CHAPTER 21 Disorders of male reproduction
Introduction
Physiology
Spermatogenesis
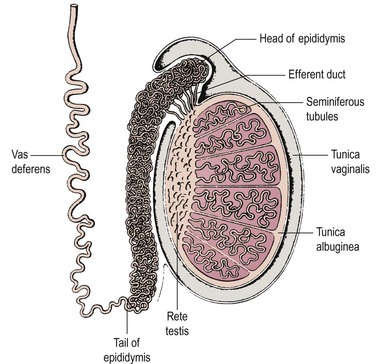
Spermatogenesis takes place in several hundred tightly coiled seminiferous tubules arranged in lobules (Figure 21.1) (Dym 1977), which constitute some 80% of testicular volume in man. Testis volume therefore reflects spermatogenesis more than testosterone production. Each tubule resembles a loop draining at both ends into a network of tubules, the rete testis, and thence into the epididymis, a single but highly coiled tube which, in turn, drains into the unconvoluted and muscular-walled vas deferens.
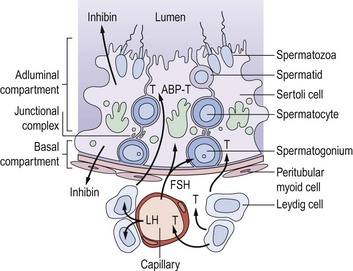
The walls of the seminiferous tubules are composed of germ cells and Sertoli cells around a central lumen, surrounded by peritubular myoid cells and a basement membrane (Figure 21.2). Spermatogenesis is a continuous sequence of closely regulated events, highly organized in space and time, whereby cohorts of undifferentiated diploid germ cells (spermatogonia) multiply and, while maintaining the population of stem cell spermatogonia, are then transformed into haploid spermatozoa. The following events can be observed in the seminiferous epithelium during normal spermatogenesis.
Sertoli cell function
Sertoli cells have extensive cytoplasm which spans the full height of the seminiferous epithelium from basement membrane to lumen (Figure 21.2). Where adjacent Sertoli cells come into contact with each other near the basement membrane, special occluding junctions are formed which divide the seminiferous epithelium into a basal (outer) compartment, which interacts with the systemic circulation, and an adluminal (inner) compartment enclosed by a functional permeability barrier, the blood–testis barrier (Figure 21.2). Spermatogonia divide by mitosis in the basal compartment, while the two reduction divisions of the spermatocytes and spermiogenesis are confined to the avascular microenvironment of the adluminal compartment created by the blood–testis barrier. The developing germ cells are therefore completely dependent on Sertoli cells for metabolic support. In response to appropriate trophic stimuli [of which follicle-stimulating hormone (FSH) and testosterone are the best described], Sertoli cells secrete a wide range of substances including growth factors and a distinctive tubular fluid high in potassium and low in protein which bathes the mature spermatozoa.
Sertoli cells contribute directly to the feedback regulation of pituitary gonadotrophin secretion. The existence of an endocrine product of the testis termed ‘inhibin’ was postulated for many decades. Inhibin B is a dimeric glycoprotein secreted by Sertoli cells which has a physiological role in the regulation of FSH secretion. Inhibin B concentrations reflect the functional activity of the seminiferous tubule and show a positive relationship with sperm production. Its production requires and reflects the interaction of the germ cell population with the Sertoli cells, and is absent in men with Sertoli cell only syndrome. The measurement of inhibin B may be of clinical value as a marker of the activity of the seminiferous epithelium (O’Connor and De Kretser 2004). Inhibin A, the product of the dominant follicle and the corpus luteum in women, is not present in the circulation in men.
Unlike the actively dividing germ cells, Sertoli cells do not proliferate in the adult testis. Spermatogenesis is a cyclical process which is critically dependent on changes in Sertoli cell function associated with the constantly changing combination of germ cells in contact with its cytoplasm. Changes in the germ cell complement in contact with any one Sertoli cell occur at a fixed sequence and interval. Thus, the synchronization of these repetitive cyclical changes in Sertoli cell function, associated with the variations in germ cell metabolic requirements as they divide and differentiate, has now become one of the central tenets of our conceptualization of normal spermatogenesis (Sharpe 1990). Although pituitary gonadotrophins provide obligatory trophic support for testicular function as a whole, the classic concept that luteinizing hormone (LH) stimulates Leydig cell steroidogenesis and FSH controls functions in the seminiferous tubules is far too simplistic in the light of our current understanding of spermatogenesis. There is now good evidence that the interstitial and tubular compartments are not functionally distinct, but that there is a close and complex inter-relationship between them. Thus, testosterone from the interstitial Leydig cells stimulates Sertoli cell functions either directly or via the peritubular cells, as does Leydig-cell-derived insulin-like factor 3, itself critical for testicular descent (Ivell et al 2005). Altered tubular/Sertoli cell function, on the other hand, can induce changes in Leydig cell steroidogenesis, although the identity of the intercompartmental regulator(s) is unknown.
Hormonal Control of Spermatogenesis
The hormonal control of spermatogenesis requires the actions of the pituitary gonadotrophins LH and FSH. There is general agreement that both LH and FSH are needed for the initiation of spermatogenesis during puberty. However, the specific roles and relative contributions of the two gonadotrophins in maintaining spermatogenesis are unclear (Liu et al 2002).
FSH initiates function in immature Sertoli cells, prior to the onset of spermatogenesis, by stimulating the formation of the blood–testis barrier and the secretion of tubular fluid and other specific secretory products via FSH receptors which activate intracellular cAMP. Once spermatogenesis is established in the adult testis, Sertoli cells become less responsive to FSH. Evidence for the non-essential role of FSH is provided by individuals with inactivating mutations of the FSH receptor. Such men have been documented to have complete spermatogenesis but with low sperm concentrations (Tapanainen et al 1997). However, it can be shown, in animals immunized against FSH and in experimentally induced hypogonadotrophic men given gonadotrophin replacement, that both testosterone (depending on LH) and FSH are required for quantitatively normal spermatogenesis in the adult (testosterone-replete) testis by determining the number of spermatogonia available by meiosis. However, testosterone on its own can maintain qualitatively normal spermatogenesis once it has been initiated (Matsumoto et al 1984). FSH therefore acts either by increasing spermatogonial mitosis or by decreasing the number of cells that degenerate at each cell division. Testosterone is essential for the subsequent stages from meiosis to spermiogenesis.
Hypothalamic–pituitary–testicular axis
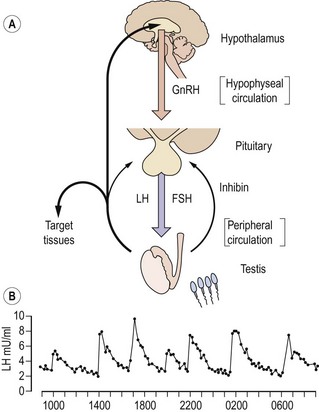
The secretion of gonadotrophins from the anterior pituitary gland is controlled by gonadotrophin-releasing hormone (GnRH) released into the pituitary portal circulation from axon terminals in the hypothalamic median eminence. These neurosecretory neurones in the medial basal hypothalamus are responsive to a wide variety of sensory inputs as well as to gonadal negative feedback. GnRH stimulates the secretion of both LH and FSH. In the adult male, GnRH is released episodically into the pituitary portal circulation at a frequency of approximately every 140 min; each volley of GnRH elicits an immediate release of LH, producing the typical pulsatile pattern of LH in the systemic circulation (Figure 21.3; Wu et al 1989). Although also secreted episodically, FSH and testosterone pulses are not apparent in normal men because of the slower secretion of newly synthesized rather than stored hormone and the longer circulating half-lives. The intermittent mode of GnRH stimulation, within a narrow physiological range of frequency, is obligatory for sustaining the normal pattern of gonadotrophin secretion. Continuous or high-frequency GnRH stimulation paradoxically desensitizes the pituitary gonadotrophin response in men as in women, because of depletion of receptors and refractoriness of postreceptor response mechanisms. It has recently been demonstrated that the secretion of GnRH is dependent on the newly-described kisspeptin (also known as ‘metastin’). Mutations of the kisspeptin receptor result in pubertal failure. Kisspeptin-containing neurones impinge directly on GnRH neurones in the hypothalamus, and there is emerging evidence that they also mediate effects of metabolic signals such as leptin on the reproductive axis (Seminara and Crowley 2008).
Testosterone exerts the major negative feedback action on gonadotrophin secretion. Its effect is predominantly to restrict the frequency of GnRH pulses from the hypothalamus to within the physiological range. Testosterone also acts on the pituitary to reduce the amplitude of the LH response to GnRH. It is now recognized that these inhibitory effects on GnRH and gonadotrophin secretion are, in part, mediated following conversion of testosterone to oestradiol by the enzyme P450 aromatase. This is demonstrated both by administration of aromatase inhibitors to normal men and by the finding that men with either mutant, non-functional oestrogen receptors or absent aromatase activity have markedly elevated gonadotrophin concentrations despite high-normal testosterone concentrations (Smith et al 1994, Morishima et al 1995). Interestingly, these men also showed marked osteoporosis, suggesting a distinct role for oestrogen in bone. Feedback inhibition of pituitary FSH synthesis is predominantly mediated by inhibin B and also by testosterone, particularly at high concentrations. The regulation of FSH secretion by inhibin in addition to testosterone results in the selective rise in FSH but not LH concentrations in men with various disorders of spermatogenesis.
The spermatozoon
The primary function of the spermatozoon is the delivery of a male pronucleus to the fertilized egg. The spermatozoon must conserve its DNA and transport it to the site of fertilization, where it must recognize and fuse with a receptive egg. The ejaculated spermatozoon must first escape from the seminal plasma in which it is deposited beside the cervix, and penetrate the barrier presented by cervical mucus. It must then travel through the uterus to the site of fertilization in the fallopian tube. During this journey, it must complete the process of functional maturation known as ‘capacitation’, an ordered series of events involving reorganization of cell surface components and changes in cellular metabolism and motility patterns, which are a prerequisite for successful fertilization. Having reached the oviduct, the male gamete must recognize the oocyte, penetrate the cumulus oophorus and bind to the zona pellucida. At this point, it must display a unique pattern of movement known as ‘hyperactivated motility’ and undergo the acrosome reaction. This process is initiated by a specific protein component of the zona pellucida (ZP3) and results in release of the contents of the acrosomal matrix, which include the serine protease acrosin and other hydrolytic enzymes including hyaluronidase. In addition, the acrosome reaction results in the generation of a fusogenic equatorial segment, which is the zone of fusion with the oocyte plasma membrane. For a review of sperm structure and function, the reader is referred to Grudzinskas (1995).
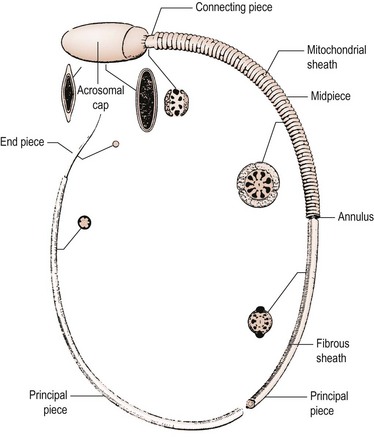
To enable it to undertake these complex functions, the human spermatozoon has developed a highly specialized morphology, with its various structural components tailored to specific functional attributes. The appearance of the spermatozoon was first described over 300 years ago by Anthony van Leeuwenhoek. In outline, the spermatozoon has a dense oval head capped by an acrosome and is propelled by a motile tail (Figure 21.4; Fawcett 1975). The head is made up largely of highly condensed nuclear chromatin constituting the haploid chromosome complement, complexed with highly basic proteins termed ‘protamines’. It is covered in its anterior half by the acrosome, a membrane-enclosed sac of enzymes including acrosin and hyaluronidase. The area of the sperm head immediately behind the acrosome (the equatorial region) is important as it is this part which attaches to and fuses with the egg. The shape of the human sperm head is highly pleomorphic, making the morphological definition of a normal sperm head extremely challenging. Behind the head may be found a cytoplasmic droplet which consists of the remains of the residual cytoplasm left after the morphological remodelling of the cell during spermiogenesis.
Sperm transport and maturation
The cervical canal is the first selective filtering barrier to meet the ejaculated sperm (Figure 21.5). This barrier is virtually complete except during midcycle, when oestrogenized cervical mucus glycoprotein fibrils form parallel chains called ‘micelles’ which permit spermatozoa with active progressive motility to swim through at a rate of 2–3 mm/min. Spermatozoa probably enter the uterine cavity from the internal os by virtue of their own motility, and appear in the uterine cavity approximately 90 min after insemination. The uterotubal junction is the second of the major physical barriers for spermatozoa. The mechanism for selectivity is not clear, but may depend on factors other than sperm motility since inert particles can pass through. Once the uterotubal junction has been successfully negotiated, a minority of sperm immediately traverse the oviduct to the ampulla; however, the majority congregate in the isthmus until ovulation has occurred. At this time, capacitated sperm showing hyperactivated movements of the tail gradually progress towards the fimbriated end, helped on by the muscular contraction of the oviduct wall and the flow of fluid in the oviduct. A maximum number of spermatozoa is present in the cervix 15–20 min following insemination and remains constant for 24 h, although a rapid decline has commenced by 48 h. Some spermatozoa may remain motile at the site of fertilization for up to 3 days (Mortimer 1995).
Male reproductive ageing
The decline in function of the endocrine output of the hypothalamo–pituitary–testicular axis with age is well established and results in a fall of approximately 50% in plasma testosterone concentrations (Vermeulen 1991). This has both central and peripheral components; thus, there is a relatively small increase in LH. The ‘andropause’ is increasingly the subject of investigation (Wu 2007), with large placebo-controlled studies of the effect of replacement under way. Changes in Sertoli cell function and spermatogenesis with ageing have received more limited investigation, but the data do suggest that there is indeed an age-related decline in function. Spermatogenesis, however, may be well maintained in elderly men.
Male Infertility
Definition and epidemiology
Infertility is commonly defined as the failure of conception after at least 12 months of unprotected intercourse (Rowe 1993), but such a definition serves to obscure the true complexity of the clinical situation. In reality, those couples who fail to achieve a pregnancy within 12–24 months include those who can be considered sterile (i.e. who will never achieve a spontaneous pregnancy) and those who are more properly termed ‘subfertile’ and who have reduced fecundability (probability of achieving a pregnancy within one menstrual cycle) and hence a prolonged time to pregnancy. Accurate assessment of the prevalence of infertility has always been difficult because of the scarcity of large-scale, population-based studies. Estimates suggest that some 14–17% of couples may be affected at some time in their reproductive lives (Hull et al 1985), with European data suggesting that as many as one in four couples who try may experience difficulties in conceiving (Schmidt 2006).
While infertility is relatively common, it is very difficult indeed to establish the relative contribution of the male partner, given the profound difficulties which exist in the accurate diagnosis of male infertility. Most studies which have attempted to evaluate the aetiology of infertility have used the conventional criteria of semen quality, promulgated by the World Health Organization (WHO) (World Health Organization 1999), to define the ‘male factor’. Although of great importance and shortly to be updated, these criteria are of limited diagnostic value, and a significant proportion of men with normal conventional criteria of semen quality will be infertile because of defects in sperm function, while a significant number of men with abnormal semen quality will have normal sperm function. Very few studies on the epidemiology of male infertility have used functional, as opposed to descriptive, diagnostic criteria. Nevertheless, one common theme to emerge is that, using the available diagnostic techniques, male factor infertility is, in many studies, the most common single diagnostic category.
Aetiology
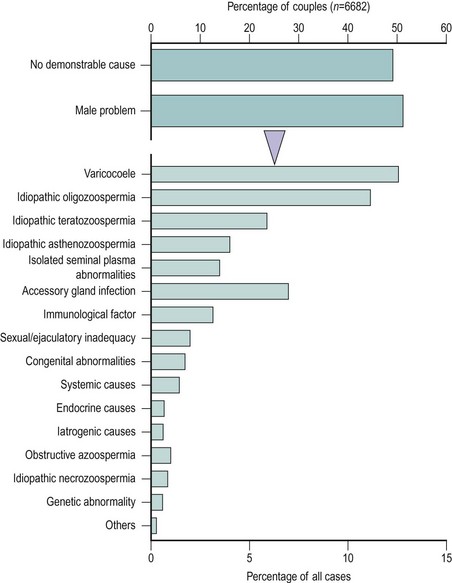
Notwithstanding the difficulties in diagnosis outlined above, WHO has proposed a scheme for the diagnostic classification of the male partner of the infertile couple (Rowe 1993) (Box 21.1). This approach is of enormous value as a basis for standardization and for comparative multicentre studies. However, many of the male diagnostic categories are of a descriptive nature (e.g. idiopathic oligozoospermia) or of controversial clinical relevance (e.g. male accessory gland infection). Moreover, recent advances in our understanding of the causes of male infertility, particularly in the area of genetic problems (Hargreave 2000), mean that this classification is in need of review. The relative frequency of the major diagnostic categories is shown in Figure 21.6, using data taken from a WHO study of over 8500 couples from 33 centres in 25 countries (Comhaire et al 1987). It can be seen that the largest single male ‘diagnostic’ category was men with seminal abnormalities of unknown cause. Beyond this, varicocoele was a relatively common pathology, as was male accessory gland infection; however, systemic, iatrogenic and endocrine causes were very infrequent.
Box 21.1
Diagnostic categories for the male partner of an infertile couple according to WHO
| No demonstrable cause | Systemic causes |
| Idiopathic oligozoospermia | Endocrine causes |
| Idiopathic asthenozoospermia | Iatrogenic causes |
| Idiopathic teratozoospermia | Congenital abnormalities |
| Idiopathic azoospermia | Acquired testicular damage |
| Obstructive azoospermia | Varicocoele |
| Isolated seminal plasma abnormalities |
Source: Rowe PG 1993 WHO Manual for the Standardized Investigation and Diagnosis of the Infertile Couple. Cambridge University Press, Cambridge, UK.
Genetic causes
Perhaps the most striking advances in our understanding of the aetiology of male infertility in the past decade have been in the area of genetics. Many of the ‘systemic’ disorders commonly associated with male infertility (see below) are now understood to have a genetic basis, and as our knowledge of the aetiology of disease expands, this will be increasingly the case. Traditionally, genetic causes of male infertility have been sought at the level of chromosomal abnormalities, with chromosomal abnormalities being detected in between 2.1% and 8.9% of men attending infertility clinics (Chandley 1994). The frequency of chromosomal abnormalities increased as sperm concentration declined, with abnormal karyotypes being found in 15% of azoospermic patients, 90% of whom had Klinefelter’s syndrome (47XXY), which accounted for half of the entire chromosomally abnormal group. In oligozoospermic patients, the incidence of chromosome abnormalities was 4%. However, it has been recognized for some time that structural anomalies of the Y chromosome, resulting in deletion of the distal fluorescent heterochromatin in the long arm, are associated with severe abnormalities of spermatogenesis. More recent studies have defined a family of genes on the Y chromosome involved in spermatogenesis, and it has become clear that a little over 10% of cases of non-obstructive azoospermia may have deletions affecting these genes. A proportion of cases of very severe oligozoospermia may have a similar aetiology. Microdeletions have been found in three non-overlapping regions of the Y chromosome, AZF a-b-c. Several genes have been described and these include RBM, DAZ, DFFRY, DBY and CDY. The abnormality most commonly reported in the literature is a microdeletion in the AZFc region and encompassing the DAZ gene. However, there is no exact correlation between DAZ deletion and the presence or absence of spermatogenesis, but this may be because of an autosomal copy of the DAZ gene (Hargreave 2000). DNA fragmentation has also been identified as the cause of defective sperm function (Tarozzi et al 2007). The ability of microassisted fertilization to overcome severe deficits in spermatogenesis has reinforced the importance of understanding and investigating genetic causes of male subfertility, as these will now be transmitted to the next generation of males.
Cryptorchidism
Undescended testis is a good example of a condition present at birth, and presumed to have its origins in intrauterine life, which is significantly associated with an increased risk of impaired spermatogenesis in later life and with an increased risk of testicular cancer (Irvine 1997). The testis which is not in a low scrotal position by the age of 2 years is histologically abnormal; spontaneous descent rarely occurs after 1 year and there is little evidence that surgical orchidopexy for an undescended testis after 2 years of age improves fertility. For these reasons, treatment should ideally be undertaken between 1 and 2 years of age. Evidence suggests that fertility may also be impaired in boys with retractile testis who experience spontaneous descent during puberty. Apart from the association with infertility, cryptorchidism is a well-established risk factor for testicular cancer, the risk of which in a patient with a history of undescended testis, whether successfully treated or not, is four to 10 times higher than in the general population.
Orchitis
Symptomatic orchitis occurs as a complication in 27–30% of males over the age of 10–11 years who suffer from mumps. In 17% of cases, orchitis is bilateral and seminiferous tubular atrophy is a common sequela of mumps orchitis, although recovery of spermatogenesis even after persistent azoospermia for 1 year has been reported (Sandler 1954). The prevalence of infertility after mumps orchitis is unknown, but fertility should only be impaired significantly if the orchitis is bilateral and occurs after puberty.
Varicocoele
The subject of varicocoele has generated controversy amongst the andrological community since the Edinburgh urologist Selby Tulloch first reported the apparently beneficial effects of treatment (Tulloch 1952). The available evidence certainly suggests that varicocoele is a common pathology and that it is more common in men with lower sperm counts. The diagnosis of visible (grade 3) and palpable (grade 2) varicocoeles is not difficult when the patient is examined in a standing position. The detection of subclinical (grade 1) varicocoeles, where spermatic vein reflux can only be detected during the Valsalva manoeuvre, requires more experience and has been aided by the use of Doppler or scrotal thermography. Prevalence figures from 5% to 25% have been reported in surveys of apparently healthy men (Hargreave 1994). In contrast, amongst men attending infertility clinics, varicocoele affects some 11% of men with normal semen and 25% of men with abnormal semen (World Health Organization 1992). The difficulty has been in establishing with certainty whether or not varicocoele affects spermatogenesis and, most importantly, whether or not treatment of varicocoele improves fertility, and if so, in which groups of men. It seems clear that varicocoele is associated with abnormal semen quality, and while the mechanism of this relationship remains to be established with certainty, abnormal testicular temperature regulation is known to be associated with varicocoele and impairment in semen quality.
Whatever the pathophysiology, there is a body of evidence suggesting that varicocoele causes progressive testicular damage, further complicating an assessment of its role in the aetiology of male infertility. Substantial controversy exists, however, over the question of whether or not the correction of varicocoele improves fertility (Evers et al 2008). Most of the appropriately designed controlled studies suggest that treatment is clearly associated with an improvement in semen quality; however, when the achievement of pregnancy is used as the endpoint, some studies find treatment to be effective while some suggest that it is of no benefit.
Occupational and environmental factors
The actively dividing male germ cells are one of the most sensitive cell types in the body with regard to the toxic effects of radiation, cytotoxic drugs and an increasing number of chemicals. Indeed, male gonadal function may be one of the most sensitive indices of overexposure to potential toxins (in the workplace, environment, foods, cosmetics and medicines) (Sharpe 2000, Bonde and Storgaard 2002). Data on occupational hazards to male reproduction remain controversial. Exposure to heavy metals, such as cadmium, lead, arsenic and zinc, has been reported to impair spermatogenesis, although the data are conflicting. Certain pesticides and herbicides have more clearly been shown to be toxic to spermatogenesis, as have some organic chemicals. The best documented modern example is the pesticide dibromochloropropane, which was responsible for azoospermic infertility in half of the male workers in a factory. There would seem to be clear evidence that occupational or environmental exposure to heat will have adverse consequences for spermatogenesis and will prolong time to pregnancy (Thonneau et al 1996). Recreational drugs such as cigarettes, alcohol and cannabis have all been linked with lower semen quality, and there is conflicting evidence on whether or not dress habit has a significant effect.
Recent data have demonstrated that male reproductive health is deteriorating, with evidence of a secular decline in semen quality (Carlsen et al 1992, Auger et al 1995, Irvine 1996), an increase in the incidence of congenital malformation of the male reproductive tract and an increase in the incidence of testicular cancer; however, there is, as yet, no evidence that these changes are having an influence on the prevalence of male infertility.
Genital tract obstruction
The most common congenital abnormality is agenesis or malformations of the Wolffian-duct-derived structures: the corpus/cauda epididymis, vas deferens and seminal vesicles. Diagnosis is usually quite easy; the scrotal vasa are not palpable and the ejaculate consists of low volumes (>1 ml) of acidic non-coagulating prostatic fluid devoid of fructose and sperm. Congenital bilateral absence of the vas deferens is associated with CFTR mutations and is found in approximately 2% of men with obstructive azoospermia (Hargreave 2000). In recent years, increasing numbers of mutations of the CFTR gene have been characterized and more than 400 have been described. In general, the more mutations tested for, the higher the percentage of men found to have mutations. As such, in more recent publications, detection rates have been almost 70–80%.
Immunological causes
Suspected immunological infertility was found in some 3% of couples in a WHO survey on the basis of the finding of 10% or more of motile spermatozoa coated with antibody using assays such as the Immunobead test or the mixed antiglobulin reaction. Whilst antisperm antibodies are found in perhaps one in six of the male partners of infertile couples, a prevalence which is higher than that for fertile controls, their effect on fertility is hard to determine. Some studies suggest that ‘antibody-positive’ couples conceive at a lower rate than those without immunological problems. Unfortunately, antibodies to sperm surface antigens are also found in fertile control populations, and current techniques do not permit the meaningful separation of cases with autoimmunity to biologically relevant epitopes (Paradisi et al 1995). Given the consensus view that assisted conception is the treatment of choice, this may not now be a clinically relevant issue.
Gonadotrophin deficiency
Gonadotrophin treatment of these syndromes is discussed below. Androgen treatment is required by hypogonadal men for long-term replacement, and is generally given by injection of testosterone esters every 3–4 weeks, aiming to maintain plasma testosterone concentrations in the physiological range. This is made more difficult by the high peaks and low troughs following administration of these preparations; monitoring of dosage should take place immediately before subsequent injection (i.e. at the trough). Other preparations include the orally active testosterone undecanoate, transdermal patches, gels and subcutaneous testosterone pellets. Undecanoate is largely confined to paediatric practice for pubertal induction as circulating concentrations tend to be low. Testosterone patches frequently cause skin irritation, although this is not a problem with the gels as these do not contain the enhancers necessary to promote absorption across the skin. New injectable esters are becoming available with a longer duration of action, with up to 3 months between administration (Srinivas-Shankar and Wu 2006). Testosterone will restore sexual interest and activity, and penile erections during and on waking from sleep. Other symptoms of testosterone deficiency include tiredness, irritability and loss of body hair. Testosterone will not induce or improve fertility, and there is no place for androgen treatment of men wishing to conceive.
Idiopathic impairment of semen quality
‘Teratozoospermia’ is the term used to describe altered morphology. Surface morphology directly reflects the maturity and functional integrity of the spermatozoa so that morphological analysis of ejaculated sperm is an important means of assessing spermatogenesis in the testis. Indeed, some workers believe that sperm morphology is the best predictor of spontaneous fertility or the outcome of IVF (Kruger et al 1995). It has been reported that morphology in the individual spermatozoon is related to movement characteristics (swimming velocity, sperm head trajectories, flagellar beat frequency) and its ability to exhibit hyperactivation. Similarly, the ability to undergo the acrosome reaction has also been shown to be significantly higher in sperm with morphologically normal, compared with abnormal, sperm heads. Ultrastructural studies have also revealed a variety of structural malformations of the acrosome complex, the most extreme example being the round-headed sperm where the acrosome is completely missing, but lesser degrees of acrosomal defects are increasingly being identified. These attempts to relate specific functional defects to recognizable structural malformations in individual spermatozoa provide evidence that morphologically abnormal sperm are also functionally impaired.
Clinical Management
Recent advances in assisted conception technology have revolutionized the management of couples with male factor infertility, and have advanced our understanding of the aetiology of male infertility by drawing attention to the major contribution of genetic factors. Paradoxically, they have also encouraged a minimalist clinical approach to the diagnosis of men with fertility problems, given the limited range of effective therapeutic options. The dangers of this approach have been highlighted in the light of existing concerns over the safety of microassisted fertilization (Cummins and Jequier 1994).
Many clinicians endeavour to manage male infertility at a distance, a request for semen analysis preceding, even substituting for, taking a history from and performing a physical examination of the male partner. Those who take this attitude do their patients a disservice; they foster the idea that the male contribution to infertility is limited and while a semen analysis will only occasionally provide the clinician with a diagnosis, a careful history and examination may identify the cause of a couple’s infertility. A number of significant features of the clinical history, together with their associated rates of azoospermia or abnormal semen quality, are shown in Table 21.1.
Table 21.1 Features in the clinical history with an influence on male fertility and their associated rates of azoospermia and abnormal semen quality
| Feature | % of cases with azoospermia | % of cases with abnormal semen |
|---|---|---|
| Diabetes mellitus | 16.7 | 60.0 |
| Bronchiectasis | 32.0 | 82.4 |
| Higher fever | 5.2 | 64.4 |
| Long-term medication | 12.3 | 52.9 |
| Urinary tract infection | 8.9 | 60.1 |
| Sexually transmitted disease | 7.6 | 50.4 |
| Epididymitis | 17.3 | 70.2 |
| Testicular injury | 12.6 | 56.9 |
| Testicular torsion | 18.2 | 88.9 |
| Unilateral maldescent | 20.8 | 65.7 |
| Testicular maldescent (bilateral) | 40.8 | 75.9 |
| Mumps orchitis (bilateral) | 22.0 | 84.4 |
| Excessive alcohol consumption | 9.6 | 51.8 |
From Comhaire FH, de Kretser D and Farley TMM Towards more objectivity in diagnosis and management of male infertility. Results of a WHO Multicentre Study. International Journal of Andrology 1987; 10(S7): 1–53.
History
Infertility is, of course, the problem of a couple and must be managed as such, with the history being taken from both partners together. The duration of the present union and the duration of infertility complained of should be established at the outset, together with the history of any pregnancies for which the individual may have been responsible. In the patient’s past medical history, areas which should receive special attention are a history of mumps virus infection, the age at which this occurred and whether or not there was an associated orchitis. As can be seen from Table 21.1, 84% of men with a history of bilateral mumps orchitis have abnormal semen and 22% are azoospermic. Diabetes mellitus and certain neurological diseases are known to be associated with ejaculatory disturbances, and a history of these or of any other systemic illness should be sought, as should a history of recent pyrexial illness as this may compromise spermatogenesis for many weeks. A history of respiratory disease should be sought carefully, including recurrent respiratory tract infections, sinusitis, bronchiectasis or cystic fibrosis, as these conditions can be associated with ciliary dysfunction and therefore with impaired sperm motility, as in Kartagener’s syndrome, or with obstructive azoospermia, as in Young’s syndrome. As many as 82% of men with a history of bronchiectasis have abnormal semen and 32% are azoospermic. Parasitic diseases, such as schistosomiasis and filiariasis, are rare but must be borne in mind as potential causes of excurrent duct obstruction and prostatovesiculitis.
Examination
Turning to the urogenital examination, the penis is examined for evidence of phimosis, hypospadias, epispadias or the characteristic plaques of Peyronie’s disease. The scrotum should be examined and the site of the testes determined, following which the testicular volume in millilitres should be determined with the aid of a Prader orchidometer (Figure 21.7) and their consistency evaluated. It is known that a clear relationship exists between testicular volume and sperm production. Any tenderness of the gonads should be noted and the epididymides carefully palpated from caput to cauda to exclude thickening, tenderness, cystic lesions, atrophy or absence of the epididymides. The vasa deferentia should next be palpated to establish that they are not congenitally absent and any thickening or induration should be noted. Scrotal swellings, such as hydrocoele or hernia, should be noted and the presence and grade of varicocoele should be established by asking the patient to perform Valsalva’s manoeuvre. The inguinal regions should be inspected for hernia, scarring or the presence of lymphadenopathy. A rectal examination should be performed to assess the state of the prostate and seminal vesicles, although this seldom provides useful information and may be omitted unless ejaculatory duct obstruction or prostatovesiculitis is suspected.
Semen analysis
The conventional criteria of semen quality have changed little since van Leeuwenhoek first described spermatozoa in the human ejaculate in 1685. A standard semen analysis, performed according to clearly established guidelines promulgated by WHO (World Health Organization 1999) (Box 21.2), provides descriptive information concerning sperm number, motility and morphology, together with aspects of the physical characteristics of the ejaculate.
Box 21.2
Normal values of semen analysis
| Volume | 2.0 ml or more |
| pH | 7.2–7.8 |
| Sperm concentration | 20 × 106/ml or more |
| Motility | 50% or more with progressive motility (grade 1 + 2) within 60 min of ejaculation |
| Morphology | Normal morphology below 15% is associated with reduced fertilization rates in the context of IVF |
| Viability | 75% or more live |
| White blood cells | Fewer than 1 × 106/ml |
| Mixed antiglobulin reaction test | Fewer than 10% spermatozoa with adherent particles |
Source: World Health Organization 1999 WHO Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction. Cambridge University Press, Cambridge.
Microscopic evaluation
Morphology
In addition to the above, a number of supplementary procedures may be undertaken in the evaluation of human semen, including semen culture, and biochemical evaluation for quantitation of acid phosphatases, citric acid and zinc (as markers of prostatic function), fructose and prostaglandins (as markers of seminal vesicular function) and free L-carnitine (as a marker of epididymal function). WHO has issued a statement of the commonly accepted normal values for the parameters discussed above (Box 21.2), although making the point that it is preferable for each laboratory to establish its own normal ranges for each variable, by evaluating semen from individuals of proven fertility amongst its own local population. It is anticipated that a revised analysis of sperm norms will be published soon by WHO.
Antisperm antibodies
The mixed agglutination reaction uses sheep red blood cells coated with rabbit antibodies to specific classes of human immunoglobulins, which will attach to motile sperm carrying immunoglobulins of the same class on their surface membrane (Bronson et al 1984). This permits the detection of immunoglobulin gamma A, G or M on the surface of the sperm head or tail. The direct test uses washed sperm from the patient, and the presence of surface-bound antibody, indicated by particulate binding in over 10% of spermatozoa, is considered to be a positive result. It depends on the availability of sufficient numbers of motile sperm in the patient’s fresh semen sample and is currently the standard screening test in most laboratories. The indirect test uses decomplemented patient serum or seminal plasma which is incubated with motile donor sperm, and is known as the ‘tray agglutination test’. Antisperm antibodies will bind to donor sperm and their presence is detected by attachment of particles to the sperm surface. The indirect test is therefore more convenient for screening larger numbers of patients. A positive screening test, however, must be substantiated by investigations to assess the biological significance of sperm antibody.
Chromosome analysis
Chromosome karyotyping should be carried out in patients with azoospermia or severe oligospermia with reduced testicular volumes and elevated FSH. Cytogenetic abnormalities, by far the most common being Klinefelter’s syndrome, may be detected in approximately 10% of this group and are of importance if treatment by ICSI is being contemplated. Although still only research, screening for microdeletions on the Y chromosome is likely to enter routine clinical practice in the near future (Krausz and Degl’Innocenti 2006).
Treatment
General measures
Although much has been written about the nature of general advice which should be given, objective evidence for its efficacy in improving fertility is sadly lacking (Bonde and Storgaard 2002). Commonly raised issues include avoidance of stress, a healthy diet and exercise. Recreational drugs such as cigarettes, excessive alcohol consumption and cannabis should certainly be withdrawn or reduced if possible. Occupational or social situations that may chronically elevate testicular temperature should be avoided. Medications that interfere with fertility, such as nitrofurantoin, anabolic steroids, sex steroids and anticonvulsants, should be avoided if possible. In patients with inflammatory bowel disease treated by sulphasalazine, changing treatment to 5-aminosalicylic acid removes the toxic agent, sulphapyridine, and leads to a rapid recovery of fertility without deterioration in disease activity. Although testicular function may improve in patients with chronic renal failure after successful transplantation, fertility impairment may be perpetuated by the continued use of immunosuppressive agents.
Medical treatment
Management of gonadotrophin deficiency
The treatment outcome of gonadotrophin induction of spermatogenesis is relatively successful, with up to 90% showing some degree of spermatogenesis. Up to 70% can be expected to achieve pregnancies (Büchter et al 1998, Liu et al 2002). Previous treatment with testosterone does not compromise the response to subsequent exogenous gonadotrophin, but there is a clear relationship between pretreatment testicular volume and duration of treatment required. Thus, spermatogenesis will generally be restored more quickly and with less frequent need for FSH in men with acquired gonadotrophin deficiency in adulthood (e.g. as a result of treatment for a pituitary tumour) than in those who have not gone through puberty spontaneously.
Management of genital tract infection
Infection of the male genital tract should be treated if present, but there is no evidence that this will improve fertility. Symptomatic urethritis responds to treatment with the appropriate antibiotics. Chronic infection of the male genital tract is more difficult to diagnose and the presence of pus cells in the semen only indicates an infection in some patients. An alkaline pH (>8.0) may occur in prostatitis due to decreased secretion of acid phosphatase by the prostate. Repeated growth on culture of the same organisms is probably of significance, as is the finding of organisms during a modified Stamey test (Meares and Stamey 1972), where the semen culture replaces the culture of expressed prostatic secretions. If treatment is felt to be warranted, the antibiotics chosen to treat prostatoseminal vesiculitis (such as doxycycline, erythromycins, cephalosporins or oflaxacin) should be secreted by the male accessory glands, and treatment should be continued for 4–12 weeks depending on the chronicity of the infection. In longer courses of treatment, it is customary to rotate the antibiotics, but overall the current tendency is towards a shorter duration (6 weeks) of treatment consisting of two antibiotics, each of which is taken for 3 weeks.
Erectile failure
The inability of the man to obtain vaginal penetration may be overcome by various pharmacological approaches, and this is particularly useful in men with neurological problems or with performance anxiety. If intracavernous agents are used, the physician should be familiar with the technique and avoid the tragedy of a priapism. Papaverine was the drug of choice for many years but this has been superseded by alprostadil, a prostaglandin E1 preparation, and more recently by oral sildenafil and other phosphodiesterase type 5 inhibitors (Hackett G, Kell P, Ralph D et al, British Society for Sexual Medicine 2008). In those men with organic impotence, the implantation of a penile prosthesis may provide a solution to the problem.
Surgical treatments
Relief of obstruction
Obstructive lesions of the seminal tract should be suspected in azoospermia or severe oligozoospermia with normally sized testes and with normal plasma FSH levels. Where surgery is undertaken, it is convenient to explore and biopsy the testes, perform vasography and correct any obstruction that is identified under the same general anaesthetic. A fuller account of the indications, techniques and outcomes of surgical treatments of male infertility may be found elsewhere (Pryor et al 1997).
Epididymal obstruction
The results of reconstructive surgery on the epididymis are varied and depend upon the cause and duration of obstruction and the expertise of the surgeon. Congenital blockages have poor outcome, as do blockages resulting from tuberculous or chlamydial infections. The overall patency rate would appear to be approximately 10–15% of operations, as in many patients there is a great deal of scarring and the epididymis is filled with inspissated material. The best results are achieved when there is localized postgonococcal obstruction in the cauda epididymis. In these instances, sperm may appear in 50% of ejaculates after surgery. A microsurgical technique would appear to give a 10% improvement in patency rates. Spermatozoa acquire motility as they pass through the epididymis, and higher pregnancy rates occur when the blockage is in the distal part of the epididymis (Schoysman and Bedford 1986).
Vasal obstruction
Vasal obstruction is less common in infertility practice and is treated by vasovasostomy. Many techniques are described but an end-to-end one-layer anastomosis with 6-0 sutures is simple and effective. Vasal obstruction most commonly occurs following vasectomy, and after vasovasostomy, patency is to be expected in 80–90% of men with a conception rate of 40–50% in 1 year. Magnification is desirable and the use of an operating microscope for vasovasostomy is excellent training for the more difficult tubulovasostomy that is necessary in epididymal obstruction. The prognosis is worse for those men with a long interval between the vasectomy and reversal (Silber 1989), but time does not prohibit any attempt to operate. The age of the female partner is of importance with regard to conception rates.
Ejaculatory duct obstruction
This is an uncommon cause of obstruction and is readily diagnosed by a low volume of semen, azoospermia or extreme oligozoospermia, and an acid pH in a man with palpable vasa. Müllerian duct cysts are amenable to treatment (Pryor et al 1997), with sperm appearing in the ejaculate of 80% of men and conception in 33% of female partners. Other forms of ejaculatory duct obstruction do less well and are probably best treated by sperm retrieval.
Assisted conception and male infertility
Early developments in IVF focused on couples with female factor infertility and particularly women suffering from bilateral tubal occlusion. Conventional IVF rapidly became established as an effective treatment option for couples with tubal disease and with unexplained infertility, but it soon became apparent that it yielded generally poor pregnancy rates for couples with male factor infertility. Although there was much discussion in the literature on the fine tuning of the IVF procedure for couples with problems in the male partner, management options for couples with poor semen quality remained very limited until the breakthrough of effective microassisted fertilization in 1992 (Palermo et al 1992).
Micromanipulation techniques
Partial zona dissection was the first micromanipulation technique studied in animal models with clinical intent, and early reports in human practice of clinical pregnancies were encouraging, suggesting that monospermic fertilization and cleavage rates could be doubled by these appraoches. However, concerns existed over the risk of polyspermy, along with doubts about appropriate case selection. Subzonal insemination (SUZI) involves the injection of spermatozoa into the privitelline space; again, initial reports of its use were encouraging, although other groups found the technique to be less successful. The developments in human microassisted fertilization culminated in ICSI, with the first human pregnancies resulting from this technique being described by the Brussels group (Palermo et al 1992). This approach involves injection of a single spermatozoon directly into the cytoplasm of the oocyte through the intact zona pellucida, and it very soon became apparent that this technique produced superior results to partial zona dissection or SUZI, with pregnancy rates of 22% per started cycle being reported (Van Steirteghem et al 1993, Abdalla et al 1995). Indeed, such has been the success of ICSI that some commentators suggest that it might be considered the treatment of choice for all cases where in-vitro conception is indicated.
A meta-analysis has concluded that for couples with normal semen, there is no evidence of any benefit, either in fertilization rates per retrieved oocyte or in pregnancy rates, between ICSI and conventional IVF. In contrast, for couples with borderline semen, ICSI results in higher fertilization rates than IVF, and couples with very poor semen will have better fertilization outcomes with ICSI than with SUZI or additional IVF (van Rumste et al 2000).
ICSI with epididymal spermatozoa
Initially, clinical ICSI was used in the treatment of couples in whom the male partner had substantially abnormal semen quality, but it was not long before the technology was applied to the significant numbers of men who present with no sperm in their ejaculates. Amongst men with obstructive azoospermia, attention focused on spermatozoa derived from the epididymis. The first pregnancies achieved with epididymal sperm were described using conventional IVF. However, fertilization rates were low, so whilst it was established that sperm from the epididymis had a degree of functional competence, this was limited (Liu et al 1994, Silber et al 1994).
As a consequence of the successful use of epididymal spermatozoa in ICSI, techniques have been described to facilitate the surgical retrieval of spermatozoa. The major approaches include microsurgical epididymal sperm aspiration (MESA) and percutaenous epididymal sperm aspiration (PESA). MESA involves a formal scrotal exploration, is commonly performed under general anaesthetic and hence is a significant surgical intervention. PESA is a widely used technique which is less invasive, can be performed under local anaesthesia and can be undertaken repeatedly. However, PESA provides less diagnostic information and the yield of spermatozoa may be lower (Girardi 1999).
ICSI with testicular spermatozoa
Without doubt, the most thorough and detailed follow-up studies of ICSI offspring have been those orchestrated by the Brussels group (Bonduelle et al 1999) who have undertaken a prospective follow-up study of 1987 children born after ICSI, aiming to compile data on karyotypes, congenital malformations, growth parameters and developmental milestones. It was found that 1.66% of karyotypes determined by prenatal diagnosis were abnormal de novo (nine each of autosomal and sex chromosomal aberrations) and 0.92% were inherited structural aberrations. Most of these were transmitted from the father. Forty-six major malformations (2.3%) were observed at birth. Seven malformations observed by prenatal ultrasound were terminated. Twenty-one (1.1%) stillbirths, including four with major malformations, occurred later than 20 weeks of pregnancy.
Several other large cohort studies have been reported, and as the database of ICSI offspring grows larger, the available evidence on the health of these offspring is generally reassuring (Leunens et al 2008). It is important, however, to appreciate the important role that genetic aetiology plays in the origins of much male subfertility and the ability of ICSI to promote the transgenerational transmission of genetic defects causing gametogenic failure. The significantly increased risk of chromosomal abnormalities in men with impaired semen quality is easily managed by the appropriate investigation and counselling which are required prior to treatment. It is less easy to be certain how to respond to the available evidence on microdeletions of the Y chromosome in men with severely impaired semen quality. The strength of the association between Y chromosome deletions and severely impaired semen quality is impressive, and it is increasingly suggested that these lesions may result in progression from oligozoospermia to azoospermia over time. It is also clear that these genetic deletions, if present, can be transmitted to offspring via ICSI. On the basis of this evidence, some authorities now advocate screening of men for Y chromosome microdeletions prior to ICSI, and advocate testing of offspring and reproductive monitoring for those found to have inherited deletions.
Changes in Male Reproductive Health
During the past two decades, a number of reports have raised serious concerns about the development of reproductive problems in animals and man. There have been controversial reports of changes in human semen quality (Carlsen et al 1992, Auger et al 1995, Irvine 1996), alongside reports of an increasing incidence of congenital malformations of the male genital tract, such as cryptorchidism and hypospadias (Kallen et al 1986, Ansell 1992) and of an increasing incidence of testicular cancer (Adami et al 1994, Wanderas et al 1995). However, there is controversy over whether or not these reported changes in male reproductive health are genuine, and if so, the causes and implications.
Testicular cancer
Although many of the changes seen in male reproductive health are controversial, there seems little argument that testicular cancer is increasing in frequency, with unexplained increases in the age-standardized incidence being observed in Europe (Adami et al 1994, Bergstrom et al 1996) and the USA (Devesa et al 1995). There would appear to be substantial geographical variation in both the incidence of testicular cancer and in the observed rate of increase (Adami et al 1994). Of note, this geographical variation may be linked with that seen in semen quality; testicular cancer is four times more common in Denmark, where studies have revealed rather low sperm counts (Jensen et al 1996), than in Finland, where semen quality appears to be better (Vierula et al 1996). Interestingly, the observed increases, both in Europe and the USA, would appear to be birth cohort related. Bergstrom et al (1996) evaluated data from Denmark, Norway, Sweden, East Germany, Finland and Poland, including data on over 30,000 cases of testicular cancer from 1945 to 1989 in men aged 20–84 years. They found considerable regional variation in both the incidence of testicular cancer and the observed rate of increase, ranging from a 2.3% increase annually in Sweden to a 5.2% increase annually in East Germany. In all six countries, birth cohort was a stronger determinant of testicular cancer risk than calendar time, such that men born in 1965 had a risk of testicular cancer that was 3.9 times [95% confidence interval (CI) 2.7–5.6; Sweden] to 11.4 times (95% CI 8.3–15.5; East Germany) higher than that for men born in 1905.
A recent study has looked in detail at the risk of testicular cancer in subfertile men (Moller and Skakkebaek 1999) using a population-based case–control design. This study found that paternity was associated with a reduced risk of testicular cancer (relative risk 0.63, 95% CI 0.47–0.85), and that prior to the diagnosis of testicular cancer, cases tended to have fewer children than expected for their age (relative risk 1.98, 95% CI 1.43–2.75). The study suggested that these observations are consistent with the hypothesis that testicular cancer and male subfertility share important aetiological factors.
Cryptorchidism and congenital malformations of the male genital tract
The incidence of congenital malformation of the male genital tract may also be changing, with increases observed in the prevalence of cryptorchidism and hypospadias. Cryptorchidism, for example, has increased by as much as 65–77% over recent decades in the UK (Ansell 1992). In contrast, some data from the USA have tended to suggest that rates of cryptorchidism have not changed (Berkowitz et al 1993), although one recent large study from the USA reported that rates of hypospadias doubled from the 1970s to the 1980s (Paulozzi et al 1997). Here too, though, regional differences have been observed, although the data are perhaps less robust than is the case with testicular cancer. One multicentre study of 8122 boys from seven malformation surveillance systems around the world concluded that, even when differences in ascertainment were taken into account, true geographical differences exist in the prevalence of hypospadias at birth (Kallen et al 1986).
Changing semen quality: historical data on normal men
In 1992, Carlsen et al (1992) reawakened concern over the possibility of secular trends in semen quality, publishing a meta-analysis of data on semen quality in normal men. The authors undertook a systematic review of available data on semen quality in normal men, published since 1930. Standard techniques applicable to meta-analysis were used to identify relevant papers, and care was taken to exclude data on infertile couples, men selected on the basis of their semen quality and data generated using non-classical approaches to semen analysis. Data were obtained on 14,947 men, published in 61 papers between 1938 and 1990. The authors observed a decline in sperm concentration from 113 × 106/ml in 1940 to 66 × 106/ml in 1990, along with a decline in the proportion of men with a sperm concentration above 100 × 106/ml. Predictably, the central message of this meta-analysis, that sperm counts had declined by approximately 50% over the past 50 years, attracted enormous attention and generated much controversy.
Since publication of Carlsen et al’s meta-analysis, several papers have presented contemporary analyses of retrospective data. Unfortunately, the available data still fail to reach a conclusion on whether or not there is any secular trend in semen quality; at least as many studies have reported evidence of deteriorating semen quality as have reported evidence of no change. A very careful reanalysis of the historical data (Carlsen et al 1992) on semen quality in normal men (Swan et al 1997) found that there was evidence of a decline in sperm concentrations in the USA of −1.5 × 106/ml/year (95% CI −1.9 to −1.1), and in Europe of −3.13 × 106/ml/year (95% CI −4.96 to −1.30), but not in non-Western countries.
Whilst the available evidence is inconclusive and circumstantial, its weight is considerable and, at the very least, it should raise concerns that deserve to be addressed by properly designed, coordinated and funded research. Delay may compromise the fertility and reproductive health of future generations (de Kretser 1996, Irvine 1996).
KEY POINTS
Abdalla H, Leonard T, Pryor J, Everett D. Comparison of SUZI and ICSI for severe male factor. Human Reproduction. 1995;10:2941-2944.
Adami HO, Bergstrom R, Mohner M, et al. Testicular cancer in nine northern European countries. International Journal of Cancer. 1994;59:33-38.
Aitken RJ, Clarkson JS, Hargreave TB, Irvine DS, Wu FCW. Analysis of the relationship between defective sperm function and the generation of reactive oxygen species in cases of oligozoospermia. J Androl. 1989;10(3):214-220.
Ansell PE. Cryptorchidism: a prospective study of 7,500 consecutive male births. Archives of Disease in Childhood. 1992;67:892-899.
Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. New England Journal of Medicine. 1995;32:281-285.
Bergstrom R, Adami HO, Mohner M, et al. Increase in testicular cancer incidence in six European countries: a birth cohort phenomenon. Journal of the National Cancer Institute. 1996;88:727-733.
Berkowitz GS, Lapinski RH, Dolgin SE, Gazella JG, Bodian CA, Holzman IR. Prevalence and natural history of cryptorchidism. Pediatrics. 1993;92:44-49.
Bonde JP, Storgaard L. How work-place conditions, environmental toxicants and lifestyle affect male reproductive function. International Journal of Andrology. 2002;25:262-268.
Bonduelle M, Camus M, De Vos A, et al. Seven years of intracytoplasmic sperm injection and follow-up of 1987 subsequent children. Human Reproduction. 1999;14(Suppl 1):243-264.
Bronson RA, Cooper GW, Rosenfeld D. Sperm antibodies: their role in infertility. Fertility and Sterility. 1984;42:171-183.
Büchter D, Behre HM, Kleisch S, Nieschlag E. Pulsatile GnRH or human chorionic gonadotrophin/human menopausal gonadotrophin as effective treatment for men with hypogonadotrophic hypogonadism: a review of 42 cases. European Journal of Endocrinology. 1998;139:298-303.
Burger HG, Baker HWG. Therapeutic considerations and results of gonadotrophic treatment in male hypogonadotrophic hypogonadism. Annals of the New York Academy of Sciences. 1982;438:447-453.
Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ (Clinical Research Ed.). 1992;305:609-613.
Chandley AC. Chromosomes. In: Hargreave T, editor. Male Infertility. London: Springer-Verlag, 1994.
Comhaire FH, de Kretser D, Farley TMM. Towards more objectivity in diagnosis and management of male infertility. Results of a WHO Multicentre Study. International Journal of Andrology. 1987;10(Suppl 7):1-53.
Cummins JM, Jequier AM. Treating male infertility needs more clinical andrology, not less. Human Reproduction. 1994;9:1214-1219.
de Kretser DM. Declining sperm counts. BMJ (Clinical Research Ed.). 1996;312:457-458.
Devesa SS, Blot WJ, Stone BJ, Miller BA, Tarone RE, Fraumeni JFJr. Recent cancer trends in the United States. Journal of the National Cancer Institute. 1995;87:175-182.
Dym M. Histology. In: Weiss L, Greep RO, editors. Histology. New York: McGraw Hill; 1977:981.
Evers JH, Collins J, Clarke J 2008 Surgery or embolisation for varicoceles in subfertile men. Cochrane Database of Systematic Reviews 3: CD000479.
Fawcett DW. The mammalian spermatozoon. Developmental Biology. 1975;44:394-436.
Girardi SK. Techniques for sperm recovery in assisted reproduction. Reproductive Medicine Review. 1999;7:131-139.
Grudzinskas JG. Gametes — the Spermatozoon. Cambridge, UK: Cambridge University Press; 1995.
Hackett G, Kell P, Ralph D, et al. British Society for Sexual Medicine guidelines on the management of erectile dysfunction. Journal of Sexual Medicine. 2008;5:1841-1865.
Hargreave TB. Varicocele. In: Hargreave TB, editor. Male Infertility. London, UK: Springer Verlag, 1994.
Hargreave TB. Genetic basis of male fertility. British Medical Bulletin. 2000;56:650-671.
Hull MGR, Glazener CMA, Kelly NJ, et al. Population study of causes, treatment, and outcome of infertility. BMJ (Clinical Research Ed.). 1985;291:1693-1697.
Irvine DS. Is the human testis still an organ at risk? BMJ (Clinical Research Ed.). 1996;312:1557-1558.
Irvine DS. Declining sperm quality: a review of facts and hypotheses. Baillières Clinical Obstetrics and Gynaecology. 1997;11:655-671.
Ivell R, Hartung S, Anand-Ivell R. Insulin-like factor 3: where are we now? Annals of the New York Academy of Sciences. 2005;1041:486-496.
Jensen TK, Giwercman A, Carlsen E, Scheike T, Skakkebaek NE. Semen quality among members of organic food associations in Zealand, Denmark. The Lancet. 1996;347:1844.
Johnsen SG. Testicular biopsy score count: a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1:2-25.
Kallen B, Bertollini R, Castilla E, et al. A joint international study on the epidemiology of hypospadias. Acta Paediatrica Scandinavica. 1986;324(Suppl):1-52.
Krausz C, Degl’Innocenti S. Y chromosome and male infertility: update 2006. Frontiers in Bioscience. 2006;11:3049-3061.
Kruger TF, du Toit TC, Franken DR, Menkveld R, Lombard CJ. Sperm morphology: assessing the agreement between the manual method (strict criteria) and the sperm morphology analyzer IVOS. Fertility and Sterility. 1995;63:134-141.
Leunens L, Celestin-Westreich S, Bonduelle M, Liebaers I, Ponjaert-Kristoffersen I. Follow-up of cognitive and motor development of 10-year-old singleton children born after ICSI compared with spontaneously conceived children. Human Reproduction. 2008;23:105-111.
Liu J, Lissens W, Silber SJ, Devroey P, Liebaers I, Van Steirteghem A. Birth after preimplantation diagnosis of the cystic fibrosis delta F508 mutation by polymerase chain reaction in human embryos resulting from intracytoplasmic sperm injection with epididymal sperm. Journal of the American Medical Association. 1994;272:1858-1860.
Liu PY, Gebski VJ, Turner L, Conway AJ, Wishart SM, Handelsman DJ. Predicting pregnancy and spermatogenesis by survival analysis during gonadotrophin treatment of gonadotrophin-deficient infertile men. Human Reproduction. 2002;17:625-633.
Matsumoto AM, Paulsen CA, Bremner WJ. Stimulation of sperm production by human luteinizing hormone in gonadotrophin-suppressed normal men. Journal of Clinical and Endocrinological Metabolism. 1984;59:882-887.
Meares EMJr, Stamey TA. The diagnosis and management of bacterial prostatitis. British Journal of Urology. 1972;44:175-179.
Moller H, Skakkebaek NE. Risk of testicular cancer in subfertile men: case–control study. BMJ (Clinical Research Ed.). 1999;318:559-562.
Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. Journal of Clinical Endocrinology and Metabolism. 1995;80:3689-3698.
Mortimer D. Sperm Transport in the Female Genital Tract. Cambridge, UK: Cambridge University Press; 1995.
O’Connor AE, De Kretser DM. Inhibins in normal male physiology. Seminars in Reproductive Medicine. 2004;22:177-185.
Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. The Lancet. 1992;340:17-18.
Paradisi R, Pession A, Bellavia E, Focacci M, Flamigni C. Characterization of human sperm antigens reacting with antisperm antibodies from autologous sera and seminal plasma in a fertile population. Journal of Reproductive Immunology. 1995;28:61-73.
Paulozzi LJ, Erickson JD, Jackson RJ. Hypospadias trends in two US surveillance systems. Pediatrics. 1997;100:831-834.
Pryor JL, Kent-First M, Muallem A, et al. Microdeletions in the Y chromosome of infertile men. New England Journal of Medicine. 1997;336:534-539.
Rowe PG. WHO Manual for the Standardized Investigation and Diagnosis of the Infertile Couple. Cambridge, UK: Cambridge University Press; 1993.
Sandler B. Recovery from sterility after mumps orchitis. BMJ (Clinical Research Ed.). 1954;2:795.
Schmidt L. Infertility and assisted reproduction in Denmark. Epidemiology and psychosocial consequences. Danish Medical Bulletin. 2006;53:390-417.
Schoysman RJ, Bedford JM. The role of the human epididymis in sperm maturation and sperm storage as reflected in the consequences of epididymovasostomy. Fertility and Sterility. 1986;46:293-299.
Seminara SB, Crowley WFJr. Kisspeptin and GPR54: discovery of a novel pathway in reproduction. Journal of Neuroendocrinology. 2008;20:727-731.
Sharpe RM. Intratesticular control of steroidogenesis. Clinical Endocrinology. 1990;33:787-807.
Sharpe RM. Lifestyle and environmental contribution to male infertility. British Medical Bulletin. 2000;56:630-642.
Silber SJ. Pregnancy after vasovasostomy for vasectomy reversal: a study of factors affecting long-term return of fertility in 282 patients followed for 10 years. Fertility and Sterility. 1989;31:309-315.
Silber SJ, Nagy ZP, Liu J, Godoy H, Devroey P, Van Steirteghem AC. Conventional in-vitro fertilization versus intracytoplasmic sperm injection for patients requiring microsurgical sperm aspiration. Human Reproduction. 1994;9:1705-1709.
Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. New England Journal of Medicine. 1994;331:1056-1061.
Srinivas-Shankar U, Wu FC. Drug insight: testosterone preparations. Nature Clinical Practice Urology. 2006;3:653-665.
Swan SH, Elkin EP, Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environmental Health Perspectives. 1997;105:1228-1232.
Tapanainen TS, Aittomaki K, Min J, Vasivko T, Huhtaniemi IT. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nature Genetics. 1997;15:205-206.
Tarozzi N, Bizzaro D, Flamigni C, Borini A. Clinical relevance of sperm DNA damage in assisted reproduction. Reproductive Biomedicine Online. 2007;14:746-757.
Thonneau P, Ducot B, et al. Heat exposure as a hazard to male fertility. Lancet. 1996;347:204-205.
Tulloch WS. A consideration of sterility factors in the light of subsequent pregnancies: subfertility in the male. Edinburgh Obstetrical Society. 1952;52:29-34.
van Rumste MM, Evers JL, Farquhar CM, Blake DA 2000 Intra-cytoplasmic sperm injection versus partial zona dissection, subzonal insemination and conventional techniques for oocyte insemination during in vitro fertilisation. Cochrane Database of Systematic Reviews 2: CD001301.
Van Steirteghem AC, Liu J, Joris H, et al. Higher success rate by intracytoplasmic sperm injection than by subzonal insemination. Report of a second series of 300 consecutive treatment cycles. Human Reproduction. 1993;8:1055-1060.
Vermeulen A. Androgens in the aging male. Journal of Clinical Endocrinology and Metabolism. 1991;73:221-224.
Vierula M, Niemi M, Keiski A, Saaranen M, Saarikoski S, Suominen J. High and unchanged sperm counts of Finnish men. International Journal of Andrology. 1996;19:11-17.
Wanderas EH, Tretli S, Fossa SD. Trends in incidence of testicular cancer in Norway 1955–1992. European Journal of Cancer. 1995;31A:2044-2048.
World Health Organization. The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. Fertility and Sterility. 1992;57:1289-1293.
World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction. Cambridge: Cambridge University Press; 1999.
Wu FC. Guideline for male testosterone therapy: a European perspective. Journal of Clinical Endocrinology and Metabolism. 2007;92:418-419.
Wu FC, Taylor PL, Sellar RE. LHRH pulse frequency in normal and infertile men. Journal of Endocrinology. 1989;123:149-158.