| Shaft fracture | Subperiosteal new bone | Metaphyseal irregularity | Generalized osteopenia | Comments | |
|---|---|---|---|---|---|
| Osteogenesis imperfecta | + | + | +/- | + | First fracture may occur before osteopenia is evident |
| Scurvy | – | + | + | + | Diet of ultra high temperature milk, few fruits and vegetables |
| Myelodysplasia/cerebral palsy | + | + | +/– | – | Disuse osteopenia in affected limbs |
| Osteomyelitis | – | + | + | – | Occasionally multifocal in infancy |
| Caffey disease | – | + | – | – | Mandible often involved |
| Congenital syphilis | – | + | + | – | Transmission from mother |
| Vitamin A intoxication | – | + | – | – | Increased intracranial pressure |
| Hypertrophic osteoarthropathy | – | + | – | – | Cardiac, pulmonary, biliary disease; intestinal transplant; tumors |
| Multiple hereditary exostoses | – | – | – | – | Rib lesions may simulate healing fracture |
| Leukemia | – | + | – | + | Variable bone changes may be seen prior to diagnosis |
| Menkes syndrome | – | + | + | +/– | Wormian bones, low serum copper, bladder, tortuous vessels |
| Copper deficiency | + | + | + | + | Low birth weight, total parenteral nutrition, anemia, neutropenia |
| Congenital indifference to pain | + | + | + | – | Normal neurologic exam |
| Metaphyseal and spondylo-metaphyseal dysplasias | – | – | + | – | Short stature |
Inherited bone dysplasias
The diagnosis of child abuse is sometimes entertained at the initial presentation of infants with inherited bone dysplasias, which are extensively catalogued in the On Line Mendelian Inheritance in Man (OMIM®) with unique phenotype MIM numbers (1, 2). The conditions which can potentially lead to the mistaken diagnosis of child abuse are those with metaphyseal irregularity and fragmentation. Langer and colleagues described a form of spondylometaphyseal dysplasia (SMD) with metaphyseal fragmentation similar to that noted with abuse, and termed this condition SMD, Sutcliffe/corner fracture type (MIM 184255) (3–5). Patients with this disorder are short, with progressive coxa vara. The condition is genetically transmitted, with either autosomal or X-linked dominant inheritance. Although the radiographic features are strikingly similar to those in child abuse, the long bones are short and dysplastic and the spine is abnormal (Fig. 7.1). With weight-bearing, remodeling, and evolution of the dysplastic features, conspicuous medial metaphyseal fragments may be associated with tibia vara (Fig. 7.2).
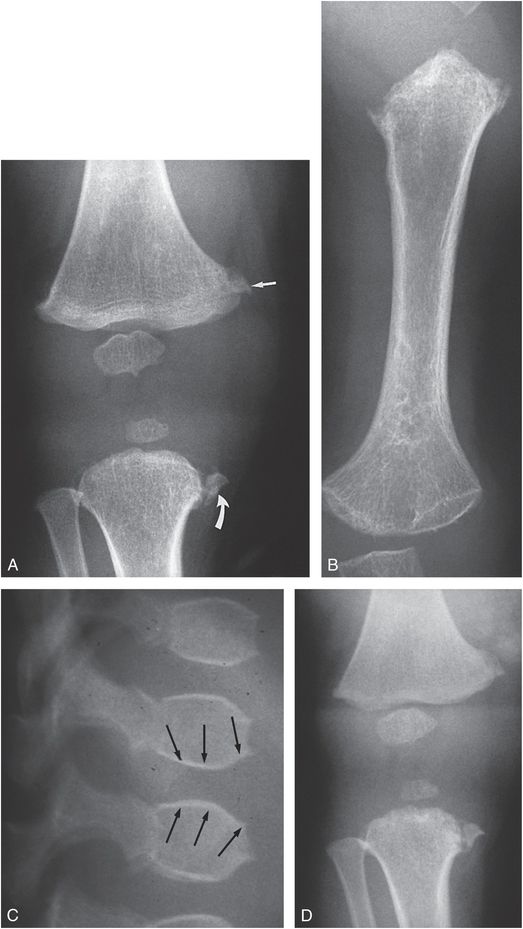
Figure 7.1 SMD, corner fracture type. A, AP view of a one-year-old child with short stature reveals metaphyseal irregularity of the proximal left tibia with an osseous fragment medially, suggesting a corner fracture (curved arrow). Metaphyseal irregularity is noted involving the distal femur, most evident medially (straight arrow). B, AP view of the left humerus demonstrates metaphyseal irregularity proximally and to a lesser extent distally. The humeral shaft is shortened. C, Lateral view of the upper lumbar spine shows a biconvex appearance to the vertebral bodies (arrows). D, AP view of the right knee at 14 months of age shows little change in the appearance of the metaphyseal fragments. True metaphyseal lesions associated with abuse are excluded owing to the lack of significant interval change. Widespread dysplastic changes were noted elsewhere.
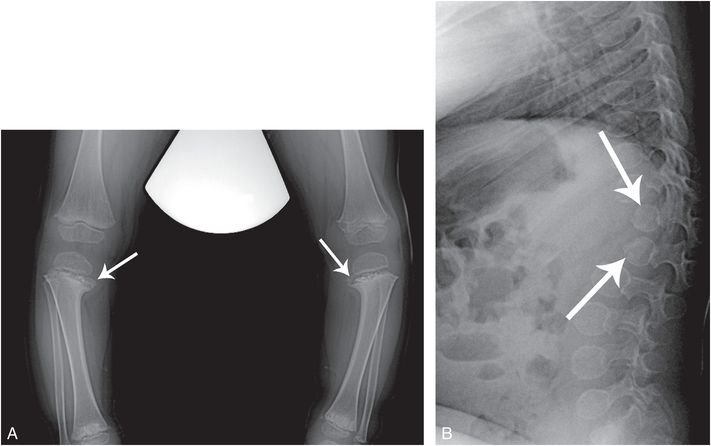
Figure 7.2 SMD, corner fracture type. A two-year-old child with short stature and focal dorso-lumbar kyphosis. A, AP standing view of the tibias shows bilateral tibia vara with a downward slope of the proximal tibial physes. There is sclerosis of the metaphyses with conspicuous osseous fragments resembling corner fractures (arrows). Lesser changes are evident in the distal tibias. B, Lateral view of the thoracolumbar spine demonstrates an acutely angulated kyphosis with hypoplastic and posteriorly situated T12 and L1 vertebral bodies (arrows).
The metaphyseal dysplasias (MD) are a heterogeneous group of skeletal disorders, and like SMD, they generally pose no diagnostic confusion with abuse (4, 5). An exception is MD, Schmid type (MIM 156500), an autosomal dominant chondrodysplasia due to a variety of mutations in the COL10A1 gene. Kleinman reported an infant with normal stature and metaphyseal changes that were initially suspected to be due to abuse (6). Skeletal survey (SS), clinical, and radiographic follow-up, and the presence of similar findings in the father and a subsequent sibling confirmed this autosomal dominant condition (Fig. 7.3). In earlier discussions, attention has been drawn to the metaphyseal and physeal changes of the proximal femurs with inflicted injury (see Chapter 3), and birth trauma will be discussed subsequently (see Chapter 11). Quite similar findings may be encountered in MD, Schmid type as well as SMD, Sutcliffe/corner fracture type, with discrete osseous fragments arising from the medial femoral and tibial metaphyses with progressive coxa vara (Fig. 7.4) (7). The other radiologic and clinical findings readily permit differentiation from traumatic injury. A missense mutation of the COL10A1 gene has also been shown to cause the rare Japanese variety of SMD. Savarirayan noted that 9.1% of cases of MD, Schmid type have mild spinal changes. Since both the MD, Schmid type and the Japanese form of SMD have similar collagen X abnormalities, the conditions are likely one and the same (MIM 156500) (1, 8).
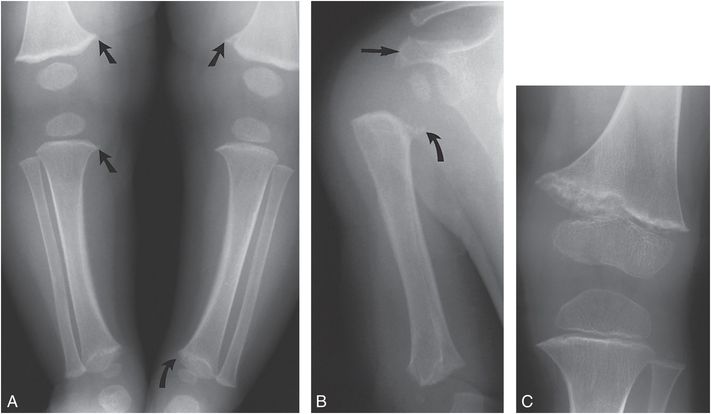
Figure 7.3 Metaphyseal dysplasia, Schmid type. A, AP view of the lower extremities in a five-month-old child with clinical suspicion of leg length discrepancy demonstrates metaphyseal irregularities (straight arrows) of the distal femurs and proximal right tibia, suggesting corner fractures. Sclerosis and irregularity is noted in the left distal tibial metaphysis (curved arrow). B, AP view of the right proximal humerus shows an irregular bony spur arising from the medial humeral metaphysis (curved arrow). The distance between the ossified epiphysis and metaphysis is increased. Irregularity and sclerosis of the distal humerus and acromion process (straight arrow) are evident. Height at this time was greater than the 95th percentile and weight was at the 50% percentile. The patient developed coxa vara and increased femoral bowing requiring osteotomy. C, AP view of the left knee at age two years demonstrates persistent irregularity and sclerosis of the distal femoral metaphysis. The diagnosis was subsequently made in the father and a younger sibling. (From Kleinman PK. Schmid-like metaphyseal chondrodysplasia simulating child abuse. AJR. 1991;156:576–8.)
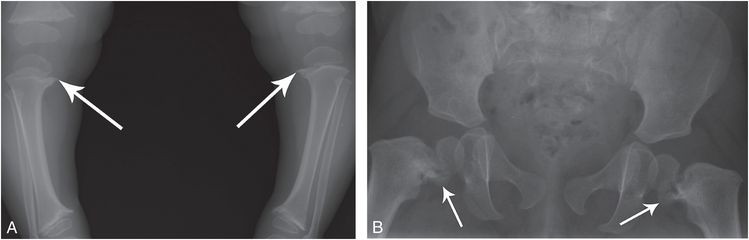
Figure 7.4 Metaphyseal dysplasia, Schmid type. A 20-month-old child with progressive bowing of the lower extremities. A, AP standing view of the lower extremities shows metaphyseal irregularity and sclerosis, most evident in the medial aspects of the proximal tibias (arrows). B, AP view of the pelvis demonstrates coxa vara with conspicuous medial metaphyseal fragments (arrows) simulating corner fractures.
Other SMD and MD forms have variable and sometimes dramatic metaphyseal irregularities/fragmentation, and these are usually associated with other distinctive radiologic, clinical, and/or laboratory findings. One such example is cartilage hair hypoplasia (CHH), also known as MD, McKusick type (MIM 250250) – the metaphyseal irregularities may be accompanied by flaring of the costochondral junctions (CCJs) (Fig. 7.5) (4, 5). In contrast to fractures of the CCJs, the rib ends are diffusely expanded and irregular.
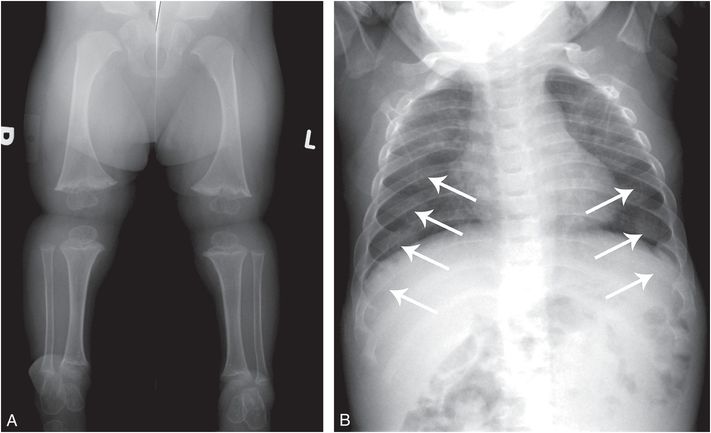
Figure 7.5 CHH. A two-year-old child with short stature. Genetic testing revealed compound heterozygous RMRP mutation consistent with CHH. A, AP view of the lower extremities shows short tibias with metaphyseal flaring, sclerosis, and irregularity. B, Frontal view of the chest shows diffusely expanded and irregular CCJs (arrows).
Osteopetrosis
Osteopetrosis refers to a variety of rare genetic disorders characterized by increased bone density (MIM 259700, 259710, 611490). Although it does not pose differential difficulties with child abuse, there are several aspects of the condition that are relevant to this discussion (4, 5). The classic severe autosomal recessive form is characterized by short stature, bone marrow failure, fractures, and compressive neuropathies. Diagnosis is readily apparent on radiographs based on dense bones, metaphyseal lucencies/irregularities, and a “bone-in bone” ossification pattern, which may persist long after the normal “bone-in-bone” pattern of young infancy resolves (Fig. 7.6).
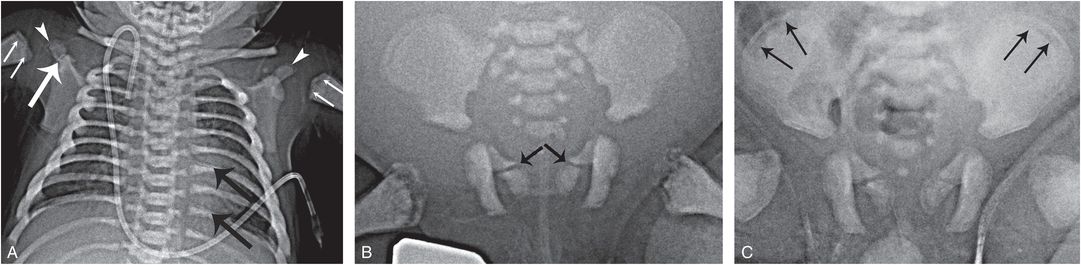
Figure 7.6 Osteopetrosis. A two-month-old infant with osteopetrosis and hypocalcemic seizures. A, AP radiograph of the chest shows diffuse increased bone density. An oblique lucency extends across the right acromion process (thick white arrow), consistent with a slightly displaced fracture. Lucent metaphyseal bands are evident in the humeri (thin arrows) with similar changes in the tips of the acromion processes (arrowheads). There is a bone-in-bone appearance of the posteromedial ribs, simulating healing rib fractures (thick black arrows). B, Two weeks later an AP view of the pelvis shows bilateral oblique lucencies extending through the SPR, suggesting fractures (arrows). Metaphyseal lucencies and irregularity are evident in the proximal femurs. C, Four weeks later, there is little change in the SPR lucencies, suggesting normal variants. Note that a bone-in-bone appearance in the iliac wings has become more accentuated (arrows).
Hypocalcemia may result in tetanic seizures, and these myoclonic/tonic episodes appear to be responsible for the rarely described acromial fractures in young infants with osteopetrosis (Fig. 7.6) (9). Acromial fractures in otherwise normal infants are highly associated with abuse (see Chapter 5), and the occurrence of these fractures in osteopetrosis, as well as neonatal tetanus (see Fig. 5.64) and other seizure disorders, suggests that abnormal tractional forces on the acromion are responsible for these distinctive injuries (10, 11). It is not unusual to encounter nonspecific long bone fractures with osteopetrosis, and there should be no confusion with inflicted injury (12, 13). The case of osteopetrosis in Fig. 7.6 also showed bilateral oblique lucencies extending through the superior pubic rami (SPR). Whether these represent fractures (see Chapter 3) or normal developmental variants (see Chapter 12) is unclear, but their persistence on follow-up suggests variants, perhaps related to the underlying disordered ossification. Figure 7.6 also illustrates lucent metaphyseal bands and a bone-in-bone appearance of the posteromedial ribs, which can simulate healing fractures.
Rachitic changes may be encountered in osteopetrosis, and these radiographic features, in conjunction with those related to vitamin D and calcium administration may result in unusual ossification patterns, apparent metaphyseal fragmentation, and SPNBF that suggest traumatic injury (Fig. 7.7) (14, 15).
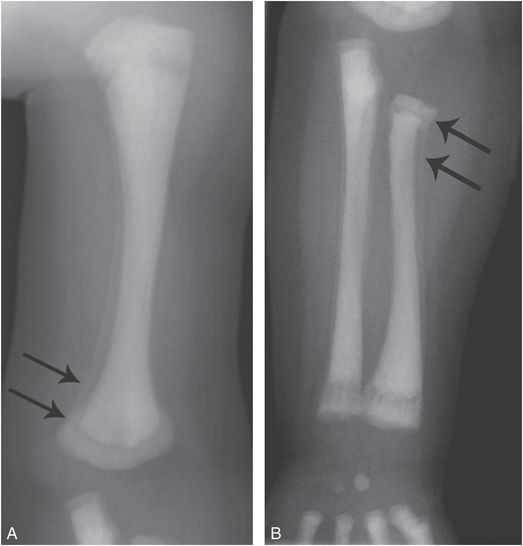
Figure 7.7 Osteopetrosis. A five-month-old infant with osteopetrosis on calcium supplementation, awaiting bone marrow transplantation. AP views of the left humerus (A) and forearm (B) show conspicuous SPNBF (arrows) along the humeral and radial shafts. There is an evolving bone-within-bone appearance with amorphous ossification in the metaphyses. The SPNBF extends to the overlapping margins of the distal humeral and proximal radial metaphyses, giving the impression of displaced fractures.
Multiple hereditary exostoses
Multiple hereditary exostoses (MHE) (MIM 133700) is an autosomal dominant condition which is usually diagnosed in children based on exophytic osseous masses, often associated with a family history of the disorder (4, 5). When the rib lesions are encountered on a chest radiograph, the findings may be misinterpreted as multiple healing rib fractures and concerns of abuse can result (Fig. 7.8). Rib radiographs with attention to bone detail, and SS are diagnostic.
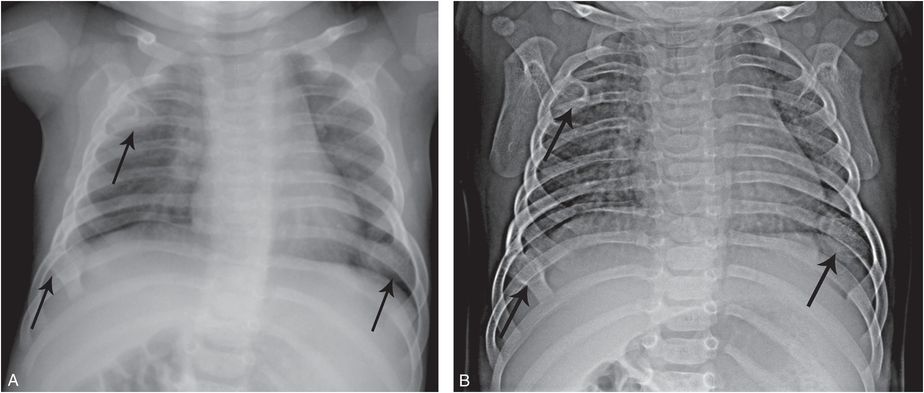
Figure 7.8 MHE masquerading as healing rib fractures. A four-month-old infant with respiratory distress. A, Frontal radiograph of the chest done elsewhere shows multiple foci of ossification (arrows) interpreted as healing anterior rib fractures resulting in transfer for suspected abuse. B, AP view of the thorax, optimized for bone detail, shows multiple exostoses (arrows). The diagnosis of MHE was confirmed with the demonstration of additional osteochondromas in the extremities (not shown).
Hereditary sensory and autonomic neuropathies
The hereditary sensory and autonomic neuropathies (HSAN) are a rare group of disorders associated with sensory dysfunction (16). There has been considerable research that provides a genetic classification of these complex and varied conditions, but much phenotypic variation occurs. Historically the diagnosis has been based on clinical evaluation combined with sensory and autonomic assessments, but genetic testing is now customary.
Congenital insensitivity to pain
Congenital insensitivity (indifference) to pain is a rare syndrome inherited as an autosomal recessive trait. It can be associated with lesions that mimic inflicted skeletal injury (17, 18). Affected children have normal intelligence and their neurologic examination results are normal except for insensitivity or indifference to painful stimuli. Light and deep touch and proprioception sensations are normal, although temperature perception is diminished in a few affected patients. Sweating is usually absent (anhidrosis). Because of the lack of pain, both minor and major trauma to the growing skeleton may go undetected until marked deformity is present. Repeated unrecognized injuries to the physis lead to epiphyseal separations and nonunion. In a child without symptoms or history of trauma, the presence of osseous injury may raise concerns of child abuse (16, 19). Images of an affected area may show fractures and epiphyseal separations in various stages of repair. In addition to shaft fractures, metaphyseal injuries are common during early childhood (20). Metaphyseal chronic osteomyelitis is also an occurrence during childhood, perhaps because of the increased incidence of skin infections secondary to the multiple burns and lacerations incurred by affected children. Imaging features of osteonecrosis/osteochondritis dissecans are common in weight-bearing joints (Fig. 7.9) (21). By adolescence, repetitive microtrauma to the pain-insensitive joint can result in neuropathic arthropathy.
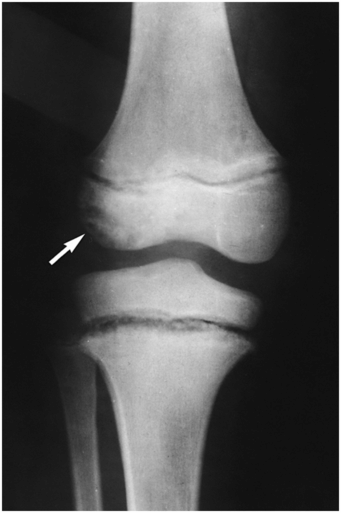
Figure 7.9 Congenital indifference to pain. AP radiograph of the knee in a nine-year-old boy shows physeal widening and metaphyseal irregularity of the proximal tibia. Osteonecrosis is present in the lateral femoral condyle (arrow).
Riley–Day syndrome
Riley–Day syndrome, or familial dysautonomia, is a rare autosomal recessive neurodevelopmental disorder that affects the central nervous system (5). It is characterized by deficits in peripheral autonomic and sensory neurons. Skeletal complications included fracture, osteomyelitis, septic arthritis, and neuropathic arthropathy. Dramatic acral osteolysis may result from self-mutilating behavior, which can be complicated by soft tissue infection and osteomyelitis (Fig. 7.10).
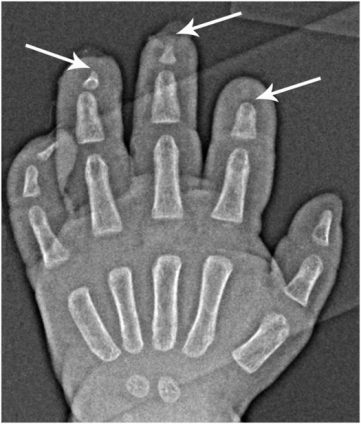
Figure 7.10 Riley–Day syndrome with acral osteolysis and osteomyelitis. PA view of the left hand of a two-year-old with Riley–Day syndrome shows variable amputation of the distal phalanges of the second to fourth fingers (arrows) due to self-mutilating behavior, complicated by cellulitis/osteomyelitis.
Myelodysplasia and other paralytic disorders
Myelodysplasia
Thoracic and lumbosacral spinal dysraphism with underlying spinal cord and nerve root abnormalities will result in variable degrees and levels of sensory loss and paralysis in the lower extremities. The lack of normal motor function results in decreased bone mineral density (BMD) and a greater tendency to fracture, particularly in wheelchair-bound children (22). With severe motor involvement, periarticular soft tissue contractures may develop in infancy, and diapering and other routine handling can result in shaft fractures. These injuries may be undetected unless gross hemorrhage and marked displacement are present or callus is eventually palpated (23, 24). In older children without total lower extremity paralysis, ambulation is often encouraged. Braces and crutches contribute to stresses to the physis and the metaphysis, resulting in Salter–Harris (SH) physeal fractures and epiphyseal separations. Repeated minor trauma leads to metaphyseal fragmentation, periosteal elevation, and hemorrhage into the physis (25). The metaphyses become dense and irregular, the physis appears wide, and SPNBF/heterotopic bone become visible during healing (Fig. 7.11).
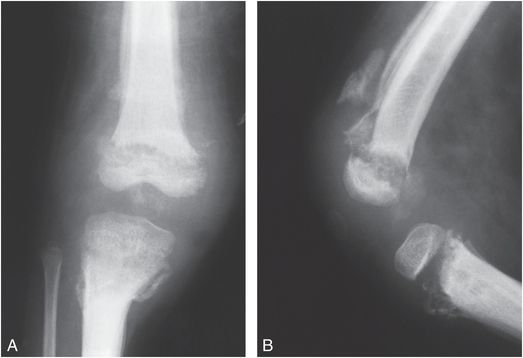
Figure 7.11 Myelodysplasia. AP (A) and lateral (B) views of the right knee in a four-year-old child with spina bifida reveal fragmentation, sclerosis, and irregularity of the metaphyseal–epiphyseal regions. The physes are widened and extensive SPNBF is present involving the femur and tibia.
These neuropathic changes are the Charcot joint equivalent in the growing skeleton. Similar, but less dramatic physeal injuries occur in myelodysplastic infants and children undergoing vigorous physical therapy for contractures. Occasionally, fractures occur with appearances that suggest the classic metaphyseal lesion (CML) noted with abuse (Fig. 7.12). Careful inspection usually reveals the fractures to be epiphyseal separations of the SH type II variety. Fractures occur below the level of neurologic involvement, affecting predominantly the femur in patients with thoracic involvement and the tibia in those with lumbar involvement. Fractures are more frequent in cases with thoracic spine defects (26). Physeal widening resolves quickly with the use of orthoses and limitation of activity. Early physeal closure and growth disturbance may be prevented by such therapy (27). Although progression to Charcot’s arthropathy is a potential complication, complete healing is possible. Since these bone changes occur in the lower extremities in children with known spinal dysraphism, and the typical underlying osseous changes of the condition are evident, differentiation from child abuse is usually simple. However, confusion with inflicted injury has been reported (28).
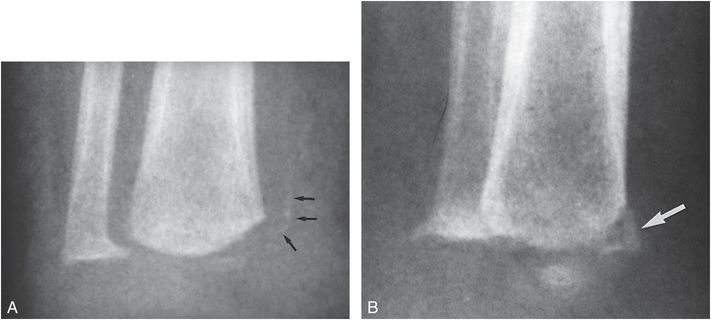
Figure 7.12 Myelodysplasia. AP (A) and oblique (B) views of the distal right tibia in an 18-month-old boy with myelodysplasia demonstrate a metaphyseal fracture. On the oblique view (B) the injury has a corner fracture appearance (large arrow). On the frontal projection (A) the osseous density (small arrows) is noted to be separated from the metaphysis, suggesting that this is a SH type II physeal injury. The child had recently undergone vigorous physical therapy for the lower extremities. (Courtesy of David G. Dissler MD.)
Cerebral palsy
Other paralytic neurologic disorders may result in osteopenia and gracile bones, predisposing to fracture. Children with cerebral palsy are at risk for fracture with routine handling and aided ambulation (29–31). Less commonly they may sustain SH physeal injuries that can be delayed in presentation (Fig. 7.13). Fractures may also occur during spastic muscle activity and children with epilepsy are at increased risk of such injuries (32). Ballal and others described a proximal femoral epiphyseal separation following seizures in 22-month-old and 9-year-old children with cerebral palsy (33). An additional case of a 5-month-old infant with a proximal femoral physeal injury following a grand mal seizure was described by Aoudi and others (34). As noted, patients with paralytic disorders are at risk for fracture when undergoing physical therapy or other strenuous manipulation of an involved extremity.
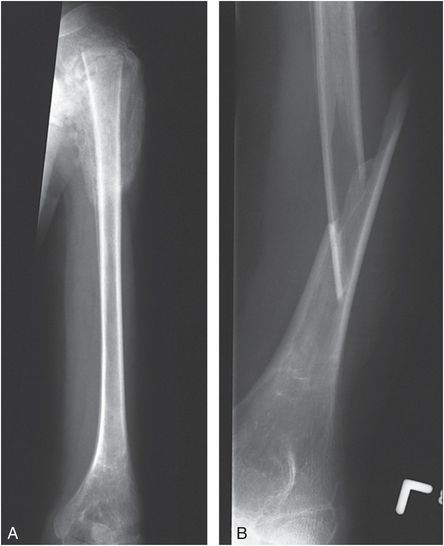
Figure 7.13 Severe spastic cerebral palsy. A, AP view of the left humerus in a 13-year-old boy with severe spastic cerebral palsy and left shoulder swelling demonstrates a proximal epiphyseal separation. The epiphysis is displaced and there is exuberant callus and SPNBF. B, During restraint in the Radiology Department for a subsequent radiograph of the humerus, the child evidenced sudden spastic contraction of the left upper extremity, and an audible snap was appreciated. This subsequent view of the humerus revealed a new angulated fracture of the humerus.
Grayev and others described tibial injuries in 8 children, 1–4 months old, who underwent serial casting for clubfoot (35). Two patients had myelodysplasia and one had arthrogryposis. All children manifested injury with periosteal new bone formation. One child with bilateral tibial metaphyseal fractures had clear evidence of abuse with 24 rib fractures. Four other children had metaphyseal fractures resembling CMLs, and three developed an area of sclerosis within the metaphysis. The authors stressed the need to consider an iatrogenic basis for metaphyseal injuries in children undergoing orthopedic treatment and casting for congenital foot/ankle deformities. They suggested that the mechanics entailed in these orthopedic manipulations provides insights into the mechanisms of similar metaphyseal injuries occurring in the setting of abuse. Kleinman described a metaphyseal fracture in a four-month-old child with arthrogryposis who was found to have distal tibial and fibular metaphyseal fractures following manipulation for clubfoot deformity (Fig. 7.14) (36). It was unclear if the fracture occurred as a result of the orthopedic manipulation or during the dorsiflexion positioning routinely performed during radiographic assessment. It should be kept in mind that children with disabilities may be at increased risk of abuse (see Chapter 23).
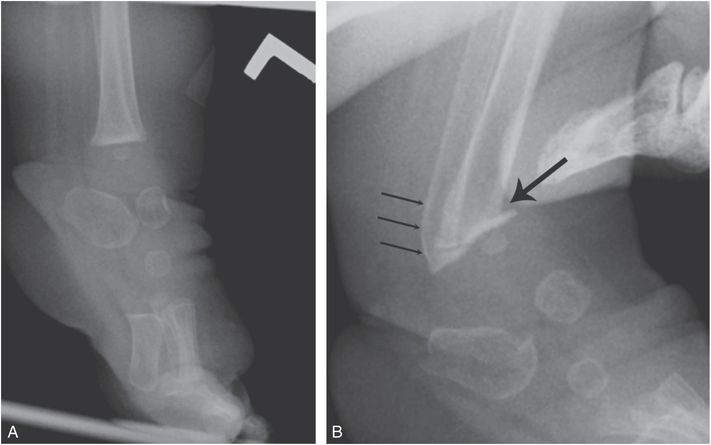
Figure 7.14 Iatrogenic metaphyseal fracture. A, Lateral view of the left ankle and foot of a four-month-old child with arthrogryposis and left clubfoot shows equinus deformity. B, Lateral view of the same foot obtained shortly after manipulation shows metaphyseal fractures of the distal tibia (thick arrow) and fibula (thin arrows). (Published with permission from Kleinman PK. Problems in the diagnosis of metaphyseal fractures. Pediatr Radiol. 2008;38(Suppl. 3):S388–94.)
Infection
Osteomyelitis and septic arthritis
Young infants with osteomyelitis and/or septic arthritis often present with irritability and reluctance to use the extremity. The shoulder and hip are common locations. Initial radiographs may be normal or misleading. Since fever may be absent, a traumatic injury may be the initial consideration. Osteomyelitis and septic arthritis of the shoulder can be particularly problematic because SPNBF may be construed as traumatic, and an apparently abnormal glenohumeral relationship suggests dislocation or a displaced SH injury (Fig. 7.15). Sonography is useful to document a normal physis and glenohumeral relationship, and can also demonstrate medullary metaphyseal destruction (osteomyelitis) with epiphyseal involvement (chondritis). Magnetic resonance imaging (MRI) elucidates the inflammatory findings.
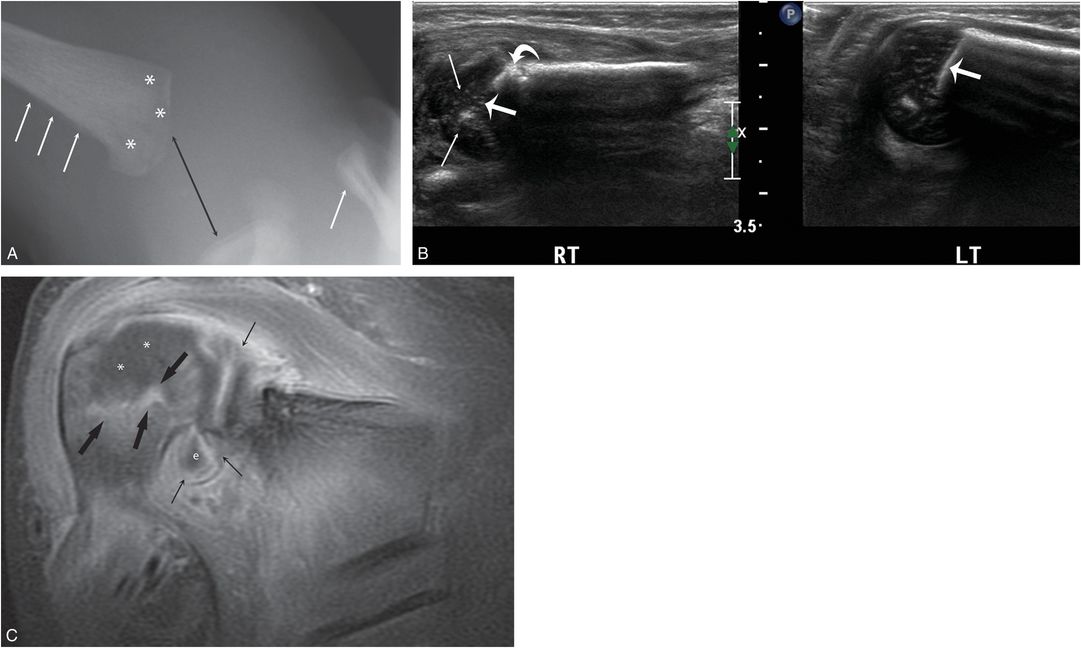
Figure 7.15 Osteomyelitis and septic arthritis. An 11-day-old infant with 1-day history of right shoulder swelling and not moving arm. Radiographs elsewhere suggested a traumatic dislocation and the infant was transferred with concerns of abuse. There was no fever. A, AP view of the right shoulder shows apparent widening of the glenohumeral articulation (black arrow). SPNBF is evident along the proximal humerus and the distal clavicle (white arrows). There is subtle permeative destruction of the humeral metaphysis (*). B, Coronal sonograms of the right and normal left shoulders demonstrate loss of the normal echogenic band of the ZPC (thick straight arrows) as well as abnormal epiphyseal cartilage echotexture (thin arrows) on the right. Note focal cortical destruction (curved arrow). C, Coronal T1W FS gadolinium-enhanced MRI shows diffuse intense synovial enhancement (thin arrows) and a joint effusion (e). Note the focal juxtaphyseal enhancement (thick arrows) and cartilage enhancement defect (*) within the epiphysis consistent with metaphyseal osteomyelitis extending to the nonossified epiphysis (chondritis). Culture of the purulent material at arthrotomy grew group B streptococcus.
A pathologic fracture through a focus of osteomyelitis may be confused with an inflicted injury involving the metaphyseal epiphyseal complex (Fig. 7.16) (37). Since the process commonly involves the epiphyseal blood vessels in infants, osteomyelitis can result in a growth disturbance and long-term orthopedic deformity (Fig. 7.17) that may be indistinguishable from a traumatic physeal injury without associated infection (see Fig. 3.14).
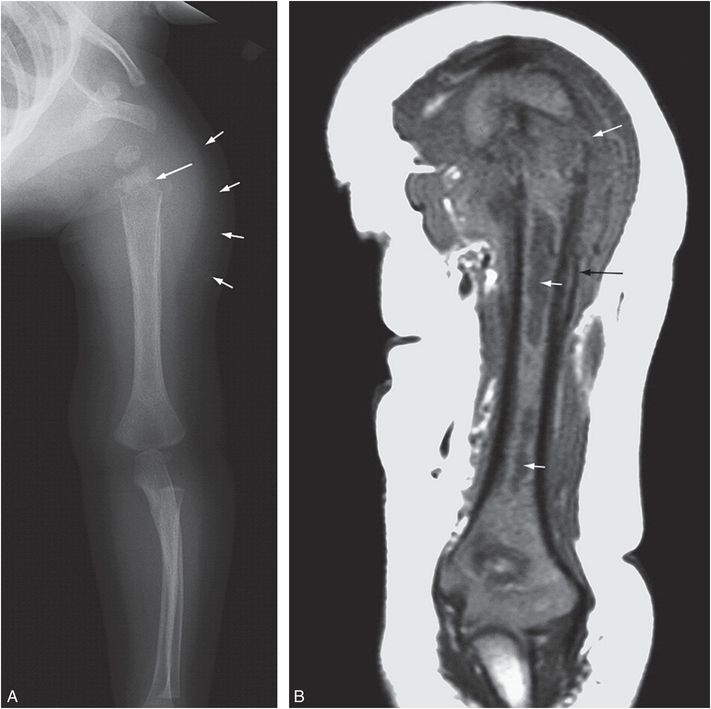
Figure 7.16 Osteomyelitis with pathologic fracture. A seven-month-old infant not moving left arm. There was no fever or history of trauma. A healing humeral fracture was noted radiographically and concerns of abuse were raised. A, AP view of the left humerus shows a transverse metaphyseal fracture (long arrow) through a region of permeative bone destruction. There is SPNBF and soft tissue swelling (short arrows). B, Coronal T1W MRI shows a displaced metaphyseal fracture (long white arrow).There is abnormal low marrow signal at the level of the fracture and at two other sites in the shaft (short arrows) consistent with abscesses. Note the SPNBF (black arrow). (With permission from Taylor MN, Chaudhuri R, Davis J, Novelli V, Jaswon MS. Childhood osteomyelitis presenting as a pathological fracture. Clin Radiol. 2008;63:348–51.)
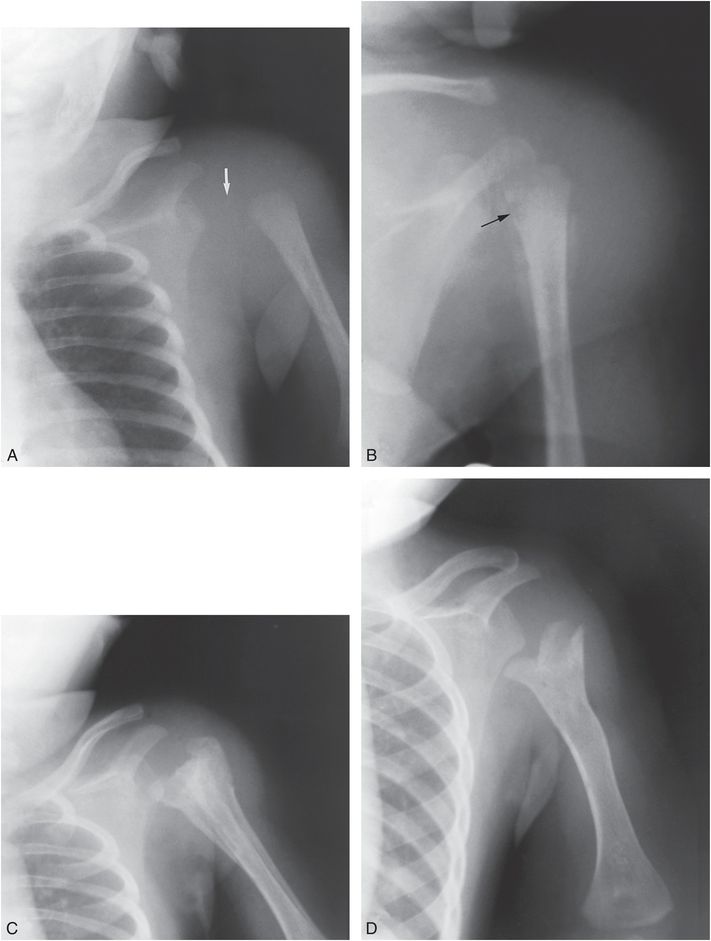
Figure 7.17 Osteomyelitis with septic arthritis. A, A three-week-old previously healthy infant presented with the acute onset of swelling of the shoulder. The joint capsule is distended, causing lateral displacement of the humeral head (arrow). In addition, there is lateral displacement of the shaft from the head as a result of a fracture through the physis. B, Four days later, after aspiration of the joint, the shaft is no longer displaced. The ossification center of the humeral head is no longer visible, because of either destruction by lytic pus or demineralization secondary to hyperemia. Lytic areas are present in the metaphysis (arrow). SPNBF cloaks the proximal shaft and is also present along the axillary border of the scapula. The metaphyseal destructive lesions distinguish osteomyelitis from child abuse. C, Changes of healing are evident one month later during treatment with IV antibiotics. Exuberant dense SPNBF (involucrum) surrounds the proximal and midhumeral shaft. The SPNBF along the scapula has become better defined as healing progresses. D, Six months later, there is complete healing of the infection with severe residual deformity. The humeral head is not well defined and the shaft is inferiorly and medially displaced. The metaphysis is wide, with a forked surface.
When osteomyelitis and septic arthritis of the hip are present in infants, the pathologic fracture can occur thorough the physis resulting in an epiphyseal separation that can also be confused with a purely traumatic injury. In neonates and young infants with a nonossified proximal femoral epiphysis, displacement of the metaphysis may also be mistaken for a hip dislocation (38, 39). Sonography provides a simple means of documenting that the separated femoral epiphysis is normally situated in the acetabulum. A similar epiphyseal separation has also been noted in the distal femur of a 45-day-old infant (38).
Ribe and Changsri described a unique case of a pathologic fracture through a focus of osteomyelitis in a fatally abused child (Fig. 7.18) (40). Radiographically, the process was accompanied by expansion of the medullary cavity with a sclerotic rim, and at autopsy, a medullary abscess with surrounding scar tissue was present, all features indicating chronicity.
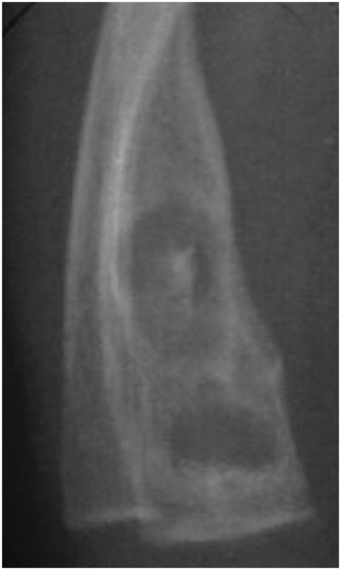
Figure 7.18 Osteomyelitis in an abused infant. A 4-month-old former 31-week premature infant was found dead in bed. Autopsy showed acute bronchopneumonia, three partially healed skull fractures, a chronic SDH, chronic intracerebral hemorrhage, RHs, multiple healing rib fractures, a fractured fibula, and a partially healed fracture of the distal right radius. The fracture of the right radius showed a medullary abscess containing pus and granulation tissue surrounded by scar tissue. (Reprinted with permission from Ribe JK, Changsri C. A case of traumatic osteomyelitis in a victim of child abuse. Am J Forensic Med Pathol. 2008;29:164–6.)
Deficiency of interleukin-1-receptor antagonist syndrome is a rare auto-inflammatory condition that can produce diffuse SPNBF and focal areas of bone destruction. The other manifestation of this life-threatening autosomal-recessive condition should prevent any confusion with inflicted skeletal injury (41).
Meningococcemia
In infantile meningococcemia with septic emboli to epiphyseal vessels, ischemia may damage the physis, causing physeal growth disturbance with resultant limb shortening and angular deformity (Fig. 7.19) (42–44). Central physeal fusion may lead to cone-shaped epiphyses and metaphyseal cupping. Vascular occlusion and osteomyelitis may cause autoamputation of multiple digits in the hands or feet. Differentiation from abuse is made on the basis of the history of fulminant sepsis in early childhood.
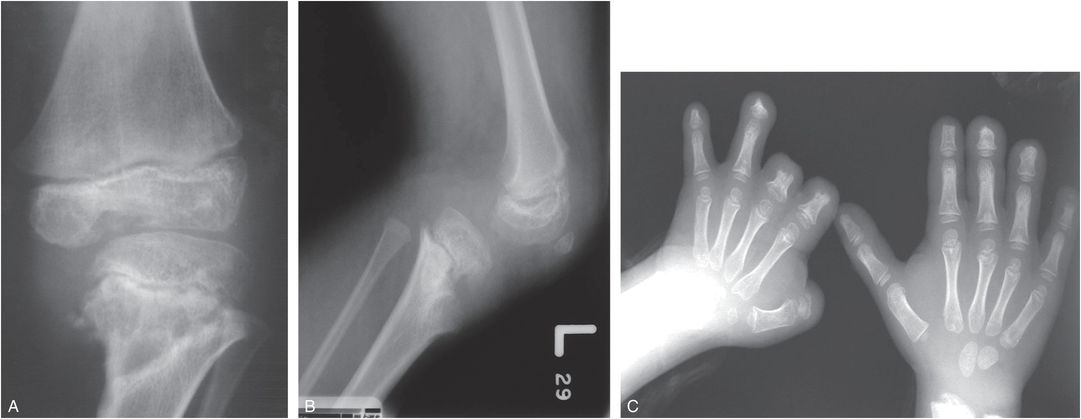
Figure 7.19 Meningococcemia with osteomyelitis, the long-term consequences. A six-year-old child with a history of meningococcemia in infancy. A, B, Frontal and lateral views of the left knee show mixed sclerosis and lucency in the distal femoral epiphysis and the proximal tibial metaphysis. There is lateral and posterior subluxation of the knee and anteromedial angular deformity of the proximal tibia related to a physeal growth disturbance. C, PA view of the hands demonstrates widespread digital amputations related to septic emboli and ischemia. (A, B, Published with permission from Kleinman PK. A regional approach to osteomyelitis of the lower extremities in children. Radiol Clin North Am. 2002;40:1033–59.)
Congenital syphilis
In recent years, there has been a substantial increase in the incidence of primary and secondary syphilis in adults in the United States, resulting in a resurgence of congenital syphilis, especially in poor urban areas (45–49). Syphilitic infection of the infant skeleton is diffuse and can involve the long bones, the skull, and even the small bones of the hands and feet in severe cases. Lesions in the spine appear to be rare. The tibia, femur, and humerus are the bones most often involved and the distribution is generally irregular. A recent report suggests that preterm neonates had more clinical evidence of infection than term neonates (49).
The spirochetes cross the placenta and are implanted in the fetal tissue. Destructive and productive changes of osteomyelitis are seen in the metaphyses and diaphyses after birth, most commonly between the first and sixth months of life. While no specific epiphyseal lesions are seen radiographically, there is delayed ossification of the proximal tibial epiphysis in approximately 30% of full-term newborns with congenital syphilis. Delayed ossification of both the distal femoral and proximal tibial ossification centers is found in 10% of euthyroid newborns with congenital syphilis (50). Syphilitic granulation tissue is found in the areas of destruction, but, in addition, nonspecific trophic changes are frequently present in the metaphyses soon after birth. Bands of decreased density are seen parallel to the physis in the long bones. SPNBF is common involving a portion of, or the entire length of a long bone (51).
The destructive lesions in the metaphyses vary in degree from small peripheral rarefactions involving the cortex to more central regions of medullary trabecular destruction (Fig. 7.20). Metaphyseal abnormalities are present in over 90% of infants with symptomatic congenital syphilis and 20% of asymptomatic newborns with positive serologic tests for treponema (47). A medial tibial metadiaphyseal defect is referred to as Wimberger’s sign. Similar lytic lesions can be seen at the same location in severe bacterial osteomyelitis – these are usually, but not invariably unifocal.
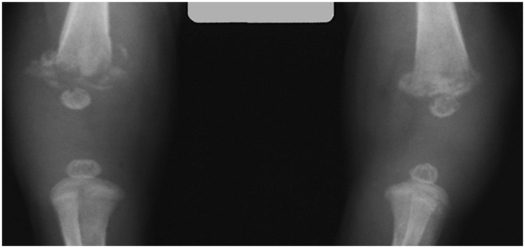
Figure 7.20 Congenital syphilis. Frontal radiograph of the lower extremities in a two-week-old infant. The distal femoral metaphyses appear fragmented, indicating pathologic fractures in areas of infection. SPNBF is seen along the lateral aspects of both distal femurs.
Pathologic fractures in the infected metaphyses are not unusual and may mimic the skeletal findings in the abused child (17, 52–54). SPNBF is also not specific for congenital syphilis and can be confused with healing trauma. The serologic test for syphilis is most helpful in confirming the diagnosis.
Vitamin deficiency and excess
Scurvy
Scurvy is caused by insufficient ingestion of vitamin C. It is much rarer than rickets (see Chapter 8), and in the pediatric age group is found primarily in older infants and young children who receive pasteurized or boiled milk formulas without vitamin supplements. The onset is seldom before age six months. Despite a voluminous literature emphasizing the distinctive clinical, laboratory, and imaging findings of scurvy, the past decade has seen continued interest in scurvy, with occasional consideration to it as a potential mimicker of abuse (55–61). It has also been described as a contributor to a fatal case of neglect (62).
Some of the radiographic changes of scurvy are attributable to the suppression of normal cellular activity. Others result from a hemorrhagic tendency caused by deficient intracellular cement in capillary endothelium. There is normal deposition of calcium in the zone of provisional calcification (ZPC), but the cortex and spongiosa become atrophic. Radiographs show relatively increased density of the ZPC and the periphery of the epiphyseal ossification centers. The spongiosa just beneath the ZPC is disorganized and appears abnormally radiolucent. Fractures occur through the ZPC and the subjacent metaphysis, and thus the pattern may suggest an epiphyseal separation. Subperiosteal and soft tissue hemorrhages are frequent and may be extensive. They are most evident radiographically during the healing phase, when large calcific cloaks are seen along the shafts of the affected long bones (Fig. 7.21) (51, 63).
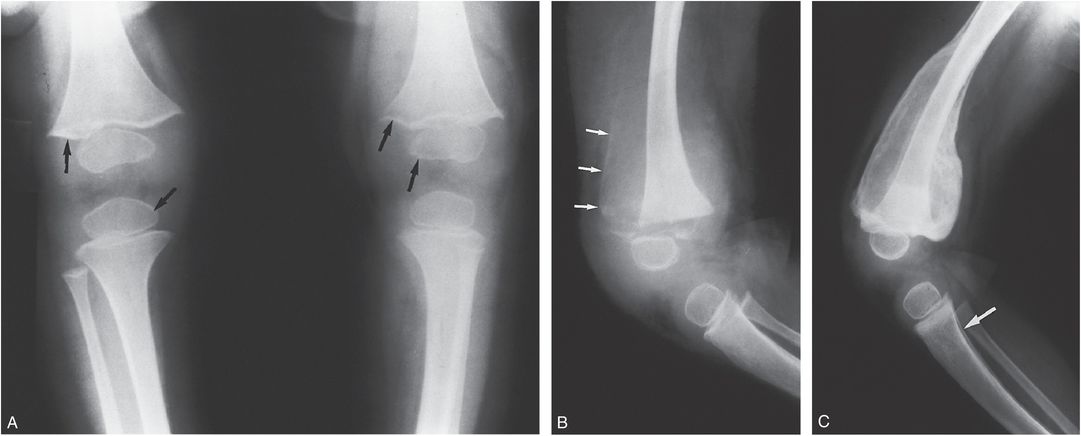
Figure 7.21 Scurvy. A, Frontal view of the knees in a toddler. The ZPC and the periphery of the epiphyses (arrows) appear relatively dense compared with the adjacent demineralized bone. The cortices are thin. Vitamin C was initiated. B, Lateral view of the left knee eight days later shows peripheral calcification (arrows) in a SH along the anterior femoral shaft. The epiphysis is displaced anteriorly by a fracture through the physis. C, A lateral view of the left knee five weeks later shows that the subperiosteal hemorrhage (SH) is densely calcified and cloaks the femoral shaft. SPNBF is noted involving the proximal tibia (arrow).
The metaphyseal changes of scurvy bear some similarity to those of child abuse. In scurvy, however, there are additional diagnostic clues. Thin cortices and osteopenia adjacent to the ZPC and the central portion of the epiphyseal ossification centers are regular features of scurvy. Similarly, the combination of a dense ZPC and ring around the epiphyseal bone are distinct features of scurvy. In most abused infants and children bone mineralization appears normal radiographically. Fractures of long bone shafts are frequent in abuse and rare in scurvy. Damage to the physis during the acute phase of scurvy may result in a growth disturbance and cone-shaped epiphyses (63).
Vitamin A intoxication
Retinoic acid, an active metabolite of vitamin A, causes increased bone resorption and SPNBF (64, 65). The early clinical findings of chronic hypervitaminosis A are nonspecific and include anorexia, irritability, and itching. Weeks to months after the initiation of excessive administration of vitamin A, hard and tender swellings are found over the extremities, and the diagnosis of child abuse may be considered when radiographs show SPNBF (Fig. 7.22). The new bone is thick and undulating. Any tubular bone may be affected, but the ulna and metatarsals are the most characteristic sites. Normal metaphyses and the absence of characteristic fractures help to distinguish hypervitaminosis A from child abuse. In acute vitamin A intoxication, there is increased intracranial pressure causing widening of the cranial sutures (Fig. 7.22B).
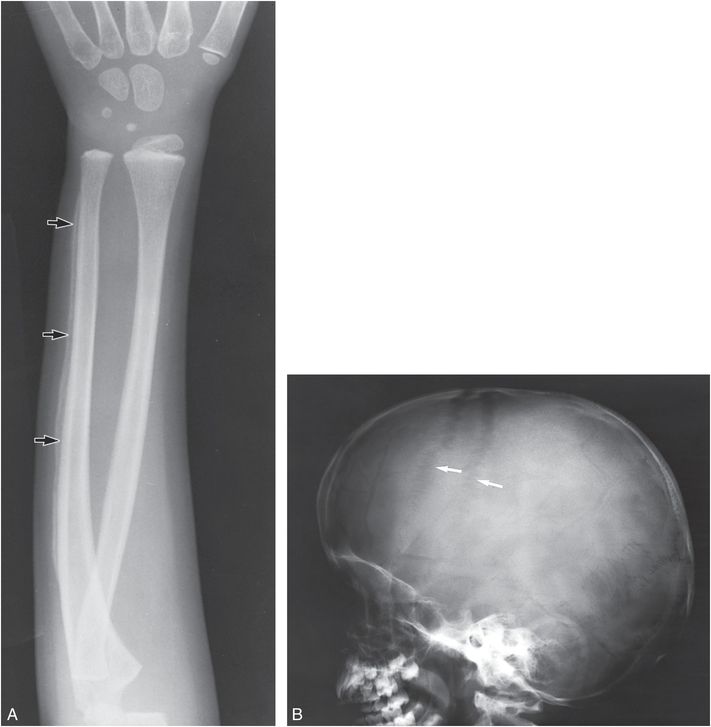
Figure 7.22 Vitamin A intoxication. A, Frontal view of the forearm in a four-year-old boy. SPNBF along the ulnar shaft is thick and undulating (arrows). B, Lateral view of the skull of the same patient. The widening of the coronal sutures (arrows) reflects increased intracranial pressure.
The cortical thickening resolves within several months after cessation of vitamin A administration. Late deformities have been documented despite the absence of radiographically demonstrable epiphyseal or metaphyseal lesions during the clinical illness. Enlargement and premature fusion of physes may cause significant limb shortening that is usually asymmetric. As described in other conditions above, cone-shaped epiphyses may be seen in vitamin A intoxication (66).
Caffey disease
Caffey disease (infantile cortical hyperostosis) is a rare disorder of young infants in which painful SPNBF and cortical thickening are found in multiple bones (4, 5, 67). The disorder has been shown to be due to a mutation in the COL1A1 gene, but it is unknown how this mutation causes the clinical and pathologic feature of the condition (68). The current incidence appears much lower than in the years following Caffey’s description. Virtually all bones can be involved, but the mandible, clavicle, and ulna are reportedly the most frequently affected sites (Figs. 7.23, 7.24). The mandible is involved in about 75% of cases. Neonatal cases have been described and onset after the age of six months is very rare. There is a tendency for the areas of SPNBF/cortical thickening to be focal and dramatic, with convex margins that rapidly transition to normal cortex. A familial distribution is not uncommon (Fig. 7.24) (69–71).
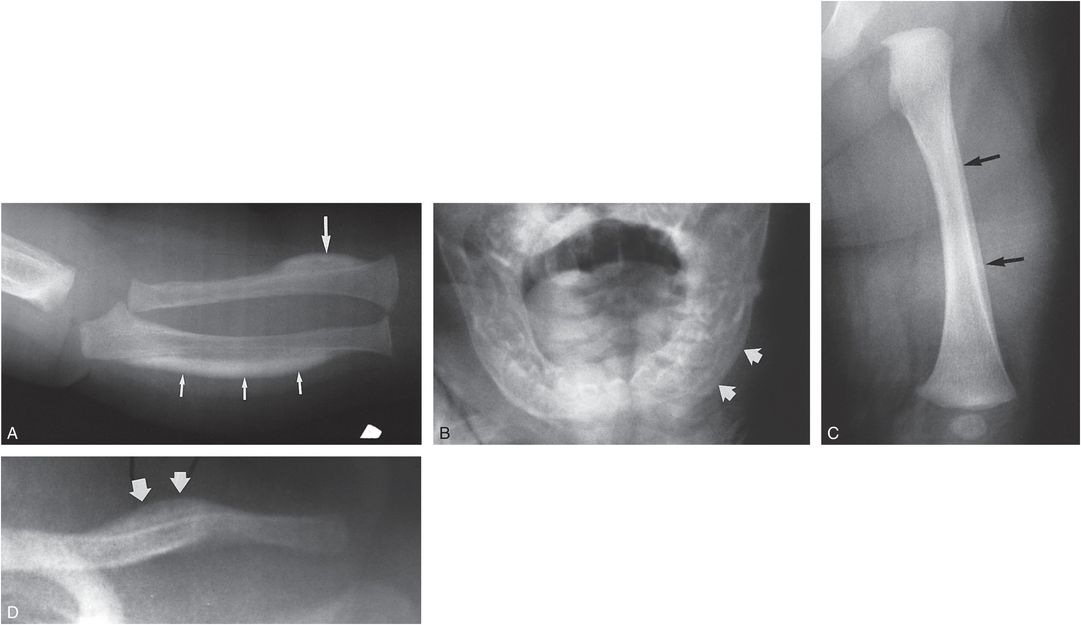
Figure 7.23 Caffey disease in twins. A, A view of the right forearm in a two-month-old infant demonstrates dense focal cortical thickening involving the lateral aspect of the distal radial shaft (large arrow). A broader zone of cortical thickening involves the ulnar diaphysis (small arrows). B, AP view of the mandible demonstrates SPNBF on the left (arrows). C, D, The twin shows less distinctive osseous change, with mature SPNBF along the left femoral shaft (arrows in C) and focal cortical thickening involving the left clavicle (arrows in D). Scattered areas of modest SPNBF were noted involving various long bones in both infants. A cousin subsequently developed typical features of Caffey disease. (Courtesy of Jacqueline Wellman MD.)
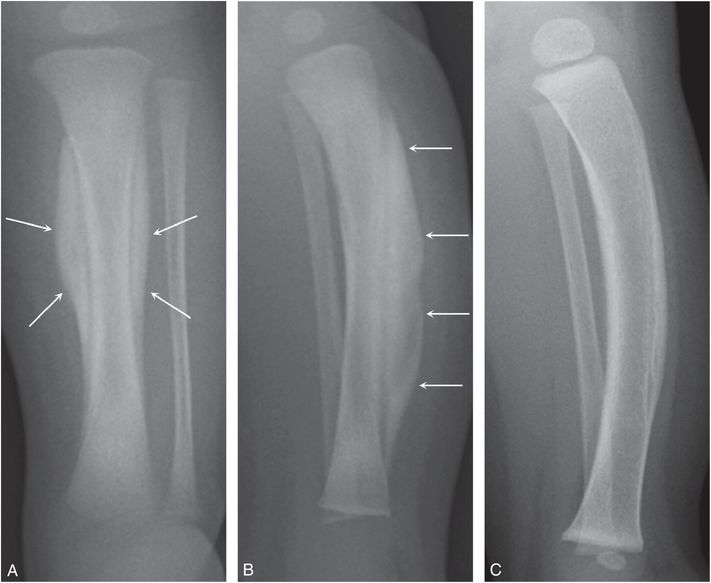
Figure 7.24 Caffey disease localized to the tibia. A 17-day-old infant with irritably, guarding and tenderness of the left lower leg. A, B, Frontal and lateral radiographs of the left lower leg demonstrate dramatic dense organized cortical thickening of the tibial diaphysis (arrows). The father and an older brother had a history of Caffey disease. C, Six months later a lateral view of the left lower leg shows healing with resultant undertubulation and anterior bowing of the tibial diaphysis.
MRI findings may be quite striking, suggesting an infectious or neoplastic process (72–74). Confusion between Caffey disease and child abuse may occur in the early stages when the SPNBF is modest and nonspecific and there are overlying soft tissue changes (75). However, the absence of fractures and metaphyseal irregularity, as well as the characteristic patterns of involvement, usually allow easy differentiation. Complete healing is the rule in Caffey disease, although undertubulation and bowing of the long bones may be slow to resolve (Fig. 7.24C). Late sequelae have been reported and are usually the result of acquired synostosis of adjacent involved bony shafts (e.g., radioulnar synostosis). The late sequelae of child abuse are usually the result of damage to the physis.
Hypertrophic osteoarthropathy and other causes of SPNBF
A wide array of neoplastic, inflammatory, and miscellaneous conditions can be associated with widespread SPNBF with otherwise normal osseous structures. Traditionally these have been loosely grouped under the heading of hypertrophic osteoarthropathy. In children, the findings are most often described with chronic pulmonary, hepatobillary, and congenital heart disease. It can also be encountered with pediatric neoplasms (76). Recently, widespread SPNBF has been noted in a child following intestinal transplantation and the features bear striking resemblance to those noted with Caffey disease (Fig. 7.25) (77). There are a variety of other causes of widespread SPNBF including prostaglandin administration, SPNBF associated with high-frequency jet ventilation, and extracorporeal membrane oxygenation (78–88).
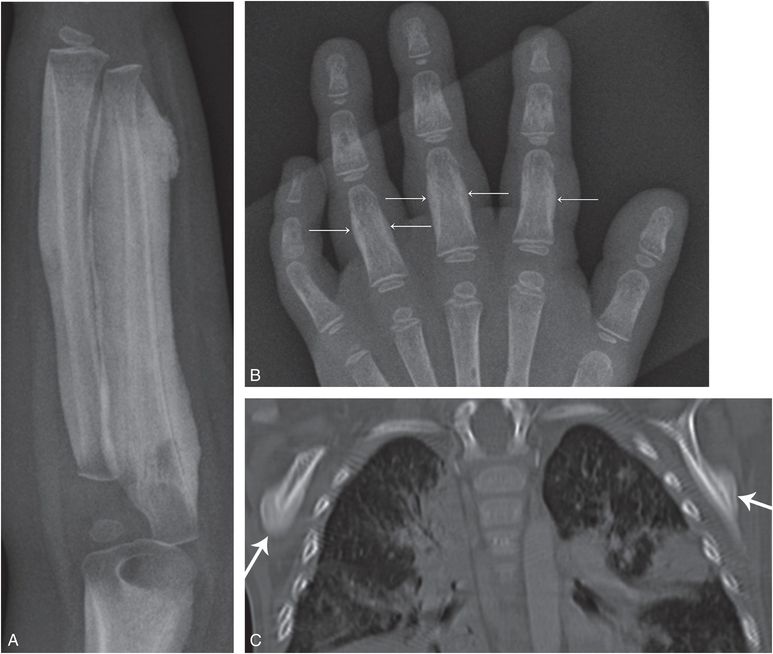
Figure 7.25 Caffey disease-like changes with intestinal transplantation. A three-year-old girl’s status post-intestinal transplantation for pseudo-obstruction. She was treated with steroids, OKT3 antithymocyte globulin, alemtuzumab, and infliximab for acute cellular rejection. A, Radiograph of the left forearm demonstrates dramatic organized cortical thickening along the humeral, radial, and ulnar shafts. B, PA view of the left hand shows SPNBF involving the second to fourth proximal phalanges (arrows). C, Coronal reformat of a chest CT shows dense cortical thickening of the scapulae (arrows). The patterns are indistinguishable from Caffey disease.
Sickle cell crisis due to SS, SC, or sickle thalassemia disease may cause bone infarction that is accompanied by pain, soft tissue swelling, and eventually SPNBF (89). Although not a differential problem in the older child, swelling of the hands and/or feet in an infant or young child due to a sickle cell vaso-occlusive crisis may raise concerns of abuse (Fig. 7.26). The lack of preceding trauma and the presence of SPNBF may heighten concerns of occult injury. Appropriate history and hematologic studies resolve the issue.
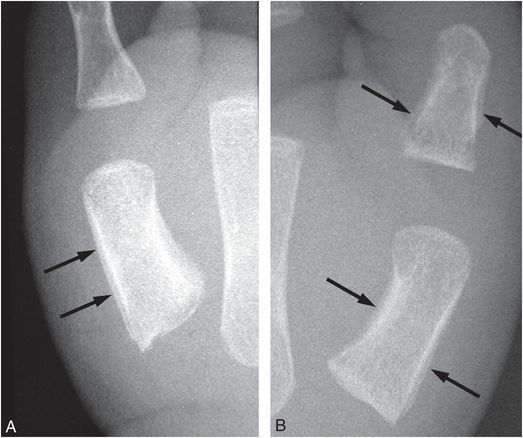
Figure 7.26 Sickle cell dactylitis. Infant with known sickle cell disease and swollen hands. A, B, Frontal radiographs of the left and right thumb shows SPNBF (arrows) along the shafts of first metacarpals bilaterally and the proximal phalanx on the left thumb.
Neoplasia
Leukemia
The osseous changes of acute leukemia in infancy are most often noted with acute lymphoblastic leukemia (ALL), and are due to a combination of growth disturbance and leukemic invasion (90). Confusion can occur with the radiologic findings of child abuse, particularly if there is cutaneous bleeding to suggest traumatic bruising (91, 92). Diffuse demineralization and SPNBF are not unusual (Fig. 7.27). Localized osteolytic lesions are usually multiple and sclerotic foci are unusual. So-called leukemic lines are narrow radiolucent metaphyseal bands that can usually be distinguished from the CMLs of child abuse by the accompanying osteopenia and the absence of bony fragments. The growth disturbances associated with leukemia and its treatment may produce a variety of radiographic alterations that superficially resemble inflicted injuries. Patients with acute myelogenous leukemia (AML) may present with a localized solid mass referred to as granulocytic sarcoma (93).
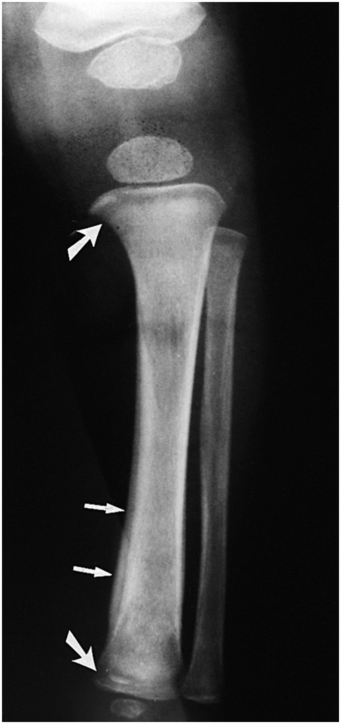
Figure 7.27 Leukemia. Frontal view of the leg of an infant with leukemia. Metaphyseal lucent bands (large arrows), SPNBF (small arrows), and osteopenia are evident.
Infantile hemangioma
When the mass effect of a solid neoplasm suggests a bruise, an inflicted injury may be considered. Infantile hemangiomas are not typically evident at birth and they enlarge in infancy. When deep, they often have a bluish hue (94). If associated osseous changes are interpreted as a fracture, concerns for abuse may be heightened (Fig. 7.28). Careful analyses of the radiographic findings, sonography, and/or contrast enhanced MRI resolve the issue.
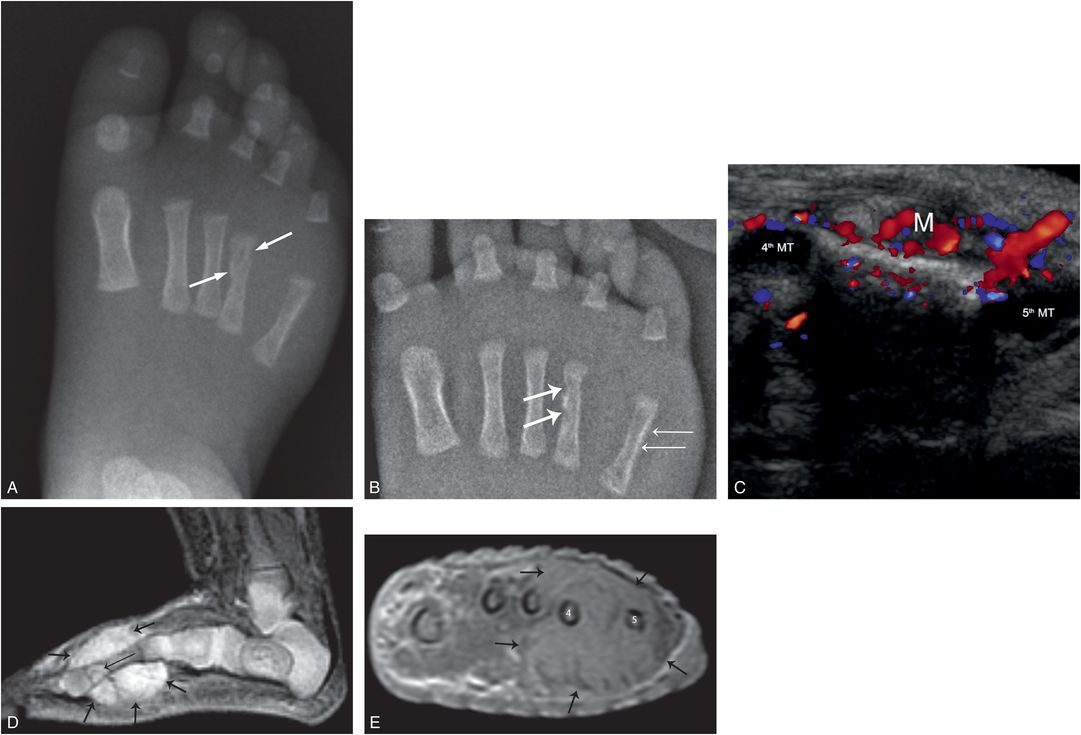
Figure 7.28 Infantile hemangioma with bony involvement. An eight-week-old former-premature infant with recently noted right-foot swelling and a reported fourth metatarsal fracture, transferred for SS and further evaluation. A, Initial frontal radiograph of the right foot shows cortical deformity with an apparent fracture of the fourth metatarsal (arrows). B, AP view of the right foot with bone detail shows cortical erosion and sclerosis of the fourth metatarsal (thick arrows), widening of the fourth/fifth metatarsal interval, and SPNBF of the fifth metatarsal (thin arrows). C, Transverse color Doppler sonogram shows a vascular mass (M) with widening of the space between the fourth and fifth metatarsals (MT). D, Sagittal STIR MRI shows a hyperintense mass (thick arrows) surrounding the fourth metatarsal. There is a focally intramedullary extension distally (thin arrow). E, Axial post-gadolinium T1W FS image at the level of the mid metatarsals shows an enhancing mass (arrows) surrounding the third-to-fifth metatarsals with increased separation of metatarsals four and five. Biopsy revealed an infantile hemangioma. A scalp hemangioma was also resected.
Drug-induced bone changes
Prostaglandin E1 may be used in the treatment of ductus-dependent congenital heart disease to maintain the patency of the ductus arteriosus. Its use frequently causes SPNBF of the ribs and long bones that resolves within six months to one year after discontinuation of the drug (85, 86). The radiologic findings are identical to those of traumatic SPNBF. The diagnosis is made on the basis of history.
Fractures and osteopenia have been reported in children treated with methotrexate for leukemia and Burkitt’s lymphoma (95). The history and presence of osteopenia allow differentiation from abuse. Such fractures are seldomly seen today because of lower doses of methotrexate in current treatment protocols. Osteopenia is also a feature of the rickets-like bone changes associated with ifosfamide, a derivative of cyclophosphamide, used in the treatment of malignant tumors, including Wilms’ tumor and Ewing’s sarcoma (96, 97). Adjuvant treatment of neuroblastoma with 13-cis-retinoic acid has led to the bony changes of hypervitaminosis A (98). Metaphyseal irregularity has been noted in children with thalassemia major treated with deferoxamine, started before three years of age (99, 100). In an MRI study of children and young adults with thalassemia a variety of abnormalities was found at the levels of epiphysis, physis, metaphysis, and metadiaphysis (101).
Copper deficiency
Copper is an essential component of several human enzyme systems that engage in oxidation reduction reactions. Disease resulting from copper deficiency is very uncommon with characteristic clinical and radiologic features. Shaw noted a total of 52 cases in his 1988 review of the literature (102). He found one or more of the following predisposing factors in each case: (1) low birth weight; (2) dietary deficiency of copper related to early total parenteral nutrition formulas; (3) copper-deficient milk; (4) antecedent malnutrition; and (5) peritoneal dialysis. The median age at presentation for full-term infants was 8.3 months (range: 5–18 months), and for low-birth-weight infants 3 months (range: 2.2–15.0 months). Clinical characteristics included psychomotor retardation, hypotonia, hypopigmentation, prominent scalp veins, pallor, and hepatosplenomegaly. Anemia and neutropenia were also noted. Plasma copper and ceruloplasmin levels were decreased.
Distinctive radiologic features have been noted, including osteopenia, metaphyseal cupping, sickle-shaped metaphyseal spurs, SPNBF, and fractures that may result in epiphyseal separation (Fig. 7.29) (102–105). When viewed at an obliquity, the prominent peripheral spurs can produce a “bucket handle” appearance (Fig. 7.29A). Rib and skull fractures are not features of copper deficiency and well-documented cases with fractures show gross abnormalities radiographically (102, 104, 106). In early communications, Paterson suggested that copper deficiency was responsible for the radiologic and clinical alterations in numerous infants with radiologic features suggesting abuse, but he provided no convincing radiologic or laboratory data to support this view (107–109). Paterson’s views with respect to copper deficiency and abuse have been severely criticized (104, 110–114).
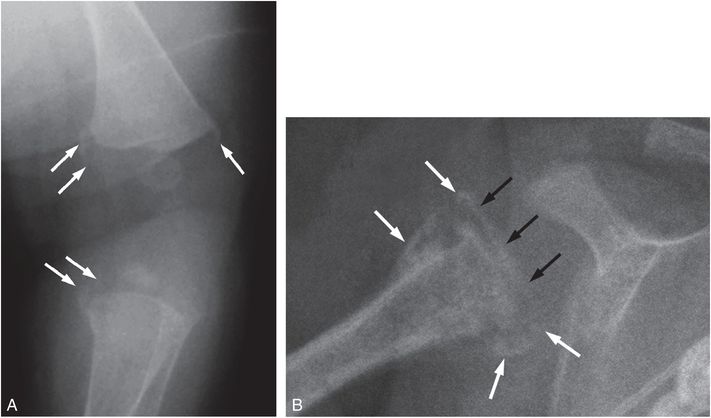
Figure 7.29 Copper deficiency. Former 24-week gestation, 720-g infant with chronic lung disease receiving long-term parenteral nutrition following small bowel resection for necrotizing enterocolitis. Because of cholestatsis at 2½ months, copper supplement was reduced to 10 mg/kg per day. At five months of age metaphyseal abnormalities were noted and a child abuse consultation was obtained. Serum copper level was undetectable. A, AP oblique radiograph of the left knee at age six months shows osteopenia and curvilinear metaphyseal spurs (arrows) resulting in bucket handle appearances in the distal femur and proximal tibia (arrows). Similar findings were present on the right. B, AP radiograph of the right shoulder shows irregular mineralization of the proximal humeral metaphysis. The character of the SPNBF (white arrows) and its continuity across the physis (black arrows) indicate an epiphyseal separation. A similar finding was present on the left. (Courtesy of Stephen Done MD and Kenneth Feldman MD.)
Despite a heightened awareness of the risks of copper deficiency in very low-birth-weight infants, cases with dramatic radiographic changes are still reported, perhaps related to reduction of copper supplementation in infants with cholestasis (105). The recent literature supports the conclusion of previous authors – when an otherwise normal infant without predisposing factors manifests the radiographic stigmata of physical abuse, dietary copper deficiency is not a serious differential consideration.
Menkes disease (kinky-hair syndrome)
Metaphyseal fractures similar to those seen in child abuse are present in Menkes disease, a rare X-linked recessive disorder in which there is defective gastrointestinal absorption of copper. Serum copper and ceruloplasmin levels are reduced. Long bone metaphyses have spurs with or without fracture (Fig. 7.30A) (115). Recently, Hill et al. noted ossification defects in 4 of 35 (11%) of children with Menkes disease (116). SPNBF may be present, and osteopenia is frequent after six months of age. Large numbers of Wormian bones may be seen in the skull (Fig. 7.30B). Irregular, tortuous cerebral, and abdominal arteries have been demonstrated angiographically (117, 118). The neuroimaging features of Menkes disease are further discussed in Chapter 19. Characteristic findings in the cerebral vasculature have been demonstrated by MR angiography (MRA) (119). The osseous and central nervous system findings of Menkes disease have been confused with child abuse, particularly at presentation, but the condition is generally readily differentiated on clinical grounds (120, 121). The hair is thin, coarse, and lacking in pigment. Affected infants have seizures, psychomotor retardation, and failure to thrive (FTT). The diagnosis is confirmed by demonstration of low serum copper levels. The long-term osseous changes of the disorder have also been described (115).
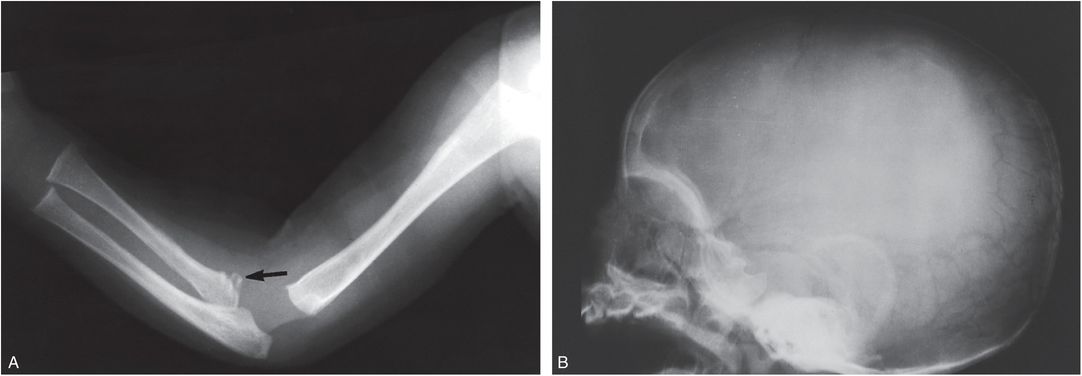
Figure 7.30 Menkes disease. A, Upper extremity of a three-month-old infant. There is a fracture involving the proximal radial metaphysis (arrow). B, Lateral view of the skull of the same infant. Numerous Wormian bones are seen posteriorly. (Courtesy of N. Genieser MD.)
Miscellaneous genetic and metabolic disorders
A variety of additional diseases manifest skeletal features that may also be noted with abuse. Metabolic disorders characterized by abnormal accumulations of chemical compounds can result in dramatic osseous abnormalities that are usually easily distinguished from traumatic lesions. Occasionally, in infancy, radiographs of limited anatomic regions may raise the possibility of previous injury. Vertebral notching and an accentuated thoracolumbar kyphosis may be the presenting features of Hunter syndrome (Fig. 7.31) (122). Because this finding can also be noted with trauma, abuse may be considered initially (see Chapter 21).
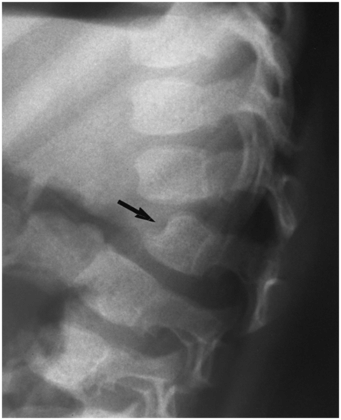
Figure 7.31 Hunter syndrome. Lateral view of the thoracolumbar junction in a 24-month-old child with short stature demonstrates an acute kyphosis at the L1–2 level. L2 demonstrates hypoplasia with an inferiorly beaked configuration (arrow). L1 appears subluxed and abnormally inclined on L2. The pattern is reminiscent of vertebral notching with abuse (see Chapter 21), and occult previous trauma was initially considered in this case. In an infant the appearance of L2 might suggest a normal developmental variant (see Chapter 12). This child demonstrated other radiologic features, clinical findings, and laboratory abnormalities of Hunter syndrome.
Neurofibromatosis type 1 (NF1) may be associated with a tibial, and sometimes fibular dysplasia, leading to fracture and pseudoarthrosis (123). The findings may occur in the absence of other features of NF1. The deformity usually prompts radiographic assessment; however, an acute fracture through the dysplastic tibia can present with acute/subacute symptoms (Fig. 7.32). In the absence of trauma, radiographs may raise concerns of an inflicted injury. The imaging features of pseudoarthrosis of the tibia are distinctive with anterolateral bowing and focal narrowing of the anteroposterior (AP) diameter of the diaphysis with endosteal sclerosis. As with many of the mimics of abuse, the true diagnosis becomes obvious when a systematic analysis of high-quality radiographs is carried out.
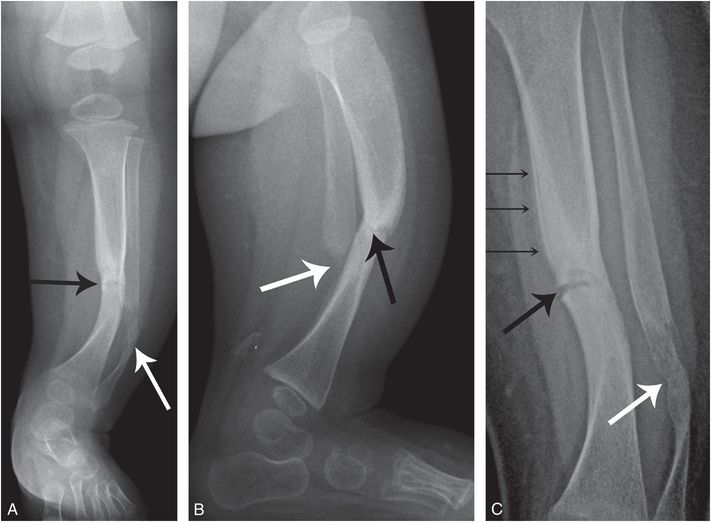
Figure 7.32 Pseudoarthrosis of the tibia and fibula. Six-month-old with increasing fussiness and screaming with diaper change. Radiographs elsewhere revealed left lower leg fracture and the child was referred with concerns of abuse. Initial frontal (A) and lateral (B) radiographs of the left lower leg show lateral bowing of the tibia and fibula. The tibia shows endosteal sclerosis with abrupt narrowing of the AP dimension and a faint transverse lucency in the midshaft (black arrows). The fibula is dysplastic in appearance with a displaced fracture (white arrows). C, High-detail AP radiograph from the SS shows mild medial distraction of the fracture (thick black arrow) with faint SPNBF (thin arrows), indicating a recent fracture through the dysplastic tibia. The fibula shows irregular ossification (thick white arrow). There was no family history of NF1. At 10 months of age, there was one café-au-lait macule measuring more than 0.5 cm – insufficient for diagnosis of NF1.
In addition to some of the disorders above, and ostogenesis imperfecta (see Chapter 9), there is a wide variety of conditions associated with diffuse osseous changes that may lead to fracture, and Lachman has provided a comprehensive list of disorders associated with fragile bones (5). New distinctive conditions continue to be reported (124–127). Differentiating accidental and spontaneous fractures from inflicted injury can pose a problem in these populations. On occasion, abuse may coexist with chronic disease and in other handicapped populations (see Chapter 23). As with those conditions previously described, a thorough multidisciplinary assessment should minimize confusion with abuse.