Digestive and Respiratory Systems and Body Cavities
The initial formation of the digestive system by the lateral folding of the endodermal germ layer into a tube is described in Chapter 6. From its beginnings as a simple tubular gut, development of the digestive system proceeds on several levels, including molecular patterning, elongation and morphogenesis of the digestive tube itself, inductions and tissue interactions leading to the formation of the digestive glands, and the biochemical maturation of the secretory and absorptive epithelia associated with the digestive tract. Clinical Correlations 15.1 to 15.3, later in the chapter, discuss malformations associated with the digestive system.
Formation of the respiratory system begins with a very unimposing ventral outpocketing of the foregut. Soon, however, this outpocketing embarks on a unique course of development while still following some of the basic patterns of epithelial-mesenchymal interactions characteristic of other gut-associated glands. Initially, the digestive and respiratory systems form in a common body cavity, but functional considerations later necessitate the subdivision of this primitive body cavity into thoracic and abdominal components. Later in the chapter, Clinical Correlation 15.4 presents malformations associated with the respiratory system, and Clinical Correlation 15.5 discusses malformations related to other body cavities.
Digestive System
Chapter 6 describes the formation of the primitive endodermal digestive tube, which is bounded at its cephalic end by the oropharyngeal membrane and at its caudal end by the cloacal plate (see Fig. 6.20). Because of its intimate relationship with the yolk sac through the yolk stalk, the gut can be divided into a foregut, an open-bottomed midgut, and a hindgut.
Patterning the Gut
Patterning of the broad foregut area occurs through the inhibition of Wnt signals (Fig. 15.1). The foregut domain is then marked by the expression of the transcription factors, Sox-2, Hhex, and Foxa-2. In contrast, a mix of activity by Wnts, FGFs, and bone morphogenetic proteins (BMPs), along with retinoic acid, represses foregut identity and maintains the regional identity of the hindgut. This is marked by the expression of the transcription factor Cdx-2 throughout the broad hindgut and the later expression of Pdx-1 in the midgut as this region emerges as a separate entity. Cdx-2 acts upstream of a broad range of Hox activity (Fig. 15.2) that is expressed throughout the gut. The activity of specific signaling molecules is associated with important transition points along the gut. FGF-4 is strongly expressed near the foregut-midgut boundary (around the duodenal-jejunal junction), and FGF-10 is associated with the establishment of the cecum.
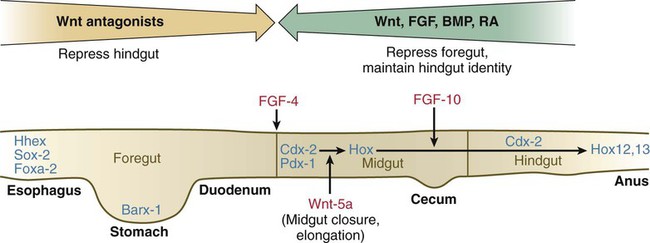
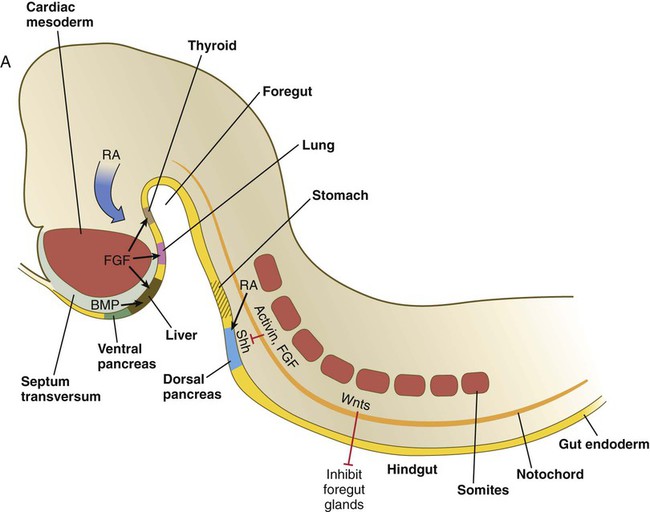
See text for details. Red letters, signaling molecules; blue letters, transcription factors.
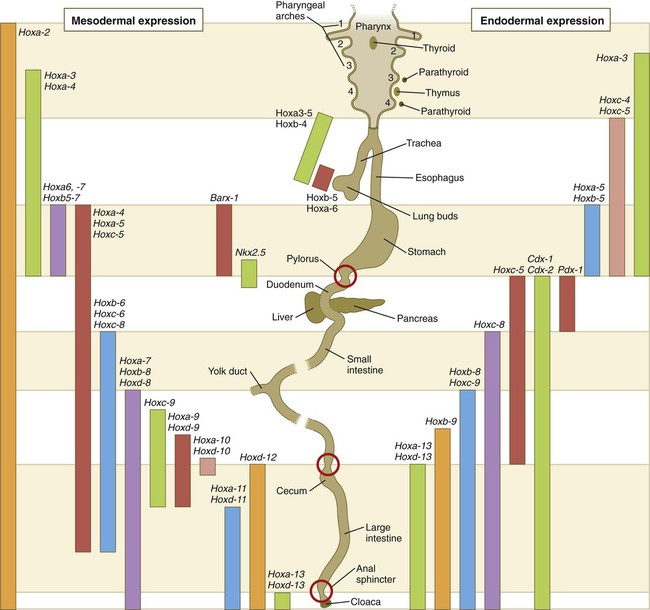
Expression along the gut endoderm (right) and in the gut-associated mesoderm (left). The circles represent areas where sphincters are located.
Largely through the action of Cdx-2, the orderly expression of homeobox-containing genes then takes over in the regional patterning of the digestive system (see Fig. 15.2).
By the end of the first month, small endodermal diverticula, which represent primordia of the major digestive glands, can be identified (Fig. 15.3). (Development of the pharynx and its glandular derivatives is discussed in Chapter 14.) The digestive glands and respiratory structures grow in complex branching patterns, resembling fractals, as the result of continuous epithelial-mesenchymal interactions. These interactions also occur in the developing digestive tube itself, with specific regional mesenchymal influences determining the character of the epithelium lining that part of the digestive tract.
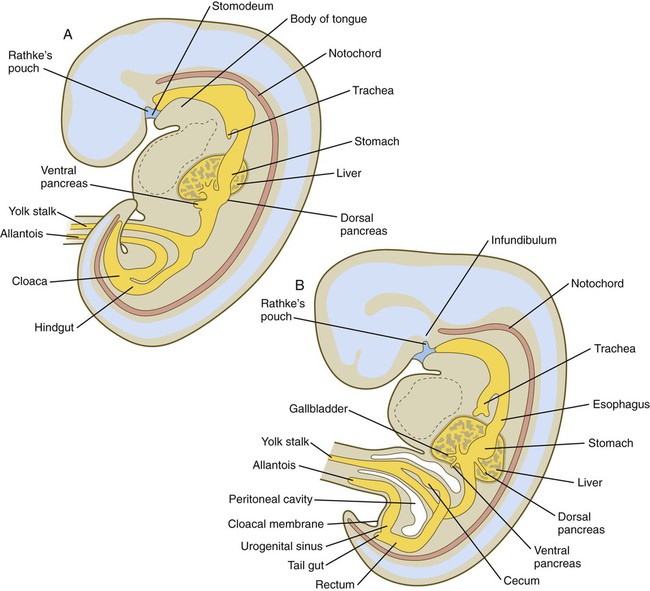
A, Early in the fifth week. B, Early in the sixth week.
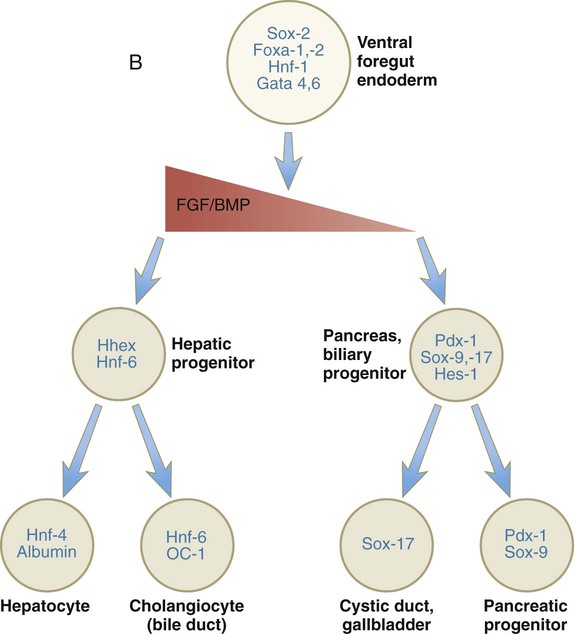
During neurulation, as the head bends sharply to create the foregut, the ventral foregut endoderm is closely opposed to two mesodermal masses: the cardiac mesoderm and the primordium of the septum transversum (see Fig. 15.4). High levels of FGF, secreted by the cardiac mesoderm, and also retinoic acid induce the formation of the liver, lung bud, and thyroid (see Fig. 15.4). BMP-4 from the mesoderm of the septum transversum is also required for liver induction. By contrast, endodermal movements carry the preventral pancreas cells far enough from the cardiac mesoderm to expose them to a low level of FGF, thus allowing the ventral pancreas to develop. For the dorsal pancreas to develop, locally produced sonic hedgehog (shh) must be inactivated by activin and FGF emanating from the notochord. In addition, retinoic acid from the somitic mesoderm is needed for induction of the dorsal pancreas. Meanwhile, in the hindgut, the actions of Wnt and other signaling molecules repress the expression of genes, such as Hhex and Pdx1, which are essential for formation of the liver and pancreas, respectively.
Induction of these organs is marked by the activation of transcription factors specific for the organ and stage of development of that organ. Some of these factors are schematically represented in Figure 15.4B.
Formation of the Esophagus
Just caudal to the most posterior pharyngeal pouches of a 4-week-old embryo, the pharynx becomes abruptly narrowed, and a small ventral outgrowth (lung bud) appears (see Fig. 6.20). The region of foregut just caudal to the lung bud is the esophagus. This segment is initially very short, with the stomach seeming to reach almost to the pharynx. In the second month of development, during which the gut elongates considerably, the esophagus assumes nearly postnatal proportions in relation to the location of the stomach.
Although the esophagus grossly resembles a simple tube, it undergoes a series of striking differentiative changes at the tissue level. In its earliest stages, the endodermal lining epithelium of the esophagus is stratified columnar. By 8 weeks, the epithelium has partially occluded the lumen of the esophagus, and large vacuoles appear (Fig. 15.5). In succeeding weeks, the vacuoles coalesce, and the esophageal lumen recanalizes, but with multilayered ciliated epithelium. During the fourth month, this epithelium finally is replaced with the stratified squamous epithelium that characterizes the mature esophagus.
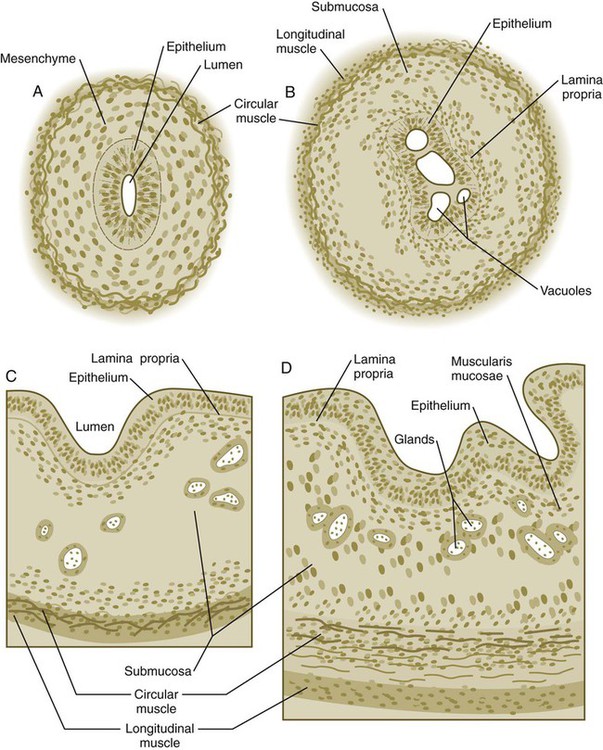
A, At 7 weeks. B, At 8 weeks. C, At 12 weeks. D, At 34 weeks.
The cross-sectional structure of the esophagus, similar to that of the rest of the gut, is organized into discrete layers. The innermost layer (mucosa) consists of the epithelium, derived from endoderm, and an underlying layer of connective tissue, the lamina propria (see Fig. 15.5C and D). A thick layer of loose connective tissue (submucosa) separates the mucosa from the outer layers of muscle (usually smooth muscle, with the exception of the upper esophagus). This radial organization is regulated by the epithelial expression of shh, acting through its receptor, patched, and BMP-4. Shh inhibits the formation of smooth muscle in the submucosal layer of the esophagus. Farther from the source of the endodermal shh, smooth muscle can differentiate in the outer wall of the intestine. How the developing smooth muscle layer of the mucosa (muscularis mucosae) escapes this inhibitory influence is also unclear. Intestinal mesenchyme can spontaneously differentiate into smooth muscle in the absence of an epithelium (which produces shh). Because in humans the muscularis mucosae differentiates considerably later than the outer muscular layers, it is possible that inhibitory levels of shh are reduced by that time.
Formation of the Stomach
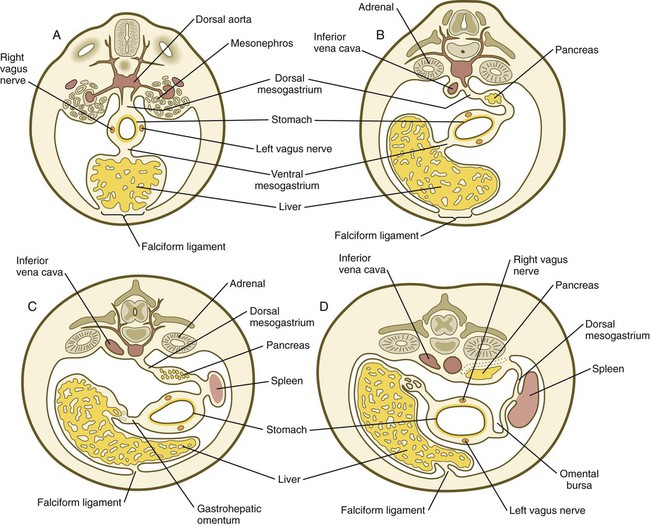
Very early in the formation of the digestive tract, the stomach is recognizable as a dilated region with a shape remarkably similar to that of the adult stomach (see Fig. 15.3). The early stomach is suspended from the dorsal body wall by a portion of the dorsal mesentery called the dorsal mesogastrium. It is connected to the ventral body wall by a ventral mesentery that also encloses the developing liver (Fig. 15.6).
When the stomach first appears, its concave border faces ventrally, and its convex border faces dorsally. Two concomitant positional shifts bring the stomach to its adult configuration. The first is an approximately 90-degree rotation about its craniocaudal axis so that its originally dorsal convex border faces left, and its ventral concave border faces right. The other positional shift consists of a minor tipping of the caudal (pyloric) end of the stomach in a cranial direction so that the long axis of the stomach is positioned diagonally across the body (Fig. 15.7).
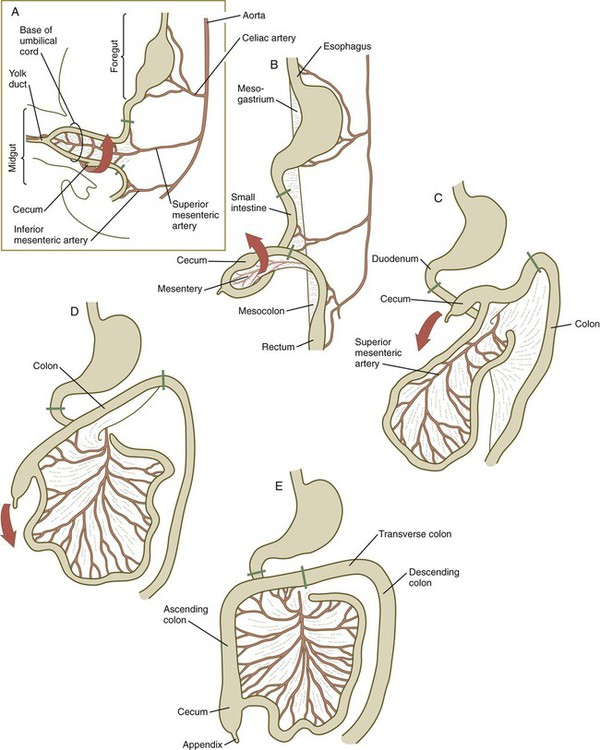
A, At 5 weeks. B, At 6 weeks. C, At 11 weeks. D, At 12 weeks. E, Fetal period. Areas between the green lines represent the midgut, which is supplied by the superior mesenteric artery.
During rotation of the stomach, the dorsal mesogastrium is carried with it, thus leading to the formation of a pouchlike structure called the omental bursa (bursa is a Latin word meaning “sac” or “pouch”). Both the spleen and the tail of the pancreas are embedded in the dorsal mesogastrium (see Fig. 15.6). Another viewpoint suggests that the right pneumatoenteric recess, a projection from the pleural cavity into the dorsal mesogastrium, persists as the omental bursa.
As the stomach rotates, the dorsal mesogastrium and the omental bursa that it encloses enlarge dramatically. Soon, part of the dorsal mesogastrium, which becomes the greater omentum, overhangs the transverse colon and portions of the small intestines as a large, double flap of fatty tissue (Fig. 15.8). The two sides of the greater omentum ultimately fuse and obliterate the omental bursa within the greater omentum. The rapidly enlarging liver occupies an increasingly large portion of the ventral mesentery.
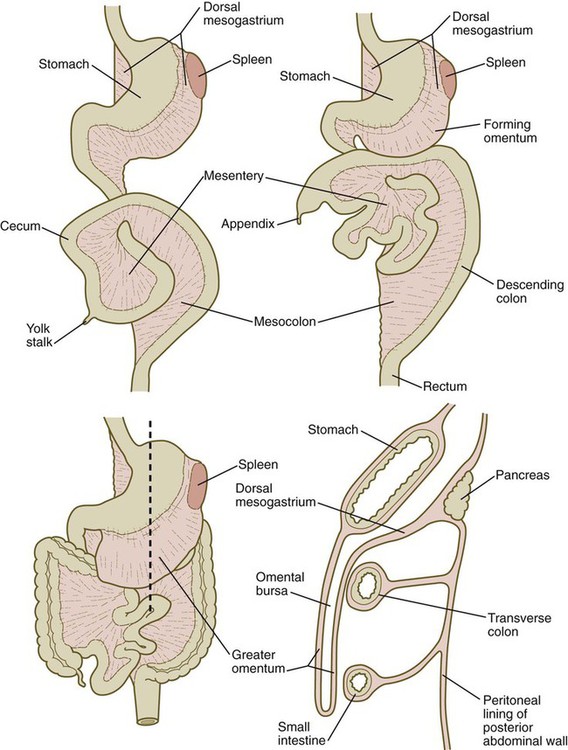
A section through the level of the dashed line (lower left) is shown (lower right).
Clinical Correlation 15.1 presents malformations of the esophagus and stomach.
Development of the Spleen
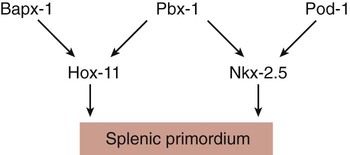
Development of the spleen is not well understood. Initially, two bilaterally symmetrical organ fields are reduced by regression to one on the left. The spleen is first recognizable as a mesenchymal condensation in the dorsal mesogastrium at 4 weeks and is initially closely associated with the developing dorsal pancreas. Initiation of splenic development requires the cooperative action of a basic helix-loop-helix protein (Pod-1) and a homeobox-containing protein (Bapx-1), acting through another transcription factor, Pbx-1. Such a combination is emerging as a common theme in the initiation of development of several organs. These substances act on the downstream molecules Nkx 2.5 and the oncogene Hox-11 (T-cell leukemia homeobox-1) in early splenic development (Fig. 15.9).
Formation of the Intestines
The intestines are formed from the posterior part of the foregut, the midgut, and the hindgut (Table 15.1). Table 15.2 summarizes the chronology of major stages in the development of the digestive tract. Two points of reference are useful in understanding the gross transformation of the primitive gut tube from a relatively straight cylinder to the complex folded arrangement characteristic of the adult intestinal tract. The first is the yolk stalk, which extends from the floor of the midgut to the yolk sac. In the adult, the site of attachment of the yolk stalk is on the small intestine about 2 feet cranial to the junction between small and large intestine (ileocecal junction). On the dorsal side of the primitive gut, an unpaired ventral branch of the aorta, the superior mesenteric artery, and its branches feed the midgut (see Fig. 15.7). The superior mesenteric artery itself serves as a pivot point about which later rotation of the gut occurs.
Table 15.1
Derivatives of Regions of the Primitive Gut
| Blood Supply | Adult Derivatives |
| Foregut | |
| Celiac artery (lower esophagus to duodenum) | Pharynx |
| Esophagus | |
| Stomach | |
| Upper duodenum | |
| Glands of pharyngeal pouches, respiratory tract, liver, gallbladder, pancreas | |
| Midgut | |
| Superior mesenteric artery | Lower duodenum |
| Jejunum and ileum | |
| Cecum and vermiform appendix | |
| Ascending colon | |
| Cranial half of transverse colon | |
| Hindgut | |
| Inferior mesenteric artery | Caudal half of transverse colon |
| Descending colon | |
| Rectum | |
| Superior part of anal canal |
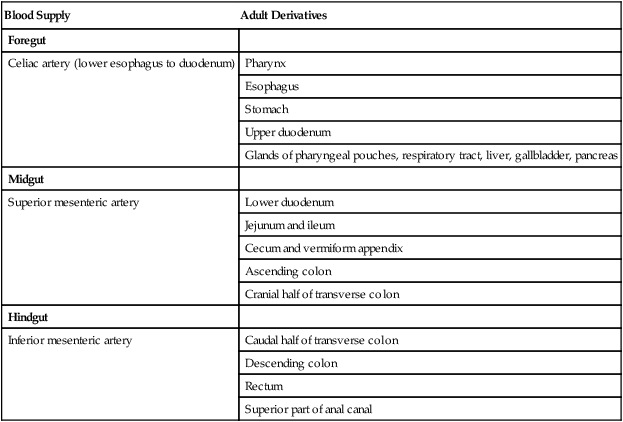
Table 15.2
Timelines in Development of the Digestive System
| Normal Time (wk) | Developmental Events |
| 3 | Tubular gut beginning to form; early induction of major digestive glands |
| 4 | Most of gut tubular; primordia of liver, dorsal and ventral pancreas, and trachea visible; rupture of oropharyngeal membrane |
| 5 | Expansion and early rotation of stomach; intestinal loop beginning to form; cecum and bile duct evident |
| 6 | Rotation of stomach completed, prominent intestinal loop; appearance of allantois and appendix; urorectal septum beginning to subdivide cloaca into rectum and urogenital sinus |
| 7 | Herniation of intestinal loop; rapid growth of liver; fusion of dorsal and ventral pancreas; cloacal septation complete |
| 8 | Counterclockwise rotation of herniated intestinal loop; recanalization of intestine; early penetration of parasympathetic neuronal precursors from cranial neural crest into gut |
| 9 | Return of herniated gut into body cavity; differentiation of epithelial types in intestinal lining |
| 11 | Villi appearing in small intestine; differentiation of goblet cells |
| 16 | Villi lining entire intestine (including colon) |
| 20 | Peyer’s patches seen in small intestine |
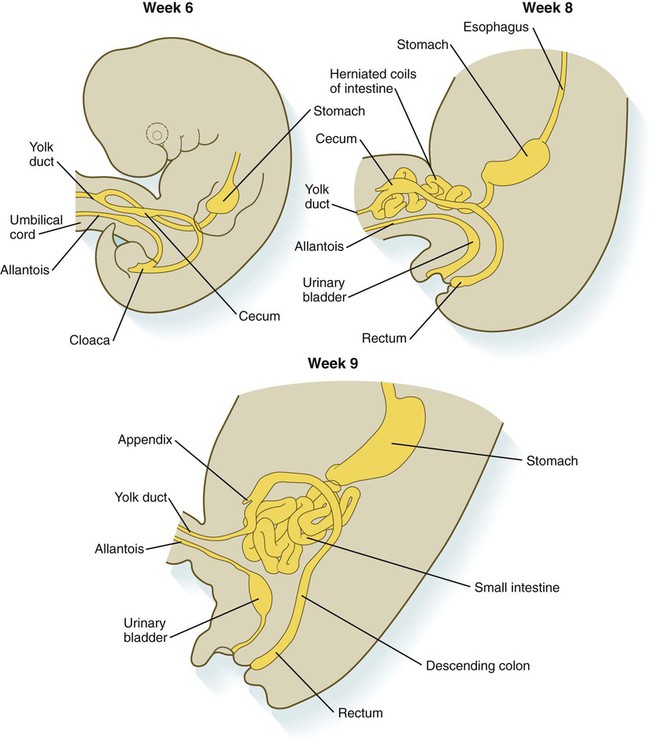
By 5 weeks, rapid growth of the gut tube causes it to buckle out in a hairpinlike loop. The growth in length results in large part from the effect of FGF-9, which is produced by the epithelium and stimulates proliferation of the fibroblasts in the intestinal walls. The major change that causes the intestines to assume their adult positions is a counterclockwise rotation of the caudal limb of the intestinal loop (with the yolk stalk attachment and superior mesenteric artery as reference points) around the cephalic limb from its ventral aspect. The main consequence of this rotation is to bring the future colon across the small intestine so that it can readily assume its C-shaped position along the ventral abdominal wall (see Fig. 15.7). Behind the colon, the small intestine undergoes great elongation and becomes packed in its characteristic position in the abdominal cavity.
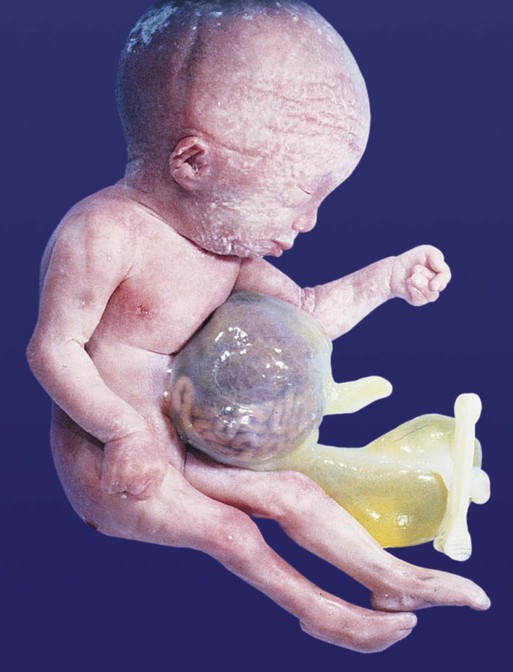
The rotation and other positional changes of the gut occur partly because the length of the gut increases more than the length of the embryo. From almost the first stages, the volume of the expanded gut tract is greater than the body cavity can accommodate. Consequently, the developing intestines herniate into the body stalk (the umbilical cord after further development) (Fig. 15.11). Intestinal herniation begins by 6 or 7 weeks of embryogenesis. By 9 weeks, the abdominal cavity has enlarged sufficiently to accommodate the intestinal tract, and the herniated intestinal loops begin to move through the intestinal ring back into the abdominal cavity. Coils of small intestine return first. As they do, they force the distal part of the colon, which was never herniated, to the left side of the peritoneal cavity, thus establishing the definitive position of the descending colon. After the small intestine has assumed its intra-abdominal position, the herniated proximal part of the colon also returns, with its cecal end swinging to the right and downward (see Fig. 15.7).
During these coilings, herniations, and return movements, the intestines are suspended from the dorsal body wall by a mesentery (Fig. 15.12). Experimentation has shown that looping of the intestine is caused principally by tension-compression relationships between the intestine and its dorsal mesentery. When the intestine is separated from the mesentery, the normal looping does not occur. As the intestines assume their definitive positions within the body cavity, their mesenteries follow. Parts of the mesentery associated with the duodenum and colon (mesoduodenum and mesocolon) fuse with the peritoneal lining of the dorsal body wall.
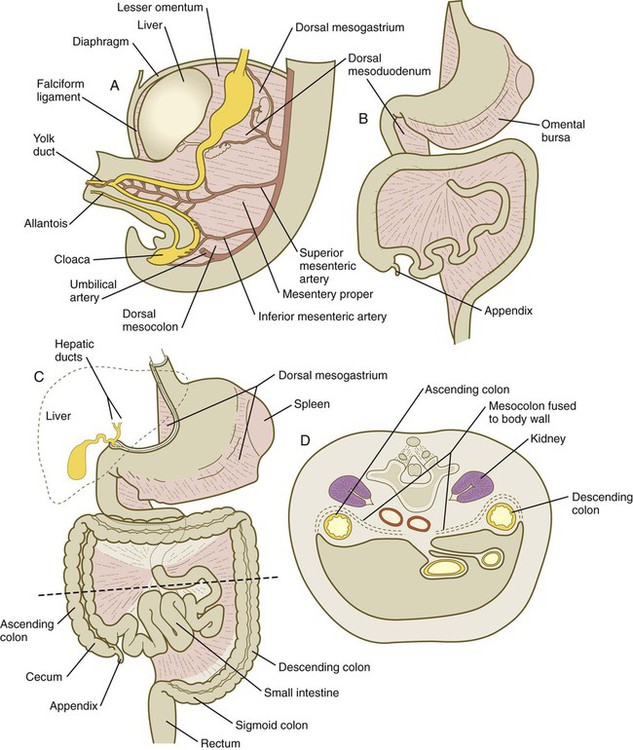
A, At 5 weeks. B, In the third month. C, During the late fetal period. D, Cross section through the dashed line in C. In C, the shaded areas represent regions where the mesentery is fused to the dorsal body wall.
Starting in the sixth week, the primordium of the cecum becomes apparent as a swelling in the caudal limb of the midgut (see Fig. 15.7). In succeeding weeks, the cecal enlargement becomes so prominent that the distal small intestine enters the colon at a right angle. The sphincterlike boundary at the cecum between the small and large intestines, similar to that in other regions of the gut, is regulated by a high concentration of Cdx-2 and a sequence of Hox gene expression. In mice, deletion of Hoxd4, Hoxd8 to Hoxd11, and Hoxd13 results in the absence of this region. When the overall pattern has been set by combinations of Hox genes, cecal development depends on an interaction between FGF-9 produced by the cecal epithelium and FGF-10 produced by the overlying mesoderm.
Partitioning of the Cloaca
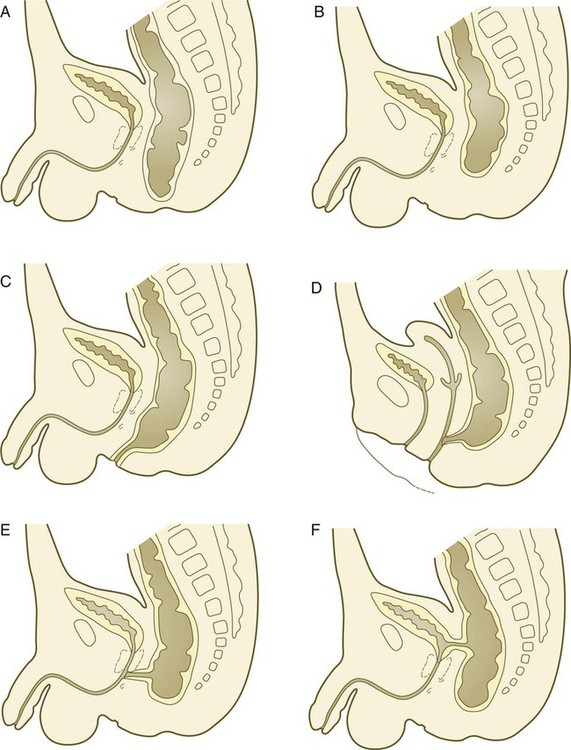
In the early embryo, the caudal end of the hindgut terminates in the endodermally lined cloaca, which, in lower vertebrates, serves as a common termination for the digestive and urogenital systems. The cloaca also includes the base of the allantois, which later expands as a common urogenital sinus (see Chapter 16). A cloacal (proctodeal) membrane consisting of apposed layers of ectoderm and endoderm acts as a barrier between the cloaca and an ectodermal depression known as the proctodeum (Fig. 15.13). A shelf of mesodermal tissue called the urorectal septum is situated between the hindgut and the base of the allantois. During weeks 6 and 7, the urorectal septum advances toward the cloacal membrane. At the same time, lateral mesodermal ridges extend into the cloaca.
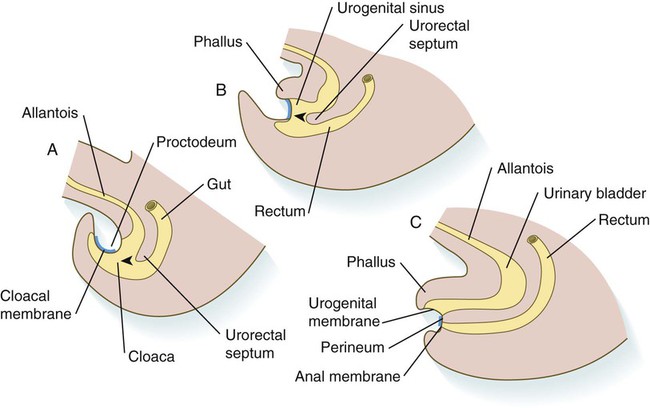
A, In the fifth week. B, In the sixth week. C, In the eighth week. The arrowheads indicate the direction of growth of the urorectal septum.
The combined ingrowth of the lateral ridges and growth of the urorectal septum toward the cloacal membrane divide the cloaca into the rectum and urogenital sinus (see Fig. 15.13B). Double mutants of Hoxa13 and Hoxd13 result in the absence of cloacal partitioning, along with hypodevelopment of the phallus (genital tubercle). In addition, they lead to the absence of the smooth muscle component of the anal sphincter. According to classic embryology, the urorectal septum fuses with the cloacal membrane, thus dividing it into an anal membrane and a urogenital membrane before these membranes break down (see Fig. 15.13C). Other research suggests that the cloacal membrane undergoes apoptosis and breaks down without its fusion with the urorectal septum. The area where the urorectal septum and lateral mesodermal folds fuse with the cloacal membrane becomes the perineal body, which represents the partition between the digestive and urogenital systems.
Histogenesis of the Intestinal Tract
With the formation of villi, pitlike intestinal crypts form at the bases of the villi. Toward the bottom of the crypts are epithelial intestinal stem cells, which, in response to Wnt signaling, have a high rate of mitosis and serve as the source of epithelial cells for the entire intestinal surface (Fig. 15.14). Despite the presence of four to six stem cells per crypt, it has been shown that each crypt is monoclonal (i.e., all the existing cells are descendants of a single stem cell from earlier in development). Toward the top of a crypt, shh and Indian hedgehog (Ihh) signaling stimulates the activity of BMP. This BMP has two main functions. It counteracts the effects of Wnt and thus keeps proliferation deep in the crypt, and it also facilitates cellular differentiation. Aided in part by an ephrin-eph gradient, progeny of the stem cells make their way up the wall of the crypt as multipotential transit amplifying cells, which, under the influence of the Delta-Notch system, begin to differentiate into the four main mature cell types of the intestinal epithelium (see Fig. 15.14). These cells then both differentiate and migrate toward the tip of the villus, until after about 4 days they die and are shed into the intestinal lumen as they are replaced below by new epithelial cells derived from the crypts. Human intestinal epithelial cells develop the intrinsic capacity for apoptosis by 18 to 20 weeks of gestation.
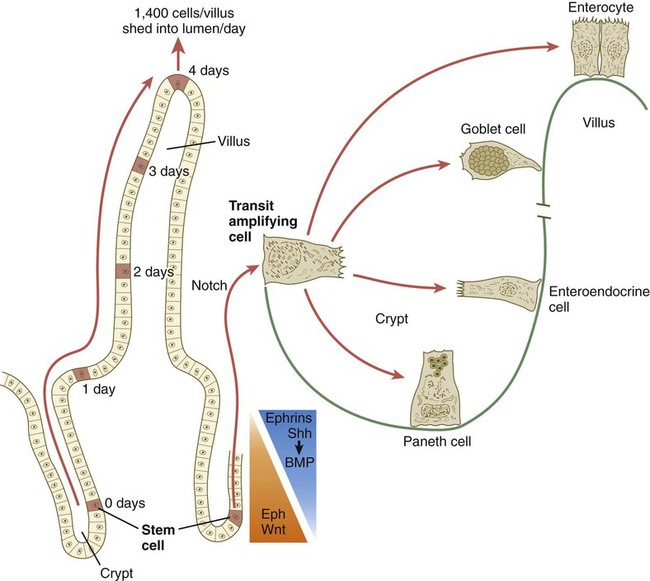
The time scale shows the typical course of migration of daughter cells starting with their generation from the stem cell population to their being shed from the villus into the intestinal lumen.
Histodifferentiation of the intestinal tract is not an isolated property of the individual tissue components of the intestinal wall. During the early embryonic period and sometimes into postnatal life, the epithelial and mesodermal components of the intestinal wall communicate by inductive interactions. In region-specific manners, these interactions involve hedgehog signaling (shh for the foregut and midgut and Ihh for the hindgut) from the endodermal epithelium. BMP signaling from the mesoderm is involved in positioning the crypts and villi in the small intestine and the glands of the colon. Interspecies recombination experiments show that the gut mesoderm exerts a regional influence on intestinal epithelial differentiation (e.g., whether the epithelium differentiates into a duodenal or colonic phenotype). When regional determination is set, however, the controls for biochemical differentiation of the epithelium are inherent. This pattern of inductive influence and the epithelial reaction are similar to those outlined earlier for dermal-epidermal interactions in the developing skin (see Chapter 9).
Although the intestine develops many functional capabilities during the fetal period, no major digestive function occurs until feeding begins after birth. The intestines of the fetus contain a greenish material called meconium (see Fig. 18.9), which is a mixture of lanugo hairs and vernix caseosa sloughed from the skin, desquamated cells from the gut, bile secretion, and other materials swallowed with the amniotic fluid.
Formation of Enteric Ganglia
As outlined in Chapter 12, the enteric ganglia of the gut are derived from neural crest. Pax-3–expressing cells from the vagal neural crest migrate into the foregut and spread in a wavelike fashion throughout the entire length of the gut. Slightly later, cells from the sacral crest enter the hindgut and intermingle with cells derived from the vagal neural crest. The migratory properties of vagal crest cells are much more pronounced than are those of cells from the sacral crest. Initially, the vagal crest cells migrate throughout the mesenchyme of the gut, but as the smooth muscle of the intestines begins to differentiate, the migrating vagal crest cells become preferentially distributed between the smooth muscle and the serosa, where the myenteric plexuses form. They are absent from the connective tissue of the submucosa because of the inhibiting effects of shh, secreted by the epithelial cells. During migration through the gut, the population of neural crest cells undergoes a massive expansion until the number of enteric neurons ultimately exceeds the number of neurons present in the spinal cord. Glial cells also differentiate from neural crest precursors in the gut, but the environmental factors that contribute to the differentiation of neural crest cells in the gut wall remain poorly understood.
Clinical Correlation 15.2 presents malformations of the intestinal tract.
Glands of the Digestive System
The glands of the digestive system arise through inductive processes between the early gut epithelium and the surrounding mesenchyme. The various glandular epithelia have considerably different requirements in the types of mesenchyme that can support their development. In tissue recombination experiments, pancreatic epithelium undergoes typical development when it is juxtaposed with mesenchyme from almost any source. The development of salivary gland epithelium is supported by mesenchyme from lung or accessory sexual glands, but not by many other types of mesenchyme. Inductive support of hepatic (liver) epithelium follows a distinctive pattern. Normal epithelial development is supported by mesenchyme derived from lateral plate or intermediate mesoderm, but axial mesenchyme (either somitic or neural crest) fails to support hepatic differentiation. The inductive properties of certain glandular mesenchymes may be correlated with different modes of vascularization of these mesenchymes (see p. 414).
Formation of the Liver
After initial induction by the cardiac mesoderm and the septum transversum (see Fig. 15.4), the gut-derived hepatic endoderm thickens to form a pseudostratified epithelium (Fig. 15.21). The nuclear dynamics within the pseudostratified epithelium parallel those in the early neural tube (see Fig. 11.4). The nuclei undergo DNA synthesis (S phase of the cell cycle) in the basal position near the basal lamina surrounding the liver bud. Then the nuclei migrate to the apical (luminal) position, where they undergo mitotic division. The pathways and fates of the daughter cells have not yet been clearly determined. The transition to the pseudostratified stage requires the activity of the homeobox gene Hhex, without which the liver does not form (see Fig. 15.21).
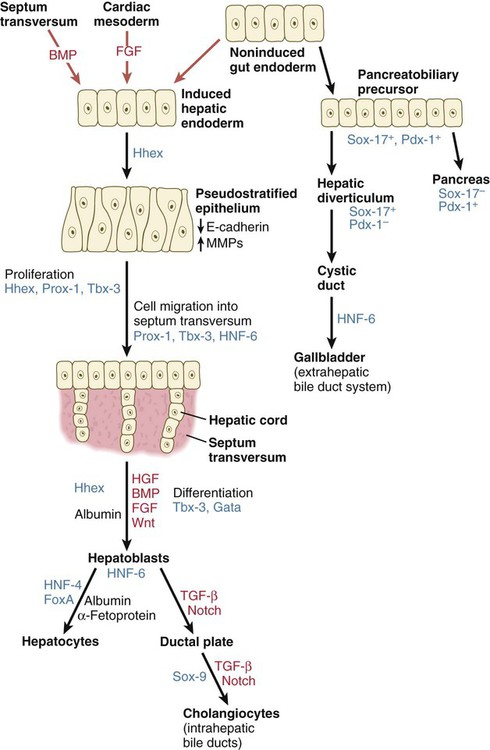
Growth factors are printed in red, and transcription factors in blue. BMP, bone morphogenetic protein; FGF, fibroblast growth factor; HGF, hepatic growth factor; HNF, hepatic nuclear factor; TGF, transforming growth factor.
The cells of the hepatic cords (hepatoblasts) are bipotential: they can form either hepatic parenchymal cells (hepatocytes) or intrahepatic bile duct cells (cholangiocytes). Guided by the transcription factors hepatic nuclear factor-4 (HNF-4) and FoxA, some hepatoblasts differentiate into hepatocytes, which begin to express molecules (e.g., albumin and α-fetoprotein) characteristic of mature hepatic parenchymal cells. Other hepatoblasts, under the influence of TGF-β and Notch, gather as a single layer around branches of the portal vein as a ductal plate (Fig. 15.22A). Through mechanisms still not well understood, two ductlike structures (future bile ducts) begin to form around each vein. Initially, the cells comprising the walls of the ducts are hybrid in character (Fig. 15.22B). Cells closest to the vein have the characteristics of cholangiocytes, whereas those on the opposite face more closely resemble hepatocytes. Eventually, all the cells lining the bile duct become full-fledged cholangiocytes. These bile ducts branch to form networks leading toward the edges of the hepatic lobes and constitute the intrahepatic component of the bile duct system.
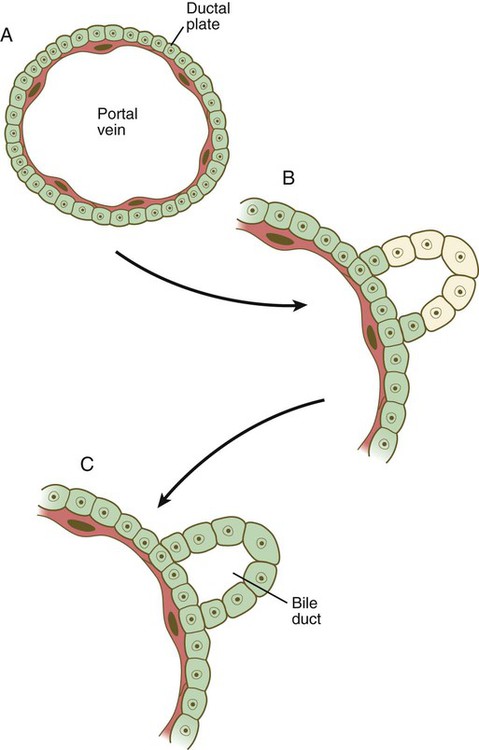
A, A layer of hepatic endodermal cells forms a ductal plate around a portal vein. B, In early stages in the formation of a bile duct, the cells at the outer perimeter have the characteristics of hepatocytes, whereas the inner cells exhibit a cholangiocyte phenotype. C, A differentiated bile duct, with all cells having the cholangiocyte phenotype. (Based on Lemaigre FP: Prog Mol Biol Transl Sci 97:103-126, 2010.)
The other major component of the bile duct system, consisting of the larger hepatic ducts, the cystic duct, the gallbladder, and the common bile duct, arises outside the main body of the liver and is called the extrahepatic biliary tree. Its precursor cells arise as a component of a common pancreatobiliary precursor (see Fig. 15.21), which is located caudal to the prehepatic endoderm. Expressing Sox-17 and Pdx-1, these cells are bipotential, like those of the hepatic diverticulum. Some of these cells cease expressing Sox-17, but continue to express Pdx-1 and go on to form the ventral pancreas. Others, which lose Pdx-1 expression but continue to express Sox-17, become precursors of the extrahepatic biliary tree. They extend to form the cystic duct, and a dilatation from that foreshadows the further development of the gallbladder (Fig. 15.23). How the intrahepatic and extrahepatic bile ducts become connected remains unclear.
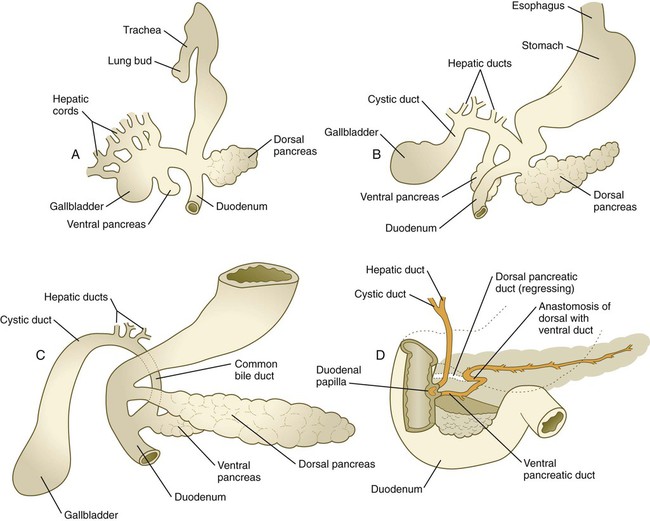
A, In the fifth week. B, In the sixth week. C, In the seventh week. D, In the late fetus, showing fusion of the dorsal and ventral pancreatic ducts and regression of the distal portion of the dorsal duct.
The entire liver soon becomes too large to be contained in the septum transversum, and it protrudes into the ventral mesentery within the abdominal cavity. As it continues to expand, the rapidly growing liver remains covered by a glistening, translucent layer of mesenteric tissue that now serves as the connective tissue capsule of the liver. Between the liver and the ventral body wall is a thin, sickle-shaped piece of ventral mesentery: the falciform ligament. The ventral mesentery between the liver and the stomach is the lesser omentum (see Fig. 15.6).
Development of Hepatic Function
A major function of the embryonic liver is the production of blood cells. After yolk sac hematopoiesis, the liver is one of the chief sites of intraembryonic blood formation (see Fig. 17.2). Hematopoietic cells, seeding the liver from origins in other sites, appear in small clusters among the hepatic parenchymal cells. As the liver matures, the intrahepatic environment no longer supports blood cell development, and hematopoiesis migrates to other sites in the fetus.
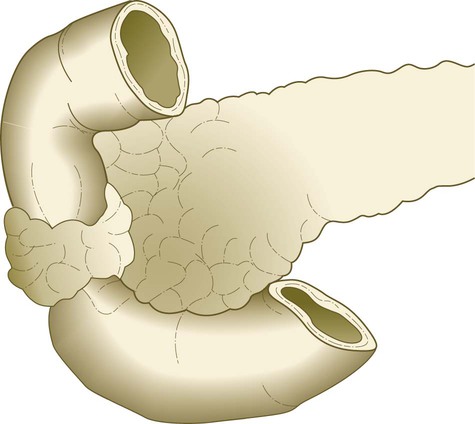
Formation of the Pancreas
The pancreas begins as separate dorsal and ventral primordia within the duodenal endoderm (see Fig. 6.20D). Early development of each of these two primordia is under different molecular controls. As discussed previously, ventral endoderm of the hepatic diverticulum is patterned to differentiate into ventral pancreatic tissue by a default mechanism in areas where liver induction does not occur. In primitive vertebrates, pancreatic function is distributed in cells within the foregut, rather than in a discrete gland, and some investigators have speculated that the ventral pancreatic bud represents the extension of this system, whereas the discrete dorsal pancreas is an evolutionary newcomer.
The dorsal pancreas is induced from the dorsal gut endoderm by activin and FGF signals emanating from the notochord, which early in development is directly opposed to the endoderm (Fig. 15.24). Shh activity in the dorsal endoderm must be repressed, or pancreatic differentiation does not occur. Initiation of development of the ventral pancreas is under a different set of developmental controls and heavily depends on the activity of the transcription factor Ptf-1a. During the earliest stages of dorsal pancreatic bud formation, the pancreatic progenitor cells express the transcription factors Pdx-1 and Hoxb-9. If Pdx-1 expression is eliminated by targeted mutagenesis, development of the pancreatic bud ceases. From this point, different environmental signals and intracellular responses result in the differentiation of two lineages of cells: the pancreatic exocrine cells and the pancreatic endocrine cells (see Fig. 15.24).
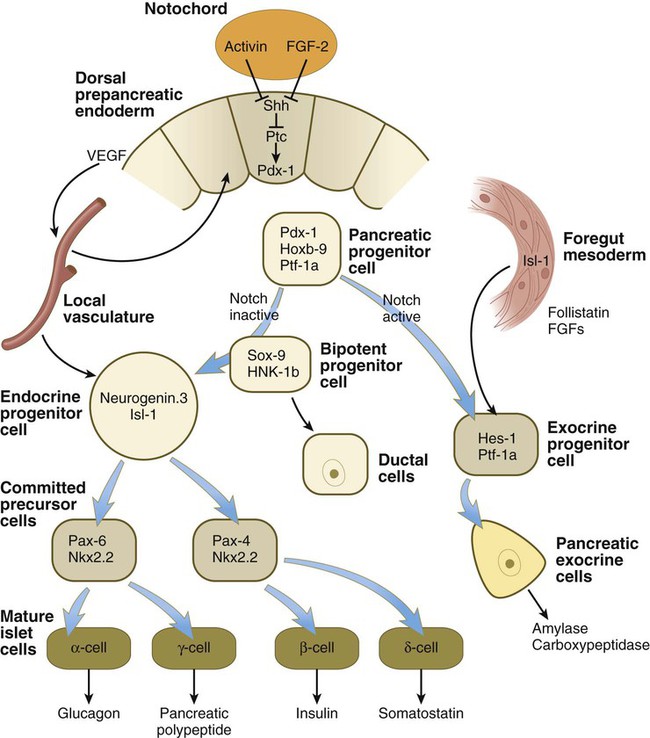
FGF, fibroblast growth factor; Shh, sonic hedgehog; VEGF, vascular endothelial growth factor.
Both the dorsal pancreas and the ventral pancreas possess a large duct. After fusion of the two pancreatic primordia, the main duct of the ventral pancreas makes an anastomotic connection with the duct of the dorsal pancreas. The portion of the dorsal pancreatic duct between the anastomotic connection and the duodenum normally regresses, leaving the main duct of the ventral pancreas (duct of Wirsung) the definitive outlet from the pancreas into the duodenum (see Fig. 15.23).
In certain pancreatic progenitor cells, the action of the signaling molecules, follistatin, and several FGFs emanating from the surrounding mesoderm, in combination with the activation of the Notch receptor system (see p. 69), results in the differentiation of many of the pancreatic cells along the exocrine pathway. These cells, which secrete digestive hormones, such as amylase and carboxypeptidase, are ultimately responsible for the gross morphogenesis of the pancreas. During outgrowth of the pancreatic primordia, the exocrine cells assume the form of sequentially budding cords. From these cellular cords, the acini and their ducts differentiate. Tissue recombination experiments, conducted in vitro and in vivo, have shown that the presence of mesenchyme is necessary for the formation of acini, but that ducts can form in the absence of mesenchyme if the endodermal precursor cells are exposed to a gel rich in basement membrane material.
Differentiation of the acini is divided into three phases (Fig. 15.25). The first, called the predifferentiated state, occurs while the pancreatic primordia are first taking shape. A population of pancreatic progenitor cells that exhibits virtually undetectable levels of digestive enzyme activity is established. As the pancreatic buds begin to grow outward, the epithelium undergoes a transition into a second, protodifferentiated state. During this phase, the exocrine cells synthesize low levels of many hydrolytic enzymes that they will ultimately produce. After the main period of outgrowth, the pancreatic acinar cells pass through another transition before attaining a third, differentiated state. By this time, these cells have acquired an elaborate protein-synthesizing apparatus, and the inactive forms of the polypeptide digestive enzymes are stored in the cytoplasm as zymogen granules. Glucocorticoid hormones from the fetal adrenal cortex stimulate increased production of certain digestive enzymes.
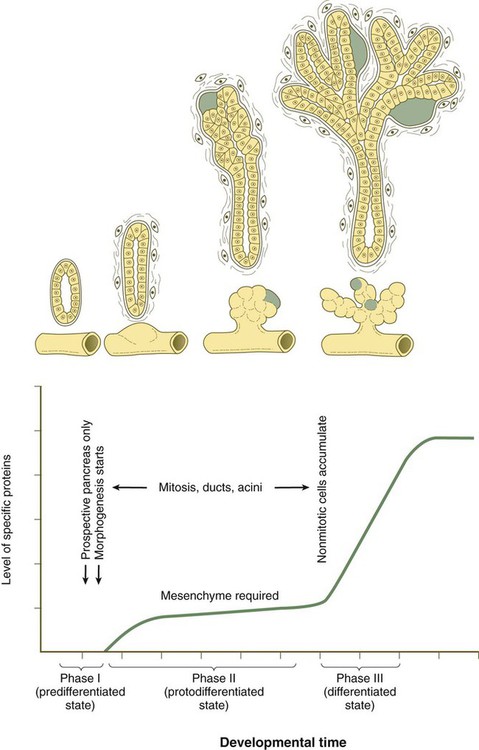
The green areas represent primitive islets. (Adapted from Pictet R, Rutter W: Handbook of physiology, section 7: Endocrinology, vol 1, Washington, D.C., 1972, American Physiological Society, pp 25-66.)
Development of the islets of Langerhans follows a different course from that of the acini. The islets of Langerhans are formed from groups of epithelial cells that break away from the acinar epithelial cells during the second (protodifferentiated) phase of acinar cell development. In a pathway not involving activation of the Notch system, but requiring signals from the local vasculature, a bipotential precursor cell has the potential to become either an endocrine cell or a ductal cell (see Fig. 15.24). Those cells entering the endocrine lineage as endocrine progenitor cells express the transcription factors neurogenin-3 and Isl-1. The endocrine progenitor cells give rise to two types of progenies (committed precursor cells), each of which is characterized by the expression of a different Pax gene. One type, which differentiates at 8 to 9 weeks, gives rise to α and γ cells, which produce glucagon and pancreatic polypeptide. The other type, which differentiates later, gives rise to β and δ cells, which produce insulin and somatostatin. During the second phase of pancreatic differentiation (protodifferentiated state), the levels of glucagon synthesis considerably exceed the levels of insulin. By the third phase of pancreatic development, secretory granules are evident in the cytoplasm of most islet cells. Insulin and glucagon are present in the fetal circulation by the end of the fifth month of gestation.
Clinical Correlation 15.3 presents anomalies of the liver and pancreas.
Respiratory System
The location of the future respiratory system in the ventral foregut is indicated by a zone of expression of the transcription factor Nkx 2.1, which also marks the site of formation of the thyroid gland. The dorsal wall of the foregut in this area is characterized by Sox-2 expression. Specification (induction) of the respiratory region is mediated by Wnt and FGF signals from the adjacent mesoderm. Late in the fourth week, paired lung buds begin to protrude from the posterior part of the respiratory endoderm (Fig. 15.28A). Dorsal to the lung buds, a pair of lateral mesodermal ridges begins to grow inward. Under the influence of Wnt signaling, these ridges fuse in a posterior-to-anterior direction. As they do so, they create a septum that separates the newly forming trachea from the esophagus. Through a series of interactions with the surrounding mesoderm, the early respiratory diverticulum (trachea plus lung buds) elongates, and the lung buds begin a set of 23 bifurcations that continue into postnatal life.
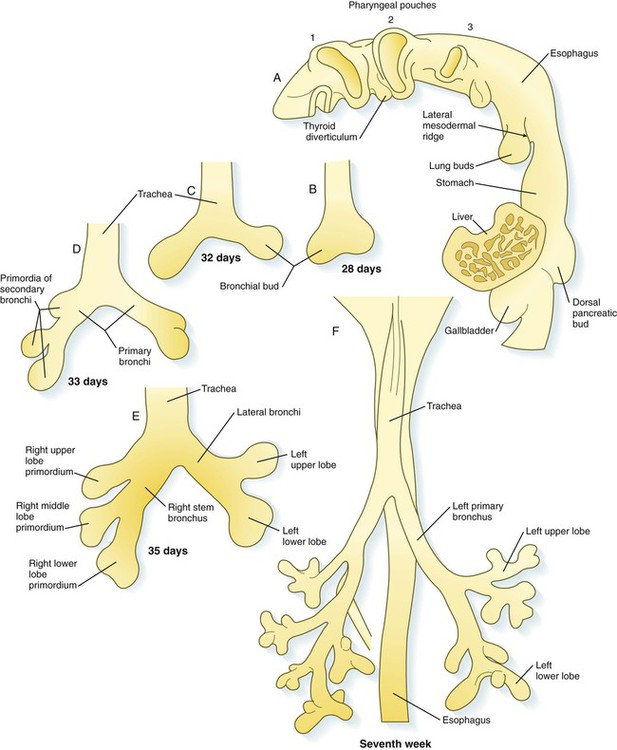
A, Lateral view of the pharynx, showing the respiratory diverticulum in a 4-week-old embryo. B, At 4 weeks. C, At 32 days. D, At 33 days. E, At the end of the fifth week. F, Early in the seventh week.
Formation of the Trachea and Bronchial Tree
At the initial appearance of the respiratory diverticulum, a pair of bronchial buds appears at its end (Fig. 15.28B). It now seems that the precursors of the trachea and lung buds are derived from separate sources of cells, and that the lung buds give rise to the bronchi and distal respiratory tree. The straight portion of the respiratory diverticulum is the primordium of the trachea. The bronchial buds, which ultimately become the primary bronchi, give rise to additional buds—three on the right and two on the left. These buds become the secondary, or stem, bronchi, and their numbers presage the formation of the three lobes of the right lung and the two lobes of the left (see Fig. 15.28). From this point, each secondary bronchial bud undergoes a long series of branchings throughout embryonic and fetal life.
The basic principles underlying pulmonary branching are similar to those operating in the development of the salivary glands and pancreas. At points of branching, epithelial cell proliferation is reduced, and the deposition of types I, III, and IV collagen, fibronectin, and proteoglycans stabilizes the morphology of the branching point and more proximal ductal regions. Heightened epithelial cell proliferation characterizes the rapidly expanding portions of the epithelial buds (Fig. 15.29).
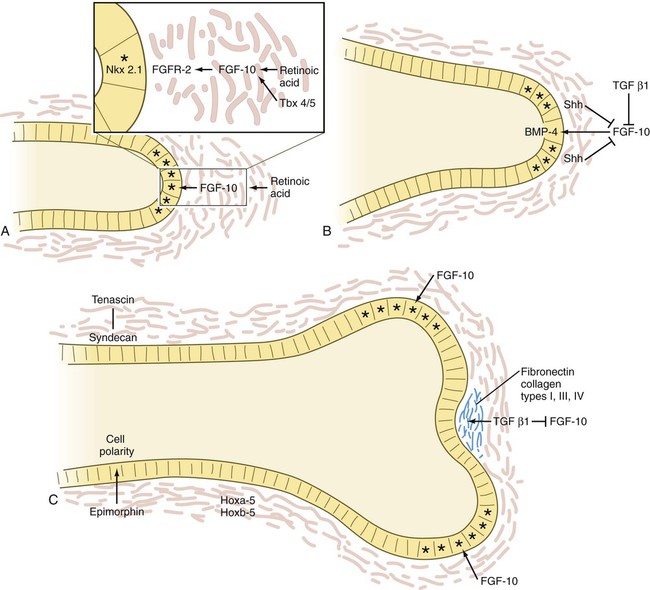
A, The tip of an elongating respiratory duct. Fibroblast growth factor-10 (FGF-10) secretion in the mesenchyme stimulates the growth of the tip of the epithelial duct toward it. B, The prelude to branching. Inhibition of FGF-10 signaling at the tip of the duct leads to stabilization of that area. C, Cleft formation. Extracellular matrix molecules are deposited in the newly forming cleft, and two new centers of outgrowth, stimulated by FGF-10 signaling, indicate the start of branching. Asterisk indicates dividing cells. BMP, bone morphogenetic protein; FGFR, fibroblast growth factor receptor; Shh, sonic hedgehog; TGF, transforming growth factor.
The activities of many molecules contribute to lung morphogenesis. More than 50 genes are involved in morphogenesis of the tracheal system in Drosophila, which shows remarkable parallels to the mammalian respiratory system. A prime mover in generating branching is FGF-10, which, in response to the action of retinoic acid* and Tbx-4 and Tbx-5, is produced by the mesenchyme off the tip of a growing bud in the respiratory system. In FGF-10 knockout mice, budding of the developing lungs fails to occur. FGF-10 acts as a signaling center, by stimulating cell proliferation in the epithelium at the tip of the bud and causing the epithelium to grow out toward the source of the FGF-10 (see Fig. 15.29A). Apical epithelial proliferation is also promoted by the expression of the transcription factor Nkx 2.1 in these cells.
Branching is initiated with the stimulation of secretion of BMP-4 in the apical epithelial cells; this inhibits their proliferation. Simultaneously, shh, which is also produced by the epithelium, stimulates proliferation of the mesenchymal cells off the tip and inhibits the formation of FGF-10 (see Fig. 15.29B). These mesenchymal cells begin to produce TGF-β1, which, in addition to inhibiting FGF-10 production along with shh, promotes the synthesis of extracellular matrix molecules just distal to the apical epithelial cells. These molecules, including fibronectin and collagen types I, III, and IV, stabilize the formerly growing epithelial tip.
While the cell proliferation in the epithelial tip is reduced and the cells become bound by newly secreted extracellular matrix molecules, FGF-10 is secreted by the mesenchyme lateral to the old apex, where the concentrations of shh and TGF-β1 are reduced to less than the inhibitory level (see Fig. 15.29C). This activity sets up two new signaling centers on either side of the original one, and the cycle of apical epithelial proliferation begins anew. As the new apical growth centers mature, FGF-10 signaling is again inhibited, and each of the two existing tips begins its own branching cycle. The concurrent presence of the epithelial cell–associated proteoglycan syndecan is important for maintaining the stability of epithelial sheets along the ducts. Interacting with the extracellular matrix protein tenascin, syndecan is found along already formed ducts, but not in areas where branching is occurring in terminal saccular regions of the developing airway (see Fig. 15.29).
Stages in Lung Development
Embryonic Stage (Weeks 4 to 7)
The embryonic stage includes the initial formation of the respiratory diverticulum up to the formation of all major bronchopulmonary segments. During this period, the developing lungs grow into and begin to fill the bilateral pleural cavities. These structures represent the major components of the thoracic body cavity above the pericardium (Fig. 15.30).
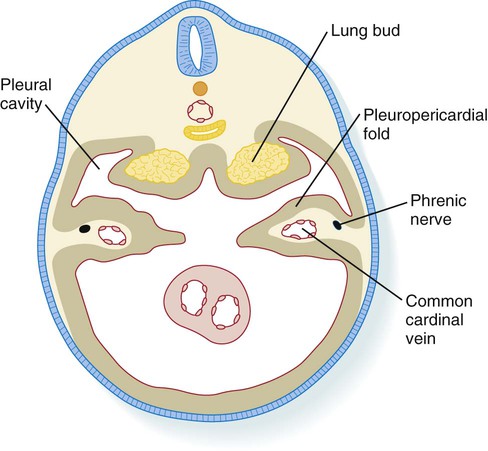
The pleuropericardial folds separate the future pleural from the pericardial cavities.
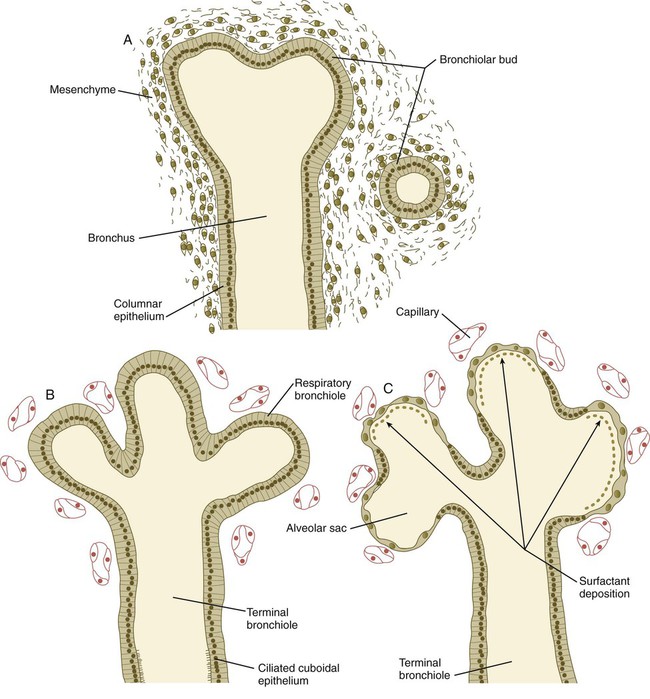
Pseudoglandular Stage (Weeks 8 to 16)
The pseudoglandular stage is the period of major formation and growth of the duct systems within the bronchopulmonary segments before their terminal portions form respiratory components. The histological structure of the lung resembles that of a gland (Fig. 15.31), thus providing the basis for the designation of this stage. During this period, the pulmonary arterial system begins to form. The elongating vessels parallel the major developing ducts.
Canalicular Stage (Weeks 17 to 26)
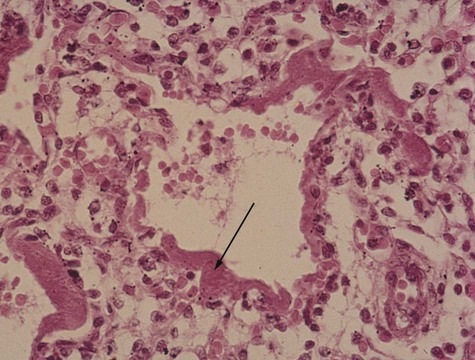
The canalicular stage is characterized by the formation of respiratory bronchioles as the result of budding of the terminal components of the system of bronchioles that formed during the pseudoglandular stage. An array of many different cell types forms along the developing respiratory tree. A gradient of BMP-4 and Wnt signaling, which is highest at the distal tips of the branches, prevents the distal cells from forming phenotypes more characteristic of the larger branches of the bronchial tree. The other major events during this stage are the intense ingrowth of blood vessels into the developing lungs and the close association of capillaries with the walls of the respiratory bronchioles (see Fig. 15.31). Occasionally, a fetus born toward the end of this period can survive with intensive care, but respiratory immaturity is the principal reason for poor viability.
Terminal Sac Stage (Weeks 26 to Birth)
During the terminal sac stage, the terminal air sacs (alveoli) bud off the respiratory bronchioles that largely formed during the canalicular stage. The epithelium lining the alveoli differentiates into two types of cells: type I alveolar cells (pneumocytes), across which gas exchange occurs after birth; and type II alveolar (secretory epithelial) cells. Type II alveolar cells form pulmonary surfactant, the material that spreads over the surface of the alveoli to reduce surface tension and facilitate expansion of the alveoli during breathing. Research involving specific markers of the epithelial cells has shown that type II cells form first in the alveolar lining. After proliferation, some type II cells flatten, lose their characteristic secretory function, and undergo terminal differentiation into type I pneumocytes. Other type I cells may differentiate directly from a pool of epithelial precursor cells in the early alveolar lining. With increasing amounts of pulmonary surfactant being formed, the fetus has a correspondingly greater chance of survival if born prematurely. In the fetus, the respiratory passageways in the lungs are filled with fluid (see Chapter 18). During the last 4 weeks of pregnancy, greatly increased formation of alveoli results in an exponential increase in the respiratory surface area of the lung. These weeks are sometimes referred to as the alveolar period of lung development.
Body Cavities
Formation of the Common Coelom and Mesentery
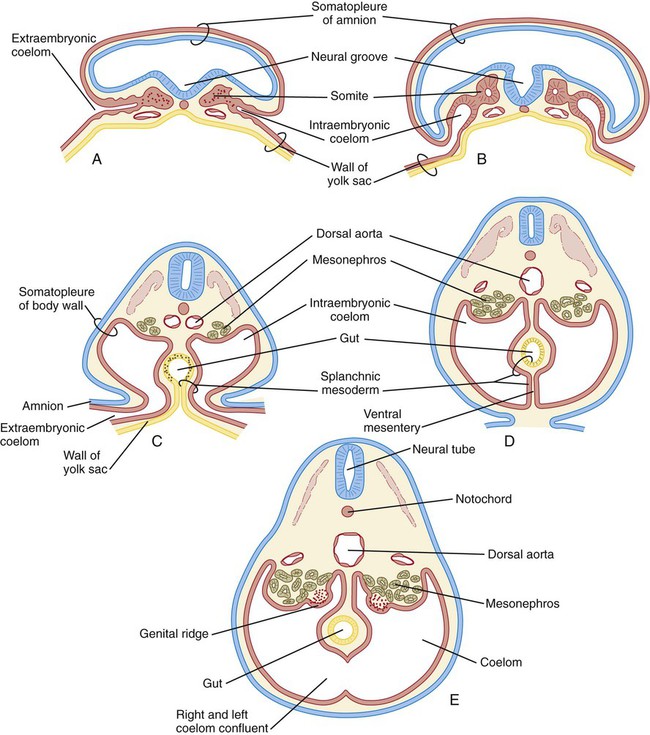
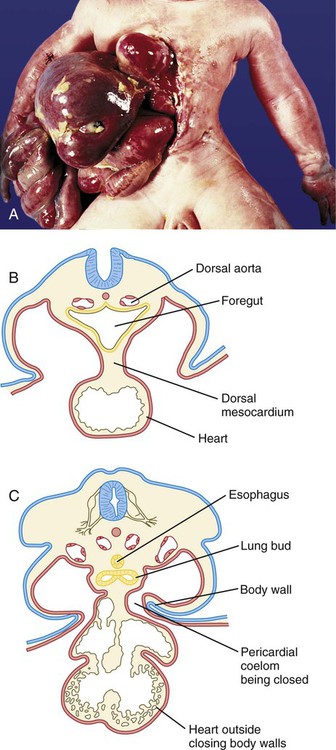
As the lateral plate mesoderm of the early embryo splits and then folds laterally, the space between the somatic and splanchnic layers of mesoderm becomes the common intraembryonic coelom (Fig. 15.34). The same folding process that results in the completion of the ventral body wall and the separation of the intraembryonic from the extraembryonic coelom also brings the two layers of splanchnic mesoderm around the newly formed gut as the primary (common) mesentery. The primary mesentery suspends the gut from the dorsal body wall as the dorsal mesentery and attaches it to the ventral body wall as the ventral mesentery. This placement effectively divides the coelom into right and left components. Soon, however, most of the ventral mesentery breaks down and causes a confluence of the right and left halves of the coelom. In the region of the developing stomach and liver, the ventral mesentery persists, thus forming the ventral mesogastrium and the falciform ligament of the liver (see Fig. 15.6). Further cranially, the tubular primordium of the heart is similarly supported by a dorsal mesocardium and briefly by a ventral mesocardium, which soon breaks down.
Formation of the Septum Transversum and Pleural Canals
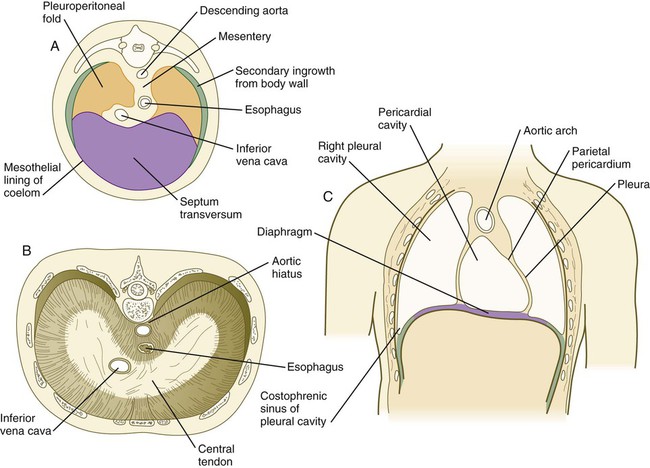
A major factor in division of the common coelom into thoracic and abdominal components is the septum transversum. This septum grows from the ventral body wall as a semicircular shelf, which separates the heart from the developing liver (Fig. 15.35). During its early development, a major portion of the liver is embedded in the septum transversum. Ultimately, the septum transversum constitutes a significant component of the diaphragm (see p. 367).
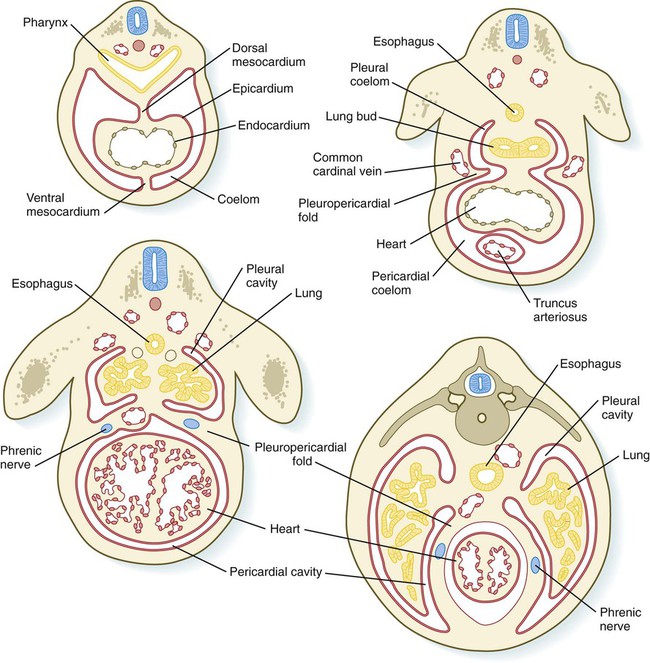
The expanding septum transversum serves as a partial partition between the pericardial and the peritoneal portions of the coelom. By the time the expanding edge of the septum transversum reaches the floor of the foregut, it has almost cut the common coelom into two parts. Two short channels located on either side of the foregut connect the two major parts (Fig. 15.36). Initially known as the pleural (pericardioperitoneal) canals, these channels represent the spaces into which the developing lungs grow. The pleural canals enlarge greatly as the lungs increase in size and ultimately form the pleural cavities.
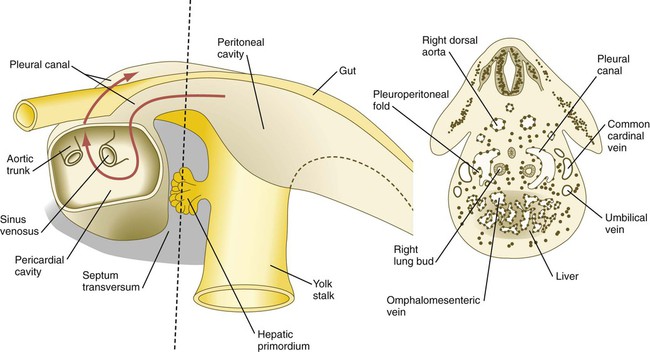
The red arrow passes from the left pleural cavity into the pericardial cavity and then into the right pleural canal. The dashed line on the left represents the level of the cross section on the right.
The pleural canals are partially delimited by two paired folds of tissue: the pleuropericardial and pleuroperitoneal folds. The pleuropericardial folds (see Fig. 15.30) are ridges of tissue associated with the common cardinal veins, which bulge into the dorsolateral wall of the coelom as they arch toward the midline of the thoracic portion of the coelom and enter the sinus venosus of the heart (Fig. 15.37). Initially, the pleuropericardial folds are not large and cause only a narrowing at the junction of the pericardial cavity and pleural canals. As the lungs expand, however, the folds form prominent shelves that meet at the midline and form the fibrous (parietal) layer of the pericardium.
At the caudal ends of the pleural canals, another pair of folds, the pleuroperitoneal folds, becomes prominent as the expanding lungs push into the mesoderm of the body wall. The pleuroperitoneal folds occupy successively greater portions of the pleural canal until they fuse with the septum transversum and the mesentery of the esophagus, thereby effectively obliterating the pleural canal (Fig. 15.38). Cells from the pleuroperitoneal folds continue into the abdominal cavity and contribute to the connective tissue that connects the liver and the right adrenal gland. All connections between the abdominal cavity and the thoracic cavity are eliminated.
Formation of the Diaphragm
The diaphragm, which separates the thoracic from the abdominal cavity in adults, is a composite structure derived from several embryonic components (see Fig. 15.38). The large ventral component of the diaphragm arises from the septum transversum, which fuses with the ventral part of the esophageal mesentery. Converging on the esophageal mesentery from the dorsolateral sides are the pleuroperitoneal folds. These components form the bulk of the diaphragm. As the lungs continue to grow, their caudal tips excavate additional space in the body wall. The body wall mesenchyme separated from the body wall proper becomes a third component of the diaphragm by forming a thin rim of tissue along its dorsolateral borders. In keeping with their motor innervation by the phrenic nerve, arising from the third to fifth cervical roots, the cellular precursors of the diaphragmatic musculature shift caudally into the body cavity from their site of origin in the cervical somites.
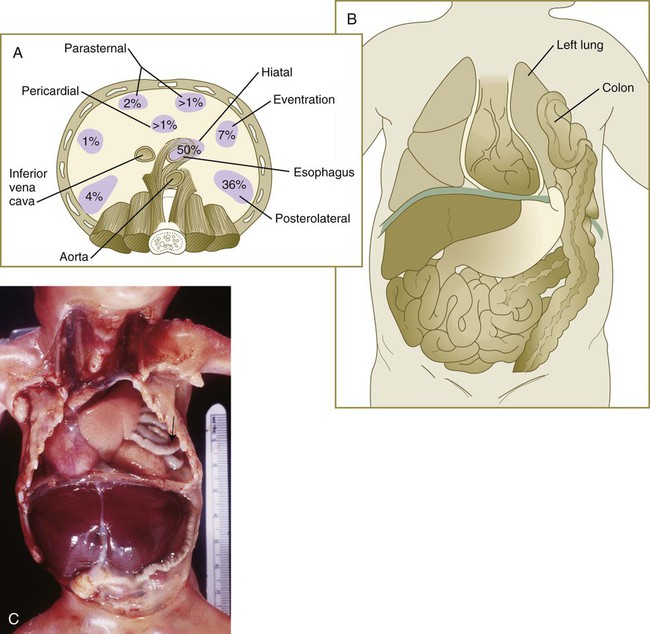
Clinical Correlation 15.5 presents malformations of the body cavities, diaphragm, and body wall.
Summary
 The digestive system arises from the primitive endodermally lined gut tube, which is bounded cranially by the oropharyngeal membrane and caudally by the cloacal membrane. The gut is divided into foregut, midgut, and hindgut segments, with the midgut opening into the yolk sac. Specification of the various regions of the gut tract depends on a pattern set up by tightly regulated combinations of Hox genes. Development of virtually all parts of the gut depends on epithelial-mesenchymal interactions. Responding to such interactions, primordia of the respiratory system, the liver, the pancreas, and other digestive glands bud out from the original gut tube.
The digestive system arises from the primitive endodermally lined gut tube, which is bounded cranially by the oropharyngeal membrane and caudally by the cloacal membrane. The gut is divided into foregut, midgut, and hindgut segments, with the midgut opening into the yolk sac. Specification of the various regions of the gut tract depends on a pattern set up by tightly regulated combinations of Hox genes. Development of virtually all parts of the gut depends on epithelial-mesenchymal interactions. Responding to such interactions, primordia of the respiratory system, the liver, the pancreas, and other digestive glands bud out from the original gut tube.
 The esophagus takes shape as a simple tubular structure between the pharynx and stomach. At one stage, the epithelium occludes the lumen of the esophagus; the lumen later recanalizes. The developing stomach is suspended from a dorsal and ventral mesogastrium. Through two types of rotation, the stomach attains its adult position. Common malformations of the stomach include the following: pyloric stenosis, which interferes with emptying of the stomach; and ectopic gastric mucosa, which can produce ulcers in unexpected locations.
The esophagus takes shape as a simple tubular structure between the pharynx and stomach. At one stage, the epithelium occludes the lumen of the esophagus; the lumen later recanalizes. The developing stomach is suspended from a dorsal and ventral mesogastrium. Through two types of rotation, the stomach attains its adult position. Common malformations of the stomach include the following: pyloric stenosis, which interferes with emptying of the stomach; and ectopic gastric mucosa, which can produce ulcers in unexpected locations.
 As they grow, the intestines form a hairpinlike loop that herniates into the body stalk. Further growth of the small intestine causes small intestinal loops to accumulate in the body stalk. As the intestines retract into the body cavity, they rotate around the superior mesenteric artery. This rotation results in the characteristic positioning of the colon around the small intestine in the abdominal cavity. During these changes in position, parts of the dorsal mesentery fuse with the peritoneal lining of the dorsal body wall. In the posterior part of the gut, the urorectal septum partitions the cloaca into the rectum and urogenital sinus.
As they grow, the intestines form a hairpinlike loop that herniates into the body stalk. Further growth of the small intestine causes small intestinal loops to accumulate in the body stalk. As the intestines retract into the body cavity, they rotate around the superior mesenteric artery. This rotation results in the characteristic positioning of the colon around the small intestine in the abdominal cavity. During these changes in position, parts of the dorsal mesentery fuse with the peritoneal lining of the dorsal body wall. In the posterior part of the gut, the urorectal septum partitions the cloaca into the rectum and urogenital sinus.
 During its differentiation, the lining of the intestinal tract passes through phases of (1) epithelial proliferation, (2) cellular differentiation, and (3) biochemical and functional maturation. Similar to the esophagus, the small intestine goes through a period of occlusion of the lumen by the epithelium. At later stages, intestinal crypts located at the base of villi contain epithelial stem cells, which supply the entire intestinal epithelial surface with various epithelial cells.
During its differentiation, the lining of the intestinal tract passes through phases of (1) epithelial proliferation, (2) cellular differentiation, and (3) biochemical and functional maturation. Similar to the esophagus, the small intestine goes through a period of occlusion of the lumen by the epithelium. At later stages, intestinal crypts located at the base of villi contain epithelial stem cells, which supply the entire intestinal epithelial surface with various epithelial cells.
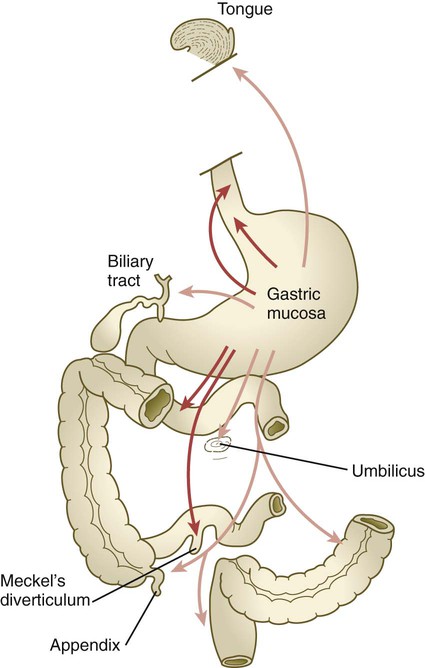
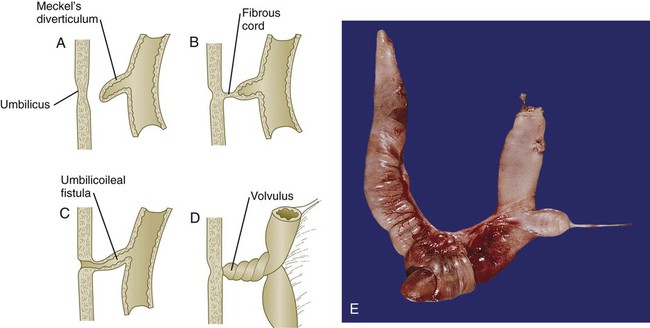
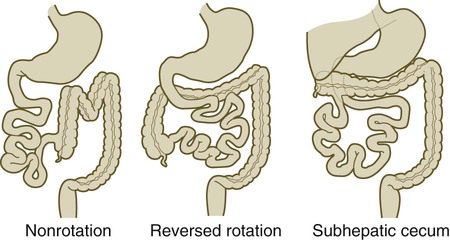
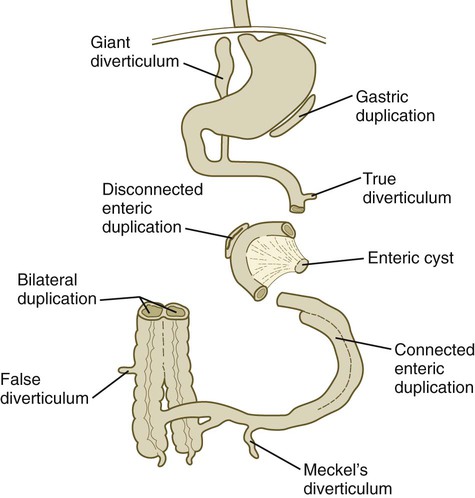
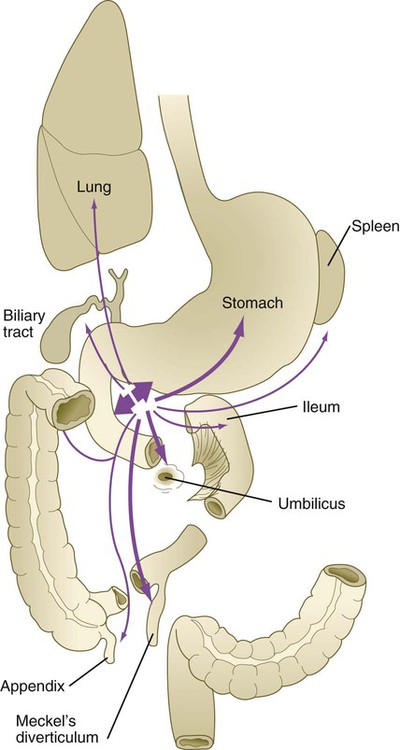
 The intestinal tract is subject to a variety of malformations, including local stenosis, atresia, duplications, diverticula, and abnormal rotation. Incomplete resorption of the vitelline duct can give rise to Meckel’s diverticulum, vitelline duct ligaments, cysts, or fistulas. Omphalocele is the failure of the intestines to return to the body cavity from the body stalk. Aganglionic megacolon is caused by the failure of parasympathetic neurons to populate the distal part of the colon. Failure of the anal membrane to break down (imperforate anus) may be associated with fistulas connecting the digestive tract to various regions of the urogenital system.
The intestinal tract is subject to a variety of malformations, including local stenosis, atresia, duplications, diverticula, and abnormal rotation. Incomplete resorption of the vitelline duct can give rise to Meckel’s diverticulum, vitelline duct ligaments, cysts, or fistulas. Omphalocele is the failure of the intestines to return to the body cavity from the body stalk. Aganglionic megacolon is caused by the failure of parasympathetic neurons to populate the distal part of the colon. Failure of the anal membrane to break down (imperforate anus) may be associated with fistulas connecting the digestive tract to various regions of the urogenital system.
 Digestive glands arise as epithelial diverticula from the gut. Their formation and further outgrowth are based on inductive interactions with the surrounding mesenchyme. The primordium of the liver arises in the septum transversum, but as it expands, it protrudes into the ventral mesentery. As it develops, the liver acquires the capacity to synthesize and secrete serum albumin and to store glycogen, among other biochemical functions. The pancreas grows out as dorsal and ventral pancreatic buds, which ultimately fuse to form a single pancreas. Within the pancreas, the epithelium forms exocrine components, which secrete digestive enzymes, and endocrine components (islets of Langerhans), which secrete insulin and glucagon.
Digestive glands arise as epithelial diverticula from the gut. Their formation and further outgrowth are based on inductive interactions with the surrounding mesenchyme. The primordium of the liver arises in the septum transversum, but as it expands, it protrudes into the ventral mesentery. As it develops, the liver acquires the capacity to synthesize and secrete serum albumin and to store glycogen, among other biochemical functions. The pancreas grows out as dorsal and ventral pancreatic buds, which ultimately fuse to form a single pancreas. Within the pancreas, the epithelium forms exocrine components, which secrete digestive enzymes, and endocrine components (islets of Langerhans), which secrete insulin and glucagon.
 The respiratory system arises as a ventral outgrowth from the gut just caudal to the pharynx. Through epithelial-mesenchymal interactions, the tip of the respiratory diverticulum undergoes up to 23 sets of dichotomous branchings. Other interactions with the surrounding mesenchyme stabilize the tubular parts of the respiratory tract by inhibiting branching. Lung development goes through several stages: (1) the embryonic stage, (2) the pseudoglandular stage, (3) the canalicular stage, (4) the terminal sac stage, and (5) the postnatal stage.
The respiratory system arises as a ventral outgrowth from the gut just caudal to the pharynx. Through epithelial-mesenchymal interactions, the tip of the respiratory diverticulum undergoes up to 23 sets of dichotomous branchings. Other interactions with the surrounding mesenchyme stabilize the tubular parts of the respiratory tract by inhibiting branching. Lung development goes through several stages: (1) the embryonic stage, (2) the pseudoglandular stage, (3) the canalicular stage, (4) the terminal sac stage, and (5) the postnatal stage.
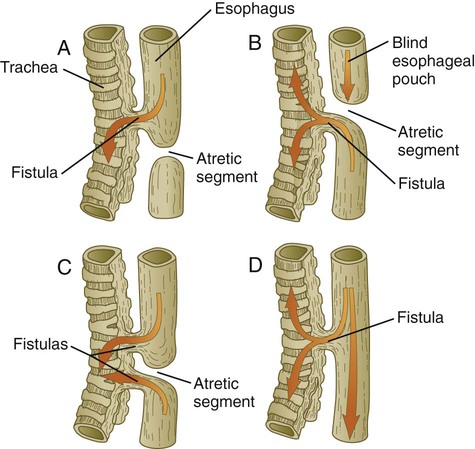
 Important malformations of the respiratory tract include tracheoesophageal fistulas, which result in abnormal connections between the trachea and esophagus. Atresia of components of the respiratory system is rare, but anatomical variations in the morphology of the lungs are common. Respiratory distress syndrome, commonly seen in premature infants, is related to insufficiencies in the formation of pulmonary surfactant by type II alveolar cells.
Important malformations of the respiratory tract include tracheoesophageal fistulas, which result in abnormal connections between the trachea and esophagus. Atresia of components of the respiratory system is rare, but anatomical variations in the morphology of the lungs are common. Respiratory distress syndrome, commonly seen in premature infants, is related to insufficiencies in the formation of pulmonary surfactant by type II alveolar cells.
 In its most basic condition, the intraembryonic coelom is separated into right and left components by the dorsal and ventral mesenteries, which suspend the gut. Except for the region of the stomach and liver, the ventral mesentery disappears. In the region of the heart, the dorsal mesocardium persists, and the ventral mesocardium disappears.
In its most basic condition, the intraembryonic coelom is separated into right and left components by the dorsal and ventral mesenteries, which suspend the gut. Except for the region of the stomach and liver, the ventral mesentery disappears. In the region of the heart, the dorsal mesocardium persists, and the ventral mesocardium disappears.
 The septum transversum divides the coelom into thoracic and abdominal regions, which are connected by pleural canals. The developing lungs grow into the pleural canals, which are partially delimited by paired pleuropericardial and pleuroperitoneal folds. The definitive diaphragm is formed from (1) the septum transversum, (2) the pleuroperitoneal folds, and (3) ingrowths from body wall mesenchyme.
The septum transversum divides the coelom into thoracic and abdominal regions, which are connected by pleural canals. The developing lungs grow into the pleural canals, which are partially delimited by paired pleuropericardial and pleuroperitoneal folds. The definitive diaphragm is formed from (1) the septum transversum, (2) the pleuroperitoneal folds, and (3) ingrowths from body wall mesenchyme.
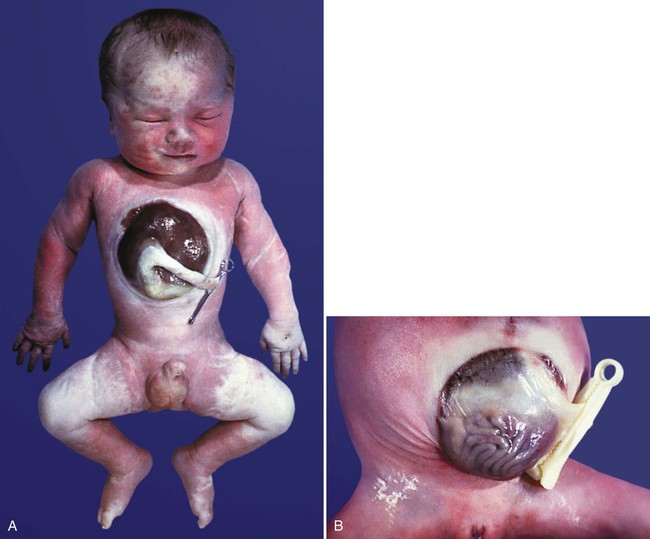
 Quantitative deficiencies in ventral body wall tissue can result in abnormalities ranging from failure of sternal fusion to ectopia cordis in the thorax and omphalocele to gastroschisis or exstrophy of the bladder or both in the abdomen. Defects in the diaphragm are diaphragmatic hernias and can result in herniation of the intestines into the thoracic cavity.
Quantitative deficiencies in ventral body wall tissue can result in abnormalities ranging from failure of sternal fusion to ectopia cordis in the thorax and omphalocele to gastroschisis or exstrophy of the bladder or both in the abdomen. Defects in the diaphragm are diaphragmatic hernias and can result in herniation of the intestines into the thoracic cavity.