Chapter 45 Developmental Language Disorders
Introduction
Developmental language disorder (DLD; also known as Specific Language Impairment – SLI) is a condition in which a child with normal intelligence and hearing fails to develop language in an age-appropriate fashion. DLD is a clinical diagnosis, based on the presence of a normal nonverbal IQ, evidence of expressive and/or receptive language at least 1.5 standard deviations below the mean for age, and absence of other specific conditions such as autism, mental retardation, metabolic or genetic disorders, or severe environmental deprivation. The prevalence of DLD varies widely in different reports with a range of 1–11 percent [Bishop, 2002a; Shriberg et al., 1999]. Box 45-1 lists normal language milestones as a baseline for assessing a child’s developing language competence.
Pathophysiology
The underlying neural dysfunction that causes DLD is not known. Studies over the past 30 years have suggested that DLD is the result of a deficit in processing rapid auditory information. Evidence for this hypothesis comes from research showing that children with DLD have difficulty discriminating both nonspeech and speech sounds that are presented rapidly or very briefly in time [Tallal et al., 1993; Tallal and Benasich, 2002]. Some investigators argue for a speech-specific processing deficit [Mody et al., 1997], while others believe that the deficit is more general and that processing of all auditory information is slowed in DLD, whether the information presented is linguistic or nonlinguistic (e.g., environmental sounds) [Cummings and Ceponiene, 2010]. Studies using event-related brain potentials (ERPs) have demonstrated clear differences between DLD and control children, with language-impaired individuals having delayed N400 responses to incongruous picture–word combinations [McArthur et al., 2009].
MRI studies have shown relatively subtle differences between the brains of DLD and controls. Some children and adults with DLD (as well as relatives of DLD probands) do not have the typical planum temporale asymmetry pattern [Jackson and Plante, 1996; Gauger et al., 1997]. The absence of the typical planum asymmetry may be the result of aberrant neurogenesis, which leads to reduced cell development in the perisylvian regions or atypical patterns of cell death. Atypical right-biased asymmetries have also been reported in the prefrontal region [Jernigan et al., 1991]. An extra sulcus in the inferior frontal gyrus was associated with a history of DLD [Clark and Plante, 1998] in a group of 41 neurologically normal adults. DLD children may have decreased white-matter volumes in a left-hemispheric network comprising the motor cortex, the dorsal premotor cortex, the ventral premotor cortex, and the planum polare in the superior temporal gyrus [Jancke et al., 2007]. Rare reports document right hemisphere abnormalities in the DLD child that are suggestive of a right hemisphere contribution to language acquisition [Plante et al., 2001]. Trauner et al. [2000], in a series of 35 children with DLD, found evidence of structural abnormalities in one-third of the children. These included ventricular enlargement (n = 5), central volume loss (3), and white-matter abnormalities (4). The findings were consistent with bilateral white-matter disruption, and suggested that connectivity between different brain regions important for language development might be disrupted in DLD.
Other structural differences in brain development have been observed in some individuals with specified DLD types. Perisylvian abnormalities of varying degrees and associated with language disorders of varying severity have been reported, particularly in verbal dyspraxia and the phonological syntactic syndromes (see below for nosology). Complete opercular agenesis has been reported in association with suprabulbar palsy (Worster–Drought syndrome). Polymicrogyria has also been reported in the perisylvian region in children with DLD. Patients with the most extensive disease have the greatest language impairments, while those with posterior parietal polymicrogyria have milder symptoms [Guerreiro et al., 2002; Nevo et al., 2001]. One form of DLD, semantic pragmatic syndrome, has been reported in patients with agenesis of the corpus callosum and with hydrocephalus, which supports a possible localization in the subcortex and its connections or a disconnection effect. Consistent with this, callosal size may be decreased in some children with DLD [Preis et al., 2000]. In the KE family (see below) the caudate nucleus and inferior frontal gyrus are reduced in size bilaterally, while the left frontal opercular region (pars triangularis and anterior insular cortex) and the putamen bilaterally have a greater volume of gray matter [Watkins et al., 2002a]. An insufficient dosage of a critical forkhead transcription factor during embryogenesis may lead to malformations of regions of the brain necessary for speech and language development [Lai et al., 2001].
Metabolic imaging suggests abnormalities in the left temporal region and may vary by DLD subtype. Some children with DLD may be right hemisphere language-dominant [Bernat and Altman, 2003]. Whitehouse and Bishop [2008] compared language organization in four groups of adults – those with persisting SLI, those with a history of SLI, those on the autistic spectrum (ASD) with language impairment, and matched controls – using functional transcranial Doppler ultrasonography (fTCD), which assesses blood flow through the middle cerebral arteries. The participants were asked to generate words starting with a given letter silently and then later were required to verbalize these words. All of the participants in the SLI-history group and the majority of participants in the ASD (81.8 percent) and typical (90.9 percent) groups had greater activation in the left compared to the right middle cerebral arteries, while the majority of participants in the persistent SLI group had bilateral (27 percent) or right hemisphere (55 percent) language function. The investigators suggest that atypical language dominance may be a marker of persisting SLI. All 17 of the DLD children studied by Im et al. [2007] had grossly normal magnetic resonance imaging (MRI); however, 87.5 percent had decreased metabolic activity on positron emission tomography (PET) studies, most frequently in the thalamus, but also in both frontal, temporal, and right parietal areas, and significantly increased metabolism in both occipital areas as compared to a control group. Children with SLI showed significantly lower cerebral blood flow (CBF) values in the right parietal region and in subcortical regions compared to an attention-deficit hyperactivity disorder (ADHD) group. In addition, the DLD group had symmetric CBF distributions in the left and right temporal regions, whereas the ADHD group showed the usual asymmetry with left-sided hemispheric predominance in the temporal regions. The findings provide further evidence of anomalous neurodevelopment with deviant hemispheric lateralization as an important factor in the etiology of SLI. They also point to the role of subcortical structures in language impairment in childhood [Ors et al., 2005].
Although DLD is often referred to as “specific” language impairment, individuals with this disorder often have other accompanying problems, including abnormalities in gross and fine motor function [Trauner et al., 2000; Visscher et al., 2007]. These findings are indicative of more global neurological dysfunction and may reflect the fact that language disorders do not occur in isolation, but are merely the most prominent symptom of a more widespread neural network malfunction. Stuttering is a disorder involving the rhythm and fluency of speech production, as opposed to language. The stutterer knows what s/he wishes to say but cannot get the words out without significant dysfluency and hesitation. The neurobiological basis of stuttering has been the subject of a number of recent studies [Brown et al., 2005; Watkins et al., 2008]. During speech production, regardless of fluency or auditory feedback, stutterers showed overactivity relative to controls in the anterior insula, cerebellum, and midbrain bilaterally, and underactivity in the ventral premotor, rolandic opercular and sensorimotor cortex bilaterally, and Heschl’s gyrus on the left. These results are consistent with a recent meta-analysis of functional imaging studies in developmental stuttering. Overactivity occurred in the midbrain, at the level of the substantia nigra, and extended to the pedunculopontine nucleus, red nucleus, and subthalamic nucleus. This overactivity is consistent with suggestions in previous studies of abnormal function of the basal ganglia or excessive dopamine in stutterers. Underactivity of the cortical motor and premotor areas was associated with articulation and speech production. Analysis of the diffusion data revealed that the integrity of the white matter underlying the underactive areas in ventral premotor cortex was reduced in people who stutter. The white matter tracts in this area, via connections with posterior superior temporal and inferior parietal cortex, provide a substrate for the integration of articulatory planning and sensory feedback, and via connections with primary motor cortex, a substrate for execution of articulatory movements. These data would lead to the conclusion that stuttering is a disorder related primarily to disruption in the cortical and subcortical neural systems supporting the selection, initiation, and execution of motor sequences necessary for fluent speech production [Watkins et al., 2002b]. A recent meta-analysis of these studies identified three “neural signatures” of stuttering: people who stutter show more activity than fluent-speaking controls in the cerebellar vermis and in the right anterior insular cortex, with an “absence” of activity in the auditory cortices in the superior temporal lobe [Brown et al., 2005]. These abnormal levels of activity were observed during speech production, regardless of the presence or absence of stuttered speech during scan acquisition. Surprisingly, the meta-analysis did not reveal abnormal levels of activity in the basal ganglia circuitry, despite early imaging work on small samples showing abnormal metabolism.
Factors Associated with DLD
As with many neurodevelopmental disorders, there is a higher incidence of DLD in males. One recent study showed a ratio of 1.66:1 males to females in a group of children with communication disorders [McLeod and McKinnon, 2007]. The cause for the gender differences is not known. A number of biological and environmental risk factors for DLD have been identified. Box 45-2 lists a number of disorders associated with language impairment. Low birth weight and prematurity, as well as prenatal exposure to drugs (e.g., cocaine) and to cigarettes, adversely affect language development [Lewis et al., 2007]. Although frequent episodes of otitis media have been suggested as causing language impairment, there is little evidence from controlled studies to indicate a causal relationship. Intermittent hearing loss may interfere with language development in at-risk children, but is not likely to cause long-term language issues in otherwise normally developing children. Other environmental factors that may potentially adversely affect language development have been studied. For example, early and excessive TV exposure has been associated with language delay. Children who started watching television before 12 months of age and watched television for more than 2 hours per day were approximately six times more likely to have language delays than children without such early TV exposure [Chonchaiya et al., 2008]. Such associations, however, do not indicate causation.
Box 45-2 Disorders Known to be Associated with Language Delay/Impairment
Language impairment is seen in association with specific neurological and genetic disorders [Nass and Frank, 2010]. For example, perisylvian polymicrogyria (or congenital bilateral perisylvian syndrome) is a disorder of defective neuronal migration that has a spectrum of neurological impairments that include severe epilepsy and cognitive impairment. In some children with this condition, language impairment is the most prominent feature [Brandao-Almeida et al., 2008]. Language impairment has been described in children with neurofibromatosis type 1, although those individuals often have other cognitive deficits and learning disabilities as well. Language impairment is also prominent in a number of chromosomal disorders, including Down, Klinefelter’s, and fragile X syndromes. Epileptic encephalopathies, particularly Landau–Kleffner syndrome (LKS), may present with language impairment as an isolated or primary symptom [Kleffner and Landau, 2009]. Children with LKS typically have greater receptive than expressive language dysfunction, although they may become completely aphasic and mute in the course of the disease process. Rolandic epilepsy, often considered to be “benign”, may be complicated by DLD and learning disabilities [Lillywhite et al., 2009].
Genetics
Heritability rates for DLDs run as high as 0.5 [Byrne et al., 2009], but they are very variable and are affected by the criteria used to diagnose DLD. The median incidence rate for language difficulties in the families of children with language impairment runs as high as 35 percent, compared with a median incidence rate of 11 percent in control families [Stromswold, 1998]. Increased monozygotic versus dizygotic twin concordance rates indicate that heredity, not just shared environment, is responsible for familial clustering [Bartlett et al., 2002; Stromswold, 2001]. Heritability is substantially higher if DLD is identified based on referral to speech and language pathology services [Bishop and Hayiou-Thomas, 2008] than if it is identified by language test scores. Childhood language disorders that are demonstrated in population screening are likely to have different phenotypes and different etiologies than clinically referred cases. It is also possible, however, that familial cases are more likely to come to the attention of specialists since the parents are more attuned to the problem and recognize it earlier in subsequent children.
Studies using genome-wide scanning have implicated a number of gene loci, but the same loci have not been found in a reproducible fashion [SLI Consortium, 2002; Grigorenko, 2009]. One exceptional family stands out. In the three-generation KE family, half the members are affected with a severe speech and language disorder that is transmitted as an autosomal-dominant monogenic trait involving the FOXP2 forkhead-domain gene [Watkins et al., 2002b]. Notably, however, a recent screening of 270 4-year-olds with DLD was negative for the FOXP2 mutation [Meaburn et al., 2002]. It is unlikely that there will prove to be specific genes whose function would be restricted to forming the genetic basis for speaking and language acquisition. It is much more likely that there are many genes that contribute to a variety of functions, and that these genes form networks that are recruited in the process of forming language-related representations during language acquisition [Grigorenko, 2009]. The definition of the phenotype forming the basis for specific genetic studies is crucial. To date, in addition to the KE family phenotype, nonword repetition has proven the most useful phenotype in molecular genetic research [SLI Consortium, 2004; Vernes et al., 2008]. The issue of pleiotropy, or the impact of the same genes on multiple phenotypes, has also been discussed in the literature on DLD, given the substantive overlap in regions of linkage for a variety of developmental disorders, such as speech and sound disorders (SSD) and developmental dyslexia [Miscimarra et al., 2007; Stein et al., 2004], and SLI and autism [Tager-Flusberg and Joseph, 2003]. Once again, whether these are true examples of pleiotropy or outcomes of the imprecision of phenotype definitions is yet to be determined [Grigorenko, 2009].
Diagnosis
Box 45-3 lists warning signs that suggest a DLD during the first 3 years. Language delay can be diagnosed very early, whether the delay is primarily expressive only or mixed receptive and expressive. Before the age of 2 years, however, delay may not always equal disorder. Research on late-talking toddlers reveals a lack of homogeneity within the population of children with a vocabulary delay at 2 years of age. In a recent study, only about 40 percent of children retained the diagnosis of DLD at ages 3 and 4 years [Dale et al., 2003]. This is particularly true if the early language delay was primarily expressive. Children with receptive language impairments are more likely to have a persistent DLD. The pattern of receptive language development is highly predictable during the elementary school years. In a study of 184 children age-assessed at three time points – 7 years, 8 years, and 11 years of age – receptive language disorder was associated with declining rates of language growth over time [Law et al., 2008]. Thus, concern for poor language prognosis should be heightened when receptive language deficits are identified.
Box 45-3 Warning Signs of a Developmental Language Disorder
(Modified with permission from Nelson NW. Childhood Language Disorders in Context: Infancy through Adolescence. New York: Macmillan, 1993; Hall N. Developmental language disorders. Semin Pediatr Neurol 1997;4:77–85.)
Another basis sometimes suggested as a means of screening for DLD is the presence of a large discrepancy between nonverbal intelligence and language capabilities [Klee et al., 2000; Aram et al., 1992]. However, this means of identification of DLD may have variable results, depending on the tests used. Various discrepancy criteria have been used to identify children with developmental language disorders [Bishop, 2004]. In one study of young children clinically designated as having a developmental language disorder, the diagnosis was suspected only 40–60 percent of the time, using variations of the Stanford–Binet IQ test/Test of Language Development discrepancy score. A nonverbal IQ/specific language test performance discrepancy criterion of 1 standard deviation (e.g., Wechsler Performance IQ versus the Peabody Picture Vocabulary Test, Token Test, Rapid Automatized Naming, or Sentence Repetition) identified 34 percent of very low birth weight 7-year-olds and 45 percent of controls as having a developmental language disorder. A 2 standard deviation discrepancy yielded 14 percent and 19 percent frequency in the two groups, respectively [Aram et al., 1992]. Evaluating a group of adolescents, Miller and Gilbert [2008] had similar problems using discrepancy scores to diagnose SLI. They too found that nonverbal IQ scores are dependent on the test being used and the group being tested. The discrepancies were greater in those with SLI than in those with normal language. Thus, using the difference between a language measure and a particular nonverbal IQ measure may result in diagnosis with one test and not another, and potentially different diagnoses (DLD or not) in the same child. Both under- and over-diagnosis occur using seemingly standardized criteria over a broad age range. Thus, this method of assessing for DLD is not the recommended approach.
Nosology of Developmental Language Disorders
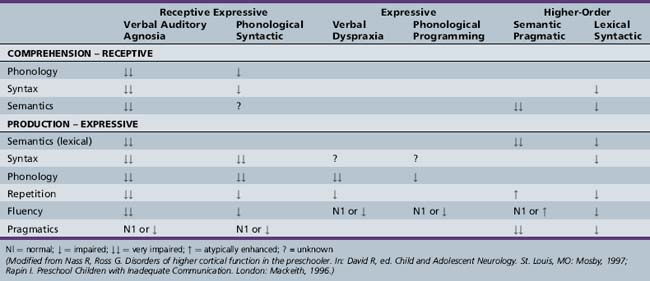
The proper nosology of the DLDs is debated. The DSM-IV-TR [APA, 2000] subtypes of communication disorders (expressive language disorder, mixed receptive–expressive language disorder, phonological disorder, stuttering, and communication disorder, Not Otherwise Specified (NOS)) are rather nonspecific. The subtypes discussed here are more specific and focus on psycholinguistic features. The subtypes are named for the linguistic areas that are most problematic [Rapin, 1996; Table 45-1 and glossary of terms, Box 45-4) Depending on subtype, DLDs vary in their characteristic features, etiology, prognosis, and treatment response.
Box 45-4 Glossary of Terms used in Describing Linguistic Functions
| Functors | The small words of the language, like prepositions, conjunctions, etc. These are also called closed-class words because they are limited in number |
| Lexicon | The words in a language; the dictionary of word meanings |
| Mean length of utterance (MLU) | The number of morphemes per utterance |
| Morpheme | The smallest meaningful unit in a language, occurring either in a word or as a word. A compound word, like “compounding,” is made up of three morphemes: com-pound-ing. Prefixes, suffixes and inflected endings like-ed, -s, and -ly are also morphemes |
| Phoneme | A distinct sound unit in a language. In English, there are 46: 9 vowels and 37 consonants |
| Phonology | The rules a speaker follows when combining speech sounds |
| Pragmatics | The communicative intent of speech rather than its content, e.g. asking a question at the right time and in the right way |
| Prosody | The melody of language: the tone of voice used to ask questions, for example, or show emotion |
| Semantics | The meaning of words; their definition |
| Syntax | The grammar of a language; the acceptable relationship between words in a sentence |
Articulation and Expressive Fluency Disorders
Stuttering and Cluttering
Stuttering is a disorder in the rhythm of speech. The speaker knows what to say, but is unable to say it because of an involuntary, repetitive prolongation or cessation of a sound. Some degree of dysfluency is common as language skills evolve during the preschool years, particularly as the mean length of utterance (MLU) reaches 6–8 words between ages 3 and 4 years. However, stuttering, in contrast to developmental dysfluency, is probably a linguistic disorder (errors occur at grammatically important points in the sentence), as well as a motor planning problem [Logan, 2002]. Stuttering is often a genetic trait. Although the cause of developmental stuttering is unknown, the main theories are anomalous dominance and abnormalities of interhemispheric connections [Foundas et al., 2001]. Stuttering occurs more frequently in children with other DLDs and with mental retardation [Gordon, 2002]. Cluttering, by contrast, as seen in fragile X syndrome, is characterized by incomplete sentences and short outbursts of two- to three-word phrases, along with echolalia, palilalia (compulsive repetition reiterated with increasing rapidity and decreasing volume), perseveration, poor articulation, and stuttering.
Stuttering may provide a clue to the genetics of language disorders. For example, the typically observed onset of stuttering is between the ages of 3 and 6 years, and reports indicate natural, unassisted recovery rates of 75 percent [Yairi and Ambrose, 1999]. Thus, the prevalence of stuttering as a lifetime disorder is much lower than its incidence (0.5–1 percent vs. 4–5 percent, respectively; Bloodstein, 1995; Felsenfeld, 2002]. Yet childhood stuttering is a significant risk factor for other DLDs that develop later in life, even if stuttering disappears. It is possible that this “continuity” of developmental transformation is due to the presence of particular dimensions of the complex phenotypes common to all DLDs [Grigorenko, 2009].
Phonological Programming Disorder
Children with the phonological programming disorder have fluent speech, and their MLU approaches normal. Despite initially poor intelligibility, serviceable speech is expected. Language comprehension is relatively preserved. Most such children show delayed rather than deviant phonology, and improve 1–7 years after their preschool diagnosis. It is debatable whether this disorder is a severe articulation problem or a mild form of verbal dyspraxia [Shriberg and Kwiatkowski, 1994]. The fact that patients with the phonological programming disorder have more difficulty learning manual signs than do controls supports an association with dyspraxia [Bradford and Dodd, 1994; Bishop, 2002a]. A pre-remediation paired associate learning task may help select the best remediation method for each child because some are better with symbols and some with signs [Pearce et al., 1987]. An adult aphasia equivalent does not exist.
Verbal Dyspraxia
The speech of children with verbal dyspraxia [Nevo et al., 2001], also called dilapidated speech [Ferry et al., 1975], is extremely dysfluent. These children are unable to convert an abstract phonological representation into a set of motor commands to the articulators [Bishop, 1992], or, put in other terms they have a deficit in phonology–motor conversion [Thoonen et al., 1997]. Utterances are short and laboriously produced. Phonology is impaired and includes inconsistent omissions, substitutions, and distortions of speech sounds. Consonant substitutions can be divided into substitutions by place of articulation, manner of articulation, and voicing. Children with dysarthria make voicing errors that distort, while children with dyspraxia make place substitution errors [Maassen, 2004]. In conversation, they make phrasal errors [Maassen, 2004]. Syntactic skills are difficult to assess in the face of dysfluency. Language comprehension is relatively preserved, but many children do have noticeable receptive language problems. Many require speech and language therapy for prolonged periods. Children with verbal dyspraxia who do not develop intelligible speech by age 6 years are unlikely to acquire it later. The frequency with which nonverbal praxis deficits – buccal-lingual dyspraxia (e.g., positioning muscles of articulation) and generalized dyspraxia or clumsiness – coexist with verbal dyspraxia is unknown. The presence of a more diffuse disorder of praxis has significant therapeutic implications because children with verbal dyspraxia may depend on signing and writing skills for communication [Shriberg et al., 1997]. Developmental coordination disorder (DCD) is commonly comorbid with speech/language learning disabilities [Visscher et al., 2007]. Young children who are in early intervention programs for speech/language delays may have significant coordination difficulties that will become more evident at kindergarten age, when motor deficits begin to impact self-care and academic tasks [Gaines and Missiuna, 2007]. The use of nonspeech oral motor treatments (NSOMTs) in the management of apraxia is controversial. At this time there is no evidence to support their use [Powell, 2008]. Although often accompanied by other neurological symptoms, verbal dyspraxia most resembles the adult aphasia called aphemia.
Disorders of Receptive and Expressive Language
Phonological Syntactic Syndrome
Phonological syntactic syndrome (also called mixed receptive expressive disorder, expressive disorder, and nonspecific formulation-repetition deficit) is probably the most common DLD [Korkman and Hakkinen-Rihu, 1994]. The high frequency of this subtype is consistent with the view that impaired inflectional morphology is a hallmark of DLD [Rice and Wexler, 1996]. The phonological disturbances consist of omissions, substitutions, and distortions of consonants and consonant clusters in all word positions. The production of unpredictable and unrecognizable sounds makes speech impossible to understand. The syntactic impairment consists of a lack of functors and an absence of appropriate inflected endings. Plurals, third person singulars, past tense, auxiliary verb be, the and a, infinitives – to, and case markings on pronouns are particularly vulnerable. Grammatical forms are atypical, not just delayed. Whereas a typically developing young child may say “baby cry” or “a baby crying,” children with the phonological syntactic syndrome produce deviant constructions, such as “the baby is cry.” Telegraphic speech is common. The presence or absence of difficulties in other language areas is variable. Overall, comprehension is relatively, although not wholly, spared. Semantic skills tend to be intact. Repetition, pragmatics, and prosody may be normal. Autistic children with this DLD subtype produce a significant amount of jargon.
Verbal Auditory Agnosia
Despite intact hearing, meaningful language is not understood by children with verbal auditory agnosia (VAA) (also called generalized low performance and global dysfunction). VAA may occur on a developmental basis, and as an acquired disorder, the Landau–Kleffner syndrome [Billard et al., 2009]. VAA is common in low-functioning children with autism. VAA best supports the theory that DLDs result from difficulty with processing basic sensory information entering the nervous system in rapid succession [Tallal and Benasich, 2002].
Higher-Order Language Disorders
Semantic Pragmatic Syndrome
Children with the semantic pragmatic syndrome (also called repetition strength and comprehension deficit, language without cognition, and cocktail party syndrome in children with hydrocephalus usually with accompanying meningomyeloceles) are fluent speakers, even verbose. Vocabulary is often large and somewhat formal. Parents are often encouraged by the child’s sizeable vocabulary, only to find later that the verbosity did not indicate superior cognitive skills. Many children have trouble with meaningful conversation and informative exchange of ideas. They talk to talk. Pragmatic skills are lacking. Children with semantic pragmatic syndrome often show deficits in prosody; their speech has a monotonous, mechanical, or sing-song quality. They cannot convey the additional pragmatic intentions that prosody affords, such as speaking with the proper emotion or indicating by tone of voice that they are asking a question. Comprehension may be impaired. Phonological and syntactic skills are generally intact [Rapin and Allen, 1998]. Semantic pragmatic syndrome is often seen in higher-functioning autistic children [Bishop, 2002a].
Lexical Syntactic Syndrome
The lexical syntactic syndrome is relatively common, occurring in approximately 15 percent of children with DLD. Speech is generally dysfluent, even to the point of stuttering, because of word-finding difficulties and poor syntactic skills, with many hesitancies and false starts. Both literal and semantic paraphasias are common. Lexical deficits may not be specific to this DLD subtype. Most children with DLD have delays in word acquisition and less lexical diversity than their age-matched counterparts. Verbs appear to be the most difficult lexical category for them to learn. Some have questioned whether their difficulties with verbs cause collateral damage, i.e., contribute to syntactic problems [Leonard and Deevy, 2004]. Syntax is immature but not deviant. Phonology is spared, and therefore speech is intelligible. Repetition is generally better than spontaneous speech. In conversation, idiom use is better than spontaneous speech. During narratives fourth-graders with DLD evidence higher disruption rates at phrase boundaries than do their age-matched peers, reflecting the lexical and syntactic deficits in children with DLD [Guo et al., 2008]. Pragmatics may be impaired, particularly when this syndrome occurs in autistic children. Comprehension is generally acceptable, although complex questions and other linguistic forms taxing higher-level receptive syntactic skills are often deficient.
Outcome of Developmental Language Disorders
The occurrence of a DLD, even when it appears to resolve, may affect later social-emotional adjustment, educational achievement, and vocational choices. Short- and long-term behavioral, social-emotional, and psychiatric problems are associated with early language problems [Irwin et al., 2002; Jerome et al., 2002]. Language delays were not associated with behavior problems among toddlers, except that toddlers aged 18–30 months with language delays appeared to show elevated social withdrawal relative to typically developing toddlers [Rescorla et al., 2007]. Using data from Child Behavior Checklists completed by teachers and parents, Coster et al. [1999] found that behavior problems were common in DLD children in elementary school, occurring in 32 percent of their sample, and those problems tended to be internalizing rather than externalizing.
Emotional and behavioral problems often surface as DLD children enter school. In school-age children with speech and language problems, the frequency of ADHD ranges from 30 to 49 percent, and the frequency of behavioral and emotional problems ranges from 10–22 percent to 50 percent [Beitchman et al., 1996; Cantwell and Baker, 1987]. The incidence of dyslexia and other learning disabilities in children earlier diagnosed with DLD is up to 50 percent in some studies [Eisenmajer et al., 2005]. Children with early language impairment had higher rates of anxiety disorder (particularly social phobia) and antisocial personality disorder in young adulthood compared with nonimpaired children. The majority of participants with anxiety disorders had a diagnosis of social phobia. Trends were found toward associations between language impairment and overall and antisocial personality disorder rates [Beitchman et al., 2001]. In some, but not all, studies the biggest differentiating factor between those with and those without a psychiatric diagnosis was the degree of language deficit [Cantwell and Baker, 1987; Beitchman et al., 2001]. In preschool children with DLD, nonverbal intelligence is the best single predictor of overall long-term outcome, and severity of language problems is the best predictor of later language skills. Preschool language skills are the best single predictor of later reading ability and disability [Aram and Nation, 1980]. Even children with good receptive skills who speak late may be at risk for continuing subtle language difficulties and later reading and language-based academic difficulties [Rescorla et al., 1997]. Thus, both screening and follow-up studies of children with DLD are important. Persisting, although often subtle, language or communication problems in adolescence and beyond have been reported in as many as 90 percent of individuals with earlier diagnosed DLD [Conti-Ramsden et al., 2001; Rescorla, 2002, Young et al., 2002]. Follow-up of 112 individuals with DLD into adult life demonstrated lower levels of functioning in the areas of communication, educational attainment, and occupational status compared with their typical peers [Johnson et al., 2009]. Interestingly, the adults with DLD did not perceive their quality of life to be worse than that of their peers. Such studies, however, do indicate the need for continued surveillance of individuals with DLD and adequate guidance in terms of academic and career choices.
Evaluation of the Child with Suspected DLD
The differential diagnosis of a child with language impairment is listed in Box 45-5. The work-up of the child with a DLD (Box 45-6) must include an assessment of hearing and an assessment of overall level of cognitive functioning. A number of metabolic disorders can present with isolated language disorders, so in some circumstances a metabolic screen is appropriate [Vodopiutz et al., 2007]. Mitochondrial disorders, as well as organic acidemias, may have language impairment as their primary feature, particularly in the first few years of life. Mucopolysaccharidosis type III (Sanfilippo Syndrome), as well as juvenile Tay–Sachs disease, may present early with what appears to be an isolated language delay. Those children will eventually develop progressive neurologic deterioration, but initially such conditions should be considered in the differential diagnosis of a child with unexplained language delay. Similarly, some other syndromes present with predominantly language issues in association with their distinctive dysmorphic features [Nass and Frank, 2010]. Children with congenital cleft palate and those with velocardiofacial syndrome may have specific language impairments not explained solely by the facial deformity [Goorhuis-Brouwer et al., 2003; Priester and Goorhuis-Brouwer, 2008].
Box 45-6 Evaluation of a Child with Suspected Language Disorder
 Complete neurodevelopmental and family history and neurological examination (including social interaction and communicative behaviors)
Complete neurodevelopmental and family history and neurological examination (including social interaction and communicative behaviors) Office developmental screen (e.g., Denver Developmental Test [Frankenburg et al., 1992], McArthur Communicative Development Inventory [Fenson et al., 1993]; Children’s Communication Checklist [Norbury et al., 2004]; Peabody Picture Vocabulary Test [Dunn and Dunn, 2001]; Preschool language scale [Zimmerman et al., 2011]; Early Language Milestones [Coplan et al., 2006]) may be helpful
Office developmental screen (e.g., Denver Developmental Test [Frankenburg et al., 1992], McArthur Communicative Development Inventory [Fenson et al., 1993]; Children’s Communication Checklist [Norbury et al., 2004]; Peabody Picture Vocabulary Test [Dunn and Dunn, 2001]; Preschool language scale [Zimmerman et al., 2011]; Early Language Milestones [Coplan et al., 2006]) may be helpfulTreatment
Whether intensive early therapy changes the long-term outcome to an appreciable degree remains to be determined [Tyler, 2002; Forrest, 2002; Warren and Yoder, 2004]. Treatment of language-disordered preschool children varies according to the kind of language impairment, as well as its degree of severity. Children with a moderate to severe language impairment, who suffer associated social, cognitive, and behavioral difficulties, are best treated in a therapeutic nursery or special education preschool for language-impaired children. Mildly impaired children can often do well in a regular nursery program combined with individual speech-language therapy. Play materials are used by the speech-language therapist with the preschool child in a directive way. Every activity becomes a language activity, in that the child’s actions are given words by the therapist. Play activities are also a helpful way to engage children with severe expressive difficulties. Pleasurable activities involving the mouth, such as blowing bubbles, or initiating mouth movements and sounds, as well as nonvocal imitative games, such as hand clapping, have been found to foster language acquisition. Formal language work typically begins at the phonologic level, involving repetition of sounds and sound sequences to encourage fluency. Treatment of receptive disorders often necessitates the use of visual modalities, such as signs and gesture. Less severe disorders of comprehension are addressed through practiced structuring of conversations with the child. Developmental language-disordered children with severe comprehension deficits rarely progress in treatment as well as children with primary expressive disorders.
Another focus for language remediation is driven by different theoretical frameworks [Dunn, 1997]. One approach involves identifying specific linguistic deficits (e.g., problems with morphology) and targeting them for remediation. Another approach involves identifying specific DLD subtypes and addressing them in remediation [Law, 2004]. This approach means, for example, that a child’s level of comprehension is taken into account in selecting a strategy for improving language production. A third approach aims to detect a core cognitive processing deficit to be targeted for intervention. A fourth approach emphasizes the neuropsychological profile. In contrast to the other approaches, which are deficit-centered, the neuropsychological approach defines and uses children’s strengths to remediate their weaknesses; it also takes into account the child’s temperament and neurodevelopmental status to determine their learning styles and develop optimal methods for remediating targeted deficits. To date, no formal study has compared the efficacy of these approaches.
In the classroom, some accommodations may be necessary in order for the child with DLD to succeed. Children with DLD may have associated ADHD and/or learning disabilities, and may require additional help from a resource specialist or tutor. They may require additional time for giving reports and for taking tests. Whenever possible, presentation of oral information should be accompanied by visual aids. In support of previous research, children with DLD have problems with inferencing, linking directly observed or stated information to likely outcomes. They also have limited working memory capacity, and they are more likely to make errors related to inattention. In a recent study, narrative abilities of 6-year-old children were linked to their verbal working memory [Dodwell et al., 2008]. The information the DLD group heard was harder to access than information they had been able to generate themselves based on a series of pictures. Such findings suggest that children with DLD are likely to be at a disadvantage in classroom situations, particularly for information presented aurally and if the information is complex. The use of pictorial aids may help them encode the information. They may also benefit from having information broken into manageable (shorter) units.
References
![]() The complete list of references for this chapter is available online at www.expertconsult.com.
The complete list of references for this chapter is available online at www.expertconsult.com.
Aram D.M., Morris R., Hall N.E. The validity of discrepancy criteria for identifying children with developmental language disorders. J Learn Disabil. 1992;25:549.
Aram D.M., Nation J. Preschool language disorders and subsequent language and academic difficulties. J Commun Disord. 1980;13:159.
Bartlett C.W., Flax J.F., Logue M.W., et al. A major susceptibility locus for specific language impairment is located on 13q21. Am J Hum Genet. 2002;71:45-55.
Beitchman J.H., Wilson B., Brownlie E.B., et al. Long-term consistency in speech/language profiles: II. Behavioral, emotional, and social outcomes. J Am Acad Child Adolesc Psychiatry. 1996;35:815-825.
Beitchman J.H., Wilson B., Johnson C.J., et al. Fourteen-year follow-up of speech/language-impaired and control children: psychiatric outcome. J Am Acad Child Adolesc Psychiatry. 2001;40:75-82.
Bernat B., Altman N. Speech delay in children: A functional MR imaging study. Radiology. 2003;229:651-658.
Billard C., Fluss J., Pinton F. Specific language impairment versus Landau-Kleffner syndrome. Epilepsia. 2009;50(Suppl 7):21-24.
Bishop D.V.M. Motor immaturity and specific speech and language impairment: evidence for a common genetic basis. Am J Med Genet Neuropsychiatr Genet. 2002;114:56-63.
Bishop D.V.M. Putting language genes in perspective. Trends Genet. 2002;18:57-59.
Bishop D.V.M. SLI: diagnostic dilemmas. In: Verhoeven L., van Balkom H., editors. Classification of Developmental Language Disorders. Mahwah, NJ: Lawrence Erhbaum; 2004:309-320.
Bishop D.V.M. The underlying nature of SLI. J Child Psychol Psychiatry. 1992;33:3-66.
Bishop D.V.M., Hayiou-Thomas M.E. Heritability of specific language impairment depends on diagnostic criteria. Genes Brain Behav. 2008;7:365-372.
Bloodstein O. A handbook on stuttering. Chicago: National Easter Seal Society; 1995.
Bradford A., Dodd B. The motor planning abilities of phonologically disordered children. Eur J Disord Commun. 1994;29:349-369.
Brandão-Almeida I.L., Hage S.R., Oliveira E.P., et al. Congenital bilateral perisylvian syndrome: familial occurrence, clinical and psycholinguistic aspects correlated with MRI. Neuropediatrics. 2008;39(3):139-145.
Brown S., Ingham R.J., Ingham J.C., et al. Stuttered and fluent speech production: an ALE meta-analysis of functional neuroimaging studies. Human Brain Map. 2005;25:105-117.
Brown L., Sherbenou R.J., Johnsen S.K. Test of Nonverbal Intelligence, Fourth Edition (TONI-4). San Antonio, TX: Pearson; 2010.
Byrne B., Coventry W., Olson Richard K., et al. Genetic and environmental influences on aspects of literacy and language in early childhood: Continuity and change from preschool to Grade 2. J Neurolinguistics. 2009;22:219-236.
Cantwell D., Baker L. Prevalence and type of psychiatric disorders and developmental disorders in three speech and language groups. J Commun Disord. 1987;20:151.
Chonchaiya W., Pruksananonda C. Television viewing associates with delayed language development. Acta Paediatr. 2008;97(7):977-982.
Clark M., Plante E. Morphology of the inferior frontal gyrus in developmentally language-disordered adults. Brain Lang. 1998;61:288-303.
Conti-Ramsden G., Botting N., Simkin Z., et al. Follow-up of children attending infant language units: outcomes at 11 years of age. Int J Lang Commun Disord. 2001;36(2):207-219.
Coster F.W., Goorhuis-Brouwer S.M., Nakken H., et al. Specific language impairments and behavioural problems. Folia Phoniatr Logop. 1999;51:99-107.
Coplan J. Early Language Milestone Scale – Second Edition (ELM Scale-2). Shreveport, LA: DysphagiaPlus; 2006.
Cummings A., Ceponiene R. Verbal and nonverbal semantic processing in children with developmental language impairment. Neuropsychologia. 2010;48(1):77-85.
Dale P.S., Price T.S., Bishop D.V.M., et al. Outcomes of early language delay. I: Predicting persistent and transient delay at 3 and 4 years. J Speech Lang Hear Res. 2003;46:544-560.
Dodwell K., Bavin E.L. Children with specific language impairment: An investigation of their narratives and memory. Int J Lang Commun Disord. 2008;43(2):201-218.
Dunn M.. Remediation of children with DLD. Nass R., Rapin I., editors. Seminars in Pediatric Neurology. 1997:135-142.
Dunn L.M., Dunn D.M. Peabody Picture Vocabulary Test, Fourth Edition (PPVT™-4). San Antonio, TX: Pearson; 2009.
Eisenmajer N., Ross N., Pratt C. Specificity and characteristics of learning disabilities. J Child Psychol Psychiatry. 2005;46(10):1108-1115.
Felsenfeld S. Finding susceptibility genes for developmental disorders of speech: the long and winding road. J Commun Disord. 2002;35:329-345.
Fenson L., Dale P., Reznick J.S., et al. The MacArthur Communicative Development Inventories: User?s Guide and Technical Manual. San Diego, CA: Singular Publishing Group; 1993.
Ferry P., Hall S., Hicks J. Dilapidated speech: developmental verbal apraxia. Dev Med Child Neurol. 1975;17:749-756.
Forrest K. Are oral-motor exercises useful in the treatment of phonological/articulatory disorders? Semin Speech Lang. 2002;23(1):15-26.
Foundas A.L., Bollich A.M., Corey D.M., et al. Anomalous anatomy of speech-language areas in adults with persistent developmental stuttering. Neurology. 2001;57(2):207-215.
Frankenburg W.K., Dodds J., Archer P., et al. The Denver II: A Major Revision and Restandardization of the Denver Developmental Screening Test. Pediatrics. 1992;89:91-97.
Gaines R., Missiuna C. Early identification: are speech/language-impaired toddlers at increased risk for developmental coordination disorder. Child Care Health Dev. 2007;33(3):325-332.
Gauger L.M., Lombardino L.J., Leonard C.M. Brain morphology in children with specific language impairment. J Speech Lang Hear Res. 1997;40:1272-1284.
Goorhuis-Brouwer S.M., Dikkers F.G., Robinson P.H., et al. Specific language impairment in children with velocardiofacial syndrome: Four case studies. Cleft palate Craniofac J. 2003;40:190-196.
Gordon N. Stuttering: incidence and causes. Dev Med Child Neurol. 2002;44(4):278-281.
Grigorenko E.L. Speaking genes or genes for speaking? Deciphering the genetics of speech and language. J Child Psychol Psychiatry. 2009;50:116-125.
Guerreiro M.M., Hage S.R., Guimaraes C.A., et al. Developmental language disorder associated with polymicrogyria. Neurology. 2002;59(2):245-250.
Guo L.Y., Tomblin J.B., Samelson V. Speech disruptions in the narratives of English-speaking children with specific language impairment. J Speech Lang Hear Res. 2008;51(3):722-738.
Im S.-H., Park E.S., Kim D.Y., et al. The neuroradiological findings of children with developmental language disorder. Yonsei Med J. 2007;48(3):405-411.
Irwin J.R., Carter A.S., Briggs-Gowan M.J. The social-emotional development of “late-talking” toddlers. J Am Acad Child Adolesc Psychiatry. 2002;41(11):1324-1332.
Jackson T., Plante E. Gyral morphology in the posterior Sylvian region in families affected by developmental language disorder. Neuropsychol Rev. 1996;6(2):81-94.
Jancke L., Siegenthaler T., Preis S., et al. Decreased white-matter density in a left-sided fronto-temporal network in children with developmental language disorder: evidence for anatomical anomalies in a motor-language network. Brain Lang. 2007;102(1):91-98.
Jernigan T.L., Hesselink J.R., Sowell E., et al. Cerebral structure on magnetic resonance imaging in language- and learning-impaired children. Arch Neurol. 1991;48(5):539-545.
Jerome A.C., Fujiki M., Brinton B., et al. Self-esteem in children with specific language impairment. J Speech Lang Hear Res. 2002;45:700-714.
Johnson C.J., Beitchman J.H., Brownlie E.B. Twenty-year follow-up of children with and without speech-language impairments: Family, educational, occupational, and quality of life outcomes. Am J Speech Lang Pathol. 2009. Jul 30 (epub ahead of print)
Klee T., Pearce K., Carson D. Improving the positive predictive value of screening for developmental language disorders. J Speech Lang Hear Res. 2000;43:821-831.
Kleffner F.R., Landau W.M. The Landau-Kleffner syndrome. Epilepsia. 2009;50(Suppl 7):3.
Korkman M., Hakkinen-Rihu P. A new classification of DLD. Brain Lang. 1994;47:96-104.
Lai C.S., Fisher S.E., Hurst J.A., et al. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519-523.
Law J. The close association between classification and intervention for children with primary language impairment. In: Verhoeven L., van Balkom H., editors. Classification of Developmental Language Disorders. Mahwah, NJ: Lawrence Erhbaum; 2004:401-410.
Law J., Tomblin J.B., Zhang X. Characterizing the growth trajectories of language-impaired children between 7 and 11 years of age. J Speech Lang Hear Res. 2008;51(3):739-749.
Leonard L., Deevy P. Lexical deficits in specific language disorders. In: Verhoeven L., van Balkom H., editors. Classification of Developmental Language Disorders. Mahwah, NJ: Lawrence Erhbaum; 2004:209-217.
Lewis B.A., Kirchner H.L., Short E.J., et al. Prenatal cocaine and tobacco effects on children’s language trajectories. Pediatrics. 2007;120(1):78-85.
Lillywhite L., Saling M., Harvey S., et al. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia. 2009;50(10):2276-2284.
Logan K.J. The effect of syntactic structure upon speech initiation times of stuttering and nonstuttering speakers. J Fluency Disord. 2002;28:17-35.
Maassen B. Speech output disorders. In: Verhoeven L., van Balkom H., editors. Classification of Developmental Language Disorders. Mahwah, NJ: Lawrence Erhbaum; 2004:173-191.
McArthur G., Atkinson C., Ellis D. Atypical brain responses to sounds in children with specific language and reading impairments. Devel Sci. 2009;12:768-783.
McLeod S., McKinnon D.H. Prevalence of communication disorders compared with other learning needs in 14 500 primary and secondary school students. Int J Lang Commun Disord. 2007;42(S1):37-59. March
Meaburn E., Dale P.S., Craig I.W., et al. Language-impaired children: No sign of the FOXP2 mutation. Neuroreport. 2002;13(8):1075-1077.
Miller C., Gilbert E. Comparison of performance on two nonverbal intelligence tests by adolescents with and without language impairment. J Commun Disord. 2008;41:358-371.
Miscimarra L., Stein C., Millard C., et al. Further evidence of pleiotropy influencing speech and language: Analysis of the DYX8 region. Hum Hered. 2007;63(1):47-58.
Mody M., Studdert-Kennedy M., Brady S. Speech perception deficits in poor readers: auditory processing or phonological coding? J Exp Child Psychol. 1997;64(2):199-231.
Nass R., Frank Y. Cognitive and behavioral complications of pediatric disorders. NY: Oxford University Press; 2010.
Nevo Y., Segev Y., Gelman Y., et al. Worster-Drought and congenital perisylvian syndromes–a continuum. Pediatr Neurol. 2001;24(2):153-155.
Norbury C.F., Nash M., Baird G., et al. Using a parental checklist to identify diagnostic groups in children with communication impairment: a validation of the Children’s Communication Checklist – 2. Int J Lang Commun Disord. 2004;39(3):345-364.
Ors M., Ryding E., Lindgren M., et al. SPECT findings in children with specific language impairment. Cortex. 2005;41:316-326.
Pearce P.S., Darwish H.Z., Gaines B.H. Visual symbol and manual sign learning by children with phonologic programming deficit syndrome. Dev Med Child Neurol. 1987;29:743-750.
Plante E., Boliek C., Mahendra N., et al. Right hemisphere contribution to developmental language disorder. Neuroanatomical and behavioral evidence. J Commun Disord. 2001;34:415-436.
Powell T.W. The use of nonspeech oral motor treatments for developmental speech sound production disorders: interventions and interactions. Lang Speech Hear Serv Sch. 2008;39(3):374-379.
Preis S., Steinmetz H., Knorr U., et al. Corpus callosum size in children with developmental language disorder. Brain Res Cogn Brain Res. 2000;10(1–2):37-44.
Priester G.H., Goorhuis-Brouwer S.M. Speech and language development in toddlers with and without cleft palate. Int J Pediatr Otorhinolaryngol. 2008;72:801-806.
Rapin I. Preschool Children with Inadequate Communication. London: Mackeith Press; 1996.
Rapin I., Allen D. Syndromes in developmental dysphasia and adult aphasia. In: Plum F., editor. Language, Communication and the Brain. New York: Raven Press, 1988.
Rescorla L. Language and reading outcomes to age 9 in late-talking toddlers. J Speech Lang Hear Res. 2002;45(2):360-371.
Rescorla L., Roberts J., Dahlsgaard K. Late talkers at 2: Outcome at age 3. J Speech Hear Res. 1997;40(3):556-566.
Rescorla L., Ross G.S., McClure S. Language delay and behavioral/emotional problems in toddlers: findings from two developmental clinics. J Speech Lang Hear Res. 2007;50(4):1063-1078.
Rice M.L., Wexler K. Toward tense as a clinical marker of Specific Language Impairment in English-speaking children. J Speech Hear Res. 1996;39:1239-1257.
Shriberg L.D., Aram D.M., Kwiatkowski J. Developmental apraxia of speech: I. Descriptive and theoretical perspectives. J Speech Lang Hear Res. 1997;40(2):273-285.
Shriberg L.D., Kwiatkowski J. Developmental phonological disorders. I: A clinical profile. J Speech Hear Res. 1994;37:1100-1126.
Shriberg L.D., Tomblin J.B., McSweeny J.L. Prevalence of speech delay in 6-year-old children and comorbidity with language impairment. J Speech Lang Hear Res. 1999;42:1461-1481.
SLI Consortium. Highly significant linkage to the SLI1 locus in an expanded sample of individuals affected by SLI. Am J Hum Genet. 2004;74:1225-1238.
SLI ConsortiumNewbury D.F., Cleak J.D., Ishikawa Brush Y. A genome wide scan identifies two novel loci involved in specific language impairment. Am J Hum Genet. 2002;70:384-398.
Stromswold K. Genetics of spoken language disorders. Hum Biol. 1998;70(297):211-212.
Stromswold K. The heritability of language: A review and meta-analysis of twin, adoption, and linkage studies. Language. 2001;77:647-723.
Tager-Flusberg H., Joseph R.M. Identifying neuro-cognitive phenotypes in autism. Philos Trans R Soc Lond B Biol Sci. 2003;358(1430):303-314.
Tallal P., Benasich A.A. Developmental language learning impairments. Dev Psychopathol. 2002;14:559-579.
Tallal P., Miller S., Fitch R.H. Neurobiological basis of speech: a case for the preeminence of temporal processing. Ann N Y Acad Sci. 1993;682:27-47.
Thoonen G., Maassen B., Gabreels F. Towards a standardized assessment procedure for developmental apraxia of speech. Eur J Disord Commun. 1997;32:37-60.
Trauner D.A., Wulfeck B., Tallal P., et al. Neurologic and MRI profiles of language impaired children. Devel Med Child Neurol. 2000;42:470-475.
Tyler A.A. Language-based intervention for phonological disorders. Semin Speech Lang. 2002;23:69-82.
Vernes C., Newbury D.F., Abrahams B.S., et al. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359:2337-2345.
Visscher C., Houwen S., Scherder E., et al. Motor profile of children with developmental speech and language disorders. Pediatrics. 2007;120(1):e158-e163.
Vodopiutz J., Item C.B., Hausler M., et al. Severe speech delay as the presenting symptom of guanidinoacetate methyltransferase deficiency. J Child Neurol. 2007;22(6):773-774.
Warren S., Yoder P. Early intervention for young children with language impairment. In: Verhoeven L., van Balkom H., editors. Classification of Developmental Language Disorders. Mahwah, NJ: Lawrence Erhbaum; 2004:367-384.
Watkins K.E., Vargha-Khadem F., Ashburner J., et al. MRI analysis of an inherited speech and language disorder: structural brain abnormalities. Brain. 2002;125:465-478.
Watkins K.E., Dronkers N.F., Vargha-Khadem F. Behavioural analysis of an inherited speech and language disorder: comparison with acquired aphasia. Brain. 2002;125:452-464.
Watkins K., Smith S., Davis S., et al. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2008;131:50-59.
Whitehouse A., Bishop D.V.M. Cerebral dominance for language function in adults with specific language impairment or autism. Brain. 2008;131:3193-3200.
Yairi E., Ambrose N.G. Early childhood stuttering I: persistency and recovery rates. J Speech Lang Hear Res. 1999;42:1097-1112.
Young A.R., Beitchman J.H., Johnson C., et al. Young adult academic outcomes in a longitudinal sample of early identified language impaired and control children. J Child Psychol Psychiatry. 2002;43:635-645.
Zimmerman I.L., Steiner V.G., Pond R.E. Preschool Language Scale, Fourth Edition (PLS-4). San Antonio, TX: Pearson; 2011.