Chapter 31 Dopaminergic Therapy of Restless Legs Syndrome
Since Akpinar’s original 1982 report1 on the dramatic effects of levodopa on restless legs syndrome (RLS) symptoms, there have been numerous, independent studies that have validated these findings and that have substantially verified the effectiveness of all dopaminergic agents in treating RLS. However, despite current research supporting the notion that dopaminergic agents represent the most effective agents for treating RLS, the dopaminergic agents do have their limitations and have not turned out to be the long-term panacea that RLS patients had hoped for. However, at the current time there is no other class of agents that can be said to be as consistently and broadly effective (>90% efficacy) as the dopaminergic agents. In this chapter, the relevant pharmacology of the dopaminergic drugs, the research support for treatment efficacy, management issues, and side effect profiles are discussed.
Pertinent Pharmacology of Dopaminergic Agents
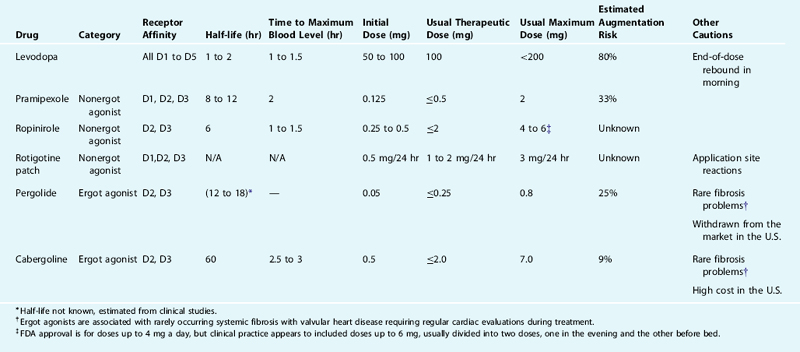
For more details about the biochemical basis of dopaminergic neuropharmacology, which is relevant to understanding the actions of the drugs to be discussed below, see Chapter 11. The effects of the various dopaminergic medications reflect both their kinetics of uptake and elimination and their relative effects on the dopaminergic system. These are presented in Table 31-1 for each of the dopaminergic medications currently used to treat RLS. Levodopa is taken up by the dopamine producing cells and decarboxylated into dopamine adding to the dopamine produced in the cells available for release. Because the feedback mechanisms regulating dopamine production and release do not respond to levodopa, the drug has little immediate effect until after it is converted into dopamine, which potentially activates all five of the dopamine receptors. The dopamine agonists act on the dopamine receptor and differ in terms of relative affinity for each of the five dopamine receptors. Because the medications currently used to treat RLS all activate D2 receptors, they also activate the presynaptic autoreceptors serving to decrease dopamine production and release. This presynaptic activation may predominate at lower doses or initially after taking the medication. All the dopamine agonists currently used for the treatment of RLS have D2 and D3 activation, but only pergolide has significant D1 activation.
Dopaminergic Medications for the Treatment of Restless Legs Syndrome
Levodopa in Restless Legs Syndrome
Levodopa was first reported as a treatment for RLS in 1982.1 Since then, 10 double-blind crossover controlled trials of levodopa with a dopa decarboxylase inhibitor have been reported, two studying the effect of the medication on RLS alone,2,113 four on PLMS alone,4–7 and four on both.8–11 One study included only uremic patients,7 one study included only narcoleptics,4 two studies included patients with either uremic or idiopathic RLS,8,12 and the remaining six studied patients with idiopathic RLS. Of these, five trials have had a sample size of eight or fewer,4,5,7,9,113 two had a sample size of 10 to 20,2,6 and three had a sample size of 30 to 32 patients.8,10,12 Despite the low power of most of the studies, all showed benefit for the drug over placebo in at least some parameters. RLS symptoms were assessed subjectively in different ways, but all studies showed a statistically significant benefit of levodopa over placebo. In the largest published study,8 physician ratings of RLS severity at sleep onset improved 77% over baseline with 100 to 200 mg levodopa at bedtime compared with 36% improvement with placebo, while ratings of RLS during the night improved 57% compared with 11% with placebo. Periodic limb movement indices (PLMI) also improved significantly with the drug (47% improvement over baseline compared with a 7% worsening with placebo).8 However, this effect was restricted to the first half of the night when immediate-release levodopa was administered before sleep. A significant beneficial effect of levodopa was demonstrated on the first night of use, thus allowing for the drug to be used intermittently. One study examined the effects of administering a combination of immediate release and slow release levodopa before sleep to patients with persistent symptoms in the second half of the night.10 The quality of sleep, intensity of RLS, and PLMI significantly improved in the second half of the night with the combination compared with immediate-release levodopa alone. The effects on objective sleep measures have been more varied, with contradictory effects reported on sleep latency and percentage slow wave sleep.5,6,11 Frequent episodic K-alpha complexes have been noted to commonly occur with PLM and these persist unchanged despite reduction in PLMI with levodopa.7
Long-term effects of levodopa therapy have been studied in a number of clinical series comprising 7 to 51 patients followed for 4 weeks to more than 2 years.3,13–20 The percentage of patients who discontinued medication varied between 13% and 65%,3,14,19,21 depending on the duration of follow-up and the availability of alternative effective agents at the time of the study. The major problem encountered was daytime augmentation (Table 31-2). This occurred in 82% of 30 patients with RLS treated with a mean of 267 mg levodopa followed for at least 2 months.13 Of 33 levodopa failures in a study of 51 patients, 85% were caused by augmentation.13 In a 1-year extension of a controlled trial of immediate- and slow-release levodopa, 8 of 23 patients discontinued the drugs because of augmentation and RLS severity during the day increased in an unspecified number of the remainder.19 Factors increasing the risk of augmentation include a dose of 200 mg or more levodopa and the onset of RLS symptoms before 6 P.M. before medication.13 A minority of patients with PLMS alone treated with levodopa will develop RLS earlier in the day.13 If augmentation is treated with additional doses of levodopa earlier in the day, further augmentation often occurs, sometimes with catastrophic increases in symptoms.13 Except for mild cases, levodopa should be discontinued if augmentation develops and a dopamine agonist or other class of medication introduced.
| A. Basic features (all of which need to be met) |
Reprinted with permission from Garcia-Borreguero D, Allen RP, Kohnen R, et al. Diagnostic standards for dopaminergic augmentation of restless legs syndrome: Report from a World Association of Sleep Medicine—International Restless Legs Syndrome Study Group Consensus Conference at the Max Planck Institute. Sleep Med 2007;8:520-530.
An additional adverse effect of levodopa is that of morning rebound. This phenomenon, characterized by an increase in symptoms in the morning, may be related to the short half-life of levodopa. It occurs in 19% to 35% of patients13–15 and may be more common if an additional dose of levodopa is administered during the night.13 The short duration of action of direct release levodopa and tendency to rebound effects were confirmed in a polysomnograph study: PLMS were significantly reduced in the first third of the night, but increased in the last third compared with baseline.16 The frequency of tolerance (the need for an increase in the dose with time to treat the same symptoms) is hard to determine because of the varying natural history of the disorder and the complicating effects of augmentation and rebound. Side effects from the drug were not severe in the long-term studies, with nausea, lightheadedness, and diarrhea occasionally limiting therapy.3,13,18,19
In summary, levodopa is effective in controlling RLS, but its use is severely limited by the development of daytime augmentation and morning rebound. Current guidelines suggest that its use should be limited to RLS that does not occur sufficiently frequently to require daily medication.22
Dopaminergic Agonists in Restless Legs Syndrome
Ergot Agonists
Bromocriptine
Bromocriptine was the first dopamine agonist to be studied in RLS patients. A small double-blind randomized cross over study of 6 RLS patients taking a mean of 7.5 mg bromocriptine before sleep for 30 days demonstrated improvement in symptoms, decreased PLMS, increased total sleep time and sleep efficiency. Nasal stuffiness and lightheadedness occurred in one patient.23 Seven patients treated successfully with bromocriptine were included in a clinical series of 49 patients.14 No further studies have been reported and the drug is rarely used for RLS today.
Pergolide
Four randomized, controlled trials of pergolide in the treatment of RLS have been reported, three for idiopathic RLS24–26 and one for RLS in uremia.27 Sample sizes ranged from 8 to 83, and treatment duration, from 10 days to 6 weeks. The larger trials showed an improvement in RLS symptomatology of 87% over baseline compared with 13% with placebo (based on a 10-point self-rating scale at bedtime)26 and 52% compared with 7% (using the International Restless Legs Scale).25 In one study, the PLM arousal index fell from baseline by 83% compared with 20% with placebo,25 whereas another demonstrated a 93% reduction in PLM arousal index compared with placebo, sustained for 7 hours of sleep.26 Significant changes in total sleep time and sleep efficiency were noted in some studies24,26 but not in others.25,27 The average daily dose used ranged from 0.25 to 0.4 mg.
Information is available from open-label, follow-up studies of 10 to 31 patients using pergolide for a mean of 6 to 25 months.13,28–33 The mean daily dose used ranged from 0.1 to 0.67 mg with the higher doses reported in studies in which domperidone was added to control nausea.31–33 The percentage of patients continuing to use the drug throughout the study varied between 55% and 93%, with all but two studies32,33 reporting a range of 70% to 80%. Augmentation did occur but at a lower frequency than with levodopa. Augmentation occurred in 15% to 38% of patients in four different studies, each lasting a mean of 12 or more months.13,28,30,31 In addition, augmentation was generally mild and, unlike with levodopa, could usually be controlled with an additional dose of the drug earlier in the day.28 Like levodopa, pergolide has been reported to induce RLS when used to treat PLMS alone.34 Nausea occurred frequently (35% to 41%)28,25,26,31 but could easily be controlled by the addition of domperidone (in countries where it is available). Other side effects included orthostatic hypotension, nasal stuffiness, insomnia, constipation, and peripheral edema.
An additional potential complication of pergolide therapy is the development of ergot-related pleuropulmonary, cardiac valvular, pericardial, or retroperitoneal fibrosis.35,36 Although most frequently found in patients taking pergolide for Parkinson’s disease,115 similar complications have been reported in patients treated with higher doses of pergolide (1.5 and 3.5 mg daily) for RLS.37,38 A case control study of 46 patients on pergolide for Parkinson’s disease suggested a 2- to 3-fold increased risk of abnormal valves in the pergolide group and a 14-fold increased risk of tricuspid regurgitation.39 Other studies have shown relative risk ratios of valvulopathy in association with pergolide therapy for Parkinson’s disease of 2.3 and 7.1.40,41 Cumulative dose appears to be a risk factor for the development of valvulopathy. Pergolide was withdrawn from the U.S market in 2007; in countries where it is still available, it should be rarely prescribed and regular echocardiograms should be performed.
Cabergoline
Cabergoline is an ergot-based dopamine agonist with an extremely long elimination half-life of 65 to 110 hours.42 It has been investigated for the treatment of RLS in three open-label trials42–44 and in two multicenter double-blind placebo-controlled trials.11,45 Two of the open-label trials studied small numbers of patients (9 and 12),43,44 while the third reported on 302 patients from multiple sites.42 The duration of follow-up ranged between 3 and 12 months. All three open-label studies commenced therapy with 0.5 mg cabergoline in the evening. Mean effective doses ranged from 1.3 to 2.1 mg. The two studies using the International Restless Legs Syndrome Study Group Severity Scale (IRLS) showed a reduction of 64 and 75% in RLS severity compared with baseline.42,44 In one study using follow-up polysomnography, PLMI fell by 87%.43 In the largest study, 18% of patients discontinued the drug in the first 6 months, the majority due to side effects.42 Side effects thought to be drug related occurred in 48% of patients, the majority in the first month of therapy. Nausea was the commonest (17%), with dizziness, fatigue, and edema also noted. Possible daytime augmentation within 6 months was reported in 3%, but another much smaller study reported no augmentation at 1 year.44
In the first controlled study, 85 patients were randomly assigned to one of four treatment conditions: placebo and 0.5, 1.0, and 2.0 mg of cabergoline taken once daily. Dose was titrated up over the first 2 weeks of the study and then held constant until week 5 at which point efficacy was evaluated using the validated IRLS.46 Patients were then entered into a longer-term (48 weeks) open-label trial with 6 weeks of dose adjustment to efficacy and then maintained for 42 weeks. In the double-blind phase, the average IRLS score decreases from baseline were 3.3 for placebo and 13.1, 13.5, and 15.7 for doses of 0.5, 1.0, and 2.0 mg (approximately 43% to 55% decreases). Both the 1- and the 2-mg doses produced statistically significant improvement compared with placebo (P <.05) but not the lowest dose (P >.05). The number of adverse effects were low and only nausea occurred appreciably more often than for placebo. Augmentation occurred in about 9% of those continuing in the long-term open-label study.45
In the second controlled trial conducted in 51 European centers,11 Cabergoline at fixed doses of 2 or 3 mg/day was compared with levodopa at doses of 200 or 300 mg/day over a 30-week period. The primary outcome measure was the IRLS, which declined by 16.1 points in the cabergoline group (n = 178) and by 9.5 points in the levodopa group (n = 183). More patients in the levodopa than the cabergoline group dropped out due to loss of efficacy (14.2% versus 7.9%) or augmentation (9.8% versus 4.0%), the second primary end point. More side effects occurred in the cabergoline group, such as gastrointestinal symptoms (55.6% versus 30.0%), and the authors concluded that cabergoline was not as well tolerated.
In a third controlled trial that focused on polysomnography,47 the PLMS-associated arousal index decreased and the sleep efficiency increased compared with placebo. Subjective measures, including IRLS, QoL-RLS, and RLS-6 day and night measures, were all improved as well.
Fibrosing reactions were not evaluated in these studies; cabergoline, nonetheless, like pergolide, has been associated in high doses with occasional ergot-related systemic fibrosis.48,49 Studies of cabergoline in Parkinson’s disease have shown relative risk ratios of 1.7-12.96 for the occurrence of cardiac valvulopathies.40,41,50 The dose range associated with valvulopathy was 2 to 7 mg in one study with total cumulative dose being a risk factor.50 If cabergoline is used for RLS, regular echocardiograms are essential.
Alpha-dihydroergocryptine
A single 4 week open-label trial of alpha-dihydroergocryptine (DHEC) in 16 patients has been reported,51 with results similar to those seen with other ergot-based agonists. The dose was titrated from 5 mg daily to a mean of 23 mg daily. Using a visual analogue scale, RLS severity at night fell by 64% from baseline. Side effects were noted in 87% of patients, predominantly nausea. No augmentation was noted over the short duration of the study.
Lisuride
This drug, which is not available in the United States, is approved in Europe to treat Parkinson’s disease, hyperprolactinemia, and migraine headaches (off-label). Lisuride is an ergot-related dopamine agonist (the second available dopamine agonist after bromocriptine) that has been available in Europe for 20 years in pill form and, more recently, as a transdermal patch. It has a short half-life of about 2 hours and is metabolized in the liver. Due to its short half-life and variable bioavailability, this drug is better suited to treat RLS by the transdermal route as opposed to its oral form. Despite it being an ergot-related dopamine drug, there is little evidence that it causes fibrosis, perhaps because it antagonizes the 5-HT2B receptor. Two small studies have been published. Oral lisuride was studied at a dose of 0.3 mg daily either as monotherapy or add-on therapy with L-dopa with improvement of PLM and CGI.52 Lisuride patches, one (3 mg lisuride) or two (6 mg listuride) applied every other day, were studied in another trial with improvement on open label and double blind segments of the trial after lisuride run-in.52,109 A large-scale multicenter study of lisuride and placebo reported only in abstract revealed increasing benefit with patches releasing 0.1 to 0.4 mg/day that benefited both nighttime and daytime measures of the RLS-6 scales.53
Terguride
A single 2- to 8-week open-label study of nine patients receiving terguride (transdihydrolisuride) for RLS has been reported.54 The effective dose range was 0.25 to 0.5 mg daily. One patient discontinued the drug due to abdominal pain and one developed augmentation. The IRLS fell by a mean of 42% from baseline.
Nonergot Agonists
Pramipexole
Extensive randomized, double-blind placebo controlled trials of pramipexole in RLS have been undertaken and these have been reviewed by the U.S. Food and Drug Administration, which in 2006 approved the use of pramipexole for treatment of moderate to severe RLS. Two large (N = 344 or 345) double-blinded, placebo-controlled trials evaluated efficacy and safety of pramipexole treatment of primary RLS with a randomization giving twice as many subjects on pramipexole as placebo. Both studies used the clinical global improvement and the change from baseline in IRLS as primary end points. One study conducted in Europe110 with individually titrated doses ranging from 0.125 to 0.75 (median pramipexole dose of 0.35 mg/day) reported response after 6 weeks of treatment. The mean IRLS score decreases were 5.7 for placebo and 12.3 for pramipexole. The percentage much/very much improved was 32.5% on placebo and 62.9% on pramipexole. In the second study conducted in the United States,114 patients were randomized to placebo or one of three fixed pramipexole doses of 0.25, 0.5, and 0.75 mg/day. Responses evaluated after 12 weeks of treatment showed decreases on the IRLS of 9.3 for placebo versus 12.8 for 0.25 mg, 13.8 for 0.5 mg, and 14.1 for 0.75 mg doses. The percentage much/very much improved on the CGI was 51.2% for placebo vs. 74.7% for 0.25, 67.9% for 0.5 mg and 72.9% for 0.75 mg.33 This study showed significant improvement in the IRLS for the 0.5 compared with the 0.25 mg dose but there was no significant added treatment benefit for the highest dose of 0.75 mg. In another study, 150 RLS patients who had responded to pramipexole were randomly switched to either placebo or continuing on their pramipexole and followed for another 3 months. The percentage of patients with predefined worsening of RLS scale and also a decrease in Clinical Global Improvement was 85.5% for placebo and 20.5% for pramipexole treatment.56 A third study was a dose-finding parallel-group polysomnography study in which a total of 109 patients were randomly administered one of the following: placebo, 0.125 mg, 0.25 mg, 0.5 mg, or 0.75 mg for 3 weeks. The PLMI was reduced in all four drug groups by about 80% compared with placebo. The IRLS was lower for all drug doses compared with placebo, with the higher doses showing more than 50% reduction.57 In these three larger trials, pramipexole was well tolerated with the usual adverse effects for a dopamine agonists (mostly nausea).
At least 10 open-label studies of pramipexole treatment of RLS have been reported.58–66108 The four largest studies included 52 to 195 patients each with mean follow-ups of 21 to 39 months.61,62,65,66 In three studies, between 37% and 76% of these patients had received prior levodopa, whereas in the largest,66 no patient had received prior dopaminergic therapy. The mean dose after initial stabilization for most patients was in the range of 0.3 to 0.6 mg daily. Efficacy was assessed using various quantitative measures. In one study, 94% of patients reported a partial or complete response,62 while another demonstrated a 75% improvement on a physician’s global impression scale.65 Side effects, although common, were generally mild and self limited and only occasionally led to discontinuation of the drug. Nausea, insomnia and lightheadedness were the most common, although edema and nasal congestion were occasionally described. Peripheral edema, reversible on discontinuation of the drug, has been reported to occur in about 17% of patients taking pramipexole, predominantly in high doses for Parkinson’s disease.67 Sleepiness is uncommon in RLS patients treated with pramipexole62,63 and no “sleep attacks” have been reported. This is in contrast to descriptions of sudden sleep without warning occurring while driving, described in some patients taking high doses of the drug for Parkinson’s disease.68–70
The risk of augmentation with pramipexole has been assessed in a number of long-term case series studies. In three large studies lasting a mean of 21 to 31 months, augmentation rates of 22% to 33% were noted.62,65,66 In two studies, the mean duration of treatment before the start of augmentation was 9 and 14 months. In contrast to the augmentation seen with levodopa, but similar to that seen with pergolide, adjustment of the timing of the dose or an additional dose given earlier in the day resulted in satisfactory alleviation of the symptoms in most patients. At most, one patient in each study needed to discontinue the drug as a result of augmentation. Tolerance, defined as the need for an increase in dose due to the reappearance of RLS symptoms after the time medication was administered, occurred in 46% of patients in a study lasting a mean of 21 months (dose increased from a mean of 0.43 mg to 0.89 mg daily).65 In another study, the mean daily dose after an average of 27 months had increased from 0.38 to 0.63 mg, with 39% of patients not having an increased dose.62 About 38% of patients in longer-term studies were placed on twice or three times daily doses.62,65 No cases of early morning rebound have been reported. In the largest series, 19% to 54% of patients were given an additional medication for RLS during the course of the study, such as a benzodiazepine, an opioid, or gabapentin.61,62,65
Ropinirole
Extensive randomized, double-blind placebo-controlled trials of ropinirole in RLS have been undertaken, and these have been reviewed by the U.S. Food and Drug Administration, which in 2005 approved the use of ropinirole for treatment of moderate to severe RLS. The four major trials reported include three with parallel groups71–73 and one with a crossover design,74 together studying 638 subjects. The three parallel-group trials followed similar methodology. Each trial lasted 12 weeks with the dose of ropinirole being titrated upward in an unforced paradigm from 0.25 mg to a maximum of 4 mg administered in a single dose 1 to 3 hours before bed. The primary end point was the IRLS in two studies71–73 and the PLMI on polysomnography in the third.71 The mean dose used in the 3 studies was remarkably similar (1.8 or 1.9 mg). The IRLS scores were significantly lower with ropinirole compared with placebo, but the scale proved unexpectedly sensitive to placebo effect. Thus, the degree of difference between the placebo and treatment groups was only 3.072 and 2.573 points of a possible 40. In the third study, ropinirole resulted in a significant 70% decrease in PLMS while asleep and 61% decrease in PLMW while awake compared with placebo.71 However, the IRLS score, measured as a secondary outcome, did not significantly differ between the drug and placebo groups, probably due to the strong placebo effect and the fact that the study was not powered to detect treatment differences with this measure. The only crossover study74 used a forced titration protocol to 6 mg daily, allowing decreases only for drug intolerance. Thus, the mean daily dose was higher than in the other studies (4.6 mg). The IRLS was significantly lower with ropinirole compared with placebo (12 point or 47% difference). Side effects in the four studies more common with ropinirole than placebo were headache,71,73 nausea,71–74 and dizziness.74 Augmentation was not reported.
It deserves note that in these large studies quality of life and sleep were both evaluated and both found to improve.72,73 Thus, this treatment not only reduced symptoms but also reduced the morbidity associated with RLS. Sleepiness during the day was reported to decrease on treatment72 in contrast to sleepiness problems that have been reported for use of the dopamine agonists in Parkinson’s disease.68–70
Several other small single-blind, or open-label, controlled trials have been reported with similar results.75–77 One study compared slow-release levodopa therapy (mean dose, 190 mg) with ropinirole (mean dose, 1.45 mg) in 11 RLS patients on hemodialysis and found ropinirole statistically superior using the IRLS.75 No adverse effects were reported after 6 weeks of therapy. Another study showed no difference between ropinirole and gabapentin used in idiopathic RLS but significant improvements from baseline in both groups.78 A few small open label studies with 5 to 16 subjects79–82 followed for a mean of 1 to 12 months have reported continuing efficacy, using a variety of outcome measures. Augmentation has not been systematically assessed with ropinirole alone.
Rotigotine
This fairly short half-life dopamine agonist is administered via a transdermal patch delivering the medication at a nearly constant rate as long as the patch is worn and replaced every 24 hours. The dopamine transdermal delivery has been evaluated for treatment of RLS in a multiple-fixed-dose, double-blind, placebo-controlled short-term study with 7 days of treatment involving wearing the patch 24 hours a day.83a Sixty-three patients were evaluated in nine German centers with three fixed doses of the medication (1.125 mg, 2.25 mg, and 4.5 mg daily) and placebo. The relative improvements compared with placebo on the IRLS were 1.3, 3.6, and 6.3 for each of the doses, respectively. The adverse effects were considered minimal and skin tolerability of the patch differed little between placebo and active medication patches. The constant use of this medication produces a steady state of medication availability avoiding the more fluctuating levels that occur with most oral medications.
Rotigotine has been further investigated in a number of large, multicenter trials, some of which have been reported in abstract. In one published dose-finding study,55 five doses between 0.5 mg and 4 mg (estimated 24-hour drug delivery) were tested. All doses except for the 0.5 mg dose showed significant benefit compared with placebo on the primary end point (IRLS). There was a dose-response effect in which the 3 mg dose showed the greatest benefit (mean decrease of −17.5 points on the IRLS compared with placebo change of −9.2).
Piribidel
The use of piribidel in a single case of RLS was reported as early as 1987.2 However, only one additional open-label study of 13 patients is available.83 Eleven of the patients responded with a mean 75% improvement in RLS symptoms on a 10-point scale. The mean dose was 120 mg at bedtime and the beneficial effect persisted for a mean of 10 months. Domperidone was administered with each dose to prevent nausea.
Other Dopaminergic Agents in Restless Legs Syndrome
Amantadine
Amantadine (100 to 300 mg per day) has been evaluated for treatment of RLS in one small (21 patients) uncontrolled, open-label study.85 Patients had doses titrated for maximum treatment benefit without significant adverse effects. They were rated for degree of response from 0% (no improvement) to 100% (complete improvement) at the end of dose titration. Eleven of 21 (52%) reported some positive response and 9 (42%) remained on this treatment for several months, but most patients discontinued the treatment apparently for lack of efficacy. Adverse effects of drowsiness, fatigue, and insomnia were each reported by two or three patients. Thus, amantadine appears to have limited therapeutic benefit for RLS.
Selegiline
One polysomnographic, open-label study reported that 31 patients with periodic leg movements in sleep (PLMS) showed significantly decreased PLMS on selegiline without any alerting effects of this medication disturbing sleep.86 No study on selegiline treatment of RLS has been published at this time and its efficacy or safety for RLS treatment is unknown.
Practical Treatment Considerations in the Use of Dopaminergic Agents
The Medical Advisory Board of the International RLS Study Group published in 2004 a consensus algorithm for the management of RLS, classifying the disorder into three categories.22 Intermittent RLS is defined as RLS troublesome enough to require treatment but not occurring frequently enough to necessitate daily therapy. Daily RLS is defined as RLS frequent and troublesome enough to require daily therapy. Refractory RLS is defined as daily RLS treated with a dopamine agonist with inadequate initial response despite adequate doses, response that has become inadequate with time despite increasing doses, intolerable side effects, or augmentation not controllable with additional earlier doses of the drug.
The mainstays of treatment of daily RLS are the nonergot dopamine agonists. Although gabapentin and low-potency opioids are alternative agents favored by some authorities, an agonist should always be tried if the other drugs fail or are poorly tolerated. Pramipexole and ropinirole are equally acceptable agents. As discussed, ropinirole and pramipexole have both been studied with multiple large controlled trials, but more long-term follow-up information is available for pramipexole. The two drugs have not been compared head-to-head in a single study, but clinical experience suggests that both are effective. Dopamine agonists should be prescribed initially in a low dose, once daily 2 hours before major RLS symptoms commence, with small increases as needed every 2 to 3 days. Considerably lower doses are used compared with the treatment of Parkinson’s disease (see Table 31-1). It should be recalled that the therapeutic dose of ropinirole is usually about 4 times that of pramipexole. Augmentation is often initially treatable with adjustment of the timing of the drug or an additional dose earlier in the day. However, in many patients augmentation will increase further with time, eventually requiring a change to a different agent. Staying within the recommended dose levels given in Table 31-1 serves to reduce the risk of severe augmentation. Thus, these medications should be considered to have a therapeutic dose range not to be exceeded in general treatments.
One of the possible approaches to refractory RLS is to change to another dopamine agonist. There is often variability in the response of individual patients to different agents with respect to efficacy, side effects, tolerance, and rate of augmentation. If pramipexole was initially used, then ropinirole should be tried next, and vice versa. Very occasionally, it may be reasonable to use pergolide in countries in which it is still available, with the patient understanding the considerable risk of side effects, especially fibrosing reactions. If domperidone is available, this should be initially prescribed with pergolide to prevent nausea. Cabergoline and possibly the rotigotine patch may also be tried, especially if agents with a shorter half-life have resulted in rapid development of augmentation. In the United States, the drug cabergoline is approved by the Food and Drug Administration only for the treatment of hyperprolactinemia in a twice-weekly dose and should only be used for RLS with very careful consideration of the significant risk of inducing valvulopathy, especially at higher doses and longer durations of treatment.
Three useful evidence-based reviews and accompanying practice parameters regarding the management of RLS have been published by the American Academy of Sleep Medicine87–90 and the European Federation of Neurological Societies.91 However, papers published later than 2004 are not included. It should also be noted that only the dopamine agonists ropinirole and pramipexole have to date been approved by the U.S. Food and Drug Administration and the European authorities for the treatment of RLS. Levodopa has also been approved in Germany, Switzerland, and Austria. Thus, all other drugs are used “off label” as of mid-2007. All uses of the approved drugs, except for once daily use to treat moderate to severe primary RLS, are also off label as of November 2007, although this may change in the near future.
Common Adverse Effects
Customary adverse effects of dopaminergic treatment include nausea, headache, dizziness, stuffy nose, and peripheral edema. Nausea is the most common side effect, occurring in as many as 40% of the subjects tested in the treatment trails. It is usually short-lived, only infrequently causing discontinuation of medication. Headaches, dizziness, lightheadedness, and stuffy nose were much less common and also rarely problems leading to ending treatment. Peripheral edema is known to occur with these medications, although it was not generally reported in these mostly short-term studies where patients were carefully screened to minimize medical problems. Unlike the other customary adverse effects, peripheral edema may emerge sometimes months or years after starting treatment and may then require medication adjustment. One longer-term evaluation of pramipexole use in a clinical setting reported peripheral edema occurred in 17% of the patients, predominantly those on higher doses.67 The delayed onset of this adverse effect may reflect the development of some other factor, such as decreased activity, interacting with the medication to produce the peripheral edema.
Augmentation
The paradoxical worsening or augmentation of RLS symptoms caused by dopaminergic treatment remains the biggest significant reason for discontinuing or decreasing the dose of these medications and attempting other types of treatment. To date, with the exception of tramadol, which has catecholaminergic activity,92,93 there have been no drugs in the other classes used to treat RLS implicated in causing augmentation. Augmentation manifests with earlier onset of RLS in the evening or afternoon than was the case before starting treatment. In addition, other indications of RLS augmentation include shorter period of rest required to provoke the RLS symptoms, more intense expression of symptoms when they occur, decreased duration of benefit from the medication and RLS symptoms spreading to other body parts (e.g., arms).13,94 Clinical criteria for defining augmentation (see Table 31-2) were established at a 2006 workshop of the International RLS Study Group workshop and require either (1) a paradoxical response to medication (greater benefit with smaller dose) or (2) either (a) a 4-hour advance in the usual time of onset of RLS symptoms or (b) a 2-hour advance and at least one of four other features including: decreased duration of rest provoking RLS symptoms, decreased duration of medication benefit, spreading of symptoms to other body parts, or greater severity of symptoms including PLM commencing during waking or becoming more severe95 (Table 31-2). But at the heart of the clinical presentation is a sense by the patient or the physician that the disease is progressing: there is a worsening of symptoms. This may be seen following months or years of sustained improvements in RLS at the same dose of medication.
Augmentation has been reported for levodopa and all dopamine agonists evaluated in reasonably large longer duration studies at rates ranging from 9% to 80%. A study of 83 patients treated with a variety of dopamine agonists (including 52 with pramipexole) found a high overall augmentation rate of 48% over a mean follow-up of 39 months.61 Family history of RLS and an absence of peripheral neuropathy predicted the development of augmentation. In contrast, a study of 60 patients using pramipexole for at least 6 months found an augmentation rate of only 8%.58 The discrepancy from the other studies is most probably due to the shorter duration of follow-up, as augmentation continues to develop for at least 2 years after starting the drug.62 In a 30-week double-blind study of cabergoline (2 to 3 mg/day) compared with L-Dopa (200 to 300 mg/day), 4% of the cabergoline and 10% of the L-Dopa group dropped out due to augmentation.11 But there is also difficulty operationally defining augmentation and differentiating tolerable augmentation from severe augmentation that requires discontinuation of the provocative medication; this difficulty may result in variability in reporting the incidence of augmentation from study to study.
As shown in Table 31-1, the rates of augmentation differ between the drugs. Augmentation appears to occur more commonly for medications with shorter half-lives and may be related to the on-off nature of stimulation of the dopamine system provided by daily use of shorter half-life dopamine agonists. It may also be that the longer half-life medications actually mask the development of augmentation with the only indication of its presence being the need to continue to increase the dose. In the major double-blind placebo-controlled cabergoline study about 75% of the patients continued to increase their medications during a 47-week open-label extension to doses above those in the initial 5-week treatment period, but the initial doses in that study were fixed and not titrated to efficacy. The dose increases, nonetheless, occurred even for those in the highest fixed dose.64 It is unclear whether or not there may have been some augmentation contributing to the need to increase the doses during longer-term treatment.
Augmentation is considered a problem that generally develops within the first couple of years of treatment. Silber and colleagues62 provided a survival curve for occurrence of augmentation during treatment of 49 patients followed over 4 to 46 months. In that study augmentation continued to develop even after 30 months of treatment (Fig. 31-1). The delayed onset of the augmentation may reflect the nature of the changes in the RLS biology adjusting to continued dopaminergic stimulation and accordingly it may be that the more severe the augmentation, the more likely it will be observed earlier in treatment. Certainly the dose of the medication appears to have some relation to augmentation.13
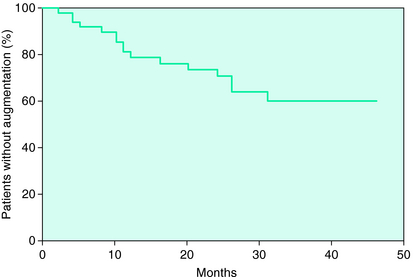
FIGURE 31-1. Augmentation occurrence during longer-term treatment on a dopamine agonist (pramipexole).62
The high prevalence of augmentation for treatment with levodopa may reflect the short half-life of the medication, but it also may relate to the marked differences in its pharmacology: it directly increases dopamine in the neurons. One study found that prior augmentation with levodopa predicted the occurrence of augmentation with pramipexole,65 whereas another study did not.62 Even though occurrence of augmentation with levodopa may increase the risk of augmentation with a dopamine agonist, this is only a relative increase and does not occur in most situations. Thus, at the first signs of augmentation on levodopa, a switch of medications to the dopamine agonists provides a reasonable management to avoid morbid augmentation. A better policy given the high risk of augmentation on levodopa is to avoid using it except intermittently (less than twice a week) and only at very low doses (e.g., 100 mg).
Assessment of severity of RLS becomes an important consideration for management of the problem. Mild augmentation with dopamine agonists may be managed with a single addition of a dose earlier in the day. If this can be done once, without exceeding the dosage guidelines as given in Table 31-1 and without having to make further dose adjustments, then it may provide a reasonable approach to augmentation. Morbid augmentation, however, can only be treated by withdrawal of the dopaminergic medication and after a difficult 4 to 7 days off medication starting a different class of medication to treat the residual RLS symptoms. It is unclear if the use of alternate medications such as opioids are particularly helpful during the dopamine withdrawal period but they are sometimes used. It is important to avoid oversedating the patient during the initial withdrawal period, because no matter what they are given, they will still have to get up and move around with risk of falling.
Given the importance of determining the severity of augmentation, a scale for assessing it has been developed and validated in an augmentation study using 6 months of treatment on levodopa plus benserazide. This augmentation severity rating scale (ASRS) has reasonable psychometric properties (test-retest reliability r = 0.72, inter-rater agreement r = 0.86) and is fairly easy to administer and score.96 It is designed to provide a standard for evaluation of the degree of augmentation that can be used in multiple studies and hopefully in future clinical trials. The ASRS, like the IRLS, is not designed to diagnose augmentation, but to be applied to those who may suffer from augmentation to assess its severity. To formalize diagnosis, a structured diagnostic interview, the Augmentation Diagnostic Interview (ADI), is under development. In a preliminary validation study, the ADI (then termed the “Structured Interview to Diagnose Augmentation”) was administered by two independent trained raters to each patient in whom the treating physician suspected possible augmentation. The outcome of the interview was compared with the diagnosis on augmentation of an independent expert with extensive experiences with augmentation.97 Subjects were RLS patients on current dopaminergic treatment from two centers in Austria and Spain (n = 24). Sensitivity was 92% and specificity was 91%, the interrater reliability was high (κ = 0.92).
Newly Identified Adverse Effects
These include significant daytime sleepiness with sudden-onset of sleepiness and compulsive behaviors such as gambling. Neither of these was reported in the clinical treatment trials but they have been reported to rarely occur with clinical dopamine treatment of RLS.
Sleepiness and Sleep Attacks
The problem of sleepiness with dopaminergic treatment in Parkinson’s disease has been extensively studied with indications both that the problem results from Parkinson’s disease itself and that it also occurs or is exacerbated with dopaminergic treatment. The reports of spells of sudden-onset of sleepiness while on higher doses of dopaminergic agonists68–70 raised some concerns about driving safety, but higher doses of dopaminergic agents are given for more significant Parkinson’s disease and thus this may not be primarily a drug effect. Because virtually all Parkinson’s disease patients are treated with dopaminergic medications it becomes difficult to separate a medication effect from the symptoms occurring with the disorder itself. This is not the case for RLS where the disorder itself is not associated with profound sleepiness and the patients can often be treated with non-dopaminergic medications. Bassetti and coworkers98 described one case of an RLS patient reporting spells of sudden onset of sleepiness and an abnormal multiple sleep latency test (average sleep latency of 5 min 20 sec, no sleep onset REM sleep) occurring shortly after his pergolide dose had been decreased with symptoms resolving on a change to pramipexole treatment. This appears to be more a problem of withdrawal than continued treatment. There has been one retrospective case series of 85 RLS patients randomly selected from those being treated with dopamine agonists in one large treatment center who were evaluated for subjective reports of excessive sleepiness and sleep attacks.99 These patients were treated with one of several possible dopamine agonists: pergolide, pramipexole, or ropinirole. Seven patients (8%) reported excessive daytime sleepiness disrupting their normal functioning but none reported sleep attacks. In another retrospective review of 60 patients treated with pramipexole, three (5%) reported excessive daytime sleepiness, but the Epworth Scores on two of these were only 4 and 11, with 10 or less usually considered as normal and 11 as only mild sleepiness.62 None of these patients reported sleep attacks nor did they discontinue the medication owing to the sleepiness problems. Two clinical treatment trials evaluating subjective sleepiness through 12 weeks of treatment with ropinirole found decreased daytime somnolence compared with baseline and placebo.72,73 The measure used in these clinical trials, however, did not clearly distinguish feelings of fatigue and tiredness from sleepiness.
It is somewhat puzzling that there are not more reports of sleepiness with dopaminergic treatment. RLS is associated with profound sleep loss72,73 without profound excessive daytime sleepiness100 and thus the disease includes some mechanism compensating for the expected sleepiness effects of profound chronic sleep loss. Treatment of the disease would be expected to alter this compensatory mechanism but this may only occur after a period of continued treatment. Moreover, the improved sleep at night and the initial pleasure in being able to relax or take a nap in the daytime may initially mask any development of sleepiness with continued treatment. Unfortunately the studies to date tend to cover too short a treatment period and tend to confound fatigue and tiredness with sleepiness. In one questionnaire study, it was found that untreated RLS patients were more sleepy than controls, and treatment tended to reduce the risk of sudden onset of sleep in the treated patients.101
Compulsive Behaviors and Gambling
Dopamine agonist treatment of Parkinson’s disease patients has been linked to compulsive eating,102 excessive gambling,103 compulsive shopping, and pathologic hypersexuality,104 with resolution of these problems associated with reduction of the dose of dopamine agonists. In one study, the lifetime prevalence of pathologic gambling in Parkinson’s disease patients was 3.4% and the current prevalence 1.7%.104 Seven percent of patients on pramipexole and 12% of patients on ropinirole were affected. In a similar study, the lifetime prevalence of pathologic hypersexuality in Parkinson’s disease was 2.4% and the current prevalence of 2.0%.105 The current prevalence of compulsive shopping was 0.7%. Thus, the total lifetime prevalence for any impulse control disorder developing in Parkinson’s disease patients on dopamine agonists was as high as 13.7%.
It has been conjectured that this problem results mostly from activation of D3 receptors and that genetic and/or environmental factors modulate sensitivity to this effect. Presumably some individuals prone to these behaviors may experience significant increase or release of the behavior even when treated at the relatively lower doses used for treating RLS. Moreover, the dopamine agonists most commonly used to treat RLS have reasonably high affinity for D3 receptor. Three RLS patients with impulse control disorders developing after initiation of dopamine agonists have been reported at doses of 0.75 mg pramipexole.106 A similar case has been reported in a subsequent case report.107 Studies of the frequency of this complication are needed. In the meantime, RLS patients prescribed dopamine agonists should be warned about the possibility of the impulse control disorders, including pathologic gambling, obsessive shopping, and other excessive activities. Increased libido and resultant increased sexual activity may also be mentioned.
1. Akpinar S. Treatment of restless legs syndrome with levodopa plus benserazide [letter]. Arch Neurol. 1982;39:739.
2. Akpinar S. Restless legs syndrome treatment with dopaminergic drugs. Clin Neuropharmacol. 1987;10:69-79.
3. von Scheele C, Kempi V. Long-term effect of dopaminergic drugs in restless legs. Arch Neurol. 1990;47:1223-1224.
4. Walker SL, Fine A, Kryger MH. L-Dopa/carbidopa for nocturnal movement disorders in uremia. Sleep. 1996;19:214-218.
5. Boivin DB, Montplaisir J, Poirier G. The effects of L-Dopa on periodic leg movements and sleep organization in narcolepsy. Clin Neuropharmacol. 1989;12:339-345.
6. Kaplan PW, Allen RP, Buchholz DW, et al. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717-723.
7. Montplaisir J, Boucher S, Gosselin A, et al. Persistence of repetitive EEG arousals (K-alpha complexes) in RLS patients treated with L-DOPA. Sleep. 1996;19:196-199.
8. Benes H, Kurella B, Kummer J, et al. Rapid onset of action of levodopa in restless legs syndrome: A double-blind, randomized, multicenter, crossover trial. Sleep. 1999;22:1073-1081.
9. Brodeur C, Montplaisir J, Godbout R, et al. Treatment of restless legs syndrome and periodic movements during sleep with L-dopa: A double-blind, controlled study. Neurology. 1998;38:1845-1848.
10. Collado-Seidel V, Kazenwadel J, Wetter TC, et al. A controlled study of additional SR-L-dopa in L-dopa-responsive restless legs syndrome with late-night symptoms. Neurology. 1999;52:285-290.
11. Trenkwalder C, Benes H, Grote L, et al. Cabergoline compared to levodopa in the treatment of patients with severe restless legs syndrome: Results from a multi-center, randomized, active controlled trial. Mov Disord. 2007;22:696-703.
12. Trenkwalder C, Stiasny K, Pollmacher T, et al. L-Dopa therapy of uremic and idiopathic restless legs syndrome: A double-blind, crossover trial. Sleep. 1995;18:681-688.
13. Allen RP, Earley CJ. Augmentation of the restless legs syndrome with carbidopa/levodopa. Sleep. 1996;19:205-213.
14. Becker PM, Jamieson AO, Brown WD. Dopaminergic agents in restless legs syndrome and periodic limb movements of sleep: Response and complications of extended treatment in 49 cases. Sleep. 1993;16:713-716.
15. Guilleminault C, Cetel M, Philip P. Dopaminergic treatment of restless legs and rebound phenomenom. Neurology. 1993;43:445.
16. Montplaisir J, Godbout R, Poirier G, et al. Restless legs syndrome and periodic movements in sleep: Physiopathology and treatment with L-dopa. Clin Neuropharmacol. 1986;9:456-463.
17. Saletu M, Anderer P, Hogl B, et al. Acute double-blind, placebo-controlled sleep laboratory and clinical follow-up studies with a combination treatment of RR-L-dopa and SR-L-dopa in restless legs syndrome. J Neural Transm. 2003;110:611-626.
18. Sandyk R, Bernick C, Lee SM, et al. L-Dopa in uremic patients with the restless legs syndrome. Int J Neurosci. 1987;35:233-235.
19. Trenkwalder C, Collado Seidel V, Kazenwadel J, et al. One-year treatment with standard and sustained-release levodopa: Appropriate long-term treatment of restless legs syndrome? Mov Disord. 2003;18:1184-1189.
20. Walters AS, Mandelbaum DE, Lewin DS, et al. Dopaminergic therapy in children with restless legs/periodic limb movements in sleep and ADHD. Dopaminergic Therapy Study Group. Pediatr Neurol. 2000;22:182-186.
21. Earley CJ, Allen RP. Pergolide and carbidopa/levodopa treatment of the restless legs syndrome and periodic leg movements in sleep in a consecutive series of patients. Sleep. 1996;19:801-810.
22. Silber MH, Ehrenberg BL, Allen RP, et al. An algorithm for the management of restless legs syndrome. Mayo Clin Proc. 2004;79:916-922.
23. Walters AS, Hening WA, Kavey N, et al. A double-blind randomized crossover trial of bromocriptine and placebo in restless legs syndrome. Ann Neurol. 1988;24:455-458.
24. Earley CJ, Yaffee JB, Allen RP. Randomized, double-blind, placebo-controlled trial of pergolide in restless legs syndrome. Neurology. 1998;51:1599-1602.
25. Trenkwalder C, Hundemer HP, Lledo A, et al. Efficacy of pergolide in treatment of restless legs syndrome: The PEARLS Study. Neurology. 2004;62:1391-1397.
26. Wetter TC, Stiasny K, Winkelmann J, et al. A randomized controlled study of pergolide in patients with restless legs syndrome. Neurology. 1999;52:944-950.
27. Pieta J, Millar T, Zacharias J, et al. Effect of pergolide on restless legs and leg movements in sleep in uremic patients. Sleep. 1998;21:617-622.
28. Silber MH, Shepard JW Jr, Wisbey JA. Pergolide in the management of restless legs syndrome: an extended study. Sleep. 1997;20:878-882.
29. Noel S, Korri H, Vanderheyden JE. Low dosage of pergolide in the treatment of restless legs syndrome. Acta Neurol Belg. 1998;98:52-53.
30. Staedt J, Hunerjager H, Ruther E, et al. Pergolide: Treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). Long-term follow-up on pergolide. J Neural Transm. 1998;105(2-3):265-268.
31. Stiasny K, Wetter TC, Winkelmann J, et al. Long-term effects of pergolide in the treatment of restless legs syndrome. Neurology. 2001;56:1399-1402.
32. Trenkwalder C, Wetter TC, Stiasny K, et al. [Restless legs syndrome and periodic limb movements in sleep]. Nervenarzt. 2001;72:425-436.
33. Winkelmann J, Wetter TC, Stiasny K, et al. Treatment of restless leg syndrome with pergolide—An open clinical trial. Mov Disord. 1998;13:566-569.
34. Santamaria J, Iranzo A, Tolosa E. Development of restless legs syndrome after dopaminergic treatment in a patient with periodic leg movements in sleep. Sleep Med. 2003;4:153-155.
35. Agarwal P, Fahn S, Frucht SJ. Diagnosis and management of pergolide-induced fibrosis. Mov Disord. 2004;19:699-704.
36. Shaunak S, Wilkins A, Pilling JB, et al. Pericardial, retroperitoneal, and pleural fibrosis induced by pergolide. J Neurol Neurosurg Psychiatry. 1999;66:79-81.
37. Danoff SK, Grasso ME, Terry PB, et al. Pleuropulmonary disease due to pergolide use for restless legs syndrome. Chest. 2001;120:313-316.
38. Pritchett AM, Morrison JF, Edwards WD, et al. Valvular heart disease in patients taking pergolide. Mayo Clin Proc. 2002;77:1280-1286.
39. Baseman DG, O’suilleabhain PE, Reimold SC, et al. Pergolide use in Parkinson disease is associated with cardiac valve regurgitation. Neurology. 2004;63:301-304.
40. Junghanns S, Fuhrmann JT, Simonis G, et al. Valvular heart disease in Parkinson’s disease patients treated with dopamine agonists: A reader-blinded monocenter echocardiography study. Mov Disord. 2006;2:234-238.
41. Schade R, Andersohn F, Suissa S, et al. Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med, 356:29-38.
42. Benes H, Heinrich CR, Ueberall MA, et al. Long-term safety and efficacy of cabergoline for the treatment of idiopathic restless legs syndrome: Results from an open-label 6-month clinical trial. Sleep. 2004;27:674-682.
43. Stiasny K, Robbecke J, Schuler P, et al. Treatment of idiopathic restless legs syndrome (RLS) with the D2-agonist cabergoline—An open clinical trial. Sleep. 2000;23:349-354.
44. Zucconi M, Oldani A, Castronovo C, et al. Cabergoline is an effective single-drug treatment for restless legs syndrome: Clinical and actigraphic evaluation. Sleep. 2003;26:815-818.
45. Stiasny-Kolster K, Benes H, Peglau I, et al. Effective cabergoline treatment in idiopathic restless legs syndrome. Neurology. 2004;63:2272-2279.
46. The International Restless Legs Syndrome Study Group. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121-132.
47. Oertel WH, Benes H, Bodenschatz R, et al. Efficacy of cabergoline in restless legs syndrome: A placebo-controlled study with polysomnography (CATOR). Neurology. 2006;67:1040-1046.
48. Horvath J, Fross RD, Kleiner-Fisman G, et al. Severe multivalvular heart disease: A new complication of the ergot derivative dopamine agonists. Mov Disord. 2004;19:656-662.
49. Ling SC, Wilkinson JD, Hollman AS, et al. The evolution of liver disease in cystic fibrosis. Arch Dis Child. 1999;81:129-132.
50. Yamamoto M, Uesugi T, Nakayama T. Dopamine agonists and cardiac valvulopathy in Parkinson disease: a case-control study. Neurology. 2007;67:1225-1229.
51. Tergau F, Wischer S, Wolf C, et al. Treatment of restless legs syndrome with the dopamine agonist alpha-dihydroergocryptine. Mov Disord. 2001;16:731-735.
52. Benes H, Deissler A, Rodenbeck A, et al. Lisuride treatment of restless legs syndrome: First studies with monotherapy in de novo patients and in combination with levodopa in advanced disease. J Neural Transm. 2006;113:87-92.
53. Benes H, Högl B, Palla F, Kohnen R. Transdermal lisuride provides early and sustained improvement in quality of sleep and daytime tiredness in RLS patients [abstract]. Sleep Med. 2007;8:S58.
54. Sonka K, Pretl M, Kranda K. Management of restless legs syndrome by the partial D2-agonist terguride. Sleep Med. 2003;4:455-457.
55. Oertel WH, Benes H, Garcia-Borreguero D, et al. Efficacy of rotigotine transdermal system in severe restless legs syndrome: A randomized, double-blind, placebo-controlled, six-week dose-finding trial in Europe. Sleep Med. 2008;9:228-239.
56. Trenkwalder C, Stiasny-Kolster K, Kupsch A, et al. Controlled withdrawal of pramipexole after 6 months of open-label treatment in patients with restless legs syndrome. Mov Disord. 2006;21:1404-1410.
57. Partinen M, Hirvonen K, Jama L, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: A polysomnographic dose-finding study—The PRELUDE study. Sleep Med. 2006;7:407-417.
58. Ferini-Strambi L. Restless legs syndrome augmentation and pramipexole treatment. Sleep Med. 2002;3(suppl):S23-S25.
59. Lin SC, Kaplan J, Burger CD, et al. Effect of pramipexole in treatment of resistant restless legs syndrome. Mayo Clin Proc. 1998;73:497-500.
60. Montplaisir J, Denesle R, Petit D. Pramipexole in the treatment of restless legs syndrome: A follow-up study. Eur J Neurol. 2000;7(supp. 1):27-31.
61. Ondo W, Romanyshyn J, Vuong KD, et al. Long-term treatment of restless legs syndrome with dopamine agonists. Arch Neurol. 2004;61:1393-1397.
62. Silber MH, Girish M, Izurieta R. Pramipexole in the management of restless legs syndrome: An extended study. Sleep. 2003;26:819-821.
63. Stiasny K, Moller JC, Oertel WH. Safety of pramipexole in patients with restless legs syndrome. Neurology. 2000;55:1589-1590.
64. Stiasny-Kolster K, Oertel WH. Low-dose pramipexole in the management of restless legs syndrome. An open label trial. Neuropsychobiology. 2004;50:65-70.
65. Winkelman JW, Johnston L. Augmentation and tolerance with long-term pramipexole treatment of restless legs syndrome (RLS). Sleep Med. 2004;5:9-14.
66. Montplaisir J, Fantini ML, Desautels A, et al. Long-term treatment with pramipexole in restless legs syndrome. Eur J Neurol. 2006;13:1306-1311.
67. Tan EK, Ondo W. Clinical characteristics of pramipexole-induced peripheral edema. Arch Neurol. 2000;57:729-732.
68. Frucht S, Rogers JD, Greene PE, et al. Falling asleep at the wheel: Motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology. 1999;52:1908-1910.
69. Paus S, Brecht HM, Koster J, et al. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson’s disease. Mov Disord. 2003;18:659-667.
70. Arnulf I. Excessive daytime sleepiness in parkinsonism. Sleep Med Rev. 2005;9:185-200.
71. Allen R, Becker PM, Bogan R, et al. Ropinirole decreases periodic leg movements and improves sleep parameters in patients with restless legs syndrome. Sleep. 2004;27:907-914.
72. Trenkwalder C, Garcia-Borreguero D, Montagna P, et al. Ropinirole in the treatment of restless legs syndrome: Results from the TREAT RLS 1 study, a 12 week, randomised, placebo controlled study in 10 European countries. J Neurol Neurosurg Psychiatry. 2004;75:92-97.
73. Walters AS, Ondo WG, Dreykluft T, et al. Ropinirole is effective in the treatment of restless legs syndrome. TREAT RLS 2: A 12-week, double-blind, randomized, parallel-group, placebo-controlled study. Mov Disord. 2004;19:1414-1423.
74. Adler CH, et al. Ropinirole for restless legs syndrome: A placebo-controlled crossover trial. Neurology. 2004;62:1405-1407.
75. Pellecchia MT, Vitale C, Sabatini M, et al. Ropinirole as a treatment of restless legs syndrome in patients on chronic hemodialysis: An open randomized crossover trial versus levodopa sustained release. Clin Neuropharmacol. 2004;27:178-181.
76. Saletu B, Gruber G, Saletu M, et al. Sleep laboratory studies in restless legs syndrome patients as compared with normals and acute effects of ropinirole. 1. Findings on objective and subjective sleep and awakening quality. Neuropsychobiology. 2000;41:181-189.
77. Saletu M, Anderer P, Saletu B, et al. Sleep laboratory studies in restless legs syndrome patients as compared with normals and acute effects of ropinirole. 2. Findings on periodic leg movements, arousals and respiratory variables. Neuropsychobiology. 2000;41:190-199.
78. Happe S, Sauter C, Klosch G, et al. Gabapentin versus ropinirole in the treatment of idiopathic restless legs syndrome. Neuropsychobiology. 2003;48:82-86.
79. Estivill E, de la Fuente V. The efficacy of ropinirole in the treatment of chronic insomnia secondary to restless legs syndrome: Polysomnography data. Rev Neurol. 1999;29:805-807.
80. Estivill E, de la Fuente V. Uso de ropinirol como tratamientodel síndrome de piernas inquieta. Rev Neurol. 1999;28:962-963.
81. Galvez-Jimenez N, Khan T. Ropinirole and restless legs syndrome. Mov Disord. 1999;14:890-892.
82. Ondo W. Ropinirole for restless legs syndrome. Mov Disord. 1999;14:138-140.
83. Evidente VG. Piribedil for restless legs syndrome: A pilot study. Mov Disord. 2001;16:579-581.
83a Stiasny-Kolster K, Kohnen R, Schollmayer E, et al. Patch application of the dopamine agonist rotigotine to patients with moderate to advanced stages of restless legs syndrome: A double-blind, placebo-controlled pilot study. Mov Disord. 2004;19:1432-1438.
84. Inoue Y, Mitani H, Nanba K, et al. Treatment of periodic leg movement disorder and restless leg syndrome with talipexole. Psychiatry Clin Neurosci. 1999;53:283-285.
85. Evidente VG, Adler CH, Caviness JN, et al. Amantadine is beneficial in restless legs syndrome. Mov Disord. 2000;15:324-327.
86. Grewal M, Hawa R, Shapiro C. Treatment of periodic limb movements in sleep with selegiline HCl. Mov Disord. 2002;17:398-401.
87. Chesson ALJr, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep. 1999;22:961-968.
88. Hening W, Allen R, Earley C, et al. The treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Review. Sleep. 1999;22:970-999.
89. Hening WA, Allen RP, Earley CJ, et al. An update on the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004;27:560-583.
90. Littner MR, Kushida C, Anderson WM, et al. Practice parameters for the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004;27:557-559.
91. Vignatelli L, Billiard M, Clarenbach P, et al. EFNS guidelines on management of restless legs syndrome and periodic limb movement disorder in sleep. Eur J Neurol. 2006;13:1049-1065.
92. Earley CJ, Allen RP. Restless legs syndrome augmentation associated with tramadol. Sleep Med. 2006;7:592-593.
93. Vetrugno R, La Morgia C.D, Angelo R, et al. Augmentation of restless legs syndrome with long-term tramadol treatment. Mov Disord. 2007;22:424-427.
94. Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: Diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101-119.
95. Garcia-Borreguero D, Allen RP, Kohnen R, et al. Diagnostic standards for dopaminergic augmentation of restless legs syndrome: Report from a World Association of Sleep Medicine—International Restless Legs Syndrome Study Group Consensus Conference at the Max Planck Institute. Sleep Med. 2007;8:520-530.
96. Garcia-Borreguero D, Kohnen R, Hogl B, et al. Validation of the Augmentation Severity Rating Scale (ASRS): A multicentric, prospective study with levodopa on restless legs syndrome. Sleep Med. 2007;8:455-463.
97. Högl B, Garcia Borreguero D, Gschliesser V, et al. On the development of the “Structured Interview for Diagnosis of Augmentation during RLS treatment” (RLS-SIDA): First experiences [abstract]. Sleep. 6(supp. 2), 2005.
98. Bassetti C, Clavadetscher S, Gugger M, et al. Pergolide-associated ‘sleep attacks’ in a patient with restless legs syndrome. Sleep Med. 2002;3:275-277.
99. Gelevert A, de Groen J. Restless legs syndrome: Excessive daytime sleepiness caused by dopamine-agonists. Sleep. 2003;26:A329-A330.
100. Saletu B, Anderer P, Saletu M, et al. EEG mapping, psychometric, and polysomnographic studies in restless legs syndrome (RLS) and periodic limb movement disorder (PLMD) patients as compared with normal controls. Sleep Med. 2002;3(suppl):S35-S42.
101. Moller JC, Korner Y, Cassel W, et al. Sudden onset of sleep and dopaminergic therapy in patients with restless legs syndrome. Sleep Med. 2006;7:333-339.
102. Nirenberg MJ, Waters C. Compulsive eating and weight gain related to dopamine agonist use. Mov Disord. 2006;21:524-529.
103. Dodd ML, Klos KJ, Bower JH, et al. Pathological gambling caused by drugs used to treat Parkinson disease. Arch Neurol. 2005;62:1377-1381.
104. Voon V, Hassan K, Zurowski M. Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology. 2006;66:1750-1752.
105. Voon V, Hassan K, Zurowski M, et al. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67:1254-1257.
106. Tippmann-Peikert M, Silber MH, Park JP, et al. Pathologic gambling in patients with restless legs syndrome treated with dopaminergic agonists. Neurology. 2007;68:301-303.
107. Quickfall J, Suchowersky O. Pathological gambling associated with dopamine agonist use in restless legs syndrome. Parkinsonism Relat Disord 2007;13:535-536.
108. Becker PM, Ondo W, Sharon D. Encouraging initial response of restless legs syndrome to pramipexole. Neurology. 1998;51:1221-1223.
109. Benes H. Transdermal lisuride: short-term efficacy and tolerability study in patients with severe restless legs syndrome. Sleep Med. 2006;7:31-35.
110. Oertel WH, Stiasny-Kolster K, Bergtholdt B, et al. Efficacy of pramipexole in restless legs syndrome: a six-week, multicenter, randomized, double-blind study (effect-RLS study). Mov Disord. 2007;22:213-219.
111. Silber M.H, Shepard JWJr, Wisbey JA. Pergolide in the management of restless legs syndrome: an extended study. Sleep. 1997;20:878-882.
112. Trenkwalder C, Brandenburg U, Hundemer H, et al. A randomized long-term placebo-controlled multicenter trial of pergolide in the treatment of restless legs syndrome with central evaluation of polysomnographic data. Neurology. 2001;58:A5.
113. von Scheele C. Levodopa in restless legs. Lancet. 1986;2:426-427.
114. Winkelman JW, Sethi KD, Kushida CA, et al. Efficacy and safety of pramipexole in restless legs syndrome. Neurology. 2006;67:1034-1039.
115. Zanettini R, Antonini A, Gatto G, et al. Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med. 2007;365:39-46.