Chapter 79 Determination of Brain Death in Infants and Children
The diagnosis of brain death in infants and children can be established after careful review of the medical history and performance of a detailed neurologic examination. Although criteria in children follow those established in adults, there are no universally accepted worldwide criteria for brain death determination in adults [Haupt and Rudolf, 1999; Wijdicks, 2001, 2002]. In the United States and most other countries, the 1987 pediatric guidelines [Report of Special Task Force, 1987] have been employed, with general agreement regarding their usefulness [Banasiak and Lister, 2003; Farrell and Levine, 1993; Kaufman, 1989], although criticisms about their validity and specificity, particularly in young infants, have been raised [Freeman and Ferry, 1988; Shewmon, 1988; Volpe, 1987]. These guidelines have recently been revised and will be described in detail in this chapter [Nakagawa et al., 2011].
Historical Perspective
A state beyond coma, or coma dépassé, was proposed in 1959 by Mollaret and Goulon to describe a premorbid clinical condition with loss of sensation, motor activity, consciousness, and vegetative functions [Mollaret and Goulon, 1959]. In 1968, an ad hoc committee of the Harvard Medical School faculty recommended clinical guidelines that subsequently shaped development of brain death concepts [Beecher, 1968]. The committee proposed that brain death could be defined by coma, apnea, lack of spontaneous or purposeful movements, and loss of selective cranial nerve functions, including the corneal reflex, pupillary response to light, pharyngeal gag and swallowing, yawning, vocalization, and failure of eye deviation to caloric stimulation of the tympanic membranes. Failure of improvement, sustained over 24 hours of observation, established a diagnosis of brain death. Two caveats were that the patient was normothermic and that drugs capable of maintaining coma were excluded. Two isoelectric electroencephalograms (EEGs) performed 24 hours apart were considered confirmatory, but not essential, in the declaration of brain death. In 1971, Mohandas and Chou emphasized the importance of determining the etiology of coma and documenting the persistence of apnea [Mohandas and Chou, 1971].
The Conference of Medical Royal College and Faculties in the United Kingdom proposed the following definition in 1976: “Permanent functional death of the brainstem constitutes brain death” [Conference, 1976]. Irreversible structural brainstem damage was defined carefully and believed to be clinically diagnosable without confirmatory EEG recordings. A follow-up report in 1979 recommended that brainstem death be considered death [Conference, 1979]. Subsequently a working group convened by the Royal College of Physicians further defined the criteria of brainstem death [Criteria, 1995]. In 1980, the National Institute of Neurologic and Communicative Disorders and Stroke (NINCDS) Collaborative Study of Brain Death reported on the outcome of 503 adult comatose and apneic patients, including neuropathologic brain studies and EEGs [NINCDS, 1980]. The combination of loss of pupillary light reflex, corneal reflex, oculocephalic reflex (doll’s-eye phenomenon), and oculovestibular reflex (caloric eye deviation) was highly predictive of death. Apnea, coma, and absence of brainstem reflexes, combined with an electrocerebral silent EEG, were highly associated with pathology in a brain that had experienced long-term respirator exposure (“respirator brain”). Exceptions were rarely noted (i.e., presence of respirator brains in patients with persistent biologic EEG activity and, conversely, lack of this pathology in patients with electrocerebral silence). EEG use was recommended in confirming brain death when the clinical neurologic examination was equivocal. An isoelectric EEG, despite its rare failure always to diagnose brain destruction, was predictive of a fatal outcome, except in two patients with drug intoxication. The report emphasized that a patient was not considered brain-dead if the EEG was not isoelectric. About 30 percent of the population had drug toxicity, despite the absence of a history of ingestion; 28 percent had measurable amounts of drugs that would suppress EEG activity. The report concluded that drug screening and EEG monitoring were mandatory before declaring brain death. Subsequent reports in adults [Grigg et al., 1987] and children [Ashwal and Schneider, 1979] documented that EEG activity could persist in unequivocally brain-dead patients.
In 1981, a U.S. presidential commission was convened to establish guidelines for the determination of brain death [President’s Commission, 1981]. These guidelines emphasized diagnosis based on the irreversible cessation of function of the entire brain. This report recognized that adult criteria might not be applicable to children because of developmental factors, a possible greater tolerance to asphyxia, and the recognition that infants and children occasionally exhibited significant recovery despite prolonged coma. The age of 5 years was selected as the earliest age suitable for the application of adult criteria, although this age selection reflected no known biologic phenomena.
In 1995, the American Academy of Neurology published criteria for the determination of brain death in adults [Practice Parameter, 1995]. These criteria were recently revised, recommending that only one examination be required, that ancillary testing was not necessary, and that the diagnosis could be made solely on clinical criteria [Wijdicks et al., 2010]. It was suggested, however, that if the examination was equivocal, additional neurodiagnostic testing could be done. In both the 1995 and 2010 parameters, it was stated that these recommendations were for individuals older than age 18 years. Box 79-1 summarizes the recommendations section of the 2010 American Academy of Neurology parameter that provided practical (non-evidenced-based) guidance for determination of brain death in adults.
Box 79-1 2010 American Academy of Neurology Recommendations for the Diagnosis of Brain Death in Adults
The determination of brain death can be considered to consist of four steps:
II The Clinical Evaluation (Neurologic Assessment)
B Absence of Brainstem Reflexes
 Absence of ocular movements using oculocephalic testing and oculovestibular reflex testing
Absence of ocular movements using oculocephalic testing and oculovestibular reflex testing
 Absence of the pharyngeal and tracheal reflexes
Absence of the pharyngeal and tracheal reflexes
C Apnea
 Absence of a breathing drive
Absence of a breathing drive
Procedure
 Reduce positive end-expiratory pressure (PEEP) to 5 cm H2O (oxygen desaturation with decreasing PEEP may suggest difficulty with apnea testing)
Reduce positive end-expiratory pressure (PEEP) to 5 cm H2O (oxygen desaturation with decreasing PEEP may suggest difficulty with apnea testing) If pulse oximetry oxygen saturation remains >95%, obtain a baseline blood gas (PaO2, PaCO2, pH, bicarbonate, base excess)
If pulse oximetry oxygen saturation remains >95%, obtain a baseline blood gas (PaO2, PaCO2, pH, bicarbonate, base excess) Preserve oxygenation (e.g., place an insufflation catheter through the endotracheal tube and close to the level of the carina and deliver 100% O2 at 6 L/min)
Preserve oxygenation (e.g., place an insufflation catheter through the endotracheal tube and close to the level of the carina and deliver 100% O2 at 6 L/min) Look closely for respiratory movements for 8–10 minutes. Respiration is defined as abdominal or chest excursions and may include a brief gasp
Look closely for respiratory movements for 8–10 minutes. Respiration is defined as abdominal or chest excursions and may include a brief gasp Abort if oxygen saturation measured by pulse oximetry is <85 percent for >30 seconds. Retry procedure with T-piece, continuous positive airways pressure (CPAP) 10 cm H2O, and 100% O2 at 12 L/min
Abort if oxygen saturation measured by pulse oximetry is <85 percent for >30 seconds. Retry procedure with T-piece, continuous positive airways pressure (CPAP) 10 cm H2O, and 100% O2 at 12 L/min If no respiratory drive is observed, repeat blood gas (PaO2, PaCO2, pH, bicarbonate, base excess) after approximately 8 minutes
If no respiratory drive is observed, repeat blood gas (PaO2, PaCO2, pH, bicarbonate, base excess) after approximately 8 minutesIII Ancillary Tests
 In clinical practice, electroencephalography (EEG), cerebral angiography, nuclear scan, transcranial Doppler, computed tomographic angiography, and magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) are currently used ancillary tests in adults. Most hospitals will have the logistics in place to perform and interpret an EEG, nuclear scan, or cerebral angiogram, and these three tests may be considered the preferred tests. Ancillary tests can be used when uncertainty exists about the reliability of parts of the neurologic examination or when the apnea test cannot be performed. In some protocols, ancillary tests are used to shorten the duration of the observation period
In clinical practice, electroencephalography (EEG), cerebral angiography, nuclear scan, transcranial Doppler, computed tomographic angiography, and magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) are currently used ancillary tests in adults. Most hospitals will have the logistics in place to perform and interpret an EEG, nuclear scan, or cerebral angiogram, and these three tests may be considered the preferred tests. Ancillary tests can be used when uncertainty exists about the reliability of parts of the neurologic examination or when the apnea test cannot be performed. In some protocols, ancillary tests are used to shorten the duration of the observation period The interpretation of each of these tests requires expertise. In adults, ancillary tests are not needed for the clinical diagnosis of brain death and cannot replace a neurologic examination. Physicians ordering ancillary tests should appreciate the disparities between tests and the potential for false-positives (i.e., the test suggests brain death, but the patient does not meet clinical criteria). Rather than ordering ancillary tests, physicians may decide not to proceed with the declaration of brain death if clinical findings are unreliable
The interpretation of each of these tests requires expertise. In adults, ancillary tests are not needed for the clinical diagnosis of brain death and cannot replace a neurologic examination. Physicians ordering ancillary tests should appreciate the disparities between tests and the potential for false-positives (i.e., the test suggests brain death, but the patient does not meet clinical criteria). Rather than ordering ancillary tests, physicians may decide not to proceed with the declaration of brain death if clinical findings are unreliableIV Documentation
 The time of brain death is documented in the medical records. Time of death is the time at which the arterial PCO2 reached the target value. In patients with an aborted apnea test, the time of death is when the ancillary test has been officially interpreted. A checklist is filled out, signed, and dated. Federal and state law requires the physician to contact an organ procurement organization following determination of brain death
The time of brain death is documented in the medical records. Time of death is the time at which the arterial PCO2 reached the target value. In patients with an aborted apnea test, the time of death is when the ancillary test has been officially interpreted. A checklist is filled out, signed, and dated. Federal and state law requires the physician to contact an organ procurement organization following determination of brain death(From Wijdicks et al. Evidence-based guideline update: determining brain death in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010 Jun 8;74[23]:1911–1918.)
Seeking to remedy the lack of criteria for infants and children, multiple professional neurologic and pediatric societies convened a task force in 1987 to propose a pediatric standard for brain death determination [Report of Special Task Force, 1987]. A history of the etiology and reversibility of coma was emphasized, as was the loss of brainstem function and apnea. Age-dependent observation periods of 12–48 hours were recommended. Ancillary EEG or radionuclide scanning was recommended in children younger than 1 year of age. Infants less than 7 days of age were excluded because of a lack of supportive literature. Later studies confirmed the validity of applying these specific guidelines in newborns of greater than 34 weeks of conceptual life [Ashwal and Schneider, 1989]. More recently, the Society of Critical Care Medicine, the American Academy of Pediatrics, and the Child Neurology Society convened a committee to re-evaluate and revise pediatric brain death criteria. These revised recommendations for the diagnosis of brain death in infants and children were published in 2011 (Box 79-2 and Box 79-3).
1 Determination of Brain Death
 Determination of brain death in neonates, infants and children relies on a clinical diagnosis that is based on the absence of neurologic function with a known irreversible cause of coma. Coma and apnea must coexist to diagnose brain death. This diagnosis should be made by physicians who have evaluated the history and completed the neurologic examinations
Determination of brain death in neonates, infants and children relies on a clinical diagnosis that is based on the absence of neurologic function with a known irreversible cause of coma. Coma and apnea must coexist to diagnose brain death. This diagnosis should be made by physicians who have evaluated the history and completed the neurologic examinations2 Prerequisites for Initiating a Brain Death Evaluation
3 Number of Examinations, Examiners, and Observation Periods
4 Apnea Testing
5 Ancillary Studies
6 Declaration of Death
(Nakagawa TA et al. Guidelines for the determination of brain death in infants and children: an update of the 1987 Task Force recommendations. Crit Care Med 2011; in press.)
Box 79-3 Neurologic Examination Criteria for Brain Death in Infants and Children from the 2011 Guidelines
Legal Definition of Brain Death
The American Medical Association and the American Bar Association supported universal enactment of the Uniform Determination of Death Act published in 1980 [Uniform, 1980]. The Act defined death, stating that “an individual who has sustained either (1) irreversible cessation of circulatory and respiratory functions; or (2) irreversible cessation of all functions of the brain including the brainstem is dead.” Subsequently, all states of the United States have enacted legislation accepting death by this definition. Brain death can be declared despite heart-lung ventilator dependence.
Epidemiology
Incidence of Brain Death
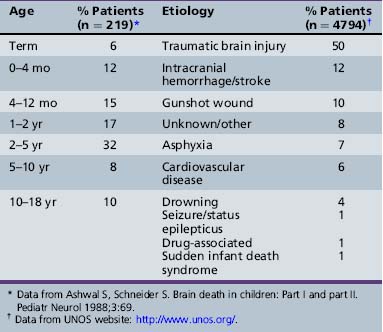
The overall incidence of brain death in children is unknown. In the United States, data are available concerning the number of brain-dead children who become organ donors (see United Network Organ Sharing [UNOS] website at http://www.unos.org/), and more recent studies have suggested that approximately 55 percent of children declared brain-dead become organ donors [Sheehy et al., 2003]. Combining such data suggests there are approximately 1800 children per year in the United States declared brain-dead, with most being between 11 and 17 years of age (Figure 79-1). Accurate data for neonates and infants younger than 1 year of age are not available. There also was a decrease between 1993 and 2003 in the number of brain-dead children who were organ donors (from 1137 to 886 [22 percent]), suggesting that the total number of children who are annually being declared brain-dead has decreased (see UNOS website). This decrease likely is due to the 26 percent decline in the total number of pediatric deaths since the 1990s (data from the National Center for Health Statistics [website http://www.cdc.gov/nchs/]).
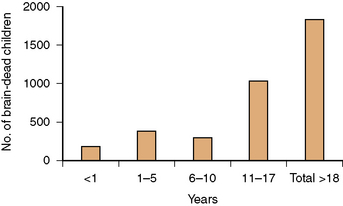
Fig. 79-1 Number of children declared brain-dead by age in the United States.
(Data from the United Network Organ Sharing website: http://www.unos.org/.)
Studies from pediatric intensive care units in the 1990s reported that the incidence of brain death in older infants and children ranges from 0.65 to 1.2 percent of admissions [Ashwal and Schneider, 1999]. In one study, the mortality rate in the pediatric intensive care unit was 8.7 percent, with 22 percent of these children declared brain-dead [Ryan et al., 1993]. The mortality rate in the neonatal intensive care unit was 5.6 percent, with none of the infants declared brain-dead. Another study reported the percentage of brain deaths in relation to the number of overall deaths to be 31.4 percent in children older than 1 month of age and 6.3 percent in neonates [Parker et al., 1995]. Similar findings from other countries have been reported [Goh et al., 1999; Gotay-Cruz and Fernandez-Sein, 2002; Lopez-Herce et al., 2000; Martinot et al., 1998]. Data from Loma Linda University Children’s Hospital indicate that the percentage of brain deaths in relation to the number of overall deaths is 28 percent and 2.1 percent in the pediatric and neonatal intensive care units, respectively. In some pediatric intensive care units, the percentage of patients diagnosed as brain-dead, compared with all deaths, is even higher (37 percent to 38 percent) [Martinot et al., 1995; Mejia and Pollack, 1995].
Etiologies of Brain Death
Table 79-1 provides information on the age distribution of infants and children in whom brain death has been diagnosed and characterization by disease categories that were responsible for the cerebral insult. Brain death most commonly occurs in adolescents and less so in infants younger than 1 year of age. Closed head injury was the most common clinical presentation leading to brain death (50 percent), followed by intracranial hemorrhage and stroke (12 percent). Asphyxial injury usually occurred as a complication of septic or hemorrhagic shock, with unexplained out-of-hospital cardiac arrest, or from strangulation or suffocation. Sudden infant death syndrome was a rare cause of brain death. Brain death secondary to meningitis was seen in patients who developed massive cerebral edema with herniation within 2–24 hours of admission. Miscellaneous causes of brain death involve rare metabolic diseases, perioperative insults to the central nervous system, acute hydrocephalus, and a variety of other rare disorders that ultimately affect the brain.
Outcome after Diagnosis of Brain Death
Most children are removed from life support or undergo organ donation within a 2-day time period when the diagnosis of brain death is confirmed [Ashwal and Schneider, 1987]. Some children are continued on ventilator support until cardiac arrest occurs, and their “survival” has averaged about 17 days. In rare cases, children have been maintained on ventilator support for periods ranging from 6 months to 5 years [Shewmon, 1998]. There have been no reports of children recovering neurologic function who met adult brain-death criteria on neurologic examination [Moshe and Alvarez, 1986; Rowland et al., 1983]. These criteria include an absence of spontaneous activity; specific cranial nerve dysfunction; and cardiac response to ocular compression (vagal bradycardia response), decerebrate or decorticate posturing, and apnea.
Neurologic Evaluation
Reports indicate that a marked variability exists in the knowledge and use of the 1987 pediatric brain-death guidelines [Ashwal, 1991; Mejia and Pollack, 1995]. A national survey of 16 pediatric intensive care units found that apnea testing either was not performed (25 percent) or was deemed “controversial” (22 percent) in children diagnosed as brain-dead, and in many of these infants and children, appropriate confirmatory testing was not done [Mejia and Pollack, 1995]. Similar findings were noted in a survey of neurosurgeons and the criteria they employed in evaluating children with head injury for brain death [Chang et al., 2003]. Likewise, a survey of pediatric attending physicians and residents in the United States found a high percentage (39–58 percent) who could not define brain death correctly or recognize that brain death could be diagnosed without confirmatory testing in children older than 1 year of age [Harrison and Botkin, 1999].
A more recent study has also documented the wide variability in clinical practice for determining brain death [Mathur et al., 2008]. Of 142 of 277 children referred to OneLegacy who became organ donors, the number of brain-death examinations performed was 0 (4 patients), 2 (122 patients), 3 (14 patients), or 4 (2 patients). Recommended intervals between examinations were followed for 18 percent of patients greater than 1 year of age and for no younger patients. A mean of only 5.5 of 14 examination elements was completed by neurologists and pediatric intensivists, and 5.8 by neurosurgeons. No apnea testing was recorded in 60 percent of patients, and an inadequate increase in PaCO2 levels occurred in more than half the patients. EEG was performed as a confirmatory test in only 26 percent of patients, and cerebral blood flow (CBF) determination was performed in 74 percent. This study clearly demonstrated the wide variability in clinical practice for following the 1987 guidelines, and that documentation is incomplete for most patients.
Clinical Examination
Cerebral Unresponsivity
The neurologic evaluation for brain death is difficult in a comatose patient, particularly in infants and children. It is necessary to standardize the basal conditions under which the patient is examined. The patient should be normothermic, and pharmacologic agents capable of producing coma or neuromuscular blocking agents must be excluded [Kennedy and Kiloh, 1996; Tobin, 1996]. Assessment for cerebral unresponsivity, loss of cranial nerve function, and determination of apnea (tested with a hypercapneic stimulus) must be performed and documented. Confounding circumstances always increase the complexity of the evaluation. Cerebral unresponsivity or coma can be quantitated using the Glasgow Coma Scale score, which ranges from 3 (no response) to 15 (normal) using assigned scores for eye opening, motor activity, and vocalization (see Chapter 73) [Jennett and Bond, 1975]. The scale should be modified, substituting socialization for verbalization for infants and children who are too young to have developed speech. The predictive value of the duration of unresponsivity in infants and young children may be less trustworthy than in adults. In young children, recovery may occur when unresponsiveness has occurred for prolonged periods, and even when serious structural nervous system abnormalities are present [Booth et al., 2004; Haque et al., 2008; Mandel et al., 2002; Carter and Butt, 2005]. If the neurologic evaluation is uncertain or inconsistent in children younger than 1 year of age, supportive laboratory documentation, including EEG and cerebral blood flow determinations, should be considered.
Brainstem Examination
Cessation of brainstem reflexes is generally accepted as the hallmark of brain failure. The NINCDS reported, however, that 141 of 459 comatose patients who died retained one or more brainstem reflexes [NINCDS, 1980]. No single preserved brainstem reflex could discriminate the preservation of any other brainstem reflex. The combination of pupillary light response and oculocephalic and oculovestibular reflexes had the greatest specificity, but this combination included 4 percent of patients who retained other brainstem reflexes. Box 79-4 outlines the procedures used to assess the cold caloric (oculovestibular) response. The 2011 pediatric guidelines recommends that doing cold caloric testing is sufficient and that oculocephalic testing (head turning) is not necessary; they also state that, because some individuals could have spinal cord injury, such testing might pose additional risk [Nakagawa et al., 2011].
Premature infants of less than 32 weeks’ conceptual age do not have completely developed cranial nerve function. Clinicians examining these infants should be aware of the development of the different cranial nerve reflexes during gestation (Table 79-2). Newborns often present obstacles to examination. Ear canals are frequently small and may be plugged, pupils are small, adhesive tape obscures the face and extremities, and neuromuscular blocking agents alter the examination. The 2011 pediatric guidelines include recommendations for term infants from 37 weeks’ gestation. Recommendations for preterm infants younger than 37 weeks were not made because of insufficient evidence. Fortunately, brain death rarely occurs in preterm infants, so from a practical clinical standpoint, clinicians will likely not have to confront the issue of trying to diagnose brain death in this population.
| Developmental Reflex | Gestational Age (Weeks) when Reflex is Elicitable |
|---|---|
| Suck, root, gag | 32–34 |
| Auditory response | 30–32 |
| Pupillary response to light | 30–32 |
| Oculocephalic response | 28–32 |
| Corneal response | 28–32 |
| Moro response | 28–32 |
| Grasp response (palmar) | 30–32 |
| Alertness | 30–32 |
| Apnea response to PaCO2 stimulus | 33 |
(Some data from Fanaroff et al. The respiratory system. In: Fanaroff A, Martin RJ, eds. Neonatal-perinatal medicine: Diseases of the fetus and newborn. St. Louis, Mosby, 1987.)
Number of examinations, examiners and observation periods
Number of Examinations and Examiners
The revised 2011 guidelines have tried to address issues related to the duration of time for which a pediatric patient should be observed before the official declaration of brain death, as well as the number of examinations needed and who should be doing the examination [Nakagawa et al., 2011]. The 1987 guidelines recommended observation periods between brain-death examinations based upon age and the results of neurodiagnostic testing [Report of Special Task Force, 1987]. Two examinations and EEGs separated by at least 48 hours were recommended for infants of 7 days to 2 months. Two examinations and EEGs separated by at least 24 hours were recommended for children of 2 months to 1 year. A repeat EEG was not necessary if a cerebral radionuclide scan or cerebral angiography demonstrated no flow or visualization of the cerebral arteries. For children older than 1 year, an observation period of 12 hours was recommended and ancillary testing was not required when an irreversible cause existed. The observation period in this age group could be decreased if there was documentation of electrocerebral silence or absent CBF. The general consensus was that the younger the child, the longer the waiting period should be, unless ancillary studies supported the clinical diagnosis of brain death; if so, the observation period could be shortened.
Whereas the recently revised 2011 guidelines for declaring brain death in adults (see Box 79-1) recommended only one examination, the revised pediatric guideline recommend continued performance of two examinations separated by an observation period. In addition, the adult guidelines also stated that, because recommendations as to the length of observation periods have varied extensively throughout the world and the United States, and because no detailed studies on serial examinations in adult patients who have been declared brain-dead have been done, there was insufficient evidence to determine the minimally acceptable observation period to ensure that neurologic functions have ceased irreversibly.
Duration of Observation Periods
A literature review of 171 children diagnosed as brain-dead found that 47 percent had ventilator support withdrawn, on average, 1.7 days after the diagnosis of brain death was made [Ashwal and Schneider, 1987]. Seventy-nine children (46 percent) in whom support was continued after declaration of brain death suffered a cardiac arrest an average of 22.7 days later. The remaining children died by an unknown mechanism (5 percent), or made an incomplete (1 percent) or complete recovery (0.5 percent). The age range of the children in this study included preterm and term neonates, and older infants and children up to age 18 years. These data and the reports of more recent studies [Ashwal and Schneider, 1987; Parker et al., 1995] suggest that there is likely no biological justification for using different durations of observation to diagnose brain death in infants greater than 1 month of age. In fact, there are no reports of children recovering neurologic function after meeting adult brain-death criteria based on neurologic examination findings [Ashwal, 2001]. Although some authors have reported apparent reversibility of brain death, further review of these cases reveals that these children would not have fulfilled brain-death criteria by currently accepted U.S. medical standards [Joffe et al., 2009].
Based on the above data, currently available literature, and clinical experience, the 2011 committee recommended that the observation period between examinations should be 24 hours for neonates (37 weeks up to 30 days), and 12 hours for infants and children (>30 days to 18 years). The first examination determines that the child has met neurologic examination criteria for brain death. The second examination confirms brain death, based on an unchanged and irreversible condition. Timing of the first clinical brain-death examination, reduction of the duration of the observation period, and use of ancillary studies are discussed in separate sections of this guideline [Nakagawa et al., 2011].
Apnea Testing
Documentation of apnea, under controlled conditions, is the most important determination in clinically evaluating brain death. Virtually all protocols recommend a period of unassisted ventilation, allowing hypercapnia maximally to stimulate the respiratory effort. The first formal observation period (3 minutes) was recommended by the Ad Hoc Harvard Medical School Faculty [Beecher, 1968]. The Conference of Royal Colleges and Faculties suggested administering a mixture of 5 percent carbon dioxide and 95 percent oxygen for 5 minutes while withholding respirator support, and then supplying 100 percent tracheal oxygen [Conference, 1976]. The President’s Commission recommended 100 percent oxygen ventilation for 10 minutes, followed by passive 100 percent tracheal oxygen for a period long enough to achieve a PCO2 of at least 60 mmHg [President’s Commission, 1981].
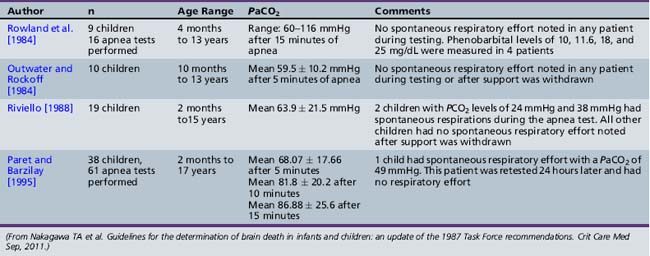
The normal physiologic apneic threshold (minimum PCO2 at which respiration begins) depends on many factors, and can be altered by anesthetic agents, narcotics, sedatives, and certain disease states. Schafer and Caronna described three patients, suspected of being brain-dead, who developed spontaneous respiration at PCO2 levels between 45 mmHg and 50 mmHg, which suggested a maximal end point of 60 mmHg [Schafer and Caronna, 1978]. Ropper and co-workers studied seven brain-dead patients and reported a PCO2 of 44 mmHg as a maximal excitatory end point [Ropper et al., 1981], but this has not been studied in children.
Table 79-3 summarizes data collected by the 2011 Pediatric Task Force on Brain Death from previous studies of apnea testing in children. The maximal PCO2 apneic threshold in children is probably similar to that in adults. Outwater and Rockoff studied 10 patients, age 10 months to 15 years, in whom they increased mean PCO2 from 34.4 mmHg to 59.5 mmHg over 5 minutes while supplying 100 percent tracheal oxygen [Outwater and Rockoff, 1984]. The PO2 exceeded 200 mmHg during the test period. Rowland and colleagues performed 16 apnea tests on 9 patients, age 4 months to 13 years, 4 of whom had detectable phenobarbital levels between 10 and 25 mg/dL [Rowland et al., 1984]. These patients received 100 percent oxygen for 10 minutes. Oxygen then was delivered at 6 L/min through a catheter into the length of the endotracheal tube with the ventilator turned off during the 15-minute study period. These patients were moderately hyperventilated before the apnea test, with a mean PCO2 of 28 mmHg. The PCO2 increased 4.4 mmHg, 3.4 mmHg, and 2.6 mmHg per minute at 5, 10, and 15 minutes. Arterial PCO2 at the end of 15 minutes ranged from 40 to 116 mmHg, and by 15 minutes, 14 of 16 patients had PCO2 levels greater than 60 mmHg. Two patients had PCO2 levels of 100 mmHg and 116 mmHg, with pH determinations of 6.92 and 6.98. Arterial PO2 remained greater than 100 mmHg, and in 12 of 16 patients it was greater than 200 mmHg. Mild alterations of pulse or blood pressure, or both, also were observed in 6 patients but were reversible. Both studies concluded that apnea test periods of 5–10 minutes would result in PCO2 levels exceeding 60 mmHg without danger of hypoxemia.
Apnea challenges, when initiated at PCO2 levels of 40–50 mmHg, have a predictive PCO2 linear increase [Paret and Barzilay, 1995]. When the initial PCO2 was 40–51 mmHg in 11 patients, the minute PCO2 increase was 5.1–6.7 mmHg. During the pretest period, the PCO2 was stabilized for 10 minutes. Most studies recommend that PCO2 levels be determined at 5-minute intervals and continued up to 15 minutes if the PCO2 has not reached 60 mmHg and the PO2 has not decreased to less than 50 mmHg. Apnea testing of patients who are hypothermic or receiving medications that suppress respiration is not valid for documenting brainstem failure. It still can be performed under such circumstances, however, because the presence of respiratory effort would eliminate brain death as a diagnosis.
Several case reports involving only a few patients have raised related issues concerning apnea testing in young infants and children that also apply to newborns. Ammar and co-workers reported five children, age 9 months to 7 years, with severe brainstem dysfunction, including loss of pupillary reflexes and apnea, that was due to surgically resectable brainstem lesions with return of spontaneous respirations and substantial neurologic function [Ammar et al., 1993]. None of these children was considered brain-dead before surgery, although they were severely compromised. These investigators correctly pointed out that treatment of compressive brainstem lesions might reverse severe neurologic deficits that mimic brain death. A second report concerns a 3-month-old infant who met the 1987 task force criteria for brain death, but who, on day 43 after admission, developed 2–3 breaths per minute [Okamoto and Sugimoto, 1995]. The infant died 71 days after presentation. At issue is whether this case should be considered a return of respiratory function. An editorial commentary on this case by Fishman stated, “The return of no clinical neurologic function other than two to three ineffectual respirations/minute due to the survival of only a small group of neurons in the medulla should not be considered an example of reversible deficits and failure of current brain death criteria” [Fishman, 1995]. A third case report described a 4-year-old male with a posterior fossa mass, who became brain-dead 4 days after admission [Vardis and Pollack, 1998]. His first apnea test had a PCO2 of 91 mmHg, at which time he developed spontaneous respirations. A repeat apnea test the following day found an initial PCO2 of 67 mmHg, but no respiratory effort was detected; he began to breathe spontaneously when challenged again, and the PCO2 was 71 mmHg. This last patient raises the question as to whether the carbon dioxide threshold may be age-dependent or disease-dependent. Because of the limited number of patients in these few case reports, additional research into these issues is required.
Technique for performing apnea testing
Apnea testing, as recommended in the 2011 pediatric guidelines, should be performed in children only after a full explanation to the parents is provided, and permission for the procedure is obtained. Rarely, parents refuse to allow an apnea challenge. On these rare occasions, carbon dioxide augmentation at 1 L/min, preceded by 100 percent preoxygenation, increases the PCO2 to 60 mmHg within 2 minutes. At this time, a respirator can be interrupted for 3–5 minutes as a substitute test of apnea [Lang, 1995].
The following summarizes the recommendations for performing apnea testing in term newborns, infants, and children, as reported in the 2011 pediatric brain death guidelines (Box 79-5). This includes normalization of the pH and PaCO2, measured by arterial blood gas analysis, maintenance of core temperature >35°C, normalization of blood pressure appropriate for the age of the child, and correcting for factors that could affect respiratory effort as a prerequisite to testing. The patient must be preoxygenated, using 100 percent oxygen for 5–10 minutes, prior to initiating this test. Intermittent mandatory mechanical ventilation should be discontinued once the patient is well oxygenated and a normal PaCO2 has been achieved. The patient can then be changed to a T piece attached to the endotracheal tube (ETT), or a self-inflating bag valve system, such as a Mapleson circuit connected to the ETT. Tracheal insufflation of oxygen using a catheter inserted through the ETT has also been used; however, caution is warranted to ensure adequate gas excursion and to prevent barotrauma. High gas-flow rates with tracheal insufflation may also promote CO2 washout, preventing adequate PaCO2 rise during apnea testing. Continuous positive airway pressure (CPAP) ventilation has been used during apnea testing. Many current ventilators automatically change from a CPAP mode to mandatory ventilation and deliver a breath when apnea is detected. It is also important to note that spontaneous ventilation has been falsely reported to occur while patients were maintained on CPAP, despite having the trigger sensitivity of the mechanical ventilator reduced to minimum levels. Physicians performing apnea testing should continuously monitor the patient’s heart rate, blood pressure, and oxygen saturation while observing for spontaneous respiratory effort throughout the entire procedure. PaCO2, measured by blood gas analysis, should be allowed to rise to ≥20 mmHg above the baseline PaCO2 level and ≥60 mmHg. If no respiratory effort is observed from the initiation of the apnea test to the time the measured PaCO2 is ≥60 mmHg and ≥20 mmHg above the baseline level, the apnea test is consistent with brain death. The patient should be placed back on mechanical ventilator support and medical management should continue until the second neurologic examination and apnea test confirming brain death is completed. If oxygen saturations fall below 85 percent, hemodynamic instability limits completion of apnea testing, or a PaCO2 level of ≥60 mmHg cannot be achieved, the infant or child should be placed back on ventilator support with appropriate treatment to restore normal oxygen saturations, normocarbia, and hemodynamic parameters. In this instance, another attempt to test for apnea may be performed at a later time, or an ancillary study may be pursued to assist with determination of brain death. Evidence of any respiratory effort is inconsistent with brain death, and the apnea test should be terminated and the patient placed back on ventilatory support.
Ancillary Neurodiagnostic Studies
Virtually all guidelines have stressed that the historical events leading to coma, when combined with the clinical triad of coma, absence of brainstem reflexes, and a failed apnea challenge, are fundamental to brain-death diagnosis. Many physicians are uncomfortable with a clinical diagnosis and have sought an absolute laboratory test to confirm brain death. A universally applicable, valid, and reliable study is a laudable, but probably unattainable, goal [Shewmon, 1987]. All brain cells do not die simultaneously; highly selective tests lack the sensitivity to report absolutely that the brain has failed and such failure is permanent. The combination of clinical evaluation and pertinent diagnostic studies (EEG, CBF studies) can yield a sound clinical decision. However, as outlined in the following sections, data acquired over the past two decades have clearly shown that a substantial number of adult and pediatric patients may have EEG activity or demonstrable CBF when they meet clinical criteria for irreversible brain death. Because of such observations and general consensus, the 2011 pediatric guidelines recommended that ancillary studies are not required to establish brain death and should not be viewed as a substitute for the neurologic examination [Nakagawa et al., 2011]. Ancillary studies were recommended for use to assist the clinician in making the diagnosis of brain death:
The 2011 guidelines also recommended that, similar to the neurologic examination, hemodynamic and temperature parameters should be normalized prior to obtaining EEG or CBF studies. Pharmacologic agents that could affect the results of testing should be discontinued (Table 79-4) and levels determined as clinically indicated. The guidelines recommended that low to midtherapeutic levels of barbiturates should not preclude the use of EEG testing, and that radionuclide CBF study could be utilized in patients with high-dose barbiturate therapy to demonstrate absence of CBF.
Table 79-4 Medications Administered to Critically Ill Pediatric Patients and Recommendations for Time Interval to Testing after Discontinuation*
| Medication | Elimination Half-Life | |
|---|---|---|
| Infants/Children | Neonates | |
| INTRAVENOUS INDUCTION, ANESTHETIC, AND SEDATIVE AGENTS | ||
| Thiopental | Adults: 3–11.5 hr (Shorter half-life in children) | |
| Ketamine | 2.5 hr | |
| Etomidate | 2.6–3.5 hr | |
| Midazolam | 2.9–4.5 hr | 4–12 hr |
| Propofol | 2–8 min Terminal half-life 200 min (range 300–700 min) |
|
| Dexmedetomidine | Terminal half-life 83–159 min | Infants have faster clearance |
| ANTIEPILEPTIC DRUGS | ||
| Phenobarbital | Infants: 20–133 hr† Children: 37–73 hr† |
45–500 hr† |
| Pentobarbital | 25 hr† | |
| Phenytoin | 11–55 hr† | 63–88 hr† |
| Diazepam | 1 month to 2 years: 40–50 hr 2 years–12 years: 15–21 hr 12 years–16 years: 18–20 hr |
50–95 hr |
| Lorazepam | Infants: 40.2 hr (range 18–73 hr) Children: 10.5 hr (range 6–17 hr) |
40 hr |
| Clonazepam | 22–33 hr | |
| Valproic acid | Children >2 months: 7–13 hr† Children 2–14 years: mean 9 hr; range 3.5–20 hr |
10–67 hr† |
| Levetiracetam | Children 4–12 years: 5 hr | |
| Intravenous Narcotics | ||
| Morphine sulfate | Infants 1–3 months: 6.2 hr (5–10 hr) 6 months to 2.5 years: 2.9 hr (1.4–7.8 hr) Children: 1–2 hr |
7.6 hr (range 4.5–13.3 hr) |
| Meperidine | Infants <3 months: 8.2–10.7 hr (range 4.9–31.7 hr) Infants 3–18 months: 2.3 hr Children 5–8 years: 3 hr |
23 hr (range 12–39 hr) |
| Fentanyl | 5 months to 4.5 years: 2.4 hr (mean) 0.5–14 years: 21 hr (range 11–36 hr for long-term infusions) |
1–15 hr |
| Sufentanil | Children 2–8 years: 97 ± 42 min | 382–1162 min |
| MUSCLE RELAXANTS | ||
| Succinylcholine | 5–10 min Prolonged duration of action in patients with pseudocholinesterase deficiency or mutation |
|
| Pancuronium | 110 min | |
| Vecuronium | 41 min | 65 min |
| Atracurium | 17 min | 20 min |
| Rocuronium | 3–12 months: 1.3 ± 0.5 hr 1 to <3 years: 1.1 ± 0.7 hr 3 to <8 years: 0.8 ± 0.3 hr Adults: 1.4–2.4 hr |
|
* Metabolism of pharmacologic agents may be affected by organ dysfunction and hypothermia. Physicians should be aware of total amounts of administered medication that can affect drug metabolism and levels.
† Elimination half-life does not guarantee therapeutic drug levels for longer-acting medications or medications with active metabolites. Drug levels should be obtained to ensure that levels are in a low to midtherapeutic range prior to neurologic examination to determine brain death. In some instances, this may require waiting several half-lives and rechecking serum levels of the medication before conducting the brain-death examination.
(From Nakagawa TA et al. Guidelines for the determination of brain death in infants and children: an update of the 1987 Task Force recommendations. Crit Care Med Sep, 2011.)
Electroencephalogram
Despite limitations of the EEG, especially in regard to measurement of brainstem activity, many physicians tend to equate an isoelectric EEG (electrocerebral silence [ECS]) with brain death. Despite the obvious dangers in depending only on the EEG, the isoelectric EEG, combined with the clinical triad of coma, absent brainstem reflexes, and apnea, remains commonly used for determining brain death. It is widely available, has bedside portability, is noninvasive, is relatively inexpensive, and can be interpreted by most neurologists. The American Electroencephalographic Society Guidelines have developed criteria for brain death recordings [American Electroencephalographic Society, 1986, 1994]. The recording should be isoelectric for a minimum of 30 minutes and show no electrical activity beyond 2 µV at a sensitivity of 2 µV/mm, with filter settings at 0.1 or 0.3 second at 70 Hz. Telephone transmission of EEGs is unacceptable for brain-death determinations. Technical problems in performing EEGs have been reviewed previously [Bennett et al., 1976; Chatrian, 1986; Moshe, 1989; Schneider, 1989] and summarized here.
Electroencephalogram in Pediatric Brain Death
Pediatric patients present unique difficulties in interpretation of the EEG because of shorter interelectrode distances, greater electrocardiogram contamination, reduced cortical potentials in premature infants, and delayed metabolism of barbiturates. Reversible ECS may occur with the use of central nervous system-depressant drugs, hypothermia, cardiovascular shock, or metabolic encephalopathies. In children, the most common medications causing the reversible loss of brain electrocortical activity include barbiturates, benzodiazepines, narcotics, and certain intravenous (thiopental, ketamine, midazolam) and inhalation (halothane and isoflurane) anesthetics (see Table 79-4). Phenobarbital is the most common drug responsible for reversible ECS because it is widely used for seizure control. Previous studies have suggested that phenobarbital levels greater than 25 µg/mL might suppress EEG activity to the point of ECS, and that levels less than 20–25 µg/mL are unlikely to cause electrocerebral silence or affect apnea testing or the examination of brainstem reflexes [Ashwal and Schneider, 1989]. A study in 92 children reported data suggesting that therapeutic levels of phenobarbital (i.e., 15–40 µg/mL) do not affect the EEG [LaMancusa et al., 1991]. The authors also recommended that physicians and families do not have to wait the 3.7 days for subtherapeutic (<15 mg/mL) or the 6 days for therapeutic (15–40 µg/mL) barbiturate levels to be completely metabolized to consider withdrawal of life support. Others have reported that absent brainstem reflexes could not be correlated to thiopentone levels [Grattan Smith and Butt, 1993]. A study performed in adults found residual postmortem toxic brain levels of barbiturates despite absent premorbid serum levels [Saito et al., 1995].
A core temperature greater than 32.2°C (>90°F) has been regarded as a prerequisite for reliably determining brain death by EEG [Hicks and Poole, 1981]. In adults, ECS does not occur until the temperature decreases to less than 20°C (68°F). In children, suppression of EEG activity does not appear until 24°C (75.2°F), and complete loss of EEG activity does not occur until the temperature is less than 18°C (<64.4°F) [Jorgensen and Malchow-Moller, 1978]. The average temperature when EEGs are obtained for confirmation of brain death is 97.3°F ± 1.4°F. This finding suggests that hypothermia is not likely to be a significant problem for pediatric EEG interpretation [Nash et al., 2011].
Reversible ECS may occur soon after a child has had a cardiac arrest [Schmitt et al., 1993]. Several infants in whom the initial EEG was observed 8–10 hours after a cardiac arrest had ECS, but a repeat study 12–24 hours later demonstrated diffuse low-voltage activity. Several of these infants survived in a vegetative or minimally conscious state; most died as a result of other complications of the acute catastrophic insult. Similar observations have been reported in adults [DeOliveira et al., 1984].
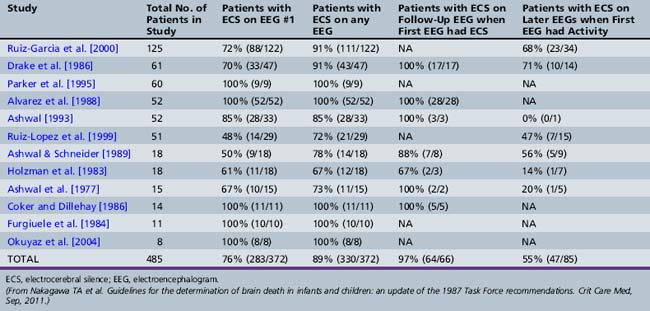
Extensive literature exists documenting persistent EEG activity in brain-dead infants, children, and adults [Ashwal and Schneider, 1979; Grigg et al., 1987]. Table 79-5 summarizes data from the 2011 guidelines on the EEG results of 12 studies in 485 suspected brain-dead children in all age groups [Nakagawa et al., 2011]. The data show that 76 percent of all children who were evaluated with EEG for brain death on the first EEG had ECS. Multiple EEGs increased the yield to 89 percent. For those children who had ECS on their first EEG, 64 of 66 patients (97 percent) had ECS on a follow-up EEG. The first exception was a neonate who had a phenobarbital level of 30 μg/mL when the first EEG was performed. The second exception was a 5-year-old head trauma patient who was receiving pentobarbital and pancuronium at the time of the initial EEG.This patient also had a CBF study performed, demonstrating flow. In retrospect, these two patients would not have met currently accepted standards for brain death, based on pharmacologic interference with EEG testing. Additionally, of those patients with EEG activity on the first EEG, 55 percent had a subsequent EEG that showed ECS. The remaining 45 percent either had persistent EEG activity or additional EEGs were not performed. All died (spontaneously or by withdrawal of support). Only one patient survived from this entire group of 485 patients: a neonate with an elevated phenobarbital level, whose first EEG showed photic response, and who survived severely neurologically impaired.
Since Green and Lauber’s report in 1972 of two infants who had return of some EEG activity after an initial ECS recording [Green and Lauber, 1972], there have only been a few additional infants reported in whom EEG activity returned [Ashwal, 1997; Ashwal et al., 1977; DeOliveria et al., 1984; Juguilon and Reilly, 1982; Kohrman and Spivack, 1990; Pezzani et al., 1986; Toffol et al., 1987]. A previous report, based on most of these cases, estimated that the return of EEG activity when the initial study was isoelectric was approximately 0.02 percent [Ashwal, 1997]. There are presumably additional unreported cases in which EEG activity returned, but these additional cases are unlikely to substantially affect this extremely low ratio. Concerns about the return of EEG activity have been overemphasized. In addition, if one considers issues related to subsequent clinical recovery of the patients in whom EEG activity returned, none of the patients made significant clinical recoveries. The lack of absolute correlation between EEG findings and other supportive ancillary brain-death tests limits reliance on the EEG as a single confirmatory test [Paolin et al., 1995]. Despite these well-documented drawbacks, the EEG remains extremely valuable when combined with the neurologic examination and other confirmatory studies [Schneider, 1989]. However, it should be noted that the EEG is being used less commonly than measurement of CBF as an ancillary study in brain-death determinations.
Measurements of Cerebral Perfusion
Several technologies are available for estimating or measuring CBF (Table 79-6), cerebral perfusion, or cerebral blood velocity. Their use in children has followed after application and testing in adults. New and older technologies have been applied in the search for an ideal test. Each advance in technology needs to be carefully scrutinized in terms of availability, cost, 24-hour accessibility, portability, patient risk, invasiveness, and simultaneous outcomes comparison with established techniques. The clinician who is requested to perform brain-death determinations should be aware of individual institutional resources. Concerns exist about the validity and reliability of several of these methods in preterm and newborn infants, in whom CBF values are quite low, and whether these methods possess the sensitivity to detect very low flow values [Altman et al., 1988; Greissen, 1986].
Table 79-6 Principal Methods of Assessing Cerebral Blood Flow in Brain-Dead Patients
| Method | Measurement | Absent Circulation Indicated by |
|---|---|---|
| Cerebral angiography | High-pressure and rapid, intra-arterial injection of contrast agent | Absence of intracranial filling of large cerebral arteries and their major branches |
| Radionuclide imaging | Intravenous rapid bolus injection of radionuclide isotope | Absence of radionuclide detection in distribution of large vessels and in brain parenchyma |
| HMPAO SPECT | Similar to radionuclide imaging but with different detection system and isotope | Absence of activity on dynamic scintigraphy or by delayed isotope uptake |
| CT angiography | Use of conventional CT scanning, combined with use of rapid infusion of contrast agent, to visualize intracranial vessels | Relatively new method with data from adult studies that show lack of filling of intracranial arteries |
| MRI and MR angiography | Use of conventional MRI to detect red blood cells within intracranial arteries as an indicator of blood-vessel structure | MRI demonstrates edema MR angiography demonstrates lack of filling of intracranial blood vessels |
| Transcranial Doppler | Measures blood velocity from large intracranial vessels during diastole and systole | Serial changes with loss of diastolic flow, flow reversal, and loss of systolic flow |
| Digital subtraction angiography | Imaging that provides visualization of cerebral vessels | Absence of contrast material within large cerebral vessels |
| Xenon CT | Provides quantitative measure of cerebral blood flow by inhalation of xenon gas detected with cranial CT | Cerebral blood flow values <5–10 mL/min/100 g indicate absence of cerebral circulation |
CT, computed tomography; HMPAO, hexamethylpropylene amine oxime; MRI, magnetic resonance imaging; SPECT, single-photon emission computed tomography.
Cerebral Angiography
Direct visualization of the cerebral circulation with angiography that shows the complete cessation of CBF has long been accepted as the standard for comparing all other neuroimaging modalities for brain-death confirmation. Near-simultaneous comparisons of multiple techniques confirm the sensitivity of this long-held but seldom-challenged concept [Bradac and Simon, 1974; Conrad and Sinha, 2003; Paolin et al., 1995]. The technique should be considered only for unresolvable clinical situations, however, because of its limitations, which include patient transfer, invasiveness, limited radiologic personnel, and prolonged study time. Few cases have been reported in which this invasive method has been performed in brain-dead children to validate the absence of cerebral circulation [Ashwal and Schneider, 1979].
Radionuclide Imaging
Several radionuclide techniques have been used in brain-dead patients since the 1970s to determine noninvasively whether there has been cessation of cerebral circulation [Conrad and Sinha, 2003]. With most methods, circulation was assessed during an early “dynamic” phase, and later by examining static images for cerebral uptake of the specific radionuclide (usually technetium-99m pertechnetate, technetium-99m glucoheptonate, or technetium-99m DTPA) (Figure 79-2). During the dynamic phase, a bolus of the radionuclide was injected rapidly, and isotopic cranial images were obtained similar to that of a carotid arteriogram. Usually in the arterial phase, cerebral activity was detectable within several seconds, and from the time of peak cerebral activity, sagittal sinus activity was observed within 6–8 seconds [Thompson et al., 1987]. Various terms have been used to describe the early phase of this study, including radioisotopic bolus angiography and dynamic brain scintigraphy. If activity was not detectable in this early phase, CBF was considered absent, resulting from either low cardiac output or very high intracranial pressure (as is seen with brain death). Because most tracers have a half-life of several hours, the static phase of a radionuclide imaging study usually was done later, looking for the absence or presence of diffuse parenchymal isotopic uptake. Currently, most centers are using single-photon emission computed tomography scanning and technetium-99m hexamethylpropylene amine oxime (HMPAO) as the isotopic agent [Abdel Dayem et al., 1989; Bonetti et al., 1995; Galaske et al., 1988; Mrhac et al., 1995; Spieth et al., 1995; Valle et al., 1993; Wieler et al., 1993; Wilson et al., 1993]. This agent is more lipophilic, is not dependent on the quality of the bolus injection, and enables more precise static imaging of parenchymatous brain perfusion in contrast to conventional dynamic imaging because of retention in the intact brain parenchyma. With this technology, the isotope can be injected in the intensive care unit, and better planar images can be obtained at a later time using a mobile camera or whenever the patient can be moved to the nuclear medicine department.
Fig. 79-2 Technetium cerebral blood flow study.
(Courtesy of Dr. Eloy Schulz, Department of Radiology, Loma Linda University Medical Center.)
Multiple studies in adults and children have documented radionuclide imaging to be accurate and reproducible, and it has been compared favorably with other methods of detecting the presence or absence of CBF [Conrad and Sinha, 2003; Drake et al., 1986; Goodman et al., 1985; Holtzman et al., 1983; Kaukinen et al., 1995; Parker et al., 1995; Ruiz-Garcia et al., 2000; Schneider, 1989; Schwartz et al., 1984; Wieler et al., 1993]. This technique is particularly useful when hypothermia or elevated barbiturate levels prevent valid EEG interpretation [Holtzman et al., 1983]. Premature and full-term newborns, despite having extremely reduced CBF compared with older children, can be readily evaluated using radionuclide measurements [Ashwal et al., 1989; Greissen, 1986]. Hospital nuclear medicine departments should be aware of improved advances in radiopharmaceuticals [Spieth et al., 1995; Wilson et al., 1993] and the ability to detect flow in the posterior fossa circulation [Spieth et al., 1995], and the fact that ventricular drainage procedures may give a false-negative indication that CBF is absent [Hansen et al., 1993].
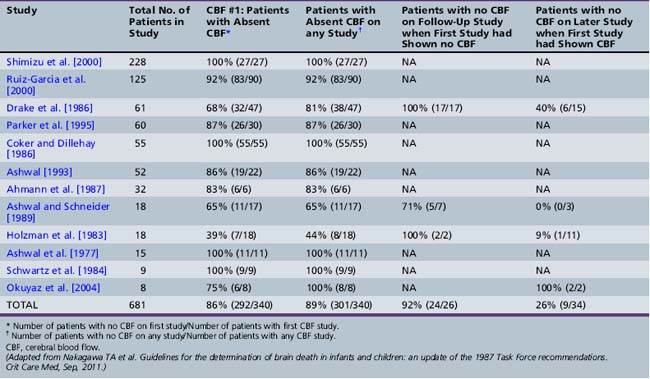
Table 79-7, from the 2011 Task Force report, summarizes CBF data from 12 studies in 681 suspected brain-dead children in all age groups [Nakagawa et al., 2011]. Different but well-standardized and conventional radionuclide cerebral angiography methods were used. Absent CBF was found in 86 percent of children who were clinically brain-dead, and the yield did not significantly change if more than one CBF study was done (89 percent). Table 79-7 also summarizes follow-up data on children whose subsequent CBF study showed no flow. Some 24 of 26 patients (92 percent) had no flow on follow-up CBF studies when the first study showed absent flow. The two exceptions, in whom flow developed later, were newborns. The first newborn had minimal flow on the second study and ventilator support was discontinued. The other newborn developed flow on the second study and had some spontaneous respirations and activity. A phenobarbital level 2 days after the second CBF study with minimal flow was 8 μg/mL. In those patients with preserved CBF on the first CBF study, 26 percent (9 of 34) had a second CBF study that showed no flow. The remaining 74 percent either had preserved flow or no further CBF studies were done, and all but one patient died (either spontaneously or by withdrawal of support). Only one patient survived with severe neurologic impairment from this entire group of patients – the same neonate as noted previously, with no CBF on the first study but presence of CBF on the second study.
Computed Tomographic Angiography and Perfusion
Computed tomographic angiography (CT-a) and perfusion (CTP) combines conventional CT with rapid bolus infusion of a contrast agent to examine the vascular structure and flow of any organ system. Several recent studies have examined its use in adults who met clinical and EEG brain-death criteria, and have demonstrated very good sensitivity and specificity when compared to other methods used to estimate cerebrovascular hemodynamics in brain-dead patients [Berenguer et al., 2010; Escudero et al., 2009]. However, there have been several case reports documenting the fact that CT-a may not show findings confirming the absence of CBF [Quesnel et al., 2007]. Some investigators have attempted to develop simplified scoring systems by counting a prespecified number of intracranial arteries to document the absence of CBF [Combes et al., 2007]. CT-a is a promising method to use to determine the absence of intracranial circulation, but studies have not yet been published in pediatric brain-dead patients.
Magnetic Resonance Imaging and Magnetic Resonance Angiography
MRI and MR angiography have also been used to evaluate the cerebral circulation in adults who have met clinical criteria for brain death [Karantanas et al., 2002]. Characteristic features seen in most brain-dead (but not comatose) patients include the following:
Similar findings have been described by other investigators, although the loss of gray–white matter differentiation is not a consistent finding [Lee et al., 1995; Matsumura et al., 1996]. In another study, MR angiography also was performed, and nonvisualization above the level of the supraclinoid portion of the internal carotid arteries was found [Ishii et al., 1996]. Abnormalities of diffusion-weighted imaging also have been observed in adult patients who are brain-dead [Lovblad et al., 2000]. Presumably, similar neuroimaging findings would be present in children, but this has not yet been reported.
Transcranial Doppler
Transcranial Doppler ultrasonography has advocates because of its obvious portability and noninvasiveness and isolette accessibility [Ahmann et al., 1987; Feri et al., 1994; Manno, 1997; McMenamin and Volpe, 1983]. More recent studies document a high rate of false-negative test results and lower levels of specificity and sensitivity, which suggest significant limitations for use of this technique for brain-death confirmation [Chiu et al., 2003; de Freitas et al., 2003; Dosemeci et al., 2004; Rodriguez et al., 2002]. In most of the more recently reported series, several patients who had Doppler findings typically seen with brain death recovered, and patients who were brain-dead frequently did not have the previously described progression of Doppler abnormalities [Chiu et al., 2003; de Freitas et al., 2003; Dosemeci et al., 2004; Rodriguez et al., 2002]. Doppler studies have been used on at least one occasion to confirm the diagnosis of fetal brain death in a 23-week gestational age infant [Otsubo et al., 1999].
Digital Subtraction Angiography
Digital subtraction angiography is another technique that has been used to assess the intracranial circulation. This technique can be performed intravenously [Van Bunnen et al., 1989] or by intra-arterial injection [Albertini et al., 1993]. A small amount of nonionic contrast material is injected while digital subtraction imaging of the cerebral vasculature is done, similar to conventional cerebral angiography. This procedure allows visualization of contrast within the major intracranial vessels; lack of such visualization indicates absence of cerebral blood flow. There are few reports of this technique in children and only one case report in a neonate diagnosed with brain death [Albertini et al., 1993]. A report using intravenous digital subtraction angiography in 110 patients with clinical signs of brain death observed that the first study documented absent contrast enhancement in 105 patients [Van Bunnen et al., 1989]. Repeat studies in the remaining five patients within several hours also confirmed the cessation of CBF. Digital subtraction angiography has not been used much in children and is not readily available.
Xenon Computed Tomography
Stable xenon CT and 133-xenon CT are examples of reliable and well-documented tests that are seldom available because of cost and limited personnel [Pistoia et al., 1991]. Xenon CT allows quantitative and regional measurement of CBF. In brain-dead adults, CBF values of 1.6 ± 2 mL/min/100 g have been reported [Darby et al., 1987]. Previous studies found an average CBF of 1.3 ± 1.6 mL/min/100 g in 10 brain-dead children, compared with 33.5 ± 16.3 mL/min/100 g in 11 profoundly comatose children [Ashwal et al., 1989]. This finding compares favorably with average CBF values of 12 mL/min/100 g using 133-xenon CT [Greissen, 1986] and 7–11 mL/min/100 g using positron emission tomography in preterm infants who were not brain-dead [Altman et al., 1988].
Positron Emission Tomography
The results of positron emission tomography (see Chapter 11) have been reported in only a few brain-dead patients [Medlock et al., 1993; Meyer, 1996; Vander Borght et al., 2001]. Because of limited availability, cost, and lack of comparison studies, positron emission tomography offers no advantages over the more standardized methods previously discussed. Meyer reported an 18-year-old brain-dead adolescent whose dynamic positron emission tomography study, performed 7 days after a severe post-traumatic closed head injury, found no intracerebral uptake or retention of tracer and was considered consistent with the diffuse absence of brain metabolism [Meyer, 1996]. Medlock and associates reported a 2-month-old brain-dead infant with preserved CBF, whose positron emission tomography scan on the 11th day after injury revealed a normal glucose metabolic gradient between gray and white matter [Medlock et al., 1993]. Postmortem examination revealed widespread necrosis with mononuclear cell infiltrates throughout all cerebral cortical layers. The persistence of glucose metabolism was thought to be associated with the presence of inflammatory microglial cells and suggested that the persistence of CBF and glucose metabolism in brain-dead children might not indicate neuronal survival.
Magnetic Resonance Spectroscopy
In the 1980s and 1990s, phosphorus (31P) and proton (1H) MR spectroscopy were used to measure aspects of brain metabolic activity noninvasively (see Chapter 11). Studies in neonates, older infants, and children have reported significant abnormalities using these techniques, with loss of metabolic activity associated with severe acute central nervous system insults and with poor long-term outcomes [Holshouser et al., 1997; Martin et al., 1996]. 31P-MR spectroscopy has been reported in 3 infants, 4 children, and 17 adults who met adult criteria for brain death. Spectra in these patients indicated the absence of adenosine triphosphate (ATP) and phosphocreatine. The inorganic phosphate and phosphodiester peaks were still detectable [Kato et al., 1991]. Another 31P-MR spectroscopy study involving three brain-dead adults found similar spectral abnormalities [Aichner et al., 1992]. These findings suggest that MR spectroscopy could provide another technique to assess the complete loss of cerebral function objectively. The advantage of MR spectroscopy is that it can be done in conjunction with MRI, which allows acquisition of anatomic and metabolic data that could clarify the etiology of the central nervous system insult and determine whether there is irreversible neuronal loss. Terk and colleagues reported a term brain-dead infant whose 31P spectra on days 11 and 18 demonstrated three distinct ATP peaks and several other peaks, however, which suggested the persistence of metabolic activity [Terk et al., 1992]. Although no definite reasons could be ascertained for this finding, unlike the study of Kato and associates, they suggested that any proposed spectral signature for brain death would need to be modified.
Comparison of EEG and CBF studies
Table 79-8 summarizes the comparative diagnostic yield of EEG vs. CBF determinations in children who had both studies done as part of the initial brain-death evaluation that was reported in the 2011 pediatric guidelines [Nakagawa et al., 2011]. Data from the 12 studies cited in Tables 79-6 and 79-7 were stratified by three age groups:
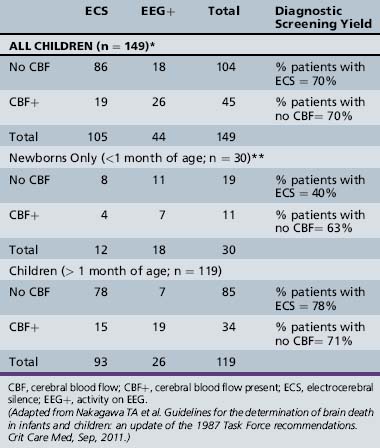
The data in Tables 79-6 and 79-7 show that the yield from the initial CBF studies was higher (86 percent) than from the initial EEG (76 percent), but no differences were present for any CBF study (89 percent) vs. any EEG study (89 percent). In contrast, the data in Table 79-8 for all children show that, when both studies are initially performed, the diagnostic yield is the same (70 percent had ECS; and 70 percent showed absent CBF). The diagnostic yield for children greater than 1 month of age was similar for both tests (EEG with ECS, 78 percent; no CBF, 71 percent). For newborns, EEG with ECS was less sensitive (40 percent) than absence of CBF (63 percent) when confirming the diagnosis of brain death, but even in the CBF group, the yield was low.
Evoked Potentials
Brainstem auditory potentials have been studied extensively as a brain-death determinant [Guerit, 1992; Litscher et al., 1995; Lutschg et al., 1983; Machado et al., 1991; Ruiz-Garcia et al., 2000; Ruiz-Lopez et al., 1999; Steinhart and Weiss, 1985]. The portability, rapidity, and noninvasiveness seem ideal, but multiple studies have raised significant doubts as to the reliability of brainstem auditory responses in brain-death determinations [Dear and Godfrey, 1985; De Merlier and Taylor, 1986; Flannery, 1999; Schmitt et al., 1993; Steinhart and Weiss, 1985; Taylor et al., 1983]. Somatosensory-evoked potentials also have been used for the confirmation of brain death [Beca et al., 1995; Ruiz-Garcia et al., 2000; Ruiz-Lopez et al., 1999; Wagner, 1996; Ying et al., 1992], but there are insufficient data to assess whether it is superior to brainstem-evoked responses for brain-death determination. From a practical perspective, most clinicians evaluating infants and children for brain death do not rely on evoked potentitals as an ancillary test for brain death; rather, testing remains useful in children who survive catastrophic brain injury as a prognostic indicator of poor outcome.
Brain Death in Newborns
The revised 2011 pediatric brain death guidelines state that brain death can be diagnosed in term infants of more than 37 weeks’ gestation, provided the physician is aware of the limitations of the clinical examination and ancillary studies in this age group [Nakagawa et al., 2011]. Recommendations for preterm neonates of less than 37 weeks’ gestation were not made because of insufficient data in this age group.
As previously noted, preterm infants and term infants of less than 7 days of age were excluded from the 1987 pediatric brain death guidelines [Report of Special Task Force, 1987]. Several years after the publication of these guidelines, data on 18 brain-dead neonates were published, and it was suggested that brain death could be diagnosed in full-term newborn infants and preterm infants of more than 34 weeks’ gestational age within the first week of life [Ashwal and Schneider, 1989]. Because a newborn has patent sutures and an open fontanel, increases in intracranial pressure after acute injury are not as significant as in older patients. The usual cascade of events in older patients after acute brain injury, which results in increasing intracranial pressure and reduced cerebral perfusion, is less likely to occur in a newborn. Consequently, absent cerebral blood flow is less likely, as is the development of ECS (as reviewed previously in the section on neurodiagnostic testing). The use of whole-brain death criteria might not be appropriate in a newborn. It might be more medically justifiable to use brainstem death as the major criterion for brain death in newborns. In many situations, the issue of whether a neonate is brain-dead or just catastrophically and irreversibly brain-injured is resolved by the decision of physicians caring for the neonate with the understanding and agreement of the family to redirect to compassionate care.
Epidemiology
It has been estimated that there are about 550 newborns diagnosed each year as brain-dead out of a total of 3,900,089 live births [Ashwal, 1997]. Etiologies of brain death based on data from 88 newborns less than 1 month of age included hypoxic-ischemic encephalopathy (61 percent), birth trauma (8 percent), central nervous system malformations (6 percent), central nervous system hemorrhage (6 percent), infection (7 percent), sudden infant death syndrome (7 percent), nonaccidental trauma (4 percent), and metabolic disorders (1 percent).
Clinical Examination
Limited data are available regarding the clinical examination for brain death in preterm and term infants. Aspects of performing the neurological examination in this age group are reviewed in Chapter 4. It has been recognized that examination of preterm infants of less than 37 weeks’ gestation to determine if they meet brain death criteria may be difficult because of the possibility that some of the brainstem reflexes may not be completely developed (see Table 79-2), and that it is also difficult to assess the level of consciousness in a critically ill, sedated, and intubated neonate. The revised guidelines emphasized the importance of carefully and repeatedly examining term newborns, with particular attention to examination of brainstem reflexes and apnea testing, and that, as with older children, assessment of neurologic function in the term newborn might be unreliable immediately following an acute catastrophic neurologic injury or cardiopulmonary arrest; therefore, a period of 24 hours or longer was recommended before evaluating the term newborn for brain death.
Duration of Observation
The 2011 guidelines recommend a 24-hour observation period between the two neurological examinations for term infants (>37 weeks’ gestation). Discussions of specific issues regarding certainty of diagnosis, immaturity of the nervous system, and the effects of development on the pathophysiology and diagnosis of brain death in preterm and term infants have been published previously [Coulter, 1987; Freeman and Ferry, 1988; Kohrman, 1993; Volpe, 1987] and updated [Ashwal, 1997]. For the most part, the task-force recommendations concerning the duration of observation of brain death in children of different ages were based on expert opinion and consensus rather than being evidence-based. Data from 87 newborns allowed an estimation of the duration of coma after the insult until brain death was initially diagnosed (37 hours), duration of time before brain-death confirmation (75 hours), and duration of time to transplantation (20 hours) [Ashwal, 1997]. The average duration of brain death in these patients was about 95 hours (i.e., 4 days). Recovery of brainstem function was not observed in any of the patients, despite a variety of EEG and CBF results. Further analysis examining the duration of brain death was carried out on the 53 neonates whose organs were donated for transplantation. The total duration of brain death (including time to transplantation) averaged 2.8 days in neonates less than 7 days of age. In neonates 1–3 weeks old, the duration was approximately 5.2 days. The data suggest that a 24- to 48-hour observation period in neonates less than 7 days of age should be sufficient to confirm the diagnosis of brain death.
Apnea Testing
Neonatal studies reviewing PaCO2 thresholds for apnea are limited. However, data from 35 neonates who were ultimately determined to be brain-dead revealed a mean PaCO2 of 65 mmHg, suggesting that the threshold of 60 mmHg is also valid in the newborn [Ashwal, 1997]. Apnea testing in the term newborn may be complicated by observations that treatment with 100 percent oxygen may inhibit the potential recovery of respiratory effort, and that bradycardia may precede hypercarbia and limit the ability to perform apnea testing.
Ancillary Studies
EEG and radionuclide imaging techniques, as in older infants, children, and adults, remain the most commonly used ancillary studies to confirm the diagnosis of brain death. The 1987 task force guidelines on pediatric brain death did recommend two examinations and EEGs 48 hours apart in neonates 7 days to 2 months old, but made no statement concerning the use of cerebral perfusion techniques because of limited data [Report of Special Task Force, 1987]. Likewise, no recommendations were made for infants less than 7 days old or preterm infants.
Ancillary studies performed in the newborn under 30 days of age are limited [Ashwal, 1989]. As summarized in Table 79-8, ancillary studies in this age group are less sensitive in detecting the presence or absence of brain electrical activity or CBF than in older children. Of the two studies, detection of absence of CBF (63 percent) was more sensitive than demonstration of ECS (40 percent) in confirming the diagnosis of brain death; however, even in the CBF study group, the sensitivity was low.
Because of the significant physiologic and cerebrovascular differences in the neonatal response to injuries resulting in brain death, previous studies have observed a much higher incidence of newborns with EEG activity or cerebral perfusion [Ashwal, 1989]. In addition, some newborns with ECS demonstrated preserved CBF, and conversely, others without CBF showed EEG activity. In neonates, even though CBF and mean arterial blood pressures are much lower, increases in intracranial pressure after acute injury are less dramatic. In a neonate in whom the cerebral metabolic rate is lower, cerebral perfusion is likely to persist to the extent that, even when the neonate is brain-dead, CBF can be detected, and some neuronal viability persists because of this low-flow state. Data on 30 newborns who had EEGs and radionuclide perfusion studies revealed that one-third (4 of 12) with ECS showed evidence of CBF, and 58 percent (11 of 19) of newborns with absent CBF had evidence of EEG activity [Ashwal, 1997]. These data suggest that, in this age group, clinical rather than laboratory data may be more appropriate, provided that a sufficient period of observation is allowed.
Determination of Brain Death in the Comatose Pediatric Patient
An algorithmic approach to the evaluation of a comatose child to determine whether the child is brain-dead is presented in Figure 79-3. The question of whether a comatose child is brain-dead arises within the first days of hospitalization. Occasionally, this issue occurs later because the long-term use of sedative and paralytic agents does not permit the necessary screening to assess for the presence of coma and loss of brainstem function, especially the loss of pupillary reflexes that triggers the question as to whether a child is brain-dead. In most neonatal and pediatric intensive care units, intensivists, in combination with neurologists and neurosurgeons, go through the process of diagnosing brain death. This assessment must include determination of the proximate cause of coma, with review of all neuroimaging, neurophysiologic, metabolic, and toxicology screening studies that were performed to determine the etiology of the global cerebral insult and to determine whether the insult is or is not irreversible. Serum and urine studies should include toxicology screens and serum electrolytes, glucose, calcium, urea nitrogen, creatinine, liver enzymes, lactate, pyruvate, and ammonia determinations [Kennedy and Kiloh, 1996; Tobin, 1996]. If the history suggests a metabolic disorder, serum amino acid and urine organic acid studies should be considered. Neuromuscular blocking agents and all sedative agents also should be discontinued.
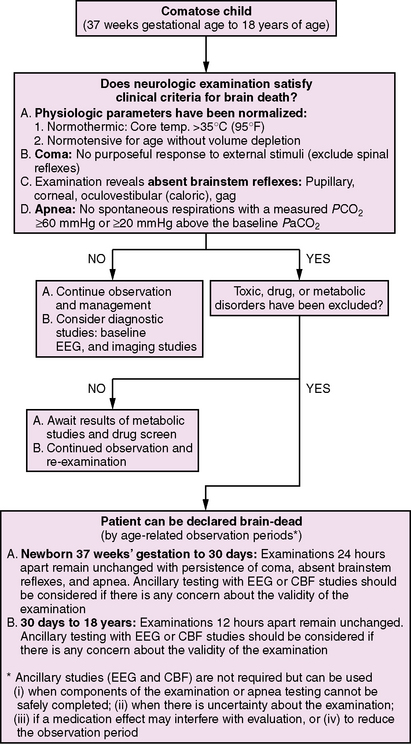
Fig. 79-3 Algorithm for the evaluation for brain death in a comatose child.
(This algorithm is based on the revised 2011 Pediatric Brain Death guidelines.)
As part of the examination, it is necessary to ensure that the core body temperature and blood pressure are within the normal range. The presence of purposeful movements in response to external stimuli or spontaneous (not spinal-related) movements, posturing, or occurrence of seizures would obviate the diagnosis of brain death. A careful examination and documentation of the absence of brainstem reflexes is crucial and may be difficult to perform, particularly in an intubated neonate or young infant. Generally, parents should be allowed to observe the process, and nursing and respiratory therapy staff should be informed of the findings. As previously discussed, the duration of observation to confirm the diagnosis of brain death depends on the child’s age, as do recommendations for confirmatory testing (see Figure 79-3). The 2011 guidelines recommend that children of 37 weeks’ gestation to 30 days require 24 hours of observation, and children of 30 days to 18 years require 12 hours to confirm the diagnosis of brain death. There are no consensus criteria for preterm neonates; the diagnosis can be made in preterm infants, presumably after a longer period of observation (i.e., 48–72 hours) based on available data [Ashwal and Schneider, 1989].
Confirmatory studies, depending on the child’s age, are recommended as outlined in Figure 79-3. These tests are readily available in all hospitals and are helpful in establishing the diagnosis of brain death. Results of these tests provide physicians with an opportunity to discuss with the child’s family the concept of brain death and the loss of electrocortical activity and CBF. The tests also allow the physician to explain how the cause of injury ultimately caused brain death to develop.
Discussions with Family Members and Staff
Most pediatric brain-death determinations are done on patients who were normal children just hours or days before the injury, and even after extensive discussions with family members, the concept of brain death remains unclear for many [Siminoff et al., 2003]. Grieving parents may not understand the futility of continued respirator support and may not accept that continuing care cannot benefit the child. Without being confrontational, physicians caring for the child should discuss the prognosis with parents. Frequently, a conference with family members and members from the health-care team (e.g., physicians, nurses, therapists, social workers, clergy) is helpful in educating the family and assuring them that the health-care professionals are in agreement with the diagnosis and recommendations. Caregivers, particularly nursing and respiratory technicians, can be overlooked regarding their own need for emotional support and the grief they share with parents [House and Benitez, 1992; Stoyles, 1995].
Organ Donation
The National Organ Transplant Act of 1987 requires that every hospital in the United States develop a protocol for requesting organ donation. After declaration of brain death, parents of a potential donor should be approached regarding donation [Williams et al., 2003]. Refusal from parents with whom donation has been explored should be respected. If the child is not a potential donor (sepsis, severe ischemia), it is important to discuss the reason why donation is not suitable, so that parents do not believe donation was neglected.
Strategies for retrieval of organs should be in place in the pediatric intensive care unit [Dutie et al., 1993; Tsai et al., 2000]. In many centers, the intensivist caring for the patient is the individual who may first declare the patient brain-dead and initiate the discussion regarding organ donation, so it is important and prudent to have independent confirmation of brain death by a second physician. Brain-dead child abuse victims represent a significant percentage of brain-death diagnoses and, as such, require medicolegal evaluation before organ donation can proceed [Zenel and Goldstein, 2002].
Maintenance of brain-dead patients requires careful management in anticipation of organ donation [Lutz-Dettinger et al., 2001]. Maintenance of temperature [Goldstein et al., 1993] and provision of adequate fluids and supplemental oxygen to achieve satisfactory perfusion are necessary, as is consideration for hormone replacement [Rosendale et al., 2003] with corticosteroids [Arita et al., 1993], vasopressin [Pennefather et al., 1995], and thyroxine [Roels et al., 2000]. Diabetes insipidus, hypernatremia, hyperglycemia, and coagulopathies commonly occur in brain-dead children and require special management if transplantation is being considered [Lutz-Dettinger et al., 2001]. By developing a careful balance between the care of a child who is considered to be brain-dead and the desire to retrieve organs for donation, physicians can help grieving families and improve the opportunity to increase the number of organs that are being donated to save the lives of children with many different forms of severe, disabling, and life-threatening diseases.
References
![]() The complete list of references for this chapter is available online at www.expertconsult.com.
The complete list of references for this chapter is available online at www.expertconsult.com.
Abdel Dayem H.M., Bahar R.H., Sigurdsson G.H., et al. The hollow skull: A sign of brain death in Tc-99m HM-PAO brain scintigraphy. Clin Nucl Med. 1989;14:912.
Ahmann P.A., Carrigan T.A., Carlton D., et al. Brain death in children: Characteristic common carotid arterial velocity patterns measured with pulsed Doppler ultrasound. J Pediatr. 1987;110:723.
Aichner F., Felber S., Birbamer G., et al. Magnetic resonance: A noninvasive approach to metabolism, circulation, and morphology in human brain death. Ann Neurol. 1992;32:507.
Albertini A., Schonfeld S., Hiatt M., et al. Digital subtraction angiography – a new approach to brain death determination in the newborn. Pediatr Radiol. 1993;23:195.
Altman D.I., Powers W.J., Perlman J.M., et al. Cerebral blood flow requirements for brain viability in newborn infants is lower than in adults. Ann Neurol. 1988;24:218.
Alvarez L.A., Moshe S.L., Belman A.L., et al. EEG and brain death determination in children. Neurology. 1988;38(2):227-230.
American Electroencephalographic Society. Guideline three: Minimum technical standards for EEG recording in suspected cerebral death. J Clin Neurophysiol. 1994;11:10.
American Electroencephalographic Society. Guidelines in EEG 1-7 (revised 1985). J Clin Neurophysiol. 1986;3:131.
Ammar A., Awada A., Al-Luwami I. Reversibility of severe brain stem dysfunction in children. Acta Neurochir. 1993;124:86.
Arita K., Uozumi T., Oki S., et al. The function of the hypothalamo-pituitary axis in brain dead patients. Acta Neurochir (Wien). 1993;123:64.
Ashwal S. Brain death in the newborn. Clin Perinatol. 1989;16:501.
Ashwal S. Are we diagnosing brain death in newborns accurately? J Heart Lung Transplant. 1991;10:863.
Ashwal S. Brain death in early infancy. J Heart Lung Transplant. 1993;12(6 Pt 2):S176-S178.
Ashwal S. Brain death in the newborn. Clin Perinatol. 1997;24:859.
Ashwal S. Clinical diagnosis and confirmatory testing of brain death in children. In: Wijdick E., editor. Brain Death. Philadelphia, PA: Lippincott William & Wilkins; 2001:91-114.
Ashwal S., Schneider S. Failure of electroencephalography to diagnose brain death in comatose patients. Ann Neurol. 1979;6:512.
Ashwal S., Schneider S. Brain death in children: Part I. Pediatr Neurol. 1987;3:5-11. Part II, Pediatr Neurol 3:69–77, 1987
Ashwal S., Schneider S. Brain death in the newborn: Clinical, EEG and blood flow determinations. Pediatrics. 1989;84:429.
Ashwal S., Schneider S. Determination of brain death in infants and children. In: Swaiman K.F., Ashwal S., editors. Pediatric neurology: Principles and practice. St. Louis: Mosby; 1999:969.
Ashwal S., Schneider S., Thompson J. Xenon computed tomography cerebral blood flow in determination of brain death in children. Ann Neurol. 1989;25:539.
Ashwal S., Smith A.J.K., Torres F., et al. Radionuclide bolus angiography: A technique for verification of brain death in infants and children. J Pediatr. 1977;91:722.
Banasiak K.J., Lister G. Brain death in children. Curr Opin Pediatr. 2003;15:288.
Beca J., Cox P.N., Taylor M.J., et al. Somatosensory evoked potentials for prediction of outcome in acute severe brain injury. J Pediatr. 1995;126:44.
Beecher H.K. A definition of irreversible coma: Report of the ad hoc committee of Harvard Medical School to examine the definition of brain death. JAMA. 1968;205:337.
Bennett D.R., Hughes J.R., Korein J., et al. Atlas of electroencephalography in coma and cerebral death. New York: Raven Press; 1976.
Berenguer C.M., Davis F.E., Howington J.U. Brain death confirmation: comparison of computed tomographic angiography with nuclear medicine perfusion scan. J Trauma. 2010;68(3):553-559.
Bonetti M.G., Ciritella P., Valle G., et al. 99mTc HM-PAO brain perfusion SPECT in brain death. Neuroradiology. 1995;37:365.
Booth C.M., Boone R.H., Tomlinson G., et al. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291(7):870-879.
Bradac G.B., Simon R.S. Angiography in brain death. Neuroradiology. 1974;7:25.
Carter B.G., Butt W. A prospective study of outcome predictors after severe brain injury in children. Intensive Care Med. 2005;31(6):840-845.
Chang M.Y., McBride L.A., Ferguson M.A. Variability in brain death declaration practices in pediatric head trauma patients. Pediatr Neurosurg. 2003;39:7.
Chatrian G.E. Electrophysiologic evaluation of brain death: A critical appraisal. In: Aminoff M.J., editor. Electrodiagnosis in clinical neurology. New York: Churchill-Livingstone, 1986.
Chiu N.C., Shen E.Y., Ho C.S. Outcome in children with significantly abnormal cerebral blood flow detected by Doppler ultrasonography: Focus on the survivors. J Neuroimaging. 2003;13:53.
Coker S.B., Dillehay G.L. Radionuclide cerebral imaging for confirmation of brain death in children: the significance of dural sinus activity. Pediatr Neurol. 1986;2:43-46.
Combes J.C., Chomel A., Ricolfi F., et al. Reliability of computed tomographic angiography in the diagnosis of brain death. Transplant Proc. 2007;39(1):16-20.
Conference of Medical Royal Colleges and their Faculties in the UK. Diagnosis of death. BMJ. 1976;2:1187.
Conference of Medical Royal Colleges and their Faculties in the UK. Diagnosis of death. BMJ. 1979;1:332.
Conrad G.R., Sinha P. Scintigraphy as a confirmatory test of brain death. Semin Nucl Med. 2003;33:312.
Coulter D.L. Neurologic uncertainty in newborn intensive care. N Engl J Med. 1987;316:840.
Criteria for the diagnosis of brain stem death. Review by a working group convened by the Royal College of Physicians and endorsed by the Conference of Medical Royal Colleges and their Faculties in the United Kingdom. J R Coll Physicians Lond. 1995;29:381.
Darby J.M., Yonas H., Gur D., et al. Xenon-enhanced computed tomography in brain death. Arch Neurol. 1987;44:551.
Dear P.R.F., Godfrey D.J. Neonatal auditory brainstem response cannot reliably diagnose brainstem death. Arch Dis Child. 1985;60:17.
de Freitas G.R., Andre C., Bezerra M., et al. Persistence of isolated flow in the internal carotid artery in brain death. J Neurol Sci. 2003;210:31.
De Merlier J.L., Taylor M.J. Evoked potentials in comatose children: Auditory brain stem responses. Pediatr Neurol. 1986;2:31.
DeOliveira W.M., DeOliveira M.L.J., Pereira I.E., et al. Reavaliacao dos criterios clinicos e electroencefalograficos de determinacao da morte cerebral na crianca. Arq Neuropsiquiatr. 1984;42:25.
Dosemeci L., Dora B., Yilmaz M., et al. Utility of transcranial Doppler ultrasonography for confirmatory diagnosis of brain death: Two sides of the coin. Transplantation. 2004;77:71.
Drake B., Ashwal S., Schneider S. Determination of cerebral death in the pediatric intensive care unit. Pediatrics. 1986;78:107.
Dutie S.E., Peterson B.W., Cutler J., et al. Successful organ donation in victims of child abuse. Clin Transplant. 1993;9:415.
Escudero D., Otero J., Marqués L., et al. Diagnosing brain death by CT perfusion and multislice CT angiography. Neurocrit Care. 2009;11(2):261-271.
Fanaroff A., Martin R.J., Miller M.J. The respiratory system. In: Fanaroff A., Martin R.J., editors. Neonatal-perinatal medicine: Diseases of the fetus and newborn. St. Louis: Mosby, 1987.
Farrell M.M., Levine D.L. Brain death in the pediatric patient: Historical, sociological, medical, religious, cultural, legal, and ethical considerations. Crit Care Med. 1993;21:1951.
Feri M., Ralli L., Felici M., et al. Transcranial Doppler and brain death diagnosis. Crit Care Med. 1994;22:1120.
Fishman M.A. Validity of brain death criteria in infants. Pediatrics. 1995;96:513.
Flannery A.M. Brain death and evoked potentials in pediatric patients. Crit Care Med. 1999;27:264.
Freeman J.M., Ferry P.C. New brain death guidelines in children: Further confusion. Pediatrics. 1988;81:301.
Furgiuele T.L., Frank L.M., Riegle C., et al. Prediction of cerebral death by cranial sector scan. Crit Care Med. 1984;12(1):1-3.
Galaske R.G., Schober O., Heyer R. 99mTc-HM-PAO and 1231-amphetamine cerebral scintigraphy: A new, noninvasive method in determination of brain death in children. Eur J Nucl Med. 1988;14:446.
Goh A.Y., Lum L.C., Chan P.W., et al. Withdrawal and limitation of life support in paediatric intensive care. Arch Dis Child. 1999;80:424.
Goldstein B., DeKing D., DeLong D.J., et al. Autonomic cardiovascular state after severe brain injury and brain death in children. Crit Care Med. 1993;21:228.
Goodman J.M., Heck L.L., Moore B.D. Confirmation of brain death with portable isotope angiography: A review of 204 consecutive cases. Neurosurgery. 1985;16:492.
Gotay-Cruz F., Fernandez-Sein A. Pediatric experience with brain death determination. P R Health Sci J. 2002;21:11.
Grattan Smith P.J., Butt W. Suppression of brainstem reflexes in barbiturate coma. Arch Dis Child. 1993;69:151.
Green R., Lauber A. Recovery of activity in young children after ECS. J Neurol Neurosurg Psychiatry. 1972;35:103.
Greissen G. Cerebral blood flow in preterm infants during the first week of life. Acta Paediatr Scand. 1986;75:43.
Grigg G., Kelly M., Celesia G., et al. Electroencephalographic activity after brain death. Arch Neurol. 1987;44:948.
Guerit J.M. Evoked potentials: A safe brain-death confirmatory tool? Eur J Med. 1992;1:233.
Hansen A.V., Lavin P.J., Moody E.B., et al. False-negative cerebral radionuclide flow study, in brain death, caused by a ventricular drain. Clin Nucl Med. 1993;18:502.
Haque I.U., Udassi J.P., Zaritsky A.L. Outcome following cardiopulmonary arrest. Pediatr Clin North Am. 2008;55(4):969-987.
Harrison A.M., Botkin J.R. Can pediatricians define and apply the concept of brain death? Pediatrics. 1999;103:e82.
Haupt W.F., Rudolf J. European brain death codes: a comparison of national guidelines. J Neurol. 1999;246:432.
Hicks R.C., Poole J.L. Electroencephalographic changes with hypothermia and cardiopulmonary bypass in children. J Thorac Cardiovasc Surg. 1981;81:781.
Holshouser B.A., Ashwal S., Luh G.Y., et al. Proton MR spectroscopy after acute central nervous system injury: outcome prediction in neonates, infants and children. Radiology. 1997;202:487.
Holzman B.H., Curless R.G., Seakianakis G.N., et al. Radionuclide cerebral perfusion scintigraphy in determination of brain death in children. Neurology. 1983;33:1027.
House M.A., Benitez N. Multiple organ retrieval: Impact on staff and institutions. Semin Perioper Nurs. 1992;1:51.
Ishii K., Onuma T., Kinoshita T., et al. Brain death: MR and MR angiography. Am J Neuroradiol. 1996;17:731.
Jennett B., Bond M. Assessment of outcome after severe brain damage, a practical scale. Lancet. 1975;1:480.
Joffe A.R., Kolski H., Duff J., et al. A 10 month old with reversible findings of brain death. Pediatr Neurol. 2009;41:378-382.
Jorgensen E.O., Malchow-Moller A. Cerebral prognostic signs during cardiopulmonary resuscitation. Resuscitation. 1978;6:217.
Juguilon A.C., Reilly E.L. Development of EEG activity after ten days of electrocerebral inactivity: Ten days of electrocerebral inactivity: A case report in a premature neonate-hydranencephaly or massive ventricular enlargement. Clin Electroencephalogr. 1982;13:233.
Karantanas A.H., Hadjigeorgiou G.M., Paterakis K., et al. Contribution of MRI and MR angiography in early diagnosis of brain death. Eur Radiol. 2002;12:2710.
Kato T., Tokumaru A., O’uchi T., et al. Assessment of brain death in children by means of P-31 MR spectroscopy: Preliminary note. Radiology. 1991;179:95.
Kaufman H.H. Pediatric brain death and organ/tissue retrieval: Medical, ethical, and legal aspects. New York: Plenum; 1989.
Kaukinen S., Makela K., Hakkinen V.K., et al. Significance of electrical brain activity in brain-stem death. Intensive Care Med. 1995;21:76.
Kennedy M., Kiloh N. Drugs and brain death. Drug Saf. 1996;14:171.
Kohrman M.H. Brain death in neonates. Semin Neurol. 1993;13:116.
Kohrman M.H., Spivack B.S. Brain death in infants: Sensitivity and specificity of current criteria. Pediatr Neurol. 1990;6:47.
LaMancusa J., Cooper R., Vieth R., et al. The effects of the falling therapeutic and subtherapeutic barbiturate blood levels on electrocerebral silence in clinically brain-dead children. Clin Electroencephalogr. 1991;22:112.
Lang C.J. Apnea testing by artificial CO2 augmentation. Neurology. 1995;45:966.
Lee D.H., Nathanson J.A., Fox A.J., et al. Magnetic resonance imaging of brain death. Can Assoc Radiol J. 1995;46:174.
Litscher G., Schwartz G., Kleinert R. Brainstem auditory evoked potential monitoring: Variations of stimulus artifact in brain death. Electroencephalogr Clin Neurophysiol. 1995;96:413.
Lopez-Herce J., Sancho L., Martinon J.M. Study of paediatric intensive care units in Spain. Spanish Society of Paediatric Intensive Care. Intensive Care Med. 2000;26:62.
Lovblad K.O., Bassetti C., Basssetti C. Diffusion-weighted magnetic resonance imaging in brain death. Stroke. 2000;31:539.
Lutschg J., Pfenninger J., Lundin H.P., et al. Brainstem auditory evoked potentials and early somatosensory evoked potentials in neuro-intensively treated comatose children. Am J Dis Child. 1983;137:421.
Lutz-Dettinger N., de Jaeger A., Kerremans I. Care of the potential pediatric organ donor. Pediatr Clin North Am. 2001;48:715.
Machado C., Valdes P., Garcia Tigera J., et al. Brain-stem auditory evoked potentials and brain death. Electroencephalogr Clin Neurophysiol. 1991;80:392.
Mandel R., Marinot A., Delepoulle F. Prediction of outcome after hypoxic-ischemic encephalopathy: A prospective clinical and electrophysiologic study. J Pediatr. 2002;141:45. –5
Manno E.M. Transcranial Doppler ultrasonography in the neurocritical care unit. Crit Care Clin. 1997;13:79.
Martin E., Buchli R., Ritter S., et al. Diagnostic and prognostic value of cerebral 31P magnetic resonance spectroscopy in neonates with perinatal asphyxia. Pediatr Res. 1996;40:749.
Martinot A., Grandbastien B., Leteurtre S., et al. No resuscitation orders and withdrawal of therapy in French paediatric intensive care units. Groupe Francophone de Reanimation et d’Urgences Pediatriques. Acta Paediatr. 1998;87:769.
Martinot A., Lejeune C., Hue V., et al. Modality and causes of 259 deaths in a pediatric intensive care unit. Arch Pediatr. 1995;2:735.
Mathur M., Petersen L., Stadtler M., et al. Variability in pediatric brain death determination and documentation in southern California. Pediatrics. 2008;121(5):988-993.
Matsumura A., Meguro K., Tsurushima H., et al. Magnetic resonance imaging of brain death. Neurol Med Chir (Tokyo). 1996;36:166.
McMenamin J.B., Volpe J.J. Doppler ultrasonography in the determination of neonatal brain death. Ann Neurol. 1983;14:302.
Medlock M.D., Hanigan W.C., Cruse R.P. Dissociation of cerebral blood flow, glucose metabolism, and electrical activity in pediatric brain death: Case report. J Neurosurg. 1993;79:752.
Mejia R.E., Pollack M.M. Variability in brain death determination practices in children. JAMA. 1995;274:550.
Meyer M.A. Evaluating brain death with positron emission tomography: Case report on dynamic imaging of 18F-fluorodeoxyglucose activity after intravenous bolus injection. J Neuroimaging. 1996;6:117.
Mohandas A., Chou S.N. Brain death: A clinical pathological study. J Neurosurg. 1971;35:211.
Mollaret P., Goulon M. Le coma depasse. Rev Neurol. 1959;101:3.
Moshe S.K., Alvarez L.A. Diagnosis of brain death in children. J Clin Neurophysiol. 1986;3:239.
Moshe S.L. Usefulness of EEG in the evaluation of brain death in children: The pros. Electroencephalogr Clin Neurophysiol. 1989;73:272.
Mrhac L., Zakko S., Parikh Y. Brain death: The evaluation of semi-quantitative parameters and other signs in HMPAO scintigraphy. Nucl Med Commun. 1995;16:1016.
Nakagawa T.A., Ashwal S., Mathur M., et al. Guidelines for the determination of brain death in infants and children: an update of the 1987 Task Force recommendations. Crit Care Med Sep. 2011.
Nash K.B., Bonifacio S.L., Glass H.C., et al. Video-EEG monitoring in Newborns with Hypoxic-Ischemic Encephalopathy Treated with Hypothermia. Neurology. 2011. in press
National Institute of Neurologic and Communicative Disorders and Stroke. The NINCDS collaborative study of brain death. Monograph No. 24, NIH Pub. No. 81-2286. Bethesda, MD: NINCDS; 1980.
Okamoto K., Sugimoto T. Return of spontaneous respiration in an infant who fulfilled current criteria to determine brain death. Pediatrics. 1995;96:518.
Okuyaz C., Gucuyener K., Karabacak N.I., et al. Tc-99m-HMPAO SPECT in the diagnosis of brain death in children. Pediatr Int. 2004;46:711-714.
Orrison W.W.Jr, Champlin A.M., Kesterson O.L., et al. MR “hot nose sign” and “intravascular enhancement sign” in brain death. Am J Neuroradiol. 1994;15:913.
Otsubo Y., Yoneyama Y., Sawa R., et al. Fetal brain death and Dandy-Walker malformation. Prenat Diagn. 1999;19:777.
Outwater K.M., Rockoff M.A. Apnea testing to confirm brain death in children. Crit Care Med. 1984;12:357.
Paolin A., Manuali A., Di Paola F., et al. Reliability in diagnosis of brain death. Intensive Care Med. 1995;21:657.
Paret G., Barzilay Z. Apnea testing in suspected brain dead children – physiological and mathematical modelling. Intensive Care Med. 1995;21:247.
Parker B.L., Frewen T.C., Levin S.D., et al. Declaring pediatric brain death: Current practice in a Canadian pediatric critical care unit. Can Med Assoc J. 1995;153:909.
Pennefather S.H., Bullock R.E., Mantle D., et al. Use of low dose arginine vasopressin to support brain-dead organ donors. Transplantation. 1995;59:58.
Pezzani C., Radvanyi-Bouvet M.F., Relier J.P., et al. Neonatal electroencephalography during the first twenty-four hours of life in full-term newborn infants. Neuropediatrics. 1986;17:11.
Pistoia F., Johnson D.W., Darby J.M., et al. The role of xenon CT measurements of cerebral blood flow in the clinical determination of brain death. Am J Neuroradiol. 1991;12:97.
Practice parameters for determining brain death in adults (summary statement). The Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1995;45(5):1012-1014.
President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. Guidelines for the determination of death. JAMA. 1981;246:2184.
Quesnel C., Fulgencio J.P., Adrie C., et al. Limitations of computed tomographic angiography in the diagnosis of brain death. Intensive Care Med. 2007;33(12):2129-2135.
Report of special task force. Guidelines for the determination of brain death in children. Ad Hoc Task Force consisting of representatives from the American Academy of Pediatrics, American Academy of Neurology, Child Neurology Society, American Neurological Association, American Bar Association, and the NINCDS. Pediatrics. 1987;80:298.
Rivello J.J., Sapin J.I., Brown L.W., et al. Hypoxemia and hemodynamic changes during the hypercarbia stimulation test. Pediatr Neurol. 1988;4:213-218.
Rodriguez R.A., Cornel G., Alghofaili F., et al. Transcranial Doppler during suspected brain death in children: Potential limitation in patients with cardiac “shunt”. Pediatr Crit Care Med. 2002;3:153.
Roels L., Pirenne J., Delooz H., et al. Effect of triiodothyronine replacement therapy on maintenance characteristics and organ availability in hemodynamically unstable donors. Transplant Proc. 2000;32:1564.
Ropper A.H., Kennedy S.K., Russell L. Apnea testing in the diagnosis of brain death. J Neurosurg. 1981;55:942.
Rosendale J.D., Kauffman H.M., McBride M.A., et al. Hormonal resuscitation yields more transplanted hearts, with improved early function. Transplantation. 2003;75:1336.
Rowland R.W., Donnelly J.H., Jackson A.H., et al. Brain death in the pediatric intensive care unit. Am J Dis Child. 1983;137:547.
Rowland T.W., Donnelly J.H., Jackson A.H. Apnea documentation for determination of brain death in children. Pediatrics. 1984;74:505.
Ruiz-Garcia M., Gonzalez-Astiazaran A., Collado-Corona M.A., et al. Brain death in children: Clinical, neurophysiological and radioisotopic angiography findings in 125 patients. Childs Nerv Syst. 2000;16:40.
Ruiz-Lopez M.J., Martinez de Azagra A., Serrano A., et al. Brain death and evoked potentials in pediatric patients. Crit Care Med. 1999;27:412.
Ryan C.A., Byrne P., Kuhn S., et al. No resuscitation and withdrawal of therapy in a neonatal and a pediatric intensive care unit in Canada. J Pediatr. 1993;123:534.
Saito T., Takeichi S., Nakajima Y., et al. Influence of antemortem medication on the determination of brain death. Nippon Hoigaku Zasshi. 1995;49:484.
Schafer J.A., Caronna J.J. Duration of apnea needed to confirm brain death. Neurology. 1978;28:661.
Schmitt B., Simma B., Burger R., et al. Resuscitation after severe hypoxia in a young child: Temporary isoelectric EEG and loss of BAEP components. Intensive Care Med. 1993;19:420.
Schneider S. Usefulness of EEG in the evaluation of brain death in children: The cons. Electroencephalogr Clin Neurophysiol. 1989;73:276.
Schwartz J.A., Baxter J., Brill D.R. Diagnosis of brain death in children by radionuclide cerebral imaging. Pediatrics. 1984;73:14.
Sheehy E., Conrad S.L., Brigham L.E., et al. Estimating the number of potential organ donors in the United States. N Engl J Med. 2003;349:667.
Shewmon D.A. The probability of an inevitability: The inherent impossibility of validating criteria for brain death or “irreversibility” through clinical studies. Stat Med. 1987;6:535.
Shewmon D.A. Commentary on guidelines for the determination of brain death in children. Ann Neurol. 1988;24:789.
Shewmon D.A. Chronic “brain death”: Meta-analysis and conceptual consequences. Neurology. 1998;51:1538.
Shimizu N., Shemie S., Miyasaka E., et al. Preliminary report: use of clinical criteria for the determination of pediatric brain death and confirmation by radionuclide cerebral blood flow. Masui. 2000;49(10):1126-1132.
Siminoff L.A., Mercer M.B., Arnold R. Families’ understanding of brain death. Prog Transplant. 2003;13:218.
Spieth M., Abella E., Sutter C., et al. Importance of the lateral view in the evaluation of suspected brain death. Clin Nucl Med. 1995;20:965.
Steinhart C.M., Weiss I.P. Use of brainstem auditory evoked potentials in pediatric brain death. Crit Care Med. 1985;13:560.
Stoyles H.A. Management of multi organ donor. Axone. 1995;16:97.
Taylor M.J., Houston B.D., Lowry N.J. Recovery of auditory brainstem responses after a severe hypoxic ischemic insult. N Engl J Med. 1983;309:1169.
Terk M.R., Gober J.R., DeGiorgio C., et al. Brain death in the neonate: Assessment with P-31 MR spectroscopy. Radiology. 1992;182:582.
Thompson J.R., Ashwal S., Schneider S., et al. Comparison of cerebral blood flow measurements by xenon computed tomography and dynamic brain scintigraphy in clinically brain dead children. 13th Symposium Neuroradiologicum. Acta Radiol Suppl (Stockh). 1987;369:675.
Tobin B. Drugs and brain death: A matter of “practical certainty”. Clin Exp Pharmacol Physiol. 1996;23:S44.
Toffol G.J., Lansky L.L., Hughes J.R., et al. Pitfalls in diagnosing brain death in infancy. J Child Neurol. 1987;2:134.
Tsai E., Shemie S.D., Cox P.N., et al. Organ donation in children: Role of the pediatric intensive care unit. Pediatr Crit Care Med. 2000;1:156.
Uniform determination of death act #7180 of the health and safety code. Uniform Law Annotated. 1980;12:310.
Valle G., Ciritella P., Bonetti M.G., et al. Considerations of brain death on a SPECT cerebral perfusion study. Clin Nucl Med. 1993;18:953.
Van Bunnen Y., Delcour C., Wery D., et al. Intravenous digital subtraction angiography: A criteria of brain death. Ann Radiol (Paris). 1989;32:279.
Vander Borght T., Laloux P., Maes A., et al. Guidelines for brain radionuclide imaging: Perfusion single photon computed tomography (SPECT) using Tc-99m radiopharmaceuticals and brain metabolism positron emission tomography (PET) using F-18 fluorodeoxyglucose. The Belgian Society for Nuclear Medicine. Acta Neurol Belg. 2001;101:196.
Vardis R., Pollack M.M. Increased apnea threshold in a pediatric patient with suspected brain death. Crit Care Med. 1998;26:1917.
Volpe J.J. Commentary on brain death determination in the newborn. Pediatrics. 1987;80:293.
Wagner W. Scalp, earlobe and nasopharyngeal recordings of the median nerve somatosensory evoked p14 potential in coma and brain death: Detailed latency and amplitude analysis in 181 patients. Brain. 1996;119:1507.
Wieler H., March K., Kaisar K.P., et al. Tc-99m HmPAO cerebral scintigraphy: A reliable, noninvasive method for determination of brain death. Clin Nucl Med. 1993;18:104.
Wijdicks E.F. Brain death worldwide: Accepted fact but no global consensus in diagnostic criteria. Neurology. 2002;58:20.
Wijdicks E.F., Varelas P.N., Gronseth G.S., et alAmerican Academy of Neurology. Evidence-based guideline update: determining brain death in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74(23):1911-1918.
Wijdicks E.F.M., editor. Brain death. Philadelphia: Lippincott, 2001.
Williams M.A., Lipsett P.A., Rushton C.H., et al. The physician’s role in discussing organ donation with families. Crit Care Med. 2003;31:1568.
Wilson K., Gormon L., Seeby J.B. The diagnosis of brain death with Tc-99m HmPAO. Clin Nucl Med. 1993;18:428.
Ying Z., Schmid U.D., Schmid J., et al. Motor and somatosensory evoked potentials in coma: Analysis and relation to clinical status and outcome. J Neurol Neurosurg Psychiatry. 1992;55:470.
Zenel J., Goldstein B. Child abuse in the pediatric intensive care unit. Crit Care Med. 2002;30(Suppl 11):S515.