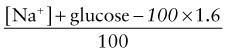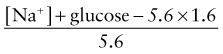Chapter 583 Diabetes Mellitus
583.1 Introduction and Classification
DM is not a single entity but rather a heterogeneous group of disorders in which there are distinct genetic patterns as well as other etiologic and pathophysiologic mechanisms that lead to impairment of glucose tolerance. A classification of diabetes and other categories of glucose intolerance is presented in Web Table 583-1. Three major forms of diabetes and several forms of carbohydrate intolerance are identified.
Web Table 583-1 ETIOLOGIC CLASSIFICATIONS OF DIABETES MELLITUS
* Patients with any form of diabetes may require insulin treatment at some stage of the disease. Such use of insulin does not, of itself, classify the patient.
Type 2 Diabetes Mellitus
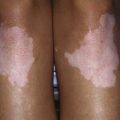
The presentation of T2DM is typically more insidious than that with T1DM. In contrast to patients with T1DM who are usually ill at the time of diagnosis, children with T2DM often seek medical care because of excessive weight gain and fatigue as a result of insulin resistance and/or an incidental finding of glycosuria during routine physical examination. A history of polyuria and polydipsia is relatively uncommon in these patients. The incidence of T2DM in children has increased by more than 10-fold in many diabetes centers, in part as a result of the epidemic of childhood obesity (Chapter 44). Pediatric T2DM may account for as many as 30% of the new cases of diabetes, especially in obese African and Mexican-American adolescents. Acanthosis nigricans (dark pigmentation of skin creases/flexural areas), a sign of insulin resistance, is present in the majority of patients with T2DM and is accompanied by a relative hyperinsulinemia at the time of the diagnosis (Chapter 644). However, the serum insulin elevation is usually disproportionately lower than that of age-, weight-, and sex-matched nondiabetic children and adolescents, suggesting a state of insulin insufficiency. In some individuals, it may represent slowly evolving T1DM.
Other Specific Types of Secondary Diabetes
Web Table 583-2 details the current criteria for the diagnosis of DM. It should be noted that a fasting blood glucose that exceeds 125 mg/dL (6.9 mmol/L) is the accepted criterion for the diagnosis of diabetes.
Web Table 583-2 DIAGNOSTIC CRITERIA FOR IMPAIRED GLUCOSE TOLERANCE AND DIABETES MELLITUS
| IMPAIRED GLUCOSE TOLERANCE (IGT) | DIABETES MELLITUS (DM) |
|---|---|
| Fasting glucose 100-125 mg/dL (5.6-7.0 mmol/L) | Symptoms* of DM plus random plasma glucose ≥200 mg/dL (11.1 mmol/L) |
| or | |
| 2-hr plasma glucose during the OGTT ≥140 mg/dL, but <200 mg/dL 11.1 mmol/L) |
Fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) or 2-hr plasma glucose during the OGTT ≥200 mg/dL |
OGTT, oral glucose tolerance test.
* Symptoms include polyuria, polydipsia, and unexplained weight loss with glucosuria and ketonuria.
From Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, Diabetes Care 20(Suppl 1):S5, 1999.
Impaired Glucose Tolerance
In the absence of pregnancy, IGT is not a clinical entity but rather a risk factor for future diabetes and cardiovascular disease. This may be observed as an intermediate stage in any of the disease processes listed in Web Table 583-1. IGT is often associated with the insulin resistance syndrome (also known as syndrome X or metabolic syndrome), which consists of insulin resistance, compensatory hyperinsulinemia to maintain glucose homeostasis, obesity (especially abdominal or visceral obesity), dyslipidemia of the high-triglyceride or low- or high-density lipoprotein type, or both, and hypertension (Chapter 44). Insulin resistance is directly involved in the pathogenesis of T2DM. IGT appears as a risk marker for this type of diabetes at least in part because of its correlation with insulin resistance. The diagnostic criteria for IGT are presented in Web Table 583-2.
583.2 Type 1 Diabetes Mellitus (Immune Mediated)
Epidemiology
T1DM accounts for about 10% of all diabetes, affecting 1.4 million in the USA and over 15 million in the world. While it accounts for most cases of diabetes in childhood, it is not limited to this age group; new cases continue to occur in adult life and approximately 50% of individuals with T1DM present as adults. The incidence of T1DM is highly variable among different ethnic groups. The overall age-adjusted incidence of type 1 DM varies from 0.7/100,000 per year in Karachi (Pakistan) to over 40/100,000 per year in Finland (Fig. 583-1). This represents a more than 400-fold variation in the incidence among 100 populations. The incidence of T1DM is increasing in most (but not all) populations and this increase appears to be most marked in populations where the incidence of autoimmune diseases was historically low. Data from Western European diabetes centers suggest that the annual rate of increase in T1DM incidence is 2-5%, whereas some central and eastern European countries demonstrate an even more rapid increase. The rate of increase is greatest among the youngest children. In the USA, the overall prevalence of diabetes among school-aged children is about 1.9/1,000, increasing from a prevalence of 1/1,430 children at 5 yr of age to 1/360 children at 16 yr. Among African Americans, the occurrence of T1DM is 30-60% of that seen in American whites. The annual incidence of new cases in the USA is about 14.9/100,000 of the child population. It is estimated that 30,000 new cases occur each year in the USA, affecting 1 in 300 children and as many as 1 in 100 adults during the lifespan. Rates are similar or higher in most Western European countries and significantly lower in Asia and Africa. But while incidence rates are much higher in European populations, the absolute number of new cases is almost equal in Asia and Europe because the population base is so much larger in Asia. Thus it is estimated that of the 400,000 total new cases of type 1 diabetes occurring annually in all children under age 14 yr in the world, about half are in Asia even though the incidence rates in that continent are much lower, because the total number of children in Asia is larger.
Genetics
MHC/HLA Encoded Susceptibility to Type 1 Diabetes Mellitus
The MHC is a large genomic region that contains a number of genes related to immune system function in humans. These genes are further divided into HLA class I, II, III, and IV genes. Class II genes are the ones most strongly associated with risk of T1DM, but as genetic studies become more detailed it is becoming apparent that some of the risk associated with various HLA types is due to variation in genes in HLA classes other than class II. Overall, genetic variation in the HLA region can explain 40-50% of the genetic risk of T1DM (Fig 583-2).
Environmental Factors
Pathogenesis and Natural History of Type 1 Diabetes Mellitus
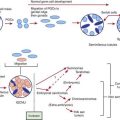
In type 1A diabetes mellitus, a genetically susceptible host develops autoimmunity against his or her own β cells. What triggers this autoimmune response remains unclear at this time. In some (but not all) patients, this autoimmune process results in progressive destruction of β cells until a critical mass of β cells is lost and insulin deficiency develops. Insulin deficiency in turn leads to the onset of clinical signs and symptoms of T1DM. At the time of diagnosis, some viable β cells are still present and these may produce enough insulin to lead to a partial remission of the disease (honeymoon period) but over time, almost all β cells are destroyed and the patient becomes totally dependent on exogenous insulin for survival (Fig. 583-3). Over time, some of these patients develop secondary complications of diabetes that appear to be related to how well-controlled the diabetes has been. Thus, the natural history of T1DM involves some or all of the following stages:
Initiation of Autoimmunity
Genetic susceptibility to T1DM is determined by several genes (see section on genetics), with the largest contribution coming from variants in the HLA system. But it is important to keep in mind that even with the highest risk haplotypes, most carriers will NOT develop T1DM. Even in monozygotic twins, the concordance is 30-65%. A number of factors including prenatal influences, diet in infancy, viral infections, lack of exposure to certain infections, even psychologic stress, have been implicated in the pathogenesis of T1DM, but their exact role and the mechanism by which they trigger or aggravate autoimmunity remains uncertain (Fig. 583-4). What is clear is that markers of autoimmunity are much more prevalent than clinical T1DM, indicating that initiation of autoimmunity is a necessary but not a sufficient condition for T1DM. Whatever the triggering factor, it seems that in most cases of T1DM that are diagnosed in childhood, the onset of autoimmunity occurs very early in life. In a majority of the children diagnosed before age 10 yr, the 1st signs of autoimmunity appear before age 2 yr. Development of autoimmunity is associated with the appearance of several autoantibodies. Insulin associated antibodies (IAA) are usually the 1st to appear in young children, followed by glutamic acid decarboxylase 65 kd (GAD65) and tyrosine phosphatase insulinoma-associated 2 (IA-2) antibodies. The earliest antibodies are predominantly of the IgG1 subclass. Not only is there “spreading” of autoimmunity to more antigens (IAA, and then GAD 65 and IA-2) but there is also epitope spreading within 1 antigen. Initial GAD65 antibodies tend to be against the middle region or the carboxyl-terminal region, while amino-terminal antibodies usually appear later and are less common in children.
Preclinical Autoimmunity with Progressive Loss of β-Cell Function
In some, but not all patients, the appearance of autoimmunity is followed by progressive destruction of β cells. Antibodies are a marker for the presence of autoimmunity, but the actual damage to the β cells is primarily T-cell mediated (Fig. 583-5). Histologic analysis of the pancreas from patients with recent-onset T1DM reveals insulitis, with an infiltration of the islets of Langerhans by mononuclear cells, including T and B lymphocytes, monocytes/macrophages, and natural killer (NK) cells. In the NOD mouse, a similar cellular infiltrate is followed by linear loss of β cells until they completely disappear. But it appears that the process in human T1DM is not necessarily linear and there may be an undulating downhill course in the development of T1DM.
Pathophysiology
Insulin performs a critical role in the storage and retrieval of cellular fuel. Its secretion in response to feeding is exquisitely modulated by the interplay of neural, hormonal, and substrate-related mechanisms to permit controlled disposition of ingested foodstuff as energy for immediate or future use. Insulin levels must be lowered to then mobilize stored energy during the fasted state. Thus, in normal metabolism, there are regular swings between the postprandial, high-insulin anabolic state and the fasted, low-insulin catabolic state that affect liver, muscle, and adipose tissue (Table 583-1). T1DM is a progressive low-insulin catabolic state in which feeding does not reverse but rather exaggerates these catabolic processes. With moderate insulinopenia, glucose utilization by muscle and fat decreases and postprandial hyperglycemia appears. At even lower insulin levels, the liver produces excessive glucose via glycogenolysis and gluconeogenesis, and fasting hyperglycemia begins. Hyperglycemia produces an osmotic diuresis (glycosuria) when the renal threshold is exceeded (180 mg/dL; 10 mmol/L). The resulting loss of calories and electrolytes, as well as the persistent dehydration, produce a physiologic stress with hypersecretion of stress hormones (epinephrine, cortisol, growth hormone, and glucagon). These hormones, in turn, contribute to the metabolic decompensation by further impairing insulin secretion (epinephrine), by antagonizing its action (epinephrine, cortisol, growth hormone), and by promoting glycogenolysis, gluconeogenesis, lipolysis, and ketogenesis (glucagon, epinephrine, growth hormone, and cortisol) while decreasing glucose utilization and glucose clearance (epinephrine, growth hormone, cortisol).
TABLE 583-1 INFLUENCE OF FEEDING (HIGH INSULIN) OR OF FASTING (LOW INSULIN) ON SOME METABOLIC PROCESSES IN LIVER, MUSCLE, AND ADIPOSE TISSUE*
| HIGH PLASMA INSULIN (POSTPRANDIAL STATE) | LOW PLASMA INSULIN (FASTED STATE) | |
|---|---|---|
| Liver | Glucose uptake | Glucose production |
| Glycogen synthesis | Glycogenolysis | |
| Absence of gluconeogenesis | Gluconeogenesis | |
| Lipogenesis | Absence of lipogenesis | |
| Absence of ketogenesis | Ketogenesis | |
| Muscle | Glucose uptake | Absence of glucose uptake |
| Glucose oxidation | Fatty acid and ketone oxidation | |
| Glycogen synthesis | Glycogenolysis | |
| Protein synthesis | Proteolysis and amino acid release | |
| Adipose tissue | Glucose uptake | Absence of glucose uptake |
| Lipid synthesis | Lipolysis and fatty acid release | |
| Triglyceride uptake | Absence of triglyceride uptake |
* Insulin is considered to be the major factor governing these metabolic processes. Diabetes mellitus may be viewed as a permanent low-insulin state that, untreated, results in exaggerated fasting.
Diagnosis
Diabetic Ketoacidosis
DKA is the end result of the metabolic abnormalities resulting from a severe deficiency of insulin or insulin effectiveness. The latter occurs during stress as counter-regulatory hormones block insulin action. DKA occurs in 20-40% of children with new-onset diabetes and in children with known diabetes who omit insulin doses or who do not successfully manage an intercurrent illness. DKA may be arbitrarily classified as mild, moderate, or severe (Table 583-2), and the range of symptoms depends on the depth of ketoacidosis. There is a large amount of ketonuria, an increased ion gap, a decreased serum bicarbonate (or total CO2) and pH, and an elevated effective serum osmolality, indicating hypertonic dehydration.
Treatment
Insulin Therapy
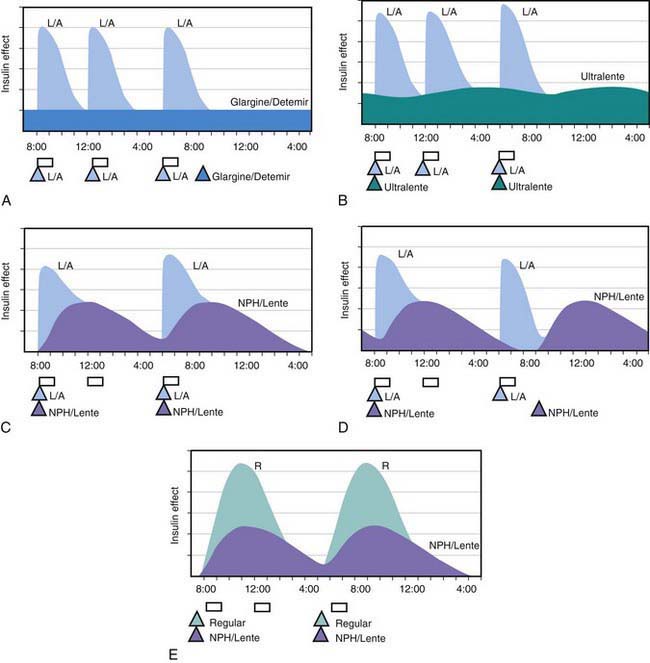
All preanalog insulins form hexamers, which must dissociate into monomers subcutaneously before being absorbed into the circulation. Thus, a detectable effect for regular (R) insulin is delayed by 30-60 min after injection. This, in turn, requires delaying the meal 30-60 min after the injection for optimal effect—a delay rarely attained in a busy child’s life. R has a wide peak and a long tail for bolus insulin (Figs. 583-6 and 583-7). This profile limits postprandial glucose control, produces prolonged peaks with excessive hypoglycemic effects between meals, and increases the risk of nighttime hypoglycemia. These unwanted between-meal effects often necessitate “feeding the insulin” with snacks and limiting the overall degree of blood glucose control. NPH and Lente insulins also have inherent limits because they do not create a peakless background insulin level (see Fig. 583-7C-E). This produces significant hypoglycemic effect during the midrange of their duration. Thus, it is often difficult to predict their interaction with fast-acting insulins. When R is combined with NPH or Lente (see Fig. 583-7E), the composite insulin profile poorly mimics normal endogenous insulin secretion. There are broad areas of excessive insulin effect alternating with insufficient effect throughout the day and night. Lente and Ultralente insulins have been discontinued and are no longer available.
Lispro (L) and aspart (A), insulin analogs, are absorbed much quicker because they do not form hexamers. They provide discrete pulses with little if any overlap and short tail effect. This allows better control of post-meal glucose increase and reduces between-meal or nighttime hypoglycemia (see Fig. 583-7A). The long-acting analog glargine (G) creates a much flatter 24-hr profile, making it easier to predict the combined effect of a rapid bolus (L or A) on top of the basal insulin, producing a more physiologic pattern of insulin effect (see Fig. 583-7A). Postprandial glucose elevations are better controlled, and between-meal and nighttime hypoglycemia are reduced.
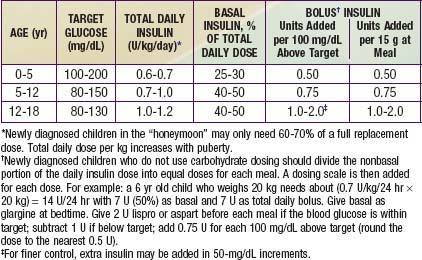
Ultralente (UL) given twice a day provided a reasonable basal profile (see Fig. 593-6C) and was quite effective when used with lispro or aspart (see Fig. 583-7B). Since UL is no longer available, G may be given every 12 hr in young children if a single daily dose of G does not produce complete 24-hr basal coverage. The basal insulin glargine should be 25-30% of the total dose in toddlers and 40-50% in older children. The remaining portion of the total daily dose is divided evenly as bolus injections for the 3 meals. A simple 3- or 4-step dosing schedule is begun based on the blood glucose level (Table 583-3). As soon as the family is taught to calculate the carbohydrate content of meals, bolus insulin can be more accurately dosed by both the carbohydrate content of the meal as well as the ambient glucose (see Table 583-3).
Ketoacidosis
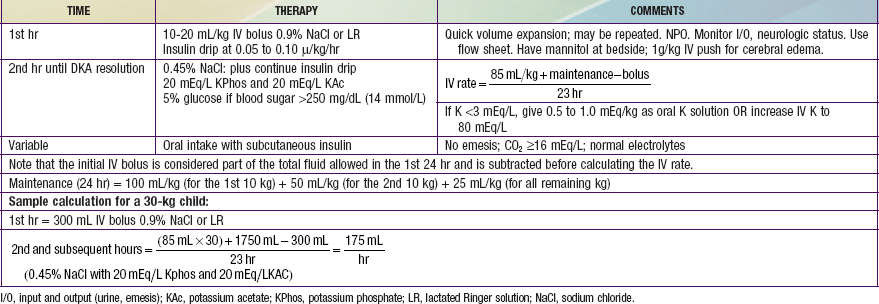
Reversal of DKA is associated with inherent risks that include hypoglycemia, hypokalemia, and cerebral edema. Any protocol must be used with caution and close monitoring of the patient. Adjustments based on sound medical judgment may be necessary for any given level of DKA (Table 583-4).
Table 583-4 DIABETIC KETOACIDOSIS (DKA) TREATMENT PROTOCOL
Hyperglycemia and Dehydration
Calculation of fluid deficits using clinical signs is difficult in children with DKA because intravascular volume is better maintained in the hypertonic state. For any degree of tachycardia, delayed capillary refill, decreased skin temperature, or orthostatic blood pressure change, the child with DKA will be more dehydrated than the child with a normotonic fluid deficit. The protocol in Table 583-4 corrects a deficit of 85 mL/kg (8.5% dehydration) for all patients in the 1st 24 hr. Children with mild DKA rehydrate earlier and can be switched to oral intake, whereas those with severe DKA and a greater volume deficit require 30-36 hr with this protocol. This more gradual rehydration of the child with severe DKA is an inherent safety feature. The initial intravenous bolus (20 mL/kg of glucose-free isotonic sodium salt solution such as Ringer lactate or 0.9% sodium chloride) for all patients ensures a quick volume expansion and may be repeated if clinical improvement is not quickly seen. This bolus is given as isotonic saline because the patient is inevitably hypertonic, keeping most of the initial infusion in the intravascular space. Subsequent fluid is hypotonic to repair the free water deficit, to allow intracellular rehydration, and to allow a more appropriate replacement of ongoing hypotonic urine losses.
where glucose is in mg/dL, or
where glucose is in mmol/L.
Nutritional Management
Total recommended caloric intake is based on size or surface area and can be obtained from standard tables (Tables 583-5 and 583-6). The caloric mixture should comprise approximately 55% carbohydrate, 30% fat, and 15% protein. Approximately 70% of the carbohydrate content should be derived from complex carbohydrates such as starch; intake of sucrose and highly refined sugars should be limited. Complex carbohydrates require prolonged digestion and absorption so that plasma glucose levels increase slowly, whereas glucose from refined sugars, including carbonated beverages, is rapidly absorbed and may cause wide swings in the metabolic pattern; carbonated beverages should be sugar free. Priority should be given to total calories and total carbohydrate consumed rather than its source. Carbohydrate counting has become a mainstay in the nutrition education and management of patients with DM. Each carbohydrate exchange unit is 15 g. Patients and their families are provided with information regarding the carbohydrate contents of different foods and food label reading. This allows patients to adjust their insulin dosage to their mealtime carbohydrate intake. The use of carbohydrate counting and insulin to carbohydrate ratios and the use of fast-acting insulin analogs and long-acting basal insulin (detemir and glargine) provide many children with less rigid meal planning. Flexibility in the use of insulin in relation to carbohydrate content of food improves the quality of life.
| AGE | kcal REQUIRED/kg BODY WEIGHT* |
|---|---|
| CHILDREN | |
| 0-12 mo | 120 |
| 1-10 yr | 100-75 |
| YOUNG WOMEN | |
| 11-15 yr | 35 |
| ≥16 yr | 30 |
| YOUNG MEN | |
| 11-15 yr | 80-55 (65) |
| 16-20 yr | |
| Average activity | 40 |
| Very physically active | 50 |
| Sedentary | 30 |
Numbers in parentheses are means.
* Gradual decline in calories per unit weight as age increases.
From Nutrition guide for professionals: diabetes education and meal planning, Alexandria, VA, and Chicago, IL, 1988, The American Diabetes Association and The American Dietetic Association.
Table 583-6 SUMMARY OF NUTRITION GUIDELINES FOR CHILDREN AND/OR ADOLESCENTS WITH TYPE 1 DIABETES MELLITUS
| NUTRITION CARE PLAN |
| Promotes optimal compliance. |
| Incorporates goals of management: normal growth and development, control of blood glucose, maintenance of optimal nutritional status, and prevention of complications. Uses staged approach. |
From Connell JE, Thomas-Doberson D: Nutritional management of children and adolescents with insulin-dependent diabetes mellitus: a review by the Diabetes Care and Education Dietetic Practice Group, J Am Diet Assoc 91:1556, 1991.
The intake of fat is adjusted so that the polyunsaturated : saturated ratio is increased to about 1.2 : 1.0, in contrast to the estimated American average of 0.3 : 1.0. Dietary fats derived from animal sources are, therefore, reduced and replaced by polyunsaturated fats from vegetable sources. Substituting margarine for butter, vegetable oil for animal oils in cooking, and lean cuts of meat, poultry, and fish for fatty meats, such as bacon, is advisable. The intake of cholesterol is also reduced by these measures and by limiting the number of egg yolks consumed. These simple measures reduce serum low-density lipoprotein cholesterol, a predisposing factor to atherosclerotic disease. Less than 10% of calories should be derived from saturated fats, up to 10% from polyunsaturated fats, and the remaining fat-derived calories from monounsaturated fats. Table 583-6 summarizes current nutritional guidelines.
Monitoring
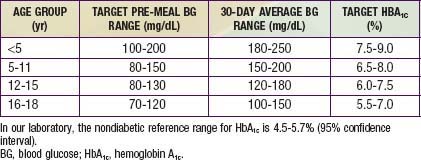
Daily blood glucose monitoring has been markedly enhanced by the availability of strips impregnated with glucose oxidase that permit blood glucose measurement from a drop of blood. A portable calibrated reflectance meter can approximate the blood glucose concentration accurately. Many meters contain a memory “chip” enabling recall of each measurement, its average over a given interval, and the ability to display the pattern on a computer screen. Such information is a useful educational tool for verifying degree of control and modifying recommended regimens. A small, spring-loaded device that automates capillary bloodletting (lancing device) in a relatively painless fashion is commercially available. Parents and patients should be taught to use these devices and measure blood glucose at least 4 times daily—before breakfast, lunch, and supper and at bedtime. When insulin therapy is initiated and when adjustments are made that may affect the overnight glucose levels, SMBG should also be performed at 12 A.M. and 3 A.M. to detect nocturnal hypoglycemia. Ideally, the blood glucose concentration should range from approximately 80 mg/dL in the fasting state to 140 mg/dL after meals. In practice, however, a range of 60-220 mg/dL is acceptable, based on age of the patient (Table 583-7). Blood glucose measurements that are consistently at or outside these limits, in the absence of an identifiable cause such as exercise or dietary indiscretion, are an indication for a change in the insulin dose. If the fasting blood glucose is high, the evening dose of long-acting insulin is increased by 10-15% and/or additional fast-acting insulin (lispro or aspart) coverage for bedtime snack may be considered. If the noon glucose level exceeds set limits, the morning fast-acting insulin (lispro or aspart) is increased by 10-15%. If the pre-supper glucose is high, the noon dose of fast-acting insulin is increased by 10-15%. If the pre-bedtime glucose is high, the pre-supper dose of fast-acting insulin is increased by 10-15%. Similarly, reductions in the insulin type and dose should be made if the corresponding blood glucose measurements are consistently below desirable limits.
Glycosylated Hemoglobin (HbA1c)
A reliable index of long-term glycemic control is provided by measurement of glycosylated hemoglobin. HbA1c represents the fraction of hemoglobin to which glucose has been nonenzymatically attached in the bloodstream. The formation of HbA1c is a slow reaction that is dependent on the prevailing concentration of blood glucose; it continues irreversibly throughout the red blood cell’s life span of approximately 120 days. The higher the blood glucose concentration and the longer the red blood cell’s exposure to it, the higher is the fraction of HbA1c, which is expressed as a percentage of total hemoglobin. Because a blood sample at any given time contains a mixture of red blood cells of varying ages, exposed for varying times to varying blood glucose concentrations, an HbA1c measurement reflects the average blood glucose concentration from the preceding 2-3 mo. When measured by standardized methods to remove labile forms, the fraction of HbA1c is not influenced by an isolated episode of hyperglycemia. Consequently, as an index of long-term glycemic control, a measurement of HbA1c is superior to measurements of glycosuria or even multiple blood glucose determinations. (The latter can reveal important fluctuations but may not accurately reflect the average overall glycemic control.) It is recommended that HbA1c measurements be obtained 3-4 times per yr to obtain a profile of long-term glycemic control. The more consistently lower the HbA1c level, and hence the better the metabolic control, the more likely it is that microvascular complications such as retinopathy and nephropathy will be less severe, delayed in appearance, or even avoided altogether. Depending on the method used for determination, HbA1c values may be spuriously elevated in thalassemia (or other conditions with elevated hemoglobin F) and spuriously lower in sickle cell disease (or other conditions with high red blood cell turnover). Although values of HbA1c may vary according to the method used for measurement, in nondiabetic individuals, the HbA1c fraction is usually less than 6%; in diabetics, values of 6-7.9% represent good metabolic control, values of 8.0-9.9%, fair control, and values of 10% or higher, poor control. Adjustments in target HbA1c should be made for younger children (see Table 583-7).
Hypoglycemic Reactions
Hypoglycemia is the major limitation to tight control of glucose levels. Once injected, insulin absorption and action are independent of the glucose level, thus creating a unique risk of hypoglycemia from an unbalanced insulin effect. Insulin analogs may help reduce but cannot eliminate this risk. Most children with T1DM can expect mild hypoglycemia each week, moderate hypoglycemia a few times each year, and severe hypoglycemia every few years. These episodes are usually not predictable, although exercise, delayed meals or snacks, and wide swings in glucose levels increase the risk. Infants and toddlers are at higher risk because they have more variable meals and activity levels, are unable to recognize early signs of hypoglycemia, and are limited in their ability to seek a source of oral glucose to reverse the hypoglycemia. The very young have an increased risk of permanently reduced cognitive function as a long-term sequela of severe hypoglycemia. For this reason, a more relaxed degree of glucose control is necessary until the child matures (see Table 583-7).
Hypoglycemia can occur at any time of day or night. Early symptoms and signs (mild hypoglycemia) may occur with a sudden decrease in blood glucose to levels that do not meet standard criteria for hypoglycemia in nondiabetic children (Chapter 86). The child may show pallor, sweating, apprehension or fussiness, hunger, tremor, and tachycardia, all due to the surge in catecholamines as the body attempts to counter the excessive insulin effect. Behavioral changes such as tearfulness, irritability, aggression, and naughtiness are more prevalent in children. As glucose levels decline further, cerebral glucopenia occurs with drowsiness, personality changes, mental confusion, and impaired judgment (moderate hypoglycemia), progressing to inability to seek help and seizures or coma (severe hypoglycemia). Prolonged severe hypoglycemia can result in a depressed sensorium or strokelike focal motor deficits that persist after the hypoglycemia has resolved. Although permanent sequelae are rare, severe hypoglycemia is frightening for the child and family and can result in significant reluctance to attempt even moderate glycemic control afterward.
Eating Disorders
Management During Infections
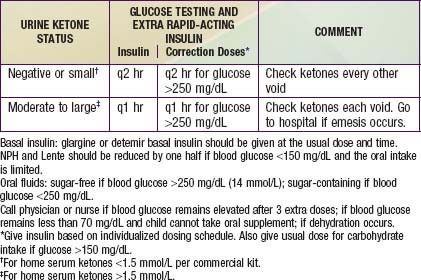
Although infections are no more common in diabetic children than in nondiabetic ones, they can often disrupt glucose control and may precipitate DKA. In addition, the diabetic child is at increased risk of dehydration if hyperglycemia causes an osmotic diuresis or if ketosis causes emesis. Counter-regulatory hormones associated with stress blunt insulin action and elevate glucose levels. If anorexia occurs, however, lack of caloric intake increases the risk of hypoglycemia. Although children younger than 3 yr tend to become hypoglycemic and older children tend toward hyperglycemia, the overall effect is unpredictable. Therefore, frequent blood glucose monitoring and adjustment of insulin doses are essential elements of sick day guidelines (Table 583-8).
Management During Surgery
Maintaining glucose control and avoiding DKA are best accomplished with IV insulin and fluids. A simple insulin adjustment scale based on the patient’s weight and blood glucose level can be used in most situations (Table 583-9). The IV insulin is continued after surgery as the child begins to take oral fluids; the IV fluids can be steadily decreased as oral intake increases. When full oral intake is achieved, the IV may be capped and subcutaneous insulin begun. When surgery is elective, it is best performed early in the day, allowing the patient maximal recovery time to restart oral intake and subcutaneous insulin therapy. When elective surgery is brief (less than 1 hr) and full oral intake is expected shortly afterward, one may simply monitor the blood glucose hourly and give a dose of insulin analog according to the child’s home glucose correction scale. If glargine or detemir is used as the basal insulin, a full dose is given the evening before planned surgery. If NPH or Lente is used, one half of the morning dose is given before surgery. The child should not be discharged until blood glucose levels are stable and oral intake is tolerated.
Table 583-9 GUIDELINES FOR INTRAVENOUS INSULIN COVERAGE DURING SURGERY
| BLOOD GLUCOSE LEVEL (mg/dL) | INSULIN INFUSION (U/kg/hr) | BLOOD GLUCOSE MONITORING |
|---|---|---|
| <120 | 0.00 | 1 hr |
| 121-200 | 0.03 | 2 hr |
| 200-300 | 0.06 | 2 hr |
| 300-400 | 0.08 | 1 hr† |
| 400 | 0.10 | 1 hr† |
An infusion of 5% glucose and 0.45% saline solution with 20 mEq/L of potassium acetate is given at 1.5 times maintenance rate.
Long-Term Complications: Relation to Glycemic Control
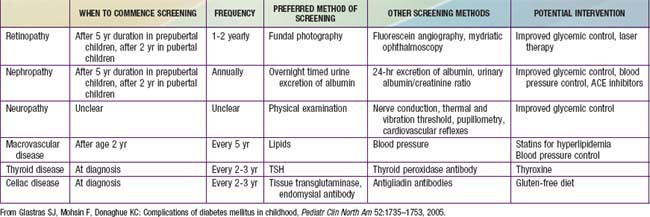
The increasingly prolonged survival of the diabetic child is associated with an increasing prevalence of complications. Complications of DM can be divided into 3 major categories—(1) microvascular complications, specifically, retinopathy and nephropathy; (2) macrovascular complications, particularly accelerated coronary artery disease, cerebrovascular disease, and peripheral vascular disease; and (3) neuropathies, both peripheral and autonomic, affecting a variety of organs and systems (Table 583-10). In addition, cataracts may occur more frequently.
Guidelines suggest that diabetic patients have an initial dilated and comprehensive examination by an ophthalmologist shortly after the diagnosis of diabetes is made in patients with T2DM, and within 3-5 yr after the onset of T1DM (but not before age 10 yr). Any patients with visual symptoms or abnormalities should be referred for ophthalmologic evaluation. Subsequent evaluations for both T1DM and T2DM patients should be repeated annually by an ophthalmologist who is experienced in diagnosing the presence of diabetic retinopathy and is knowledgeable about its management (see Table 583-10).
Screening for diabetic nephropathy is a routine aspect of diabetes care (see Table 583-10). The American Diabetes Association (ADA) recommends yearly screening for individuals with T2DM and yearly screening for those with T1DM after 5 yr duration of disease (but not before puberty). Twenty-four hour AER (urinary albumin and creatinine) or timed (overnight) urinary AER are acceptable techniques. Positive results should be confirmed by a 2nd measurement of AER because of the high variability of albumin excretion in patients with diabetes. Short-term hyperglycemia, exercise, urinary tract infections, marked hypertension, heart failure, and acute febrile illness can cause transient elevation urinary albumin excretion. There is marked day-to-day variability in albumin excretion, so at least 2 of 3 collections done in a 3- to 6-mo period should show elevated levels before microalbuminuria is diagnosed and treatment is started. Once albuminuria is diagnosed, a number of factors attenuate the effect of hyperfiltration on kidneys: (1) meticulous control of hyperglycemia, (2) aggressive control of systemic blood pressure, (3) selective control of arteriolar dilation by use of angiotensin-converting enzyme (ACE) inhibitors (thus decreasing transglomerular capillary pressure), and (4) dietary protein restriction (because high protein intake increases renal perfusion rate). Tight glycemic control will delay the progression of microalbuminuria and slow the progression of diabetic nephropathy. Previous extensive therapy of diabetes has a persistent benefit for 7-8 yr and may delay or prevent the development of diabetic nephropathy.
Pancreas and Islet Transplantation and Regeneration
583.3 Type 2 Diabetes Mellitus
Natural History
T2DM is considered a polygenic disease aggravated by environmental factors, such as low physical activity and excessive caloric intake. Most patients are obese, though the disease can occasionally be seen in normal weight individuals. Asians in particular appear to be at risk for T2DM at lower degrees of total adiposity. Some patients may not necessarily meet overweight or obese criteria for age and gender despite abnormally high percentage of body fat in the abdominal region. Obesity, in particular, central obesity, is associated with the development of insulin resistance. In addition, patients who are at risk for developing T2DM exhibit decreased glucose-induced insulin secretion. Obesity does not lead to the same degree of insulin resistance in all individuals and even those who develop insulin resistance do not necessarily exhibit impaired β-cell function. Thus, many obese individuals have some degree of insulin resistance, but compensate for it by increasing insulin secretion. But those who are unable to adequately compensate for insulin resistance by increasing insulin secretion, develop impaired glucose tolerance and impaired fasting glucose (usually, thought not always, in that order). Hepatic insulin resistance leads to excessive hepatic glucose output (failure of insulin to suppress hepatic glucose output), while skeletal muscle insulin resistance leads to decreased glucose uptake in a major site of glucose disposal. Over time hyperglycemia worsens, a phenomenon that has been attributed to the deleterious effect of chronic hyperglycemia (glucotoxicity) or chronic hyperlipidemia (lipotoxicity) on β-cell function and is often accompanied by increased triglyceride content and decreased insulin gene expression. At some point, blood glucose elevation meets the criteria for diagnosis of T2DM (see ![]() Web Table 583-2 on the Nelson Textbook of Pediatrics website at www.expertconsult.com), but most patients with T2DM remain asymptomatic for months to years after this point because hyperglycemia is moderate and symptoms are not as dramatic as the polyuria and weight loss accompanying T1DM. Even weight gain may continue. The prolonged hyperglycemia may be accompanied by the development of microvascular and macrovascular complications. In time, β-cell function can decrease to the point that the patient has absolute insulin deficiency and becomes dependent on exogenous insulin. In T2DM, insulin deficiency is rarely absolute, so patients usually do not need insulin to survive. Nevertheless, glycemic control can be improved by exogenous insulin. DKA is uncommon in patients with T2DM, but may occur and is usually associated with the stress of another illness such as severe infection and may resolve when the stressful illness resolves. DKA tends to be more common in African-American patients than in other ethnic groups. Although it is generally believed that autoimmune destruction of pancreatic β cells does not occur in T2DM, autoimmune markers of T1DM—namely, GAD65, ICA512, and IAA—may be positive in up to one third of the cases of adolescent T2DM. The presence of these autoimmune markers does not rule out T2DM in children and adolescents. At the same time, due to the general increase in obesity, the presence of obesity does not preclude the diagnosis of T1DM. While the majority of newly diagnosed diabetics can be confidently assigned a diagnosis of T1DM or T2DM, a few exhibit features of both types and are difficult to classify.
Web Table 583-2 on the Nelson Textbook of Pediatrics website at www.expertconsult.com), but most patients with T2DM remain asymptomatic for months to years after this point because hyperglycemia is moderate and symptoms are not as dramatic as the polyuria and weight loss accompanying T1DM. Even weight gain may continue. The prolonged hyperglycemia may be accompanied by the development of microvascular and macrovascular complications. In time, β-cell function can decrease to the point that the patient has absolute insulin deficiency and becomes dependent on exogenous insulin. In T2DM, insulin deficiency is rarely absolute, so patients usually do not need insulin to survive. Nevertheless, glycemic control can be improved by exogenous insulin. DKA is uncommon in patients with T2DM, but may occur and is usually associated with the stress of another illness such as severe infection and may resolve when the stressful illness resolves. DKA tends to be more common in African-American patients than in other ethnic groups. Although it is generally believed that autoimmune destruction of pancreatic β cells does not occur in T2DM, autoimmune markers of T1DM—namely, GAD65, ICA512, and IAA—may be positive in up to one third of the cases of adolescent T2DM. The presence of these autoimmune markers does not rule out T2DM in children and adolescents. At the same time, due to the general increase in obesity, the presence of obesity does not preclude the diagnosis of T1DM. While the majority of newly diagnosed diabetics can be confidently assigned a diagnosis of T1DM or T2DM, a few exhibit features of both types and are difficult to classify.
Epidemiology
The epidemic of T2DM in children and adolescents parallels the emergence of the obesity epidemic (Chapter 44). Although obesity itself is associated with insulin resistance, diabetes does not develop until there is some degree of failure of insulin secretion. Thus, when measured, insulin secretion in response to glucose or other stimuli is always lower in persons with T2DM than in control subjects matched for age, sex, weight, and equivalent glucose concentration.
Clinical Features
In the USA, T2DM in children is more likely to be diagnosed in Native American, Hispanic American, and African American youth, with the highest incidence being reported in Pima Indian youth, where its prevalence in the 15-19 yr age group is 5%. While cases may be seen as young as 6 yr of age, most are diagnosed in adolescence and incidence increases with increasing age. Family history of T2DM is present in practically all cases. Typically, these patients are obese and present with mild symptoms of polyuria and polydipsia, or are asymptomatic and detected on screening tests. But presentation with diabetic ketoacidosis occurs in up to 10% of cases and may be higher in African Americans. Physical examination frequently reveals the presence of acanthosis nigricans, most commonly on the neck and in other flexural areas. Other findings may include striae and an increased waist-hip ratio. Laboratory testing reveals elevated HbA1c levels and HbA1c values are higher at diagnosis among minority youth. Hyperlipidemia characterized by elevated triglycerides and low-density lipoprotein (LDL) cholesterol levels are commonly seen in patients with T2DM at diagnosis. Therefore lipid screening is indicated in all new cases of T2DM. Since hyperglycemia develops slowly and patients may be asymptomatic for months or years after they develop T2DM, screening for T2DM is recommended in high-risk children (Table 583-11) and many patients are diagnosed upon routine screening. The ADA recommends that all youth who are overweight and have at least 2 other risk factors be tested for T2DM beginning at age 10 yr or at the onset of puberty and every 2 yr after that. These risk factors include family history of T2DM in first- or second-degree relatives, history of gestational diabetes in the mother, belonging to certain ethnic groups (i.e., Native Americans, African American, Hispanic, or Asian/Pacific Islander) and having signs of insulin resistance (e.g., acanthosis nigricans, hypertension, dyslipidemia, or polycystic ovary syndrome). The current recommendation is to use fasting blood glucose as a screening test, but some authorities now recommend that HbA1c be used as a screening tool and it has the advantage that a fasting sample is not required. In borderline or asymptomatic cases, the diagnosis may be confirmed using a standard glucose tolerance test, but this test is not required if typical symptoms are present or fasting plasma glucose is clearly elevated on 2 separate occasions.
Table 583-11 TESTING FOR TYPE 2 DIABETES IN CHILDREN
* Clinical judgment should be used to test for diabetes in high-risk patients who do not meet these criteria.
From American Diabetes Association: Type 2 diabetes in children and adolescents, Diabetes Care 23:386, 2000. Reproduced by permission.
Treatment
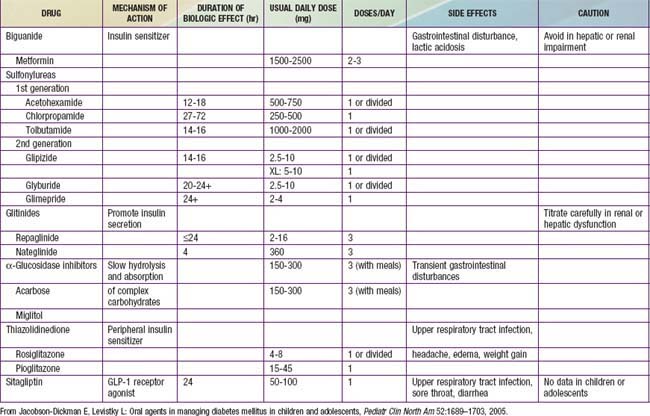
When lifestyle interventions fail to normalize blood glucose, oral hypoglycemic agents are introduced for management of persistent hyperglycemia (Table 583-12). Patients who present with DKA or with markedly elevated HbA1c (>9.0%) will require treatment with insulin using protocols similar to those used for treating T1DM. Once blood glucose levels are under control most cases can be managed with oral hypoglycemic agents and lifestyle changes, but some patients will continue to require insulin therapy.
Complications
In the SEARCH study of diabetes in youth, 92% of the patients with T2DM had 2 or more elements of the metabolic syndrome (hypertension, hypertriglyceridemia, decreased HDL, increased waist circumference), including 70% with hypertension. In addition, the incidence of microalbuminuria and diabetic retinopathy appears to be higher in T2DM than it is in T1DM. In the SEARCH study, the incidence of microalbuminuria among patients who had T2DM of LESS than 5 yr duration was 7-22%, while retinopathy was present in 18.3%. Thus, all adolescents with T2DM should be screened for hypertension and lipid abnormalities and screening for microalbuminuria and retinopathy may be indicated even earlier than it is in T1DM. Sleep apnea and fatty liver disease are being diagnosed with increasing frequency and may necessitate referral to the appropriate specialists. Complications associated with all forms of diabetes and recommendations for screening are noted in Table 583-10 while Table 583-13 lists additional conditions particularly associated with T2DM.
Table 583-13 MONITORING FOR COMPLICATIONS AND CO-MORBIDITIES
| CONDITION | SCREENING TEST | COMMENT |
|---|---|---|
| Hypertension | Blood pressure | |
| Fatty liver | AST, ALT, possibly liver ultrasound | |
| Polycystic ovary syndrome | Menstrual history, assessment for androgen excess with free/total testosterone, DHEA | |
| Microalbuminuria | Urine albumin concentration and albumin/creatinine ratios | |
| Dyslipidemia | Fasting lipid profile (total, LDL, HDL cholesterol, triglycerides) | Obtain at diagnosis and every 2 yr |
| Sleep apnea | Sleep study to assess overnight oxygen saturation |
From Liu L, Hironaka K, Pihoker C: Type 2 diabetes in youth, Curr Probl Pediatr Adolesc Health Care 34:249–280, 2004.
Prevention
The difficulties in achieving good glucose control and preventing diabetes complications make prevention a compelling strategy. This is particularly true for T2DM, which is clearly linked to modifiable risk factors (obesity, a sedentary lifestyle). The Diabetes Prevention Program (DPP) was designed to prevent or delay the development of T2DM in adult individuals at high risk by virtue of impaired glucose tolerance (IGT). DPP results demonstrated that intensified lifestyle or drug intervention in individuals with IGT prevented or delayed the onset of T2DM. The results were striking. Lifestyle intervention reduced diabetes incidence by 58%; metformin reduced the incidence by 31% compared with placebo. The effects were similar for men and women and for all racial and ethnic groups. Lifestyle interventions are believed to have similar beneficial effects in obese adolescents with IGT. Screening is indicated for at-risk patients (see Table 583-11).
Impaired Glucose Tolerance
The performance of the glucose tolerance test should be standardized according to currently accepted criteria. These include at least 3 days of a well-balanced diet containing approximately 50% of calories from carbohydrates, fasting from midnight until the time of the test in the morning, and a dose of glucose for the test of 1.75 g/kg but not more than 75 g. Plasma samples are obtained before ingestion of the glucose and at 1, 2, and 3 hr thereafter. The arbitrarily designated response to the test that identifies IGT is a fasting plasma glucose value of less than 126 mg/dL and a value at 2 hr of more than 140 mg/dL but less than 200 mg/dL (see ![]() Web Table 583-2 on the Nelson Textbook of Pediatrics website at www.expertconsult.com). Determination of serum insulin responses during the glucose tolerance test is not a prerequisite for reaching a diagnosis; the magnitude of the response, however, may have prognostic value.
Web Table 583-2 on the Nelson Textbook of Pediatrics website at www.expertconsult.com). Determination of serum insulin responses during the glucose tolerance test is not a prerequisite for reaching a diagnosis; the magnitude of the response, however, may have prognostic value.
583.4 Other Specific Types of Diabetes
Genetic Defects of β-Cell Function
Maturity-Onset Diabetes of Youth
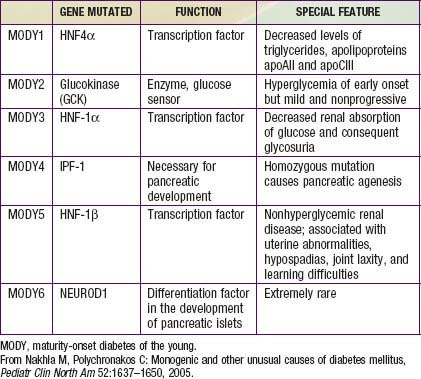
Several forms of diabetes are associated with monogenic defects in β-cell function. Before these genetic defects were identified, this subset of diabetics was diagnosed on clinical grounds and described by the term MODY or maturity-onset diabetes of youth. This subtype of DM consists of a group of heterogeneous clinical entities that are characterized by onset between the ages of 9 and 25 yr, autosomal dominant (AD) inheritance, and a primary defect in insulin secretion. Strict criteria for the diagnosis of MODY include diabetes in at least 3 generations with AD transmission and diagnosis before age 25 yr in at least 1 affected subject. Now that the genetic basis and mechanism of these disorders is better understood, the term MODY is used for dominantly inherited monogenic defects of insulin secretion. The ADA groups these disorders together under the broader category of “genetic defects of β-cell function.” Six of these defects typically meet the clinical criteria for the diagnosis of MODY and are listed in Table 583-14. Just 2 of them (MODY2 and MODY3) account for 80% of the cases in this category in European populations, but the distribution may be different in other ethnic groups. Except for MODY2 (which is due to mutations in the enzyme glucokinase), all other forms are due to genetic defects in various transcription factors (see Table 583-14).
Diabetes Mellitus of the Newborn
Permanent Neonatal Diabetes Mellitus (PNDM)
IPEX Syndrome: Mutations in the FOXP3 (Forkhead box P3) gene lead to severe immune dysregulation and rampant autoimmunity. Autoimmune diabetes develops in >90% of cases, usually within the 1st few weeks of life and is accompanied by enteropathy, failure to thrive and other autoimmune disorders (Chapter 120.5).
Autoimmune Diseases
Chronic lymphocytic thyroiditis (Hashimoto thyroiditis) is frequently associated with T1DM in children (Chapter 560). As many as 1 in 5 insulin-dependent diabetic patients have thyroid antibodies in their serum; the prevalence is 2-20 times greater than in control populations. Only a small proportion of these patients, however, acquire clinical hypothyroidism; the interval between diagnosis of diabetes and thyroid disease averages about 5 yr. Periodic palpation of the thyroid gland is indicated in all diabetic children; if the gland feels firm or enlarged, serum measurements of thyroid antibodies and thyroid-stimulating hormone (TSH) should be obtained. A confirmed TSH level of greater than 10 µU/mL indicates existing or incipient thyroid dysfunction that warrants replacement with thyroid hormone. Deceleration in the rate of growth may also be due to thyroid failure and is, in itself, a reason for securing serum measurements of thyroxine and TSH concentrations.
Celiac disease, which is due to hypersensitivity to dietary gluten, is another autoimmune disorder that occurs with significant frequency in children with T1DM (Chapter 330.2). It is estimated that about 7% of children with T1DM develop celiac disease within the 1st 6 yr of diagnosis, and the incidence of celiac disease is significantly higher in children under 4 yr of age and in girls. Young children with T1DM and celiac disease usually present with gastrointestinal symptoms (abdominal cramping, diarrhea, and gastroesophageal reflux), growth failure due to suboptimal weight gain, and unexplained hypoglycemic reactions due to nutrient malabsorption; adolescents may remain asymptomatic. The diagnosis of celiac disease is considered if serum antiendomysial and/or tissue transglutaminase antibody titers are positive in the presence of normal serum total IgA level. The diagnosis is confirmed on endoscopic evaluation and biopsy of small bowel revealing characteristic atrophy of intestinal villi. Therapy consists of a gluten-free diet, which will alleviate gastrointestinal symptoms and may reduce glycemic excursions.
Endocrinopathies
The endocrinopathies listed in ![]() Web Table 583-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com are only rarely encountered as a cause of diabetes in childhood. They may accelerate the manifestations of diabetes in those with inherited or acquired defects in insulin secretion or action.
Web Table 583-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com are only rarely encountered as a cause of diabetes in childhood. They may accelerate the manifestations of diabetes in those with inherited or acquired defects in insulin secretion or action.
Genetic Syndromes Associated with Diabetes Mellitus
A number of rare genetic syndromes associated with IDDM or carbohydrate intolerance have been described (see Web Table 583-1 on the ![]() Nelson Textbook of Pediatrics website at www.expertconsult.com). These syndromes represent a broad spectrum of diseases ranging from premature cellular aging, as in the Werner and Cockayne syndromes (Chapter 84) to excessive obesity associated with hyperinsulinism, resistance to insulin action, and carbohydrate intolerance, as in the Prader-Willi syndrome (Chapters 75 and 76). Some of these syndromes are characterized by primary disturbances in the insulin receptor or in antibodies to the insulin receptor without any impairment in insulin secretion. Although rare, these syndromes provide unique models to understand the multiple causes of disturbed carbohydrate metabolism from defective insulin secretion or from defective insulin action at the cell receptor or postreceptor level.
Nelson Textbook of Pediatrics website at www.expertconsult.com). These syndromes represent a broad spectrum of diseases ranging from premature cellular aging, as in the Werner and Cockayne syndromes (Chapter 84) to excessive obesity associated with hyperinsulinism, resistance to insulin action, and carbohydrate intolerance, as in the Prader-Willi syndrome (Chapters 75 and 76). Some of these syndromes are characterized by primary disturbances in the insulin receptor or in antibodies to the insulin receptor without any impairment in insulin secretion. Although rare, these syndromes provide unique models to understand the multiple causes of disturbed carbohydrate metabolism from defective insulin secretion or from defective insulin action at the cell receptor or postreceptor level.
Epidemiology, Etiology, Genetics, Pathology, Classification, and Prevention
Achenbach P, Bonifacio E, Koczwara K, et al. Natural history of type 1 diabetes. Diabetes. 2005;54(Suppl 2):S25-S31.
Ali K, Harnden A, Edge JA. Type 1 diabetes in children. BMJ. 2011;342:d294.
Altobelli E, Petrocelli R, Verrotti A, et al. Infections and risk of type I diabetes in childhood: a population-based case-control study. Eur J Epidemiol. 2003;18:425-430.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31(Suppl 1):S55-S60.
Betts PR, Mulligan J, Ward P, et al. Increasing body weight predicts the earlier onset of insulin-dependent diabetes in childhood: testing the ‘accelerator hypothesis.’. Diabet Med. 2005;2:144-151.
Cardwell CR, Carson DJ, Patterson CC. No association between routinely recorded infections in early life and subsequent risk of childhood-onset type 1 diabetes: a matched case-control study using the UK General Practice Research Database. Diabet Med. 2008;25:261-267.
Cardwell CR, Stene LC, Joner G, et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia. 2008;51:726-735.
Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360:1646-1654.
Dabelea D. The accelerating epidemic of childhood diabetes. Lancet. 2009;373:1999-2000.
Dabelea D, Bell RA, D’Agostino RBJr, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716-2724.
Diamond Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857-866.
Diabetes Prevention Trial-Type 1 Diabetes Study Group. Effects of oral insulin in relatives of patients with type 1 diabetes mellitus. Diabetes Care. 2005;28:1068-1076.
Dunger DB, Todd JA. Prevention of type 1 diabetes: what next? Lancet. 2008;372:1710-1711.
EURODIAB Substudy 2 Study Group. Infections and vaccinations as risk factors for childhood type I (insulin-dependent) diabetes mellitus: a multicentre case-control investigation. Diabetologia. 2000;43:47-53.
Frohlich-Reiterer EE, Kaspers S, Hofer S, et al. Anthropometry, metabolic control, and follow-up in children and adolescents with type 1 diabetes mellitus and biopsy-proven celiac disease. J Pediatr. 2011;158:589-593.
Graves PM, Barriga KJ, Norris JM, et al. Lack of association between early childhood immunizations and beta-cell autoimmunity. Diabetes Care. 1999;22:1694-1697.
Groop L. Open chromatin and diabetes risk. Nat Genet. 2010;42:190-192.
Hämäläinen AM, Knip M. Autoimmunity and familial risk of type 1 diabetes. Curr Diab Rep. 2002;2:347-353.
Harlan DM, Lee MM. Infant formula, autoimmune triggers, and type 1 diabetes. N Engl J Med. 2010;363:1961-1963.
Hober D, Same F. Enteroviruses and type 1 diabetes. BMJ. 2010;341:c7072.
Hoppu S, Ronkainen MS, Kimpimäki T, et al. Insulin autoantibody isotypes during the prediabetic process in young children with increased genetic risk of type I diabetes. Pediatr Res. 2004;55:236-242.
Hummel M, Bonifacio E, Schmid S, et al. Brief communication: early appearance of islet autoantibodies predicts childhood type 1 diabetes in offspring of diabetic parents. Ann Intern Med. 2004;140:882-886.
Karavanaki K, Tsoka E, Liacopoulou M, et al. Psychological stress as a factor potentially contributing to the pathogenesis of Type 1 diabetes mellitus. J Endocrinol Invest. 2008;31:406-415.
Knip M, Virtanen SM, Seppä K, et al. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med. 2010;363(20):1900-1908.
Li L, Yi Z, Tisch R, et al. Immunotherapy of type 1 diabetes. Arch Immunol Ther Exp (Warsz). 2008;56:227-236.
Lindley S, Dayan CM, Bishop A, et al. Defective suppressor function in CD4+CD25+ T-cells from patients with type diabetes. Diabetes. 2005;54:92-99.
Mohr SB, Garland CF, Gorham ED, et al. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia. 2008;51:1391-1398.
Mortensen HB, Hougaard P, Swift P, et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care. 2009;32:1384-1390.
Myliwska J, Zorena K, Myliwiec M, et al. The -174GG interleukin-6 genotype is protective from retinopathy and nephropathy in juvenile onset type 1 diabetes mellitus. Pediatr Res. 2009;66:3341-3345.
Nanto-Salonen K, Kupila A, Simell S, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomized controlled trial. Lancet. 2008;372:1746-1755.
Narula P, Porter L, Langton J, et al. Gastrointestinal symptoms in children with type 1 diabetes screened for celiac disease. Pediatrics. 2009;124:e489-e495.
Nejentsev S, Howson JM, Walker NM, et al. Wellcome Trust Case Control Consortium, Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature. 2007;450:887-892.
Newgard CB, Attie AD. Getting biological about the genetics of diabetes. Nat Med. 2010;16:388-391.
Norris JM, Yin X, Lamb MM, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298:1420-1428.
Patterson CC, Dahlquist GG, Gyurus E, et al. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027-2033.
Pawlowicz M, Birkholz D, Niedzwiecki M, et al. Difficulties or mistakes in diagnosing type 1 diabetes in children?—demographic factors influencing delayed diagnosis. Pediatr Diabetes. 2009;10:542-549.
Poulsen P, Grunnet LG, Pilgaard K, et al. Increased risk of Type 2 diabetes in elderly twins. Diabetes. 2009;58:1350-1355.
Quinn M, Fleishman A, Rosner B, et al. Characteristics at diagnosis of type 1 diabetes in children younger than 6 years. J Pediatr. 2006;148:366-371.
Raman VS, Loar RW, Renukuntla VS, et al. Hyperglycemia and diabetes mellitus in children with pancreatitis. J Pediatr. 2011;158:612-616.
Redondo MJ, Jeffrey J, Fain PR, et al. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med. 2008;359:2849-2850.
Rewers M, Norris J, Dabelea D. Epidemiology of type 1 diabetes mellitus. In Eisenbarth GS, editor: Immunology of type 1 diabetes mellitus, ed 2, Boston: Kluwer Academic Publishing Group, 2004.
Richardson SJ, Willcox A, Bone AJ, et al. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52:1143-1151.
Rosenbauer J, Herzig P, Giani G. Early infant feeding and risk of type 1 diabetes mellitus—a nationwide population-based case-control study in pre-school children. Diabetes Metab Res Rev. 2008;24:211-222.
Sadauskaite-Kuehne V, Ludvigsson J, Padaiga Z, et al. Longer breastfeeding is an independent protective factor against development of type 1 diabetes mellitus in childhood. Diabetes Metab Res Rev. 2004;20:150-157.
Samuelsson U, Sadauskaite V, Padaiga Z, et al. A fourfold difference in the incidence of type 1 diabetes between Sweden and Lithuania but similar prevalence of autoimmunity. Diabetes Res Clin Pract. 2004;66:173-181.
Selvin E, Steffes MW, Gregg E, et al. Performance of A1C for the classification and prediction of diabetes. Diabetes Care. 2011;34(1):84-89.
Smyth DJ, Plagnol V, Walker NM, et al. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med. 2008;359:2767-2776.
Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between type I and type II diabetes. Diabetologia. 2001;44:914-922.
Whincup PH, Kaye SJ, Owen CG, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886-2897.
Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ. 2011;342:d35.
Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93:512-517.
Aly TA, Ide A, Jahromi MM, et al. Extreme genetic risk for type 1A diabetes. Proc Natl Acad Sci U S A. 2006;103:14074-14079.
Gillespie K, Bain SC, Barnett AH, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364:1699-1700.
Gullstrand C, Wahlberg J, Ilonen J, et al. Progression to type 1 diabetes and autoantibody positivity in relation to HLA-risk genotypes in children participating in the ABIS study. Pediatr Diabetes. 2008;9(3 Pt 1):182-190.
Huopio H, Otonkoski T, Vauhkonen I, et al. A new subtype of autosomal dominant diabetes attributable to a mutation in the gene for sulfonylurea receptor 1. Lancet. 2003;361:301-307.
Mayer-Davis EJ, Bell RA, Dabelea D, et al. The many faces of diabetes in American youth: type 1 and type 2 diabetes in five race and ethnic populations: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(Suppl 2):S99-S101.
Siljander HT, Veijola R, Reunanen A, et al. Prediction of type 1 diabetes among siblings of affected children and in the general population. Diabetologia. 2007;50:2272-2275.
TRIGR Study Group. Study design of the Trial to Reduce IDDM in the Genetically at Risk (TRIGR). Pediatr Diabetes. 2007;8:117-137.
Velho G, Froguel P. Genetic, metabolic and clinical characteristics of maturity onset diabetes of the young. Eur J Endocrinol. 1998;138:233-239.
Zamani M, Cassiman JJ. Reevaluation of the importance of polymorphic HLA class II alleles and amino acids in the susceptibility of individuals of different populations to type 1 diabetes. Am J Med Genet. 1998;76:183-194.
Writing Group for the SEARCH for Diabetes in Youth Study Group. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716-2724.
Amiel SA, Alberti KG. Inhaled insulin. BMJ. 2004;328:1215-1216.
DeWitt DE, Dugdale DC. Using new insulin strategies in the outpatient treatment of diabetes. JAMA. 2003;289:2265-2269.
Dunger DB, Sperling MA, Acerini CL, et al. European Society for Pediatric Endocrinology/Lawson Wilkins Pediatric Endocrine Society consensus statement on diabetic ketoacidosis in children and adolescents. Pediatrics. 2004;113:e133-e140.
Edge JA, Jakes RW, Roy Y, et al. The UK case-control study of cerebral edema complicating diabetic ketoacidosis in children. Diabetologia. 2006;49:2002-2009.
Felner EI, White PC. Improving management of diabetic ketoacidosis in children. Pediatrics. 2001;108:735-740.
Fiordalisi I, Novotny WE, Holbert D, et al. An 18-yr prospective study of pediatric diabetic ketoacidosis: an approach to minimizing the risk of brain herniation during treatment. Critical Care Management Group. Pediatr Diabetes. 2007;8:142-149.
Glaser NS, Wootton-Gorges SL, Marcin JP, et al. Mechanism of cerebral edema in children with diabetic ketoacidosis. J Pediatr. 2004;145:164-171.
Green SM, Rothrock SG, Ho JD, et al. Failure of adjunctive bicarbonate to improve outcome in severe pediatric diabetic ketoacidosis. Ann Emerg Med. 1998;31:41-48.
Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
Hom J, Sinert R. Evidence-based emergency medicine/critically appraised topic. Is fluid therapy associated with cerebral edema in children with diabetic ketoacidosis? Ann Emerg Med. 2008;52:69-75.
Kuppermann N, Park J, Glatter K, et al. Prolonged QT interval corrected for heart rate during diabetic ketoacidosis in children. Arch Pediatr Adolesc Med. 2008;162:544-549.
Lawrence SE, Cummings EA, Gaboury I, et al. Population-based study of incidence and risk factors for cerebral edema in pediatric diabetic ketoacidosis. J Pediatr. 2005;146:688-692.
2006 Medical Letter: Insulin glulisine (Apidra): a new rapid-acting insulin. Med Lett. 2006;48:33-34.
Muir AB, Quisling RG, Yang MCK, et al. Cerebral edema in childhood diabetic ketoacidosis: natural history, radiographic findings, and early identification. Diabetes Care. 2004;27:1541-1546.
Okuda Y, Adrogue J, Field JB, et al. Counterproductive effects of sodium bicarbonate in diabetic ketoacidosis. J Clin Endocrinol Metab. 1996;81:314-320.
Management of Type 1 Diabetes in Children
Alemzadeh R, Berhe T, Wyatt DT. Flexible insulin therapy with glargine insulin improved glycemic control and reduced severe hypoglycemia among preschool-aged children with type 1 diabetes mellitus. Pediatrics. 2005;115:1320-1324.
Alemzadeh R, Ellis JN, Holzum MK, et al. Beneficial effects of continuous subcutaneous insulin infusion and flexible multiple daily insulin regimen using insulin glargine in type 1 diabetes mellitus. Pediatrics. 2004;114:e91-e95.
Alemzadeh R, Palma-Sisto P, Holzum MK, et al. Continuous subcutaneous insulin infusion attenuated glycemic instability in preschool children with type 1 diabetes mellitus. Diabetes Technol Ther. 2007;9:339-347.
Arslanian S, Ohki Y, Becker DJ, et al. The dawn phenomenon: comparison between normal and insulin-dependent diabetic adolescents. Pediatr Res. 1992;31:203-206.
Berhe T, Postellon D, Wilson B, et al. Feasibility and safety of insulin pump therapy in children aged 2 to 7 years with type I diabetes: a retrospective study. Pediatrics. 2006;117:2132-2137.
Bolli GB, Gerich JE. The “dawn phenomenon”—a common occurrence in both non-insulin and insulin-dependent diabetes mellitus. N Engl J Med. 1984;310:746-750.
Bolli GB, Gottesman IS, Campbell PJ, et al. Glucose counter regulation and waning of insulin in the Somogyi phenomenon (posthypoglycemic hyperglycemia). N Engl J Med. 1984;311:1214-1219.
Chase HP, Lutz K, Pencek R, et al. Pramlintide lowered glucose excursions and was well-tolerated in adolescents with type 1 diabetes: results from a randomized, single-blind, placebo-controlled, crossover study. J Pediatr. 2009;155:369-373.
de Kort H, de Koning EJ, Rabelink TJ, et al. Islet transplantation in type 1 diabetes. BMJ. 2011;342:d217.
Durward A, Ferguson LP, Taylor D, et al. The temporal relationship between glusoce-corrected serum sodium and neurological status in severe diabetic ketoacidosis. Arch Dis Child. 2011;96:50-57.
Kishiyama CM, Burdick PL, Cobry EC, et al. A pilot trial of pramlintide home usage in adolescents with type 1 diabetes. Pediatrics. 2009;124:1344-1347.
Litton J, Rice A, Friedman N, et al. Insulin pump therapy in toddlers and preschool children with type 1 diabetes mellitus. J Pediatr. 2002;141:490-495.
Maniatis AK, Klingensmith GJ, Slover RH, et al. Continuous subcutaneous insulin infusion therapy in children and adolescents: an option for routine diabetes care. Pediatrics. 2001;107:351-356.
Raman VS, et al. New potential adjuncts to treatment of children with type 1 diabetes mellitus. Pediatr Res. 2009;65:370-374.
Rami B, Schober E. Postprandial glycaemia after regular and lispro insulin in children and adolescents with diabetes. Eur J Pediatr. 1997;156:838-840.
Regan FM, Dunger DB. Use of new insulins in children. Arch Dis Child. 2006;91:ep47-ep53.
Taplin CE, Cobry E, Messer L, et al. Prevening post-exercise nocturnal hypoglycemia in children with type 1 diabetes. J Pediatr. 2010;157:784-788.
Weinzimer S, Ahern JH, Doyle EA, et al. Persistence of benefits of continuous subcutaneous insulin infusion in very young children with type 1 diabetes: a follow-up report. Pediatrics. 2004;114:1601-1605.
Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568-1576.
Wilson D, Buckingham B, Kunselman EL, et al. A two-center randomized controlled feasibility trial of insulin pump therapy in young children with diabetes. Diabetes Care. 2005;28:15-19.
Zeitler P, Haqq A, Rosenbloom A, et al. Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158:9-14.
Long-Term Outcome of Childhood Diabetes: Relation of Control to Development of Complications
1996 The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45:1289-1298.
Bojestig M, Arnqvist HJ, Karlberg BE. Glycemic control and prognosis in type 1 diabetic patients with microalbuminuria. Diabetes Care. 1996;19:313-317.
Cleary PA, Orchard TJ, Genuth S, et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2006;55:3556-3565.
Leslie ND, Sperling MA. Relation of metabolic control to complications in diabetes mellitus. J Pediatr. 1986;108:491-497.
Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676-1685.
Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329:304-309.
Rosenbloom AL. Skeletal and joint manifestations of childhood diabetes. Pediatr Clin North Am. 1984;31:569-589.
Sandman DD, Shore AC, Tooke JE. Relation of skin capillary pressure in patients with insulin-dependent diabetes mellitus to complications and metabolic control. N Engl J Med. 1992;327:760-764.
Wang PH. Tight glucose control and diabetic complications. Lancet. 1993;342:129.
Type 2 Diabetes and Diseases and Syndromes Associated with Diabetes
Adler AI, Shine BS, Chamnan P, et al. Genetic determinants and epidemiology of cystic fibrosis-related diabetes: results from a British cohort of children and adults. Diabetes Care. 2008;31:1789-1794.
Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA1C in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481-488.
Barrett TG, Bundey SE. Wolfram (DIAMOAD) syndrome. J Med Genet. 1997;34:838-841.
Chiefari E, Tanyolac S, Paonessa F, et al. Functional variants of the HMGA1 gene and type 2 diabetes mellitus. JAMA. 2011;305(9):903-912.
Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1C levels in patients with type 2 diabetes. JAMA. 2010;304(20):2253-2262.
The Healthy Study Group. A school-based intervention for diabetes risk prevention. N Engl J Med. 2010;363(5):443-452.
Holst JJ, Deacon CF, Vilsbøll T, et al. Glucagon-like peptide-1, glucose homeostasis and diabetes. Trends Mol Med. 2008;14:161-168.
Krook A, Brueton L, O’Rahilly S. Homozygous nonsense mutation in the insulin receptor gene in an infant with leprechaunism. Lancet. 1993;342:277-278.
The Lancet. More insights on the dysglycaemia–cardiovascular connection. Lancet. 2010;375:2195-2196.
The Lancet. The day of diabetes: coming soon to a person near you. Lancet. 2010;376:1513.
Madsbad S. Treatment of type 2 diabetes with incretin-based therapies. Lancet. 2009;373:438-439.
McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363(24):2339-2350.
The Medical Letter. Bromocriptine (cycloset) for type 2 diabetes. Med Lett. 2010;52:97-98.
Morrison EY, McKenzie K. The Mauriac syndrome. West Indian Med J. 1989;38:180-182.
Mozzillo E, Franzese A, Valerio G, et al. One-year glargine treatment can improve the course of lung disease in children and adolescents with cystic fibrosis and early glucose derangements. Pediatr Diabetes. 2009;10:162-167.
Nguyen QM, Srinivasan SR, Xu JH, et al. Fasting plasma glucose levels within the normoglycemic range in childhood as a predictor of prediabetics and type 2 diabetes in adulthood. Arch Pediatr Adolesc Med. 2010;164:124-128.
Ramachandran A, Ma RCW, Snehalatha C. Diabetes in Asia. Lancet. 2010;375:408-416.
Rotig A, Cormier V, Chatelain P, et al. Deletion of mitochondrial DNA in a case of early-onset diabetes mellitus, optic atrophy, and deafness. J Clin Invest. 1993;91:1095-1098.
Scheen AJ, Radermecker RP. Addition of incretin therapy to metformin in type 2 diabetes. Lancet. 2010;375:1410-1412.
Shah S, Kublaoui BM, Oden JD, et al. Screening for type 2 diabetes in obese youth. Pediatrics. 2009;124:573-579.
Sobngwi E, Choukem SP, Agbalike F, et al. Ketosis-prone type 2 diabetes mellitus and human herpesvirus 8 infection in sub-Saharan Africans. JAMA. 2008;299:2770-2776.
Talmud PJ, Hingorani AD, Cooper JA, et al. Utility of genetic and non-genetic risk factors in prediction of type 2 diabetes: Whitehall II prospective cohort study. BMJ. 2010;340:192.
Taylor SI, Cama A, Accili D, et al. Mutations in the insulin receptor gene. Endocr Rev. 1992;13:566-595.
Van den Berg JM, Morton AM, Kok SW, et al. Microvascular complications in patients with cystic fibrosis-related diabetes (CFRD). J Cyst Fibros. 2008;7:515-519.
Vaxillaire MDP, Bonnefond A, Froguel P. Breakthroughs in monogenic diabetes genetics: from pediatric forms to young adulthood diabetes. Pediatr Endocrinol Rev. 2009;6:405-417.
Voight BF, Scott LJ, Steinthorsdottir V, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42:579-589.
Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448-453.
Weigensberg MJ, Goran MI. Type 2 diabetes in children and adolescents. Lancet. 2009;373:1743-1744.
Yamauchi T, Hara K, Maeda S, et al. A genome-wide association study in the Japanese population identifies susceptibility loci for type 2 diabetes at UBE2E2 and C2CD4A-C2CD4B. Nat Genet. 2010;42(10):864-868.
Yang W. Diagnosing diabetes using glycated haemoglobin A1c. BMJ. 2010;340:c2262.
Alcolado JC, Thomas AW. Maternally inherited diabetes mellitus: the role of mitochondrial DNA defects. Diabet Med. 1995;12:102-108.
Babenko AP, Polak M, Cavé H, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355:456-466.
Chen R, Hussain K, Al-Ali M, et al. Neonatal and late-onset diabetes mellitus caused by failure of pancreatic development: report of 4 more cases and a review of the literature. Pediatrics. 2008;121:e1541-e1547.
Geffner ME, Clare-Salzler M, Kaufman DL, et al. Permanent diabetes developing after transient neonatal diabetes. Lancet. 1993;341:1095.
Gloyn AL, Pearson ER, Antcliff AF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium channel subunit Kir6.2 and permanent neonatal disease. N Engl J Med. 2004;350:1838-1849.
Metz C, Cavé H, Bertrand AM, et al. Neonatal diabetes mellitus: chromosomal analysis in transient and permanent cases. J Pediatr. 2002;141:483-489.
Pagliara AS, Karl IE, Kipnis DB. Transient neonatal diabetes: delayed maturation of the pancreatic beta cell. J Pediatr. 1973;82:97-101.
Pearson ER, Flechtner I, Njølstad PR, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467-477.
Shield JP. Neonatal diabetes. Horm Res. 2007;68(Suppl 5):32-36.
Støy J, Greeley SA, Paz VP, et al. Diagnosis and treatment of neonatal diabetes: a United States experience. Pediatr Diabetes. 2008;9:450-459.
Turkkahraman D, Bircan I, Tribble ND, et al. Permanent neonatal diabetes mellitus caused by a novel homozygous (T168A) glucokinase (GCK) mutation: initial response to oral sulphonylurea therapy. J Pediatr. 2008;153:122-126.
Amiel SA, Tamborlane WV, Simonson DC, et al. Defective glucose counter regulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med. 1987;316:1376-1383.
Bolli GB, Fanelli CG. Unawareness of hypoglycemia. N Engl J Med. 1995;333:1771-1772.
Cryer PE, Fisher JN, Shamoon H. Hypoglycemia. Diabetes Care. 1994;17:734-755.
Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications trial. Diabetes. 1997;46:271-286.
Gschwend S, Ryan C, Atchinson J, et al. Effects of acute hyperglycemia on mental efficiency and counterregulatory hormones in adolescents with insulin-dependent diabetes mellitus. J Pediatr. 1995;126:178-184.
McCrimmon RJ, Gold AE, Deary IJ, et al. Symptoms of hypoglycemia in children with IDDM. Diabetes Care. 1995;18:858-861.
Porter PA, Keating B, Byrne G, et al. Incidence and predictive criteria of nocturnal hypoglycemia in young children with insulin-dependent diabetes mellitus. J Pediatr. 1997;130:366-372.
Silverstein JH, Gordon G, Pollock BH, et al. Long-term glycemic control influences the onset of limited joint mobility in type I diabetes. J Pediatr. 1998;132:944-947.
Wayne EA, Dean HJ, Booth F, et al. Focal neurologic deficits associated with hypoglycemia in children with diabetes. J Pediatr. 1990;117:575-577.
Pancreas and Islet Transplantation
Alejandro R, Lehmann R, Ricordi C, et al. Long-term function (6 years) of islet allografts in type 1 diabetes. Diabetes. 1997;46:1983-1989.
Couri CEB, Oliveira MCB, Stracieri ABP, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301:1573-1579.
Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293:830-835.
Kaddis JS, Olack BJ, Sowinski J, et al. Human pancreatic islets and diabetes research. JAMA. 2009;301:1580-1587.
Larsen JL, Stratta RJ. Consequences of pancreas transplantation. J Investig Med. 1994;42:622-631.
Mitanchez D, Doiron B, Chen R, et al. Glucose-stimulated genes and prospects of gene therapy for type 1 diabetes. Endocr Rev. 1997;18:520-540.
Robertson RP. Islet transplantation as a treatment for diabetes—a work in progress. N Engl J Med. 2004;350:694-705.
Ryan EA, Lakey JR, Paty BW, et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51:2148-2157.
Shapiro AMJ, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318-1330.

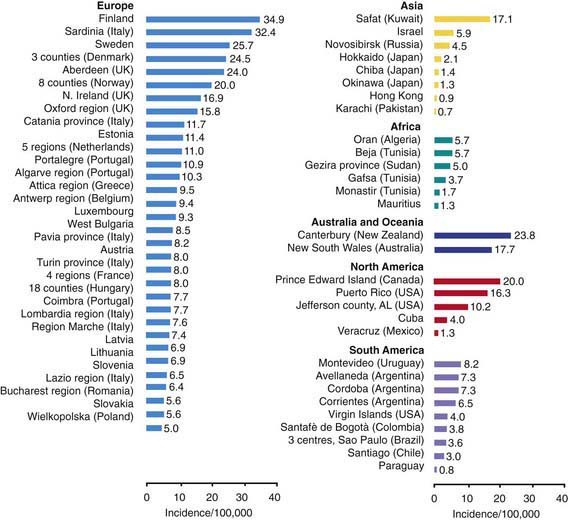
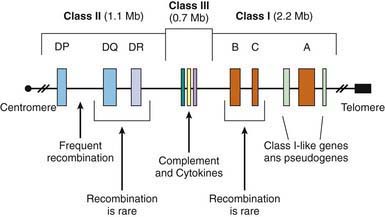
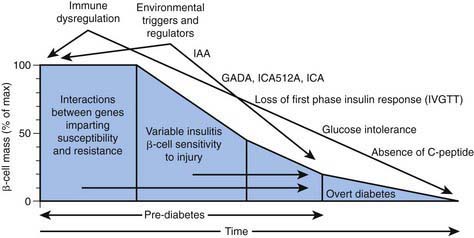
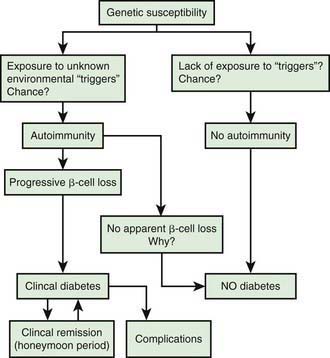
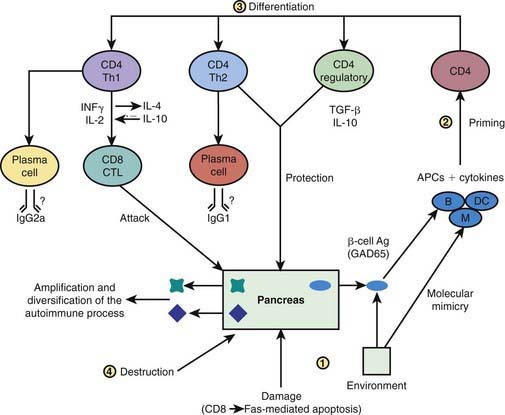
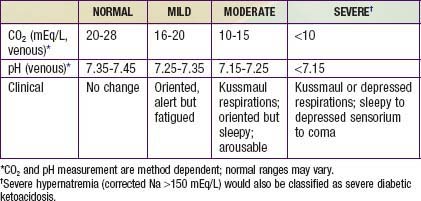
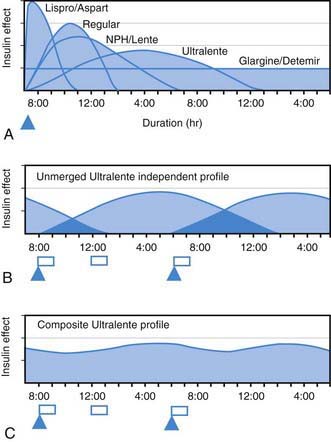
 , Injection time. B, Two Ultralente injections given at breakfast and supper. Note overlap of profiles. C, Composite curve showing approximate cumulative insulin effect for the 2 Ultralente injections. This composite view is much more useful to the patient, parents, and medical personnel because it shows important combined effects of multiple insulin injections with variable absorption characteristics and overlapping durations.
, Injection time. B, Two Ultralente injections given at breakfast and supper. Note overlap of profiles. C, Composite curve showing approximate cumulative insulin effect for the 2 Ultralente injections. This composite view is much more useful to the patient, parents, and medical personnel because it shows important combined effects of multiple insulin injections with variable absorption characteristics and overlapping durations.