CHAPTER 56 Distal Humeral Fractures–Acute Total Elbow Arthroplasty
INTRODUCTION
Distal humeral fractures are infrequent when compared with other fractures and comprise approximately 1% to 2% of all adult fractures and 10% of humeral fractures.27,29,33 The population distribution of such fractures tends to be bimodal, with a peak in the second and third decades, and with a second peak in the sixth to eighth decades.13
NONSURGICAL MANAGEMENT
Nonoperative options of soft sling or plaster cast are traditionally used in cases in which reconstruction is not an option, by virtue of patient or surgical factors. Although widely propagated as an acceptable methodology, it is documented that such a strategy does not yield consistent pain relief or consistent joint stability, with unsatisfactory results in approximately 40% of cases.17
FRACTURE FIXATION
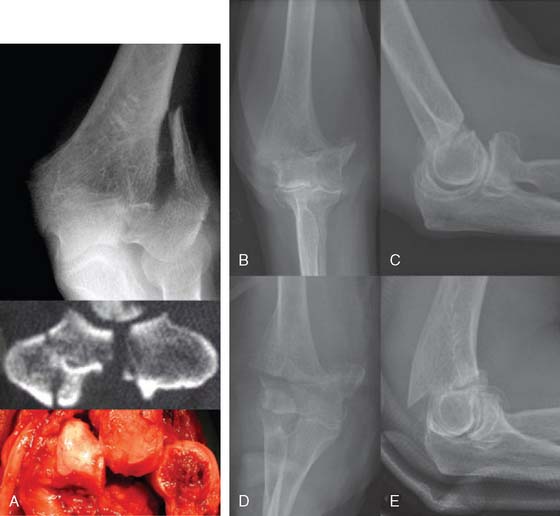
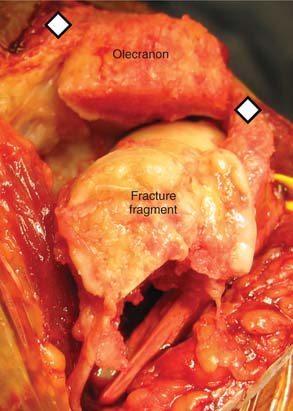
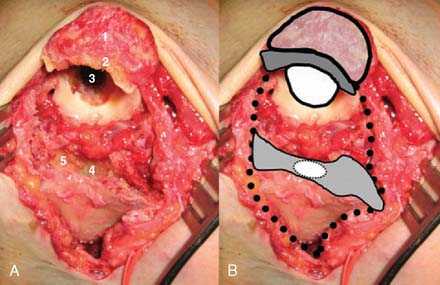
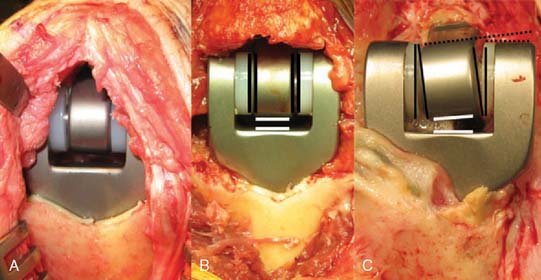
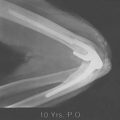
A prerequisite for reconstruction is to fix the fracture fragments with some form of rigid fixation construct that then synchronously allows stable fixation and joint motion. However, there are three main fracture factors that adversely affect the ability to reconstruct the distal humeral fracture: (1) Comminution: If the distal humeral articular surface is significantly comminuted (Fig. 56-1A), not only is it not possible to fix all the bony fragments, but the cartilage surface would also have undergone considerable injury, leading to a suboptimal bearing surface. (2) Size of fracture fragments: It is straightforward to understand the issues with a large number of small bony fragments, with a significant number made up of small osteochondral fragments. Additionally a transcondylar or very low supracondylar fracture also poses the same problem of the ability to hold the fracture sufficiently rigidly while allowing joint motion (see Fig. 56-1B). (3) Quality of bone stock: Good quality young bone stock allows better fixation than does osteopenic/osteoporotic older bone.26,31 In a young population with an average age of 35 years, distal humeral articular surface fractures treated with internal fixation achieved good and sustainable results, although 40% required secondary procedures: an average flexion arc of 106 degrees, average foream rotational arc of 165 degrees, and a Mayo Elbow Performance Score (MEPS) of 91.2 A choronologically older group of patients with distal humeral fracture fixation, but without objective proof of osteoporosis, resulted in a 75% good/excellent range of motion and 80% satisfaction,15 although other studies of the older age group found less satisfactory results.27 Helfet et al8 demonstrated that despite good surgical technique and adequate internal fixation, 2-10% of such patients develop non-unions. Furthermore, if early motion is sacrificed, a compromise made for the quality of bone stock, the prediction is a better rate of union with a stiff joint. Pajarinen and Bjorkenheim26 correlated that immobilization greater than 3 weeks was a poor prognostic factor, after open reduction and internal fixation (ORIF), for achieving a good functional score. Thereby, when the principle of rigid internal fixation with early joint mobilization cannot be adhered to, then fracture fixation, joint immobilization to union, and a secondary motion gaining procedure may have to be considered.
ACUTE TOTAL ELBOW ARTHROPLASTY
An alternative strategy that has gained a wider acceptance is the acute total elbow replacement, when the fracture is judged to be unreconstructable or reconstructable without the predictability desired to allow early motion. However, the replacement of an unreconstructable fracture involving a joint is not a new concept. Hip7 and shoulder,6,25 and less commonly knee,7,14 surgeons, have an established body of literature regarding the relevance and successful outcomes of this concept.
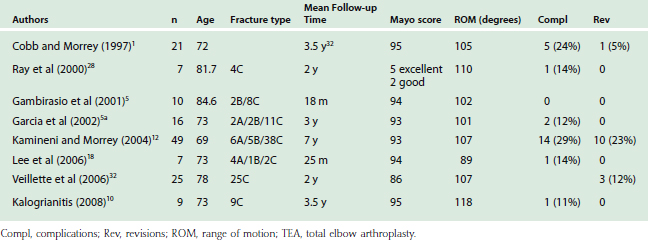
Total elbow replacements have been proven effective in the treatment of distal humeral nonunions,22 post-traumatic arthritis,16,24,30 rheumatoid arthritis,23 and chronic fracture dislocations.20 More recently, a growing literature appears to be favorable regarding this concept in the treatment of the difficult-to-reconstruct distal humeral fracture in the older age group of patients (Table 56-1). The first large series, which was reported by Cobb and Morrey,1 was a retrospective study of 21 distal humeral fractures in patients with an average age of 72 years. Ten of the cases had pre-existing rheumatoid arthritis, and the follow-up period was an average of 3.3 years (Table 56-2). The outcomes, based on the MEPS, were 15 excellent and five good results, with inadequate data in one case. Only one patient required revision arthroplasty due to a fractured ulna component (Table 56-3).
TABLE 56-1 Indications and Contraindications for Acute Elbow Arthroplasty
TABLE 56-3 Complications Associated with Acute Elbow Arthroplasty
This study was extended by Kamineni et al12 to 43 patients, and re-reported with a seven year follow-up. Nineteen patients in this cohort had pre-existing rheumatoid arthritis. The average flexion arc was 107 degrees, with an average MEPS of 13/100. Five patients required revision arthroplasty due to trauma (n = 3), aseptic loosening (n = 1), and septic loosening (n = 1). This latter study demonstrated that the results were maintained over a longer follow-up period, although the revision rate increased with time, from 5% at 3 years to 12% at 7 years.
Ray et al28 reported on seven patients with a mean age of 81 years who underwent acute total elbow arthroplasty without underlying pathology. The functional outcomes were excellent in five and good in two patients, with an average flexion arc greater than 100 degrees. Only one patient had mild pain, with the other six patients being completely pain free.
The use of unlinked prosthesis for an acute arthroplasty was reported in only one series, which focused on a group of patients with an average age of 73 years and a follow-up of 3.5 years.11 The MEPS was 95, and the average arc of flexion was 98 degrees. This helps to corroborate the concept of acute arthroplasty for fractures, independent of the choice of linked or unlinked prostheses.
When distal humeral fractures are associated with underlying rheumatoid or other inflammatory arthritides, the indications for an acute arthroplasty are more relaxed.1,11,12,28 Furthermore, fractures in the rheumatoid population should be considered a different pathology than that of a fracture in a joint that was nonpathologic before injury.1,12,28 The generally good results that can be expected in the fractured rheumatoid population was reported by Ikavalko and Lehto.10 A 100-degree arc of flexion was maintained up to a 4-year period. However, due to the low demands of this population, complications can be dealt with much more conservatively than the non-rheumatoid patient.
Whereas the indications for acute elbow joint replacement are being extended, as is expected of any new procedure, caution must be exercised when making the decision between conservative, osteosynthesis, and arthroplasty management. The importance of a correct and timely decision is highlighted by the functional outcomes of a successful osteosynthesis versus an acute arthroplasty, as opposed to a later conversion of a failed osteosynthesis to a secondary arthroplasty. Frankle et al have reported a retrospective comparison of value.4 Patients older than 65 years with C2 or C3 intra-articular distal humeral fractures were treated with either osteosynthesis or acute elbow arthroplasty. In the osteosynthesis group, there were one fair and three poor MEPS outcomes, whereas in the arthroplasty group, only excellent and good results were achieved at a 2-year follow-up. Veillette and McKee32 corroborated these findings with a multicenter prospective randomized study comparing osteosynthesis with acute elbow arthroplasty in patients older than 65 years. The MEPS revealed a significant improvement with acute arthroplasty (total elbow arthroplasty [TEA] 86 versus ORIF 73) over a 2-year follow-up. However, the Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire showed a significantly better outcome for acute arthroplasty in the first 6 months, but at a longer 2-year follow-up, the results between the two groups showed no statistical difference. However, a finding of note was that the arthroplasty group had a lower reoperation rate (12% versus 26%).
When the decision to perform an osteosynthesis is incorrect at the time of injury and the fixation fails, requiring a secondary arthroplasty, the results are generally poorer than if the primary intervention was an acute elbow arthroplasty. Mighell et al21 reported 28 cases of failed osteosynthesis converted to total elbow arthroplasty in patients with an average age of 66 years. At a 3-year follow-up, the improvement in functional outcomes (American Shoulder and Elbow Surgeons [ASES] scores) were statistically significant from 36 to 65, with improvements in pain, motion and function. These results could be interpreted as lesser to those results for a primary acute arthroplasty. In addition, 21% (6/28) required revision implant surgery, five patients required revision arthroplasty for aseptic loosening, and one required ultimate arthrodesis for septic loosening. Hence, the wrong decision at the primary intervention appears to result in a lesser functional outcome and a higher complication and revision rate.
SURGICAL TECHNIQUE
OPERATING ROOM PREPARATION
The relevant imaging of the fracture should be displayed for the operating surgeon to reference during the procedure. A torniquet is positioned on the upper arm, and its size should allow surgical access to a minimum of 10 centimeters proximal to the olecranon process for distal humeral fractures (Fig. 56-2). The torniquet should be inflated only when the whole limb is prepared and draped after the limb is elevated for 5 minutes.
PATIENT POSITIONING
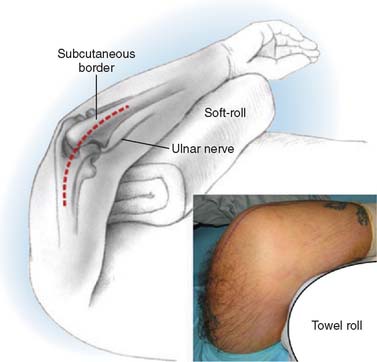
The patient is placed supine on the operating table with no other supports to restrict arm motion. The arm is placed across the chest, and rested on a bolster or roll of towels. The towel roll should be sterile in order to reposition during different parts of the procedure (Fig. 56-3).
SKIN INCISION
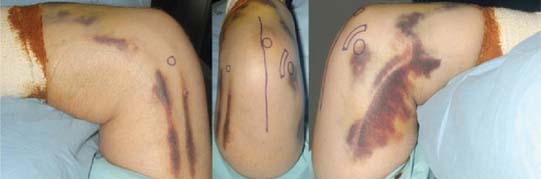
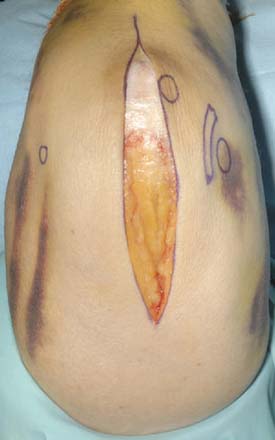
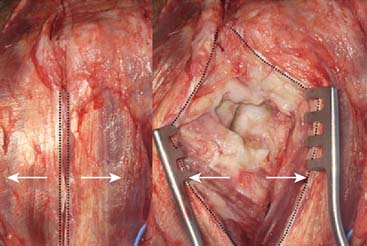
A straight skin incision 5 cm distal to and 10 cm proximal to the olecranon tip is employed (Fig. 56-4). The incision should be just medial or lateral to the tip of the olecranon in order to avoid the discomfort of a scar on a weight-bearing part of the elbow, when resting on the arm, with a potential for wound breakdown. Deepen the incision to define the superficial surface of the triceps central tendon, without breaching its surface (Fig. 56-5).
APPROACH
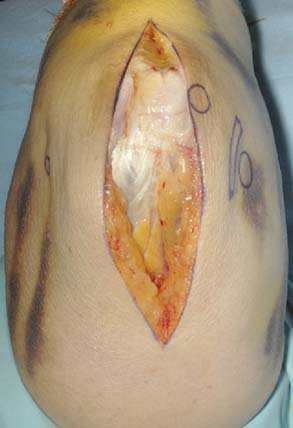
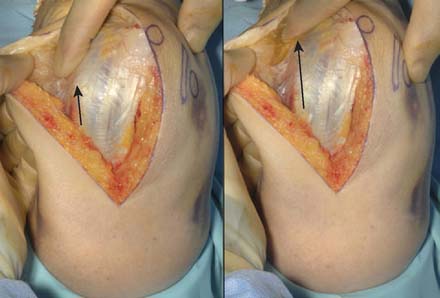
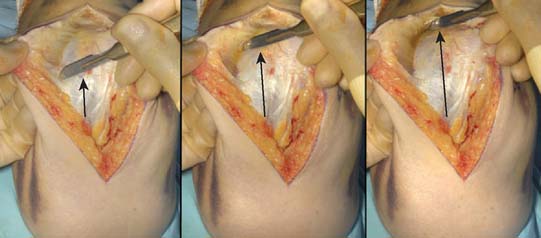
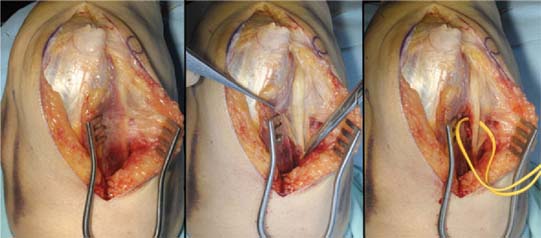
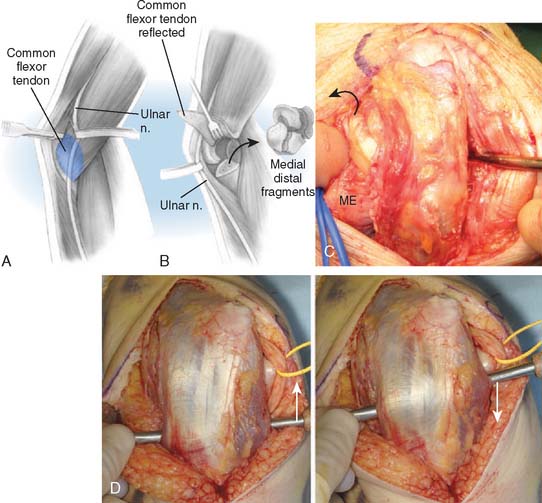
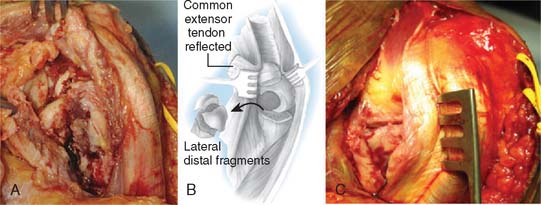
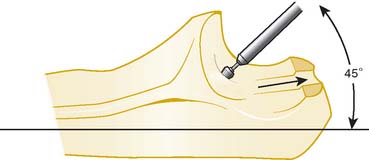
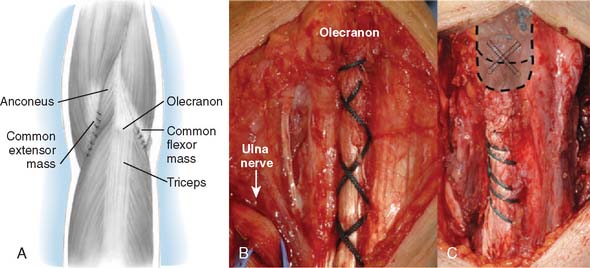
A full-thickness skin flap should be first raised up to the level of the border of the triceps muscle belly, which can often be achieved with simple finger dissection (Fig. 56-6). A method of raising the skin flaps, which are more adherent, is the flat blade technique, in which the blade is pressed against the deeper plane but angled at 45 degrees toward the superficial plane (Fig. 56-7). Because bruising and blood clots may obscure normal anatomy, at this point, the ulna nerve should be palpated gently by digital probing. The nerve should be dissected free of the cubital tunnel and released up to the first motor branch, freeing it from the medial epicondyle fragment. A vessel loop should be placed around the nerve, knotted loosely to allow easy control without an attached artery clip (Fig. 56-8). Once the nerve is protected, the remainder of the medial skin flap can be raised to allow access to the anterior aspect of the medial fragment. Once the fragments are freed, they can be removed either posterior or anterior to the nerve, depending on which situation places the nerve under least tension (Fig. 56-9). The lateral full-thickness skin flap should also be raised. Continuing the dissection distal to the epicondyle allows the anconeus–extensor carpi ulnaris interval to be identified, which will be the continuation for the lateral triceps border mobilization (Fig. 56-10). The lateral epicondyle is then enucleated of all soft-tissue attachments, and then removed through the lateral exposure (Fig. 56-11). When the fracture is a low supracondylar or transcondylar configuration, the fragment may be more easily removed, once freed medially and laterally, through the TS approach (Fig. 56-12).
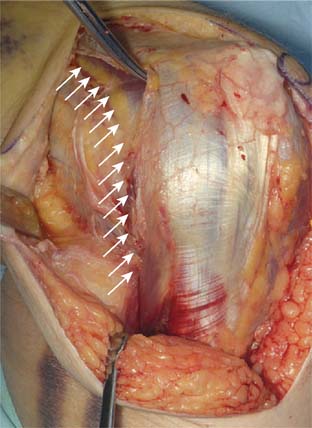
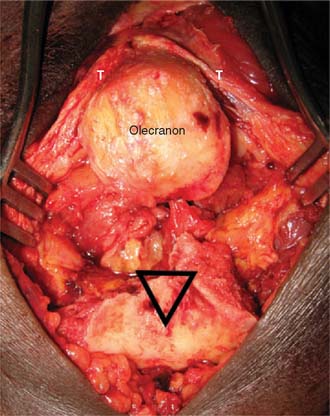
With the modified B-M approach, the forearm is now rotated toward the head of the patient in order to better expose the implantation surfaces of the humerus and ulna. The triceps, with the ulna nerve, is displaced medially. In order to improve exposure, the triceps is elevated from the medial and lateral aspects of the ulna but not from the posterior aspect. Using a triceps-sparing approach, a split is made from the apex of the olecranon, in line with the central triceps tendon fibers, for 5 to 7 cm proximally. The triceps is elevated from the posterior olecranon, sufficient to allow ulna preparation and implantation. The amount of triceps generally required to be elevated, by Sharpey fiber dissection, is 1 to 2 cm2, but no elevation is required from the medial and lateral aspects of the ulna. The humerus is then exposed through the triceps split, as is the olecranon tip (Fig. 56-13).
Humeral Preparation
No additional bony cuts are usually required once the fracture fragments are removed, with no real detriment to forearm/wrist power expected (Fig. 56-14).19 The canal is simply rasped to the correct size, and the flange of the Coonrad-Morrey humeral component should seat at the level of the coronoid fossa apex. A 6-inch humeral stem is the optimal length, but this also has to take into account the possibility of a pre-existing shoulder prosthesis. Additionally, if the patient has pre-existing shoulder pathology, with the possibility of requiring a prosthesis in the future, it is prudent to implant a 4-inch humeral stem, If the humeral fracture does not allow adequate seating of a trial implant, following canal preparation, resect additional bone.
Ulna Preparation
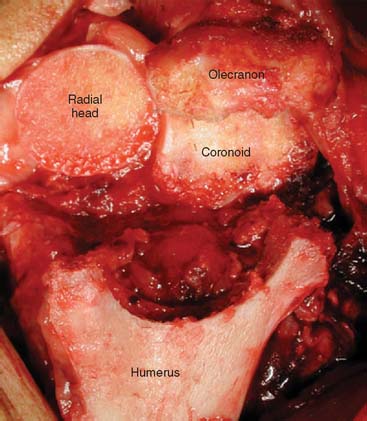
In the B-M approach, adequate exposure of the ulna is achieved by dissecting and protecting the ulnar nerve in an anteriorly displaced position and then by elevating the medial 25% of the triceps attachment. The forearm is rotated to help in exposing the medial olecranon and coronoid, and the tip of the olecranon is resected. In the TS approach, the posterior flat area of the olecranon is exposed without elevating the medial and lateral aspects of the triceps insertion. The tip of the olecranon is resected, and a clear view is achieved of the base of the coronoid and radial head when the elbow is hyperflexed (Fig. 56-15). The tip of the coronoid is also commonly resected to avoid anterior flange impingement in flexed positions (Fig. 56-16).
A high-speed olive-tipped burr is used to open the base of the coronoid process (Fig. 56-17). Extreme care should be exercised during this procedure to avoid the inadvertent injury to the ulna nerve. Tip: Hold the medial and lateral borders of the ulna between two fingers, which automatically displaces and protects the ulna nerve. When the coronoid base is sufficiently opened, ulna shaft rasping can begin. When rasping the canal, always orient the rasp parallel to the flat spot of the ulna; with the Coonrad-Morrey system, the rasp handle will be perpendicular to the flat spot. Because there is a risk of penetrating the cortices during rasping, a useful habit is to grasp or palpate the ulnar shaft with the nonrasping hand to help orient the direction of rasping in line with the rasp/burr (see Fig. 56-17).
Trial Implantation
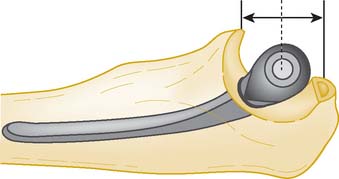
In the B-M approach, both trial components are inserted and then the components are reduced. Often, gentle distraction of the joint is prudent to avoid levering the components during the reduction. With the TS approach, the humeral component is inserted first and then, with the elbow flexed to 90 degrees, the ulnar component is inserted through the yolk of the humeral component, without the need for a reduction maneuver. The ulnar component center of rotation should coincide with the center of the greater sigmoid notch of the ulna. Once the components are inserted to the correct depth, the elbow should be examined through a full range of extension and flexion. If there is any suggestion that there is bony abutment of the olecranon process posteriorly or coronoid process anteriorly, these should be sufficiently trimmed. Any radial head abutment can also be visualized and an appropriate depth of radial head can be resected. The bone resected from the radius is useful for creating the posterior flange bone graft, and should be stored in a damp swab until humeral component insertion. Once the trials are satisfactory, they are removed and the bony canals are pulse lavaged, a cement restrictor is inserted into the humeral canal to the correct level, and the canal kept dry until cementation commences.
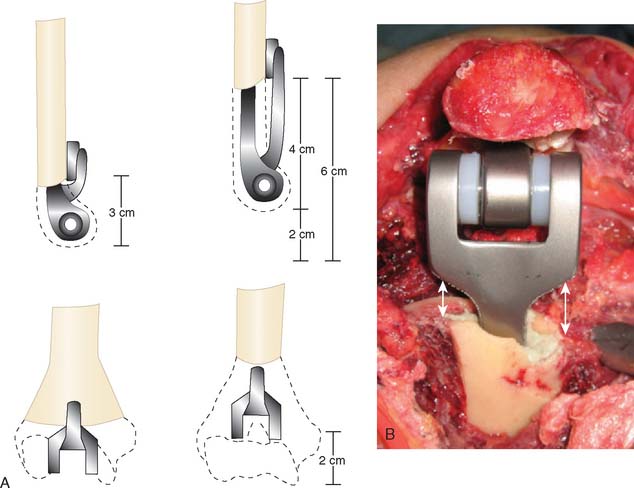
In situations in which distal humeral bone loss is greater than that would allow standard seating of the standard flange design, an extended flange may be used to regain humeral length (Fig. 56-18). However, triceps power is well maintained with humeral loss of up to 2 cm.9
Implant Insertion
A single mix of polymethylmethacylate cement with 1 g of vancomycin is vacuum mixed. Good cementing technique is of great importance.3 The cement is loaded into a cement gun, the nozzle of which was pretrimmed to 1 cm beyond the humeral stem length (Fig. 56-19), and the cement inserted into the humerus followed by the ulna. As the humerus is inserted, the bone graft, which should be gently wedge shaped (2 mm thick at the distal and 4 mm at the proximal ends), should be held on the anterior humeral cortex as the flange engages this region. With the B-M approach, the ulna is inserted first (Fig. 56-20), followed by the humerus. Once inserted to the correct depth, a reduction maneuver is performed and the locking pins are articulated. With the TS approach, the humeral component is inserted first, followed by the ulnar component through the yolk. Once the articulating pin is secure, the humeral component is finally tapped into its final position, and the elbow is extended and maintained in this position until the cement is cured (Fig. 56-21). Before setting, excess cement should be diligently removed to avoid cement particles in the articulation.
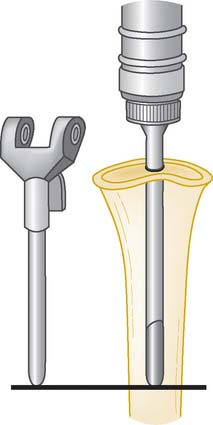
FIGURE 56-19 The cementation of the humeral and ulna canals should be approximately 1 cm distal to the prosthesis tip.
Wound Closure
The B-M approach closure consists of anteriorly isolating the ulnar nerve in the plane between the flexor muscle mass and the skin. Care to adequately resect the medial intermuscular septum should be taken, and the pocket is created with a Vicryl suture across the plane, making sure that the ulna nerve is free to mobilize during elbow motion. The flexor mass is then sutured to the medial triceps and the lateral mass to the lateral triceps margin (Fig. 56-22). With the TS approach, No. 2 ethibond is used to close the triceps tendon split with a running locking stitch, which then secures this central triceps to the olecranon through bone tunnels. The elbow is dressed with a nonadherent dressing, and a volar thermoplastic removable splint in full extension.
Postoperative Management
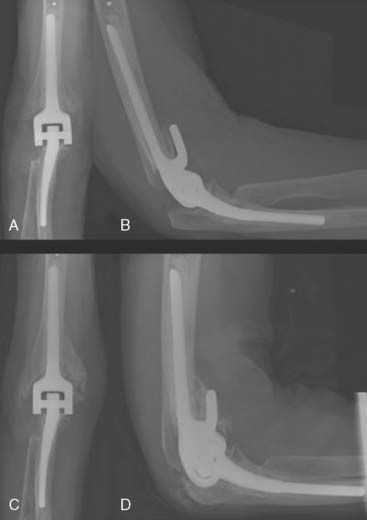
The arm is elevated for 24 hours, and the patient is typically discharged on the second or third postoperative day following evaluation of the patient’s radiographs (Fig. 56-23). A night-time volar splint is used for the first 4 weeks to encourage the maintainence of full extension. Active elbow flexion is commenced with gravity extension only for the first 4 weeks. Routine physical therapy is not needed. Normal use of the elbow can be encouraged at week 4 after operation (Fig. 56-24).
1 Cobb T.K., Morrey B.F. Total elbow arthroplasty as primary treatment for distal humeral fractures in elderly patients. J. Bone Joint Surg. Am. 1997;79:826.
2 Doornberg J.N., et al. Surgical treatment of intra-articular fractures of the distal part of the humerus. Functional outcome after twelve to thirty years. J. Bone Joint Surg. Am. 2007;89:1524.
3 Faber K.J., et al. Advanced cement technique improves fixation in elbow arthroplasty. Clin. Orthop. Relat. Res. 1997;334:150.
4 Frankle M.A., et al. A comparison of open reduction and internal fixation and primary total elbow arthroplasty in the treatment of intraarticular distal humerus fractures in women older than age 65. J. Orthop. Trauma. 2003;17:473.
5 Gambirasio R., et al. Total elbow replacement for complex fractures of the distal humerus. An option for the elderly patient. J. Bone Joint Surg Br. 2001;83:974.
5a Garcia J., Mykula R., Stanley D. Complex fractures of the distal humerus in the elderly. The role of total elbow replacement as primary treatment. J. Bone Joint Surg. Br. 2002;84:812.
6 Grassi F.A., Tajana M.S. Partial prosthetic replacement of the shoulder in fractures and fracture-dislocations of the proximal humerus. Chir. Organi Mov. 2005;90:179.
7 Greenough C.G., Jones J.R. Primary total hip replacement for displaced subcapital fracture of the femur. J. Bone Joint Surg. Br. 1988;70:639.
8 Helfet D.L., Kloen P., Anand N., Rosen H.S. Open reduction and internal fixation of delayed unions and nonunions of fractures of the distal part of the humerus. J. Bone Joint Surg. Am. 2003;85-A(1):33.
9 Hughes R.E., Schneeberger A.G., An K.N., Morrey B.F., O’Driscoll S.W. Reduction of triceps muscle force after shortening of the distal humerus: a computational model. J. Shoulder Elbow Surg. 1997;6:444.
10 Ikavalko M., Lehto M.U. Fractured rheumatoid elbow: treatment with Souter elbow arthroplasty—a clinical and radiologic midterm follow-up study. J. Shoulder Elbow Surg. 2001;10:256.
11 Kalogrianitis S., Sinopidis C., El Meligy M., Rawal A., Frostick S.P. Unlinked elbow arthroplasty as primary treatment for fractures of the distal humerus. J. Shoulder Elbow Surg. 2008;17:297.
12 Kamineni S., Morrey B.F. Distal humeral fractures treated with noncustom total elbow replacement. J. Bone Joint Surg Am. 2004;86-A:940.
13 Kasten P., Krefft M., Hesselbach J., Weinberg A.M. Kinematics of the ulna during pronation and supination in a cadaver study: implications for elbow arthroplasty. Clin. Biomech. (Bristol, Avon). 2004;19:31-35.
14 Keenan J., Chakrabarty G., Newman J.H. Treatment of supracondylar femoral fracture above total knee replacement by custom made hinged prosthesis. Knee. 2000;7:165.
15 Kocher M., Melcher G.A., Leutenegger A., Rüedi T. [Elbow fractures in elderly patients]. Swiss Surg. 1997;3:167.
16 Kozak T.K., Adams R.A., Morrey B.F. Total elbow arthroplasty in primary osteoarthritis of the elbow. J. Arthroplasty. 1998;13:837.
17 Lecestre P., Dupont J.Y., Lortat Jacob A., Ramadier J.O. [Severe fractures of the lower end of the humerus in adults (author’s transl)]. Rev. Chir. Orthop. Reparatrice Appar. Mot. 1979;65:11.
18 Lee K.T., Lai C.H., Singh S. Results of total elbow arthroplasty in the treatment of distal humerus fractures in elderly Asian patients. J. Trauma. 2006;61:889.
19 McKee M.D., Pugh D.M., Richards R.R., Pedersen E., Jones C., Schemitsch E.H. Effect of humeral condylar resection on strength and functional outcome after semiconstrained total elbow arthroplasty. J. Bone Joint Surg. Am. 2003;85-A:802.
20 Mighell M.A., Dunham R.C., Rommel E.A., Frankle M.A. Primary semi-constrained arthroplasty for chronic fracture-dislocations of the elbow. J. Bone Joint Surg. Br. 2005;87:191.
21 Mighell, M., Virani, M., Frankle, M., and Pupello, D.: Failed open reduction and internal fixation for elbow fractures converted to total elbow arthroplasty. In American Shoulder and Elbow Surgeons Closed Meeting. Chicago, 2006, p. e52.
22 Morrey B.F., Adams R.A. Semiconstrained elbow replacement for distal humeral nonunion. J. Bone Joint Surg. Br. 1995;77:67.
23 Morrey B.F., Adams R.A. Semiconstrained arthroplasty for the treatment of rheumatoid arthritis of the elbow. J. Bone Joint Surg. Am. 1992;74:479.
24 Morrey B.F., Adams R.A., Bryan R.S. Total replacement for post-traumatic arthritis of the elbow. J. Bone Joint Surg. Br. 1991;73:607.
25 Movin T., Sjoden G.O., Ahrengart L. Poor function after shoulder replacement in fracture patients. A retrospective evaluation of 29 patients followed for 2-12 years. Acta Orthop. Scand. 1998;69:392.
26 Pajarinen J., Bjorkenheim J.M. Operative treatment of type C intercondylar fractures of the distal humerus: results after a mean follow-up of 2 years in a series of 18 patients. J. Shoulder Elbow Surg. 2002;11:48.
27 Pereles T.R., Koval K.J., Gallagher M., Rosen H. Open reduction and internal fixation of the distal humerus: functional outcome in the elderly. J. Trauma. 1997;43:578.
28 Ray P.S., Kakarlapudi K., Rajsekhar C., Bhamra M.S. Total elbow arthroplasty as primary treatment for distal humeral fractures in elderly patients. Injury. 2000;31:687.
29 Riseborough E.J., Radin E.L. Intercondylar T fractures of the humerus in the adult. A comparison of operative and non-operative treatment in twenty-nine cases. J. Bone Joint Surg. Am. 1969;51:130.
30 Schneeberger A.G., Adams R., Morrey B.F. Semiconstrained total elbow replacement for the treatment of post-traumatic osteoarthrosis. J. Bone Joint Surg. Am. 1997;79:1211.
31 Sodergard J., Sandelin J., Bostman O. Mechanical failures of internal fixation in T and Y fractures of the distal humerus. J. Trauma. 1992;33:687.
32 Viellette, J., and McKee, M.: A multicenter prospective randomized controlled trial of open reduction internal fixation versus total elbow arthroplasty for displaced intra-articular distal humeral fractures in elderly patients. In ASES 23rd Annual Closed Meeting, 2006. Chicago, Illinois.
33 Zagorski J.B., Jennings J.J., Burkhalter W.E., Uribe J.W. Comminuted intraarticular fractures of the distal humeral condyles. Surgical vs. nonsurgical treatment. Clin. Orthop. Relat. Res. 1986;202:197.