CHAPTER 13 DISORDERS OF SMELL AND TASTE
The “chemical” senses of smell and taste are the human organism’s means of qualitatively analyzing the chemical composition of its immediate environment. Although impairment of smell or taste is not generally perceived to be as disabling as impairment of sight or hearing, hyposmia (impaired olfaction) or hypogeusia (impaired taste) may nevertheless cause substantial loss in quality of the esthetic and hedonistic aspects of the individual’s life, may contribute to comorbid conditions such as poor nutritional intake in the elderly, and, uncommonly, may be an early sign of a serious underlying disorder.1 Unpleasant smells or tastes may trigger recognition of toxins in food or the environment not otherwise detectable by the other senses. Smell via pheromones is an important component of courtship and mating in the life cycles of other species but to a far lesser extent in Homo sapiens, which is not known to possess a vomeronasal organ or a discrete pheromonal system.2 Loss of olfaction or gustation may go relatively unnoticed by the patient; in other cases, a perceived hypersensitivity of the chemical senses or inappropriate activation of sensory pathways may be the cause of symptoms.
DISORDERS OF SMELL
Aspects of the Anatomy and Physiology of Olfaction
The primary sensory neurons of the olfactory pathway continuously die and are continuously replaced, being generated from the basal cells of the olfactory epithelium.3 The ciliated olfactory neurons have a lifespan of between 30 and 60 days3 and can be regenerated after damage or loss.4 The olfactory epithelium secretes mucus, which bathes the sensory dendritic surface of the olfactory neurons and provides a medium to dissolve odorants. Odorant signal transduction occurs through binding to G protein–coupled olfactory receptors, of which about 350 forms occur in humans. As might be expected, congenital genetically based specific anosmias (for certain odorants) have been reported, analogous to various forms of color blindness (see Hawkes, 2002). The bipolar olfactory neurons constituting the first cranial nerve pass through the cribriform plate of the ethmoid bone and synapse in the olfactory bulb, within glomeruli, with second-order afferent neurons. Considerable convergence and information processing occur within the olfactory bulb and tract.5 Significant inhibitory and interglomerular innervation occurs within the bulb3 and is believed to modulate afferent excitatory transmission even at this level. Significant bilateral communication occurs at multiple levels within the olfactory pathways, beginning from the anterior olfactory nuclei (which are collections of neuronal bodies lying posteriorly within the olfactory tracts) and extending to the thalami and cortical olfactory areas. In humans, neurons of the olfactory tracts terminate, without synapse in the thalami, in regions collectively termed the primary olfactory cortex (lateral olfactory gyrus, piriform cortex, and periamygdaloid areas). These sites subsequently project to the hypothalamus, limbic structures, entorhinal cortex, and other areas. A minority of relays project through the dorsomedial nucleus of the thalamus. Olfactory and other areas of cortex in turn project centrifugally back to the olfactory bulbs and may modulate feedback inhibition or facilitation of afferent stimuli. Finally, the trigeminal nerve is also important in the perception of smell, mediating tactile, noxious, and pain stimuli in the nasopharynx and associated structures.6
Clinical Approach to Olfactory Disorders
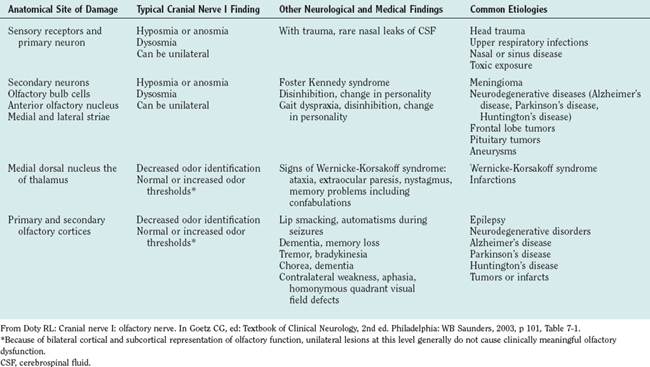
Neurological disorders of olfaction can be conveniently approached according to symptom presentation (anosmia or hyposmia; hyperosmia or dysosmia), anatomical site of dysfunction (Table 13-1), or etiology (Table 13-2).7 A careful history and examination usually allow the clinician to make a provisional diagnosis and target subsequent investigations. Upper respiratory tract infection is the most common cause of neural olfactory dysfunction, accounting for up to 33% of cases. However, in up to 40% of patients with olfactory dysfunction, no cause for their symptoms is found.8
AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
History
The nature of the olfactory abnormality, its temporal course, and its variation with differing stimuli should be clarified as precisely as the patient’s recall, cognitive state, and descriptive abilities allow. Dysosmia refers to a general distortion of smell sense, whereas troposmia refers to distortion in quality of a particular normal smell stimulus. Whether the smell concerned is pleasant, unpleasant, or excessively unpleasant (cacosmia) may be important. Complaints of nasal discharge or unilateral epistaxis are suggestive of an upper airway abnormality. Traumatic head injury is an important cause of smell and taste disorders and may account for up to 23% of cases of documented hyposmia.9 Intracranial surgery likewise may cause hyposmia. Any variation of intensity with a particular stimulus or scent, particular hyperosmia, should be noted. The possibility of pregnancy should be considered in the female patient of childbearing potential with hyperosmia or dysosmia.10 The medical history and drug history may reveal use of agents known to impair olfaction (Table 13-3). A history of smoking, alcohol consumption, and recreational drug use should be documented.11 The medical history may be notable for sinusitis or other rhinal disease,12 thyroid or other endocrine disease, hepatic or renal impairment, Parkinson’s disease13 or other neurodegenerative conditions,14 stroke, or features such as deafness or diabetes that are suggestive of a mitochondrial dis order. Association of a smell, particularly a strongly unpleasant or noxious one, with a period of amnesia, loss of awareness, or other suggestive symptoms, can indicate a focal seizure disorder.15 The patient may perceive an odor when no stimulus is present (phantosmia). A history from an observer can prove invaluable in this circumstance. Olfactory hallucinations are an uncommon manifestation of schizophrenia, being noted in approximately 6% of patients.16 A family history may also yield clues in regard to any of these conditions. Other important points include occupational or other exposure to toxins or fumes, past treatment with radiation, the psychiatric history, and past intracranial or systemic infection, including human immunodeficiency virus infection.17 Congenital hyposmia is known to be associated with immotility or absence of olfactory epithelial cilia, most commonly in association with hypogonadotropic hypogonadism (Kallman’s syndrome).18 Headache or other symptoms suggestive of an enlarging intracranial mass or of frontal region dysfunction may herald a progressive frontal, temporal, or anterior fossa tumor. It should be remembered that a significant decline in olfactory acuity is seen with aging, particularly in the sixth and seventh decades.19 Quality-of-life questionnaires have been developed to try to reproduce reliably the largely subjective perception of olfactory dysfunction.1
* Most of these agents are noted in the Physician’s Desk Reference as having adverse effects on the olfactory system.
From Doty RL, Bromley SM: Effect of drugs on olfaction and taste. Otolaryngol Clin North Am 2004; 37:1231, Box 1.
Examination
Office assessment of olfactory dysfunction by the general neurologist includes targeted neurological examination and a general medical examination, including ears and oropharynx; both examinations are guided by the history. Unilateral olfactory acuity can be assessed by sequential nasal occlusion, and a number of formal office tests of olfactory dysfunction are available (Table 13-4). The four-alternative forced-choice structure of the 40-item University of Pennsylvania Smell Identification Test (UPSIT) odor identification test may enable malingering to be detected on the basis of a performance significantly worse than that expected by chance alone, which may be useful in medicolegal cases involving trauma or toxic exposure. Formal gustatory examination should also be performed, because many causes of olfactory and taste impairment may also affect taste and smell, respectively. Endoscopic examination of the upper respiratory tract, performed by a suitably skilled and experienced clinician with appropriate equipment, should be considered, especially if a tumor or other rhinological cause is suspected.8 A Mini Mental State Examination, neuropsychological, or formal psychiatric evaluation may be indicated.
TABLE 13-4 Office Tests Commonly Used in Olfactory Assessment
Investigation of Olfactory Disorders
Computed tomography of the head, orbits, and nasopharynx is the initial imaging modality of choice.7 Lesions causing bony destruction or infiltration are readily visualized, as are most collections or intracranial masses large enough to cause neural distortion or compression. Magnetic resonance imaging and angiography with paramagnetic contrast material remain the “gold standards” for detecting subtle neuraxial lesions, small anterior fossa masses, and other nonbony structural abnormalities, such as caudate atrophy in early Huntington’s disease or focal frontal or temporal cortical epileptogenic foci. Blood and other body fluid analyses are useful for screening for specific etiologies such as infection or vitamin B12 deficiency. Electroencephalography is useful in diagnosing seizure disorders. Cerebrospinal fluid analysis and measurement of the intrathecal pressure should be performed if basal meningitis is suspected. Biopsy and histological or other examinations may be performed on suspect mass lesions. More extensive psychophysical investigation of olfactory disturbances may include threshold determination, odor discrimination, and odor memory tests, as well as odor identification tests such as the UPSIT. Odor event–related potentials and electro-olfactography are techniques generally confined to specialized clinical or research centers (see Hawkes, 2002).
Principles of Management of Olfactory Disorders
Therapy for olfactory disorders is most usefully directed at the underlying cause of dysosmia.11 Rhinological causes of olfactory dysfunction are most easily accessible. Specific treatment may include topical or systemic antihistamines or antiallergy agents, antimicrobial therapy, or invasive interventions for recurrent sinusitis, obstruction, or malignancy. Neurological causes of olfactory dysfunction often resolve if the primary site of dysfunction is at the olfactory nerve, because of regeneration of bipolar neurons from the basal layer. However, avoidance of environmental agents or cessation of medications known to induce dysosmia may not fully reverse the deficit.20 Likewise, olfactory disturbance caused by chemotherapy or radiotherapy for malignancy may or may not improve after conclusion of treatment. Smoking cessation is medically advisable for many reasons, and normosmia usually is successfully restored if the hyposmia was attributable to this cause. Treatment of intracranial causes of sensorineural dysosmia has varying success. A focal epileptogenic lesion may respond to anticonvulsants or be amenable to surgical excision, as may a frontal meningioma or other surgically accessible mass. However, deficits may be irreversible if tissue damage is permanent. Most neurodegenerative illnesses associated with olfactory disturbance are incurable. As mentioned previously, olfaction is vital to the appreciation of drink, food, and other pleasurable aspects of normal life and is important in the detection of dangerous or unpleasant odorants such as household cooking gas or spoiled food. Elderly persons especially may develop unrecognized hyposmia. They are therefore at increased risk of not recognizing such threats. Loss of smell may also be misinterpreted as anhedonia or depression. Loss of taste, too, is important in the elderly (see later discussion). Age-related hyposmia is not reversible. Counseling, heightened awareness, and physical safety measures such as gas and smoke detectors are useful interventions. An acute sense of smell is important in many professions and trades (plumber, parfumier, chef or baker, winemaker, physician), and occupational compensation may be warranted. This should always be considered in managing the patient whose sense of smell affects his or her livelihood, according to the individual laws in the state or country concerned.7 Because many cases of olfactory disturbance are not amenable to treatment or, at best, are only partially treatable, a supportive and sympathetic attitude toward the patient is always important. Appropriate education of the patient about the disorder can help greatly toward the patient’s understanding and coping with the disability.
DISORDERS OF TASTE
Aspects of the Anatomy and Physiology of Gustation
The five currently recognized tastes are salty, sour, bitter, sweet, and umami (conveniently described as the taste of glutamate).21 Smell, texture, other somatosensory perceptions (such as those elicited by menthol or chili), as well as taste, collectively contribute to the perception of flavor of ingested substances. Taste receptors are found within taste buds on the tongue, soft palate, pharynx, larynx, and upper esophagus. Individual taste receptors are responsive to all tastants with varying sensitivity, and each taste bud appears to exhibit one preferential sensitivity. The popular notion that a particular taste is “localized” onto any one area of the tongue is therefore largely inaccurate.3 Taste receptors, like olfactory bipolar cells, continuously regenerate from basal cells, being replaced approximately every 10 to 20 days. Like odorants, tastants need to be dissolved to interact with receptors, in this case in saliva. Salty (Na+) and sour (H+) tastants appear to evoke taste receptor depolarization through direct interaction with apical ion channels. Bitter and sweet tastants evoke depolarization via G protein–mediated second-messenger mechanisms, largely involving cyclic adenosine monophosphate and inositol 1,4,5-triphosphate, respectively. Taste receptors synapse with high convergence onto primary afferent neurons, which are unipolar neurons with cell bodies in the genicular ganglion of the facial nerve and the petrosal and nodose (inferior) ganglia of the glossopharyngeal and vagal nerves, respectively. Afferent fibers from the anterior two thirds of the tongue travel proximally via the chorda tympani. Afferent fibers from the posterior third of the tongue and other posterior structures travel proximally in the greater petrosal nerve branch of the facial nerve (inferior soft palate) and the lingual branch of the glossopharyngeal nerve, together with the internal laryngeal branch of the vagus nerve (epiglottis and extreme pharyngeal part of tongue). Somatosensory afferent fibers are also believed to travel proximally along similar routes, except that somatosensory fibers from the anterior two thirds of the tongue remain with the lingual branch of the mandibular nerve (V3), rather than departing with the chorda tympani. The afferent cranial nerves synapse, in descending order, caudally, ipsilaterally in the medulla of brainstem, and in the rostral third of the nucleus of the tractus solitarius with secondary gustatory neurons. At this level, taste afferent fibers are organized separately from somatosensory information. The subsequent routes of these pathways are less well delineated. Many fibers decussate at this level, and unilateral lesions of the medulla oblongata are not well described as causing taste disturbances. The pathways then ascend, largely via the central tegmental tract (not the medial lemniscus as previously thought) to the ventral posterior median nucleus of the thalamus and from there to the anteroinferior sensory and motor cortex, superior temporal gyrus, frontal operculum, and anterior insula. These pathways are, again, separate from somatosensory cortical representations of the tongue and palatal structures. Other pathways may synapse with the hypothalamus and limbic structures to mediate autonomic and emotional aspects of taste.6
Clinical Approach
The complaint of “loss of taste” (ageusia or hypogeusia) in food discrimination is related more often to an olfactory deficit (described previously) than to true impairment of gustation.22 Again, a careful history usually allows the clinician to interpret the patient’s symptom correctly. The evaluation of taste dysfunction is directed toward establishing which modalities (sweet, salty, bitter, sour umami) are preferentially impaired; whether the impairment can be localized anatomically within the sensorineural gustatory pathways as described previously; and whether a cause, treatable or otherwise, can be identified. Isolated disorders of taste caused by central nervous system lesions are rare.21 Abnormal taste (dysgeusia) is usually unpleasant; it may be strongly revolting (cacogeusia), and it may be hallucinatory (phantogeusia), as in a seizure or psychotic disorder, or real, as with a purulent nasopharyngeal infection. Hypergeusia is difficult to quantify and, more rarely, is a symptom prompting medical attention.
History
Common causes of taste dysfunction are listed in Table 13-5. They can be conveniently grouped into peripheral, central, and systemic causes. However, the anatomical site sometimes cannot be well localized. The clinician should attempt to gain as clear a description as possible of the exact nature of the patient’s taste disturbance, of any associated symptoms that may localize a lesion or suggest an underlying cause, and of recent respiratory infection. The clinician should also obtain medical and surgical histories23,24; a family history; and a careful history of prescription drug, tobacco, alcohol, and recreational drug use. Inquiry should be made into possible olfactory dysfunction. Important symptoms include xerostomia (dry mouth) or dry eyes; oral, head, facial, or neck pain; facial rash; balance difficulty, oscillopsia, or tinnitus; mouth ulcers or oral thrush; headache; and evidence of oculomotor weakness, facial weakness, or speech disturbance. Mechanical trauma causes isolated dysgeusia less frequently than it does olfactory dysfunction.9 Drugs known to be associated with taste dysfunction are listed in Table 13-3. Prior neck manipulation or trauma should prompt consideration of cervical cerebral arterial dissection.21 Symptoms suggestive of a seizure disorder in association with a powerful, intrusive, clearly abnormal taste in the absence of a tastant (phantogeusia) should prompt consideration of a focal seizure disorder. Unilaterality of taste dysfunction strongly suggests a cause below the level of the thalamus. Anhedonia should prompt consideration of a thalamic lesion or depression. Atten tion should be paid to the patient’s nutritional state, body mass index, and history of recent weight loss. Again, it should be remembered that an age-related decline in taste acuity may be seen in the normal individual.19
Examination
Office assessment of gustatory dysfunction includes examination of modality and lingual site of taste dysfunction, targeted neurological examination, and a general medical examination including nose, ears, and oropharynx. Formal otorhinolaryngological endoscopy may be necessary. Height and weight should be recorded, body mass index calculated, and attention paid to signs of protein or micronutrient malnutrition. The aims of neurological examination should be primarily to localize the anatomical site of taste disturbance if possible, and to seek evidence of a generalized process—such as sarcoidosis—that could be selectively affecting taste pathways. On oral examination, particular note should be taken of xerostomia, leukoplakia, ulcers, salivary gland enlargement, and oral hygiene. Dentures or other oral prostheses may interfere with palatal taste sensations. Psychiatric assessment may be warranted if depression or psychosis is suspected. Various methods of taste examination are described. A simple office spatial taste test is performed to assess tastant recognition of the four basic tastes (excluding umami); cotton-tipped swabs are used to apply strong sour, sweet, salty, or bitter solutions to separate areas of the patient’s tongue.25 A crude sensitivity threshold can be established by serial dilutions of the tastants down to the lowest recognizable concentration. Standardized “test strips” and other kits are commercially available with specific stimulus concentrations. It should be noted whether the deficit is unilateral or bilateral and whether the abnormality occurs on the anterior two thirds or posterior one third of the tongue. Taste examination of palatopharyngeal structures seldom adds additional useful information. Differentiation between phantom and real tastes can be made by applying topical anesthesia to the tongue. Phantogeusia can intensify after this. Formal magnitude-matching tests can be performed through comparison with another, normal, sensory modality in the patient, such as with a controlled frequency-specific aural stimulus. However, these are usually performed in specialized referral centers for taste disorders.
Investigation of Gustatory Disorders
The broad principles of investigation for taste disorders are similar to those for olfactory disorders. If a peripheral cause or localized central lesion is suspected, computed tomography and magnetic resonance imaging of the head, brain and brainstem, anterior cranial structures, internal acoustic meatus, and skull are required. Analyses of blood and body fluids, including cerebrospinal fluid analysis, are useful for a few specific causes such as infection, diabetes, or metabolic organ dysfunction. Suspect or abnormal masses should be subjected to biopsy where accessible. Directed investigation of suspected underlying causes of gustatory dysfunction is dictated by the clinical findings. Electrogustometry can be used to quantify dysgeusia objectively and assist with anatomical localization in difficult cases.26 Electrogustometry can also be used to assess glossopharyngeal nerve dysfunction. However, its use is more common in specialized otolaryngological and chemosensory disturbance clinics.
Principles of Management of Gustatory Disorders
Specific therapy for taste disturbance should be directed at the underlying cause when possible. In certain circumstances, taste disturbance may be self-limited and recover with time. Drug cessation in cases of drug-induced dysgeusia does not always reverse the disturbance, and many patients who do recover take months to years to do so.20 Prediction of dysgeusia when drug treatment begins is difficult in the individual patient. Although zinc and other micronutrient or vitamin supplementation are advocated by some authorities, it has not been shown to be of great benefit in the treatment of taste disorders outside of true deficiency states.21 The specific therapy for taste dysfunction is otherwise unsatisfactory, and many patients are left with permanent dysgeusia. Many patients with taste abnormalities, particularly elderly patients, have diabetes, congestive cardiac failure, or hypertension. Hypogeusia may prompt patients to add inordinate amounts of sugar or salt to their food, thereby potentially exacerbating these medical disorders.25 Patient education and counseling are therefore particularly important in supportive treatment of taste abnormalities in these patients. Consultation with a nutritionist may be of value. Finally, the intractable nature of these disabilities means that a supportive and sympathetic attitude toward the patient must always constitute an integral part of his or her care.
Buck LB. Smell and taste: the chemical senses. In Kandel ER, Schwartz JH, Jessell TM, editors: Principles of Neural Science, 4th ed., New York: McGraw-Hill, 2000.
Hawkes CH. Smell and Taste Complaints. Boston: Butterworth-Heinemann, 2002.
Heckmann JG, Heckmann SM, Lang CJG, et al. Neurological aspects of taste disorders. Arch Neurol. 2003;60:667-671.
Hummel T, Nordin S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngologica. 2005;125:116-121.
Mann N. Management of smell and taste problems. Cleve Clin J Med. 2002;69:329-336.
Wrobel BB, Leopold DA. Clinical assessment of patients with smell and taste disorders. Otolaryngol Clin North Am. 2004;37:1127-1142.
1 Hummel T, Nordin S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngologica. 2005;125:116-121.
2 Keverne EB. Brain evolution, chemosensory processing, and behavior. Nutr Rev. 2004;62(11):S218-S223.
3 Buck LB. Smell and taste: the chemical senses. In Kandel ER, Schwartz JH, Jessell TM, editors: Principles of Neural Science, 4th ed., New York: McGraw-Hill, 2000.
4 Upadhyay UD, Holbrook EH. Olfactory loss as a result of toxic exposure. Otolaryngol Clin North Am. 2004;37:1185-1207.
5 Buck LB. Olfactory receptors and odor coding in mammals. Nutr Rev. 2004;62(11):S184-S188.
6 Williams PL, Warwick R, Dyson M, et al, editors. Gray’s Anatomy, 37th ed., New York: Churchill Livingstone, 1989.
7 Doty RL. Cranial nerve I: olfactory nerve. In: Goetz CG, editor. Textbook of Clinical Neurology. 2nd ed. Philadelphia: WB Saunders; 2003:99-110.
8 Wrobel BB, Leopold DA. Clinical assessment of patients with smell and taste disorders. Otolaryngol Clin North Am. 2004;37:1127-1142.
9 Reiter ER, DiNardo LJ, Costanzo RM. Effects of head injury on olfaction and taste. Otolaryngol Clin North Am. 2004;37:1167-1184.
10 Nordin S, Broman DA, Olofsson JK, et al. A longitudinal descriptive study of self reported abnormal smell and taste perception in pregnant women. Chem Senses. 2004;29:391-402.
11 Mann N. Management of smell and taste problems. Cleve Clin J Med. 2002;69:329-336.
12 Raviv JR, Kern RC. Chronic sinusitis and olfactory dysfunction. Otolaryngol Clin North Am. 2004;37:1143-1157.
13 Katzenschlager R, Lees AJ. Olfaction and Parkinson’s syndromes: its role in differential diagnosis. Curr Opin Neurol. 2004;17:417-423.
14 Hawkes C. Olfaction in neurodegenerative disorder. Mov Disord. 2003;18:364-372.
15 Acharya V, Acharya J, Luders H. Olfactory epileptic auras. Neurology. 1998;51:56-61.
16 Sadock BJ, Sadock VA. Schizophrenia. In: Sadock BJ, Kaplan HI, Sadock VA, editors. Kaplan & Sadock’s Synopsis of Psychiatry. Lippincott Williams & Wilkins; 2003:471-504.
17 Heald AE, Pieper CF, Schiffman SS. Taste and smell complaints in HIV infected patients. AIDS. 1998;12:1667-1674.
18 Afzelius BA. Cilia related diseases. J Pathol. 2004;204:470-477.
19 Seiberling KA, Conley DB. Aging and olfactory and taste function. Otolaryngol Clin North Am. 2004;37:1209-1228.
20 Doty RL, Bromley SM. Effects of drugs on olfaction and taste. Otolaryngol Clin North Am. 2004;37:1229-1254.
21 Heckmann JG, Heckmann SM, Lang CJG, et al. Neurological Aspects of Taste Disorders. Arch Neurol. 2003;60:667-671.
22 Pribitkin E, Rosenthal MD, Cowart BJ. Prevalence and causes of severe taste loss in a chemosensory clinic population. Ann Otol Rhinol Laryngol. 2003;112:971-978.
23 Kamel UF. Hypogeusia as a complication of uvulopalatopharyngoplasty and use of taste strips as a practical tool for quantifying hypogeusia. Acta Otolaryngol. 2004;124:1235-1236.
24 Collet S, Eloy P, Rombaux P, et al. Taste disorders after tonsillectomy: case report and literature review. Ann Otol Rhinol Laryngol. 2005;114:233-236.
25 Brackmann DE, Fetterman BL. Cranial nerve VII: facial nerve. In: Goetz CG, editor. Textbook of Clinical Neurology. 2nd ed. Philadelphia: WB Saunders; 2003:181-194.
26 Tomita H, Ikeda M. Clinical use of electrogustometry: strengths and limitations. Acta Otolaryngol Suppl. 2002;546:27-38.