Chapter 123 Diagnostic and Therapeutic Vitrectomy for Uveitis
Introduction
Uveitis is a type of intraocular inflammation that can affect vision and may lead to legal blindness. Uveitis is primarily diagnosed upon clinical manifestation rather than laboratory findings.1,2 A patient’s medical history, course of uveitis, and therapeutic response are also important in making a diagnosis. Histopathological diagnosis of biopsy specimens is not common because of the difficulty in obtaining ocular specimens. Uveitis can be classified into autoimmune, infectious, and malignant forms based on the underlying pathogenic mechanism. Regardless of cause, accurate diagnosis and appropriate pharmacotherapy are critical for positive visual outcomes. Diagnostic vitrectomy can be helpful in discriminating between different causes of uveitis.
The vitreous, which constitutes most of the ocular volume, plays an important role in the pathogenesis of many inflammatory diseases of the eye. Ocular fluid can be a valuable tool for accurate diagnosis, and considering specimen volume requirements, vitreous samples are more useful than aqueous humor samples for analysis.3 In addition, a vitrectomy can be beneficial for the management of vitreoretinal complications in uveitis patients. This chapter, which is divided into diagnostic and therapeutic vitrectomy sections, covers the surgical indications, principles, and techniques for the management of uveitis. Novel advances in laboratory techniques will also be discussed.
Diagnostic vitrectomy
Indications
Diagnostic vitrectomy is indicated in an inflamed eye when the course or appearance of uveitis is not typical for an autoimmune disease and the presence of an infectious agent or malignant process is suspected (see also Chapter 122, Infectious endophthalmitis, and Chapter 124, Vitreous, retinal, and choroidal biopsy). If the disease fails to respond to therapy in an expected manner, the physician may suspect that the original diagnosis is incorrect; in such cases, other diagnoses need to be considered. Indications for diagnostic vitrectomy are shown in Table 123.1.
| Infectious uveitis | Endophthalmitis4: bacterial, fungal, parasitic or viral |
| Traumatic endophthalmitis including intraocular foreign body | |
| Postsurgical endophthalmitis | |
| Endogenous endophthalmitis | |
| Sterile endophthalmitis | |
| Vitritis | |
| Retinitis | |
| Choroiditis | |
| Retinal vasculitis | |
| Noninfectious uveitis | Autoimmune uveitis5 |
| Primary intraocular lymphoma6 | |
| Carcinoma metastasis7 including leukemia infiltration | |
| Choroidal melanoma8 |
Surgical principles and techniques
Preoperative preparation
From the technical viewpoint, cutting with a vitrector does not appear to cause more cellular degeneration compared with simple aspiration. Standard 3-port vitrectomy is typically performed under local anesthesia. Current studies have yet to determine if the use of 23- or 25-gauge (G) sutureless vitrectomy affects diagnostic yield compared with conventional 20G vitrectomy. Some authors advocate a lower cutting rate and higher duty cycle because larger and more intact pieces of the aspirated vitreous may have a better yield.9 However, these settings have not yet proven to be advantageous compared with traditional sampling techniques.10
Vitreous sampling
The standard technique for performing 3-port pars plana vitrectomy (PPV) involves keeping the cutter tip within the vitreous. The undiluted vitreous humor is collected through lines using the vitreous cutter connected directly to a 3 or 5 mL syringe. This syringe is manually aspirated by an assistant while the operator compresses the eyeball with his/her fingers or a cotton swab (Fig. 123.1). When the eye visibly softens, the infusion is turned on.11 In our experience, up to 1.5 mL of undiluted vitreous can be safely obtained using this technique. Other techniques, such as using continuous air or perfluorocarbon liquid (PFCL) infusion can yield large volumes of undiluted vitreous without compressing the eyeball. Air substitutes the vitreous removed from the eyeball, and this can yield vitreous samples ranging in volume from 0.6 to 1.5 mL.9,12 Perfluorocarbon-perfused vitrectomy, in which balanced salt solution is replaced with perfluorocarbon liquid during vitreous aspiration, can be a good option for preventing complications of hypotony such as suprachoroidal hemorrhage.13 Using this technique, an average of 2.24 mL of undiluted vitreous could be obtained. However, a major disadvantage, in addition to the high cost of perfluorocarbon, is that samples obtained using this technique need to be frozen to completely remove the perfluorocarbon.
Handling and preparation of vitreous samples
The overall yield of diagnostic vitrectomies varies considerably in different studies. Generally, the number of tests that can be carried out on a vitreous specimen depends on the amount of material retrieved. In addition, patient selection, surgical technique, and vitreous specimen analysis are factors that may contribute to this variation. Ideally, cytological analysis and microbiologic cultures should be performed with undiluted vitreous specimen when first obtained. The vitreous wash fluid that is collected in the vitrector cassette can be used for additional microbiological cultures. Cytokine, chemokine, and flow cytometry analysis can also be performed using the aqueous humor that is obtained. It is nevertheless important to realize that a negative cytological diagnosis or microbiological culture does not prove the absence of malignancy or infection. Polymerase chain reaction (PCR) analysis is recommended in cases where viral infection is suspected because the quantity of organisms shed into the vitreous cavity is minute, and viral culture often fails. A positive PCR result for human herpes viruses in the vitreous of patients with uveitis has been identified.14,15
Retinal or choroidal biopsy
If a vitreous biopsy does not yield a diagnosis, chorioretinal biopsy of the involved eye can be considered (see also Chapter 124, Vitreous, retinal and choroidal biopsy), depending on the location of lesions and response to therapy. Chorioretinal biopsy increases the probability of achieving a correct diagnosis. Retinal or choroidal biopsy is considered when the inflammatory process is localized primarily in the sensory retina or the retinal pigment epithelium. In such cases, if the disease only involves the retina or choroid, pathogens may not spill into the vitreous. For example, cytomegalovirus, which is difficult to culture from the vitreous, can be cultured from the retina or seen on electron microscopic examination of the retina.
An endoretinal biopsy can be helpful in diagnosing tuberculosis, sarcoidosis, and lymphoma.16 Accurate biopsy results will lead to a specific treatment for uveitis of suspected infectious or malignant origin. Histological examination of chorioretinal biopsies provides some advantages over cytological examination of vitreous specimens. In the biopsy, more material is available for immunohistochemistry; this allows for more precise classification and differentiation of the pathology of the lesion. Despite these advantages, choroidal and retinal biopsies are used as a last resort considering the severe complications that may accompany the procedure.
The surgical technique for performing a chorioretinal biopsy can either be transscleral or transvitreal, depending on the location of the lesion. In patients with panuveitis, a choroidal mass, and a detached retina, it is recommended that transvitreal endoretinal biopsy be performed at the junction of the attached and detached retina. Upon completion of the vitrectomy, the area with active disease can be identified more accurately; this area can then be biopsied. However, a biopsy specimen obtained from a retinal area in which the disease is quiescent will rarely provide diagnostic information. The preferred location for this surgical procedure is the superior and nasal retina. Intense endolaser or heavy endodiathermy at the margin of the biopsy is performed prior to long-acting gas or silicone oil tamponade.17,18 In cases in which the retina is still attached, a cannula is used to inject saline under the sensory retina to create a small bleb. The advancing edge of the lesion should be included if retinitis is suspected, because actively replicating microorganisms are most likely to be found in this location.
The retinal or chorioretinal biopsy specimen should be divided in order to permit culture, histological examination, and monoclonal antibody studies. PCR can be performed on the retinal tissue as well. However, it has been reported that retinochoroidal biopsies may be associated with the risk of retinal detachment and that false-negative results often occur in these specimens.19,20 In addition, proper positioning is essential to maintain retinal tamponade after endoretinal biopsy.
Diagnostic techniques for vitrectomy specimens
Cytological evaluation
Cytological examination reveals the phenotypes of infiltrating cells into the vitreous. Vitreous humor fluid obtained by PPV is centrifuged, and the cells are smeared onto glass slides and then either immersed in 95% ethanol for Papanicolaou (Pap) staining or left to dry for subsequent Giemsa staining. The diagnosis of a PIOL depends primarily on cytological examination of the vitreous sample. The characteristic feature, from either Giemsa or Diff Quick staining, is the presence of large B cell lymphoblasts and atypical lymphocytes with high nuclear/cytoplasm ratios amongst small round lymphocytes.21 However, diagnosis of PIOL is difficult, resulting in a high false-negative rate due to small sample volumes with low number of malignant cells, inadequate preparation of samples or carrying media, and previous administration of corticosteroids. In chronic endogenous uveitis, cytology reveals classical degenerative inflammatory cells with poor morphology, although cytological examination of the vitreous specimen can be difficult because there may be a relative lack of inflammatory cells. A cytological examination can be helpful in diagnosing sarcoidosis. Kinoshita et al. showed multinucleated giant cells in 85.7% of cases and lymphocytes and epithelioid cells in all cases of intraocular sarcoidosis.5
Histopathologic evaluation
It is recommended that the biopsy tissue be immediately processed by an ophthalmic pathologist. The biopsy specimen is generally divided into three portions: one-third is fixed for routine histopathological evaluation, including light and electron microscopic examinations. The second portion is frozen in optimal cutting temperature (OCT) embedding compound for immunopathological and molecular characterization. The third portion is sent for culture of viruses and other microorganisms or for tissue culture. If the biopsy specimen is not sufficient for all three aforementioned procedures, the tissue should be processed for frozen sections, as these can undergo routine histopathology, immunohistochemistry, and molecular analysis. Svozilkova et al. reported that the highest percentage of positive results was achieved by histopathological evaluation in uveitis.3
Microbiological culture
To diagnose infection, vitreous samples are routinely sent for Gram staining, culture, and antibiotic sensitivity tests. Currently, microbiological culture is still the “gold standard” for diagnosis of infectious uveitis. Some experts advocate immediate culture inoculation by the surgeon in the appropriate media in order to maximize organism recovery.11 However, in viral retinitis, it should be noted that viral cultures of the vitreous might reveal false-negative results because of neutralizing antibodies or low viral shedding.
Molecular analysis
The most obvious reason for performing a molecular analysis is to rule out lymphoma as the cause of a masquerade syndrome and to identify infectious causes. Molecular analysis of vitreous specimens is primarily used to study the genotypic classification of PIOL with the goal of identifying prognostic factors. Recently, it was reported that patients with bcl-2 gene translocation were significantly younger than those who lacked the translocation, suggesting the need for aggressive treatment.22 PCR is also used to detect monoclonality within the variable region of the third complementary determining region (CD3) in the immunoglobulin heavy chain gene of malignant B-cells. Single-band detection of immunoglobulin heavy chain rearrangement can be useful in PIOL.23
It is also important to detect the DNA of microorganisms in the aqueous or vitreous humor by PCR. PCR is an in vitro technique that amplifies minute quantities of nucleic acid from infectious organisms and viruses into analytic amounts.24 PCR can also be used to screen vitrectomy samples for a variety of pathogens, such as Propionibacterium acnes or Mycobacterium tuberculosis, in unresponsive or atypical uveitis cases.25,26 Microbial DNA amplification by PCR and intraocular antibody measurement has been shown to detect infection by organisms that are difficult to culture. The addition of PCR to identify bacterial DNA increased the diagnostic sensitivity from 48% to more than 80%.27 Moreover, prior short-term use of intravitreal antibiotics does not seem to affect the ability of PCR-based DNA amplification.
Flow cytometry
In addition, flow cytometry has been shown to be useful in the diagnosis of intraocular lymphoma.28 The test relies on the fact that most PIOLs are composed of monoclonal populations of B-lymphocytes that stain positive for B cell markers. Combined analysis by cytological examination and flow cytometry appears to be more sensitive for diagnosing lymphoma than using either test alone. Both techniques require a sufficient number of cells and an experienced cytopathologist.
Cytokine/chemokine measurement
B-cell malignancies can secrete high levels of IL-10, an immunosuppressive cytokine, while inflammatory conditions are associated with high levels of IL-6, a proinflammatory cytokine. Some authors have shown that PIOLs can exhibit high IL-10 levels, with IL-10:IL-6 ratios >1.29,30 Cytokine levels and IL-10:IL-6 ratios are not diagnostic of PIOL, but they can be useful adjunctive tests in corroborating the diagnosis of PIOL and determining whether there is a significant response to treatment. In contrast to molecular analysis, the measurement of cytokine levels is much easier. Therefore, measurement of IL-10 and IL-6 levels is recommended for patients with suspected PIOL in hospitals that lack a cytopathologist.
We have also shown that the intraocular cytokine or chemokine environment can vary between patients; therefore, the underlying immunopathogenesis may be different between endogenous uveitis cases.31,32 Because the vitreous is considered to be a reservoir of proinflammatory mediators that contribute to the development of many inflammatory diseases of the eye, vitreous samples can be a valuable tool for accurate diagnosis of the pathogenic mechanism. We anticipate that cytokine or chemokine analysis from vitreous samples will be used more widely to test this hypothesis in the future.
Therapeutic vitrectomy
Indications
Therapeutic vitrectomy for uveitis is conventionally indicated for: (1) media opacity causing significant visual loss33; (2) intractable cystoid macular edema (CME)34; and (3) other vitreoretinal complications, including tractional retinal detachment, rhegmatogenous retinal detachment, macular pucker, hemophthalmos, hypotony,35 or macular hole.36 Recent advances in vitrectomy techniques have expanded the indications for therapeutic vitrectomy. Vitrectomy can be used as an adjunctive procedure with intravitreal applications of anti-infectious, cytostatic drugs, or intravitreal sustained-release drug implants.37–40
Surgical principles and techniques
Preoperative meticulous control of inflammation is critical for good surgical outcome. With a few exceptions, such as rhegmatogenous retinal detachment and infectious endophthalmitis, surgery should be performed when inflammation is quiescent. Previous studies consistently demonstrate the importance of perioperative control of inflammation. Systemic corticosteroids are still the best drugs for controlling inflammation during the perioperative period. We prefer administering steroids 1 day before surgical intervention; typically, 1 mg/kg per day of steroid treatment is adequate. However, when diagnostic vitreous biopsy is also planned, ensuring that patients with suspected PIOL are not receiving corticosteroid medication at the time of vitrectomy improves the diagnostic yield for lymphoma, since lymphoma cells degenerate in response to corticosteroid treatment.41
A standard 20G, 23G, or 25G 3-port PPV is currently used. Special attention needs to be given to the removal of peripheral vitreous base and posterior cortical vitreous. Since the largest accumulation of vitreous opacities is often found in the vitreous base and remnant posterior cortical vitreous that can provide a platform for fibroglial proliferation, near-complete vitreous removal is of high importance. Triamcinolone acetonide has been used during vitrectomy as an aid to visualize residual vitreous cortex and peripheral vitreous.42 The use of surgical adjuvants is now widely accepted in clinical practice to facilitate peeling of the internal limiting membrane (ILM) during vitrectomy because membranes in uveitic eyes are very fragile and adhere to the retina. Indocyanine green (ICG) can also be used to visualize the ILM during PPV and ILM peeling.43 The ILM can also be selectively stained by brilliant blue G, which is less toxic to the retina than ICG.
Intravitreal administration of drugs at the end of therapeutic PPV may alone be sufficient to decrease inflammatory activity. Administration of intravitreal triamcinolone acetonide (IVTA) at the end of vitrectomy can reduce immediate postoperative inflammation, which has been demonstrated in patients with retinal vascular diseases and proliferative vitreoretinopathy.44,45 Antimicrobial medications can be administered at the end of vitrectomy in patients with viral, bacterial or fungal endophthalmitis. In patients with fungal endophthalmitis, intravitreal application of amphotericin B alone inhibited uveitis activity, and no exacerbation was observed during the postoperative period.3
Outcomes
Vitrectomy can be safe and effective for the management of vitreoretinal complications in patients with persistent panuveitis.36,46,47 Retinal detachment, vitreous hemorrhage or macular hole can be successfully managed with vitrectomy. Visual improvement has also been reported in most of the patients who underwent vitrectomy in uveitis cases.48 It has been suggested by several authors that PPV reduces or eliminates recurrences of inflammation because it reduces the antigen load, inflammatory mediators or toxic elements from the vitreous. Moreover, a clear vitreous cavity facilitates diffusion of intravitreally injected drugs and permits better examination of the eye postoperatively. However, although the removal of the vitreous can decrease accumulation of cells and haze during an episode of inflammation, vision would be affected by new inflammatory episodes.
Some retrospective studies suggested that PPV can reduce the overall inflammatory activity of disease, and improve refractory uveitic CME.49 Recently, a prospective randomized pilot study also reported the comparison of PPV with immunomodulatory therapy for patients with intermediate uveitis.50 A higher percentage of patients treated with PPV achieved resolution of uveitis compared with those given immunomodulatory therapy, although there was no statistically significant difference in visual outcome for either group during the 18-month follow-up.
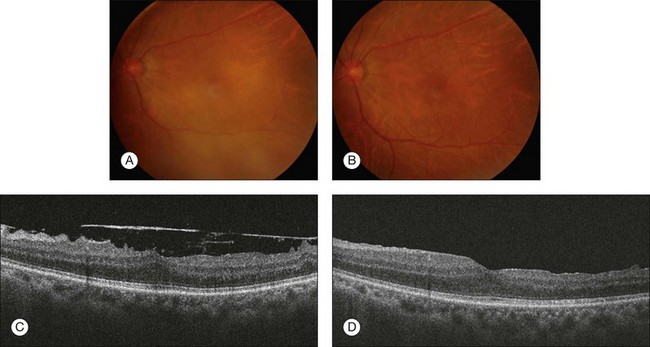
Visual improvement has been reported for vitrectomy treatments for epiretinal membranes associated with pars planitis and sarcoidosis. Epiretinal membrane peeling usually results in significant visual improvement and restoration of normal foveal contour (Fig. 123.2). However, there is a possibility of poor visual outcome associated with CME or recurrence of the membrane with persistent inflammation. Moreover, associated vascularization or scarring of the underlying retina is more common with epiretinal membranes associated with uveitis, and these can limit positive visual outcome. Outcomes of PPV with silicone oil tamponade were reported in patients with chronic hypotony due to uveitis.35 Eyes with posterior inflammation contained a dense anterior hyaloid that may be associated with ciliary body traction. If ciliary atrophy was present, intraocular pressure was restored only in eyes that received silicone oil tamponade treatment.
However, some issues still remain controversial, including whether vitrectomy reduces the activity of disease and the number of recurrences and whether vitrectomy actually reduces CME, and a large, prospective, randomized clinical trial is needed to draw a firm conclusion. In addition, clinicians should keep in mind that vitrectomy improves visual acuity and inflammatory control in some patients, but postoperative complications such as retinal detachment, vitreous hemorrhage and high intraocular pressure can limit the visual outcome.3
1 Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996;80:844–848.
2 Durani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. 2004;218:223–236.
3 Svozilkova P, Hessingerova J, Brichova M, et al. The role of pars plana vitrectomy in the diagnosis and treatment of uveitis. Eur J Ophthalmol. 2011;21:89–97.
4 Endophthalmitis Vitrectomy Study. Microbiologic factors and visual outcome in the endophthalmitis vitrectomy study. Am J Ophthalmol. 1996;122:830–846.
5 Kinoshita Y, Takasu K, Adachi Y, et al. Diagnostic utility of vitreous humor fluid cytology for intraocular sarcoidosis: A clinicopathologic study of 7 cases. Diagn Cytopathol. 2010;40:210–213.
6 Kanavi MR, Soheilian M, Bijanzadeh B, et al. Diagnostic vitrectomy (25-gauge) in a case with intraocular lymphoma masquerading as bilateral granulomatous panuveitis. Eur J Ophthalmol. 2010;20:796–798.
7 Lee EJ, Kim TW, Heo JW, et al. Natural killer/T-cell lymphoma of nasal type with intraocular involvement: case report. Eur J Ophthalmol. 2010;20:215–217.
8 Abi-Ayad N, Grange JD, Salle M, et al. Transretinal uveal melanoma biopsy with 25-gauge vitrectomy system. Acta Ophthalmol. 2011. [Epub ahead of print]
9 Tsui I, Schwartz SD, Hubschman JP. A current method to collect an undiluted vitrectomy sample. Retina. 2010;30:830–831.
10 Hubschman JP, Bourges JL, Tsui I, et al. Effect of cutting phases on flow rate in 20-, 23-, and 25-gauge vitreous cutters. Retina. 2009;29:1289–1293.
11 Margolis R. Diagnostic vitrectomy for the diagnosis and management of posterior uveitis of unknown etiology. Curr Opin Ophthalmol. 2008;19:218–224.
12 Tamai M, Nakazawa M. A collection system to obtain vitreous humor in clinical cases. Arch Ophthalmol. 1991;109:465–466.
13 Quiroz-Mercado H, Rivera-Sempertegui J, Macky TA, et al. Performing vitreous biopsy by perfluorocarbon-perfused vitrectomy. Am J Ophthalmol. 2005;140:1161–1163.
14 Lau CH, Missotten T, Salzmann J, et al. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007;114:756–762.
15 Sugita S, Shimizu N, Watanabe K, et al. Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol. 2008;92:928–932.
16 Cassoux N, Charlotte F, Rao NA, et al. Endoretinal biopsy in establishing the diagnosis of uveitis: a clinicopathologic report of three cases. Ocul Immunol Inflamm. 2005;13:79–83.
17 Johnston RL, Tufail A, Lightman S, et al. Retinal and choroidal biopsies are helpful in unclear uveitis of suspected infectious or malignant origin. Ophthalmology. 2004;111:522–528.
18 Cole CJ, Kwan AS, Laidlaw DA, et al. A new technique of combined retinal and choroidal biopsy. Br J Ophthalmol. 2008;92:1357–1360.
19 Eide N, Walaas L. Fine-needle aspiration biopsy and other biopsies in suspected intraocular malignant disease: A review. Acta Ophthalmol. 2009;83:588–601.
20 Kvanta A, Seregard S, Kopp ED, et al. Choroidal biopsies for intraocular tumors of indeterminate origin. Am J Ophthalmol. 2005;14:1002–1006.
21 Intzedy L, Teoh SC, Hogan A, et al. Cytopathological analysis of vitreous in intraocular lymphoma. Eye. 2008;22:289–293.
22 Wallace DJ, Shen D, Reed GF, et al. Detection of the bcl-2 gene t(14;18) translocation and proto-oncogene expression in primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2006;47:2750–2756.
23 Intzedy L, Teoh SC, Hogan A, et al. Cytopathological analysis of vitreous in intraocular lymphoma. Eye. 2008;22:289–293.
24 Van Gelder RN. CME review: polymerase chain reaction diagnostics for posterior segment diseases. Retina. 2003;23:445–452.
25 Lohmann CP, Linde HJ, Reischl U. Improved detection of microorganisms by polymerase chain reaction in delayed endophthalmitis after cataract surgery. Ophthalmology. 2000;107:1047–1051.
26 Ortega-Larrocea G, Bobadilla-del-Valle M, Ponce-de-Leon A, et al. Nested polymerase chain reaction for Mycobacterium tuberculosis DNA detection in aqueous and vitreous of patients with uveitis. Arch Med Res. 2003;34:116–119.
27 Garweg JG, Wanner D, Sarra GM, et al. The diagnostic yield of vitrectomy specimen analysis in chronic idiopathic endogenous uveitis. Eur J Ophthalmol. 2006;16:588–594.
28 Zaldivar RA, Martin DF, Holden JT, et al. Primary intraocular lymphoma: clinical, cytologic, and flow cytometric analysis. Ophthalmology. 2004;111:1762–1767.
29 Merle-Beral H, Davi F, Cassoux N, et al. Biological diagnosis of primary intraocular lymphoma. Br J Ophthalmol. 2004;124:469–473.
30 Ohta K, Sano K, Imai H, et al. Cytokine and molecular analysis of intraocular lymphoma. Ocul Immnol Inflamm. 2009;17:142–147.
31 Ahn JK, Yu HG, Chung H, et al. Intraocular cytokine environment in active Behçet uveitis. Am J Ophthalmol. 2006;142:429–434.
32 Kim TW, Chung H, Yu HG. Chemokine expression of intraocular lymphocytes in patients with Behcet uveitis. Ophthalmic Res. 2011;45:5–14.
33 Waters FM, Goodall K, Jones NP, et al. Vitrectomy for vitreous opacification in Fuch’s heterochromic uveitis. Eye. 2000;14:216–218.
34 Kiryu J, Kita M, Tanabe T, et al. Pars plana vitrectomy for cystoid macular edema secondary to sarcoid uveitis. Ophthalmology. 2001;108:1140–1144.
35 Kapur R, Birnbaum AD, Goldstein DA, et al. Treating uveitis-associated hypotony with pars plana vitrectomy and silicone oil injection. Retina. 2010;30:140–145.
36 Wu TT, Hong MC. Pars plana vitrectomy with internal limiting membrane removal for a macular hole associated with Behçet’s disease. Eye. 2009;23:1606–1607.
37 Martin DF, Parks DJ, Mellow SD, et al. Treatment of cytomegalovirus retinitis with an intraocular sustained-release ganciclovir implant. A randomized controlled clinical trial. Arch Ophthalmol. 1994;112:1531–1539.
38 Jaffe GJ, Ben-Nun J, Guo H, et al. Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology. 2000;107:2024–2033.
39 Rush RB, Goldstein DA, Callanan DG, et al. Outcomes of birdshot chorioretinopathy treated with an intravitreal sustained-release fluocinolone acetonide-containing device. Am J Ophthalmol. 2011;151:630–636.
40 Malone PE, Herndon LW, Muir KW, et al. Combined fluocinolone acetonide intravitreal insertion and glaucoma drainage device placement for chronic uveitis and glaucoma. Am J Ophthalmol. 2010;149:800–806. e1
41 Whitcup SM, Chan CC, Buggage RR, et al. Improving the diagnostic yield of vitrectomy for intraocular lymphoma. Arch Ophthalmol. 2000;118:446.
42 Sonoda KH, Sakamoto T, Enaida H, et al. Residual vitreous cortex after surgical posterior vitreous separation visualized by intravitreous triamcinolone acetonide. Ophthalmology. 2004;111:226–230.
43 Radetzky S, Walter P, Fauser S, et al. Visual outcome of patients with macular edema after pars plana vitrectomy and indocyanine green-assisted peeling of the internal limiting membrane. Graefes Arch Clin Exp Ophthalmol. 2004;242:273–278.
44 Lee GH, Ahn JK, Park YG. Intravitreal triamcinolone reduces the morphologic changes of ciliary body after pars plana vitrectomy for retinal vascular diseases. Am J Ophthalmol. 2008;145:1037–1044.
45 Jonas JB, Hayler JK, Panda-Jonas S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative vitreoretinopathy. Br J Ophthalmol. 2000;84:1064–1067.
46 Ahn JK, Chung H, Yu HG. Vitrectomy for persistent panuveitis in Behçet’s disease. Ocul Immunol Inflamm. 2005;13:447–453.
47 Giuliari GP, Chang PY, Thakuria P, et al. Pars plana vitrectomy in the management of paediatric uveitis: the Massachusetts Eye Research and Surgery Institution experience. Eye. 2010;24:7–13.
48 Bovey EH, Herbort CP. Vitrectomy in the management of uveitis. Ocul Immunol Inflamm. 2000;8:285–291.
49 Tranos P, Scott R, Zambarakji H, et al. The effect of pars plana vitrectomy on cystoid macular oedema associated with chronic uveitis: a randomised, controlled pilot study. Br J Ophthalmol. 2006;90:1107–1110.
50 Quinones K, Choi JY, Yilmaz T, et al. Pars plana vitrectomy versus immunomodulatory therapy for intermediate uveitis: a prospective, randomized pilot study. Ocul Immunol Inflamm. 2010;18:411–417.