Chapter 18 Direct cholangiography
Approaches, techniques, and current role
Direct Cholangiography Overview
Direct cholangiography, the introduction of contrast medium into the biliary system, can be achieved radiologically, endoscopically, or intraoperatively via surgically placed tubes. The nonoperative techniques are percutaneous transhepatic cholangiography (PTC) and endoscopic retrograde cholangiopancreatography (ERCP). Currently, noninvasive magnetic resonance imaging (MRI) and computed tomographic (CT) cholangiography have virtually eliminated the indications and need for direct cholangiography (Stroszczynski & Hünerbein, 2005; Miller et al, 2007; Bakhal et al, 2009; see Chapters 16 and 17). Today ERCP and PTC are almost exclusively performed only as part of a planned interventional procedure, such as drainage, stenting, stone extraction, or stricture dilation (see Chapters 27 and 28).
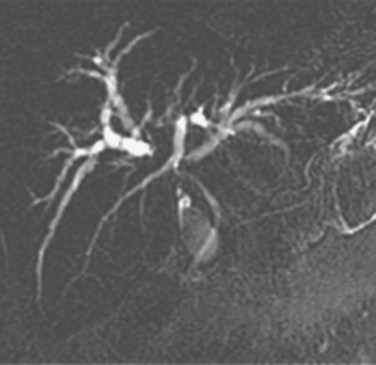
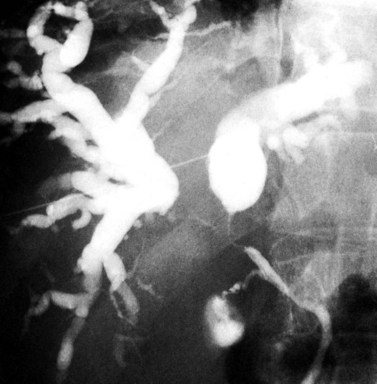
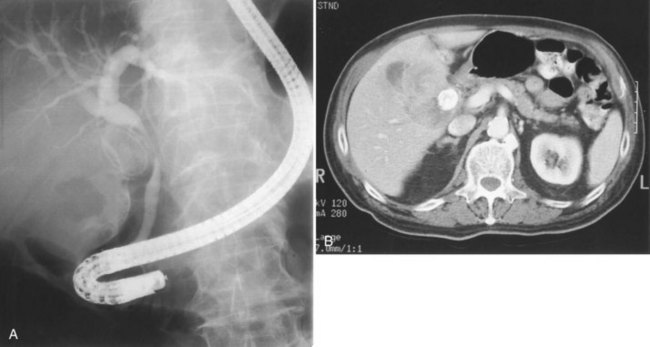
The most obvious benefits of noninvasive cholangiography are that it is painless, does not require sedation, and has few complications. Thin-section scanning techniques with multiplanar reconstruction allow for a large field of view that enables visualization of the entire biliary tree (Fig. 18.1). Reconstructed three-dimensional data sets can be displayed as a cholangiogram and manipulated in space. By contrast, direct dye injection only allows visualization of the structures in continuity with the opacified, nonisolated segments of the biliary system (Fig. 18.2). In PTC, even with multiple puncture sites and injections, simultaneous cross-sectional imaging may be required to ensure that the entire biliary tree has been opacified. In addition, noninvasive cholangiography has overall superior diagnostic accuracy compared with invasive techniques, allowing for visualization of structures contiguous to the biliary system and the bile duct wall.
Percutaneous Transhepatic Cholangiography (See Chapter 28)
History
The first report of radiologic visualization of the biliary tree was presented by Burckhardt and Müller in 1921, and cholecystocholangiography was achieved by percutaneous puncture of the gallbladder. The first report of PTC was by Huard and Do-Xuan-Hop in 1937, and cholangiography was performed with the suboptimal contrast agent Lipiodol. The use of transhepatic cholangiography as a diagnostic tool gained popularity 15 years later, after Carter and Saypol’s (1952) discussion of PTC with the use of a water-soluble contrast agent. In the ensuing years, many investigators (Flemma & Shingleton, 1966; Glenn et al, 1962; Mujahed & Evans, 1966; Seldinger, 1966; Shaldon et al, 1962; Weicher, 1964) described a variety of different methodologic details, including the use of sheathed or unsheathed needles of various sizes, different sites and directions of puncture, and various numbers of puncture trials to maximize success. The procedure remained associated with a significant risk of bile peritonitis, especially in obstructed systems, and the less frequent complication of hemorrhage. The last essential refinement in transhepatic cholangiography, the use of fine needle technique, was developed at Chiba University and was first presented by Ohto and Tsuchiya (1969) and later by Tsuchiya (1969). The value and decreased complication rate of the fine needle technique was verified by numerous authors (Benjamin et al, 1978; Ferrucci et al, 1976; Fraser et al, 1978; Gold & Price, 1980; Harbin et al, 1980; Hinde et al, 1977; Jain et al, 1977; Pereiras et al, 1977), and the fine needle technique has been generally accepted as the standard.
At present, diagnostic transhepatic cholangiography has been completely supplanted by noninvasive imaging techniques for the evaluation of the biliary tree. In 1985, Kadir reported that less than 5% of patients referred for evaluation of biliary disease required concomitant drainage procedures. With continued advancement of cross-sectional imaging techniques, including the development of MRI cholangiography (Park et al, 2004; Romagnuolo et al, 2003; Simone et al, 2004; Vaishali et al, 2004; Zhong et al, 2003) and CT cholangiography, diagnostic PTC should be performed only as part of a planned radiologic interventional procedure.
The most obvious benefits of noninvasive cholangiography are the elimination of complications and pain, but several other advantages are also important. Puncture and contrast injection techniques in high bile duct obstruction with isolated systems visualize only the portions of the biliary tree that are in continuity with the puncture site, and multiple interventions may be required to visualize the entire biliary tree if there are areas of duct isolation (see Fig. 18.2). Even with multiple punctures and contrast injections, cross-sectional imaging may be necessary sometimes to ensure that the biliary tree has been completely assessed. MRI cholangiography has the advantage of showing the entire biliary system even in the presence of isolation (see Fig. 18.1). Finally, there is the diagnostic advantage gained with cross-sectional imaging. By allowing visualization of many more organs than just the biliary system, diagnostic accuracy of cholangiography is significantly increased.
Success Rate and Accuracy
Opacification of the bile ducts is successful in 95% to 100% of patients with biliary obstruction (Mueller et al, 1981; Harbin et al, 1980; Jain et al, 1977). A success rate of 60% to 95% is reported for nondilated biliary systems. The chance of success in the nondilated system is increased by the number of needle passes performed (Jaques et al, 1980). Usually, the level of obstruction is accurately determined by careful attention to detail and liberal use of a tilt table, but the technique is less definitive for determining the cause of obstruction. No radiologic descriptions of obstructed bile ducts are pathognomonic for differentiation of benign and malignant disease (Hadjis et al, 1985; Wetter et al, 1991). Many malignant masquerades are well described, and it is not always possible to differentiate between calculous disease and papillary bile duct cancer. Other radiologic findings and cytology or histology are often required for accurate diagnosis.
Pitfalls in Interpretation
Lack of Opacification
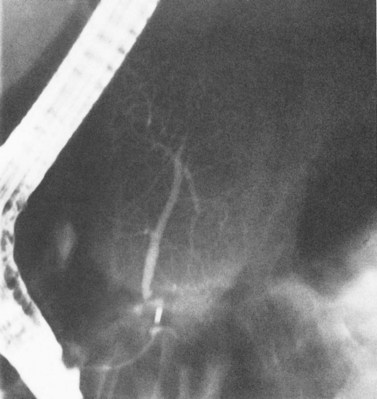
Failure to inject adequate volume of contrast agent can lead to false localization of the level of obstruction. This can occur with complete obstruction and may be recognized by the presence of a hazy margin at the level of the apparent obstruction (Fig. 18.3; Kittredge and Baer, 1975). In high bile duct obstruction, especially when associated with variant anatomy, isolated segments of the biliary tree can be visualized only by direct puncture. If direct cholangiography is incomplete, there also may be difficulty diagnosing duct injuries and bile leaks, both of which can be missed unless the proper duct is punctured and opacified.
Complications
Serious complications of PTC are rare and occur in approximately 3% of patients (Harbin et al, 1980). The most common major complications are bile leakage (1% to 2%), sepsis (2% to 3%), and hemorrhage (0.2% to 0.4%); much rarer complications include pneumothorax, biliothorax, colon puncture, and abscess formation. Many of these complications can be decreased and avoided. Ultrasound guidance and puncture below the ninth intercostal space will clearly decrease the incidence of pulmonary complications, and septic complications can be avoided by appropriate antibiotic coverage and by not overdistending the biliary tree. At times, aspiration and decompression of the biliary tree will be valuable in allowing injection of larger volumes of contrast agent without leading to sepsis.
Endoscopic Retrograde Cholangiopancreatography (See Chapter 27)
History
ERCP was first reported in 1968 by McCune and colleagues and has remained an important diagnostic modality frequently used in the management of patients with hepatobiliary and pancreatic diseases (Cotton, 1977; Cotton et al, 1972; Freeney, 1988; Oi et al, 1970). Since the 1970s, there has been continued progress in ERCP instrumentation. One major innovation was the development in the late 1980s of video, or electronic, endoscopy. Video endoscopy enables high-quality photographic and videotape documentation of important endoscopic findings and has made the training of physicians in the technique of ERCP much easier. The endoscopic skills needed to perform ERCP are more difficult to learn than the standard procedures of upper endoscopy and colonoscopy, but now many endoscopists throughout the world can perform diagnostic ERCP easily, rapidly, and safely. Working with a cooperative patient and a well-trained team, this procedure often can be completed in less than 15 minutes. Diagnostic ERCP is rapidly being replaced, however, by noninvasive imaging techniques, such as MRCP.
Technique
Age is not a contraindication to ERCP, because the procedure is safe even for selected patients in their 90s. Although children less commonly have pancreatic and biliary disease, the standard technique is often all that is necessary for children, and instrumentation is the same except in children younger than 6 months old. General anesthesia is rarely needed for children older than 11 or 12 years (Buckley & Connon, 1990). ERCP has been shown to be a valuable adjunct in the evaluation of infantile cholestasis (Wilkinson et al, 1991). In a case-control study comparing children undergoing ERCP with adults, there were no differences in the success rate or the rate of complications (Varadarajulu et al, 2004). Elderly patients can safely undergo ERCP, and they have the same risks of bleeding and perforation as younger patients do but have a lower risk of pancreatitis.
Patient comfort is maintained by the use of intravenous sedation under monitored control. Drug combinations include narcotics such as meperidine and droperidol and the benzodiazepines midazolam or diazepam. Dosages are titrated for each patient with a total dose of meperidine generally ranging from 50 and 100 mg and midazolam between 2 and 10 mg. More recently, propofol, at 190 μg/kg/min, administered by anesthesia personnel, has been replacing meperidine and a benzodiazepine. Studies have shown that propofol is more effective than sedation with midazolam, it is safe, and it is associated with a faster postprocedure recovery (Wehrman et al, 1999). In some centers, if the endoscopic procedure is expected to be difficult, or if the patient has significant comorbid medical conditions, general anesthesia is used. Excess duodenal motility is controlled by bolus injections of 0.2 to 1 mg of intravenous glucagon. Oxygen is administered by nasal cannula to avoid the hypoxemia that has been described in 40% of patients undergoing ERCP (Woods et al, 1989). The patient’s electrocardiogram, blood pressure, oxygen saturation, and overall condition are continually monitored throughout the procedure by a dedicated nurse. Antibiotics are not given routinely for diagnostic procedures except when biliary obstruction is expected. All endoscopic equipment used for this procedure, including the endoscopes, is either chemically disinfected or gas sterilized.
A side-viewing duodenoscope is used to afford excellent visualization of the ampulla of Vater. In patients with a Billroth II type gastrojejunostomy, a standard forward-viewing upper endoscope or pediatric colonoscope may be needed to successfully approach the ampulla. An initial endoscopic evaluation of the stomach and duodenum is performed before cannulation of the ampulla. The pancreatic and biliary ducts are selectively cannulated and identified, and a contrast agent, such as diluted Renografin-60, is injected into the desired duct under fluoroscopic control, with subsequent radiographic images obtained of the duct anatomy. The use of a guidewire to cannulate the ampulla without using contrast is becoming common. In one recent series, 795 (99%) of 801 ERCPs were performed without contrast to initially identify access to the common bile duct (CBD). The pancreatitis rate in this series was 1.3%, as was guidewire perforation of the bile duct, which also occurred infrequently (Adler et al, 2010). An additional way of gaining access to the difficult-to-cannulate CBD is to selectively place a pancreatic duct stent first and then attempt wire-guided cannulation of the bile duct.
A skilled endoscopist can be expected to identify the ampulla of Vater in almost all patients with normal anatomy. If the ampulla is inside a duodenal diverticulum, or if the second portion of the duodenum is stenotic, compressed, or invaded by a pancreatic mass, identification of the ampulla may be difficult. When the ampulla is found, success rates for selectively cannulating the CBD and pancreatic duct are greater than 90% (Rosch, 1985). When difficulty occurs in cannulation of the CBD, invasive maneuvers, such as precut papillotomy, can improve the success rate but with an associated increase in the complication rate (Shakoor & Geenen, 1992). In a patient with a Billroth II gastrojejunostomy, success rates for bile duct cannulation are much lower (Katon et al, 1975; Osnes & Myren, 1975).
Endoscopy and Pancreatography during Endoscopic Retrograde Cholangiopancreatography
Duodenal diverticula almost always are located near the papilla in the descending duodenum. Large juxtapapillary diverticula are clinically significant, because they are often associated with choledocholithiasis. Although it was thought that periampullary diverticula may make cannulation of the ampulla more difficult, more recent work has shown that this may not be true (Tham & Kelly, 2004). On inspection of the ampulla, anatomic variants should be looked for before attempting cannulation (Phillip et al, 1974). Occasionally, two orifices are found, and this indicates separate biliary and pancreatic duct systems. The minor ampulla is located about 1 to 2 cm above the major papilla. Impacted gallstones give rise to a characteristic distension or bulge of the papilla, and previous spontaneous passage of bile duct stones can be recognized by a fissured splitting of the papillary orifice. Previous surgical dilation of the papilla or spontaneous gallstone perforation above the papilla also produces a characteristic appearance. Occasionally, a fistulous perforation in the roof of the papilla is found as a result of a false passage produced at surgical dilation (Tanaka & Ikeda, 1983). Biliary–enteric anastomoses appear as circular or slitlike openings.
The endoscopic diagnosis of ampullary carcinoma is not difficult, especially if the tumor is exophytic. The diagnosis becomes more challenging, however, when the tumor is predominantly intramural. In such instances, the diagnosis is frequently confirmed by biopsy and cytologic specimens, after endoscopic papillotomy allows the tumor to be exposed. In a series from the Medical College of Wisconsin, the correct diagnosis was made by endoscopic biopsy in 42 of 44 patients with ampullary neoplasia and in 16 of 18 patients with invasive ampullary cancer (Komoroski et al, 1991).
Imaging the pancreatic duct can be an important adjunct to cholangiography during ERCP. Strictures, stones, and other obstructing lesions can be identified. With increasing age comes progressive atrophy and fibrosis of the pancreas. The diameter of the main pancreatic duct also increases with age, although one study found no difference in pancreatic duct length among patients younger than 40 years compared with older patients. Duct diameter throughout the pancreas was significantly greater, however, in patients over 40 (Anand et al, 1989).
Anatomic variations, such as pancreas divisum (Fig. 18.4), also may be identified on a pancreatogram. This abnormality has been described in 7.5% of ERCP procedures and can be confirmed by cannulation of the main pancreatic duct through the orifice in the minor papilla (Bernard et al, 1990).
Complications
The experience of the endoscopist is one of the most significant factors related to the development of ERCP complications (Bilbao et al, 1976). Statistics on complication rates vary accordingly. High case volume also has an impact on the complication rate. In a study conducted in Austria, endoscopists performing more that 50 ERCP procedures per year were compared with on those performing fewer than 50 per year. Those in the higher case volume group had a significantly higher success rate (86.9% vs. 80.3%; P < .001) and a lower overall complication rate (10.2% vs. 13.6%, P = .007; Kapral et al, 2008).
Acute pancreatitis after ERCP occurs in less than 7% of patients (Christensen et al, 2004; Rosch et al, 1981) and should be distinguished from transient asymptomatic hyperamylasemia, which occurs in 40% to 75% of cases (Classen & Demling, 1975). Asymptomatic hyperamylasemia disappears within 1 to 2 days, as do hypertrypsinemia (Phillip & Hagenmuller, 1983) and elevations of serum lipase and elastase 1 levels (Okuno et al, 1985). Radiographic documentation of renal excretion of the contrast agent injected into the pancreatic duct does not predict the development of acute pancreatitis (Hopper et al, 1989), and necrotizing pancreatitis, a serious complication, is observed in about 0.1% of pancreatograms. The prevalence of post-ERCP pancreatitis in children is quite low, about 2.5% in one study (Iqbal et al, 2008).
The use of nonionic contrast medium of low osmolarity has shown no advantage over the less expensive ionic contrast medium in preventing ERCP-related pancreatitis (Hannigan et al, 1985). The risk of pancreatitis is reduced by careful and gentle injection of the contrast medium into the pancreatic duct, by avoiding overdistension or “acinarization” of the pancreatic duct, and by minimizing repeated injection of the pancreatic duct when encountering difficulty in filling the CBD. Prophylactic administration of somatostatin or its analog octreotide has not been shown to prevent pancreatitis during diagnostic or therapeutic ERCP (Borsch et al, 1984; Sternlieb et al, 1992). A large, prospective, randomized, placebo-controlled study from Italy looked at the efficacy of somatostatin and gabexate in preventing post-ERCP pancreatitis; 966 patients were studied, and no difference in the rate of pancreatitis was observed between the groups (Andriulli et al, 2004). A randomized control study demonstrated that 24 hours of octreotide prophylaxis before ERCP significantly decreased the incidence of post-ERCP pancreatitis compared with a placebo group (2/100 [2%] vs. 9/101 [8.9%]; P = .03) (Thomopoulos et al, 2006). Other investigators have suggested that when injection pancreatitis is a possibility, the use of a temporary pancreatic duct stent may serve to prevent its occurrence (Singh et al, 2004). In addition, when pancreatitis has developed, the use of somatostatin does not improve its clinical course (Phillip et al, 1985).
A serious complication of ERCP is the development of postprocedure cholangitis. This condition occurs most commonly when there is a significant and high-grade bile duct obstruction. When an obstructed bile duct is identified on ERCP, it is important to treat the obstruction rapidly by nonsurgical or surgical means (Classen & Phillip, 1982). Although no studies confirm the benefit of prophylactic antibiotics, it is reasonable to administer intravenous antibiotics that are preferentially excreted from the liver into the bile until adequate biliary drainage is achieved.
One study looked at the prophylactic use of antibiotics in routine ERCPs (Sauter et al, 1990). The authors found no difference in cholangitis rates when antibiotics were used, although the frequency of procedure-related bacteremia was significantly reduced. Antibiotics added to the injected radiographic contrast medium are of no benefit (Jendrzejewski et al, 1980). In addition, it was found that endoscopic instruments used in ERCP can be the source of serious infections (Earnshaw et al, 1985). Salmonella species are the most common organisms transmitted by contaminated endoscopes (Axon, 1991), and for this reason, it is imperative to ensure that the endoscopic equipment used for ERCP is adequately disinfected or sterilized.
Direct Cholangiography and Pancreatography by Percutaneous Transhepatic Cholangiography or Endoscopic Retrograde Cholangiopancreatography
Biliary Anatomy
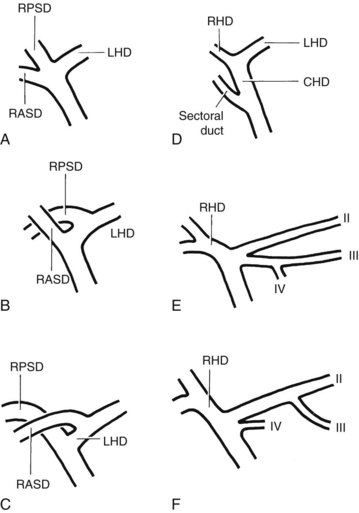
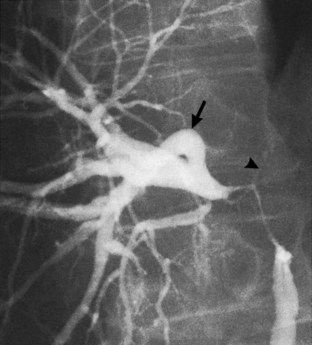
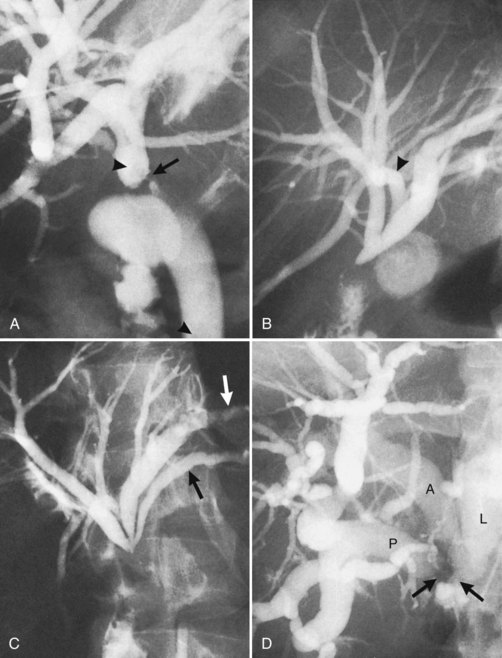
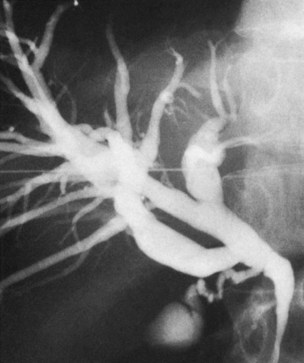
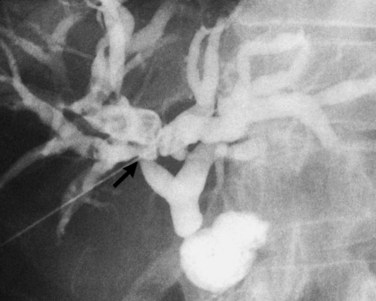
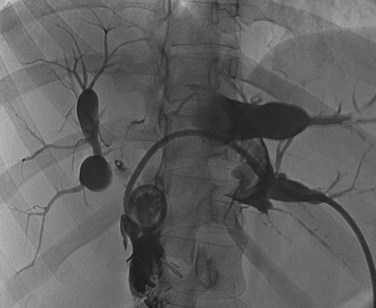
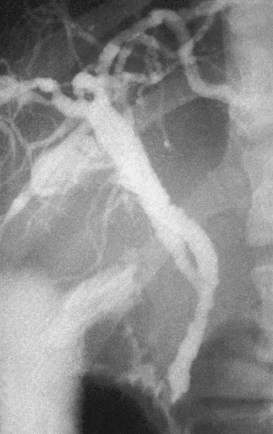
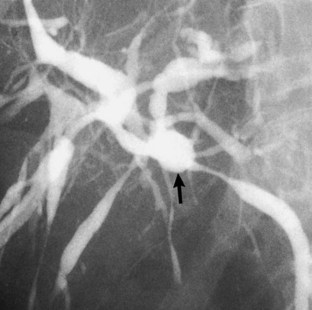
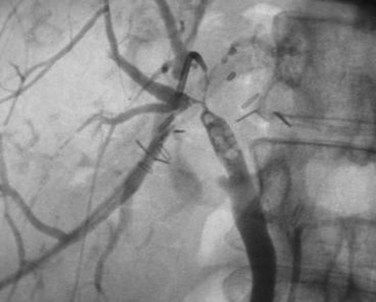
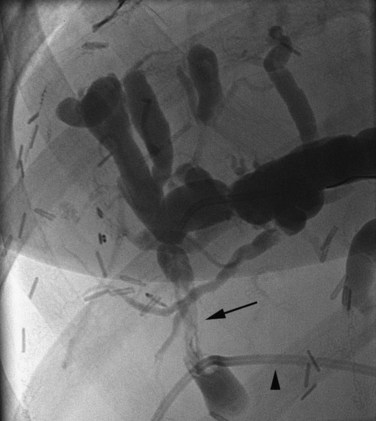
The anatomy of the bile ducts is discussed in Chapter 1A, Chapter 1B . Knowledge of the common variations of ductal branching is essential for accurate interpretations of cholangiograms, and these are shown in Figures 18.5 and 18.6; segmental nomenclature is summarized in Table 18.1. In the right lobe, the posterior segments lie more laterally than the anterior segments, so that the most lateral ducts on a cholangiogram are usually segments VI inferiorly and VII superiorly. The posterior sectoral duct is often recognizable by an arched course near the confluence (Figs. 18.7 and 18.8B). A right sectoral duct crosses to the left to join the left hepatic duct in 28% of cases according to Healey and Schroy (1953); in 22%, this is the posterior sectoral duct (see Figs. 18.6B and 18.8B), and in 6%, this is the anterior duct (see Figs. 18.6C and 18.8D). Occasionally, a right sectoral or segmental duct, posterior more often than anterior, courses inferiorly and enters the common hepatic duct directly (Fig. 18.9). The confluence of the right and left ducts takes the form of a trifurcation rather than a bifurcation in 12% of cases according to Couinaud (1957; see Fig. 18.6A).
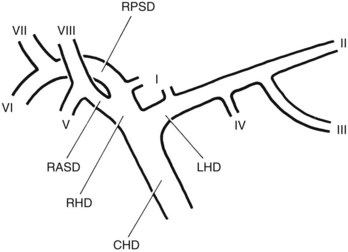
FIGURE 18.5 Standard intrahepatic ductal anatomy. The segments are numbered according to Couinaud’s description (see Table 18.1). CHD, common hepatic duct; LHD, left hepatic duct; RASD, right anterior sectoral duct; RHD, right hepatic duct; RPSD, right posterior sectoral duct.
| I | Caudate lobe |
| II | Left lateral superior segment |
| III | Left lateral inferior segment |
| IV | Left medial segment or quadrate lobe |
| V | Right anterior inferior segment |
| VI | Right posterior inferior segment |
| VII | Right posterior superior segment |
| VIII | Right anterior superior segment |
In the left lobe, the superior and inferior lateral segment ducts, segments II and III, unite in the line of, or to the right of, the umbilical fissure in 92% of cases. In the latter instance, the quadrate lobe, segment IV, may drain wholly or partially into the segment II duct. Rarely, segment II and III ducts join at or close to the confluence (see Figs. 18.6E and 18.8C), and segment IV drains directly into the common hepatic duct in 1% of cases (see Fig. 18.6F; Healey & Schroy, 1953).
The caudate ducts are often difficult to identify. Usually two or three ducts drain most commonly into the right posterior sectoral duct, right hepatic duct, or left hepatic duct (Healey & Schroy, 1953). The recognizable caudate ducts are usually a few centimeters long and drain downward or to the right.
The left hepatic duct (average length, 17 mm) is considerably longer than the right hepatic duct (average length, 9 mm) and has a longer extrahepatic course. The normal diameters of the main bile ducts as measured at PTC are shown in Table 18.2. These figures are greater than the true duct dimensions because of some distension produced by direct cholangiography together with considerable magnification occurring on any fluoroscopic “spot film.” The magnification is of the order of 40% (Nichols & Burhenne, 1984) and affects all structures in the image, including calculi, tubes, and strictures.
Table 18.2 Average Duct Diameters Measured Directly from 50 Normal Percutaneous Transhepatic Cholangiography Examinations
| Duct Diameter (mm) |
| Right hepatic = 4.7 |
| Left hepatic = 5.2 |
| Common hepatic = 6.5 |
| Common bile = 7.6 |
From Okuda K, et al, 1978: Frequency of intrahepatic arteriovenous fistula as a sequela to percutaneous needle puncture of the liver. Gastroenterology 74:1204-1207.
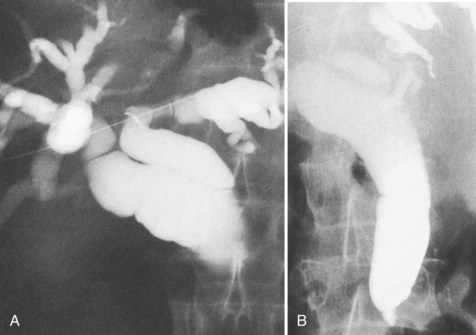
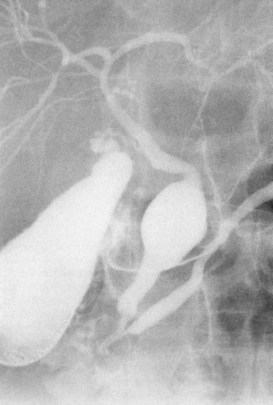
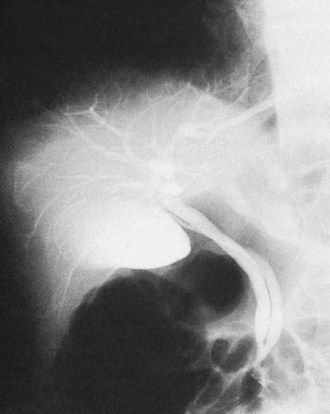
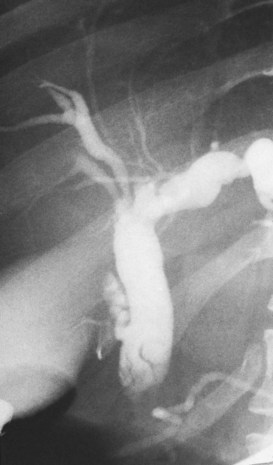
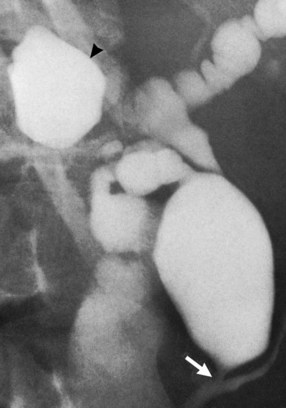
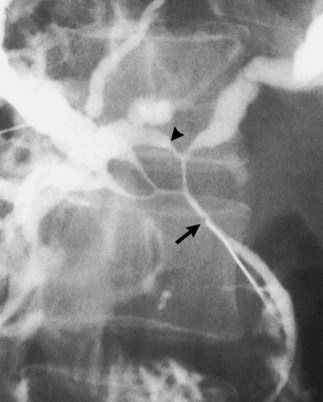
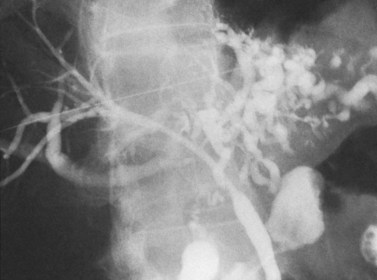
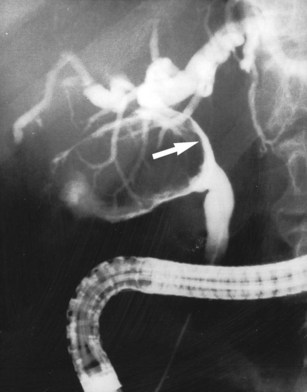
The upper limits of normal for the diameter of the extrahepatic bile ducts as measured by ERCP vary between 9 mm and 14 mm (Niederau et al, 1984). Combined radiologic and manometric studies (Poralla et al, 1985) have shown that even in the absence of extrahepatic cholestasis, the diameter of the bile ducts and the pressure difference therein increases with advancing age. The diameter of the bile ducts as measured by ultrasonography is less than those measurements obtained during ERCP (Niederau et al, 1984). Anatomic abnormalities of the hepatobiliary system include cystic dilations (Fig. 18.10) of the bile duct or of the intrahepatic bile ducts (Caroli disease; see Chapter 46). There is a wide variation in where the cystic duct joins the common hepatic duct. A low junction with a correspondingly long cystic duct (Fig. 18.11) may result in difficulties if not recognized,. This is especially true when a cholecystojejunostomy is performed as a palliative biliary bypass for carcinoma of the head of the pancreas, and the jaundice either is not relieved or recurs rapidly in the postoperative period.
Filling Defects
Air Bubbles, Blood Clots, Calculi, Primary and Secondary Bile Duct Cancers, and Parasitic Diseases
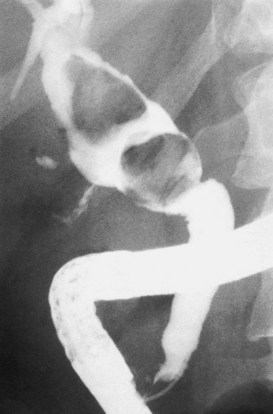
Calculous disease (see Chapters 36, 37, and 39) remains the most common filling defect in the biliary system. Distinguishing characteristics of gallstones and primary bile duct calculi include a faceted radiologic appearance and disposition to move to gravity-dependent positions. Calculi may be seen as discrete filling defects (Figs. 18.12 and 18.13) or castlike structures filling entire ducts, as occurs in oriental cholangiohepatitis, cystic diseases of the bile ducts, or even proximal to strictures of any etiology. Impacted calculi may be difficult to differentiate from strictures or tumors.
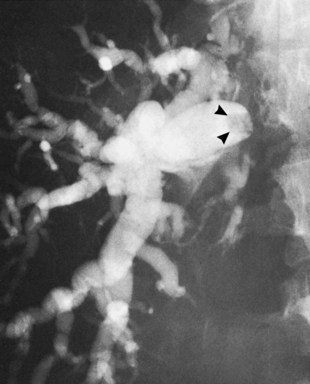
Primary bile duct cancer (see Chapter 50A, Chapter 50B, Chapter 50C, Chapter 50D ), specifically papillary cholangiocarcinomas, can also present as cholangiographic filling defects (Fig. 18.14). T-shaped filling defects may also be detected (Torok & Gores, 2001; Fig. 18.15), and papillary bile duct cancers may cause filling defects as a result of mucin production (Fig. 18.16). Other malignancies, including melanoma and intraductal metastases, such as colon cancer, are also more unusual causes of cholangiographic filling defects (Riopel, 1997).
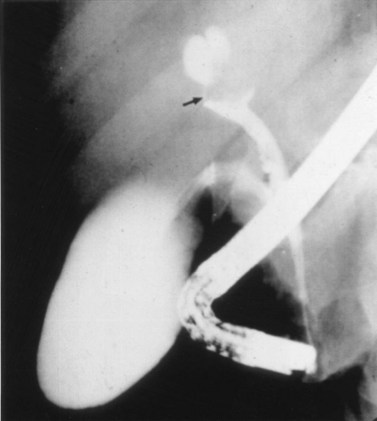
FIGURE 18.15 “Golf tee” appearance of a papillary bile duct cancer (arrow) involving the common hepatic duct.
Parasitic infections (see Chapter 45), such as with hydatid disease or with Ascaris lumbricoides or Clonorchis sinensis, are diseases with worldwide distribution that can also present cholangiographically as intraductal filling defects. A proportion of hepatic hydatid cysts (5% to 10%) rupture into the bile ducts and may simulate choledocholithiasis (see Chapter 68). Calcified cysts are easy to recognize on radiographs, and daughter cysts can cause biliary obstruction. When the calcified cyst is not obvious, biliary strictures with obstruction may be noted on cholangiogram. The biliary ducts can show considerable irregularities in caliber and extensive displacement of the intrahepatic branches secondary to the mass effect of a large hydatid cyst (Cottone et al, 1978; Dyrszka & Sanghavi, 1983; Ibrahim & Kawanishi, 1981).
Ascaris lumbricoides is a commonly seen helminth that has worldwide distribution. Its prevalence is 90% in some parts of Africa and Asia. If the worm passes through the sphincter of Oddi, it may cause acute pancreatitis or a cholestasis syndrome (Winters et al, 1984). In the acute stage, the worm occasionally may be found and extracted from the ampulla, and it can be detected in the biliary tract by cholangiography.
Eating raw meat has been associated with Clonorchis sinensis infestation. The prevalence of this disease has been estimated to be 60% of the general population of Hong Kong based on stool ova examinations. This worm can penetrate through the papilla into the bile ducts. In an ERCP study of 31 consecutive patients, the typical filamentous, wavy, or elliptical appearance of the worm in the bile ducts was believed to be pathognomonic. Other common cholangiographic findings include widely dilated extrahepatic bile ducts, which are filled with biliary sludge and stones, and intrahepatic duct strictures, which are predominantly found in the branches of the left hepatic duct (Yellin & Donovan, 1981). The eggs of C. sinensis act as a nucleus for the development of the bile duct stones (Lam et al, 1978). Naval and colleagues (1984) reported successful endoscopic biliary lavage to eliminate the eggs.
Invasion of Fasciola hepatica into the biliary tract also may cause serious lesions. In the chronic stage, F. hepatica infection can resemble sclerosing cholangitis (Hauser & Bynum, 1984). The appearance on ERCP is that of dilated bile ducts with unexplained sludge in the distal bile duct (Figs. 18.17 and 18.18).
Cystic Diseases
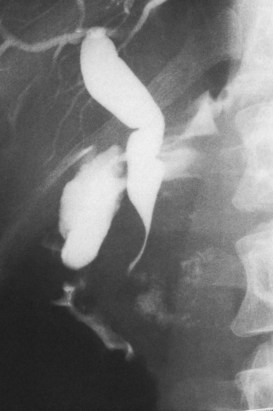
Typing of choledochal cysts (see Chapter 46) is according to the Todani et al (1977) classification. Type 1 cysts, the most common kind (80% to 90%), manifest as a fusiform dilation of the CBD, common hepatic duct (CHD), or both (see Figs. 18.10 and 18.19). A type 2 cyst (2% to 3%) is seen as a diverticular outpouching of the CBD or CHD. A type 3 cyst, also referred to as choledochocele, is a dilation of the intraduodenal (mural) portion of the distal CBD. Type 4 cysts (10% to 15%) have intrahepatic and extrahepatic cystic components in any combination, and type 5 cysts, also known as Caroli disease, is a rare form in which cysts are only seen in the intrahepatic bile ducts. Although most patients with choledochal cysts are diagnosed in infancy or childhood, 20% to 30% are diagnosed as adults. Associated calculous diseases secondary to bile stasis or stricture are frequently seen with cystic diseases. However, these must be differentiated from other more significant entities, such as bile duct cancers, because of the marked predisposition of biliary malignancies in patients with choledochal cysts (Fig. 18.20).
Bilomas, saccular intrahepatic contrast collections, may be secondary to occlusive disease or trauma, including iatrogenic trauma and trauma from transarterial chemoembolization (TACE) (Sakamito et al, 2003; Kim et al, 2001). Particularly in occlusive disease, bilomas can become infected and may appear as abscesses. Microabscesses seen with suppurative cholangitis typically appear as grapelike clusters in terminal bile ducts (Fig. 18.21).
Strictures
Bile duct strictures may be classified by their morphologic appearance or level of obstruction. Level of obstruction classification, as described by Bismuth and colleagues (1992), has significant surgical implications. When used strictly for etiologic diagnosis, however, neither level of obstruction nor morphologic appearance description is as clinically rewarding. Cholangiographic strictures are present in a diverse group of diseases. Many benign and malignant conditions can have strictures that encompass the entire gamut of cholangiographic appearances. Any combination of intrahepatic or extrahepatic distribution, shouldered or smooth, beaded or nonbeaded, long or short, and with or without associated proximal dilation has been described in most of these diseases. Diagnostic limitations of direct cholangiography are due to this great overlap in findings. Visualization of only the bile duct lumen, as achieved with direct cholangiography, offers no advantages over noninvasive imaging techniques when evaluating bile duct strictures. Direct cholangiography is generally reserved for when concomitant biliary intervention is anticipated. In much of the diagnostic spectrum of stricture disease, biopsy is frequently required to rule out malignancy.
Sclerosing cholangitis (see Chapter 41) can produce cholangiographic changes in the intrahepatic and extrahepatic biliary trees, and it is commonly seen in both (La Russo, 1984). The bile ducts in primary sclerosing cholangitis (PSC) are characterized by an annular concentric stenosis, usually multifocal, with areas of normal or mildly dilated ducts between strictures (Fig. 18.22; Rogers et al, 1972). Larger bile ducts can appear beaded (Fig. 18.23), and other radiologic findings include a “prune tree” appearance, from reduction of peripheral bile duct radicles, and diverticular outpouchings of the extrahepatic bile ducts (Gulliver et al, 1991; La Russo et al, 1984; Fig. 18.24). Ulcerative colitis is identified in as many as 70% of patients with PSC, and coexisting cholangiocarcinoma can occur in 15% of these patients (MacCarty et al, 1985; Crippin & Lindor, 1992). Detecting a cholangiocarcinoma in a background of sclerosing cholangitis is difficult. Cholangiographic features suggesting cholangiocarcinoma include progressive focal stricturing, the presence of uniform proximal duct dilation, and nodular filling defects (Li-Yeng & Goldberg, 1984). It has been suggested that evaluating these features together with cross-sectional imaging can detect many tumors (Campbell et al, 1998). Again, biopsy is generally required for definitive diagnosis.
Cholangiocarcinoma (see Chapter 50A, Chapter 50B, Chapter 50C, Chapter 50D ) is commonly seen as the classic sclerosing Klatskin type with a high-grade Y-shaped obstruction involving the right and left main bile ducts and the proximal common hepatic duct (see Fig. 18.7). Extension of stenoses to subsegmental bile ducts is frequently seen, and biliary dilation proximal to the obstruction is expected, as is a decompressed gallbladder. Klatskin tumors may cause obstruction of adjacent portal veins, resulting in hepatic atrophy that manifests itself cholangiographically as dilated, tortuous, or even beaded, crowded bile ducts (Fig. 18.25).
Like central lesions, the more common (60%) peripheral bile duct cancers are demonstrated in direct cholangiography as nonspecific stenoses or obstructions (Longmire et al, 1973; Ohto et al, 1980; Tompkins et al, 1981; Warren et al, 1972). They may also present with bilevel obstruction as a result of central lymphadenopathy. The additional diagnostic benefits of CT and MR cholangiography make these noninvasive techniques preferable for preprocedural planning and in differential diagnosis (Tompkins et al, 1990; Stroszczynski & Hünerbein, 2005).
Direct cholangiography for the diagnosis of gallbladder cancer (see Chapter 49) is rarely required. Gallbladder cancer is best diagnosed with CT, ultrasound, or MRI. Cross-sectional imaging reveals the common radiologic findings of associated calculous disease and direct extension of tumor into the adjacent hepatic parenchyma. Findings in direct cholangiography consist of high-grade stricture or occlusion of the biliary tree at any level (Fig. 18.26), typically with no visualization of the cystic duct or gallbladder.
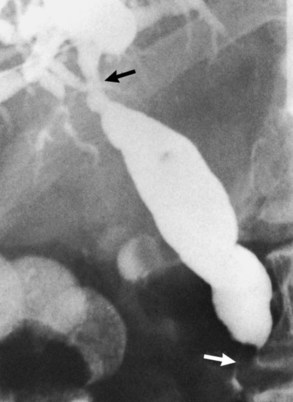
Approximately two thirds of pancreatic cancers occur in the region of the papilla of Vater and the head of the pancreas, and the bile duct is usually involved (see Chapter 58A, Chapter 58B ). Characteristic cholangiographic findings include occlusion of the distal CBD with proximal dilation. The CBD above the occlusion may have a more horizontal course (Freeney, 1988; Fig. 18.27). Cystic duct patency is often preserved, and dilation of the gallbladder may be marked. ERCP, CT, and MRI may show occlusion or stenosis of the main pancreatic duct with proximal pancreatic duct dilation. The more classic double-duct sign of simultaneous obstruction of the pancreatic duct and CBD at proximate levels is also best determined with these techniques (Fig. 18.28).
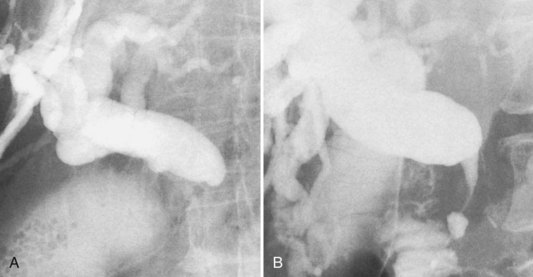
FIGURE 18.27 Two of the common appearances of carcinoma of the pancreas. A, Rounded obstruction with characteristic horizontal common bile duct. Note the coincidental stones in the distended gallbladder. B, Tapered obstruction with slight shouldering. Compare this with pancreatitis (see Figure 18.9), in which shouldering tends to be absent.
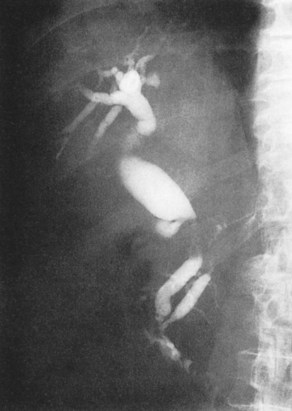
FIGURE 18.28 Double-duct sign, stenosis of the bile duct and pancreatic duct, in a case of pancreatic carcinoma.
Periampullary cancer (see Chapter 59) also causes obstruction of the CBD at the level of the papilla of Vater. The site of origin may be the duodenum or distal bile duct, which is indeterminate cholangiographically. A short-segment obstruction with proximal dilation most often identified though more central biliary abnormalities could also be seen (Fig. 18.29). A polypoid filling defect may also occasionally be seen with periampullary cancers. The filling defect, however, is not pathognomonic and must be differentiated from other possible malignancies or filling defects.
Malignant bile duct obstruction has also been described with metastatic colon, breast, lung, and gastric cancers (Fig. 18.30). Obstructions can be intrahepatic and/or extrahepatic and may be due to lymph node involvement or direct extension (Lokich et al, 1987). Bile duct obstruction from lymphoma is more commonly extrahepatic, and extrahepatic bile duct strictures can also be seen with benign lymph node enlargements such as in Castleman disease (Przkora et al, 2003).
The conundrum of “malignant masquerade” (see Chapter 42A, Chapter 42B ) present with direct cholangiography is perhaps most classically demonstrated by Mirizzi syndrome (Hadjis et al, 1985; Clemett & Lowmann, 1965; Becker et al, 1994), which occurs when a gallstone is impacted in the cystic duct or gallbladder neck, causing inflammatory obstruction of the common hepatic duct. The obstruction may be limited and focal or may extend into the intrahepatic bile ducts. On cholangiogram the syndrome is indistinguishable from cholangiocarcinoma, gallbladder cancer, or other malignant strictures (Figs. 18.31 and 18.32).
Oriental cholangiohepatitis (see Chapter 44) is generally seen in Asian populations. Cholangiography demonstrates strictured intrahepatic or extrahepatic bile ducts with markedly dilated proximal bile ducts. The dilated proximal bile ducts are packed with filling defects, which represent pigmented stones. Although all segments of the biliary tree can be involved, disease in the lateral segment of the left lobe is most common (Lim, 1991). Oriental cholangiohepatitis is characterized by recurrent episodes of cholangitis and is therefore commonly referred to as recurrent pyogenic cholangitis.
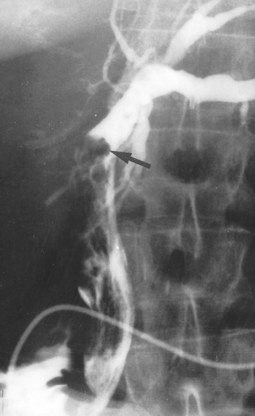
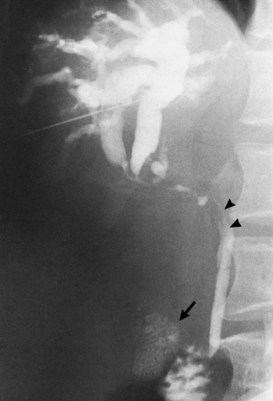
Acute pancreatitis (see Chapter 54) is usually diagnosed by combination of history, serum chemistry (amylase and lipase elevation), and cross-sectional imaging. Direct cholangiography, especially ERCP, is rarely performed during an acute episode because of the possibility of exacerbation of disease or introduction of infection. Once the episode has subsided, ERCP can be performed and may demonstrate a distal stricture and possible choledocholithiasis (Fig. 18.33). Care must be taken, however, as pancreatic cancer and periampullary cancer can not only have similar cholangiographic appearances but may coexist or even present clinically as acute pancreatitis (Mujica et al, 2000; Murakami et al, 2006; Akatsu et al, 2008).
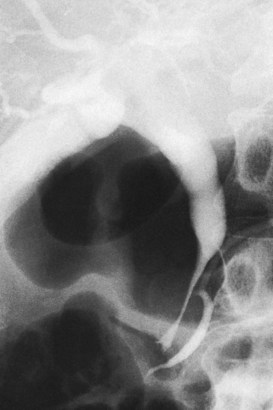
FIGURE 18.33 Acute pancreatitis with pseudocyst in the head of the pancreas (verified on operation), leading to cholestasis.
In chronic pancreatitis as in acute pancreatitis, distal bile duct stricture is the most common cholangiographic abnormality, seen in approximately 25% of patients with chronic pancreatitis (Fig. 18.34; see Chapter 42A, Chapter 42B, Chapter 55A, Chapter 55B ). Stricture length and morphology are variable, and the frequently described long, smoothly tapered stenosis occurs in only 27% to 56% of patients (Stolte et al, 1984). MRI and ERCP have greater sensitivity than PTC in chronic pancreatitis because of their ability to demonstrate pancreatic duct abnormalities such as enlargement, ectasia, beading, and the presence of chronic pseudocysts. The development of a pseudocyst can also cause pressure obstruction of more central segments of the bilinear tree.
Hepatic artery infusional chemotherapy via hepatic artery infusion pump (HAIP) and with transarterial chemoembolization have both been implicated as causes of biliary sclerosis (Kim et al, 2001; Brown et al, 1997). Stricture formation is due to either direct action of the chemotherapeutic agent or from ischemia secondary to damage of the arterial vasculature supplying the bile ducts. Chemotherapy-induced biliary sclerosis (CIBS) secondary to HAIP most commonly presents cholangiographically as high-grade obstruction of the common hepatic duct (50%) indistinguishable from a Klatskin tumor. Frequently, the area of CHD obstruction takes on a fuzzy, striated, ill-defined appearance (Fig. 18.35); peripheral stenoses may also be seen but are not always present. Biloma formation with or without associated infection (abscess) is not uncommon and may be due to peripheral bile duct necrosis.
Patients with AIDS may have bile duct inflammation from opportunistic infections such as cytomegalovirus and Cryptosporidium. The most common radiologic findings are gallbladder and bile duct wall thickening that are not demonstrated with direct cholangiography. Direct cholangiographic findings in AIDS-related cholangitis are very similar to those seen in sclerosing cholangitis: multiple strictures with intervening areas of dilation, beading, and even prune-tree appearances have been described (Farman et al, 1994; Schneiderman et al, 1987; Texidor et al, 1991; Miller et al, 1996).
Adler DG, et al. Dye-free wire-guided cannulation of the biliary tree during ERCP is associated with high success and low complication rates: outcomes in a single operator experience of 822 cases. J Clin Gastroenterol. 2010;44(3):57-62.
Akatsu T, et al. Recurrent pancreatitis caused by ampullary carcinoma and minor papilla adenoma in familial polyposis: report of a case. Surg Today. 2008;38(5):440-444.
Anand BS, et al. Effect of aging on the pancreatic ducts: a study based on endoscopic retrograde pancreatography. Gastrointest Endosc. 1989;35:210-231.
Andriulli A, et al. Somatostatin (SS) and gabextate (GM) are ineffective in preventing post-ERCP complications. Gastrointest Endosc. 2004;59:AB106. (abstract)
Axon AT. Disinfection and endoscopy: summary and recommendations. Working party report to the World Congress of Gastroenterology, Sydney. J Gastroenterol Hepatol. 1991;6:23-24.
Bakhal MS, et al. Multimodality imaging of biliary malignancies. Surg Oncol Clin N Am. 2009;2:225-239.
Becker CD, et al. Preoperative diagnosis of the Mirizzi syndrome: limitations of sonography and CT. Am J Roentgenol. 1994;143:591-596.
Benjamin IS, et al. The early use of fine needle percutaneous transhepatic cholangiography in an approach to the diagnosis of jaundice in a surgical unit. Br J Surg. 1978;65:92.
Bernard JP, et al. Pancreas divisum is a probable cause of acute pancreatitis: a report of 137 cases. Pancreas. 1990;5:248-254.
Bilbao MK, et al. Complications of endoscopic retrograde cholangiopancreatography (ERCP): a study of 1000 cases. Gastroenterology. 1976;70:314-320.
Bismuth H, et al. Management strategies in resection for hilar carcinoma. Ann Surg. 1992;215:31-38.
Borsch G, et al. Der Einfluss von Somatostatin auf die Amylasespiegel und Pankreatitisrate nach ERCP. Med Welt. 1984;35:102-109.
Brown KT, et al. Obstructive jaundice in patients receiving hepatic artery infusion chemotherapy: etiology, treatment implications, and complications after transhepatic biliary drainage. J Vasc Interv Radiol. 1997;8(2):229-234.
Buckley A, Connon JJ. The role of ERCP in children and adolescents. Gastrointest Endosc. 1990;36:369-372.
Burckhardt H, Müller W. Verusche über die Puncktion der Gallenblase und ihre Röntgendarstellung. Dtsch Chir. 1921;162:168.
Campbell WL, et al. Biliary tract carcinoma complicating primary sclerosing cholangitis: evaluation with CT, cholangiography, US, and MR imaging. Radiology. 1998;207:41-50.
Carter RF, Saypol GM. Transabdominal cholangiography. JAMA. 1952;148:253.
Christensen M, et al. Complications of ERCP: a prospective study. Gasotrointest Endosc. 2004;60:721-731.
Classen M, Demling L. Hazards of endoscopic retrograde cholangiopancreatography. Acta Hepatogastroenterol. 1975;22:1-3.
Classen M, Phillip J. Endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic papillotomy (EPT). Semin Liver Dis. 1982;2:67-74.
Clemett AR, Lowmann RB. The Roentgen features of the Mirizzi syndrome. Am J Roentgenol Therapy. 1965;94:480-483.
Cotton PB. Progress report ERCP. Gut. 1977;18:316-341.
Cotton PB, et al. Cannulation of the papilla of Vater via fiber-duodenoscope. Lancet. 1972;1:53-58.
Cottone M, et al. Endoscopic retrograde cholangiography in hepatic hydatid disease. Br J Surg. 1978;66:107.
Couinaud C. Le Foie: Études Anatomiques et Chirurgicales. Paris: Masson; 1957.
Crippin JS, Lindor KD. Primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 1992;4:261-269.
Dyrszka H, Sanghavi B. Hepatic hydatid disease: findings on endoscopic retrograde cholangiography. Gastrointest Endosc. 1983;29:248-249.
Earnshaw JJ, et al. Outbreak of Pseudomonas aeruginosa following endoscopic retrograde cholangiopancreatography. J Hosp Infect. 1985;6:95-97.
Farman J, et al. AIDS-related cholangiographic changes. Abd Imaging. 1994;94:480-483.
Ferrucci JT, et al. Fine needle transhepatic cholangiography: a new approach to obstructive jaundice. Am J Roentgenol. 1976;127:403.
Flemma RJ, Shingleton WW. Clinical experience with percutaneous transhepatic cholangiography: experience from 107 cases. Am J Surg. 1966;111:13.
Fraser GM, et al. Percutaneous transhepatic cholangiography with the Chiba needle. Clin Radiol. 1978;29:101-112.
Freeney PC. Radiology of the pancreas: two decades of progress in imaging and intervention. AJR Am J Roentgenol. 1988;150:975-981.
Glenn F, et al. Percutaneous transhepatic cholangiography. Ann Surg. 1962;156:451.
Gold RP, Price JB. Thin needle cholangiography as the primary method for the evaluation of the biliary–enteric anastamosis. Radiology. 1980;136:309-316.
Gulliver DJ, et al. Bile duct diverticula and webs: nonspecific cholangiographic features of primary sclerosing cholangitis. AJR Am J Roentgenol. 1991;157:281-285.
Hadjis NS, et al. Malignant masquerade at the hilum of the liver. Br J Surg. 1985;72:659-661.
Hannigan BF, et al. Hyperamylasemia after ERCP with ionic and non-ionic contrast media. Gastrointest Endosc. 1985;31:109-110.
Harbin WP, et al. Transhepatic cholangiography: complications and use patterns of the fine-needle technique: a multi-institution survey. Radiology. 1980;135:15-22.
Hauser SC, Bynum TE. Abnormalities on ERCP in a case of human fascioliasis. Gastrointest Endosc. 1984;30:80-81.
Healey JE, Schroy PC. Anatomy of the biliary ducts within the human liver. AMA Arch Surg. 1953;66:599-616.
Hinde GDB, et al. Percutaneous cholangiography with the Okuda needle. Gut. 1977;18:610.
Hopper KD, et al. Renal excretion of endoscopic retrograde cholangiopancreatography injected contrast: a common phenomenon. Invest Radiol. 1989;24:394-396.
Huard P, Do-Xuan-Hop. La ponction transhepatique des canaux biliares. Bull Soc Med Chir Indochine. 1937;15:1090.
Ibrahim MAH, Kawanishi H. Endoscopic retrograde cholangiography in the evaluation of complicated echinococcus of the liver. Gastrointest Endosc. 1981;27:20-22.
Iqbal CW, et al. Pediatric endoscopic injuries: incidence, management and outcomes. J Pediatr Surg. 2008;43(5):911-915.
Jain S, et al. Percutaneous transhepatic cholangiography using the “Chiba” needle: 80 cases. Br J Radiol. 1977;50:175-180.
Jaques PF, et al. The failed transhepatic cholangiogram. Radiology. 1980;134:33-35.
Jendrzejewski JW, et al. Antibiotics and ERCP: in vitro activity of aminoglycosides when added to iodinated contrast agents. Gastroenterology. 1980;78:745-748.
Kapral C, et al. Case volume and outcome of endoscopic retrograde cholangiopancreatography: results of a nationwide Austrian benchmarking project. Endoscopy. 2008;40(8):625-630.
Kadir S. Diagnostic Angiography. Philadelphia: Saunders; 1985.
Katon RM, et al. Endoscopic retrograde cholangio-pancreatography in patients with gastrectomy and gastrojejunostomy (Billroth II), a case for the forward look. Gastrointest Endosc. 1975;21:164-165.
Kim HK, et al. Ischemic bile duct injury as a serious complication post transarterial chemoembolization in patients with hepatocellular carcinoma. J Clin Gastroenterol. 2001;32(5):423-427.
Kittredge RD, Baer JW. Percutaneous transhepatic cholangiography: problems in interpretation. AJR Am J Roentgenol. 1975;125:35-46.
Komoroski RA, et al. Assessment of ampulla of Vater pathology: an endoscopic approach. Am J Surg Pathol. 1991;15:1188-1196.
Lam SK, et al. Recurrent pyogenic cholangitis: a study by endoscopic retrograde cholangiography. Gastroenterology. 1978;74:1196-1203.
La Russo NF, et al. Primary sclerosing cholangitis. N Engl J Med. 1984;310:899-903.
Lim JH. Oriental cholangiohepatitis: pathologic, clinical, and radiologic features. Am J Roentgenol. 1991;157:1-8.
Li-Yeng C, Goldberg HI. Sclerosing cholangitis: broad spectrum of radiographic features. Gastrointest Radiol. 1984;9:39-47.
Lokich JJ, et al. Biliary obstruction secondary to cancer: management guidelines and literature review. J Clin Oncol. 1987;5(6):969-981. Jun
Longmire WP, et al. Carcinoma of the extrahepatic biliary tract. Ann Surg. 1973;178:333-345.
MacCarty RL, et al. Cholangiocarcinoma complicating primary sclerosing cholangitis: cholangiographic appearances. Radiology. 1985;156:43-46.
McCune WS, et al. Endoscopic annulation of the ampulla of Vater: a preliminary report. Ann Surg. 1968;167:752-756.
Miller FH, et al. Pancreaticobiliary manifestations of AIDS. AJR. 1996;166:1269-1274.
Miller G, et al. The use of imaging in the diagnosis and staging of hepatobiliary malignancies. Surg Oncol Clin N Am. 2007;16(2):343-368.
Mueller PR, et al. Fine needle cholangiography: reflections after 450 cases. AJR Am J Roentgenol. 1981;136:85-90.
Mujahed Z, Evans JA. Percutaneous transhepatic cholangiography. Radiol Clin North Am. 1966;4:535.
Mujica R, et al. Acute pancreatitis secondary to pancreatic cancer. Study Group Participants. Pancreas. 2000;4:329-332.
Murakami Y, et al. Relapsing acute pancreatitis due to ampullary adenoma in a patient with familial adenomatous polyposis. J Gastroenterol. 2006;41(8):789-801.
Naval F, et al. Endoscopic biliary lavage in a case of Clonorchis sinensis. Gastrointest Endosc. 1984;30:292-294.
Nichols DM, Burhenne HJ. Magnification in cholangiography. AJR Am J Roentgenol. 1984;141:947-949.
Niederau C, et al. Comparison of the extrahepatic bile duct size measured by ultrasound and by different radiographic methods. Gastroenterology. 1984;87:615-621.
Ohto M, Tsuchiya Y. Non-surgically available percutaneous transhepatic cholangiography: technique and cases. Medicina (Tokyo). 1969;6:735-739.
Ohto M, et al. Ultrasonically guided percutaneous contrast medium injection and aspiration biopsy using a real-time puncture transducer. Radiology. 1980;136:171-176.
Oi I, et al. Endoscopic pancreato-cholangiography. Endoscopy. 1970;2:103-106.
Okuno M, et al. Changes in serum levels of pancreatic isoamylase, lipase, trypsin, and elastase after endoscopic retrograde pancreatography. Hepatogastroenterology. 1985;32:87-90.
Osnes M, Myren J. Endoscopic retrograde cholangiopancreatography (ERCP) in patients with Billroth II partial gastrectomies. Endoscopy. 1975;7:227-232.
Park DH, et al. Accuracy of magnetic resonance cholangiopancreatography for locating hepatolithiasis and detecting accompanying biliary strictures. Endoscopy. 2004;36:987-992.
Pereiras RJr, et al. Percutaneous transhepatic cholangiography with the “skinny” needle: a rapid, simple, and accurate method in the diagnosis of cholestasis. Ann Intern Med. 1977;86:562.
Phillip J, Hagenmuller F. Postoperative Syndrome an den Gallenwegen: das sog Postcholezystektomie-Syndrome. In: Henning, H, editor. Fortschritte der gastroenterologischen Endoskopie. Grafelfing: Demeter Verlag; 1983:47-52.
Phillip J, et al. Variations and anomalies of the papilla of Vater, the pancreas and the biliary duct system. Endoscopy. 1974;6:70-77.
Phillip J, et al. Effekt von Somatostatin auf pankreatitische Komplikationen nach ERCP und Eingriffen an der Papilla Vateri. In: Henning, H, editor. Fortschritte der gastroenterologischen Endoskopie. Grafelfing: Demeter Verlag; 1985:124-129.
Poralla T, et al. Age and sex dependency of bile duct diameter and bile duct pressure: an ERC manometry study. Z Gastroenterol. 1985;23:235-239.
Przkora R, et al. Castleman disease causing obstructive jaundice. J Hepatol. 2003;38(4):548-549. Apr
Riopel MA. Intrabiliary growth of metastatic colon adenocarcinoma: a pattern of intrahepatic spread easily confused with primary neoplasia of the biliary tract. Am J Surg Pathol. 1997;9:1030-1036. Sept
Rogers JV, et al. Sclerosing cholangitis: roentgenographic features. S Afr Med J. 1972;65:587.
Romagnuolo J, et al. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med. 2003;139:547-557.
Rosch W. Endoskopische retrograde Gallenwegsdiagnostik (ERC). Krankenhausarzt. 1985;58:27-34.
Rosch W, et al. Chronische Pankreatitis und Nachbarorgane. Fortschr Med. 1981;99:1118-1120.
Sakamito I, et al. Intrahepatic biloma formation (bile duct necrosis) after TACE. AJR. 2003;181(1):79-87.
Sauter G, et al. Antibiotic prophylaxis of infectious complications with endoscopic retrograde cholangiopancreatography: a randomized controlled study. Endoscopy. 1990;22:164-167.
Schneiderman DJ, et al. Papillary stenosis and sclerosing cholangitis in the acquired immune deficiency syndrome. Ann Int Med. 1987;106:546-549.
Seldinger SI. Percutaneous transhepatic cholangiography. Acta Radiol. 1966;253(Suppl):1-134.
Shakoor T, Geenen JE. Pre-cut papillotomy. Gastrointest Endosc. 1992;38:623-627.
Shaldon S, et al. Percutaneous transhepatic cholangiography: a modified technique. Gastroenterology. 1962;42:371.
Simone M, et al. Three-dimensional virtual cholangioscopy: a reliable tool for the diagnosis of common bile duct stones. Ann Surg. 2004;240:82-88.
Singh P, et al. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest Endosc. 2004;60:544-550.
Sternlieb JM, et al. A multicenter, randomized, controlled trial to evaluate the effect of prophylactic octreotide on ERCP-induced pancreatitis. Am J Gastroenterol. 1992;87:1561-1566.
Stolte M, et al. A special form of segmental pancreatitis: “groove pancreatitis.”. Hepatogastroenterologica. 1984;29:198-208.
Stroszczynski C, Hünerbein M. Malignant biliary obstruction: value of imaging findings. Abd Imaging. 2005;30(3):314-323.
Tanaka M, Ikeda S. Parapapillary choledochoduodenal fistula: an analysis of 83 consecutive patients diagnosed at ERCP. Gastrointest Endosc. 1983;29:88-89.
Texidor HS, et al. Cryptosporidiosis of the biliary tract in AIDS. Radiology. 1991;180:51-56.
Tham TCK, Kelly M. Periampullary duodenal diverticula, bile duct stones, and ERCP. Endoscopy. 2004;36:1050-1053.
Thomopoulos KC, et al. Twenty-four hour prophylaxis with increased dosage of octreotide reduces the incidence of post-ERCP pancreatitis. Gastrointest Endosc. 2006;64(5):726-731.
Todani T, et al. Congenital bile duct cysts: classifications, operative procedures and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263-269.
Tompkins RK, et al. Prognostic factors in bile duct carcinoma. Ann Surg. 1981;194:447-457.
Tompkins RK, et al. Changing patterns in diagnosis and management of bile duct cancer. Ann Surg. 1990;211:614-621.
Torok N, Gores GJ. Cholangiocarcinoma. Sem Gastroenterol Dis. 2001;12(2):125-132.
Tsuchiya Y. A new safe method of percutaneous transhepatic cholangiography. Jpn J Gastroenterol. 1969;63:438.
Vaishali MD, et al. Magnetic resonance cholangiography in obstructive jaundice. J Clin Gastroenterol. 2004;38:887-890.
Varadarajulu S, et al. Technical outcomes of ERCP in pediatric patients. Gastrointest Endosc. 2004;59:AB103. (abstract)
Warren KW, et al. Malignant tumors of the bile ducts. Br J Surg. 1972;59:501-505.
Wehrman T, et al. Efficacy and safety of intravenous propofol sedation during routine ERCP: a prospective, controlled study. Gastrointest Endosc. 1999;49:677-683.
Weicher KL. Percutaneous transhepatic cholangiography: technique and application. Acta Chir Scand. 1964;11(Suppl):330.
Wetter LA, et al. Differential diagnosis of sclerosing cholangitis of the common hepatic duct (Klatskin tumors). Am J Surg. 1991;161:57-63.
Wilkinson ML, et al. Endoscopic retrograde cholangiopancreatography in infantile cholestasis. Arch Dis Child. 1991;66:121-123.
Winters C, et al. Endoscopic documentation of Ascaris-induced acute pancreatitis. Gastrointest Endosc. 1984;30:83-84.
Woods SD, et al. Hypoxia and tachycardia during endoscopic retrograde cholangiopancreatography: detection by pulse oximetry. Gastrointest Endosc. 1989;35:523-525.
Yellin AE, Donovan AJ. Biliary lithiasis and helminthiasis. Am J Surg. 1981;142:128-135.
Zhong L, et al. Imaging diagnosis of pancreatico-biliary diseases: a control study. World J Gastroenterol. 2003;9:2824-2827.