Diabetic Foot and Vascular Complications
Despite numerous medical and surgical advances over the past decade, the “diabetic foot” continues to plague patients and providers across the entire spectrum of health care. Foot problems are the most common reason for hospitalization of a diabetic patient, with 15% of the nearly 21 million diabetic patients in the United States developing a foot complication severe enough to require hospitalization during their lifetime.1 Because this small group of people with diabetes (only 7% of the U.S. population) accounts for more than 60% of all nontraumatic lower-extremity amputations,2 public health initiatives have focused on aggressive treatment of the diabetic foot to halt the escalating number of amputations. However, the annual financial costs related to infection, ulceration, and amputation have increased to more than $1.5 billion nationwide despite these widespread efforts.3 Overall, the costs to society are enormous, medically, socially, and economically, and further dissemination of information is imperative to curtail the escalating vascular complications resulting from diabetes mellitus.
Pathophysiology
The origin and physiology of diabetes mellitus were discussed in previous chapters, as were the major complications of retinopathy, neuropathy, and nephropathy. Similar mechanisms underlie complications stemming from the diabetic foot, including ulceration, gangrene, ischemia, and ultimately amputation. Although glycemic control is imperative in diabetes, it is not sufficient to eliminate unwanted complications of the diabetic foot. Furthermore, it is important for clinicians to be aware of the etiologic triad leading to diabetic foot complications (i.e., neuropathy, ischemia, and infection) and to recognize that these may occur in isolation but more frequently occur in combination with one another (Fig. 29-1).
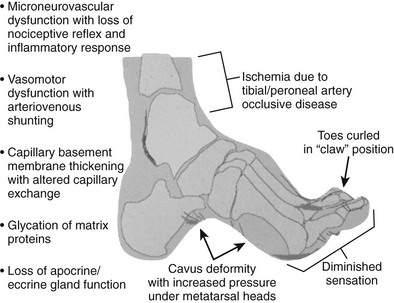
FIGURE 29-1 Pathophysiology of the diabetic foot leading to vascular complications. (Modified from LoGerfo FW: Bypass grafts to the dorsalis pedis artery. In Whittemore AD, Bandyk DF, Cronenwett JL, Hertzer NR, White RA [eds]: Advances in Vascular Surgery, vol 10. Philadelphia, Mosby, 2002, p 174.)
Neuropathy
The neuropathy stemming from diabetes mellitus has multiple manifestations within the diabetic foot because it encompasses sensory, motor, and autonomic fibers. Sensory neuropathy affects small-diameter pain and temperature fibers first, and susceptibility to injury is increased because these patients are less sensitive to pressure-related trauma and other minor skin injuries. Motor neuropathy affects the longer fibers that innervate the foot, including intrinsic foot muscles and leg muscles. Atrophy, or muscle wasting, in the intrinsic foot muscles allows the strong flexor muscles to draw up the toes in a “clawed” position, and new pressure points emerge at the tips of the toes and the prominent metatarsal heads. Limited joint mobility from glycation of scleral proteins exacerbates the situation by further changing the normal weight distribution on the foot. Last, autonomic neuropathy causes the skin to become dry through loss of sweat and oil gland function. This dry skin has a markedly increased susceptibility to skin breakdown and fissures, thus creating a portal of entry for bacteria. Additionally, diabetic patients experience a blunted neuroinflammatory response and thus are missing a crucial component of the body’s natural first-line defense against pathogens.4
Infection
Diabetic patients typically have an altered response to infectious processes owing to defects in their host immune defense system.5 Wound healing is delayed in diabetic patients as a result of abnormal cellular and inflammatory pathways involving fibroblasts, neutrophils, and advanced glycation end products (AGEs). Glycation is a nonenzymatic chemical reaction whereby sulfhydryl protein linkages are replaced by glucose, causing impairment in normal cellular and tissue functions.6 AGEs increase the stiffness of precapillary vessel walls and contribute to the development of diabetic microangiopathy.7 Furthermore, neuropathic and ischemic deficiencies in diabetic patients predispose them to infection and then unfortunately compound the problem by potentiating infection once a pathogen has been introduced.
Ischemia
Much progress has been made in identifying the cause of ischemia in the lower extremities of diabetic patients, and results have challenged long-standing misconceptions in the literature and in the medical community at large. It is imperative that practitioners continue to reject the “small-vessel disease” theory related to occlusions of the microcirculation, as espoused by a single histologic study in 1959,8 and instead embrace the notion that ischemia results from both atherosclerotic macrovascular disease and microcirculatory dysfunction.9 Diabetic patients typically suffer from tibial and peroneal arterial disease with sparing of the foot arteries, especially the dorsalis pedis and its branches (Fig. 29-2).
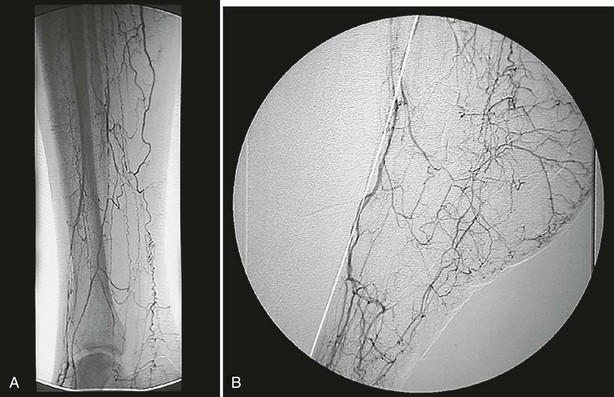
FIGURE 29-2 Intra-arterial digital subtraction arteriogram showing typical pattern of occlusive disease in a diabetic patient. A, Calf view: The posterior tibial, anterior tibial, and peroneal arteries are severely narrowed. Blood flow to the foot is entirely dependent on the small collateral vessels that are visible. B, Lateral foot view: The dorsalis pedis artery is widely patent with runoff into patent tarsal branches.
Research has shown that diabetes causes structural and functional changes within the arteriolar and capillary systems, notably thickening of the basement membrane. In spite of the thickened capillary basement membrane, no evidence reveals a decrease in capillary luminal diameter.10 A thickened membrane impairs the migration of leukocytes and hampers the normal hyperemic or vasodilatory response to injury, thus simultaneously increasing the susceptibility to injury while blunting the typical manifestations of such an injury.11 Overall, this dysfunction of the microcirculation in diabetic patients creates a functionally ischemic foot despite conditions that represent normal blood flow in healthy patients.
Besides causing specific structural changes in the microcirculation, diabetes also causes a compromise in the overall biology of the foot. When compared with nondiabetic individuals with normal biology, diabetes causes an undesirable shift in the natural balance that exists between stress/ulceration and resistance to stress/ulceration. The diabetic foot thus is more prone to ulcerate under the stress of daily life. Additionally, the presence of neuropathy usually mandates revascularization under conditions of perfusion that would not require revascularization in the absence of neuropathy. In other words, the pathophysiology underlying diabetes creates situations whereby the compromised foot requires even more perfusion than usual to resist ulceration or respond appropriately to injury (Fig. 29-3).
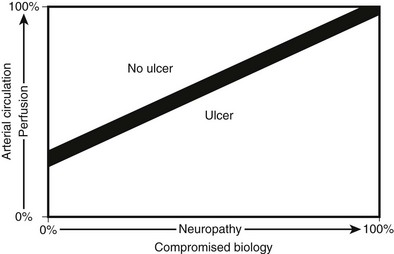
FIGURE 29-3 The relationship between ulceration, compromised biology, and arterial circulation. As perfusion decreases, even a foot with perfect biology will ulcerate. As neuropathy increases, even a well-perfused foot will ulcerate. With revascularization, the improvement in perfusion will allow healing of ulcers in diabetic patients with compromised biology.
Presentation and Diagnosis
Ulcers
The lifetime risk for acquiring foot ulcers has been estimated at 15% in diabetic patients, with an incidence of approximately 1.9% per year. Additionally, in more than 15% of diabetic patients, ulcers ultimately will lead to amputation.12 According to an American Diabetes Association consensus statement, the risk for foot ulcers is increased in diabetic patients who have had the disease for longer than 10 years, are male, have poor glycemic control, and already have other complications (cardiovascular, renal, or retinal).13 Many ulcers stem from the altered pressures created in the diabetic foot. These foot pressures are influenced by muscle atrophy, obesity, callous formation, other forms of local trauma (including improper footwear), and limited joint mobility. The presence of neuropathy, as was discussed previously, increases the risk for ulcer formation because diabetic patients might not be aware of the damage they are inflicting on their feet through their normal activities of daily living. Furthermore, impaired wound healing and blunted neuroinflammatory responses contribute to progression of the initial ulcer to a potentially more serious vascular complication.
Infection/Osteomyelitis
As was discussed earlier, diabetic patients have a blunted neuroinflammatory response and thus do not display the typical physiologic reactions to infection. In fact, the usual manifestations of infection (e.g., fever, tachycardia, elevated white blood cell count) are frequently absent in diabetic patients; therefore, these patients require extra vigilance so that providers do not overlook life-threatening conditions. Unexplained hyperglycemia should prompt an aggressive search for an infectious source in diabetic patients, because the elevated glucose might be the only sign of impending problems.14
Osteomyelitis occurs after the spread of superficial infection of the soft tissue to adjacent bone or marrow. Although numerous expensive radiologic techniques are currently available to assist clinicians, a simple metal probe usually will suffice. Grayson and coworkers revealed that if this sterile probe hits bone, then osteomyelitis can be diagnosed with a sensitivity of 66%, a specificity of 85%, and a positive predictive value of 89%.15 Plain radiographs should be obtained to determine the extent of osseous erosion, as well as to assess anatomy for surgical planning. Further scanning with magnetic resonance imaging, bone scan, or tagged white blood cell scan should be reserved for cases in which the metal probe test is equivocal, when an abscess or multifocal disease is suspected, or in patients with neuropathic osteoarthropathy, or Charcot’s foot, in whom associated bony changes and inflammatory response can be misinterpreted as osteomyelitis. The diagnosis of osteomyelitis in a foot with no skin lesions should be viewed with skepticism. Resolution of any swelling and erythema after 24 hours of bed rest without antibiotics usually will establish the diagnosis of Charcot’s osteoarthropathy and will rule out osteomyelitis. The conclusive diagnosis of osteomyelitis can be obtained by bone biopsy, but this should rarely be necessary under most circumstances.
Peripheral Vascular Disease
The diagnosis of peripheral vascular disease relies first on symptoms and physical examination and can be aided by noninvasive testing, imaging such as magnetic resonance angiography (MRA) or computed tomography (CT) angiography, and invasive contrast arteriography. Any patient without foot pulses should be considered to have arterial occlusive disease. The noninvasive vascular laboratory can be a useful adjunct for patients who have symptoms of ischemia but no obvious signs of arterial insufficiency.16 However, the ankle-brachial index can be misleading in diabetic patients because of calcification in the arterial media (Monckeberg’s sclerosis), which makes vessels difficult to compress with a blood pressure cuff. Pulse volume recordings are useful in patients with diabetes because this noninvasive test is not affected by vessel calcification; other possible modalities that are used less frequently include toe pressures and transcutaneous oxygen measurements.
On the contrary, noninvasive testing adds little to the evaluation of patients who present with obvious symptoms and signs of foot ischemia coupled with nonpalpable pulses. At this point, a vascular surgeon should be consulted, and contrast arteriography performed. The preferred technique is intra-arterial digital subtraction arteriography because it is extremely accurate for smaller vessels of the ankle and foot, even when occlusion of the tibial or peroneal arteries is present. Patients with mild to moderate renal insufficiency (glomerular filtration rate [GFR] <60 mL/min/1.73 m2) receive preprocedure and postprocedure hydration with a sodium bicarbonate intravenous fluid; those with moderate to severe renal insufficiency (GFR < 35 mL/min/1.73 m2) follow the same hydration protocol, in addition to taking an oral acetylcysteine regimen. Iso-osmolar iodixanol (Visipaque) contrast is used for all patients with GFR <60 mL/min/1.73 m2 because of its decreased likelihood of causing contrast-induced nephropathy in these patients.17 Although MRA had been used more frequently during the past decade to plan arterial reconstructions in patients with more marginal renal function,18 recent reports about nephrogenic systemic fibrosis19 have shifted clinical practice back to conventional arteriography. Moreover, we have found that conventional digital subtraction angiography (DSA) continues to provide the best quality images, and the risk for contrast-induced nephropathy can be minimized by adhering to a strict protocol as listed above.
Medical Management
As is recommended in a consensus statement regarding diabetic foot wound care, the desired outcomes besides healing foot ulcers and decreasing complications should include the following: control of infection, prevention of amputation, maintenance of health status, improved function and quality of life, and reduced costs.20 Numerous modalities are appropriate for achieving these goals, and all health care providers who treat diabetic patients should be familiar with the available options.
Prevention
Primary prevention should be the first tenet of any practitioner’s approach to the diabetic foot, but secondary prevention with meticulous ulcer care may be a more realistic goal.21 Specifically, prevention involves aggressive glycemic control; management of associated risk factors (such as smoking, hypertension, hyperlipidemia, and obesity); periodic physical examination, including a vascular examination; and, probably most important, proper foot care and hygiene strategies. The importance of daily foot inspections should be emphasized to patients and their families, with careful attention paid to calluses, fissures, red or bruised areas, and open sores or blisters. Moisturizing creams should be used on dry areas of skin, and antifungal medication should be used as needed. Astringents and heat soaks should be avoided, as should patients’ self-removal of eschar or other foot lesions. Toenails should be trimmed to prevent penetration into adjacent toes.
Determining which patients are at risk for foot infection due to both neuropathy and ischemia is an important aspect of prevention. Use of Semmes-Weinstein monofilament testing for sensory neuropathy and periodic vascular assessments (with or without the noninvasive vascular laboratory) are useful adjuncts in preventing foot complications.22 The minor costs incurred by these preventative strategies would be worth the initial investment because they prevent the major costs of future foot complications.12
Antibiotics
When infection is present in a diabetic foot ulcer, cultures should be taken, and then antibiotic therapy should be initiated. Typical organisms in superficial infection include Staphylococcus aureus, Streptococcus, and occasionally gram-negative bacilli. Deeper infections are usually polymicrobial with greater than three bacterial isolates per ulcer, including the above bacteria as well as aerobes (facultative) and anaerobes.23 The initial broad-spectrum antibiotic regimen should adequately cover the most likely offending organisms and can be narrowed once gram stain or culture/sensitivity results have been reported. Other factors to bear in mind include local bacterial resistance patterns in a specific hospital or community, individual patient characteristics (such as comorbidities and allergies), and, most important, the severity of infection. The antibiotic protocol in our tertiary care center has undergone many changes over the past 20 years. Currently, a combination of vancomycin, ciprofloxacin, and metronidazole is our empirical “first-line” choice for severe infection. Given the increasing prevalence of methicillin-resistant S. aureus (MRSA) in hospital-acquired infections, as well as in community isolates, empirical therapy with vancomycin is warranted.24
Traditional therapy for osteomyelitis was accepted as 4 to 6 weeks of intravenous antibiotics,25 but recent studies have documented a greater than 30% recurrence rate using this modality alone.26 As a result, our standard practice involves surgical debridement of infected bone with an adequate margin, followed by a shorter-duration antibiotic course. We believe that an aggressive surgical approach to osteomyelitis shortens healing time, decreases the need for long-term antibiotic therapy, limits the emergence of resistant bacteria, and reduces both inpatient and outpatient economic costs. In our experience, long-term antibiotic therapy without excision of infected bone is rarely successful in these patients.
Wound Care/Debridement
Numerous other modalities exist for wound care, such as topical growth factors, synthetic skin grafts, electrical stimulation, hyperbaric oxygen chambers, and vacuum-assisted closure. Each has its own merits, but economic constraints and patient compliance should be kept in mind in comparing these with the well-established modality of simple moist dressings. A recent randomized trial revealed the efficacy of the V.A.C. (negative-pressure wound therapy) compared with standard moist gauze dressings. In diabetic patients with partial foot amputations and adequate perfusion, V.A.C. therapy resulted in a higher proportion of healed wounds, faster healing rates, and potentially fewer re-amputations than standard care.27 Overall, health care providers must always remember that any new wound care modality is simply an adjunct to frequent clinical examinations and local wound care.
Surgical Management
Drainage Procedures
When minor debridement strategies do not effectively relieve severe infection, then it is imperative to perform more extensive drainage procedures (including partial open toe, ray, or forefoot amputation) to drain abscesses or remove necrotic tissue.28 In fact, hidden infection should be suspected in any situation in which a well-perfused foot continues to experience necrosis. Adequate drainage should be obtained, even if foot function has to be compromised initially, because function potentially can be restored later with numerous advanced wound closure techniques. Additionally, if ischemia appears to be contributing to the infection, then arteriography should be performed to determine whether perfusion is adequate, or if revascularization is necessary. Controlling active, spreading infection may delay revascularization by a few days, but longer waiting periods taken in the hope of completely sterilizing wounds are inappropriate and may result in continued necrosis or tissue loss.29 In some cases, inadequate blood flow may prevent the delivery of antibiotics, nutrients, or oxygen to the foot wound, thus requiring revascularization to correct the problem. Furthermore, bypass procedures can be performed safely in the presence of foot infection as long as sepsis has been controlled prior to surgery.30
Lower Extremity Arterial Reconstruction
Despite many preconceived notions that practitioners have about diabetic patients, research has shown that these patients tolerate revascularization extremely well and do not suffer from increased mortality or from diminished graft patency.31 The ultimate goal of any revascularization procedure should be to restore adequate perfusion distal to an occlusion to relieve symptoms and promote healing. The outflow target artery, or the site of the distal anastomosis, should be free of occlusive disease and should be in direct continuity with the arteries of the foot. Through the use of arteriography, including images of the foot arteries, appropriate inflow and outflow vessels can be identified for revascularization. Next, the surgeon needs to choose the appropriate conduit for the arterial reconstruction, which in most procedures in patients with pedal ischemia, should be autologous vein because of superior patency rates compared with prosthetic grafts.32 The decision to use saphenous vein in the reversed, in situ, or nonreversed position, as well as to use contralateral saphenous vein or arm vein when necessary, depends on the specific clinical circumstance and patient anatomy.
Occasionally, a diabetic patient may require reconstruction of the aortoiliac segment or will have disease limited to the superficial femoral artery above the knee; prosthetic grafts are well suited to achieve this goal. However, because diabetic patients more typically suffer from tibial and peroneal disease, the most effective bypass often involves restoring blood flow to the dorsalis pedis artery or the posterior tibial artery using an autologous vein as the conduit.31 In fact, numerous reports in diabetic patients have shown the durability and success of distal origin grafts that obtain inflow from the popliteal artery and bypass over a shorter length to the arteries in the foot.33,34 Although distal bypasses are technically challenging, success is attainable, especially when the surgeon maintains a flexible approach to preparation and placement of the vein graft.35
In one of the most comprehensive studies to date regarding dorsalis pedis revascularization in diabetic patients, Pomposelli and coworkers reported results from more than 1000 bypasses spanning a decade.36 Primary patency, secondary patency, and limb salvage rates were 56.8%, 62.7%, and 78.2%, respectively, at 5 years, and 37.7%, 41.7%, and 57.7%, respectively, at 10 years. Patient survival was 48.6% and 23.8% at 5 and 10 years, respectively, and perioperative mortality was only 0.9%. The popliteal artery was the source of inflow in 53.2% of patients. Overall, pedal arterial reconstruction has proved to be a safe and durable revascularization alternative in diabetic patients and should be utilized liberally to avoid ischemic diabetic foot complications.
Endovascular Procedures
Although surgical reconstruction is the current gold standard for diabetic foot revascularization, an avalanche of technology has allowed endovascular interventions to become integrated into the management strategies employed by vascular surgeons and interventionalists. Research has definitively shown that balloon angioplasty and stenting are very well suited to focal, short segment iliac stenoses or occlusions, which exist in 10% to 20% of patients with diabetes.37 Percutaneous intervention can be performed easily in the same setting as the preoperative diagnostic arteriogram.
With regard to outflow bypass procedures, the morbidity of open surgery can be quite significant and is not limited simply to local wound complications or myocardial infarctions. Readmission to the hospital, reoperation, slow time to healing, and time spent in rehabilitation must be factored into the risk-benefit analysis.38 Nicoloff et al.39 determined that the so-called “ideal outcome” (patent graft, healed wound, no additional operations in a fully ambulatory patient who can sustain independent living) was attainable only 14% to 22% of the time. When the potential pitfalls accompanying traditional surgical approaches to limb salvage are factored in, as well as the overall poor health and life expectancy of patients with peripheral vascular disease, less invasive endovascular therapy can represent an attractive alternative.
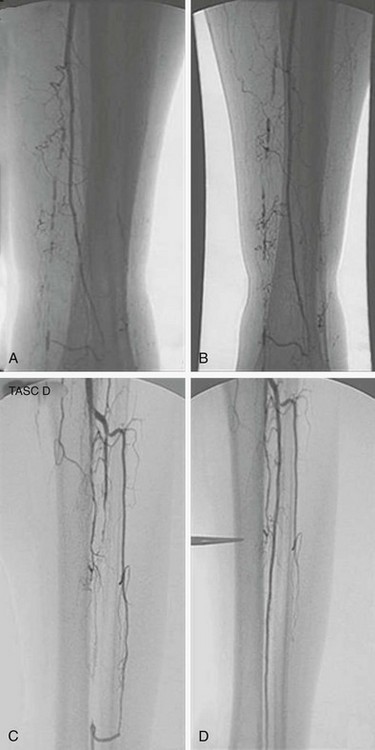
Unfortunately, the literature supporting endovascular interventions consists mainly of individual case series and retrospective reviews and does not compare with the research describing traditional open bypasses. The Transatlantic Inter-Society Consensus Working Group (TASC) initially stratified femoropopliteal and tibial lesions in 200037 and made recommendations for therapy based on lesion type (stenosis vs. occlusion), location, and length. Fig. 29-4 shows examples of typical tibial-peroneal lesions in diabetic patients and their results after balloon angioplasty. The best and most recent scientific attempt to compare open and endovascular interventions was the BASIL trial (bypass versus angioplasty in severe ischemia of the leg).40 Although only 42% of the patients in this trial were known to have diabetes, the level of ischemia was comparable with the typical disease patterns seen in this patient population. Overall, the trialists concluded that although the strategies are roughly equivalent at medium-term follow-up with regard to mortality and amputation-free survival, angioplasty should be used first for patients with significant comorbidities and with a life expectancy of less than 1 to 2 years. Longer follow-up from this trial will continue to provide statistically guided information in the years to come.
References
1. Gibbons, GW, Eliopoulos, GM. Infection of the diabetic foot. In: Kozak GP, Campbell DR, Frykberg RG, Habershaw GM, eds. Management of Diabetic Foot Problems. 2d ed. Philadelphia: WB Saunders; 1995:121–129.
2. National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics fact sheet: general information and national estimates on diabetes in the United States. http://diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm, 2005.
3. Harrington, C, Zagari, MJ, Corea, J, et al. A cost analysis of diabetic lower-extremity ulcers. Diabetes Care. 2000;23:1333–1338.
4. Parkhouse, N, Le Quesne, PM. Impaired neurogenic vascular response in patients with diabetes and neuropathic foot lesions. N Engl J Med. 1988;318:1306–1309.
5. Delamaire, M, Maugendre, D, Moreno, M, et al. Impaired leukocyte function in diabetic patients. Diabet Med. 1997;14:29–34.
6. Yan, SF, Ramasamy, R, Naka, Y, et al. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93:1159–1169.
7. Mullarkey, CJ, Brownlee, M. Biochemical basis of microvascular disease. In: Pickup JC, Williams G, eds. Chronic Complications of Diabetes. Oxford, UK: Blackwell Scientific; 1994:20–29.
8. Goldenberg, S, Alex, M, Joshi, RA, et al. Nonatheromatous peripheral vascular disease of the lower extremity in diabetes mellitus. Diabetes. 1959;8:261–273.
9. LoGerfo, FW, Coffman, JD. Vascular and microvascular disease of the foot in diabetes: implications for foot care. N Engl J Med. 1984;311:1615–1619.
10. Leinonen, H, Matikainen, E, Juntunen, J. Permeability and morphology of skeletal muscle capillaries in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982;22:158–162.
11. Rayman, G, Williams, SA, Spencer, PD, et al. Impaired microvascular hyperaemic response to minor skin trauma in type I diabetes. Br Med J. 1986;292:1295–1298.
12. Ramsey, SD, Newton, K, Blough, D, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382–387.
13. Mayfield, JA, Reiber, GE, Sanders, LJ, et al. Preventive foot care in diabetes. Diabetes Care. 2004;27:S63–S64.
14. Frykberg, RG. An evidence-based approach to diabetic foot infections. Am J Surg. 2003;186:44S–54S.
15. Grayson, ML, Gibbons, GW, Balogh, K, et al. Probing to bone in infected pedal ulcers: a clinical sign of underlying osteomyelitis in diabetic patients. JAMA. 1995;273:721–723.
16. Gahtan, V. The noninvasive vascular laboratory. Surg Clin North Am. 1998;78:507–518.
17. Jo, SH, Youn, TJ, Koo, BK, et al. Renal toxicity evaluation and comparison between Visipaque (iodixanol) and Hexabrix (ioxaglate) in patients with renal insufficiency undergoing coronary angiography: the RECOVER study: a randomized controlled trial. J Am Coll Cardiol. 2006;48(5):924–930.
18. Carpenter, JP, Baum, RA, Holland, GA, et al. Peripheral vascular surgery with magnetic resonance angiography as the sole preoperative imaging modality. J Vasc Surg. 1994;20(6):861–869.
19. Shabana, WM, Cohan, RH, Ellis, JH, et al. Nephrogenic systemic fibrosis: a report of 29 cases. AJR Am J Roentgenol. 2008;190(3):736–741.
20. American Diabetes Association. Consensus development conference on diabetic foot wound care. Diabetes Care. 1999;22:1354–1360.
21. Jeffcoate, WJ, Harding, KG. Diabetic foot ulcers. Lancet. 2003;361:1545–1551.
22. Singh, N, Armstrong, DG, Lipsky, BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228.
23. Grayson, ML. Diabetic foot infections: antimicrobial therapy. Infect Dis Clin North Am. 1995;9:143–161.
24. Lipsky, BA. Empirical therapy for diabetic foot infections: are there clinical clues to guide antibiotic selection? Clin Microbiol Infect. 2007;13(4):351–353.
25. Bamberger, DM, Daus, GP, Gerding, DN. Osteomyelitis in the feet of diabetic patients: long-term results, prognostic factors, and the role of antimicrobial and surgical therapy. Am J Med. 1987;83:653–660.
26. Tice, AD, Hoaglund, PA, Shoultz, DA. Outcomes of osteomyelitis among patients treated with outpatient parenteral antimicrobial therapy. Am J Med. 2003;114:723–728.
27. Armstrong, DG, Lavery, LA, Diabetic Foot Study Consortium. negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366(9498):1704–1710.
28. Gibbons, GW. The diabetic foot: amputations and drainage of infection. J Vasc Surg. 1987;5:791–793.
29. Pomposelli, FB, Campbell, DR. Lower extremity arterial reconstruction in patients with diabetes mellitus: Principles of treatment. In: Veves A, Giurini JM, LoGerfo FW, eds. The Diabetic Foot: Medical and Surgical Management. Totowa, NJ: Humana Press; 2002:411–428.
30. Tannenbaum, GA, Pomposelli, FB, Marcaccio, EJ, et al. Safety of vein bypass grafting to the dorsal pedal artery in diabetic patients with foot infections. J Vasc Surg. 1992;15:982–990.
31. Pomposelli, FB, Marcaccio, EJ, Gibbons, GW, et al. Dorsalis pedis arterial bypass: durable limb salvage for foot ischemia in patients with diabetes mellitus. J Vasc Surg. 1995;21:375–384.
32. Veith, FJ, Gupta, SK, Ascer, E, et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986;3:104–114.
33. Stonebridge, PA, Tsoukas, AI, Pomposelli, FB, et al. Popliteal-to-distal bypass grafts for limb salvage in diabetics. Eur J Vasc Surg. 1991;5:265–269.
34. Reed, AB, Conte, MS, Belkin, M, et al. Usefulness of autogenous bypass grafts originating distal to the groin. J Vasc Surg. 2002;35:48–55.
35. Pomposelli, FB, Jepsen, SJ, Gibbons, GW, et al. A flexible approach to infrapopliteal vein grafts in patients with diabetes mellitus. Arch Surg. 1991;126:724–729.
36. Pomposelli, FB, Kansal, N, Hamdan, AD, et al. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J Vasc Surg. 2003;37:307–315.
37. Dormandy, JA, Rutherford, RB. Management of peripheral arterial disease (PAD): TASC Working Group. J Vasc Surg. 2000;31:S1–S296.
38. Goshima, KR, Mills, JL, Hughes, JD. A new look at outcomes after infrainguinal bypass surgery: traditional reporting standards systematically underestimate the expenditure of effort required to attain limb salvage. J Vasc Surg. 2004;39:330–335.
39. Nicoloff, AD, Taylor, LM, McLafferty, RB, et al. Patient recovery after infrainguinal bypass grafting for limb salvage. J Vasc Surg. 1998;27:256–263.
40. Adam, DJ, Beard, JD, Cleveland, T, et al. BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366(9501):1925–1934.