34
Dermatomyositis
Dermatomyositis is an autoimmune connective tissue disease (AI-CTD) that may overlap with other AI-CTDs, in particular systemic sclerosis. It may be triggered by outside factors, most commonly malignancy (e.g. breast cancer, ovarian cancer) and occasionally drugs (e.g. ‘statins’) or rarely infectious agents (e.g. picornavirus).
• Often clinical and laboratory evidence of proximal inflammatory myopathy (the term polymyositis is used when the disease affects muscle only; Table 34.1); patients may report difficulty combing hair or rising from a sitting position.
Table 34.1
Revised classification system for the idiopathic inflammatory dermatomyopathies.
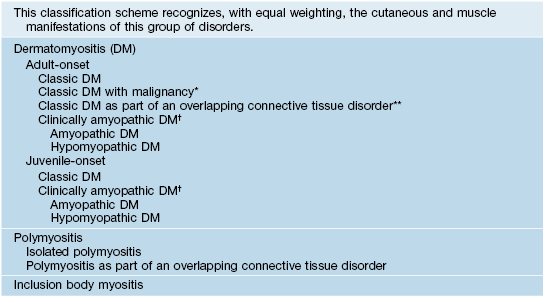
* In up to 25% of adults; ovarian, colon, breast, lung, gastric, pancreatic carcinomas (nasopharyngeal in Southeast Asian populations), and lymphoma; risk decreases to normal after 2–5 years.
** Systemic sclerosis > SLE, Sjögren’s syndrome, rheumatoid arthritis.
† Provisional = cutaneous findings without muscle weakness and with normal muscle enzymes for >6 months; confirmed = for 24 months.
• May affect the skin only (amyopathic dermatomyositis, formerly termed dermatomyositis sine myositis; see Table 34.1); Fig. 34.1 outlines an approach to the diagnosis of this form of dermatomyositis.
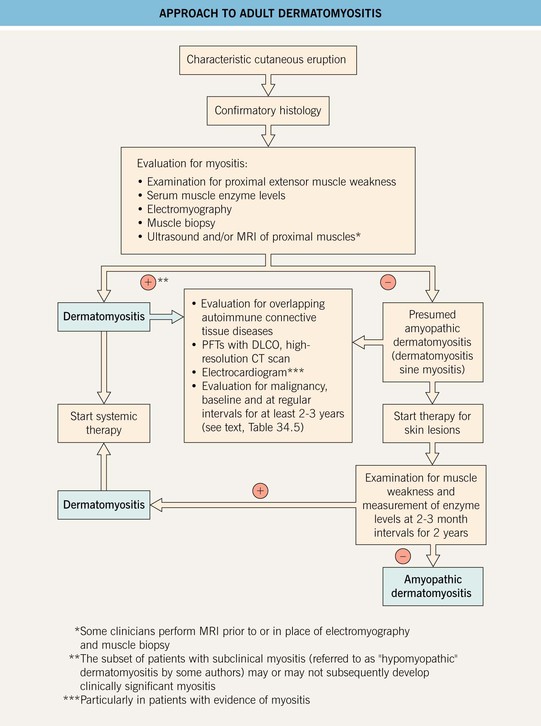
Fig. 34.1 Approach to adult dermatomyositis. The approach for children is similar but without the malignancy evaluation. Some authors classify patients with no evidence of myositis for 6 months after the onset of skin disease as having amyopathic dermatomyositis, but such individuals may go on to develop muscle disease. If planning administration of chronic systemic CS, a baseline DEXA bone density scan is recommended. CT, computed tomography; DLCO, diffusing capacity of the lung for carbon monoxide; PFTs, pulmonary function tests.
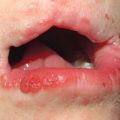
• Characteristic cutaneous findings include a violaceous hue of the upper eyelids with periorbital edema (Fig. 34.2) and nailfold telangiectasias (Fig. 34.3), as well as the cutaneous findings outlined in Table 34.2 and represented in Fig. 34.4A; these may precede systemic manifestations (Fig. 34.4B).
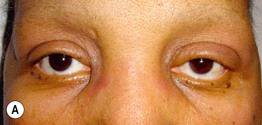
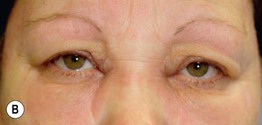
Fig. 34.2 Dermatomyositis – eyelid edema and heliotrope sign. A Inflammation of the upper eyelids can be more subtle in darkly pigmented skin; note involvement of the lateral nasal root and the cheeks. B The characteristic pink-violet color is seen with involvement of the hairline, lower forehead, upper eyelids, and cheeks; the edema is striking and involves the nasal root as well as the eyelids. B, Courtesy, Jean L. Bolognia, MD.
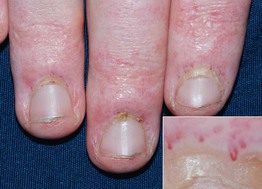
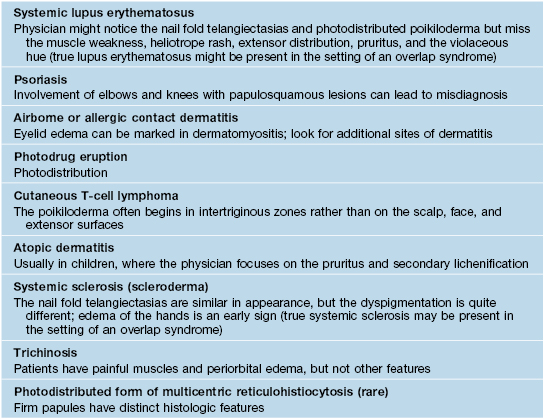
Fig. 34.3 Dermatomyositis – cuticular dystrophy and nailfold telangiectasias. The cuticles are ‘ragged’ and within the proximal nail fold, dilated capillary loops alternate with vessel dropout (insert). Atrophy, telangiectasias, and hypopigmentation are present on the fingers. Courtesy, Julie V. Schaffer, MD.
Table 34.2
Cutaneous manifestations of dermatomyositis.
For a schematic representation of the cutaneous features, see Fig. 34.4A.
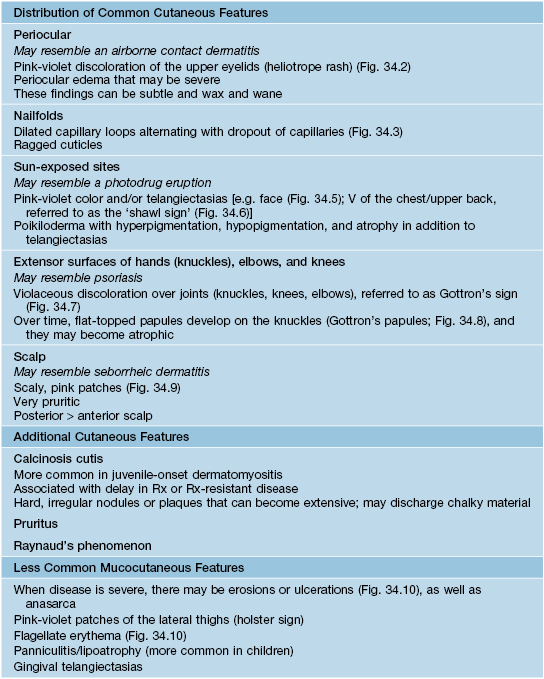
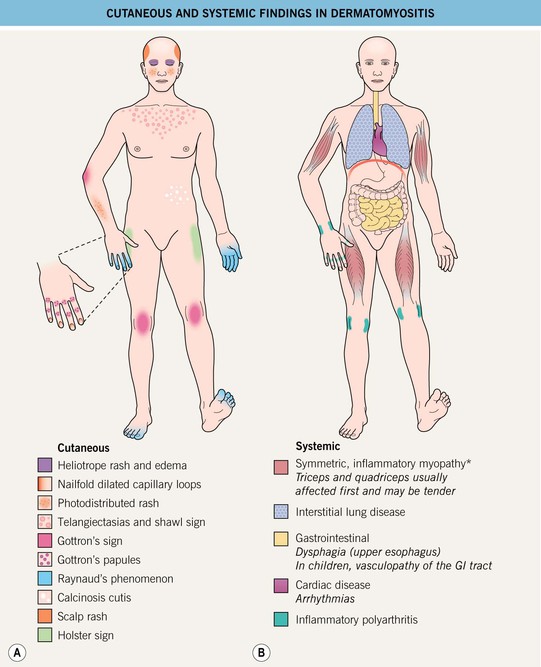
Fig. 34.4 Clinical findings in dermatomyositis. A Cutaneous. B Systemic. In addition, there may be signs and symptoms of an underlying malignancy. GI involvement may impair the absorption of oral medications. *May be absent (amyopathic dermatomyositis) or subtle (hypomyopathic dermatomyositis).
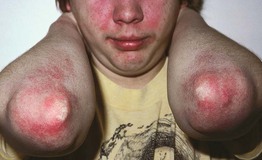
Fig. 34.5 Dermatomyositis. Misdiagnoses include psoriasis and acute cutaneous lupus erythematosus. Courtesy, Joseph L. Jorizzo, MD.
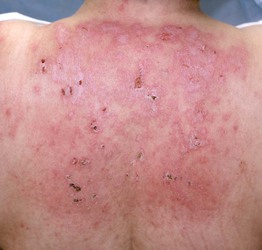
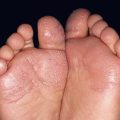
Fig. 34.6 Dermatomyositis – involvement of the upper back. The pink-violet plaques, some with associated scale, were very pruritic, as evidenced by multiple excoriations. Linear streaks of erythema are also seen. Courtesy, Jean L. Bolognia, MD.
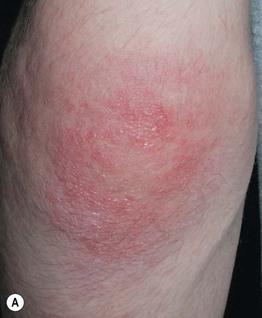
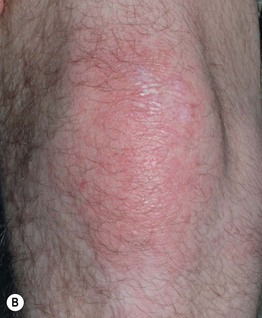
Fig. 34.7 Dermatomyositis – Gottron’s sign. Thin pink papules and plaques of the elbow (A) and knee (B). Some of the papules on the elbow are flat-topped. Courtesy, Julie V. Schaffer, MD.
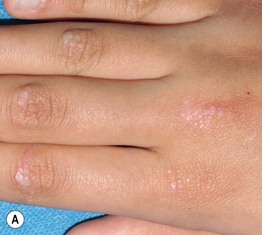
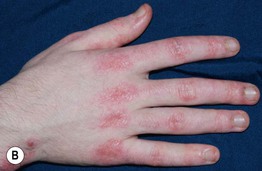
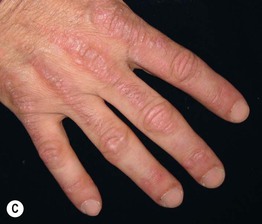
Fig. 34.8 Dermatomyositis – Gottron’s papules. A The flat-topped papules overlying the proximal interphalangeal (IP) and metacarpophalangeal (MCP) joints are subtle and were misdiagnosed as verrucae vulgaris in this child. B Obvious accentuation of skin lesions over the MCP joints, with coalescence of pink-violet flat-topped papules. C Papulosquamous plaques with a somewhat linear configuration proximally; clues against psoriasis are the Gottron’s papules of the distal IP joints, cuticular dystrophy, and nailfold telangiectasias. A, B, Courtesy, Julie V. Schaffer, MD; C, Courtesy, Ruth Ann Vleugels, MD.
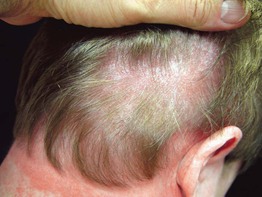
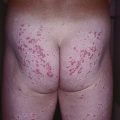
Fig. 34.9 Seborrheic-like dermatitis due to dermatomyositis. This patient presented with severe pruritus of the scalp. In addition to the scalp involvement, she had Gottron’s papules and a photodistributed poikiloderma. Courtesy, Jeffrey P. Callen, MD.
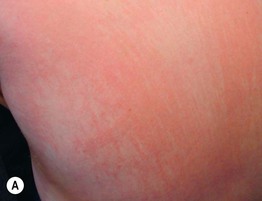
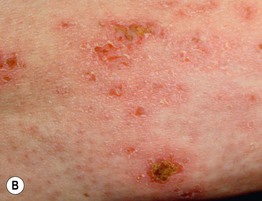
Fig. 34.10 Dermatomyositis – less common presentations. A Flagellate erythema of the posterior trunk. B Secondary changes include scale, erosions, and serous as well as hemorrhagic crusts. A, Courtesy, Ruth Ann Vleugels.
• May overlap with other AI-CTDs (1 in 5 adult cases) (Table 34.1).
• Various associated autoantibodies with clinical implications (Table 34.3); antinuclear antibodies may be negative as many autoantibodies are directed against cytoplasmic antigens.
Table 34.3
Autoantibodies associated with dermatomyositis and their clinical relevance.
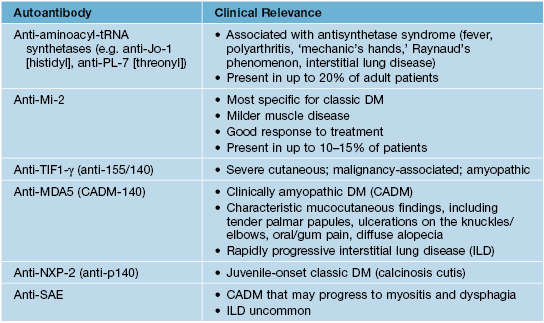
DM, dermatomyositis; Mi-2 is a DNA helicase; TIF1, transcriptional intermediary factor 1; MDA5, melanoma differentiation-associated protein 5; NXP-2, nuclear matrix protein; SAE, small ubiquitin-like modifier-activating enzyme. Patients with anti-SRP (signal recognition particle) antibodies have severe myositis and an increased risk of cardiac involvement.
• DDx can be broad (Table 34.4).
• In classic dermatomyositis with malignancy (Table 34.5), cutaneous findings may improve gradually over time in the setting of a treatment-responsive tumor.
Table 34.5
Suggested malignancy screening tests for adults with dermatomyositis.
Screening should be performed at the time of diagnosis and annually for a minimum of 3 years thereafter; history and physical examination can be performed more frequently.
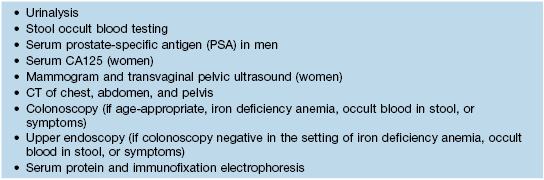
CT, computed tomography.
For further information see Ch. 42. From Dermatology, Third Edition.