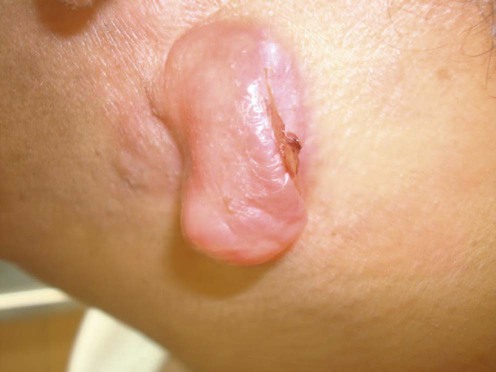
Dermatofibrosarcoma protuberans
Specific investigations
Differential expression of HMGA1 and HMGA2 in dermatofibroma and dermatofibrosarcoma protuberans: potential diagnostic applications, and comparison with histologic findings, CD34, and factor XIIIa immunoreactivity.
Li N, McNiff J, Hui P, Manfioletti G, Tallini G. Am J Dermatopathol 2004; 26: 267–72.
First-line therapies
Second-line therapies



 Mohs micrographic surgery
Mohs micrographic surgery Wide local excision
Wide local excision Imatinib mesylate (Gleevac)
Imatinib mesylate (Gleevac) Radiation
Radiation
