Disorders of Sleep
After reading this chapter you will be able to:
 Define obstructive sleep apnea (OSA).
Define obstructive sleep apnea (OSA).
 Identify why airway closure occurs only during sleep.
Identify why airway closure occurs only during sleep.
 State the long-term consequences of uncontrolled OSA.
State the long-term consequences of uncontrolled OSA.
 State how a diagnosis of OSA is made.
State how a diagnosis of OSA is made.
 Identify what groups of patients are at particular risk of OSA.
Identify what groups of patients are at particular risk of OSA.
 State what treatments are available for patients with OSA.
State what treatments are available for patients with OSA.
 Describe how continuous positive airway pressure (CPAP) works.
Describe how continuous positive airway pressure (CPAP) works.
 Identify problems associated with CPAP.
Identify problems associated with CPAP.
 Determine when bilevel pressure is useful in the treatment of OSA.
Determine when bilevel pressure is useful in the treatment of OSA.
 Identify the surgical alternatives for patients with severe OSA.
Identify the surgical alternatives for patients with severe OSA.
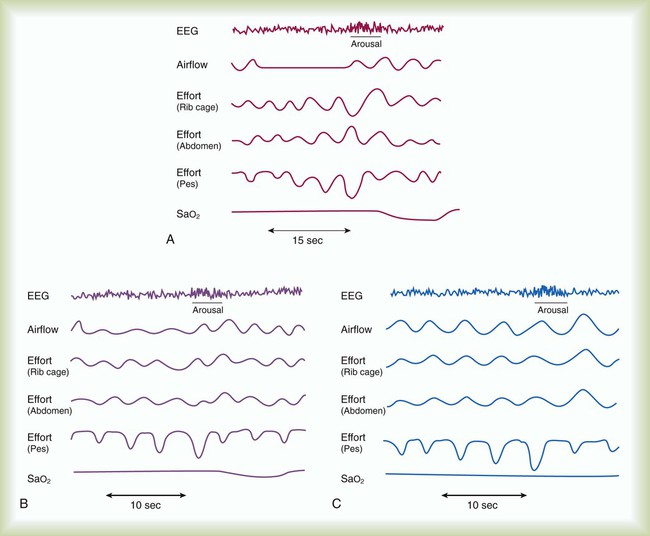
Obstructive sleep apnea (OSA) syndrome is a common clinical problem that is underdiagnosed.1 It is estimated that approximately 2% to 4% of adults have OSA.2 This prevalence is equivalent to asthma and diabetes in the general population. The spectrum of disease ranges from sleep disruption related to increased airway resistance to profound daytime sleepiness in conjunction with severe oxyhemoglobin desaturation, pulmonary hypertension, and right heart failure. The common feature in all variants of OSA syndrome is sleep disruption secondary to increased ventilatory effort that results in daytime hypersomnolence (Figure 30-1).3 Treatment decreases morbidity and mortality.
Sleep apnea is defined as repeated episodes of complete cessation of airflow for 10 seconds or longer. The events can be obstructive (caused by upper airway closure) or central (caused by lack of ventilatory effort). Primary central nervous system lesions, stroke, congestive heart failure, and high-altitude hypoxemia can diminish respiratory control and cause central apnea events.3 Central sleep apnea (CSA) is not as common as OSA. Only 10% to 15% of patients with sleep-disordered breathing are classified as having CSA.4 Mixed sleep apnea has an initial central component followed by an obstructive component (Figure 30-2).
Hypopnea is a significant decrease in breathing without complete cessation of airflow.5 Hypopnea is defined as a 30% decrease in airflow in conjunction with 4% oxygen (O2) desaturation.6 Most investigators agree that physiologically significant hypopnea is associated with a decrease in O2 saturation or arousal from sleep.7
Pathophysiology
Obstructive Sleep Apnea
The primary cause of OSA is a small or unstable pharyngeal airway. This condition can be caused by soft tissue factors, such as upper body obesity or tonsillar hypertrophy (rare in adults), and skeletal factors, such as a small or recessed chin.8 During the waking state, pharyngeal patency is maintained by increased activity of the upper airway dilator muscles. Sleep onset is associated with a decrease in the activity of these muscles. The result is airway narrowing or closure of airways that are at risk.9 In an unstable upper airway, narrowing and closure during sleep may involve multiple sites.10
Partial or complete closure of the upper airway during sleep has many serious neurobehavioral, metabolic, and cardiopulmonary consequences (Box 30-1). Compared with the general population, patients with untreated OSA have an increased risk of systemic and pulmonary hypertension, stroke, nocturnal arrhythmia, heart failure, and myocardial infarction.11,12 The repetitive cycle of upper airway closure and opening during sleep is believed to have effects on the autonomic nervous system, specifically, an increase in sympathetic tone.13 These effects are caused in part by episodes of hypoxemia and hypercapnia that are due to airway closure and hypoventilation that can occur throughout the night in patients with OSA. The arousals and microarousals during sleep also play an important role in the increase in sympathetic tone.13 Over time, increased sympathetic tone may result in systemic and modest pulmonary hypertension.14 Patients with OSA may have right ventricular hypertrophy and right heart failure if they are not treated.15,16
Obesity, especially of the upper body, has been found to correlate positively with the presence of OSA. In most instances, patients with OSA are obese with a large amount of peripharyngeal tissue and adipose tissue in the neck.17 A body mass index greater than 28 (>120% of ideal body weight normalized for height) should alert the practitioner to the possibility of OSA, particularly if the patient has excessive daytime sleepiness (EDS).2
Patients who are of normal body weight can be predisposed to OSA if they have an abnormal craniofacial configuration. Men often grow a beard to disguise such a craniofacial abnormality. If the chin is recessed (retrognathic) or small (micrognathic), the upper airway space may be narrow, and the risk of airway closure during sleep increases.2,8,14 Patients with a deviated nasal septum or trauma to the nasal passages may be predisposed to upper airway closure during sleep as a result of the increased resistive load to the upper airway. An isolated nasal abnormality is an unusual cause of OSA.
OSA may have a genetic predisposition.18 There have been reports of families in which obesity alone does not explain the increased prevalence of OSA.19 It has been postulated that craniofacial abnormalities and defects in ventilatory control explain the increased frequency of OSA in these families.
Central Sleep Apnea
Although a detailed discussion of the pathophysiology of CSA is beyond the scope of this chapter, several concepts are important to RTs. In contrast to OSA, which represents a spectrum of the same disease, CSA is a heterogeneous group of disorders. Patients have a ventilatory pattern known as periodic breathing, in which there is a waxing and waning of respiratory drive, which is reflected clinically as an increase and then a decrease in respiratory rate and tidal volume (VT). Cheyne-Stokes respiration, which often occurs in patients with congestive heart failure or stroke, is a severe type of periodic breathing characterized by a crescendo-decrescendo pattern of hyperpnea alternating with apnea. After apnea occurs, there may be an increase in central ventilatory drive and an increase in VT.3
Overlap Syndrome
Some patients with chronic obstructive pulmonary disease (COPD) have coexisting OSA. This combination is referred to as overlap syndrome.20 Patients are usually obese and have a history of smoking. They have moderate to severe nocturnal oxyhemoglobin desaturation secondary to both OSA and COPD. The worst desaturation values occur during rapid eye movement (REM) sleep and are related to the loss of accessory muscle use encountered in this physiologic state. Patients with overlap syndrome tend to have a worse prognosis and more severe blood gas abnormalities than patients with the same degree of OSA but without COPD.20 They may arrive in the intensive care unit with a “COPD exacerbation” and decompensated right heart failure. Undiagnosed OSA complicates the course at night with arousals, increased dyspnea, and O2 desaturation values resistant to supplemental O2.20
Clinical Features
Patients with sleep apnea are more commonly men (three times greater frequency than among women), are older than 40 years, and have hypertension (Box 30-2). Most patients with sleep apnea report habitual snoring that has become progressively worse.2,21 Sensations of nocturnal choking, gasping, or resuscitative snorting are frequently reported. If a bed partner observes periods of apnea, the diagnosis of OSA is highly likely.
Patients with OSA have arousals from sleep and sleep fragmentation, which can lead to fatigue, EDS, and irritability.22 Patients who have an increased frequency of awakenings and microarousals have more daytime sleepiness and greater difficulty with daytime functioning than the general population.23 Patients with OSA may have neuropsychologic deficits and impairment in vigilance.24 Compared with the general population, untreated OSA patients are at increased risk of motor vehicle accidents because of EDS.25–27
The physical examination of most patients reveals evidence of obesity, particularly in the upper body. Upper body obesity can be quantitated with neck size. A neck circumference of 42 cm (16.5 in) increases the likelihood of the diagnosis of sleep apnea.14 Examination of the oropharynx frequently reveals a long soft palate. Although tonsillar hypertrophy is common in children with sleep apnea, it is seldom found in adults. Large palatine tonsils may increase the risk of airway closure during sleep. A retrognathic or micrognathic mandible can narrow the pharyngeal airway, placing a patient of normal weight at risk of airway closure during sleep.8
The cardiovascular examination may reveal evidence of pulmonary hypertension or right heart failure (lower extremity edema).28,29 These findings are determined primarily by the hypoxic burden experienced by the patient. Pulmonary hypertension or right heart failure is more commonly encountered in patients with concomitant daytime hypoxemia. Patients with OSA and COPD or severe obesity (body mass index greater than 40) appear to be at particular risk of this complication.30 Recurrent moderate to severe oxyhemoglobin desaturation and resaturation secondary to OSA can be associated with an increased incidence of cardiac arrhythmia.31,32 Repeated nocturnal desaturation can be a cause of secondary polycythemia.14,33
OSA and poor sleep quality are also associated with metabolic syndrome independent of obesity.34,35 Metabolic syndrome includes three of the following: waist circumference 102 cm or greater in men or 88 cm or greater in women, hypertension, impaired glucose tolerance, insulin resistance, and elevated triglycerides.36,37 These interactions can also increase the patient’s cardiac risks and increased morbidity and mortality from cardiovascular disease.12,38
In the acute care setting, patients frequently present with previously undiagnosed OSA and can pose a particular challenge for diagnosis and management.39–41 A high clinical suspicion for OSA in the hospital setting is necessary because untreated or unrecognized OSA can complicate recovery from acute illness, trauma, heart failure, and recent surgery.42–45 Patients with known OSA are frequently not placed on continuous positive airway pressure (CPAP) while in the hospital or may require a temporary adjustment in pressure settings.46 Patients who are unstable for testing in a sleep laboratory can undergo portable bedside testing or empiric treatment with positive pressure if the diagnosis cannot be confirmed.47 Outpatient follow-up with confirmatory sleep evaluation is important for long-term treatment and compliance.
Laboratory Testing
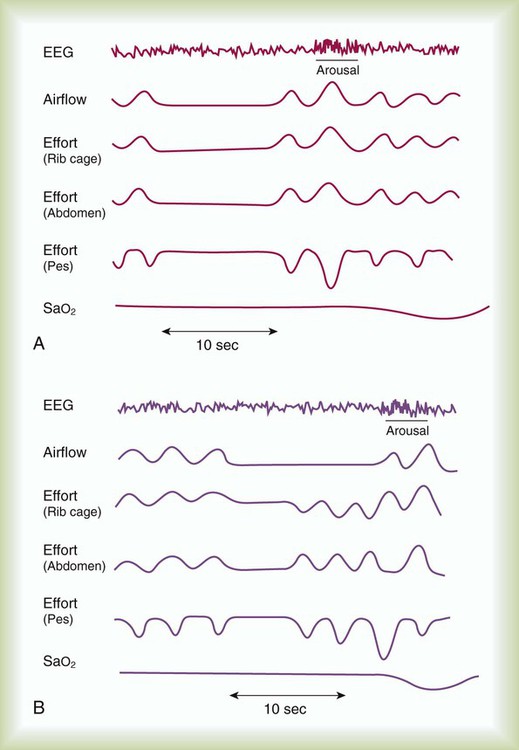
In obstructive apnea or hypopnea, airflow is absent or decreased in the presence of continued ventilatory effort. Asynchronous (paradoxical) movement of the abdomen and rib cage can be observed. O2 desaturation may or may not occur. The degree of the O2 desaturation depends on the length of the apneic event or the patient’s baseline saturation (see Figure 30-1). Respiratory effort–related arousals are characterized by increased respiratory effort, leading to arousal from sleep that does not meet the criteria of an apneic or a hypopneic event (see Figure 30-1).48,49
Measuring devices that are adequate for assessing hypopnea also are adequate for assessing apnea; however, devices used for measuring apnea cannot always detect hypopnea. The diagnosis of hypopnea may be affected by the measurement technique used. In 1999, an American Academy of Sleep Medicine (AASM) task force conducted an evidence-based review of measurement techniques for detection of hypopnea.50 The scoring system was as follows: A, good to excellent agreement with a reference standard (face mask pneumotachygraph); B, limited data, but good theoretical framework and clinical experience suggest the method is valid; C, no data, weak theoretical framework or clinical experience; and D, research or clinical experience suggests the method is invalid.
The measuring techniques were scored as follows: nasal pressure, B; respiratory inductance plethysmography (RIP) with sum of chest and abdominal signals, B; dual-channel RIP, C; single-channel RIP, C; piezoelectricity sensors, strain gauges, and thoracic impedance, D; breathing measurement signal with a desaturation or arousal, B; expired carbon dioxide (CO2), D; and thermal sensors, D. A face mask pneumotachygraph allows the greatest precision in measuring airflow, but it is poorly tolerated. Nasal pressure is a reliable way to detect hypopnea and is well tolerated by patients undergoing a diagnostic PSG.5,50
After the sleep study is completed, the sleep technologist scores it. The number of apneas and of hypopneas per hour of sleep are reported as an apnea-hypopnea index (AHI) or respiratory disturbance index (RDI). The AASM has operationally defined the severity of OSA as follows: mild, AHI 5 to 15; moderate, AHI 15 to 30; severe, AHI greater than 30. AHI less than 5 is considered within the normal range for adults. The number of arousals per hour (arousal index), percentage of each sleep stage, frequency of O2 desaturation, mean O2 saturation, and nadir of O2 saturation also are reported (Box 30-3).
Abbreviated (portable) cardiopulmonary testing has been used to confirm a diagnosis of OSA. These studies do not record the electrophysiologic signals (EEG, EOG, and EMG) required to stage and score sleep. The portable studies vary in the type and number of cardiopulmonary values recorded. Controversy exists whether portable systems are sufficient to diagnose OSA. Many variables, such as airflow, ventilatory effort, sleep stage, and O2 saturation values, may be less precise or may not be measured at all with these devices. Currently, portable monitoring for the diagnosis of OSA is acceptable in patients with high pretest probability but without significant comorbidities that may affect the accuracy of testing.51 Excerpts of American Association for Respiratory Care (AARC) Clinical Practice Guidelines for a PSG are provided in Clinical Practice Guideline 30-1.
Treatment
Management of OSA should be individualized but generally can be classified into three options: behavioral, medical, and surgical interventions.52 Behavioral therapy should be pursued in the care of all patients. Medical therapy and surgical therapy must be tailored to the individual patient. The likelihood of acceptance of and adherence to the prescribed therapeutic intervention must be considered. The goals of treatment are to normalize O2 saturation and ventilation; eliminate apnea, hypopnea, and snoring; and improve sleep architecture and continuity (Box 30-4).
Behavioral Interventions and Risk Counseling
Patients need to be informed of the risks of uncontrolled sleep apnea. Several behavioral interventions can be beneficial, including weight loss in obese patients; avoidance of alcohol, sedatives, and hypnotics; and avoidance of sleep deprivation. Although weight loss clearly influences the severity of sleep apnea, it is a difficult behavioral strategy to implement. Involvement of the patient with a dietitian or nutritionist can be helpful. Alcohol decreases the arousal threshold and as a result can increase the duration of apnea. Alcohol also reduces upper airway muscle tone, causing the airway to be more compliant and more prone to complete or partial closure.53 For these reasons, alcohol should be avoided by patients believed to have sleep apnea. Sedatives and hypnotics can decrease the stability of the upper airway and suppress certain stages of sleep.54
Positional Therapy
When a sleep study indicates that apnea and snoring occur only in the supine position, instruction on sleeping in the lateral position or head of bed elevation can be beneficial.55,56 Use of the “tennis ball” technique, in which a ball is sewn onto the back of the patient’s sleeping garment, or other positional devices that discourage the patient from rolling into the supine position can be effective in treating positional OSA.57 However, the long-term effects of positional therapy are unknown. Positional therapy is generally recommended for milder cases of positional OSA.
Medical Interventions
Positive Pressure Therapy
Continuous Positive Airway Pressure Therapy
Continuous positive airway pressure (CPAP) therapy was introduced for management of OSA in 1981.58 CPAP has become the first-line medical therapy for OSA. Numerous studies have documented the effectiveness of CPAP in decreasing the morbidity and mortality associated with OSA.9,11,59,60 For most patients, obstruction of the upper airway is abolished by CPAP pressures between 7.5 cm H2O and 12.5 cm H2O.61 The level of CPAP required for optimal management of OSA is best determined with a titration performed in the sleep laboratory.60 Attempts to use an algorithm or a prediction equation as a replacement for in-laboratory titration have not been uniformly successful.62
CPAP therapy has been shown to decrease daytime sleepiness and improve neurocognitive testing, vigilance scores, insulin sensitivity, and lipid profiles. CPAP decreases the incidence of pulmonary hypertension and right heart failure and decreases the number of ventilation-related arousals and nocturnal cardiac events. Reductions in daytime hypoxemia and hypercapnia also have been attributed to CPAP therapy.61,63–67
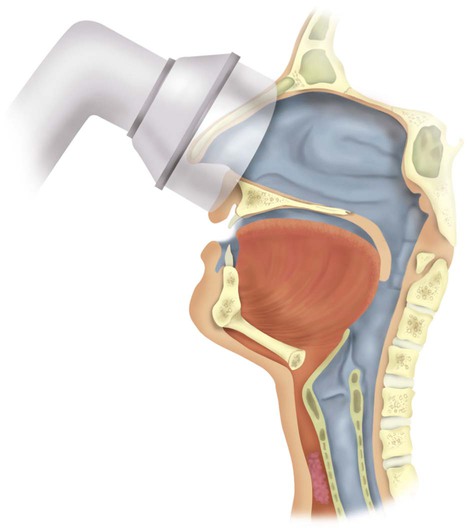
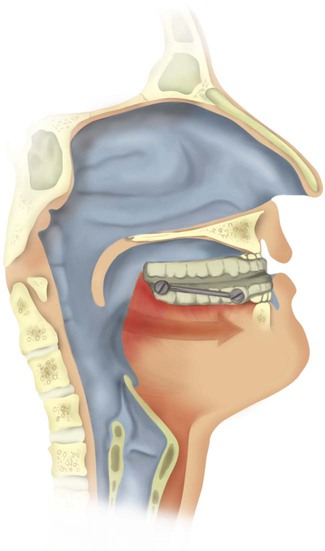
CPAP therapy primarily works by splinting the upper airway open, increasing the intraluminal pressure of the upper airway above a critical transmural pressure of the pharynx and hypopharynx that is associated with airway closure. The soft palate is effectively moved anteriorly up against the tongue, “pressurizing” the upper airway (Figure 30-3).68 CPAP allows the upper airway to be splinted open whether there is a single site (uncommon) or multiple sites (more common) of airway narrowing or closure. Investigators have found that when nasal CPAP is applied, EMG activity of the upper airway dilator muscles is decreased.69
To be successful, CPAP titration should obliterate all apneic episodes and reduce the number of hypopneic episodes for prevention of arterial O2 desaturation. Paradoxical thoracoabdominal movement and snoring should be eliminated.70 For improvement of sleep continuity, respiration-related EEG arousals and microarousals must be abolished. There is no evidence to support the misconception that a higher level of CPAP always is necessary in patients with severe sleep apnea. There is variability in the CPAP requirement to treat OSA effectively. Some patients with relatively mild elevation of the AHI need higher levels of CPAP than patients with a substantially higher AHI.71
Patients who report EDS without an increase in AHI may have repetitive 2- to 3-second transient EEG arousals during episodes of snoring. These short arousals occur during episodes of increased upper airway resistance, and although not associated with any significant arterial O2 desaturation, they may cause EDS and fatigue.72,73 This pattern is known as upper airway resistance syndrome, generally occurs in younger patients, and is characterized by respiratory effort–related arousals (see Figure 30-1). With the emergence of upper airway resistance syndrome as a clinical entity, some researchers have suggested that CPAP titrations may be suboptimal without measurement of esophageal pressure.71,74,75 Many sleep laboratories do not measure esophageal pressure. In addition, many patients refuse this type of monitoring because of perceived or real discomfort.
The contour of the inspiratory flow signal, when measured by a pressure transducer, correlates with ventilatory effort as reflected by esophageal pressure.48 When esophageal pressure is not used, nasal pressure can be useful in facilitating CPAP titrations.75 Condos and colleagues48 hypothesized that during CPAP titration, there is a period during the transition to deeper stages of sleep when there is flow limitation and increased intrathoracic pressure without EEG arousals. These investigators suggested that if this condition is not corrected, patients may have incomplete and suboptimal titrations. The clinical significance of flow limitation without EEG arousals is uncertain at the present time.
Despite numerous studies documenting the efficacy of CPAP in the treatment of patients in the sleep laboratory, clinicians have encountered difficulty with adherence of patients to CPAP therapy. Approximately 80% of patients accept CPAP, although long-term objective compliance is frequently suboptimal. Objective compliance—defined as use of the machine for more than 4 hours per night for more than 70% of observed nights—has been measured to be 46%.76,77 Severity of the AHI does not always correlate with compliance, and the benefit perceived by the patient is a better predictor. Data reported by McArdle and associates78 indicate that patients who are subjectively sleepy and have an AHI 30 or greater are likely to accept and comply with CPAP therapy. Clinic follow-up with objective compliance monitoring is essential. Compliance 1 month after the initiation of therapy is reported to be a good predictor of CPAP use at 3 months.
It is unclear whether higher levels of CPAP cause a decrease in compliance. Some patients report breathing against a continuous pressure to be uncomfortable. Discomfort with the interface and the device may also reduce acceptance and compliance.79–81 Since the introduction of CPAP, various interfaces have been designed to improve comfort and have a favorable impact on compliance. Nasal pillows or prongs, nasal masks with comfort flaps or bubbles, oronasal masks, and full-face masks are available.82–86 No studies have been conducted for direct comparison of efficacy, subjective patient comfort, or objective patient compliance with these interfaces.82 In clinical practice, some patients tolerate one interface better than another. Technician bias may affect the choice of an interface, and this may have a positive or negative impact.
Bilevel Pressure Therapy
Another form of positive pressure therapy is bilevel positive airway pressure (bilevel PAP). Bilevel PAP therapy was developed to take advantage of the fact that some patients may have different pressure requirements between inspiration and expiration.81 It was hypothesized that because a patient may have a lower expiratory pressure requirement to splint the airway open, patient acceptance and compliance would be favorably affected. Bilevel units operate on household electricity and are similar in size and appearance to conventional CPAP units. There is a difference in cost, however, with bilevel devices generally more expensive than CPAP devices.
Although patient acceptance may be slightly better with bilevel PAP, published data have shown no difference in compliance between conventional CPAP and bilevel PAP in patients who have not previously received CPAP therapy.87 However, bilevel PAP may be better tolerated by the subgroup of patients who need higher CPAP settings or who are uncomfortable exhaling against a continuous pressure.
Autotitrating Devices
A new generation of self-titrating CPAP devices has been developed to address issues of patient compliance, patient comfort, and variability of the CPAP requirement throughout the night.88–91 These devices are referred to as auto-CPAP, intelligent CPAP, or smart CPAP. These devices use a computer algorithm for adjusting the level of CPAP in response to dynamic changes in airflow or vibration secondary to snoring or both. Abnormal function manifests as snoring, hypopnea, and apnea. The average overnight pressure required to treat OSA effectively may be decreased, which may have a favorable impact on interface-related leaks. It is unknown whether these devices are capable of eliminating the need for standard CPAP titration in a sleep laboratory. Self-titrating devices may be useful in facilitating therapeutic CPAP titrations by technologists in the sleep laboratory but cannot be used as a surrogate for proper diagnostic testing.47 Further studies are needed to determine whether self-titrating CPAP devices provide any improvement over conventional CPAP units in the areas of compliance and EDS, in particular, in patients who have not previously received CPAP therapy.
Side Effects and Troubleshooting Strategies
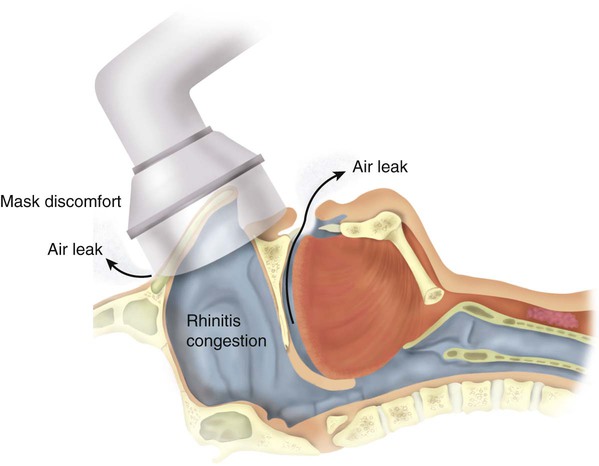
Side effects of positive pressure therapy are related to the interface and to the pressure prescribed. These effects include feelings of claustrophobia, nasal congestion, rhinorrhea, skin irritation, and nasal dryness (Figure 30-4). Claustrophobia and skin irritation can be managed by changing the interface to one that is more easily tolerated by the patient. Nasal congestion, rhinorrhea, skin irritation, and nasal dryness can be managed by use of combinations of topical nasal steroids, antihistamines, nasal saline sprays, and lotions. A humidifier can be used in-line with the machine. Heated humidification has been shown to improve compliance.92 If the patient has a sensation of too much pressure in the nose, adding a system equipped with a ramp may be beneficial.62 The ramp allows a gradual increase in pressure over 5 to 45 minutes. The ramp time is empirically determined by the prescribing physician. There is no objective evidence that a ramp feature improves patient acceptance or compliance.76
Pressure leaks are another problem RTs encounter. Most interfaces are of the nasal variety. Some patients tend to breathe partially or mainly through the mouth. The addition of a chin strap may not resolve the problem. Changing the interface to an oronasal mask may be required for effective “pressurization” of the upper airway in these patients.82
Oral Appliances
Oral appliances are devices that enlarge the airway by moving the mandible forward or by keeping the tongue in an anterior position (Figure 30-5). Patients who have mild sleep apnea and are unwilling to use CPAP may benefit from these devices. Oral appliances are worn only during sleep and come in various forms. The appliances are custom-fitted by dentists and are generally well tolerated by patients. They are overall less effective than CPAP therapy and are regarded as a second-line intervention, particularly for severe OSA.93,94
Medications
Medications have proved ineffective for most patients with sleep apnea. Benzodiazepines and other sedative-hypnotics should be avoided because they can potentiate upper airway collapse. The antidepressants protriptyline and fluoxetine have been used to manage mild sleep apnea but are ineffective in most patients.14 O2 therapy is useful for patients with oxyhemoglobin desaturation who refuse positive pressure therapy. O2 therapy can improve nocturnal desaturation but has no significant effect on ventilatory arousals and daytime sleepiness.95 O2 therapy should be used with caution by patients with concomitant severe COPD, who may retain CO2.
Surgical Interventions
Surgical alternatives can be divided into two broad categories: (1) procedures that bypass the upper airway and (2) procedures that reconstruct the upper airway (Box 30-5). Before the advent of CPAP therapy, tracheostomy was the primary therapy for severe OSA. Because of the psychosocial and medical morbidity associated with the procedure, use of tracheostomy today is limited to management of severe OSA when all other therapies have been exhausted.96,97
Palatal Surgery
Uvulopalatopharyngoplasty (UPPP) is palatal surgery performed with a standard “cold knife” technique or a laser. Portions of the soft palate, the uvula, and additional redundant tissue are removed in these procedures. The success rate of UPPP is reported to be less than 50% overall.98 The site of the physiologic obstruction cannot be predicted correctly with preoperative imaging. Laser-assisted UPPP has been marketed as an outpatient procedure; however, substantial efficacy in the management of OSA has not been documented. UPPP cannot be recommended for the management of OSA at the present time.99,100
Maxillofacial Surgery
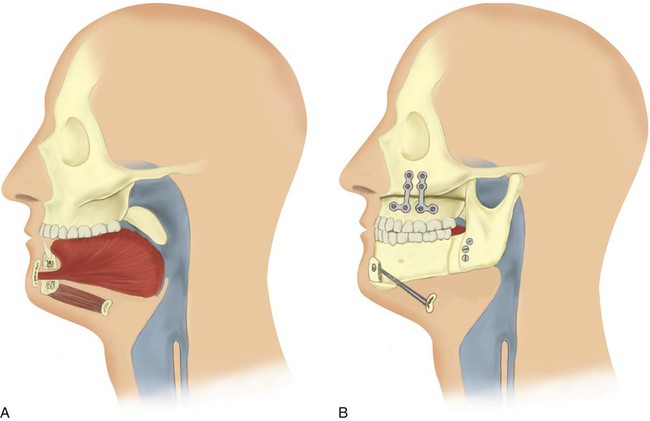
Maxillofacial surgery shows more promise for patients with OSA (Figure 30-6). Phase I surgical procedures combine UPPP with genioglossal advancement. Patients are identified preoperatively with a combination of radiologic imaging and direct visualization of the upper airway. It is beneficial to have these patients use CPAP therapy perioperatively to reduce the chronic upper airway swelling and edema present before surgery and to reduce postoperative airway edema.101 When phase I surgery is unsuccessful, phase II surgery involves advancement of the maxilla and the mandible.102 These surgical procedures are performed at only a few specialized centers. A coordinated effort by a dedicated team of otolaryngologists, oral surgeons, and sleep specialists is essential. Regardless of the surgical option chosen, a postoperative PSG should be obtained to document improvement objectively.103