitis. Food and Drug Administration. J Int Assoc Physicians AIDS Care. 1995;1:37.
89 Ganciclovir implants (Vitrasert). Treat Rev. 1996 Ap;no 21:10.
90 MacCumber MW, Sadeghi S, Cohen JA, et al. Suture loop to aid in ganciclovir implant removal. Arch Ophthalmol. 1999;117:1250–1254.
91 Schwartz SG, Flynn HW, Jr. Fluocinolone acetonide implantable device for diabetic retinopathy. Curr Pharm Biotechnol. 2011;12:347–351.
92 Brumm MV, Nguyen QD. Fluocinolone acetonide intravitreal sustained release device – a new addition to the armamentarium of uveitic management. Int J Nanomedicine. 2007;2:55–64.
93 Campochiaro PA, Hafiz G, Shah SM, et al. Sustained ocular delivery of fluocinolone acetonide by an intravitreal insert. Ophthalmology. 2010;117:1393–1399. e3
94 Kuno N, Fujii S. Biodegradable intraocular therapies for retinal disorders: progress to date. Drugs Aging. 2010;27:117–134.
95 Haller JA, Bandello F, Belfort R, Jr., et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–1146.
96 von Moos R, Thuerlimann BJ, Aapro M, et al. PEGylated liposomal doxorubicin-associated hand–foot syndrome: recommendations of an international panel of experts. Eur J Cancer. 2008;44:781–790.
97 Lorusso D, Di Stefano A, Carone V, et al. PEGylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (‘hand–foot’ syndrome). Ann Oncol. 2007;18:1159–1164.
98 Dugel PU, Eliott D, Cantrill HL, et al. I-VationTM TA: 24-month clinical results of the phase I safety and preliminary efficacy study. Invest Ophthalmol Vis Sci. 2009;50:4332.
99 Barnett PJ. Mathematical modeling of triamcinolone acetonide drug release from the I-vation intravitreal implant (a controlled release platform). Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3087–3090.
100 Thanos CG, Bell WJ, O’Rourke P, et al. Sustained secretion of ciliary neurotrophic factor to the vitreous, using the encapsulated cell therapy-based NT-501 intraocular device. Tissue Eng. 2004;10:1617–1622.
101 Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–3901.
102 Shive M, Anderson J. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24.
103 Allison SD. Analysis of initial burst in PLGA microparticles. Expert Opin Drug Deliv. 2008;5:615–628.
104 Fu K, Harrell R, Zinski K, et al. A potential approach for decreasing the burst effect of protein from PLGA microspheres. J Pharm Sci. 2003;92:1582–1591.
105 Ward MS, Khoobehi A, Lavik EB, et al. Neuroprotection of retinal ganglion cells in DBA/2 J mice with GDNF-loaded biodegradable microspheres. J Pharm Sci. 2007;96:558–568.
106 Lavik EB, Warfvinge K, Scherfig E, et al. Preservation of retinal ganglion cells via polymer microspheres delivering GDNF following low perfusion pressure in the pig. Washington, DC: Society for Neuroscience, 2005.
107 Amrite AC, Ayalasomayajula SP, Cheruvu NP, et al. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest Ophthalmol Vis Sci. 2006;47:1149–1160.
108 Kompella UB, Bandi N, Ayalasomayajula SP. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Invest Ophthalmol Vis Sci. 2003;44:1192–1201.
109 Kang SJ, Durairaj C, Kompella UB, et al. Subconjunctival nanoparticle carboplatin in the treatment of murine retinoblastoma. Arch Ophthalmol. 2009;127:1043–1047.
110 Xu J, Wang Y, Li Y, et al. Inhibitory efficacy of intravitreal dexamethasone acetate-loaded PLGA nanoparticles on choroidal neovascularization in a laser-induced rat model. J Ocul Pharmacol Ther. 2007;23:527–540.
111 Grozdanic SD, Lazic T, Kuehn MH, et al. Exogenous modulation of intrinsic optic nerve neuroprotective activity. Graefes Arch Clin Exp Ophthalmol. 2010;248:1105–1116.
112 Lemaire V, Belair J, Hildgen P. Structural modeling of drug release from biodegradable porous matrices based on a combined diffusion/erosion process. Int J Pharm. 2003;258:95–107.
113 Rabinovich-Guilatt L, Lambert G, inventors; Novagali Pharma SA, assignee. Methods for treating eye disease or conditions affecting the posterior segment of the eye. USA patent 11/444,349. 2007.
114 Kim JH, Kim JH, Kim KW, et al. Intravenously administered gold nanoparticles pass through the blood–retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology. 2009;20:505101.
115 Caldorera-Moore M, Guimard N, Shi L, et al. Designer nanoparticles: incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opin Drug Deliv. 2010;7:479–495.
116 Semete B, Booysen L, Lemmer Y, et al. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine. 2010;6:662–671.
117 Monem AS, Ali FM, Ismail MW. Prolonged effect of liposomes encapsulating pilocarpine HCl in normal and glaucomatous rabbits. Int J Pharm. 2000;198:29–38.
118 De Campos AM, Sanchez A, Gref R, et al. The effect of a PEG versus a chitosan coating on the interaction of drug colloidal carriers with the ocular mucosa. Eur J Pharm Sci. 2003;20:73–81.
119 Hironaka K, Inokuchi Y, Tozuka Y, et al. Design and evaluation of a liposomal delivery system targeting the posterior segment of the eye. J Control Release. 2009;136:247–253.
120 Inokuchi Y, Hironaka K, Fujisawa T, et al. Physicochemical properties affecting retinal drug/coumarin-6 delivery from nanocarrier systems via eyedrop administration. Invest Ophthalmol Vis Sci. 2010;51:3162–3170.
121 El Sanharawi M, Kowalczuk L, Touchard E, et al. Protein delivery for retinal diseases: from basic considerations to clinical applications. Prog Retin Eye Res. 2010;29:443–465.
122 Rothstein SN, Federspiel WJ, Little SR. A unified mathematical model for the prediction of controlled release from surface and bulk eroding polymer matrices. Biomaterials. 2009;30:1657–1664.
123 Ebendal T. Function and evolution in the NGF family and its receptors. J Neurosci Res. 1992;32:461–470.
124 Woodhall E, West AK, Chuah MI. Cultured olfactory ensheathing cells express nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neurotrophic factor and their receptors. Mol Brain Res. 2001;88:203–213.
125 Aszmann OC, Korak KJ, Kropf N, et al. Simultaneous GDNF and BDNF application leads to increased motoneuron survival and improved functional outcome in an experimental model for obstetric brachial plexus lesions. Plast Reconstr Surg. 2002;110:1066–1072.
126 Bertram J, Rauch MF, Chang K, et al. Using polymer chemistry to modulate the delivery of neurotrophic factors from degradable microspheres: Delivery of BDNF. Pharm Res. 2009;27:82–91.
127 Cao X, Schoichet M. Delivering neuroactive molecules from biodegradable microspheres for application in central nervous system disorders. Biomaterials. 1999;20:329–339.
128 Yang Y, De Laporte L, Rives CB, et al. Neurotrophin releasing single and multiple lumen nerve conduits. J Control Release. 2005;104:433–446.
129 Taylor SJ, McDonald JW, 3rd., Sakiyama-Elbert SE. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J Control Release. 2004;98:281–294.
130 Park S, Kang S, Veach AJ, et al. Self-assembled nanoplatform for targeted delivery of chemotherapy agents via affinity-regulated molecular interactions. Biomaterials. 2010;31:7766–7775.
131 Fu AS, Thatiparti TR, Saidel GM, et al. Experimental studies and modeling of drug release from a tunable affinity-based drug delivery platform. Ann Biomed Eng. 2011;39:2466–2475.
132 Lee VHL, Luo AM, Li SY, et al. Pharmacokinetic basis for nonadditivity of intraocular pressure lowering in timolol combinations. Invest Ophthalmol Vis Sci. 1991;32:2948–2957.
133 Douglas LC, Yi NY, Davis JL, et al. Ocular toxicity and distribution of subconjunctival and intravitreal rapamycin in horses. J Vet Pharmacol Ther. 2008;31:511–516.
134 Hosseini K, Matsushima D, Johnson J, et al. Pharmacokinetic study of dexamethasone disodium phosphate using intravitreal, subconjunctival, and intravenous delivery routes in rabbits. J Ocul Pharmacol Ther. 2008;24:301–308.
135 Nomoto H, Shiraga F, Kuno N, et al. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest Ophthalmol Vis Sci. 2009;50:4807–4813.
136 Liu X, Li SK, Jeong EK. Ocular pharmacokinetic study of a corticosteroid by 19F MR. Exp Eye Res. 2010;91:347–352.