Chapter 76C Distal splenorenal shunt
Background
The distal splenorenal shunt (DSRS) was developed by Warren and colleagues in 1967 to achieve selective variceal decompression to prevent recurrent variceal bleeding. Selective variceal decompression combines the benefit of a decompressive shunt to control bleeding with maintenance of portal hypertension and portal flow to the cirrhotic liver to help maintain liver function. The original, classic article on selective variceal decompression described the animal work leading up to the initial clinical data on DSRS (Warren et al, 1967). Proof of concept was shown in the animal studies and in the results of the first six patients in whom DSRS was performed. The unique prior experiences of Warren and Zeppa led to the evolution of this concept: Warren had seen that total shunts control variceal bleeding, but at the cost of liver failure, whereas Zeppa had seen devascularization procedures maintain portal perfusion, but at a cost of significant risk of rebleeding.
Over the next 4 decades, DSRS became the most widely used operation to control variceal bleeding, with a worldwide following (Orozco et al, 2007). The technique continued to evolve, with greater degrees of portal azygos disconnection improving its effectiveness (Henderson et al, 1989). However, in the 1990s, the evolution of improved endoscopic therapy with banding (see Chapter 75B), introduction of transjugular intrahepatic portosystemic shunting (TIPS; see Chapter 76E), and coming of age of liver transplantation (see Chapter 97A, Chapter 97B, Chapter 97C, Chapter 97D, Chapter 97E ) left few indications for shunt surgery for variceal bleeding (see Chapter 76A).
Indications for DSRS
• Patients with variceal bleeding refractory to endoscopic and pharmacologic therapy who have well-preserved and stable liver function
• Patients with portal hypertension and normal livers, such as those with portal vein thrombosis, who have refractory bleeding and a patent splenic vein
• Patients in some geographic locations, where there is only one chance to control bleeding, and they cannot return for the multiple visits required for management with endoscopy or TIPS
Patient Evaluation
Liver function is assessed from clinical findings and laboratory studies. Jaundice, ascites, and encephalopathy are the three clinical signs and symptoms of advanced liver disease and indicate that patients are not candidates for surgical decompression. Laboratory measurements of serum bilirubin, albumin, serum creatinine, and prothrombin time prolongation are the most useful studies to assess the status of cirrhosis. Combining the clinical and laboratory parameters to discern the Child-Turcotte-Pugh (CTP) class and/or Model for End-Stage Liver Disease (MELD) score gives an objective assessment of risk (Table 76C.1 and Box 76C.1).
Box 76C.1 Model for End-Stage Liver Disease Score for Liver Disease Severity
Score = 0.957 × loge creatinine (mg/dL) + 0.378 × loge bilirubin (mg/dL) +1.120 loge INR
1 Hepatic venous pressure gradient (HVPG)
2 Left renal vein anatomy and drainage (this is abnormal in 20% of the population)
3 Superior mesenteric, portal, and splenic vein patency, flow direction, and venous anatomy on the venous phase of the arterial study
4 Major variceal inflow paths on the same study as in number 3 above
Technique for Distal Splenorenal Shunt
The steps in DSRS are illustrated. The preferred incision is a long left subcostal incision, extended across the right rectus muscle (Fig. 76C.1). Coagulating diathermy should be used extensively in patients with portal hypertension to achieve hemostasis in dividing tissues. If present, ascites should be aspirated and cultured, and a liver biopsy specimen should be obtained to document the status of the liver at the time of the procedure.
Step 1: Exposure
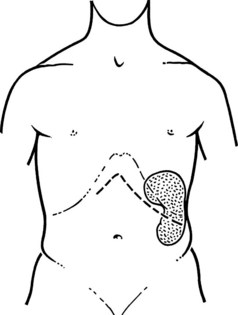
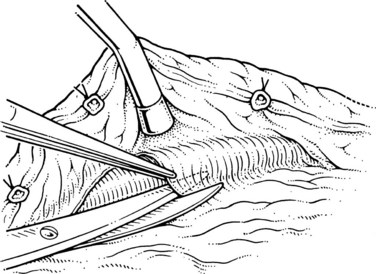
The initial steps of this procedure provide access to the vessels. The pancreas is exposed by opening the lesser sac (Fig. 76C.2) and interrupting the gastroepiploic arcade from the pylorus to the first short gastric vessels. Exposure is greatly enhanced by taking down the splenic flexure of the colon from the lower pole of the spleen. This is done most easily by initially mobilizing the left side of the splenic flexure of the colon, then identifying the plane from there back into the lesser sac at the tail of the pancreas. The splenocolic ligament is divided, giving excellent exposure to the inferior margin of the pancreas from the mesenteric vessels to the splenic hilus. The pancreas is then fully mobilized along its inferior border over its entire length, so that it is turned completely on its side; this is achieved with a combination of Bovie coagulation and ligature of tissues (Fig. 76C.3). The inferior mesenteric vein is the first venous landmark identified: in 50% of patients, this vein enters the splenic vein; in the other 50%, it enters the superior mesenteric vein. The inferior mesenteric vein should be traced up to these vessels and then divided to aid further exposure.
Step 2: Isolation of the Splenic Vein
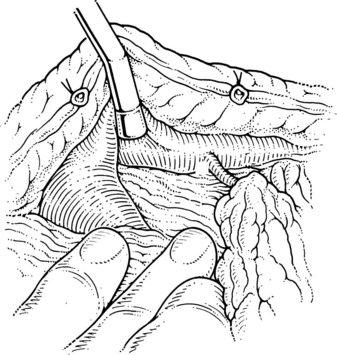
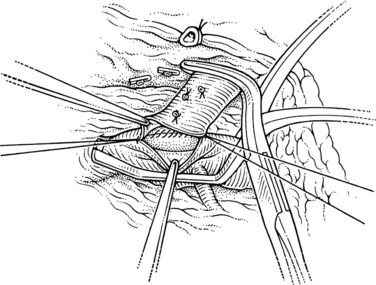
The superior mesenteric and splenic vein junction is identified, initially on its posterior surface; this is a safe plane for initial dissection. The splenic vein should be isolated along its inferior and posterior aspect with dissection occuring right on the vessel (Fig. 76C.4). At this point, the splenic, superior mesenteric, and posterior aspects of the portal vein all should be cleared (Fig. 76C.5).
When the posterior plane is free, attention turns to the anterior and more difficult plane of dissection on the splenic vein. Tributaries rarely enter the anterior surface of the portal vein; so this plane between the neck of the pancreas and the portal vein should be opened first, then the pancreas should be cautiously separated and dissected from the anterior and superior surfaces of the splenic vein. The key is to dissect the pancreas off the splenic vein rather than the other way around. This requires a delicate touch and is best achieved by spreading the tissues gently in the line of the tributaries and at right angles to the splenic vein. This isolates the tributaries, and when identified, a fine right-angle clamp is passed around them, with a 3-0 tie on the vein side and a clip on the pancreatic side (Fig. 76C.6). As much of the splenic vein as possible should be dissected in this manner before dividing the splenic vein at the superior mesenteric vein junction.
Step 4: Division of the Splenic Vein
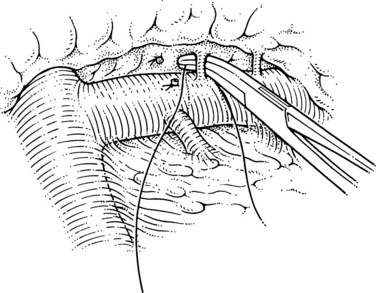
This is the step that lines up the splenic and left renal veins for anastomosis. The superior mesenteric end of the splenic vein is ligated with a 2-0 silk, and a large clip is placed flush with the vein behind that tie; this has led to the lowest incidence of thrombus within the portal vein at postoperative angiography. At this point the surgeon must judge whether enough splenic vein has been dissected free of the pancreas to allow it to come down to the left renal vein without kinking or tension. In the event that more dissection of the splenic vein is needed, this can now be performed more easily, because the vein can be manipulated downward, as shown in Figure 76C.7. The disadvantage of this maneuver is that the pressure in the splenic vein has increased with ligation, which leads to greater risk of tearing of the small tributaries.
In patients with alcoholic cirrhosis, data have shown that complete dissection of the splenic vein from the pancreas (splenopancreatic disconnection) is advantageous in leading to improved long-term maintenance of portal perfusion (Henderson et al, 1989). If this modification of DSRS is used, the complete dissection should be done at this time. Data do not support the need for entire splenopancreatic disconnection in patients with nonalcoholic liver disease.
Step 5: The Anastomosis
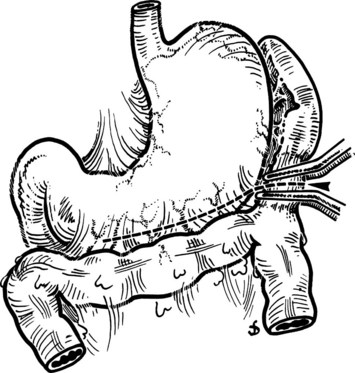
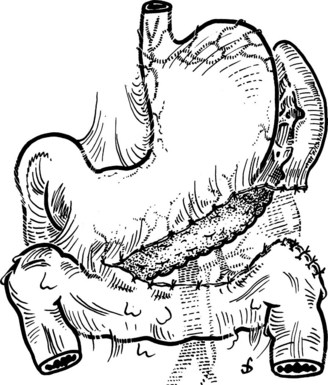
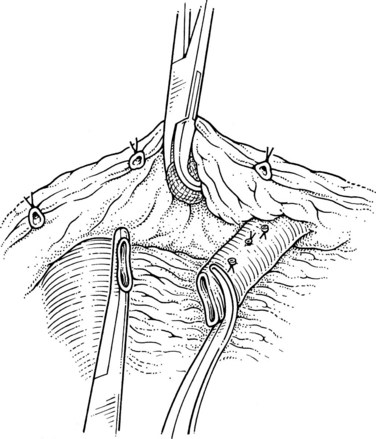
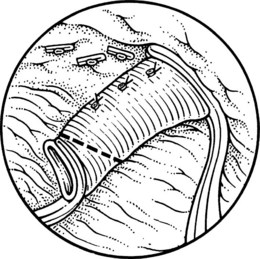
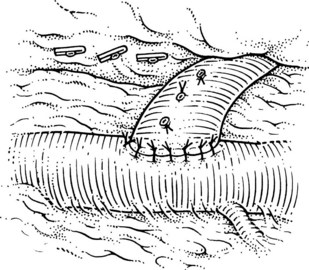
This is performed without tension, and usually the splenic vein needs to be trimmed so that when the clamps are removed, the vein is not redundant and lies without kinking. This alignment can be difficult to judge, particularly if the two veins are overlying each other. The position of the clamps and trimming of the splenic vein are shown in Figures 76C.8 and 76C.9. The left renal vein is opened over sufficient length without removing an ellipse. The posterior row of the anastomosis is completed with a running suture, with stay sutures placed at either end, and the suture is run on the inside; the anterior row is usually interrupted to avoid risk of a purse-string effect. The completed anastomosis is shown in Figure 76C.10.
Step 6: Portal Azygos Disconnection
The final step is interrupting the main paths by which the high-pressure portal vein attempts to connect to the now low-pressure splenic vein. These paths are by 1) transpancreatic collaterals, 2) collaterals along the mesocolon to the inferior ramus of the splenic vein, and 3) the left and right gastric venous systems (Henderson et al, 1985). The pancreatic siphon of large collaterals flowing through the pancreas can be prevented by splenopancreatic disconnection as described earlier. The decision whether to do this is made on a benefit-risk basis, depending on the ease of full dissection. The collaterals that develop in the mesocolon have a final common pathway in the splenocolic ligament to the lower pole of the spleen and do not develop if the splenic flexure is taken down as described earlier. The transgastric collaterals are minimized by ligating the left and right gastric veins. The right gastric vein is usually small, but it should be interrupted just above the pylorus. The left gastric (coronary) vein may be very large, and it should be clipped or divided at its junction with the portal or splenic vein. It also should be isolated immediately above the pancreas and divided at this location.
Results
Bleeding control is equal to or greater than 90% (Henderson et al, 1992; Herman, 1995; Orozco et al, 1997; Rikkers, 1998; Livingstone et al, 2006; Elwood et al, 2006). The highest risk time for rebleeding after DSRS is in the first month (Richards et al, 1987). The technical failure rate should be less than 5%, and it should be defined before hospital discharge with direct shunt catheterization as described earlier. If a technical problem occurs with the anastomosis at that time, surgical reexploration should be undertaken; if it cannot be corrected, splenectomy and devascularization are indicated. Late thrombosis of DSRS is unusual, although patients with portal vein thrombosis undergoing DSRS have a higher rate of shunt stenosis requiring balloon dilation (Warren et al, 1988). Bleeding control is significantly better than that achieved with endoscopic therapy, which is approximately 25% to 30%.
Portal perfusion is maintained at late follow-up in 90% of patients with nonalcoholic liver disease. In patients with alcoholic cirrhosis, loss of portal perfusion occurs in 50% of patients (Henderson et al, 1985). Zeppa and colleagues (1978) documented poorer survival after DSRS in alcoholic patients than in nonalcoholic patients but never proved cause and effect. The mortality rate can be improved in the latter population by complete splenopancreatic disconnection (Henderson et al, 1989). In this modification, the entire splenic vein is dissected free of the pancreas before anastomosis. Splenopancreatic disconnection also has been documented to improve survival in nonalcoholic patients.
Two major questions have arisen over the past 5 years: How often is variceal decompression needed, given improved pharmacologic and endoscopic treatment? (D’Amico et al, 2002) And is a surgical shunt ever needed in the era of TIPS? Current data show refractory bleeding through endoscopic and pharmacologic therapy in 15% to 20% of patients; most of these patients have poor liver function, and their only reasonable decompression option is liver transplantation.
The relative efficacy of surgical decompression versus TIPS has been studied in two randomized trials. One study assessed the 8-mm portacaval shunt versus TIPS, and although the surgical limb had better bleeding control, overall outcome was not significantly different (Rosemurgy et al, 2005). In a randomized trial (Henderson et al, 2006) of DSRS versus TIPS in CTP class A and B patients, the rebleeding rates were not significantly different, 6% and 11%, but 83% of TIPS patients required reintervention and dilation to achieve this outcome. Survival and encephalopathy rates were not significantly different in this study. Cost-effectiveness analysis showed a slight advantage to TIPS in this trial (Boyer et al, 2008), and it can be concluded that TIPS is equally effective as DSRS.
In summary, DSRS is an excellent operation for controlling refractory variceal bleeding in patients with portal hypertension and well-preserved liver function. Its current role is, however, minimal given the evolution of other therapeutic options, as succinctly summarized by Hector Orozco (Orozco et al, 2007): “What a pity. There have been many ideas to resolve a problem, many years of work, many patients treated, and one surgical solution that was approaching the ideal: low morbidity, low mortality, low recurrence of the hemorrhagic episodes, and long survival. Despite all of this, we threw it away because we did not know how, when, and to whom we might give it.”
Boyer TD, et al. Cost of preventing variceal rebleeding with transjugular intrahepatic portal systemic shunt and distal splenorenal shunt. J Hepatol. 2008;48:407-414.
D’Amico G, et al. Meta-analysis of trials for variceal bleeding. Hepatology. 2002;36:1023-1024.
Elwood DR, et al. Distal splenorenal shunt: preferred treatment for recurrent variceal hemorrhage in the patient with well-compensated cirrhosis. Arch Surg. 2006;141:385-388.
Henderson JM, et al. Portaprival collaterals following distal splenorenal shunt: incidence, magnitude and associated portal perfusion changes. J Hepatol. 1985;1:649-661.
Henderson JM, et al. Distal splenorenal shunt with splenopancreatic disconnection: a four-year assessment. Ann Surg. 1989;210:332-341.
Henderson JM, et al. Selective shunt in the management of variceal bleeding in the era of liver transplantation. Ann Surg. 1992;216:248-254.
Henderson JM, et al. Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: a randomized trial. Gastroenterology. 2006;130:1643-1651.
Herman RE, et al. Fifty years of surgery for portal hypertension at the Cleveland Clinic Foundation: lessons and prospects. Ann Surg. 1995;221:459-466.
Livingstone AS, et al. 507 Warren-Zeppa distal splenorenal shunts: a 34-year experience. Ann Surg. 2006;243:884-892.
Orozco H, et al. Selective shunts for portal hypertension: current role of a 21-year experience. Liver Transplant Surg. 1997;3:475-480.
Orozco H, et al. Rise and downfall of the empire of portal hypertension surgery. Arch Surg. 2007;142:219-221.
Richards WO, et al. Evaluation and treatment of early hemorrhage of the alimentary tract after selective shunt procedures. Surg Gynecol Obstet. 1987;164:530-536.
Rikkers LF. The changing spectrum of treatment for variceal bleeding. Ann Surg. 1998;228:536-546.
Rosemurgy AS, et al. H-graft portacaval shunts versus TIPS: ten-year follow-up of a randomized trial with comparison to predicted survivals. Ann Surg. 2005;241:238-246.
Warren WD, et al. Selective transplenic decompression of gastroesophageal varices by distal splenorenal shunt. Ann Surg. 1967;166:437-445.
Warren WD, et al. Management of variceal bleeding in patients with noncirrhotic portal vein thrombosis. Ann Surg. 1988;207:623-634.
Zeppa R, et al. The comparative survival of alcoholics versus non-alcoholics after distal splenorenal shunt. Ann Surg. 1978;187:510-513.