31. Discharge Planning and Follow-Up of the Neonatal Intensive Care Unit Infant*
Donna K. Daily, Angel Carter and Brian S. Carter
Parents with infants in the neonatal intensive care unit (NICU) have immediate worries about whether their newborn infant will survive but soon thereafter start having concerns about how their child will do through infancy and into adulthood. As the infant’s convalescence begins, so does discharge planning; this brings to the forefront questions about outcomes. Unfortunately, it is almost impossible to know the outcome of any individual infant at the time of discharge from the NICU. Caregivers within the NICU must be knowledgeable of the latest outcomes literature to respond to these questions and to guide parents in the importance of follow-up care.
Of the many reasons that newborns require neonatal intensive care, the most common one is preterm birth. Numerous publications report the outcomes of very-low-birth-weight (VLBW, birth weights <1500 g) and extremely-low-birth-weight (ELBW, birth weights <1000 g). 3,23,28,33,65 Recent studies have focused on the survival and outcome of even more immature infants with birth weights below 750 g, or on infants born at the limits of viability, 22 to 25 weeks’ gestation. 45,64,68,84 Late preterm infants have been a topic of renewed interest because they are at risk for a unique set of problems with adverse outcomes. 2,52 Infants with intrauterine growth restriction (IUGR) are also vulnerable to a wide range of complications requiring neonatal intensive care, especially if they are also preterm and experience extrauterine growth failure. 32,80 Full-term infants with meconium aspiration syndrome (MAS), persistent pulmonary hypertension of the newborn (PPHN), infection, congenital diaphragmatic hernia (CDH), or neonatal encephalopathy also require intensive care and are at risk for health and developmental sequelae. 121 Finally, a number of infants with multiple congenital anomalies require surgery or neonatal intensive care.
PLANNING FOR DISCHARGE
An organized, well-implemented discharge plan is the beginning of successful follow-up of a NICU graduate. A family-centered multidisciplinary team approach uses the expertise of many disciplines, along with the family, to formulate and implement the discharge and follow-up plan. The team can comprise parents, grandparents, other caregivers, physicians, nurses, case managers, dietitians, therapists, developmental specialists, and social workers.
Integrating family-centered principles into the discharge process as a continuation of family-centered care practiced throughout the NICU stay facilitates better parental adaptation to the transition to home.62 For many infants, the NICU stay has been lengthy and complex, and families may experience varying degrees of anxiety and stress as they prepare for the infant to come home. In some cases, attachment and bonding may have been affected by a long, complicated medical course. 41 A survey of preterm mothers found that symptoms of psychologic distress (fatigue, depressive mood, anxiety, physical symptoms) persisted up to a year after the birth of their premature baby. 57 Families may need extra attention paid to these issues before they can successfully attend to the discharge process. A thorough assessment of caregiver needs, environmental issues, and knowledge of their infant’s care before discharge is an important part of the planning process. Implementation of a parent educational-behavioral intervention program during the NICU stay may be one mechanism to reduce stress, depression, and anxiety and effect more positive interactions of parents with their infant, a shorter NICU stay, and shorter total hospital stay. 86 In addition, an assessment tool may be considered in efforts to quantify the discharge readiness of the family. 111
Considerations Before Discharge
INSURANCE
Many parents may need assistance to enroll their infant in their existing insurance policy or to identify the procedures necessary to apply for medical assistance. This process can take many weeks and must be accomplished before discharge in order to select a pediatrician for follow-up care. Social workers, case managers, and financial counselors are valuable resources to assist families in this process.
IDENTIFICATION OF A FOLLOW-UP PROVIDER
A pediatric health care professional to follow the infant after discharge, trained in the care of NICU graduates, should be identified before discharge. Ideally, this provider has been identified early in the admission to facilitate regular communications regarding the infant’s medical course. Immediately before discharge, a written summary should be provided, with follow-up recommendations regarding nutrition and growth, developmental surveillance, and subspecialty referrals, along with verbal notification of the discharge date. Parents should be advised to keep this summary with the infant at all times because this written documentation of the NICU stay is invaluable if the infant needs to be seen on an emergency basis shortly after hospital discharge. Providing families with a “care notebook” containing specialized forms and organizing tools can be a valuable addition to the discharge process, particularly for those with anticipated complex follow-up needs. The American Academy of Pediatrics (AAP) also provides a form for summarizing the history and current needs of children with special health care needs.
CAREGIVER EDUCATION
In a family-centered environment, families have been partners in caring for their infant throughout the hospital stay. Discharge teaching then becomes a process of reinforcing and attending to final details. In some instances, however, this teaching may be limited by the inability of the family to be present because of transportation and family or job constraints. In these cases, readiness of the caregivers and home environment should be thoroughly evaluated (Box 31-1).
BOX 31-1
• Every encounter with the parents is a teaching opportunity. Assess each individual family’s readiness for discharge.
• Inform parents verbally and in writing about the tests included in the newborn genetic screening and how they will receive the results.
• Teach parents the special nutritional needs of preterm infants after discharge, including nutritional supplementation, lactation support and intervention to promote breast feeding, and use of alternative feeding methods, if necessary.
• Teach parents the importance of maintaining their infant on home oxygen therapy (e.g., for growth and development, sleep, and feeding) at designated pulse oximetry targets until pulse oximetry studies (e.g., awake, feeding, asleep) document that the infant can tolerate weaning and discontinuing the supplemental oxygen.
• Teach parents to dress their infant appropriately to maintain adequate axillary temperature.
• Teach parents appropriate safety precautions:
• Proper positioning (supine) for sleep: “Back to Sleep”
• Proper use of car seats
• Importance of a smoke-free environment
• Never shake the baby! Dangers of shaking infants include blindness, brain damage, developmental delays, seizures, paralysis, and death
• Information, in writing, about all medications for their infant including name, action, dose, route, side effects, schedule
• Provide parents opportunity to participate in infant CPR class
• Teach parents the importance of follow-up care and appointments:
• Timely follow-up for infants with retinopathy of prematurity (ROP) provided verbally and in writing
• Timely follow-up for hearing screen and referral for re-screening
• Need for monthly RSV immunizations throughout the RSV season
• Give parents the newborn immunization record.
• Teach parents the importance of their own self-care, and assist in identifying resources for support.
HOME EQUIPMENT
When a home apnea monitor is used, a clear plan outlining the reasons for initiating home monitoring and the indications for discontinuing it should be discussed with the family and the primary care provider before discharge.5 Any necessary durable medical equipment or supplies, such as an apnea monitor, feeding pump, ventilator, suction equipment, or oxygen for home use, should be delivered to the hospital before discharge to give parents practice using the equipment. The company supplying the equipment should provide training in its use. NICU nurses or respiratory therapists should verify the parents’ understanding of the purpose of the equipment and its operation and also ensure that home caregivers for the baby have been trained in cardiopulmonary resuscitation (CPR).
ROOMING IN
Whether or not an infant is going home with equipment, giving family caregivers the opportunity to provide “independent” care of their infant with professional caregivers nearby for assistance has been shown to increase parental competence and provide confirmation of readiness for independent care at home.39 One intensive care nursery’s experience in establishing a step-down unit where mothers provided all basic care for their infant under supervision resulted in earlier discharge to home with no increases in short-term complications or readmissions. 29
DISCHARGE CRITERIA
Clearly defined discharge criteria provide both the family and the staff a point of reference from which to judge the infant’s progress. Discharge criteria should be reviewed in a multidisciplinary team meeting with the family. Setting goals that the infant, parents, and staff must accomplish before discharge helps keep everyone focused and prevents important components of the discharge process from being overlooked.
For preterm infants, the attainment of a weight of 5 pounds is no longer the criterion for discharge. Rather, the ability of a preterm or recovering neonate to maintain physiologic stability and the ability of the family to care for the infant’s physiologic and developmental needs are the criteria for discharge (Box 31-2). There are significant variations across NICUs for specific discharge criteria, with assessment of apnea and feeding behavior significantly influencing the duration of hospitalization in a healthy preterm infant. 49 The recently published AAP policy statement “Hospital Discharge of the High-Risk Neonate” provides guidance that should minimize such variations. 6
BOX 31-2
Infant
• Sustained weight gain of sufficient duration
• Maintain normal body temperature, clothed in an open bed, at normal room temperature (20 ° to 25 ° C)
• Establish and maintain competent breast or bottle feeding without cardiopulmonary problems
• Nutrition assessment and dietary management provided as indicated
• Hematologic assessment and management provided as indicated
• Documented physiologically mature and stable cardiopulmonary function of sufficient duration
• Parents have been given a report of neurodevelopmental and neurobehavioral status
• Completed metabolic, hearing, and indicated funduscopic screenings
• Appropriately immunized, including (respiratory syncytial virus [RSV] prophylaxis) and plan for subsequent injections
• A completed car seat evaluation
• A completed review of the hospital course, pending medical problems noted, and follow-up plans identified
• A home-care plan, individualized to the patient’s needs, has been provided by all disciplines
Parents, Family, and Home Environment
• Identify and assess at least two caregivers for home.
• Assess psychosocial and parenting strengths and risks.
• Consider the home environment and on-site visit as indicated.
• Review resource availability (including financial, utilities, and transportation).
• Determine caregiver availability, ability, and commitment to the following:
• Provide basic infant care: diapering, bathing, dressing, cord and circumcision care.
• Maintain infant’s thermal state: able to take temperature and dress appropriately.
• Feed infant (breast, bottle, or alternative method—nasogastric tube, gastrostomy, parenteral nutrition), and demonstrate formula preparation if required.
• Manage home feeding tube, infusion pump, intestinal stoma care, and other devices as indicated.
• Manage home monitoring, oxygen, and other equipment as indicated; address initial problem solving; demonstrate CPR and initial emergency interventions.
• Maintain safe environment, car seat, heat, electricity, telephone, transportation, smoke-free, emergency resuscitation.
• Recognize signs of illness, and identify when to call primary care provider or emergency services.
• Have support system identified to assist in infant’s care.
• Demonstrate medication administration and recognize signs of medication adverse effects (e.g., toxicity); understand importance of follow-up care, and know whom to call for questions or concerns.
Modified from American Academy of Pediatrics, Committee on Fetus and Newborn: Hospital discharge of the high-risk neonate, Pediatrics 122:1119, 2008.
TRANSFER
Some infants are not discharged home from the regional or tertiary NICU but, instead, are transferred from a regional referral center to a community unit or facility for the duration of their hospital stay. Transfer to a community hospital may be beneficial to families because they are often closer to the parents’ home (especially if the NICU is part of a regional referral center). Possible locations for transfer, as well as the criteria for transfer, should be discussed with the parents early in the hospitalization if this is an expected possibility. Communication of a comprehensive discharge plan should take place with the receiving hospital before transfer.66,79 As the capacity for back-transporting convalescing neonates to community hospitals has increased in the United States over the past 20 years, persistent issues of communication, trust, and psychosocial support remain for parents. 44
EARLY DISCHARGE
Preterm infants are often discharged between 35 and 37 weeks chronologic age.89Parental concerns at the time of discharge may include their ability to have adequate rest, their readiness to learn and assume self-care and newborn care, their readiness to parent, and availability of support systems. Concerns about the newborn may include transition from the intensive care nursery to the home care environment, ability to feed and hydrate adequately, and the early development and recognition of complications. 117 In addition, late-preterm infants (34 to 36 weeks gestational age) are sometimes discharged early (<48 hours) using criteria developed for term infants. Tomashek et al demonstrated that late-preterm, early discharged, breast-fed infants were 1.5 times more likely to require hospital-related care and 2.2 times more likely to be readmitted than term infants who were breast fed, with jaundice and infection accounting for the majority of readmissions. 132 Early discharge requires appropriate post-discharge follow-up and monitoring to prevent morbidity. Numerous studies document the positive effects of home visitation programs to assist with post-discharge education and support. 95,102,146
Screening
GENETIC SCREENING
Initial screening of sick or premature infants is performed as soon as possible after birth, before the administration of blood or antibiotics. Although it is common for these infants to have some relatively abnormal results—especially for thyroid function or amino acid profiles while on parenteral nutrition—early screening is recommended to identify in a timely manner those infants who may have an inborn metabolic disorder, congenital endocrinopathies, hemoglobinopathies, or infectious processes so that early treatment can be initiated. 12,109Subsequent screenings should take place according to an established routine, depending on state requirements. Recommendations for continued screenings after discharge should be clearly outlined in the discharge summary.
HEARING
All infants, especially those who need NICU admission for more than 5 days, should be screened for hearing loss using otoacoustic emissions or auditory brainstem response testing. This initial screening should be performed once the infant is medically stable, and if there are any concerns that warrant a secondary screen, re-screening should occur before 1 month of age. Infants who do not pass (are “referred” after secondary screening) should have a full-scale auditory diagnostic evaluation by 3 months of age. Infants with confirmed hearing loss should receive intervention by 6 months of age from an infant hearing specialist. 11,134 Those infants with an increased risk for hearing impairment should be assessed by a pediatric audiologist with a follow-up schedule outlined for the parents (Box 31-3). The goal of early detection and intervention is to maximize language, cognitive, literacy, and social development of the hearing impaired.126
BOX 31-3
• Neonatal intensive care unit (NICU) admission for more than 5 days or any of the following regardless of length of stay: extracorporeal membrane oxygenation (ECMO), assisted ventilation, exposure to ototoxic medications (gentamicin and tobramycin) or loop diuretics (furosemide/Lasix), hyperbilirubinemia requiring exchange transfusion
• Syndromes associated with hearing loss such as neurofibromatosis, osteopetrosis, and Usher syndrome
• Family history of hereditary childhood hearing loss
• Craniofacial abnormalities
• Congenital infections such as cytomegalovirus, toxoplasmosis, bacterial meningitis, syphilis, herpes, and rubella
• Physical findings (white forelock) associated with syndromes known to include hearing loss
• Neurodegenerative disorders (e.g., Hunter syndrome) or sensory motor neuropathies (e.g., Friedreich ataxia and Charcot-Marie-Tooth syndrome)
• Culture-positive postnatal infections such as bacterial and viral (especially herpes and varicella) meningitis
• Chemotherapy
• Caregiver concerns regarding hearing, speech, language, or developmental delay
Modified from American Academy of Pediatrics and Joint Committee on Infant Hearing, Year 2007 Position Statement: Principles and guidelines for early hearing detection and intervention programs, Pediatrics 120(4):898, 2007.
VISION
Development of severe retinopathy of prematurity (ROP) may still be a concern at the time of NICU discharge for infants born prematurely. Infants born at less than 32 weeks’ gestation or less than 1500 g birth weight, as well as infants with a birth weight between 1500 and 2000 g or born at more than 32 weeks with an unstable clinical course, should have a retinal screening examination with pupillary dilation.13 Follow-up of infants should be according to the ophthalmologist’s recommendations based on retinal findings. Arrangements for follow-up examinations should be made before discharge. Current research indicates that the risk to visual development in preterm infants does not end when the risk for ROP has passed. Any infant born prematurely, whether or not they develop ROP, are at increased risk for amblyopia/strabismus and refractive errors.97
IMAGING
Premature infants are at increased risk for injuries to the brain, potentially causing permanent damage. The most common form of damage and the leading cause of chronic neurologic morbidity is periventricular white matter injury. 25Identification of infants at high risk for poorer outcomes related to brain injury allows for timely referrals to early intervention therapies. A recent Cochrane review indicates that early intervention can improve cognitive outcomes up to preschool age. 127 Imaging techniques for routine screening for white matter injury have traditionally been the cranial ultrasound, although the ability of ultrasound to predict developmental outcome is inferior to magnetic resonance imaging (MRI). 90 A recent review of MRI screening to identify risks of suboptimal neurologic outcomes suggests the following be considered as indications for screening by MRI at approximately 36 weeks postmenstrual age67:
• Grade III to IV intraventricular hemorrhage
• Periventricular hemorrhagic infarction
• Cystic periventricular white matter damage
• Cerebellar hemorrhage or other abnormalities on ultrasound
• Suspected white matter abnormalities on ultrasound (echodensities/echolucencies)
• Post–hemorrhagic hydrocephalus
• Abnormal neurologic examination
• Other conditions warranting detailed neuroimaging (metabolic disorders or suspected congenital structural abnormality)
Preventive Care
IMMUNIZATIONS
Infant immunizations are recommended for all NICU infants, according to the guidelines issued by the Centers for Disease Control and Prevention (CDC) and approved by the AAP.37 Immunizations administered in the NICU should appear in the discharge summary. When immunizations have been declined by parents, this should be clearly indicated in the discharge summary, along with follow-up recommendations.
RESPIRATORY SYNCYTIAL VIRUS INFECTION PROPHYLAXIS
Respiratory syncytial virus (RSV) infection poses a risk for serious morbidity or even death for infants who were born prematurely, especially those with chronic heart or lung disease. For qualifying infants, RSV prophylaxis should be initiated with intramuscular palivizumab before discharge into the community setting during RSV season. RSV infection prophylaxis should be coordinated with the follow-up pediatrician for subsequent monthly injections.
Assessments
CAR SAFETY
All 50 states require infants to be restrained in a safety seat while riding in a motor vehicle, although laws vary from state to state. The AAP’s recent clinical report on transporting infants home states that “Infants with documented oxygen desaturation, apnea, or bradycardia in a semiupright position should travel in a supine or prone position in an Federal Motor Vehicle Safety Standard (FMVSS) 213–approved car bed after an observation period that is free of such events as described in point 1 above [e.g., ‘increased frequency of oxygen desaturation and episodes of apnea or bradycardia while sitting in car safety seats…preferably their own…for a minimum of 90 to 120 minutes or the duration of travel, whichever is longer…’].” This may need to be revised as new evidence becomes available from future research. 4,7,9
Discharge of smaller infants results in the use of car seat restraint devices that were designed for 7- to 8-pound term infants. In these devices, preterm infants may experience oxygen desaturations and apnea and bradycardia caused by head slouching and airway obstruction.105 Use of rolled diapers/blankets may be necessary to support upright posture, prevent slouching, and enable the preterm to maintain stability while in the car seat. A recent study of a foam insert that enabled the preterm’s head to be maintained in a neutral position showed a significant reduction in the rate of apneas. 133 In addition, infants with certain conditions (e.g., Down syndrome, osteogenesis imperfecta, myelomeningocele, Pierre Robin syndrome, cerebral palsy) may benefit from special-needs car restraints. 82
A car seat challenge before discharge is recommended for all infants born less than 37 weeks’ gestation; this includes “late preterm” infants (i.e., 34 to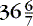 weeks) who are cared for and discharged from level I/normal newborn nurseries.7,9 Although the car seat challenge has not been standardized, certain components are common: (1) using the car seat purchased by the parents, (2) positioning the infant in the car seat immediately before discharge while on cardiorespiratory and pulse oximetry monitoring, (3) for a prescribed period of time (e.g., 30 to 90 minutes), and (4) recording respiratory/heart rates, oxygen saturations, apnea/bradycardia events. Although this is a recommended practice, limitations have been identified. First, little objective evidence supports the ability of this challenge to absolutely confirm safe travel for an infant. 60 A recent Cochrane review of the literature found no randomized controlled trials that fulfilled eligibility criteria for their review and were unable to recommend or refute a car seat challenge before discharge. 106 Further, common clinical practice has been to recommend the use of a car bed as a safe alternative to infants failing the car seat challenge, although recent studies have failed to prove any difference in apneic events. 77,119 A recommendation has been put forth regarding changing the notion of a “test” or “challenge” to a car seat “orientation” in which the emphasis would be on education on proper positioning, limiting duration of automobile travel, and close observation during travel. 60
weeks) who are cared for and discharged from level I/normal newborn nurseries.7,9 Although the car seat challenge has not been standardized, certain components are common: (1) using the car seat purchased by the parents, (2) positioning the infant in the car seat immediately before discharge while on cardiorespiratory and pulse oximetry monitoring, (3) for a prescribed period of time (e.g., 30 to 90 minutes), and (4) recording respiratory/heart rates, oxygen saturations, apnea/bradycardia events. Although this is a recommended practice, limitations have been identified. First, little objective evidence supports the ability of this challenge to absolutely confirm safe travel for an infant. 60 A recent Cochrane review of the literature found no randomized controlled trials that fulfilled eligibility criteria for their review and were unable to recommend or refute a car seat challenge before discharge. 106 Further, common clinical practice has been to recommend the use of a car bed as a safe alternative to infants failing the car seat challenge, although recent studies have failed to prove any difference in apneic events. 77,119 A recommendation has been put forth regarding changing the notion of a “test” or “challenge” to a car seat “orientation” in which the emphasis would be on education on proper positioning, limiting duration of automobile travel, and close observation during travel. 60
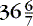 weeks) who are cared for and discharged from level I/normal newborn nurseries.7,9 Although the car seat challenge has not been standardized, certain components are common: (1) using the car seat purchased by the parents, (2) positioning the infant in the car seat immediately before discharge while on cardiorespiratory and pulse oximetry monitoring, (3) for a prescribed period of time (e.g., 30 to 90 minutes), and (4) recording respiratory/heart rates, oxygen saturations, apnea/bradycardia events. Although this is a recommended practice, limitations have been identified. First, little objective evidence supports the ability of this challenge to absolutely confirm safe travel for an infant. 60 A recent Cochrane review of the literature found no randomized controlled trials that fulfilled eligibility criteria for their review and were unable to recommend or refute a car seat challenge before discharge. 106 Further, common clinical practice has been to recommend the use of a car bed as a safe alternative to infants failing the car seat challenge, although recent studies have failed to prove any difference in apneic events. 77,119 A recommendation has been put forth regarding changing the notion of a “test” or “challenge” to a car seat “orientation” in which the emphasis would be on education on proper positioning, limiting duration of automobile travel, and close observation during travel. 60
weeks) who are cared for and discharged from level I/normal newborn nurseries.7,9 Although the car seat challenge has not been standardized, certain components are common: (1) using the car seat purchased by the parents, (2) positioning the infant in the car seat immediately before discharge while on cardiorespiratory and pulse oximetry monitoring, (3) for a prescribed period of time (e.g., 30 to 90 minutes), and (4) recording respiratory/heart rates, oxygen saturations, apnea/bradycardia events. Although this is a recommended practice, limitations have been identified. First, little objective evidence supports the ability of this challenge to absolutely confirm safe travel for an infant. 60 A recent Cochrane review of the literature found no randomized controlled trials that fulfilled eligibility criteria for their review and were unable to recommend or refute a car seat challenge before discharge. 106 Further, common clinical practice has been to recommend the use of a car bed as a safe alternative to infants failing the car seat challenge, although recent studies have failed to prove any difference in apneic events. 77,119 A recommendation has been put forth regarding changing the notion of a “test” or “challenge” to a car seat “orientation” in which the emphasis would be on education on proper positioning, limiting duration of automobile travel, and close observation during travel. 60NUTRITION AND GROWTH
Before discharge, (1) current growth trends should be reviewed; (2) either breast feeding, if desired, should be established or guidelines given for increasing and monitoring; and (3) special feeding considerations such as the use of increased calorie formula, vitamin and mineral supplementation, and use of “special” formulas, tube feedings, or home total parenteral nutrition (TPN) should be outlined.144 In studies of infants who experienced extrauterine growth restriction (EUGR), commonly defined as weight less than the 10th percentile for corrected gestational age (CGA) at discharge, the period from discharge to 30 months is shown to be a critical period for growth. 116 Nutritional intake at this time sets the trajectory for growth and neurodevelopment in childhood and adolescence. VLBW children who have low weight gain in early years of life have a higher probability of cognitive deficits; conversely, those with excessive weight gain had a higher likelihood of obesity, cardiovascular disease, and diabetes. 36
NEURODEVELOPMENT
Some premature infants may have recognized risks to their later development evident at discharge. For these infants, early evaluation of their functional neurologic status may facilitate referrals to early intervention services soon after discharge. 104 Early intervention has been shown to improve neurobehavioral development with improved cognitive outcomes and parent-child interactions. 31,98
Technology-Dependent Infants
Infants who rely on long-term technologic support are being discharged home in increasing numbers. In the past, children who were ventilator dependent, who had tracheostomies, gastrostomies, or jejunostomies, and even those who required long-term intravenous (IV) access for medications or parenteral nutrition would remain hospitalized, separated from their families and susceptible to other morbidities associated with long hospital stays (e.g., infection, delayed development, impaired mental health). With this increase of technology-dependent children discharged into the community comes a greater need for support services for the parents, providers, and the infants themselves. 142 Numerous investigators have undertaken projects to understand the impact that caring for these children has on the individual child and the family as a whole. Carnevale et al described the experiences of 12 families with technology-dependent children at home. 35 They identified key themes in this population to include (1) parental responsibility being stressful and, at times, overwhelming; (2) the devotion of significant energy in trying to “normalize” their home life; (3) living in isolation and feeling like strangers in their own communities; and (4) an overall theme of “daily living with distress and enrichment.”35
In-home nursing care, extensive parent training, and an identified primary care provider comfortable with all aspects of the child’s care are essential for successful discharge of technology-dependent children to home.70 Developing home care plans that contain emergency and resuscitation procedures, as well as parental support and respite services, is vital. 91 Parents need training around all the details of their child’s care, including early signs of illness and emergency procedures, whether or not home shift nursing will be provided. Rooming in with their infant for one or more nights is an important safety net to evaluate their abilities and confidence in caring for their child. Communication and care coordination among the subspecialties following these infants are crucial.
NEURODEVELOPMENTAL FOLLOW-UP OF HIGH-RISK INFANTS
Ideally, parents of all NICU infants would be offered comprehensive, coordinated, developmentally based, family-centered follow-up for their child through infancy and childhood (Figure 31-1). Each infant is unique, as is each infant’s family. The following are the primary objectives of follow-up care:
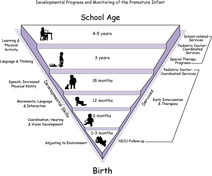 |
| FIGURE 31-1
(Courtesy Angel Carter, Brian S. Carter, and Donna K. Daily, Vanderbilt University Medical Center.)
|
• To counsel the family about their child’s development so that they are empowered to optimize the child’s health, growth, and development
• To recognize and diagnose (early) significant health conditions and neurodevelopmental disabilities to facilitate appropriate referrals for community services
• To anticipate future difficulties and needs so that optimal development is promoted and secondary complications are avoided or minimized
The ultimate goal is to promote the child’s integration into the family, school, and community.
Follow-up resources are generally limited, however, and today health insurance plans (both public and private) determine how children will access care. Consequently, criteria for developmental follow-up of NICU infants vary widely. Some high-risk infants are routinely referred to early intervention programs for developmental care, but implementation of these programs varies among states. The dynamics of development are such that periodic assessments of the child’s health and developmental progress are needed to determine whether current interventions are effective and sufficient. Parents often need guidance to better understand what to expect from their child, how to interpret their own observations of their child, and how health care and community services can support their child’s development. The AAP has emphasized that each child have a “medical home,” and especially the child with complex health and developmental needs. Unfortunately, the care of these children often is fragmented among numerous subspecialists and therapists. 10 Well-organized NICU follow-up clinics can facilitate developmental and health care of the NICU infant in coordination with the primary care provider and the family.
Developmental Milestone Attainment
In developmentally based follow-up, much of the information about the child’s development comes from a careful interview of the parent about the child’s health status and developmental milestone attainment. Noting the age of acquisition of the gross-motor, fine-motor, language, and adaptive-behavioral milestones helps determine a possible developmental delay. Parents are very good historians of their child’s current functioning and recent accomplishments, which is why eliciting a history of milestone attainment during serial clinic visits is so useful in assessing a child’s rate of development. Sometimes additional explanation may be needed to clearly determine the age of acquisition, especially language milestones.
A number of accurate screening tools are available to monitor general developmental progress or domain-specific evaluation. 58 Early delay in language and self-help milestones raises concerns about cognitive development, language disorder, or hearing impairment. 1 Many NICU developmental follow-up clinics rely on pediatric clinical psychologists to formally evaluate the cognition of high-risk infants, preferably with sequential assessments but sometimes with one assessment at a specific age (e.g., at 18 to 24 months corrected age). For infants, the Bayley Scales of Infant and Toddler Development, 3rd edition, is the most commonly used assessment tool in NICU follow-up programs in the United States. 27 For preschool-age and school-age children, several cognitive tests are available, including the Wechsler Preschool and Primary Scale of Intelligence (WPPSI), the Stanford-Binet Intelligence Scales, and the Kaufman Assessment Battery for Children (K-ABC II). 75,112,145
Correction for Degree of Prematurity
One controversy that arises in monitoring developmental scores of preterm infants is whether to correct for degree of prematurity (i.e., whether to use the child’s chronologic age, calculated from birth, or to use corrected age for degree of prematurity). The best evidence supports correcting for degree of prematurity, but whether it is best to correct throughout infancy is controversial, and there is no agreement as to when one should stop correcting for degree of prematurity. 138By convention, most practitioners correct through 2 years of age. It is necessary to be very cautious when interpreting corrected age scores at 12 months or less for ELBW infants. Parental understanding may lead to an overly optimistic outlook that will not be supported by testing at a later date.
Neurodevelopmental Examination
For high-risk infants, the standard pediatric neurologic examination is expanded to include a detailed assessment of posture, muscle tone, reflexes, postural reactions, and functional abilities. Interpretation of the examination requires a thorough understanding of the normal pattern of development over time, the examiner’s skill at assessing the infant’s performance, recognizing deviations from the norm, and determining the significance of these findings. 1,15,18,51
Abnormalities of posture, muscle tone, and reflexes are common in preterm and other high-risk NICU infants during the first year. These abnormalities include asymmetries of movement, marked extensor tone through the neck and trunk with significant shoulder retraction or elevation, hypotonia, and lower extremity hypertonia and hyperreflexia. Cerebral palsy (CP) should be considered in infants with persistent abnormalities in tone, posture, movement, and motor delay. Mild delay and neuromotor tone variation suggest transient neuromotor abnormalities. For many preterm infants, these abnormalities can no longer be elicited at 1 year but they remain at risk for later school and behavior problems. 76
NICU Follow-up Guidelines
The issue of how to do NICU developmental follow-up and how to conduct follow-up studies for high-risk infants has been complex. The methodology can lead to inadequate interpretation of published studies. The National Institute of Child Health and Human Development (NICHD), the National Institute of Neurologic Disorders and Stroke, and the CDC convened a workshop in 2002 to address these issues. Their purpose was to provide standardized guidelines for follow-up care, especially for tertiary care centers with neonatal fellowship training programs in the United States. The results of that workshop have been published and address topics such as risk factors that affect outcome, appropriate assessments, correction for prematurity, assessment tools, and research-related subjects. 138
Again, these criteria may not fit the need or focus of every program. The resources available across states, within communities, and in individual hospitals and the commitment within the NICU strongly drive follow-up programs in different communities. Programs that wish to have developmental follow-up for quality care surveillance and to provide families with information about their high-risk infant can be resourceful in developing partnerships with local early intervention programs and community physicians. Programs that address scientific questions generally need a focused approach or a research network to approach the study. 135
It is now recognized that survival to the end of the NICU stay is a very short-term outcome. It is generally recommended that NICU graduates be followed until at least 8 years of age, but most programs do not have the resources to do this. Furthermore, it has become apparent that the effects of prematurity may extend throughout the life span.64 This is important to know but clearly goes beyond the capability of most NICU follow-up programs.
NEURODEVELOPMENTAL DISABILITIES
Neurodevelopmental disabilities are a group of chronic, nonprogressive disorders of central nervous system (CNS) function that occur as result of malformation of or insult to the developing brain. 1There is a spectrum of neurodevelopmental disabilities, from the major disabilities (CP and mental retardation [MR]), to sensory impairments, and to the more subtle disorders of higher cortical functioning (Box 31-4). Preterm infants are at an increased risk for major disabilities and also for more subtle disabilities. Both may have a long-term impact on their life. 151
BOX 31-4
1. Major disability
Cerebral palsy
Global developmental delay or mental retardation
2. Sensory impairment
Hearing impairment
Visual impairment
3. Subtle disorders of higher cortical function
Language delay or disorder
Expressive language delay
Receptive and expressive language delay
Developmental coordination disorder
Fine-motor incoordination
Sensorimotor integration problems
Learning disability
Variable cognitive abilities
Visual-perceptual problems
Behavior problems
Attention deficit hyperactivity disorder
Social emotional adaptation
Modified from Accardo PJ, editor: Capute and Accardo’s neurodevelopmental disabilities in infancy and childhood, ed 3, Baltimore, Md, 2008, Paul H Brookes; Wolraich ML, editor: Disorders of development and learning, ed 3, Hamilton, Ontario, Canada, 2003, BC Decker.
The World Health Organization (WHO) has revised its definition of disability, impairment, and handicap to an International Classification of Functioning, Disability and Health (ICF) and places emphasis on the interaction of functioning and disability, health condition of the individual, and factors of the environment. The ICF is structured around the following components: (1) body functions and structure, (2) activities and participation, and (3) additional information on severity and environmental factors. 150 This view has aided our thinking not only of the specific disability but also how it affects a child’s ability to function physically and socially within his or her home and community.
Cerebral Palsy
Cerebral palsy “describes a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to non-progressive disturbances that occurred in the developing fetal or infant brain.”114Important to this current definition is the recognition of the accompanying disturbances of sensation, perception, cognition, communication, and behavior and by epilepsy and secondary musculoskeletal problems. CP is the most disabling motor impairment found in preterm infants and is difficult to diagnose with any degree of certainty before 6 to 12 months of age. It sometimes takes until the child is 2 years or older before the diagnosis becomes clear. Cerebral palsy occurs in 7% to 18% of preterm infants and is more common in the most immature infants. 33,92
CP has traditionally been classified by physiologic type (tone abnormality), topography (i.e., muscle groups involved), and severity. 1 Again, the most recent recommendation for definition and classification has been expanded and relates to the WHO ICF. 114,150 Components of the currently recommended classification stress motor abnormalities, accompanying impairments, anatomic and neuroimaging findings, and causation and timing. Because consensus has not been clear on the definition of level of severity, the Gross Motor Classification Scale is now commonly used to address motor function, and scales are being developed to more reliably measure other important areas of functioning, such as hand control, speech, and swallowing. 101,114 Persistently increased muscle tone and increased deep tendon reflexes with persistence of pathologic reflexes (e.g., Babinski) are early signs of spasticity. Variable tone with persistent primitive reflexes, often with involuntary movements, is a sign of extrapyramidal CP. The child may be 2 to 3 years old before involuntary movements are seen. Children who manifest signs of both spasticity and extrapyramidal CP have mixed CP. Extrapyramidal CP is generalized, but spasticity should be further typed according to which limbs are most significantly involved.
Spastic diplegia, the most common form of CP in preterm infants, is characterized by spasticity in both lower extremities, with mild or minimal involvement of the upper extremities.1,92Spastic hemiplegia is characterized by involvement of one side of the body, with the upper extremity more involved than the lower. Because intrauterine and perinatal strokes are usually unilateral, children who had strokes often demonstrate spastic hemiplegia. Quadriplegia is the most severe form of spastic CP, with involvement of both upper and lower extremities and the lower more severely affected than the upper. Children with neonatal encephalopathy (whether caused by hypoxia/ischemia, metabolic disorders, or other causes) who develop CP are most likely to have spastic quadriplegia or severe mixed CP.
Global Developmental Delay and Mental Retardation
Developmental delay is used to describe a deficit in any of the five developmental domains (cognition, motor, language, adaptive, social-emotional skills). Global developmental delay is used to define deficits in two or more areas of development with scores more than two deviations below norm referenced standards.123Currently in the United States, a child may receive services through his or her local school district special education program with a diagnosis of developmental delays until almost 8 years of age before the definition may be switched to mental retardation, again based on standardized testing. Standardized tests, such as the Bayley, 3rd edition, have score groupings and definitions to match, such as low average and borderline, for each domain. 27 It is during this early period of detection that early intervention services become important for the high-risk infant. 31,98,104
Mental retardation is a global impairment of cognitive functioning resulting from injury to or malformation of the developing brain that impairs the child’s ability to adapt and function in society.1,150 MR frequently manifests with an early delay in language and problem-solving abilities. A diagnosis of MR requires a comprehensive evaluation of the child, with neuropsychologic testing of intelligence and assessment of adaptive (functional) abilities, which can be reliably done only at school age.
Neuropsychologic testing includes an assessment of a child’s intelligence quotient (IQ). Intelligence is not one entity but, rather, many different abilities, including auditory and visual memory, visual-perceptual abilities, and understanding complex language concepts. The older the child is, the greater the number of functions that can be tested and therefore the more accurate the tests are in assessing intelligence. Intelligence and functional ability tests for school-age children and adults consist of a variety of subtests. Most children with MR have lower abilities for age across all domains of development, so severity of MR is easily classified. Many preterm children have a significant variability in cognitive functions, with high scores on some subtests and low scores on others, which makes them more difficult to classify and appropriate educational services more difficult to determine.
MR is classified in terms of severity, from profound (IQ below 20), severe (IQ 20 to 34), moderate (IQ 35 to 49), to mild (IQ 50 to 70). 1,150 Children with an IQ of 70 to 80 or 85 have borderline intelligence, not MR. They are capable of academic learning but may have trouble keeping up with their class. The most important characteristics of children with cognitive impairments that enhance adult functioning are interpersonal skills and ability to communicate and relate to other people.
Sensory Impairments
Hearing impairment occurs in 1% to 10% of NICU infants.136Most states require hearing screening for all newborns using a 2-step process: initial screening with otoacoustic emissions, followed by auditory evoked responses if the first test is failed.11,134Because of the risk for progressive hearing impairment, infants with congenital cytomegalovirus (CMV) infection, primary pulmonary hypertension, and congenital diaphragmatic hernia and infants treated with extracorporeal membrane oxygenation (ECMO) should have serial hearing evaluations during infancy and early childhood, as should infants with recurrent ear infections.11,14,56,110,131
Neonates demonstrate hearing thresholds similar to those of older children and adults. Even preterm infants as early as 24 to 25 weeks’ gestation demonstrate an immature brainstem waveform in response to sound stimuli, although the pattern of the waveform matures to a normal waveform as the infant reaches near-term equivalence. Infants hear and process language throughout their first year, beginning at birth.
Retinopathy of prematurity (ROP) results from injury to the very immature developing retina, which causes abnormal proliferation of blood vessels. Severe ROP, which tends to occur in the most immature and sickest preterm infants, is treated with laser to try to prevent retinal detachment and blindness. Preterm infants may develop medically related eye complications (retinal detachment, cataract, glaucoma) and are at increased risk for refractive errors, strabismus, and amblyopia.96 There are no well-delineated guidelines for eye care follow-up of the preterm infant once ROP has resolved. Infants who have structurally normal eyes, no refractive error, and a history of no or low-level regressed ROP may be dismissed by the ophthalmologist after 12 to 18 months of age but should continue to receive routine child-care eye screening based on the AAP recommendations for routine preventive care. 97 Late preterm and term infants with other neonatal complications and neurodevelopmental sequelae will often have visual impairments and need ongoing ophthalmologic follow-up.
Infants with congenital CMV infection or toxoplasmosis should be examined by ophthalmologists for chorioretinitis. Neonates symptomatic with congenital infection (e.g., CMV infection, rubella, toxoplasmosis) have a high risk (20% to 30%) of visual and/or hearing impairment. 141 Preterm infants in the NICU with varying types of postnatal sepsis syndromes have also been identified as having an increased risk for neurodevelopmental impairment. 129 If infants with neonatal encephalopathy develop disability, they tend to have severe multiple disabilities, including cortical visual impairment or processing and hearing impairment. 108
Subtle Disorders of Higher Cortical Function
Even if the NICU graduate does not develop major disability or sensory impairment, he or she remains at increased risk for the more subtle disorders of higher cortical function (seeBox 31-4). These disorders, because they are milder than the major disabilities, may not manifest in infancy or may be associated with only nonspecific symptoms in infancy (e.g., irritability, posturing, feeding problems). Diagnostic criteria, and even nomenclature, for these disorders vary widely, and few reports of preschool and school-age outcome studies have a comparison group evaluated in the identical manner.
Language delay may manifest as early as 6 to 12 months as delay or deviance (i.e., nonsequential) in language milestone acquisition. 1,147 Expressive language delay, either alone or in combination with receptive language delay, is common in preterm and other NICU infants. Every child who manifests with delayed language should have a hearing test and neuropsychologic testing to distinguish between language disorder, hearing impairment, and cognitive impairment.
Developmental coordination disorder (DCD), also referred to as minor neuromotor dysfunction, presents as mild delay or deviant motor milestone acquisition in conjunction with mild or transient neuromotor abnormalities. 33,42,76 These are children who sit by 1 year of age and walk by 2 years, although they may have an atypical pattern to their motor progress (e.g., transient low or high tone, toe-walking, persistent wide-base gait). These children generally look normal by 3 to 5 years of age, although they may continue to have some balance or motor planning problems. Fine-motor incoordination, visual-perceptual deficits, and sensorimotor inefficiencies may accompany DCD, but they may not be recognized until preschool or school age. 21,33,41 Visual-perceptual deficits, often in combination with fine-motor incoordination, are manifest by an inability to recognize and copy figures, letters, and numbers; complete puzzles and mazes; or copy block designs; or by some level of difficulty with these tasks. Fine-motor incoordination makes it difficult to button, zip, cut with scissors, draw, and write. Sensorimotor inefficiencies are characterized by difficulty following directions that include demonstrating an action (e.g., tying shoelaces) and tolerating motion through space (e.g., swinging on a swing) or different tactile sensations (e.g., clothing or food textures). For children with DCD, fine-motor incoordination or sensorimotor inefficiencies and failures in school and on the playground erode self-esteem and peer relationships.
Language disorder, visual-perceptual problems, DCD, transient neuromotor dysfunction, and variable cognitive disabilities are associated with learning disability (LD) and other school problems.1,21,33,41,147 LD means difficulty learning one or more academic subjects (reading, writing, arithmetic) in children with normal intelligence who have had adequate exposure to school. Some children have more of a learning inefficiency, in that they do well in the early grades of school but have a relative inefficiency in reading or writing that causes them trouble as the work becomes more complex. Their intelligence and resiliency help them make adaptations in learning, but they become overwhelmed in situations in which speed and accuracy are viewed as important.
Behavior problems are more common in preterm and other NICU children.19,23,28,73,93 Some children have attention deficit hyperactivity disorder (ADHD), characterized by marked distractibility, short attention span, and impulsivity. ADHD can occur with or without hyperactivity: the child may be restless, always on the move, or constantly busy or may just demonstrate difficulty paying attention and impulsiveness. One must recognize these more subtle problems as soon as possible. Counseling parents and teachers can prevent the devastating effect these “mild” disabilities have on self-esteem, peer relationships, and performance in school and at home.
Diagnosis of Disability
The major disabilities may be recognized and diagnosed in the first 2 years after birth. The more severe the disability, the sooner it may be recognized and diagnosed. Occasionally a child may have significant motor delay initially but seems to “catch up” by 1 to 2 years, with concomitant improvement in neuromotor abnormalities. These are often children with ongoing health problems (e.g., chronic lung disease [CLD]/bronchopulmonary dysplasia [BPD]) and are diagnosed with developmental coordination disorder but have a high risk for learning disability at school age. Language disorders, visual-perceptual difficulties, and fine-motor incoordination are generally recognized and diagnosed during the preschool years (ages 3 to 5 years). Specific learning disabilities and attentional difficulties cannot be diagnosed until school age, usually around 7 to 8 years of age. Mild LD or learning inefficiencies may not be recognized until middle school or high school.
Because there is so much overlap among the neurodevelopmental disabilities, whenever abnormality in one area is detected, the child should have a comprehensive, multidisciplinary evaluation of all his or her abilities. Services are now available to all children through the Individuals with Disabilities Education Act (IDEA 2004). 71 Children from birth to 3 years of age receive services through their early intervention program and after the age of 36 months, receive services through their local public school’s special education program.
PERINATAL RISK FACTORS FOR NEURODEVELOPMENTAL DISABILITY
Many conditions that require neonatal intensive care also increase risk for neurodevelopmental disability, including prematurity, PPHN, hypoxic-ischemic encephalopathy, necrotizing enterocolitis (NEC), maternal chorioamnionitis, neonatal sepsis, and IUGR. Perinatal risk factors can be used to identify NICU infants with a high risk for neurodevelopmental disability so that they can be followed closely and referred for comprehensive evaluations and early intervention programs when appropriate (Box 31-5).
BOX 31-5
• Maternal characteristics
• Socioeconomic status
• Education
• Race/ethnicity
• Obstetric/prenatal complications
• Maternal illness
• Chorioamnionitis
• Maternal ingestions (alcohol, drugs, medications)
• Congenital infection
• Multiple gestation
• Labor or delivery complications
• Placental abnormalities
• Physical characteristics
• Prematurity
• Postmaturity
• Intrauterine growth restriction
• Small for gestational age
• Macrosomia
• Gender
• Microcephaly
• Congenital anomalies
• Dysmorphic features
• Condition at birth
• Apgar scores
• Cord pH
• Meconium staining
• Need for and response to resuscitation
• Neonatal complications
• Hypoxia
• Acidosis
• Hypotension/shock
• Apnea and bradycardia
• Chronic lung disease
• Sepsis
• Meningitis
• Seizures
• Hypoxic-ischemic encephalopathy
• CNS structure and function
• Intraventricular hemorrhage
• Intraparenchymal hemorrhage or infarction
• Ventricular dilation
• Cortical atrophy
• Periventricular leukomalacia
• Burst-suppression pattern on EEG
• Abnormal neurologic examination
CNS, Central nervous system; EEG, electroencephalogram.
Modified from Taeusch W, Ballard R, Gleason C, editors: Avery’s diseases of the newborn, ed 8, Philadelphia, 2005, Saunders.
Risk does not imply causation; it is merely a marker of brain injury. As markers of brain injury, intraventricular hemorrhage (IVH), intraparenchymal hemorrhage, and white matter injury are all important neonatal risk factors. 87 Evidence of brain injury on neuroimaging studies, specifically MRI, has been strongly predictive of adverse neurodevelopmental outcomes. 22,147 There is a great deal of variability in how predictive other individual risk factors are of neurodevelopmental outcome. Very low Apgar scores, especially at 5 to 10 minutes or more after birth, are associated with later CP. These low Apgar scores are signs of severe perinatal depression. However, the signs and symptoms of neonatal encephalopathy predict CP far better than do low Apgar scores. 88 Even within a risk factor category, the degree of risk can vary. Infants who are symptomatic with congenital CMV infection at birth are far more likely to develop CP, MR, and sensory impairment than infants who are asymptomatic at birth with congenital CMV infection. 40,110Multiple risk factors increase an infant’s risk for neurodevelopmental disability, and the effects may be more than additive. As a group, preterm infants or full-term infants with IUGR tend to have lower mean IQ than full-term appropriate for gestational age (AGA) infants. 115,140 Infants with both prematurity and IUGR are vulnerable to the complications of each condition.
SPECIFIC NEURODEVELOPMENTAL OUTCOMES
This section summarizes reported neurodevelopmental outcomes for some of the most frequently encountered conditions in the NICU. A systematic approach (see Box 31-5) allows the clinician to assess the risk factors that may affect developmental outcome. Preterm infants and their medical sequelae are most commonly encountered in neonatal intensive care. However, term infants with pulmonary disease, encephalopathy, or congenital defects also may require intensive care and have significant sequelae. Finally, while we have focused our attention in recent years on smaller and smaller babies in tertiary care centers, it has become apparent that we have often neglected issues for larger “late-preterm” infants (see Chapter 5). These infants are more typically followed in level II or transitional nurseries but are not without risk. It is beyond the scope of this chapter to cover congenital malformations or genetic conditions (see Chapter 27), but it is well known that these infants often require complex multidisciplinary care and the principles of follow-up outlined previously apply to them as well.
Prematurity
For more than 50 years, the medical literature has described the neurodevelopmental outcome of preterm VLBW infants (birth weight <1500 g). With the beginning of modern neonatal intensive care in the mid-1960s, tertiary care NICUs began reporting the incidence or prevalence of major disability (i.e., CP and MR) in survivors.3 Initially, there was a great deal of variability in reported incidences because of differences in populations studied, neonatal intensive care practices, definitions of disability (e.g., whether they include children with mild CP or children with borderline intelligence), and age at follow-up. Because of these issues of study methodology, outcomes are now more frequently reported from large regional studies or research consortiums.
Among a number of studies published between 1988 and 2001, 5% to 19% of ELBW infants with birth weights below 1000 g had CP, with a mean of 10.2%. 33,81,99,118 A number of studies reporting CP by gestational age note a prevalence of CP at 8% to 21% for infants born at less than 26 weeks’ gestation. Studies of populations of VLBW and ELBW children find that their mean intelligence quotients are 0.3 to 0.6 standard deviation below that of full-term controls, or a 4-point to 9-point difference (at times up to a 17-point difference). 23 This is most meaningful when one considers how many more preterm children there are with MR or borderline intelligence. As many as 37% of ELBW children have IQs below 70. 118,136,137,139,148
A recent study of 6-year-old children who were born at less than 26 weeks gestational age found that 12% had disabling CP and 21% had moderate to severe cognitive impairment (IQ <70). 84 When compared with full-term controls, 41% of these extremely preterm children had moderate to severe cognitive impairments; one quarter had borderline intelligence; 10% had hearing loss (profound in 3%), and 7% had visual impairments (2% were blind). The lower border of viability is currently 23 to 24 weeks’ gestation and approximately 500 g birth weight. There have been rare survivors reported to have been born at 22 weeks’ gestation or at 300 to 500 g birth weight. 68 Few, if any, of the survivors at these extremes have been reported as demonstrating normal development. The largest study of infants born at less than 26 weeks’ gestation and followed to age 6 years found that 22% had severe disability, 24% had moderate disability, 34% had mild disability, and only 20% were normal.83,84
As many as 50% of VLBW children and 60% to 70% of ELBW children have school problems, including LD, failed grades, and special education requirements.23,93 Studies that have compared the outcomes of normally intelligent VLBW children with full-term control subjects have found that VLBW children have more language delay, more visual-perceptual problems, lower reading quotients, greater difficulty with arithmetic, and more problems with attention and behavior. Compared with full-term controls, ELBW children are three to five times more likely to require special resources in school, and this increases to 8 to 10 times more likely by adolescence. 64
Recent reviews and meta-analyses of outcomes of children who were born prematurely do not show improvement over time in rates of CP or presence of cognitive impairment. 30,92,99,113 While rates of CP are higher in the ELBW infant, there is also an independent increase in cognitive impairment with a correlation between cognitive impairment and birth weight or gestational age. 20,28 Advances in neuroimaging techniques are starting to correlate newborn brain injury with developmental outcome, but as yet, they are not of sufficient sensitivity and specificity to give predictive information to families. 67,149
Late Preterm Infants
In the past decade, there has been renewed interest and concern in the health and development of the larger preterm infant. While these infants make up the greater proportion of all preterm infants, their neonatal course is generally less complicated and they may actually receive their care in a level I or II nursery. Moderately-low-birth-weight infants (between 1500 and 2500 g) have been reported to have increased risk for neurodevelopmental sequelae and account for between 18% and 37% of all children with CP and 7% and 12% of children with mental retardation. 15 Even larger preterm infants, now called late preterm infants, make up 75% of all preterm births and are defined as infants born between 34 and 36 completed weeks. These infants are physiologically and metabolically immature, which predisposes them to special transitional care needs. 53 If these needs are not met or recognized, such as post-discharge hyperbilirubinemia, these infants may be placed at risk for neurodevelopmental sequelae. 2,52 Recent outcome information about this group of infants suggests that they are likely to have more problems with school readiness and that even CP and developmental delay (DD)/MR rates are increased when compared with term gestation. 2,85,103
Intrauterine Growth Restriction and Extrauterine Growth Restriction
Prenatal and postnatal growth can seriously affect neurodevelopmental outcomes of high-risk infants. The neurodevelopmental outcome of IUGR infants is strongly associated with the cause of IUGR; with the timing, severity, and duration of the insult; and with perinatal complications the IUGR infant encounters (see Box 31-4). Early severe IUGR often reflects a chromosomal anomaly, other severe genetic disorder, or congenital infection that occurred early to cause organ malformation or significant injury. Some causes of IUGR result in death (e.g., trisomy 18) or severe disability. Some carry a high risk for neurodevelopmental disability (e.g., fetal alcohol syndrome). Others are associated with only mild disability (e.g., an increased incidence of attention and behavior problems in infants born to mothers who took narcotics or cocaine during pregnancy).
One of the most common causes of IUGR is uteroplacental insufficiency, generally a diagnosis of exclusion. The fetus responds in many adaptive ways when the supply of nutrients or oxygen is limited. 16,17 There is first a decrease in subcutaneous tissue, resulting in lower birth weight, and then a decrease in length, before head and brain growth are affected (symmetric growth restriction). Nevertheless, the problem may be severe enough to overwhelm these adaptations and lead to brain injury. In addition, a chronically compromised fetus, with decreased glycogen and nutrient stores, has more difficulty with the stresses of labor and delivery, leading to perinatal depression, cold stress, hypoglycemia, and hypocalcemia. Polycythemia may result from chronic intrauterine hypoxia but may result in the complications of hyperviscosity. 115
Prospective studies of full-term infants with IUGR do not show an increased risk for major disability, but retrospective studies of CP and MR have demonstrated that more disabled children had IUGR than was expected. 3 Prospective studies of full-term IUGR school-age children compared with full-term AGA children showed that more IUGR children had language problems, learning disability, minor neuromotor dysfunction, hyperactivity, and attention and behavior problems. Postnatal growth may also be affected, but this will largely depend on whether the infant has symmetric or asymmetric IUGR. Preterm IUGR children demonstrate the disadvantages of both prematurity and IUGR, but which is more important in determining outcomes is not clear. The degree of IUGR may influence early delivery, either spontaneous or induced, because of concerns of fetal well-being. The most striking findings in studies of preterm IUGR children and preterm AGA controls are the high rates of major disability (7% to 23%) and LDs (36% to 50%). 80
Extrauterine growth restriction, that is delayed growth after birth, also occurs commonly in ill preterm newborns.36,116 The long-term effects of this early delayed growth have been unclear. 74 However, infants in the lower quartiles of growth in the NICU have higher incidences of neurodevelopmental impairment at 18 to 22 months follow-up in the NICHD-Neonatal Research Network. Although markers of illness severity such as NEC or BPD had a significant effect on outcome, poor postnatal growth itself may exert an independent effect. 47Post-discharge growth failure for preterm infants is also a common problem, especially in those with associated CLD. Transitional formulas, occasionally with caloric concentration, may be needed to optimize growth and subsequent development.46,61
Neonatal Encephalopathy
The extent and nature of an initial hypoxic-ischemic event cannot be easily determined for individual infants, leading clinicians to rely on recognizable signs and symptoms of neonatal encephalopathy to predict outcome. These signs and symptoms include the following:
• Poor feeding
• Hypotonia or extensor hypertonia
• Lethargy or hyperexcitability
• Apnea
• Seizures
• Abnormalities on neuroimaging studies, which are far more predictive than low Apgar scores
• The need for positive-pressure ventilation or CPR at birth
• Initial response to resuscitation
Many infants with congenital brain malformations or prenatal brain injury may present with perinatal cardiorespiratory depression. They do not breathe normally at birth and may require positive-pressure ventilation or further resuscitation. It is very difficult to distinguish these infants from those with encephalopathy caused by hypoxia or ischemia. Metabolic problems or neonatal sepsis may also present with these signs. Therefore the term neonatal encephalopathy is preferred over the term hypoxic-ischemic encephalopathy (HIE), because etiology cannot always be determined with certainty. However, the clinician must evaluate the history and relevant factors in each infant to address the etiology of their encephalopathy. The etiology may be important in decisions about treatment, prognosis, and follow-up, as well as for family planning.
In the absence of a confirmed specific etiology, the stages of encephalopathy described in 1976 by Sarnat and Sarnat remain highly predictive of outcome.120 Infants with stage 3 (severe) encephalopathy and coma, severe hypotonia or increased extensor tone, intermittent decerebration, decreased or absent reflexes, variable pupil reactivity, and abnormal electroencephalogram (EEG) generally will die or have multiple severe disabilities. Only 20% to 30% of infants with stage 2 (moderate) encephalopathy with lethargy or coma, mild hypotonia, overactive reflexes, seizures, abnormal EEG, and generalized parasympathetic function (constricted pupils, bradycardia, profuse secretions, and diarrhea) have multiple severe disabilities. The remainder of newborns with stage 2 encephalopathy have lower scores on tests of cognition, vocabulary, reading, spelling, and arithmetic than children with stage 1 (mild) encephalopathy (hyperalert state, jitteriness, overactivity and easily elicited reflexes, increased sympathetic function, dilated pupils, and decreased gastrointestinal motility) or healthy control children. Infants with neonatal encephalopathy should be evaluated with both EEGs and neuroimaging studies. Very-low-voltage EEG patterns (signifying little brain activity) and burst-suppression EEG patterns carry an extremely poor prognosis, as does diffuse encephalomalacia detected by MRI. 108,128 Infants with moderate encephalopathy may benefit from the newer technique of hypothermic treatment, commonly called “cooling,” (see Chapter 26) leading to increased survival without disability. The results from all published trials in North America and Europe are most promising for encephalopathies of less than a severe nature. 24,63,122 Mild encephalopathy with only a subarachnoid hemorrhage carries a good prognosis, although these children should be monitored for later subtle learning difficulties.
Infants with ischemic perinatal stroke are another group of infants with brain injury who are at risk for long-term developmental sequelae. This entity is generally distinctly different from diffuse ischemia seen in the so-called “watershed” injury of perinatal HIE. Timing of the event may also be less easily determined, but the infant may present with focal or generalized seizures or less-defined clinical signs such as poor perfusion (“dusky” or “gray” spells), respiratory distress or apnea, poor feeding, or low neuromotor tone in the first few days of life. MRI is the most reliable method of diagnostic detection in the newborn period if timed appropriately. Neurologic deficits have been reported in 50% to 75% of survivors, and hemiplegic CP is most commonly found. Later difficulties with sensory impairment or learning difficulties are also present, thus requiring long-term follow-up. 107
Persistent Pulmonary Hypertension of the Newborn/Meconium Aspiration Syndrome
PPHN and MAS often overlap clinically, and many of these infants require neonatal intensive care technologies, including inhaled nitric oxide, high-frequency ventilation, and ECMO. Full-term survivors of this care have an increased risk for major disability (2% to 26%) and milder impairments (8% to 49%), including minor neuromotor dysfunction, borderline intelligence, language delay, and attention problems.43,59 Both CLD (seen in 7% to 40%) and hearing impairment (seen in 20% to 50%) are common sequelae of PPHN or ECMO. Most recent studies of ECMO that include venovenous as opposed to venoarterial flow report a lower incidence (≈15%) of developmental disabilities. In the past decade, inhaled nitric oxide has become a more common treatment of pulmonary hypertension in the newborn, and follow-up studies indicate that these infants have a developmental disability rate of 14% to 19%. 38,50 Because the hearing impairment associated with either PPHN or ECMO may be progressive, serial hearing evaluations during infancy and early childhood are required. 56
Bronchopulmonary Dysplasia/Chronic Lung Disease
BPD or chronic lung disease (CLD) is the most common morbidity in surviving preterm infants. 55 The etiology of neonatal lung injury appears to have changed over time as methods of treatment of respiratory failure have changed and as an increasing number of extremely immature preterm infants survive. 26,72Regardless of specific cause, the risk for pulmonary hypertension, post-discharge growth failure, recurrent hospitalizations, and adverse neurodevelopmental outcomes persists in children with BPD.19,78,100,124,125 Many of these infants exhibit low tone and early motor delays, often consistent with developmental coordination disorder. 69 Cerebral palsy is highly associated with infants of extremely low birth weight, as is BPD, but varies in infants with BPD from 11% to 27% based on severity of BPD. Likewise, lower cognitive scores are related to severity of BPD and may be present in 50% of infants with severe BPD. 48,124
TRACKING HEALTH OUTCOMES: THE PRIMARY CARE PROVIDER
Primary Care Follow-up
At the time of discharge, the NICU staff must provide the primary care pediatrician and the parents with a complete and accurate history of the child’s NICU course including recommendations for ongoing care. Special health concerns, specific to the premature infant, should be closely monitored and surveillance of these should supplement the AAP guidelines for preventive, “well-child” care. These additional areas of special concern for the premature infant would include such things as the following:
• Neurodevelopmental follow-up
• Visual and hearing outcomes
• Growth, nutrition, and feeding issues
• Osteopenia of prematurity
• Dental enamel defects
• Sequelae related to issues during hospitalization including pulmonary, gastrointestinal, hematologic, and surgical conditions
Along with the subspecialty medical follow-up, the primary care provider will also need to coordinate other supportive services such as early intervention programs and developmental follow-up through an NICU follow-up program, if available.34Recommendations for specialized follow-up of the late preterm infant have also been proposed with the focus on feeding, sleeping, temperature regulation, jaundice, and infection in an effort to reduce post-hospital morbidities and rehospitalization of these infants.52,143
Growth, Nutrition, and Feeding
Premature infants are at increased risk for growth deficits after discharge—many are discharged below the body weight of their healthy term counterparts. The failure to achieve adequate growth is known as extrauterine growth restriction (EUGR) and is defined by weight less than the 10th percentile for CGA at the time of discharge.116 These infants remain at risk for long-term adverse health and neurodevelopmental outcomes. As such, early nutrition and feeding support are essential.
Recommendations from the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition support breast feeding of AGA infants or formula with long-chain polyunsaturated fatty acids for formula-fed, appropriately grown infants. 54For those infants with evidence of growth deficits present at discharge (EUGR), breast milk should be supplemented with a human milk fortifier. Formula-fed infants should receive a special post-discharge formula, with increased protein, minerals, and long-chain polyunsaturated fatty acids, for at least the first 9 months.8 Serial measurements of head circumference, weight, length, and weight/length ratio should be closely monitored. 130
Some infants do not achieve full breast feeding before discharge. It is essential, therefore, to provide clear verbal and written instruction to the breast-feeding mother about how to assess her infant’s hydration status if the transition from partial to full breast feeding is to occur at home, along with ensuring that the mother has access to medically sound breast-feeding support from the child’s pediatrician or a qualified lactation consultant.
Although premature infants are at an increased risk for childhood obesity and the metabolic syndrome associated with excess weight gain, this risk is thought to be small when compared with the risk for growth failure and should be considered with other risk factors such as parental size, adolescent weight, and lifestyle factors. 61 Nutritional status should be monitored closely to intervene for either deficient or excessive weight variations.
Medically Fragile and Chronically Ill Infants
Increasing survival of infants with post-NICU morbidities, including those associated with congenital diaphragmatic hernia (CDH), short bowel syndrome, and others, has increased the need for detailed parent training, home health care arrangements, and comprehensive, coordinated, multidisciplinary follow-up. The AAP has recently issued extensive follow-up guidelines for infants with CDH including monitoring neurodevelopment, managing pulmonary morbidities such as pulmonary hypertension and chronic lung disease, assessing hearing function at regular intervals, providing early therapies for feeding difficulties such as oral aversion and gastroesophageal reflux, and monitoring for hernia recurrence, which have been reported in 8% to 50% of CDH infants. 14 Brodsky and Ouellette recommend close monitoring of infants with a history of NEC and/or short bowel syndrome to include neurodevelopmental delays; growth, nutrition, and feeding concerns; dehydration and electrolyte imbalances; and signs of associated complications such as infection, late strictures, and cholestatic liver disease. 34
LONG-TERM NEURODEVELOPMENTAL FOLLOW-UP
During hospitalization, parents may ask questions about outcome that simply cannot be answered. Although the infant’s risk for cognitive and motor impairment should be discussed, parents must understand that the certain diagnosis of developmental delay or disability cannot be made before 12 to 24 months of age. This need to “wait and see” creates an additional burden for families that the NICU staff should help the family anticipate. Referral to a multidisciplinary developmental follow-up clinic should provide the family with ongoing information about their child’s progress and give parents the opportunity to speak with professionals about their concerns. These clinics often provide families with concrete, focused tasks to undertake with their child that may optimize infant development and help parents feel they are contributing to their child’s success.
An additional concern that has more recently surfaced is even longer–term outcome issues for high-risk children. Because many children may be seen once for a multidisciplinary evaluation (18 to 24 months corrected age) or may not be followed beyond 3 to 5 years of age in the NICU follow-up clinic, it is important that parents be informed before release from specialty follow-up care about longer-term concerns regarding health and development, especially possible challenges with academics and social-emotional issues. 64,93,94,151
Pediatric health care professionals need to be aware also that recent reports delineate some more lifelong impacts on the former NICU patient’s quality of life as a child and adolescent, daily personal and social functions, and overall experiences with health and disease. Although much of this information may be speculative and studies remain ongoing, it is nonetheless important to inform the family that their child’s long-term health and quality of life issues may relate to their child with a history of prematurity, regardless of the developmental status of the child when seen in the first few years of life.
REFERENCES
1. Accardo, P.J., Capute and Accardo’s neurodevelopmental disabilities in infancy and childhood. ed 3 ( 2008)Paul H Brookes, Baltimore, Md.
2. Adams-Chapman, I., Neurodevelopmental outcome of the late preterm infant, Clin Perinatol 33 (2006) 947.
3. Allen, M.C., Preterm outcomes research: a critical component of neonatal intensive care, Ment Retard Dev Disabil Res Rev 8 (2002) 22.
4. American Academy of Pediatrics, Transporting children with special health care needs, Pediatrics 104 (1999) 988.
5. American Academy of Pediatrics, Committee on Fetus and Newborn, Apnea, sudden infant death syndrome, and home monitoring, Pediatrics 111 (2003) 914.
6. American Academy of Pediatrics, Committee on Fetus and Newborn, Hospital discharge of the high-risk neonate, Pediatrics 122 (2008) 1119.
7. American Academy of Pediatrics, Committee on Injury and Poison Prevention, Safe transportation of newborns at hospital discharge, Pediatrics 104 (1999) 986.
8. American Academy of Pediatrics, Committee on Nutrition, Nutritional needs of the preterm infant, In: (Editor: Kleinman, R.E.) Pediatric nutrition handbooked 5 ( 2004)The Academy, Elk Grove Village, Ill.
9. American Academy of Pediatrics, Committee on Injury and Poison Prevention and Committee on Fetus and Newborn, Safe transportation of preterm and low birth weight infants at hospital discharge, Pediatrics 123 (2009) 1424.
10. American Academy of Pediatrics, Council on Children with Disabilities, Care coordination in the medical home: integrating health and related systems of care for children with special health care needs, Pediatrics 116 (2005) 1238.
11. American Academy of Pediatrics, Joint Committee on Infant Hearing, Year 2007 Position Statement, Principles and guidelines for early hearing detection and intervention programs, Pediatrics 120 (2007) 898.
12. American Academy of Pediatrics, Newborn Screening Authoring Committee, Newborn screening expands: recommendations for pediatricians and medical homes—implications for the system, Pediatrics 121 (2008) 192.
13. American Academy of Pediatrics, Section on Ophthalmology, Screening examination of premature infants for retinopathy of prematurity, Pediatrics 117 (2006) 572.
14. American Academy of Pediatrics, Section on Surgery and the Committee on Fetus and Newborn, Postdischarge follow-up of infants with congenital diaphragmatic hernia, Pediatrics 121 (2008) 627.
15. Amiel-Tison, C.; Allen, M.C.; Lebrun, F.; et al., Macropremies: underprivileged newborns, Ment Retard Dev Disabil Res 8 (2002) 281.
16. Amiel-Tison, C.; Cabrol, D.; Denver, R.; et al., Fetal adaptation to stress. I. Acceleration of fetal maturation and earlier birth triggered by placental insufficiency in humans, Early Hum Dev 78 (2004) 15.
17. Amiel-Tison, C.; Cabrol, D.; Denver, R.; et al., Fetal adaptation to stress. II. Evolutionary aspects; stress-induced hippocampal damage; long-term effects on behavior; consequences on adult health, Early Hum Dev 78 (2004) 81.
18. Amiel-Tison, C.; Gosselin, J., Neurological development from birth to six years. ( 2001)Johns Hopkins University Press, Baltimore, Md.
19. Anderson, P.J.; Doyle, L.W., Neurodevelopmental outcome of bronchopulmonary dysplasia, Semin Perinatol 30 (2006) 227.
20. Anderson, P.J.; Doyle, L.W., Cognitive and educational deficits in children born extremely preterm, Semin Perinatol 32 (2008) 51.
21. Arnaud, C.; Daubisse-Marliac, L.; White-Koning, M.; et al., Prevalence and associated factors of minor neuromotor dysfunctions at age 5 years in prematurely born children, Arch Pediatr Adolesc Med 161 (2007) 1053.
22. Arzoumanian, Y.; Mirmiran, M.; Barnes, P.D.; et al., Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants, AJNR Am J Neuroradiol 24 (2003) 1646.
23. Aylward, G.P., Cognitive and neuropsychological outcomes: more than IQ scores, Ment Retard Dev Disabil Res Rev 8 (2002) 234.
24. Azzopardi, D.; Edwards, A.D., Hypothermia, Semin Fetal Neonatal Med 12 (2007) 303.
25. Back, S.A.; Riddle, A.; McClure, M.M., Maturation-dependent vulnerability of perinatal white matter in premature birth, Stroke 38 (Suppl 2) ( 2007) 724.
26. Bancalari, E.; Claure, N., Definitions and diagnostic criteria for bronchopulmonary dysplasia, Semin Perinatol 30 (2006) 164.
27. Bayley, N., Bayley scales of infant and toddler development. ed 3 ( 2006)The Psychological Corporation, San Antonio, Tex.
28. Bhutta, A.T.; Cleves, M.A.; Casey, P.H.; et al., Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis, JAMA 288 (2002) 728.
29. Bhutta, Z.A.; Khan, I.; Salat, S.; et al., Reducing length of stay in hospital for very low birthweight infants by involving mothers in a stepdown unit: an experience from Karachi (Pakistan), BMJ 329 (2004) 1151.
30. Blair, E.; Watson, L., Epidemiology of cerebral palsy, Semin Fetal Neonatal Med 11 (2006) 117.
31. Bonnier, C., Evaluation of early stimulation programs for enhancing brain development, Acta Paediatr 97 (2008) 853.
32. Bos, A.F.; Einspieler, C.; Prechtl, H.F., Intrauterine growth retardation, general movements, and neurodevelopmental outcome: a review, Dev Med Child Neurol 43 (2001) 61.
33. Bracewell, M.; Marlow, N., Patterns of motor disability in very preterm children, Ment Retard Dev Disabil Res Rev 8 (2002) 241.
34. In: (Editors: Brodsky, D.; Ouellette, M.) Primary care of the premature infant ( 2008)Saunders, Philadelphia.
35. Carnevale, F.A.; Alexander, E.; Davis, M.; et al., Daily living with distress and enrichment: the moral experience of families with ventilator-assisted children at home, Pediatrics 117 (2006) e48.
36. Casey, P.H., Growth of low birth weight preterm children, Semin Perinatol 32 (2008) 20.
37. Centers for Disease Control and Prevention, Recommended immunization schedules for persons aged 0-18 years—United States, MMWR 56 (51&52) ( 2008) Q1.
38. Clark, R.H.; Huckaby, J.L.; Kueser, T.J.; et al., Low-dose nitric oxide therapy for persistent pulmonary hypertension: 1-year follow-up, J Perinatol 23 (4) ( 2003) 300.
39. Costello, A.; Chapman, J., Mother’s perceptions of the care-by-parent program prior to hospital discharge of their preterm infants, Neonatal Netw 17 (1998) 37.
40. Dahle, A.J.; Fowler, K.B.; Wright, J.D.; et al., Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus, J Am Acad Audiol 11 (2000) 283.
41. Davis, L.; Edwards, H.; Mohay, H.; et al., The impact of very premature birth on the psychological health of mothers, Early Hum Dev 73 (2003) 61.
42. Davis, N.M.; Ford, G.W.; Anderson, P.J.; , the Victorian Infant Collaborative Study Group, Developmental coordination disorder at 8 years of age in a regional cohort of extremely-low-birthweight or very preterm infants, Dev Med Child Neurol 49 (2007) 325.
43. Desai, S.A.; Stanley, C.; Gringlas, M.; et al., Five-year follow-up of neonates with reconstructed right common carotid arteries after extracorporeal membrane oxygenation, J Pediatr 134 (1999) 428.
44. Donohue, P.K.; Hussey-Gardner, B.; Sulpar, L.J.; et al., Parents’ perception of the back-transport of very-low-birth-weight infants to community hospitals, J Perinatol 29 (8) ( 2009) 575.
45. Doyle, L.W., Outcome at 5 years of age of children 23 to 27 weeks’ gestation: refining the prognosis, Pediatrics 108 (2001) 134.
46. Dusick, A.M.; Pindexter, B.B.; Ehrenkranz, R.A.; et al., Growth failure in the preterm infant: can we catch up?Semin Perinatol 27 (2003) 302.
47. Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; et al., Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants, Pediatrics 117 (2006) 1253.
48. Ehrenkranz, R.A.; Walsh, M.D.; Vohr, B.R.; et al., Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia, Pediatrics 116 (2005) 1353.
49. Eichenwald, E.C.; Blackwell, M.; Lloyd, J.S., Inter-neonatal intensive care unit variation in discharge timing: influence of apnea and feeding management, Pediatrics 108 (2001) 928.
50. Ellington, M.; O’Reilly, D.; Allred, E.N.; et al., Child health status, neurodevelopmental outcome, and parental satisfaction in a randomized, controlled trial of nitric oxide for persistent pulmonary hypertension of the newborn, Pediatrics 107 (2001) 1351.
51. Ellison, P.H.; Daily, D.K., The neurologic examination of the preterm and full-term neonate and of the infant, In: (Editor: David, R.B.) Child and adolescent neurologyed 2 ( 2005)Blackwell Publishing, Oxford, UK.
52. Engle, W.A.; Tomashek, K.M.; Wallman, C., Late-preterm infants: a population at risk, Pediatrics 120 (2007) 1390.
53. Escobar, G.J.; Clark, R.H.; Greene, J.D., Short-term outcomes of infants born at 35 and 36 weeks gestation: we need to ask more questions, Semin Perinatol 30 (2006) 28.
54. ESPGHAN Committee on Nutrition, Feeding preterm infants after hospital discharge: a commentary by the ESPGHAN Committee on Nutrition, J Pediatr Gastroenterol Nutr 42 (2006) 596.
55. Fanaroff, A.A.; Stoll, B.J.; Wright, L.L.; et al., Trends in neonatal morbidity and mortality for very low birthweight infants, Am J Obstet Gynecol 196 (2007) 147.e1.
56. Fligor, B.J.; Neault, M.W.; Mullen, C.H.; et al., Factors associated with sensorineural hearing loss among survivors of extracorporeal membrane oxygenation therapy, Pediatrics 115 (2005) 1519.
57. Garel, M.; Dardennes, M.; Blondel, B., Mothers’ psychological distress 1 year after very preterm childbirth: results of the EPIPAGE qualitative study, Child Care Health Dev 33 (2007) 137.
58. Glascoe, F.P., Screening for developmental and behavioral problems, MRDD Res Reviews 11 (2005) 173.
59. Glass, P.; Bulas, D.I.; Wagner, A.E.; et al., Severity of brain injury following neonatal extracorporeal membrane oxygenation and outcome at age 5 years, Dev Med Child Neurol 39 (1997) 441.
60. Greenberg, J.M., The challenge of car safety seats, J Pediatr 150 (2007) 215.
61. Greer, F.R., Post-discharge nutrition: what does the evidence support?Semin Perinatol 31 (2007) 89.
62. Griffin, T.; Abraham, M., Transition to home from the newborn intensive care unit: applying the principles of family-centered care to the discharge process, J Perinat Neonatal Nurs 20 (2006) 243.
63. Gunn, A.J.; Wyatt, J.S.; Whitelaw, A.; et al., Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy, J Pediatr 152 (2008) 55.
64. Hack, M., Young adult outcomes of very low birth weight children, Semin Fetal Neonatal Med 11 (2006) 127.
65. Hack, M.; Wilson-Costello, D.; Friedman, H.; et al., Neurodevelopment and predictors of outcomes of children with birth weights of less than 1000 g: 1992-1995, Arch Pediatr Adolesc Med 154 (2000) 725.
66. Hanrahan, K.; Gates, M.; Attar, M.A.; et al., Neonatal back transport: perspectives from parents of Medicaid-insured infants and providers, Neonatal Netw 26 (2007) 301.
67. Hart, A.R.; Whitby, E.W.; Griffiths, P.D.; et al., Magnetic resonance imaging and developmental outcome following preterm birth: review of current evidence, Dev Med Child Neurol 50 (2008) 655.
68. Hoekstra, R.E.; Ferrara, T.B.; Couser, R.J.; et al., Survival and long-term neurodevelopmental outcome of extremely premature infants born at 23-26 weeks’ gestation age at a tertiary center, Pediatrics 113 (2004) e1.
69. Holsti, L.; Granau, R.; Whitfield, M.F., Developmental coordination disorder in extremely low birth weight children at nine years, J Dev Behav Pediatr 23 (2002) 9.
70. Hummel, P.; Cronin, J., Home care of the high-risk infant, Adv Neonatal Care 4 (2004) 354.
71. Individuals with Disabilities Education Act (IDEA), ( 2004) ; website:http://idea.ed.gov/.
72. Jobe, A.H., The new BPD, NeoReviews 7 (2006) e531.
73. Johnson, S., Cognitive and behavioural outcomes following very preterm birth, Semin Fetal Neonatal Med 12 (2007) 363.
74. Kan, E.; Roberts, G.; Anderson, P.J.; , the Victorian Infant Collaborative Study Group, The association of growth impairment with neurodevelopmental outcome at eight years of age in very preterm children, Early Hum Dev 84 (2008) 409.
75. Kaufman, A.S.; Kaufman, N.L., Kaufman assessment battery for children. ed 2 ( 2004)AGS Publishing, Circle Pines, Minn.
76. Khadilkar, V.; Tudehope, D.; Burns, Y.; et al., The long-term neurodevelopmental outcome for very low birthweight (VLBW) infants with “dystonic” signs at 4 months of age, J Paediatr Child Health 29 (6) ( 1993) 415.
77. Kinane, T.B.; Murphy, J.; Bass, J.L.; et al., Comparison of respiratory physiologic features when infants are placed in car safety seats or car beds, Pediatrics 118 (2006) 522.
78. Kobaly, K.; Schluchter, M.; Minich, N.; et al., Outcomes of extremely low birth weight (<1 kg) and extremely low gestational age (<28 weeks) infants with bronchopulmonary dysplasia: effects of practice changes in 2000 to 2003, Pediatrics 121 (2008) 73.
79. Lainwala, S.; Perritt, R.; Poole, K.; et al., Neurodevelopmental and growth outcomes of extremely low birth weight infants who are transferred from neonatal intensive care units to level I or level II nurseries, Pediatrics 119 (2007) e1079.
80. Leonard, H.; Nassar, N.; Bourke, J.; et al., Relation between intrauterine growth and subsequent intellectual disability in a ten-year population cohort of children in Western Australia, Am J Epidemiol 167 (2007) 103.
81. Lorenz, J.M., The outcome of extreme prematurity, Semin Perinatol 25 (2001) 348.
82. Lovette, B., Safe transportation for children with special needs, J Pediatr Health Care 22 (2008) 323.
83. Marlow, N., Neurocognitive outcome after very preterm birth, Arch Dis Child Fetal Neonatal Ed 89 (2004) F224.
84. Marlow, N., Outcome following extremely preterm birth, Curr Obstet Gynaecol 16 (2006) 141.
85. McCormick, M.C.; Brooks-Gunn, J.; Buka, S.L.; et al., Early intervention in low birth weight premature infants: results at 18 years of age for the Infant Health and Development Program, Pediatrics 117 (2006) 771.
86. Melnyk, B.M.; Feinstein, N.F.; Alpert-Gillis, L.; et al., Reducing premature infants’ length of stay and improving parents’ mental health outcomes with the Creating Opportunities for Parent Empowerment (COPE) neonatal intensive care unit program: a randomized, controlled trial, Pediatrics 118 (2006) e1414.
87. Ment, L.R.; Vohr, B.R., Preterm birth and the developing brain, Lancet Neurol 7 (2008) 378.
88. Mercuri, E.; Rutherford, M.; Barnett, A.; et al., MRI lesions and infants with neonatal encephalopathy: is the Apgar score predictive?Neuropediatrics 33 (2002) 150.
89. Merritt, T.A.; Pillers, D.; Prows, S.L., Early NICU discharge of very low birth weight infants: a critical review and analysis, Semin Neonatol 8 (2003) 95.
90. Mirmiran, M.; Barnes, P.D.; Keller, K.; et al., Neonatal brain magnetic resonance imaging before discharge is better than serial cranial ultrasound in predicting cerebral palsy in very low birth weight preterm infants, Pediatrics 114 (2004) 992.
91. Montagnino, B.A.; Mauricio, R.V., The child with a tracheostomy and gastrostomy: parental stress and coping in the home—a pilot study, Pediatr Nurs 30 (2004) 373.
92. Msall, M.E., The panorama of cerebral palsy after very and extremely preterm birth: evidence and challenges, Clin Perinatol 33 (2006) 269.
93. Msall, M.E.; Park, J.J., The spectrum of behavioral outcomes after extreme prematurity: regulatory, attention, social, and adaptive dimensions, Semin Perinatol 32 (2008) 42.
94. Msall, M.E.; Tremont, M.R., Measuring functional outcomes after prematurity: developmental impact of very low birth weight and extremely low birth weight status on childhood disability, Ment Retard Dev Disabil Res Rev 8 (2004) 258.
95. Norr, K.F.; Crittenden, K.S.; Lehrer, E.L.; et al., Maternal and infant outcomes at one year for a nurse-health advocate home visiting program serving African Americans and Mexican Americans, Public Health Nurs 20 (2003) 190.
96. O’Connor, A.R.; Fielder, A.R., Visual outcomes and perinatal adversity, Semin Fetal Neonatal Med 12 (2007) 408.
97. O’Connor, A.R.; Fielder, A.R., Long term ophthalmic sequelae of prematurity, Early Hum Dev 84 (2008) 101.
98. Ohgi, S.; Fukuda, M.; Akiyama, T.; et al., Effect of an early intervention programme on low birthweight infants with cerebral injuries, J Paediatr Child Health 40 (2004) 689.
99. O’Shea, M., Cerebral palsy, Semin Perinatol 32 (2008) 35.
100. O’Shea, T.M.; Goldstein, D.J.; deRegnier, R.A.; et al., Outcome at 4 to 5 years of age in children recovered from neonatal chronic lung disease, Dev Med Child Neurol 38 (1996) 830.
101. Palisano, R.; Rosenbaum, P.; Walter, S.; et al., Development and reliability of a system to classify gross motor function in children with cerebral palsy, Dev Med Child Neurol 39 (1997) 214.
102. Paul, I.M.; Phillips, T.A.; Widome, M.D.; et al., Cost-effectiveness of postnatal home nursing visits for prevention of hospital care for jaundice and dehydration, Pediatrics 114 (2004) 1015.
103. Petrini, J.R.; Dias, T.; McCormick, M.C.; et al., Increased risk of adverse neurological development for late preterm infants, J Pediatr 154 (2009) 169.
104. Picciolini, O.; Gianni, M.L.; Vegni, C.; et al., Usefulness of an early neurofunctional assessment in predicting neurodevelopmental outcome in very low birthweight infants, Arch Dis Child Fetal Neonatal Ed 91 (2006) F111.
105. Pilley, E.; McGuire, W., The car seat: a challenge too far for preterm infants?Arch Dis Child Fetal Neonatal Ed 90 (2005) F452.
106. Pilley, E.; McGuire, W., Pre-discharge “car seat challenge” for preventing morbidity and mortality in preterm infants, Cochrane Database Syst Rev 1 (2008); CD005386.
107. Raju, T.N.; Nelson, K.G.; Ferriero, D.; , the NICH-NINDS Perinatal Stroke Workshop Participants, Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke, Pediatrics 120 (2007) 609.
108. Rennie, J.M.; Hagmann, C.F.; Robertson, N.J., Outcome after intrapartum hypoxic ischemia at term, Semin Fetal Neonat Med 12 (2007) 398.
109. Rinaldo, P.; Lim, J.S.; Tortorelli, S.; et al., Newborn screening of metabolic disorders: recent progress and future developments, Nestle Nutr Workshop Ser Pediatr Program 62 (2008) 81.
110. Rivera, L.B.; Boppana, S.B.; Fowler, K.B.; et al., Predictors of hearing loss in children with symptomatic congenital cytomegalovirus infection, Pediatrics 110 (2002) 762.
111. Robison, M.; Pirak, C.; Morrell, C., Multidisciplinary discharge assessment of the medically and socially high-risk infant, J Perinat Neonatal Nurs 13 (2000) 67.
112. Roid, G.H., Stanford-Binet Intelligence Scales. ed 5 ( 2003)Riverside Publishing, Itasca, Ill.
113. Romantseva, L.; Msall, M.M., Advances in understanding cerebral palsy syndromes after prematurity, NeoReviews 7 (2006) 575.
114. Rosenbaum, P.; Paneth, N.; Leviton, A.; et al., A report: the definition and classification of cerebral palsy—April 2006, Dev Med Child Neurol 49 (Suppl 109) ( 2006) 8.
115. Rosenberg, A., The IUGR newborn, Semin Perinatol 32 (2008) 219.
116. Ruth, V.A., Extrauterine growth restriction: a review of the literature, Neonatal Netw 27 (2008) 177.
117. Saenz, P.; Cerda, M.; Cordobes, J.L.; et al., Psychological stress of parents of preterm infants enrolled in an early discharge program from the neonatal intensive care unit: a prospective randomized trial, Arch Dis Child Fetal Neonatal Ed 94 (2008) F98.
118. Saigal, S.; Szatmari, P.; Rosenbaum, P.; et al., Cognitive abilities and school performance of extremely low birth weight children and matched term control children at age 8 years: a regional study, J Pediatr 118 (1991) 751.
119. Salhab, W.A.; Khattak, A.; Tyson, J.E.; et al., Car seat or car bed for very low birth weight infants at discharge home, J Pediatr 150 (2007) 224.
120. Sarnat, H.B.; Sarnat, M.S., Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study, Arch Neurol 3 (1976) 696.
121. Schiariti, V.; Klassen, A.F.; Hoube, J.S.; et al., Perinatal characteristics and parents’ perspective of health status of NICU graduates born at term, J Perinatol 28 (2008) 368.
122. Shankaran, S., Neonatal encephalopathy: treatment with hypothermia, Neurotrauma 26 (2009) 437.
123. Shevell, M.; Ashwal, S.; Donley, D.; et al., Practice parameter: evaluation of the child with global developmental delay—report of the Quality Standards Subcommittee of the American Academy of Neurology and The Practice Committee of the Child Neurology Society, Neurology 60 (2003) 367.
124. Short, E.J.; Kirchner, H.L.; Asaad, G.R.; et al., Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: analysis using a severity-based classification system, Arch Pediatr Adolesc Med 161 (2007) 1082.
125. Singer, L.; Yamashita, T.; Lilien, L.; et al., A longitudinal study of developmental outcome of infants with bronchopulmonary dysplasia and very low birth weight, Pediatrics 100 (1997) 987.
126. Sleifer, P.; da Costa, S.S.; Coser, P.L.; et al., Auditory brainstem response in premature and full-term children, Int J Pediatr Otorhinolaryngol 71 (2007) 1449.
127. Spittle, A.J.; Orton, J.; Doyle, L.W.; et al., Early developmental intervention programs post hospital discharge to prevent motor and cognitive impairments in preterm infants, Cochrane Database Syst Rev 2 (2007); CD005495.
128. Spitzmiller, R.E.; Phillips, T.; Meinzen-Derr, J.; et al., Amplitude-integrated EEG is useful in predicting neurodevelopmental outcome in full-term infants with hypoxic-ischemic encephalopathy: a meta-analysis, J Child Neurol 22 (2007) 1069.
129. Stoll, B.J.; Hansen, N.I.; Adams-Chapman, I.; et al., Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection, JAMA 292 (2004) 2357.
130. Sullivan, M.C.; McGrath, M.M.; Hawes, K.; et al., Growth trajectories of preterm infants: birth to 12 years, J Pediatr Health Care 22 (2008) 83.
131. Temple, R.O.; Pass, R.F.; Boll, T.J., Neuropsychological functioning in patients with asymptomatic congenital cytomegalovirus infection, J Dev Behav Pediatr 21 (2000) 417.
132. Tomashek, K.M.; Shapiro-Mendoza, C.K.; Weiss, J.; et al., Early discharge among late preterm and term newborns and risk of neonatal morbidity, Semin Perinatol 30 (2006) 61.
133. Tonkin, S.L.; McIntosh, C.G.; Nixon, G.M.; et al., Can we reduce episodes of haemoglobin desaturation in full-term babies restrained in car seats?Acta Paediatr 97 (2008) 105.
134. U.S. Preventive Services Task Force, Universal screening for hearing loss in newborns: U.S. Preventive Services Task Force recommendation statement, Pediatrics 122 (2008) 143.
135. Vohr, B.R., How should we report early childhood outcomes of very low birth weight infants?Semin Fetal Neonatal Med 12 (2007) 355.
136. Vohr, B.R.; Msall, M.E., Neuropsychological and functional outcomes of very low birth weight infants, Semin Perinatol 21 (1997) 202.
137. Vohr, B.R.; Wright, L.L.; Dusick, A.M.; et al., Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993-1994, Pediatrics 105 (6) ( 2002) 1216.
138. Vohr, B.R.; Wright, L.L.; Hack, M.; et al., Follow-up care of high-risk infants, Pediatrics 114 (2004) 1377.
139. Vohr, B.R.; Wright, L.L.; Poole, W.K.; et al., Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks’ gestation between 1993 and 1998, Pediatrics 116 (2005) 635.
140. Walker, D.M.; Marlow, N., Neurocognitive outcome following fetal growth restriction, Arch Dis Child Fetal Neonatal Ed 93 (2008) F322.
141. Wallon, M.; Kodjikian, L.; Binquet, C.; et al., Long-term ocular prognosis in 327 children with congenital toxoplasmosis, Pediatrics 113 (2004) 1567.
142. Wang, K.K.; Barnard, A., Technology-dependent children and their families: a review, J Adv Nurs 45 (2004) 36.
143. Wang, M.L.; Dorer, D.J.; Fleming, M.P.; et al., Clinical outcomes of near-term infants, Pediatrics 114 (2004) 372.
144. Washington State Department of Health, Low birth weight neonatal intensive care unit graduate: critical elements of care. ( 2005) ; website:www.medicalhome.org.
145. Wechsler, D., The Wechsler Preschool and Primary Scale of Intelligence-III. ( 2002)The Psychological Corporation, San Antonio, Tex.
146. Willis, V., Parenting preemies, Adv Neonatal Care 8 (2008) 221.
147. Wolraich, M.L., Disorders of development and learning. ed 3 ( 2003)BC Decker, Hamilton, Ontario, Canada.
148. Wood, N.S.; Marlow, N.; Costeloe, K.; et al., Neurologic and developmental disability after extremely preterm birth. EPICure Study Group, N Engl J Med 343 (2000) 378.
149. Woodward, L.J.; Anderson, P.J.; Austin, N.C.; et al., Neonatal MRI to predict neurodevelopmental outcomes in preterm infants, N Engl J Med 355 (2006) 685.
150. World Health Organization, International Classification of Functioning, Disability and Health (ICF). ( 2001)The Organization, Geneva.
151. Zwicker, J.G.; Harris, S.R., Quality of life of formerly preterm and very low birth weight infants from preschool age to adulthood: a systematic review, Pediatrics 121 (2008) 366.
RECOMMENDED RESOURCES
American Academy of Pediatrics (AAP), Policies/recommendations Website:http://aappolicy.aappublications.org/.
In: (Editors: Brodsky, D.; Ouellette, M.A.) Primary care of the premature infant ( 2008)Saunders, Philadelphia.
Critical elements of care for the low birth weight neonatal intensive care graduate, guidelines for care and follow-up of NICU graduates, Website:www.medicalhome.org.
Davis, D.; Stein, M.T., Parenting your premature baby and child: the emotional journey. ( 2004)Fulcrum, Golden, Colo.
Developmental and Behavioral Pediatrics, Information on pediatrics and developmental screening, Website:www.dbpeds.org/.
Individuals with Disabilities Education Act (IDEA), Website:http://idea.ed.gov/.
In: (Editors: Kenner, C.; McGrath, J.) Developmental care of newborns and infants ( 2004)Mosby, St Louis.




