CHAPTER 62 Diabetic macular edema
This chapter discusses the pathogenesis and treatment of diabetic macular edema in terms of ocular and systemic factors (Boxes 62.1 and 62.2).
Pathophysiology of diabetic macular edema
Systemic factors
Macular edema manifests clinically as retinal thickening that results from chronic breakdown of the blood–retinal barrier. Extracellular fluid accumulates in the outer plexiform layer and extends into the inner retina with increasing severity. The pathophysiologic factors that govern the formation of edema in the macula are similar to those that contribute to edema elsewhere, such as in the brain. That is, tissue edema occurs when the ability of the blood vessels and the surrounding tissue to regulate leakage are overcome either by increased intralumenal hydrostatic pressure or increased extravascular (tissue) concentration of albumin. This relationship is classically described in Starling’s Law of the Capillary, which states that fluid filtration is related to the forces that drive fluid across the vessel wall (blood pressure, intravascular fluid volume, and interstitial fluid osmotic pressure) minus the forces that promote fluid reabsorption (plasma oncotic pressure). The importance of this concept has been related to macular edema in diabetes by Kristinsson and co-workers1. This physiologic principle is useful to guide investigations into macular edema and the evaluation and treatment of patients.
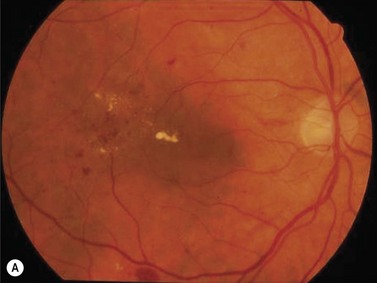
The primary systemic factor that contributes to disruption of the normal autoregulatory process of the retinal vasculature is systemic arterial hypertension. Epidemiologic studie have confirmed that hypertension aggravates existing retinopathy and increases the risk of developing retinopathy 2. This point is illustrated in Figure 62.1. The patient whose eye is illustrated is a 65-year-old woman with 10 years of type 2 diabetes, and her blood pressure was 150/105 mmHg. The eye has diffusely narrowed retinal arterioles, cotton wool spots, and hemorrhages. The macula is thickened, and lipid exudates threaten the fovea, and visual acuity was 20/40. The other eye had a similar appearance.
Patients with hyperlipidemia may also have an increased tendency for macular edema formation when serum lipoproteins accumulate in the retina and exert the osmotic influences that draw water out of retinal vessels and cause retinal thickening. Data from the Early Treatment Diabetic Retinopathy Study (ETDRS) showed a twofold increase in risk of macular edema in visual loss in patients with total serum cholesterol greater than 240 mg/dl3.
The issue of glycemic control as an isolated factor in the development of macular edema is difficult to determine because insulin affects numerous aspects of cellular metabolism. However, in the Diabetes Control and Complications Trial (DCCT), the risk of macular edema was approximately 50% greater in the group that received conventional treatment than in the group under intensive treatment. The overall risk of developing macular edema in the intensively treated patients in the secondary intervention group (those patients who had mild to moderate retinopathy at the beginning of the study) was 27%4.
Diabetic nephropathy is also associated with increased incidence and progression of retinopathy in general and macular edema in particular. This correlation of diabetic nephropathy and retinopathy is known as the diabetic ‘renal–retinal syndrome’. The risk of macular edema is greatest in patients with overt proteinuria5, and systolic hypertension and higher glycosylated hemoglobin are also important factors in the Wisconsin Epidemiologic Study of Diabetes6. Nephropathy may aggravate macular edema via hypertension, fluid overload, hypoproteinemia, hyperlipidemia, or increased difficulty with metabolic control. Diabetic macular edema is a risk factor for increased mortality from heart disease7.
Several reports have suggested that thiazolidinedione (glitazone) drugs that are used widely as effective agents for type 2 diabetes may increase the risk of diabetic macular edema8,9. Therefore, patients should be questioned about the use of these medications if they have a recent onset of DME associated with systemic edema but without cardiac or renal failure.
Ocular risk factors
The combination of cataract and diabetic retinopathy is a common and clinically challenging problem. Cataract extraction, even with an intact posterior capsule and a posterior chamber intraocular lens, frequently increases the severity of retinopathy and macular edema7. The reasons for this are unclear, but may relate to release of inflammatory mediators in the perioperative period. The risk of macular edema after cataract extraction increases with increasing severity of overall retinopathy, and with increasing age of the patients. In a series of 109 patients, Benson and co-workers10 found that only 48% of patients undergoing extracapsular cataract extraction achieved 20/40 or better final acuity, and 28% tested at 20/200 or less. Only 65% improved two or more lines of acuity. A more recent study11 showed that cataract surgery accelerates the overall progression of retinopathy after 1 year.
An additional risk factor for the development of macular edema is intensive panretinal photocoagulation12. Macular edema is particularly likely to be increased after panretinal photocoagulation if the treatment is applied in a single session or close to the macula. Avoidance of the macular region during laser surgery may reduce this risk, and this adverse event appears to be much less common now than when reported initially.
Contraction of the posterior hyaloid face of the vitreous can exert traction on the macula, and this traction may contribute to the formation of macular edema, even in eyes that have received focal laser treatment13.
Treatment of macular edema
The evaluation of both systemic and ocular factors must be included for optimal management of diabetic macular edema (See Box 62.2).
Systemic treatment
The primary factor to evaluate is metabolic control. As mentioned above, the risk of macular edema was significantly lower in the intensively treated group than in the conventional treatment group in the DCCT. However, no study has directly addressed the effects of improving metabolic control in patients with existing macular edema. In general, glycemic levels should be as low as can be achieved and tolerated by the patient without undue risk of hypoglycemia. A hemoglobin A1C value less than or equal to 6% is a desirable goal, even if difficult for many patients to attain. In patients who have poor metabolic control, it is generally advisable to improve the metabolic control gradually over 6–12 months, because rapid tightening of control can cause transient worsening of retinopathy, usually made manifest by increasing ‘cotton wool’ spots or intraretinal microvascular abnormalities; rarely, neovascularization develops. In the DCCT transient worsening occurred in 22% of the intensively treated group and 13% of the conventionally treated group14.
Blood pressure in persons with diabetes should be 130/80 mmHg or less15. Blood pressure control significantly lowers the risk of progression of incipient diabetic nephropathy to overt nephropathy, and is reasonable to apply in patients with retinopathy as well. The United Kingdom Prospective Diabetes Study (UKPDS) showed that blood pressure control reduced the risk of retinopathy progression by one-third16. Hypertension is also associated with increased risk of retinopathy, even in children and young adults with type 1 diabetes17, although there is currently no evidence that proves blood pressure reduction in patients with type 1 diabetes reduces the risk of retinopathy. However, treatment with the renin angiotensin system inhibitors losartan and enalapril reduces the risk of retinopathy (but not nephropathy) independently of blood pressure18.
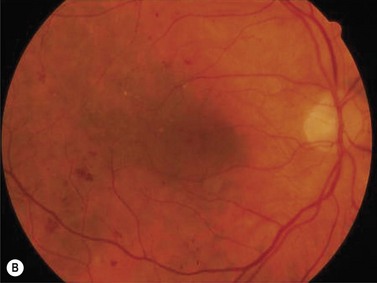
Serum lipids should also be evaluated and treated in accordance with guidelines for the reduction of stroke and myocardial infarction19. LDL cholesterol should be less than 70–100 mg/dl and non-HDL <100 mg/dl. The availability of improved medications for the control of hyperglycemia, hyperlipidemia, and hypertension over the last several years makes this possible. Identification and treatment of systemic risk factors can have substantial benefits in macular edema, even that which is clinically significant, as demonstrated in Figure 62.2. By identifying these systemic risk factors, the ophthalmologist has the opportunity to improve the visual prognosis, and the prognosis for life as well. To have this impact, the ophthalmologist must view diabetic macular edema as a systemic as well as ocular disease. This approach can facilitate collaboration with primary care physicians, diabetologists, and other specialists involved in the care of persons with diabetes.
Ocular treatment
The ETDRS12 established the role of macular photocoagulation for patients with ‘clinically significant macular edema’. Clinically significant macular edema is defined as ‘retinal thickening at or within 500 μm of the center of the macula, hard exudate at or within 500 μm of the center of the macula associated with retinal thickening, or retinal thickening one disc area or larger within one disc diameter of the center of the macula’. Focal laser surgery reduces the risk of moderate visual loss (doubling of the visual angle) by approximately 50%. The benefit of this treatment was independent of the initial visual acuity, from 20/20 to 20/200. Treatment involves direct ablation of microaneurysms with or without a ‘grid’ pattern to areas of retinal thickening. Focal treatment is more effective at reducing macular thickening than is grid treatment and is the preferred approach20. Foveal ischemia may coexist with macular edema and contribute to visual loss and, because it is associated with a worse visual prognosis, a preoperative fluorescein angiogram helps in the assessment of the foveal circulation. Patients with mild irregularity or enlargement of the foveal avascular zone may benefit from macular photocoagulation, but those with highly irregular avascular zones or greater than one disc diameter of foveal ischemia often will have less benefit, and their vision may be more likely to deteriorate after photocoagulation.
For focal treatment of diabetic macular edema, the spot size ranges from 50 to 100 μm in diameter, and the burns should cause light blanching of the microaneurysms or underlying pigment epithelium21. The power setting will necessarily be higher in patients with hazy media or light fundus pigmentation. The required power may also vary with the degree of retinal thickening. For example, a burn duration of 0.1 second usually allows an adequate burn and is short enough to prevent spread of the lesion if the patient moves the eye, but if the retina is thickening the laser energy is scattered, and higher power or longer duration exposures may be required. The wavelength of the laser light has not been shown to result in clinically important differences in results, despite theoretical considerations of absorption of blue-green light by macular xanthophyll pigment. The ophthalmologist should try to treat all of the detectable sites of leakage but avoid involvement of the foveal avascular zone. Two to three months may be required before it is possible to determine the response of an eye to macular photocoagulation, and if clinically significant thickening remains after 3–6 months, then additional treatment is probably indicated. A recent clinical trial comparing focal laser with intravitreal triamcinolone showed that the effects of focal laser on visual acuity and macular thickness continued to accrue over 2 years22.
In eyes that have coexisting proliferative diabetic retinopathy or severe non-proliferative retinopathy, panretinal photocoagulation may be applied concurrently. If the risk of immediate severe visual loss from vitreous hemorrhage appears low, then it is probably reasonable to apply macular photocoagulation first. Eyes that have vitreous hemorrhage or active neovascularization, however, may be treated with concurrent panretinal and macular photocoagulation. Panretinal photocoagulation improves retinal vascular autoregulation23 and macular edema in selected cases, particularly those with florid neovascularization24,25. Panretinal photocoagulation applied initially in the midperiphery, and then to the more posterior retina if needed, is associated with a lower risk of macular edema than panretinal photocoagulation applied to the entire retina simultaneously26.
Safe and effective pharmacotherapy for DME has been a goal for many years. The approaches have included drugs with pleiotropic actions on inflammation and vascular integrity such as corticosteroids, and targeted agents directed at single molecules. Steroids induce endothelial cell tight junction protein expression27,28 and reduce intravitreal inflammatory molecules29. Numerous case series suggested that intravitreal triamcinolone could improve diabetic macular edema, and one controlled clinical trial30 supported this conclusion. However, in the patients studied in the Diabetic Retinopathy Clinical Research Network trial, triamcinolone was inferior to focal photocoagulation22. Moreover, repeated intravitreal corticosteroid injections carry a very high risk of cataract (54%) and glaucoma (44%)30,31. Intravitreal dexamethasone implants have benefits after 6 months of treatment32 and longer-term results are pending.
Vascular endothelial growth factor (VEGF) is a potent endothelial cell permeabilizing agent that disrupts endothelial tight junctions33. Recent studies have demonstrated a moderate improvement of visual acuity in eyes treated with ranibizumab or bevacizumab34,35. As of this date (August, 2010) no benefit on DME has been shown to result from inhibiting inflammatory cytokines such as tumor necrosis factor.
1 Kristinsson JK, Gottfredsdottir MS, Stefansson E. Retinal vessel dilatation and elongation precedes diabetic macular oedema. Br J Ophthalmol. 1997;81:274-278.
2 Klein R, Klein BE, Moss SE, et al. Is blood pressure a predictor of the incidence or progression of diabetic retinopathy? Arch Intern Med. 1989;149:2427-2432.
3 Chew EY, Klein ML, Ferris FL3rd, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996;114:1079-1084.
4 Anonymous. Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Ophthalmology. 1995;102:647-661.
5 Klein R, Moss SE, Klein BE. Is gross proteinuria a risk factor for the incidence of proliferative diabetic retinopathy? Ophthalmology. 1993;100:1140-1146.
6 Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116:497-503.
7 Hirai FE, Knudtson MD, Klein BE, et al. Clinically significant macular edema and survival in type 1 and type 2 diabetes. Am J Ophthalmol. 2008;145:700-706.
8 Fong DS, Contreras R. Glitazone use associated with diabetic macular edema. Am J Ophthalmol. 2009;147:583-586. e581
9 Ryan EHJr, Han DP, Ramsay RC, et al. Diabetic macular edema associated with glitazone use. Retina. 2006;26:562-570.
10 Benson WE, Brown GC, Tasman W, et al. Extracapsular cataract extraction with placement of a posterior chamber lens in patients with diabetic retinopathy. Ophthalmology. 1993;100:730-738.
11 Hong T, Mitchell P, de Loryn T, et al. Development and progression of diabetic retinopathy 12 months after phacoemulsification cataract surgery. Ophthalmology. 2009;116:1510-1514.
12 Meyers SM. Macular edema after scatter laser photocoagulation for proliferative diabetic retinopathy. Am J Ophthalmol. 1980;90:210-216.
13 Ghazi NG, Ciralsky JB, Shah SM, et al. Optical coherence tomography findings in persistent diabetic macular edema: the vitreomacular interface. Am J Ophthalmol. 2007;144:747-754.
14 Anonymous. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
15 Anonymous. Standards of medical care in diabetes – 2009. Diabetes Care. 2009;32(Suppl 1):S13-S61.
16 Matthews DR, Stratton IM, Aldington SJ, et al. Risks of progression of retinopathy and vision loss related to diabetes mellitus: UKPDS 69. Arch Ophthalmol. 2004;122:1631-1640.
17 Gallego PH, Craig ME, Hing S, et al. Role of blood pressure in development of early retinopathy in adolescents with type 1 diabetes: prospective cohort study. BMJ. 2008;337:a918.
18 Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40-51.
19 Brunzell JD, Davidson M, Furberg C, et al. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811-822.
20 Fong DS, Strauber SF, Aiello LP, et al. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125:469-480.
21 Anonymous. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study Report no. 4. The Early Treatment Diabetic Retinopathy Study Research Group. International Ophthalmology Clinics. 1987;27:265-272.
22 Anonymous. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447-1449. 1449; e1441–510
23 Grunwald JE, Brucker AJ, Petrig BL, et al. Retinal blood flow regulation and the clinical response to panretinal photocoagulation in proliferative diabetic retinopathy. Ophthalmology. 1989;96:1518-1522.
24 Gardner TW, Eller AW, Friberg TR. Reduction of severe macular edema in eyes with poor vision after panretinal photocoagulation for proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 1991;229:323-328.
25 Gaucher D, Fortunato P, Lecleire-Collet A, et al. Spontaneous resolution of macular edema after panretinal photocoagulation in florid proliferative diabetic retinopathy. Retina. 2009;9:1282-1288.
26 Blankenship GW. A clinical comparison of central and peripheral argon laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1988;95:170-177.
27 Felinski EA, Cox AE, Phillips BE, et al. Glucocorticoids induce transactivation of tight junction genes occludin and claudin-5 in retinal endothelial cells via a novel cis-element. Exp Eye Res. 2008;86:867-878.
28 Erickson KK, Sundstrom JM, Antonetti DA. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis. 2007;10:103-117.
29 Brooks HLJr, Caballero SJr, Newell CK, et al. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004;122:1801-1807.
30 Gillies MC, Sutter FK, Simpson JM, et al. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533-1538.
31 Grover D, Li TJ, Chong CC. Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst Rev:CD005656, 2008.
32 Kuppermann BD, Blumenkranz MS, Haller JA, et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007;125:309-317.
33 Antonetti DA, Barber AJ, Khin S, et al. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Diabetes. 1998;47:1953-1959.
34 Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609-614.
35 Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117:1078-1086.