CHAPTER 171 Dorsal Root Entry Zone Lesions
Management of chronic pain has graduated over the past few decades to a multidisciplinary approach, and neurosurgical methods, the mainstay of the neurosurgeon’s armamentarium, have evolved from mostly ablative to mostly neuromodulatory. Neuroaugmentative procedures such as spinal cord stimulation have gained increasing acceptance in recent years, largely because of their minimal invasiveness. Additionally, the substantial and prolonged pain relief obtained with these procedures, their cost-effectiveness, the ability to repeatedly reprogram implant technology, and the rarity of complications have all influenced the adoption of neuromodulation as the current surgical pain treatment of choice. Ablative procedures, in particular, percutaneous approaches, nevertheless remain a standard form of treatment of certain conditions such as trigeminal neuralgia. More traditional destructive procedures, such as anterolateral cordotomy, remain an option for patients with certain cancer-related pain such as Pancoast’s syndrome or breakthrough pain resistant to pharmacotherapy. Dorsal root entry zone (DREZ) lesions, although not as frequently performed as a few decades ago, remain a potent form of therapy for management of pain from brachial plexus avulsion and other spinal cord injuries (SCIs) and several types of cancer pain.1,2 Nucleus caudalis DREZ has been shown to be effective in the treatment of anesthesia dolorosa, atypical facial pain, postherpetic pain, and some deafferentation pain.3
Pain Mechanism
Pain-signaling impulses originating in peripheral nociceptors are transmitted by first-order neurons, which have their cell bodies in the dorsal root ganglion. These fibers are either small myelinated (Aδ) or unmyelinated (C) axons that carry impulses from polymodal nociceptors or nociceptive-specific terminals, respectively.4 Second-order neurons, with cell bodies in the dorsal horn of the spinal cord, cross the midline and ascend in the contralateral spinothalamic tract to relay in the thalamus, from where third-order neurons project to the postcentral gyrus (via the internal capsule).
The “H”-shaped configuration of the gray matter of the spinal cord was first described by Rolando in 1824.5 Subsequently, Rexed described discrete laminae in the central gray matter of the spinal cord. The significance of this arrangement in the dorsal horn is that unmyelinated C fibers synapse in laminae I and II whereas the small myelinated Aδ fibers synapse in laminae I, II, and V. C fibers project to the spinoreticulodiencephalic tract (also known as the paleospinothalamic tract), whereas Aδ fibers project to the anterolateral white funiculus (also known as the neospinothalamic tract).4,6–9
Abnormal electrical activity has been documented in the dorsal horn in experimental animal models of brachial plexus avulsion and other SCIs. The concept of DREZ lesions involves surgical destruction of these second-order neurons of the ascending nociceptive pathway. Destruction of the DREZ and superficial dorsal horn is thought to abolish this abnormal electrical activity and thus help relieve pain.10,11
Historical Perspectives
Attempts at DREZ lesions were first made in 1972 in Lyon, France, by Sindou.2,12 He successfully used a technique of microcoagulation of the dorsal rootlets in a patient with Pancoast’s syndrome and termed the procedure selective posterior rhizotomy. Further attempts at the procedure were made by Nashold and colleagues in 1974 with radiofrequency (RF) thermocoagulation.2,13 They coined the term DREZ operation and championed the merits of the procedure. Later, success was reported with lasers by Levy and coworkers, Powers and associates, and Stranjalis and Torrens14–18 and with ultrasonic techniques for DREZ lesions by Dreval and Kandel and associates.19,20 In recent years, Spaic and coauthors have advocated a microsurgical technique of incising the dorsolateral sulcus and suctioning the dorsal horn, but it would appear that pain control rates with this technique are not statistically different (P < .5) from the standard procedure.21
Anatomic and Physiologic Considerations
In his classic paper, Sindou noted that in the dorsal horn of the spinal cord, afferent fibers are segregated spatially according to their size and destination.2,12 About 1 mm outside the spinal cord, the large and small fibers in the dorsal roots, which are arranged somewhat randomly in the afferent nerve fibers, undergo reorientation. Each dorsal root divides into 4 to 10 rootlets, each 0.25 to 1.5 mm in diameter and varying according to level.2,12 The small myelinated and unmyelinated fibers carrying nociceptive impulses course to the lateral side, which makes them appropriately situated to enter into the tract of Lissauer, where they run for one or two segments before penetrating the gray matter of the spinal cord.4,22,23 Because the nociceptive fibers preferentially terminate in lamina I and the substantia gelatinosa (laminae II and III), they are amenable, by virtue of their superficial position in the spinal cord, to surgical lesions without substantial risk of damage to other ascending or descending pathways.7,23–25 In a human cadaveric study of DREZ lesions in the cervicothoracic region, it was found that the average number of rootlets of the C5 to T1 roots was approximately 7.76 + 3.2, with C6 having the most rootlets. The average angle of the inferior rootlets with the longitudinal axis of the spinal cord at each root level from C5 to T1 decreased gradually from 65.6 ± 3.68 to 19.8 ± 2.98 degrees. On cross-sectional studies it was noted that the average length of the dorsal horn was 3.47 ± 0.31 mm (range, 3.10 to 3.75 mm) and the average width was 1.35 ± 0.07 mm (1.20 to 1.45 mm). The angle between the longitudinal axis of the dorsal horn and the sagittal plane of the spinal cord decreased gradually from 29 to 43 degrees, with an average of 35.9 ± 1.28 degrees.26
The dorsal horn essentially consists of central projections of primary neurons, intrinsic dorsal horn neurons, and ascending and descending tracts. It is theorized that the final mechanism underlying pain after SCI is deafferentation, which leads to permanent anatomic and neurochemical changes in the intrinsic dorsal horn neurons and thereby results in chronic pain. It is also possible that the modulating effect of the descending inhibitory impulses on the dorsal horn neurons is modified or lost in such cases.23,27,28 Woolf proposed a classification of the different physiologic states (modes) of the dorsal horn: mode 1—control state; mode 2—suppressed state (as in neurostimulation); mode 3—sensitized state; and mode 4—reorganized state.29 Mode 4 represents a potentially irreversible reorganization of the circuitry of the dorsal horn region. A shift from mode 1 to mode 4 occurs when the highly structured organization of the dorsal horn is modified by degenerative or traumatic changes, which leads to alteration of sensory processing in the dorsal horn. Loss of afferent input to the dorsal horn neurons may also result in upregulation of postsynaptic receptors (denervation hypersensitivity) and adversely affect segmental modulatory (inhibitory) mechanisms, thereby dramatically altering processing of input in the dorsal horn.4,22,23,27 Regeneration may also occur at these sites, and it has been documented that the large myelinated fibers that normally terminate in the deeper laminae may grow into lamina II (the site of C-fiber terminals).4,23 The formation of new synapses would therefore affect the way that the central nervous system processes low-threshold mechanical impulses and lead to their perception as pain. DREZ lesions may interrupt the mechanism of such pain by ablation of the dorsal horn structures involved in generating nociceptive impulses as a result of deafferentation. When Falci and colleagues performed electrophysiologic studies on the DREZ for intraoperative guidance in patients with SCI, they documented spontaneous electrical hyperactivity (higher voltage and frequencies) in the region.10 Using this as a guide to localization of the DREZ, they were able to achieve 100% pain relief in 88% of their patients.
Indications
Since introduction of the technique, DREZ lesions have been applied to treat a wide range of pain conditions, most of which are a form of central or deafferentation pain caused by different conditions ranging from brachial plexus avulsion, SCI, phantom limb, and other complex regional syndromes. DREZ lesions have also been applied to treat pain conditions caused by herpes zoster, cancer, and even spasticity.11,30–32
Despite a plethora of study data, specific application of DREZ lesions and safety guidelines are lacking.33
Brachial Plexus Injury Pain
Avulsion of the dorsal root and the resultant gliosis lead to scar formation in the dorsal horn and substantia gelatinosa. Such scar formation may give rise to loss of the inhibitory effects of large-caliber sensory fibers and to spontaneous activity in nociceptive-specific and wide–dynamic range (polymodal) neurons, which may be responsible for perception of pain after brachial plexus lesions.34,35
Brachial plexus injuries are frequently associated with avulsion of the dorsal or ventral root, or both, from their associated entry zones into the spinal cord. This leads to the production of deafferentation pain in up to 20% to 30% of patients with brachial plexus injuries.36 Brachial plexus avulsion pain may begin immediately after the trauma, but its onset can also occur months to years later. Pain projection depends primarily on the extent of the injury and the number of avulsed roots involved; it may include the whole upper limb but usually radiates to the forearm and hand.
Multiple groups have extensive experience with the application of DREZ lesions to treat pain from brachial plexus avulsion injury. Sindou and coauthors reported that 42% of patients had complete avulsion of all plexus roots; they also noticed scarring, arachnoiditis, and atrophy of the spinal cord, which in some cases was rotated. They reported that 66% of patients had good to excellent outcomes, with 71% demonstrating an improvement in daily activities (mean follow-up was 6 years).37 Friedman and colleagues reported similar results: 77% of patients had a 75% reduction in pain intensity.38,39 Prestor reported that 47.6% of patients had complete resolution of pain whereas 80.9% had at least 70% improvement. He noticed that better results were obtained in patients who underwent surgery more than 1 year after injury and reported poor results in patients previously treated with pain management surgery such as cordotomy or myelotomy.11 In a long-term follow-up study, Chen and associates reported early good results in 80% of patients operated on by thermocoagulation; however, this number dropped to 60% (5-year follow-up) and 50% (10-year follow-up).40,41 Samii and colleagues reported a 50% reduction in pain in 86% of patients immediately postoperatively, in 68% after 3 months, in 63% after 6 months, and in 62% in long-term follow-up. They found no correlation between the number of roots avulsed or the extent of the DREZ coagulation procedure performed and the degree of pain reduction, between the duration of pain and outcome after DREZ, or between pain onset and time to surgery.42 Dreval applied ultrasound to perform DREZ lesioning and operated on 127 patients. He reported that 96% of patients had immediate improvement and 87% had a good result at follow-up (average follow-up of 47.5 months).19
Spinal Cord Injury Pain
The incidence of all types of chronic pain after SCI has been variously reported to be between 18% and 94%.43–45 This variability would seem to be dependent on the population surveyed, the survey method, and pain types included (mild and moderate). An incidence of pain of around 65% after SCI is a good first approximation.46 In a longitudinal study of the prevalence and characteristics of pain in the first 5 years after SCI, Siddall and associates found that musculoskeletal pain was the most commonly experienced type of pain (59%).47
Reports vary on the incidence of neuropathic pain after SCI, with estimates varying between 10% and 25%.33,48,49 Pain may occur immediately after injury or be delayed for several years and usually has a burning character.50,51 Neuropathic pain after SCI has been divided into “at level” and “below level.” The incidence of at-level and below-level neuropathic pain has variously been reported to be 13% to 51% and 27% to 42%, respectively, after SCI.47,52 Werhagen and coauthors reported that the incidence of neuropathic injury pain is lower in younger patients (26% in patients younger than 20 years at time of injury versus 58% in patients older than 20 years). Furthermore, below-level pain is more common in SCI patients younger than 40 years, whereas at-level pain is more prevalent in patients older than 40 years at the time of injury.47,52
Sindou and associates reported good long-term results in 65% of patients with segmental pain and 0% improvement in those with infrasegmental pain. A good result was obtained in 88% of patients with predominantly paroxysmal pain, whereas only 26% with continuous pain had good results.31 Nashold and Bullitt reported good results in 77%,50 whereas Sampson and coworkers reported that 54% of patients were pain free after 3 years’ follow-up and noted better results in patients with incomplete injuries.53 Richter and Seitz reported good results with DREZ lesions in the cervical spinal cord for brachial plexus avulsion and poor results in paraplegic patients with infrasegmental pain; patients with cervical pain experienced a 30% recurrence rate.54 Kanpolat and coauthors reported good results in 47% of patients with segmental pain.30 Friedman and Nashold reported a 50% improvement over a 6-month to 6-year interval in patients with segmental pain rather than diffuse pain.39
Selection of patients for DREZ lesions is critical in those with SCI pain. Studies such as the ones just cited have shown that patients with pain in the segmental region after spinal cord trauma experience a better response than do those who have diffuse pain or pain that is below the injured segment.30,31,38,51,55 Caution should be exercised when performing DREZ lesions in patients with incomplete SCI to avoid aggravation of weakness by performance of an overly deep or aggressive lesion. In the case of complete SCI, a deep lesion can, of course, be more safely performed.
Phantom Limb Pain
Good results have been reported when the pain has a paroxysmal character or allodynia (perception of pain from what would ordinarily be a nonpainful stimulus) is a prominent feature. Good results can also be achieved in posttraumatic cases.51 Patients with phantom limb pain who also have nerve root avulsion may also have good results. Stump pain is not usually relieved unless it is of a paroxysmal type.11 Saris and colleagues operated on patients with pain from a phantom limb secondary to trauma, gangrene, and cancer and reported complete relief of the pain in 36%.56 Malin and Winkelmuller also reported good results in patients with peripheral nerve injuries that were associated with root avulsion.57 Prestor reported that DREZ lesions for peripheral nerve injuries and phantom limb pain were generally unsatisfactory but may have a role in select cases.11
Cancer Pain
Attempts at DREZ lesions were first made in 1972 for Pancoast-Tobias syndrome by Sindou.2,12 Since then, several other options to treat cancer pain have emerged, such as midline myelotomy, image-guided cordotomy, and intrathecal pump administration of opioids, which has limited the use of DREZ lesioning to treat cancer pain.
Sindou and associates reported good results in 87% of patients undergoing treatment at the cervical or cervicothoracic level and in 78% undergoing treatment at the lumbar level.51,58 Kanpolat and coauthors reported pain relief in 60% of patients with neuropathic pain of cancer origin.30
Postherpetic Neuralgia
Prestor performed DREZ lesions in 4 patients with postherpetic neuralgia and achieved an excellent outcome in 3. These patients all had pain confined to a segmental area that could be localized.11 Friedman and Nashold reported good pain relief in 8 of 12 patients in short-term follow-up,59 and good pain relief persisted in long-term follow-up in 25%.36 Kanpolat and colleagues operated on only 2 patients and achieved complete pain relief in both.30
Spasticity
The spasticity-relieving effect of DREZ lesions is probably due to interruption of both the myotonic monosynaptic reflex and the nociceptive polysynaptic reflex; these reflexes are excitatory to the dorsal horn somatosensory relay center, which has been cut off from higher central control. Conversely, DREZ lesions may preserve the inhibitory, lemniscal intersegmental input on the dorsal horn.60–65
Sindou and coworkers studied the effect and outcome of DREZ lesions in patients with spasticity. They performed DREZ lesions from C5 to T1 on patients with spastic hemiparesis and reported good outcomes in shoulder and elbow tone yet disappointing results in hand and finger tone.66,67 In patients with spastic paraplegia, Sindou and coauthors reported 87% improvement in tone, which allowed easy passive movement. Patients with both pain and spasticity also had an 88% improvement in pain. Sindou’s group noted that spasticity secondary to SCI had a better outcome than that caused by multiple sclerosis. Patients with spasticity of cerebral origin showed the least improvement and highest complication rate. Postoperative sensory disturbance was noted in all patients and was severe in 20%.64,66–68
Other Indications
Kahilogullari and coauthors reported performing cervical DREZ lesions on a patient with dystonia after thalamotomy and campotomy and achieved a good outcome.69
Teixeira and associates used cervical DREZ lesions to treat patients with postradiation plexopathy. The patients had complete relief except for one, in whom the pain recurred. Transient urinary retention and motor weakness were also observed.70
Operative Technique
Preoperative Considerations
Prescreening of patients for DREZ lesions is critical to ensure no confusion between musculoskeletal pain and neuropathic pain. It is important to determine that the patient has been treated appropriately with pharmacologic measures for neuropathic pain, including gabapentin, pregabalin, and antidepressants, before DREZ lesions are considered. Finnerup and coworkers observed that only 7% of patients suffering from symptoms suggestive of neuropathic pain have actually been prescribed drugs considered to be effective in the management of neuropathic pain.43
In patients with brachial plexus injuries, pain develops in the deafferentated arm in up to 90%; at 3 years after injury, pain is mild in 51% and severe in 40%.71 However, more than 90% of patients with avulsion injuries may suffer significant pain.37,72 Use of brachial plexus grafting and nerve transfers should be also be considered because it has been reported that up to 80% of patients may be pain free or nearly pain free at 2 years’ follow-up after nerve reconstruction.73,74
Anesthesia, Premedication, and Positioning
The standard DREZ operation is a microsurgical procedure performed with the patient under general anesthesia in the prone position. If the cervical region is to be accessed, the head should be secured by pin fixation for easier positioning and adequate access to the operative site. The “Concorde” position with the head and neck flexed may also be used to avoid loss of cerebrospinal fluid (CSF).2 The prophylactic use of preoperative intravenous steroids at doses corresponding to those used for the management of acute SCI has been advocated. The use of fluoroscopy helps mark the site of surgical intervention on the skin surface.
Surgical Procedure
A laminectomy or hemilaminectomy is performed that corresponds to the vertebral level of the spinal cord segment involved, as determined by a dermatomal pain pattern. It is prudent to obtain exposure of the cord up to two segments proximal to the involved upper segmental level to assist in localization of the dorsolateral sulcus by visualization of the entry point of the normal rootlets rostral to the injury. In most cases of brachial plexus avulsion injury, the laminectomy should extend from the upper border of C3 up to and including C7, with undercutting of the T1 lamina.11,37,75 In cases of brachial plexus avulsion, the DREZ can be localized by the presence of pitting on the dorsolateral aspect of the spinal cord, where the dorsal rootlets have been avulsed. Partially avulsed roots can be identified by a change in root color or size.2 Comparison with the contralateral side and tracing the dorsolateral sulcus by identifying the rostral and caudal normal rootlets can also help localize the DREZ.37 Sindou has reported DREZ angles at various levels: 30 degrees at C6, 26 degrees at T4, 37 degrees at T12, and 36 degrees at L3.2
Dorsal horn postsynaptic potentials may help in identification of spinal cord segments,10,75 and somatosensory evoked potentials can be monitored to confirm continued integrity of the dorsal column fibers. Microelectrode recording in the injured segments has also been reported to be useful in the performance of DREZ lesions.10
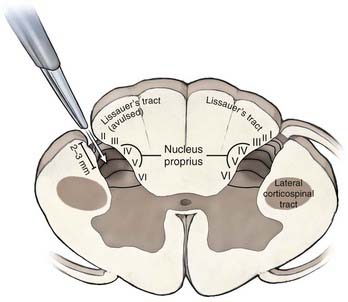
After the pia of the DREZ is incised with an arachnoid knife, microsurgical lesions are made by thermal coagulation with fine-tipped bipolar forceps at a low setting. The lesions are made in “staccato” fashion along the DREZ (4- to 5-mm lesions, with a gap of a few millimeters between each lesion). This allows DREZ lesioning without causing additional and uncontrolled injury from eversion of spinal cord tissue along a long pial incision. A lesional depth of 2 to 3 mm in the dorsal horn is sufficient to produce a lesion in the nucleus proprius (laminae IV to V) because the superficial layers of Lissauer’s tract and the superficial dorsal horn may already have been avulsed. The DREZ under any retained rootlet at the level of interest may be disrupted further by partial sectioning of the ventrolateral aspect of the rootlet.2,76
Special Considerations
In DREZ lesions for pain from brachial plexus avulsion injury, a hemilaminectomy from C3 to T1 with preservation of the spinous processes allows sufficient exposure. The ventral roots can be stimulated to confirm the segments (C5—deltoid; C6—biceps; C7—triceps; C8 to T1—small muscles of the hand).11,37
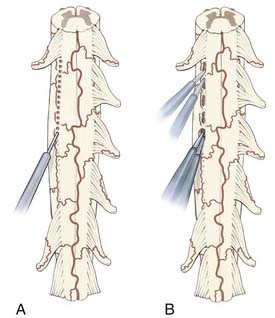
For the RF lesion method proposed by Nashold and colleagues, a 0.5-mm-diameter insulated stainless steel electrode with a 2-mm tip is used, and lesions are made with a current strength of 35 to 40 mA at less than 75°C for 10 to 15 seconds. A “chain-link” pattern is used, with 2- to 3-mm intervals between adjacent lesions (Fig. 171-1A). The lesion usually extends 1 to 2 mm beyond the tip of the electrode. Nashold and associates have also recommended measuring impedance during surgery after they determined that the impedance of damaged spinal cord was less than 1200 Ω whereas that of a normal spinal cord reaches 1500 Ω.2,7576
An alternative to the RF procedure is to use bipolar cautery with the power set at 20 to 25 and extend the DREZotomy for root avulsion pain by first coagulating over the surface along a zone that is formed by a combination of several landmarks: (1) a line directly connecting the last intact dorsal rootlet rostrally with the first visible intact rootlet distally, (2) the “pits” left by the avulsed roots, and (3) a small vein that typically runs just lateral to the DREZ, which may still be present. It is a combination of these available landmarks that delineates the line for the DREZ lesions in each individual case. DREZ lesions are made through the coagulated zone with a microknife to a depth of 2 mm, and then the bipolar tips are inserted into the myelotomy to a depth of 2 mm, opened slightly, and coagulation current applied for several seconds. Each lesion measures 5 to 7 mm along the DREZ, and lesions are made in segments such that 1 to 2 mm of intact pia is left between the lesions to prevent eversion of spinal cord tissue from a long linear myelotomy. The zone between each lesion is similarly coagulated by placing one tip of the bipolar cautery into adjacent lesions and applying coagulating current for several seconds (Fig. 171-1B). The object of a DREZ lesion is to coagulate strictly within the dorsal horn, approximately to the level of the nucleus proprius (laminae IV to V). Precise placement of the lesion is critical to avoid injury to the dorsal column medially and the corticospinal tract laterally (Fig. 171-2).
Carbon dioxide (CO2), argon, and Nd:YAG (neodymium: yttrium-aluminium-garnet [Nd:Y3Al5O12]) lasers have all been used to create DREZ lesions.14,16,18 Levy and colleagues advocated a pulse duration of 0.1 second, 20-W power, and one to two pulses to create a 2-mm-deep lesion at an angle of 45 degrees to the DREZ. For SCI pain, Stranjalis and Torrens used an Nd:YAG laser to create lesions at 1-mm intervals to a depth of 2 mm at a power output of 10 W/sec, with the lesions extending 3 cm above and below the involved segment.18 In his analysis of lesions made by RF and lasers, Young reported that although lasers produced a V-shaped lesion, RF lesions were mostly spherical.77 He also noted that RF resulted in better pain control, but at the cost of a higher complication rate.
Nashold and coworkers had used intraoperative ultrasonography to localize and drain intraspinal cysts after root avulsion.49 Dreval, at the Moscow Central Institute, reported using ultrasound at 44 KHz and an amplitude of 15 µm to 50 pm to create a “sulcomyelotomy” along the dorsolateral sulcus at an angle of projection of 25 degrees medially and ventrally.19 His procedure keeps vessels crossing the sulcus intact.
Spaic and coauthors reported a technique of microsurgical suctioning of the dorsal horn in place of the coagulation procedure.21 They advocated use of a 0.8-mm suction tip to suction the dorsal horn under microscopic visualization after opening the dorsolateral sulcus. According to these authors, the dorsal horn can be visualized at 20× magnification as a band of gray substance that gradually reaches the massive central gray matter of the cord.
Complications
Complications of DREZ lesions, as with all procedures, can include the general complications of infection, meningitis, CSF leak, and hematoma formation. Other complications specifically related to the procedure can also occur and may be caused by injury to neighboring structures such as the dorsal column, pyramidal tract, or dorsal spinocerebellar tract. Neurological complications tend to be more common in DREZ surgery performed on the thoracic spine because of the narrow dorsal horn.38,59 Such complications include dysesthesia, hypoesthesia, ataxia, motor weakness, and urogenital dysfunction, all of which may be either temporary or permanent.33,51
Edgar and coworkers operated on 112 patients with SCI and reported sensory deficit in 2, motor deficit in 3, CSF leak in 3, pulmonary embolism in 2, and myoclonus in 5.78 Freidman and colleagues operated on 54 patients and reported CSF leak in 9, epidural hematoma in 1, weakness in 2, bladder insufficiency in 2, and new dysesthesia in 2.39 Chen and Tu used RF-induced DREZ lesions to treat 500 patients with brachial plexus injuries; ataxia developed in 25% but improved within 3 months.41 Sindou and Jeanmonod operated on 53 patients with spasticity and found that 48% had worse trophic skin changes, which at follow-up improved in just 10.9%. They attributed this complication to (1) surgical stress as a result of poor nutritional condition, (2) decreased sensation on dermatomes affected by the DREZ lesion, and (3) progression and decompensation of underlying disease (mainly multiple sclerosis). They also noted that 12.5% had increased bladder dysfunction and 3.8% experienced CSF leaks.68 Prestor operated on 40 patients with deafferentation pain and reported that transient sensory loss developed in 15%, mainly in the form of proprioceptive deficit secondary to injury to the dorsal spinocerebellar tract, which improved within 8 weeks. Permanent superficial and deep sensory disturbance with weakness was observed in 1 patient (2.5%).11
Some differences in the rate of complications between alternative methods of performing DREZ lesions have been reported. Young found more deficits with RF when performed on an area of 0.5 × 2 mm than with laser or RF on an area of 0.25 × 2 mm.79
Conclusion
The frequency with which DREZ lesions are performed to control pain is unknown. There have been no class I or II trials to prove the efficacy of DREZ lesions for neuropathic pain. Case series data consistently report good results in patients with brachial plexus avulsion pain and in some patients with SCI. Patients with postherpetic neuralgia or peripheral neuropathic pain tend to have a poor response to DREZ lesions.17,37,38,79,80
Kanpolat Y, Tuna H, Bozkurt M, et al. Spinal and nucleus caudalis dorsal root entry zone operations for chronic pain. Neurosurgery. 2008;62:235-242.
McHugh JM, McHugh WB. Pain: neuroanatomy, chemical mediators, and clinical implications. AACN Clin Issues. 2000;11:168-178.
Nashold BSJr, Ostdahl RH. Dorsal root entry zone lesions for pain relief. J Neurosurg. 1979;51:59-69.
Prestor B. Microsurgical junctional DREZ coagulation for treatment of deafferentation pain syndromes. Surg Neurol. 2001;56:259-265.
Samii M, Bear-Henney S, Ludemann W, et al. Treatment of refractory pain after brachial plexus avulsion with dorsal root entry zone lesions. Neurosurgery. 2001;48:1269-1275.
Siddall PJ, McClelland JM, Rutkowski SB, et al. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249-257.
Sindou M. Dorsal root entry zone lesions. In: Burchiel KJ, editor. Surgical Management of Pain. New York: Thieme; 2002:701-713.
Sindou MP, Blondet E, Emery E, et al. Microsurgical lesioning in the dorsal root entry zone for pain due to brachial plexus avulsion: a prospective series of 55 patients. J Neurosurg. 2005;102:1018-1028.
Spaic M, Markovic N, Tadic R. Microsurgical DREZotomy for pain of spinal cord and cauda equina injury origin: clinical characteristics of pain and implications for surgery in a series of 26 patients. Acta Neurochir (Wien). 2002;144:453-462.
Woolf C. The Dorsal horn: state-dependent sensory processing and the generation of pain. In: Wall PD, Melzack R, editors. Textbook of Pain. New York: Churchill Livingstone; 1994:101-112.
Xiang JP, Liu XL, Xu YB, et al. Microsurgical anatomy of dorsal root entry zone of brachial plexus. Microsurgery. 2008;28:17-20.
Young RF. Clinical experience with radiofrequency and laser DREZ lesions. J Neurosurg. 1990;72:715-720.
1 Meyerson BA. Neurosurgical approaches to pain treatment. Acta Anaesthesiol Scand. 2001;45:1108-1113.
2 Sindou M. Dorsal root entry zone lesions. In: Burchiel KJ, editor. Surgical Management of Pain. New York: Thieme; 2002:701-713.
3 Gorecki JP. Dorsal root entry zone and brainstem ablative procedures. In: Winn HR, editor. Youmans Neurological Surgery, Vol 3. Philadelphia: WB Saunders; 2004:3045-3058.
4 Mense S. Basic neurobiologic mechanisms of pain and analgesia. Am J Med. 1983;75:4-14.
5 Rolando L. Ricerche anatomiche sulla structura del midollo spinale. In: Dizionario Periodico di Medicinia. Torino, Italy: Staperia Reale; 1824.
6 D’Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101:8-16.
7 Willis WD. Nociceptive pathways: anatomy and physiology of nociceptive ascending pathways. Philos Trans R Soc Lond B Biol Sci. 1985;308:253-270.
8 Cross SA. Pathophysiology of pain. Mayo Clin Proc. 1994;69:375-383.
9 Rexed B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952;96:414-495.
10 Falci S, Best L, Bayles R, et al. Dorsal root entry zone microcoagulation for spinal cord injury–related central pain: operative intramedullary electrophysiological guidance and clinical outcome. J Neurosurg. 2002;97:193-200.
11 Prestor B. Microsurgical junctional DREZ coagulation for treatment of deafferentation pain syndromes. Surg Neurol. 2001;56:259-265.
12 Sindou M. Study of the dorsal root entery zone: implications for pain surgery. Lyon, France: University of Lyon Press; 1972. M.D. Thesis
13 Nashold B, Urban B, Zorub D. Phantom pain relief by focal destruction of substantia gelatinosa of Rolando. In: Bonica JJ, Albe Fessard D, editors. Advances in Pain Research and Therapy, Vol 1. New York: Raven Press; 1976:959-963.
14 Levy WJ, Nutkiewicz A, Ditmore QM, et al. Laser-induced dorsal root entry zone lesions for pain control. Report of three cases. J Neurosurg. 1983;59:884-886.
15 Powers SK. Laser-induced dorsal root entry zone lesions for pain control. J Neurosurg. 1984;60:871-872.
16 Powers SK, Adams JE, Edwards MS, et al. Pain relief from dorsal root entry zone lesions made with argon and carbon dioxide microsurgical lasers. J Neurosurg. 1984;61:841-847.
17 Powers SK, Barbaro NM, Levy RM. Pain control with laser-produced dorsal root entry zone lesions. Appl Neurophysiol. 1988;51:243-254.
18 Stranjalis G, Torrens M. Dorsal root entry zone lesion performed with Nd:YAG laser. Br J Neurosurg. 1997;11:238-240.
19 Dreval ON. Ultrasonic DREZ-operations for treatment of pain due to brachial plexus avulsion. Acta Neurochir (Wien). 1993;122:76-81.
20 Kandel EI, Ogleznev K, Dreval ON. [Destruction of the entry zone of the posterior roots as a method of treating chronic pain in traumatic damage to the brachial plexus.]. Zh Vopr Neirokhir Im N N Burdenko. 1987:20-27.
21 Spaic M, Markovic N, Mikicic D, et al. The DREZ surgical treatment of chronic pain in traumatic paraplegia. Indian J Neurotrauma. 2005;2:111-116.
22 Bishop B. Pain: its physiology and rationale for management. Part I. Neuroanatomical substrate of pain. Phys Ther. 1980;60:13-20.
23 McHugh JM, McHugh WB. Pain: neuroanatomy, chemical mediators, and clinical implications. AACN Clin Issues. 2000;11:168-178.
24 Weiss N, Lawson H, Greenspan J, et al. Studies of the human ascending pain pathways. Thalamus Relat Syst. 2005;3:71-86.
25 Bishop B. Pain: its physiology and rationale for management. Part II. Analgesic systems of the CNS. Phys Ther. 1980;60:21-23.
26 Xiang JP, Liu XL, Xu YB, et al. Microsurgical anatomy of dorsal root entry zone of brachial plexus. Microsurgery. 2008;28:17-20.
27 Besson JM. The neurobiology of pain. Lancet. 1999;353:1610-1615.
28 Bishop B. Pain: its physiology and rationale for management. Part III. Consequences of current concepts of pain mechanisms related to pain management. Phys Ther. 1980;60:24-37.
29 Woolf C. The dorsal horn: state-dependent sensory processing and the generation of pain. In: Wall PD, Melzack R, editors. Textbook of Pain. New York: Churchill Livingstone; 1994:101-112.
30 Kanpolat Y, Tuna H, Bozkurt M, et al. Spinal and nucleus caudalis dorsal root entry zone operations for chronic pain. Neurosurgery. 2008;62:235-242.
31 Sindou M, Mertens P, Wael M. Microsurgical DREZotomy for pain due to spinal cord and/or cauda equina injuries: long-term results in a series of 44 patients. Pain. 2001;92:159-171.
32 Sindou M, Mifsud JJ, Rosati C, et al. Microsurgical selective posterior rhizotomy in the dorsal root entry zone for treatment of limb spasticity. Acta Neurochir Suppl (Wien). 1987;39:99-102.
33 Denkers MR, Biagi HL, Ann O’Brien M, et al. Dorsal root entry zone lesioning used to treat central neuropathic pain in patients with traumatic spinal cord injury: a systematic review. Spine. 2002;27:E177-184.
34 Nashold BSJr, Ostdahl RH. Dorsal root entry zone lesions for pain relief. J Neurosurg. 1979;51:59-69.
35 Nashold BSJr, Ostdahl RH. Pain relief after dorsal root entry zone lesions. Acta Neurochir Suppl (Wien). 1980;30:383-389.
36 Thomas DG. Brachial plexus injury: deafferentation pain and dorsal root entry zone (DREZ) coagulation. Clin Neurol Neurosurg. 1993;95(suppl):S48-S49.
37 Sindou MP, Blondet E, Emery E, et al. Microsurgical lesioning in the dorsal root entry zone for pain due to brachial plexus avulsion: a prospective series of 55 patients. J Neurosurg. 2005;102:1018-1028.
38 Friedman AH, Bullitt E. Dorsal root entry zone lesions in the treatment of pain following brachial plexus avulsion, spinal cord injury and herpes zoster. Appl Neurophysiol. 1988;51:164-169.
39 Friedman AH, Nashold BSJr. DREZ lesions for relief of pain related to spinal cord injury. J Neurosurg. 1986;65:465-469.
40 Chen HJ, Lu K, Yeh MC. Combined dorsal root entry zone lesions and neural reconstruction for early rehabilitation of brachial plexus avulsion injury. Acta Neurochir Suppl. 2003;87:95-97.
41 Chen HJ, Tu YK. Long term follow-up results of dorsal root entry zone lesions for intractable pain after brachial plexus avulsion injuries. Acta Neurochir Suppl. 2006;99:73-75.
42 Samii M, Bear-Henney S, Ludemann W, et al. Treatment of refractory pain after brachial plexus avulsion with dorsal root entry zone lesions. Neurosurgery. 2001;48:1269-1275.
43 Finnerup NB, Johannesen IL, Sindrup SH, et al. Pain and dysesthesia in patients with spinal cord injury: a postal survey. Spinal Cord. 2001;39:256-262.
44 Norrbrink Budh C, Lund I, Hultling C, et al. Gender related differences in pain in spinal cord injured individuals. Spinal Cord. 2003;41:122-128.
45 Woolsey RM. Chronic pain following spinal cord injury. J Am Paraplegia Soc. 1986;9:39-41.
46 Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord. 2001;39:63-73.
47 Siddall PJ, McClelland JM, Rutkowski SB, et al. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249-257.
48 Martin J, Davis L. Studies upon spinal cord injuries: 1. The development of automatic micturition. Ann Surg. 1947;126:472-477.
49 Nashold BSJr, Vieira J, el-Naggar AO. Pain and spinal cysts in paraplegia: treatment by drainage and DREZ operation. Br J Neurosurg. 1990;4:327-335.
50 Nashold BSJr, Bullitt E. Dorsal root entry zone lesions to control central pain in paraplegics. J Neurosurg. 1981;55:414-419.
51 Sindou MP, Mertens P. Surgery in the dorsal root entry zone for pain. Semin Neurosurg. 2004;15:221-232.
52 Werhagen L, Budh CN, Hultling C, et al. Neuropathic pain after traumatic spinal cord injury—relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord. 2004;42:665-673.
53 Sampson JH, Cashman RE, Nashold BSJr, et al. Dorsal root entry zone lesions for intractable pain after trauma to the conus medullaris and cauda equina. J Neurosurg. 1995;82:28-34.
54 Richter HP, Seitz K. Dorsal root entry zone lesions for the control of deafferentation pain: experiences in ten patients. Neurosurgery. 1984;15:956-959.
55 Spaic M, Markovic N, Tadic R. Microsurgical DREZotomy for pain of spinal cord and cauda equina injury origin: clinical characteristics of pain and implications for surgery in a series of 26 patients. Acta Neurochir (Wien). 2002;144:453-462.
56 Saris SC, Iacono RP, Nashold BSJr. Dorsal root entry zone lesions for post-amputation pain. J Neurosurg. 1985;62:72-76.
57 Malin JP, Winkelmuller W. Phantom phenomena (phantom arm) following cervical root avulsion. Effect of dorsal root entry zone thermocoagulation. Eur Arch Psychiatry Neurol Sci. 1985;235:53-56.
58 Sindou M, Goutelle A. Surgical posterior rhizotomies for the treatment of pain. In: Krayenbühl H, editor. Advances and Technical Standards in Neurosurgery, Vol 10. Wein: Springer-Verlag; 1983:147-183.
59 Friedman AH, Nashold BSJr. Dorsal root entry zone lesions for the treatment of postherpetic neuralgia. Neurosurgery. 1984;15:969-970.
60 Dimitrijevic MR, Nathan PW. Studies of spasticity in man. I. Some features of spasticity. Brain. 1967;90:1-30.
61 Dimitrijevic MR, Nathan PW. Studies of spasticity in man. 2. Analysis of stretch reflexes in spasticity. Brain. 1967;90:333-358.
62 Dimitrijevic MR, Nathan PW. Studies of spasticity in man. 3. Analysis of reflex activity evoked by noxious cutaneous stimulation. Brain. 1968;91:349-368.
63 Eccles JC, Eccles RM, Magni F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol. 1961;159:147-166.
64 Sindou M. Microsurgical DREZotomy (MDT) for pain, spasticity, and hyperactive bladder: a 20-year experience. Acta Neurochir (Wien). 1995;137:1-5.
65 Jeanmonod D, Sindou M. Somatosensory function following dorsal root entry zone lesions in patients with neurogenic pain or spasticity. J Neurosurg. 1991;74:916-932.
66 Sindou M, Mifsud JJ, Boisson D, et al. Selective posterior rhizotomy in the dorsal root entry zone for treatment of hyperspasticity and pain in the hemiplegic upper limb. Neurosurgery. 1986;18:587-595.
67 Sindou MP, Mertens P. Neurosurgery for spasticity. Stereotact Funct Neurosurg. 2000;74:217-221.
68 Sindou M, Jeanmonod D. Microsurgical DREZ-otomy for the treatment of spasticity and pain in the lower limbs. Neurosurgery. 1989;24:655-670.
69 Kahilogullari G, Ugur HC, Savas A, et al. Management of a hemidystonic patient with thalamotomy, campotomy and cervical dorsal root entry zone operation. Stereotact Funct Neurosurg. 2005;83:180-183.
70 Teixeira MJ, Fonoff ET, Montenegro MC. Dorsal root entry zone lesions for treatment of pain related to radiation-induced plexopathy. Spine. 2007;32:E316-319.
71 Bruxelle J, Travers V, Thiebaut JB. Occurrence and treatment of pain after brachial plexus injury. Clin Orthop Relat Res. 1988;237:87-95.
72 Parry CB. Pain in avulsion lesions of the brachial plexus. Pain. 1980;9:41-53.
73 Berman JS, Birch R, Anand P. Pain following human brachial plexus injury with spinal cord root avulsion and the effect of surgery. Pain. 1998;75:199-207.
74 Bertelli JA, Ghizoni MF. Pain after avulsion injuries and complete palsy of the brachial plexus: the possible role of nonavulsed roots in pain generation. Neurosurgery. 2008;62:1104-1113.
75 Tomas R, Haninec P. Dorsal root entry zone (DREZ) localization using direct spinal cord stimulation can improve results of the DREZ thermocoagulation procedure for intractable pain relief. Pain. 2005;116:159-163.
76 Rawlings CE3rd, el-Naggar AO, Nashold BSJr. The DREZ procedure: an update on technique. Br J Neurosurg. 1989;3:633-642.
77 Young RF. Laser versus radiofrequency lesions of the DREZ. J Neurosurg. 1986;64:341.
78 Edgar RE, Best LG, Quail PA, et al. Computer-assisted DREZ microcoagulation: posttraumatic spinal deafferentation pain. J Spinal Disord. 1993;6:48-56.
79 Young RF. Clinical experience with radiofrequency and laser DREZ lesions. J Neurosurg. 1990;72:715-720.
80 Rath SA, Braun V, Soliman N, et al. Results of DREZ coagulations for pain related to plexus lesions, spinal cord injuries and postherpetic neuralgia. Acta Neurochir (Wien). 1996;138:364-369.