Chapter 135 Decompressive Craniectomy for Traumatic Brain Injury
Introduction
Traumatic brain injury (TBI) is a significant cause of death and disability, accounting for an estimated 294,000 hospitalizations and 52,000 deaths annually in the United States alone according to 2006 Centers for Disease Control and Prevention data.1 Although neurosurgeons play an integral role in the management of TBI, much controversy exists regarding the use of surgical therapies, especially decompressive craniectomy. Current guidelines for the treatment of severe TBI recommend decompressive craniectomy as a salvage therapy for medically refractory intracranial hypertension,2 but little class I evidence exists comparing decompressive craniectomy to nonsurgical management for the treatment of TBI in adults.3,4 Many reviews have been written discussing the relevant data for and against the use of decompressive craniectomy for TBI, with the overwhelming conclusion being that randomized controlled trials are needed to resolve these disputes. The goal of this chapter is to summarize the data pertaining to the use decompressive craniectomy for TBI and to discuss the various issues associated with this procedure, including outcomes, techniques, and complications.
Historical perspective
Evidence of surgical decompression performed to treat TBI goes as far back as Ancient Egypt and Ancient Greece, and the indications for its use included TBI, epilepsy, headache, and mental illness.5 The concept of using surgical decompression to treat elevated intracranial pressure (ICP) was introduced to modern neurosurgery by Kocher6 and Cushing7,8 at the beginning of the 20th century. Discussion of surgical decompression for TBI in the literature did not become widespread, however, until the late 1960s and 1970s after a series of studies examining surgical decompression for various TBI-related entities. At that time, the benefit of surgical decompression was not clear. Jamieson and Yelland9 reported encouraging results for decompression of traumatic epidural hematomas, with a mortality rate of just 16% in a series of 167 patients. Surgical techniques used in their series ranged from subtemporal and suboccipital decompressions to “local” craniectomies, trephines, and osteoplastic craniotomies. Subsequent review of surgical management of traumatic subdural hematomas by the same authors, however, showed worse outcomes. In a series of 555 surgically managed subdural hematomas, 317 of the patients underwent a decompressive craniectomy, with an associated 43% mortality rate.10 Similarly, surgically managed intraparenchymal hematomas were associated with a 24% mortality rate,11 although the outcomes associated with various surgical techniques were not clearly described.
In the late 1960s and early 1970s, several groups began to study specific decompressive techniques for the treatment of severe TBI. Kjellberg et al.12 reported using a bifrontal decompressive craniectomy with duraplasty for intractable cerebral edema in 73 patients, 50 of whom had sustained TBI. They reported an 18% survival rate (22% among TBI patients), as well as an additional 16 patients who showed neurological improvement postoperatively but died as a result of other medical complications. Similarly, Venes et al13 reported 13 TBI patients with intractable cerebral edema treated with wide decompressive bifrontal craniectomies. Although the mortality rate was only 31%, only one patient (a 2½-year-old child with moderately severe TBI) returned to normal neurological function postoperatively with the exception of a seizure disorder. A wide decompressive hemicraniectomy (DHC) with durotomy was described by Ransohoff et al.14 for the treatment of subdural hematoma. They reported a 40% survival rate, and 28% of patients returning to normal activities. These outcomes were much improved over an 85% mortality rate among TBI patients treated with burr holes or routine craniectomies.14 A follow-up study by the same group, however, showed much worse outcomes for traumatic subdural hematomas treated with decompressive craniectomy, with only a 10% survival rate.15 The authors attributed this difference to the presence of concomitant brain stem or subcortical injury. Britt et al16 reported a cohort of 42 patients who underwent decompression of an acute traumatic subdural hematoma, 34 of whom had their bone flaps left off because of cerebral edema. Although Britt et al. did not separate outcomes based on craniectomy or craniotomy, they did report an overall 55% mortality rate (only 36% mortality within 30 days of decompression) and stated that their results led them to standardize the use of a large DHC for the treatment of traumatic subdural hematomas. Because poor outcomes from subdural and epidural hematomas were partially attributed to underlying contusions, Yamaura et al.17 studied the use of DHC for severe traumatic contusions. Twenty-four percent of their patients were converted to bilateral hemicraniectomies if the “unilateral opening did not seem sufficient in its decompressive effect.” They reported an overall 36% mortality rate and advocated for the use of bilateral hemicraniectomies. In radiographic follow-up of patients who had undergone hemicraniectomy, Morantz et al.18 confirmed the benefits of DHC on the resolution of midline shift and removal of hematomas. In addition to functional outcomes, Hase et al.19 examined the effect of decompressive surgery on the elasticity (change in pressure divided by change in volume) of the brain and found that decompression decreases elasticity, thus reducing pressure variations with changes in intracerebral volume.
In an attempt to further understand the pathophysiology underlying posttraumatic cerebral edema and the role of surgical decompression, several groups have developed experimental models. Moody et al.20 studied a model of TBI using epidural balloon compression in dogs. Ten dogs received no surgical decompression, and all died within 12 hours of injury. Autopsies revealed pontine hemorrhages. A second group of dogs underwent a DHC. Although none of the surgically treated dogs regained consciousness, decompression resulted in less pontine injury. However, the hemicraniectomy also resulted in hemorrhagic infarction, necrosis, and edema at the site of the craniectomy. Cooper et al.21 studied surgical decompression in a dog model of cold-injury-induced cerebral edema. Hemicraniectomy lowered ICP but resulted in significantly greater cerebral edema. This effect was attributed to possible reduction in the interstitial pressure within the brain after decompression, resulting in a greater hydrostatic pressure gradient between the intravascular and interstitial spaces.
Overall, these studies demonstrate an increasing interest in the utility of decompressive craniectomy for TBI and provide evidence for a possible benefit regarding mortality rate after surgical decompression in severe TBI but questioned whether the morbidity and quality of life attained are justifiable. Importantly, the complex nature of TBI was recognized, and many authors acknowledged the need for a systems-based approach to the management of TBI that encompasses more than just the surgical aspects of management.22 In addition, the Glasgow Coma Scale (GCS)23 and the Glasgow Outcome Scale (GOS)24 were developed, allowing for more uniform characterization and generalization of TBI patients across centers. For the next several decades, however, few studies were published regarding the use of decompressive craniectomy in TBI.
Current Evidence
More recently, there has been renewed interested in defining the use and benefits of decompressive craniectomy, with an ever increasing number of publications on the topic over the past 20 years. Gower et al.25 began to reexplore decompressive craniectomy by reporting 10 patients treated with salvage subtemporal decompression among a series of 115 severe TBI patients. They demonstrated a mortality rate of 40% among decompressive craniectomy patients compared to 82% mortality among patients treated medically (with pentobarbital-induced coma). They also showed a 34% reduction in ICP after decompressive craniectomy. Gaab et al.26 applied both unilateral and bilateral frontoparietal temporal craniectomies and dural enlargement in patients with medically refractory cerebral hypertension and showed only a 13.5% mortality rate, with 78% achieving a GOS score of 4 or 5.
Polin et al.27 compared 35 patients with malignant posttraumatic cerebral edema treated with bifrontal decompressive craniectomy to matched controls from the Traumatic Coma Data Bank. They reported 23% mortality and 37% good outcomes (GOS score 4 or 5), a significant improvement compared to matched controls. They also demonstrated significantly lower postoperative ICP compared to controls who did not have surgery. In addition, Polin et al. identified young age and early timing of decompressive craniectomy (within 48 hr of injury) as possible favorable prognostic factors. Kleist-Welch Guerra et al.28 likewise demonstrated promising results in a prospective study of 57 patients with severe TBI and medically refractory cerebral edema, 31 of whom were treated with a unilateral craniectomy and the remainder with bilateral craniectomies. Kleist-Welch Guerra et al. reported 19% mortality and 58% favorable outcomes and advocated for the use of decompressive craniectomy before barbiturate-induced coma. In a retrospective review of DHC for severe TBI, Münch et al.29 reported higher mortality (52%) but similar favorable outcomes (41%). Similarly to Polin et al., they identified young age and early DHC as favorable prognostic factors. They also demonstrated various improvements in computed tomography (CT) characteristics after DHC, including decreased midline shift and increased visibility of the mesencephalic cisterns. These studies prompted a series of investigations into the role of decompressive craniectomy in the treatment of TBI, including indications, techniques, and prognostic factors,30–55 which culminated in a recent randomized controlled trial of surgical decompression for TBI56 as well as multiple ongoing randomized trials.
Indications and Timing
The most common indication for decompressive craniectomy in the setting of TBI has been salvage therapy for medically refractory cerebral hypertension.28,30,41,48,51,53,56,57 In this setting, ICP monitoring is utilized to guide medical management. Typical medical therapy protocols include a combination of head-of-bed elevation, cerebrospinal fluid (CSF) drainage, sedation, hyperventilation, paralysis, hyperosmolar therapy, and barbiturate-induced coma.2 Several groups, however, have also employed decompressive craniectomy at the time of initial hematoma evacuation based on the intraoperative finding of cerebral swelling.29,31,33,40,43,44,46,47,58–61 Both indications have been supported by TBI management guidelines,2,3 but one of the chief controversies in the use of decompressive craniectomy for TBI is the most appropriate timing of decompression after injury. Several studies have compared the use of early (typically within 24 hr of injury and in conjunction with hematoma evacuation) to late (more than 24 to 48 hr after injury, typically to treat medically refractory cerebral hypertension). Patients within the early and late groups, of course, are distinct populations and cannot be generalized to one another. As such, comparisons of decompressive craniectomy for each group have had mixed results, with some studies reporting superior outcomes after early decompressive craniectomy,29,47,59 some reporting worse outcomes,44 and others reporting no difference.30,60 Coplin et al.33 sought to evaluate the benefit of early decompressive craniectomy at the time of initial hematoma evacuation by comparing TBI patients with traumatic mass lesions who underwent either craniotomy or craniectomy. They found no significant differences between the two groups, despite worse injuries in the craniectomy group. Similarly, Woertgen et al.54 compared craniotomy to craniectomy for treatment of acute traumatic subdural hematoma and also found no significant difference in outcomes. As Coplin et al. also reported, the craniectomy patients in the Woertgen et al. study were found to have worse injuries intraoperatively, prompting surgeons to leave off the bone flap. The fact that both studies found similar outcomes despite worse injuries in the craniectomy groups suggests that early decompressive craniectomy, or possibly even prophylactic decompressive craniectomy, at the time of initial hematoma evacuation may in fact provide benefit.
Prognostic Factors
Several studies have examined various potential prognostic factors in an attempt to better define the patient population that will benefit most from decompressive craniectomy. The two most common, and most easily obtained, factors studied have been age and preoperative GCS score. Early studies excluded older patients, with age cut-offs as low as 30 years,28 but several studies ultimately evaluated age as a prognostic factor. Several studies have reported a correlation between age and outcomes,27,29,32,43–46,48,54,62 which is not surprising, given that age has been shown to correlate with outcome after TBI in general.63 Other decompressive craniectomy studies, however, have found no correlation.30,61 De Bonis et al.64 recently summarized the decompressive craniectomy literature with regard to age as a prognostic factor, citing studies that showed a correlation with age as well as studies that did not, and concluded that there are no strong data to support an effect of age on outcome and that the age cut-offs reported in many studies are arbitrary. At our institution, we typically do not include age in making the decision whether to proceed with decompressive craniectomy. However, on the basis of our own data suggesting that old age does correlate with poor outcomes after decompressive craniectomy for TBI, we do temper our expectations of outcomes in older patients.
GCS score is, of course, another commonly used prognostic factor in TBI,65,66 and several decompressive craniectomy studies have shown a positive correlation between preoperative GCS score and GOS score.26,32,45,52,61,62 The correlation of GCS score with outcome is likely complicated, with evidence that the motor score alone may be more prognostic than the total GCS score.67 In addition, confounding factors such as the use of alcohol and other drugs as well as the timing of any acute changes in GCS score must be considered to accurately interpret a preoperative GCS score. Response of ICP to decompression has also been shown to be a possible postoperative prognostic factor, with patients who continue to have high ICP after decompressive craniectomy being more likely to have poor functional outcomes.27,62
Preoperative Evaluation
Current guidelines recommend decompressive craniectomy as a salvage therapy for medically refractory elevated ICP in the setting of TBI.2 In this situation, patients typically already have undergone cranial imaging and ICP monitoring. Imaging studies should be reviewed to determine the most appropriate technique and laterality for decompression. Skull fractures should be identified to anticipate potential bleeding sources and to avoid cautery injury to the exposed brain tissue during the soft-tissue dissection. Because cervical spine injuries are also often associated with TBI, it is important to determine the stability of the cervical spine to avoid any cervical injuries during positioning in the operating room. Decompressive craniectomy can also be used as a prophylactic measure during emergent evacuation of mass lesions if the development of elevated ICP is deemed likely based on computed tomographic scan findings or the intraoperative appearance of the brain.31,58 When performed correctly, decompressive craniectomy can reduce ICP, increase cerebral blood flow and oxygenation, and reduce therapeutic intensity levels,30,37,53,57,59,68 potentially preventing cerebral herniation and death.
Decompressive craniectomy is most often performed in the setting of impending life-threatening cerebral herniation. It is therefore important that family members as well as the treating surgeons and physicians be aware of the patient’s dire prognosis to avoid unrealistic expectations. Decompressive craniectomy is a well-established treatment for elevated ICP, but it is unable to reverse most preexisting cerebral injuries resulting from the initial insult. This is especially true for older patients and for patients with persistently low GCS scores after the time of initial injury, who may be at greatest risk for a severely disabled or persistent vegetative state outcome. Thus, whenever possible, a realistic assessment of prognosis and potential for recovery in a specific case should be discussed prior to performing decompressive craniectomy.69 In emergent cases, however, this discussion is not always possible prior to surgery.
Technique
Several techniques have been employed for surgical decompression after TBI, but two main techniques are currently used for the treatment of medically refractory cerebral edema: DHC and the bifrontal (Kjellberg) craniectomy.70,71 To date, no study has directly compared the efficacy of these techniques, but surgeons in the majority of studies cited herein have used one or both techniques. Surgeon preference is the most common reason for choosing one over the other, although the DHC bone flap can used for traumatic hematoma evacuations, giving the surgeon the option of replacing the bone flap or leaving it off if the brain appears swollen. For each technique, the head is typically placed on a foam or rubber donut and not pinned. Rigid fixation with pins is generally not recommended in the setting of trauma, unless it is certain that there are no skull fractures. It should be noted that skull fractures parallel to the plane of CT imaging can be missed. Cervical spine precautions should also be followed if the cervical spine has not yet been cleared. Care should be taken not to compress the jugular veins, whether with tight cervical collar placement, over-rotation of the head, or placement of central venous lines on the side of the injury (if at all avoidable), as these can all further increase ICP.
Decompressive Hemicraniectomy
For unilateral DHC,70,71 the patient is placed supine with a small rolled towel underneath the ipsilateral shoulder and the head turned toward the contralateral side. Prior to prepping and draping the patient, the midline should be clearly marked and then draped outside the surgical field. Accidental transgression of the superior sagittal sinus during the craniectomy can have devastating consequences, especially in a trauma situation, when patients are often coagulopathic. Marking the midline can prevent such complications. We place the towel on the ipsilateral side of the midline to provide additional protection against crossing it.
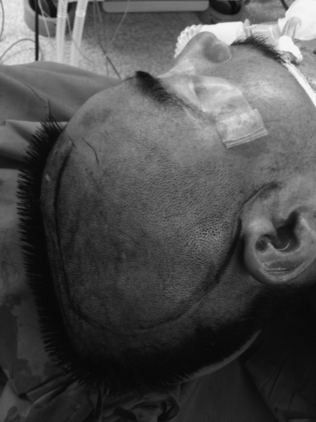
Once the site is prepped and draped, a large reverse question mark incision is made starting at the level of the zygoma and curving posteriorly above the ear, over the parieto-occipital region, then superiorly and anteriorly, approximately 2 cm lateral to the midline, and stopping just behind the hairline (Fig. 135-1). The posterior extent of the incision should be more than 15 cm behind the keyhole to allow for an adequate craniectomy flap. If possible, care should be taken to protect the superficial temporal artery to preserve blood supply to the skin flap. The incision should be extended through the subcutaneous tissue, including the temporalis muscle, down to the cranium. The resultant myocutaneous flap can then be reflected anteriorly and fixed with scalp hooks (Fig. 135-2). The temporalis dissection should be carried down to the zygoma to adequately expose the temporal bone and maximize the temporal decompression.
An ideal hemicraniectomy flap should have an anteroposterior dimension of at least 15 cm and should extend down as far as possible toward the floor of the temporal fossa. Several studies have linked larger craniectomy flap size to improved ICP control and outcomes.39,49,72 Smaller craniectomy flaps may actually cause further brain damage by compression of the cortical surface and veins as the brain herniates out through the craniectomy.73 Medially, the craniectomy should extend to only about 2 to 3 cm from the midline to prevent damage to any draining veins and arachnoid granulations near the superior sagittal sinus. Preferences for the location and number of burr holes vary, but typically three burr holes are made: one at the keyhole, one more inferiorly in the temporal bone and posterior to the sphenoid bone, and one superoposteriorly in the parietal bone. The flap is then turned using a cutting drill bit with a footplate. In the setting of an emergent operation with an underlying epidural or subdural hematoma, an additional burr hole can be made over the hematoma to allow for rapid decompression prior to turning the craniectomy flap.
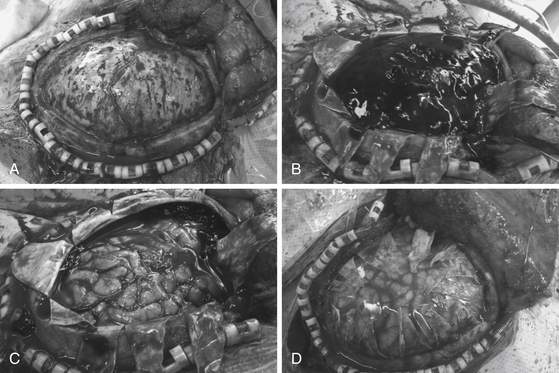
In a DHC performed for elevated ICP, the dural opening is just as important as the craniectomy. Before proceeding with the dural opening, however, it is important to achieve hemostasis with the bone and epidural space with bone wax and dural tack-up stitches, respectively. A swollen brain can quickly herniate through the dural opening and hinder primary scalp closure, so it is sometimes necessary to close skin soon after the dura has been opened. Large amounts of bleeding arising from the middle cranial fossa warrant further attention and usually come from the middle meningeal artery or the sphenoid wing. In this situation, a slightly more conservative temporal craniectomy can provide bone to which the dura can be tacked to help tamponade such bleeding. The dural opening is made with radial incisions in a stellate fashion, which maximizes the area available for cerebral decompression (Fig. 135-3).
Bifrontal Craniectomy
In contrast to DHC, the bifrontal craniectomy12,27,70,71 allows for anterior swelling of the brain and is often used in situations of bifrontal contusions or diffuse cerebral edema. The patient is placed in a supine position with the head in a donut. The head is shaved past the coronal suture, and the midline and coronal suture are marked out. Once the patient is prepped and draped, an incision is made beginning at the level of the zygomatic arch just anterior to the tragus on one side, coursing back 2 to 3 cm behind the coronal suture, and down the contralateral zygomatic arch. The incision is taken down through galea and temporalis muscle to bone. The myocutaneous flap is then brought forward over the orbital rim. Care should be taken to dissect out the supraorbital nerves from the supraorbital notch on either side. If the supraorbital notch is closed, a small osteotome can be used to open it so that the supraorbital nerve can be freed. This allows further advancement of the myocutaneous flap. A series of symmetric burr holes are then made in the bilateral keyholes, bilateral squamous portions of the temporal bone, and straddling the superior sagittal sinus just posterior to the coronal suture. Some authors advocate drilling burr holes adjacent to the anteriormost portion of the superior sagittal sinus as well. The superior sagittal sinus can be dissected away from the overlying bone by passing a Penfield 3 between the posterior and anterior burr holes. The burr holes are then connected with a cutting drill bit and footplate, making sure to cross the superior sagittal sinus last. Once the bone flap is elevated, bleeding from the superior sagittal sinus can be controlled with strips of Gelfoam. The temporal portion of the craniectomy is then extended downward to the floor of the middle fossa. Hemostasis from the bone edges and epidural space is then obtained with bone and dural tack-up stitches.
Recently, Cooper et al.56 published a randomized control trial comparing bifrontal decompressive craniectomy to standard medical therapy for the treatment of medically refractory cerebral edema after TBI. They reported overall worse outcomes in patients undergoing decompressive craniectomy; however, the craniectomy group had worse injuries, and no significant differences were found when the two study groups were controlled for pupillary reactivity at admission. In addition, their technique for bifrontal craniectomy did not include division of the anterior superior sagittal sinus and falx, so these results cannot be generalized to the technique we describe herein.
Dural Opening and Wound Closure
For both dural opening and wound closure, the dura should be opened slowly in a controlled fashion to prevent rapid changes in ICP. Cardiovascular collapse can occur if ICP is rapidly reversed because of loss of the catecholamine support that comes with a Cushing response. It is important that the anesthesiologist be aware of this possibility and has the patient adequately prepared hemodynamically for dural opening to prevent this problem. To prevent compression of large cortical veins as the brain herniates against the dura, Csokay et al.74 have advocated for placing roles of Gelfoam between the dura and cortex alongside each large cortical vessel traversing underneath the dura.
Following dural opening, underlying hematomas or contusions can then be removed (Fig. 135-3C). It is often impossible to close the dura, primarily because of cerebral swelling. Although there are proponents of duraplasty with temporalis fascia or pericranium, at our institution, we prefer to leave the durotomy open and simply overlay a dural substitute such as Gelfilm (Pfizer, New York, NY, USA) (Fig. 135-3D). Such materials also prevent adhesion of the brain to the overlying periosteum and galea and facilitate future dissection if a cranioplasty is performed. Overlaying a dural substitute also allows one to proceed with skin closure much more quickly than if a dural graft were sewn in place.
Complications
Although potentially lifesaving in the setting of TBI, decompressive craniectomies are not benign procedures, and complication rates as high as 50% to 55% have been quoted.75–77 Potential complications attributable to decompressive craniectomy include abnormalities in CSF absorption (subdural hygroma and hydrocephalus), expansion of hematomas after decompression, syndrome of the trephined, and infection. Subsequent cranioplasty also carries the risk of infection, cerebral swelling, and resorption of the bone flap.76
Cerebrospinal Fluid Absorption Disorders
Abnormalities in CSF absorption are common and typically manifest as subdural hygromas or effusions78 as well as ventriculomegalic hydrocephalus.79 Theories as to why this occurs include ruptured arachnoid creating a one-way valve for CSF flow, pressure gradients between hemispheres due to reduction of ICP and decompression of one hemisphere, and alterations in the brain’s shape as a result of surgery.78 The greatest concern with abnormalities of CSF is the risk of CSF leak, which can lead to wound complications, infection, and ultimately prolongation or hindrance of a patient’s recovery. A simple CSF leak should initially be treated with ventriculostomy and oversewing of the wound but may ultimately require shunting (either subdural-peritoneal or ventriculoperitoneal).78 In our experience, shunting in this population is best done with a programmable shunt because their CSF diversion needs are likely to change with time (typically starting with low pressure requirements and gradually requiring higher pressures), especially when the bone flap is replaced. Use of an antisiphon device should also be considered to allow for therapeutic siphoning. Alternatively, if the patient has not developed a CSF leak, replacement of the bone flap may be needed either to reverse the CSF absorption problem or at least to provide enough ICP to allow for successful shunting.80
Expanding Hematomas
TBI is a dynamic process, and new or existing mass lesions can develop postoperatively, especially given the high incidence of coagulopathy and platelet dysfunction in TBI patients.81 Rapid time to surgical decompression is a risk factor for expansion of hematomas.82 Evolution of both contusions and extra-axial hematomas can occur after the tamponading effects of cerebral edema, and elevated ICP has been relieved by decompressive craniectomy.35 For these reasons, we highly recommend postoperative imaging, especially in the setting of no ICP monitoring, as is often the case with early decompressive craniectomies.
Syndrome of the Trephined
The phrase “syndrome of the trephined” roughly encompasses a variety of symptoms that can develop following craniectomy, including fatigue, headache, mood disturbances, and even motor weakness.83,84 The mechanism underlying this syndrome is unknown but has been associated with CSF flow abnormalities, direct atmospheric pressure on the brain, and disturbances in cerebral blood flow.84 This disorder often resolves with replacement of the bone flap,85,86 and there is no evidence that it is harmful or that delay of cranioplasty can result in long-term consequences.
Future Directions
Despite an extensive body of literature describing decompressive craniectomy for TBI, there is still little class I evidence to support its use.4 Taylor et al.87 conducted a randomized controlled trial of early bilateral temporal craniectomy (without durotomy) for increased ICP in head-injured children, and they found that craniectomy led to greater reductions in ICP and noted a trend toward improved functional outcomes. Cooper et al.56 recently published the results of the Decompressive Craniectomy (DECRA) trial, a multicenter randomized controlled trial. They showed equivocal results after comparing bifrontal craniectomy to standard medical management for cerebral edema. There are currently several additional randomized controlled trials underway. The Randomized Evaluation of Surgery with Craniectomy for Uncontrollable Elevation of intracranial pressure (RESCUEicp)88 is a multicenter European study in which TBI patients were randomized to medical therapy (i.e., barbiturate-induced coma) versus decompressive craniectomy as a third-tier treatment for elevated ICP. Decompressive craniectomy consists of two options: DHC for unilateral hemispheric swelling or bifrontal craniectomy for bilateral hemispheric swelling (with the option of dividing the falx). It is clear that decompressive craniectomy can improve several clinical variables, but it remains to be determined if this lifesaving procedure truly improves functional outcomes.
Aarabi B., Hesdorffer D.C., Simard J.M., et al. Comparative study of decompressive craniectomy after mass lesion evacuation in severe head injury. Neurosurgery. 2009;64:927-940.
Albanèse J., Leone M., et al. Decompressive craniectomy for severe traumatic brain injury: evaluation of the effects at one year. Crit Care Med. 2003;31:2535-2538.
Bullock M.R., Povlishock J.T., Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, AANS/CNS Joint Section on Neurotrauma and Critical Care. Guidelines for the management of severe traumatic brain injury. 3rd ed, J Neurotrauma, 2007;24(suppl 1):S1-S106 https://www.braintrauma.org/pdf/protected/Guidelines_Management_2007w_bookmarks.pdf Accessed December 16, 2011
Bullock M.R., Chesnut R., Ghajar J., et al. Guidelines for the surgical management of traumatic brain injury author group: acknowledgments. Neurosurgery. 2006;58(suppl 3):S2.
Cooper D.J., Rosenfeld J.V., Murray L., et al. DECRA Trial Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493-1502. A published erratum appears in N Engl J Med. 2011;365:2040
Coplin W.M., Cullen N.K., Policherla P.N., et al. Safety and feasibility of craniectomy with duraplasty as the initial surgical intervention for severe traumatic brain injury. J Trauma. 2001;50:1050-1059.
Flint A.C., Manley G.T., Gean A.D., et al. Post-operative expansion of hemorrhagic contusions after unilateral decompressive hemicraniectomy in severe traumatic brain injury. J Neurotrauma. 2008;25:503-512.
Gaab M.R., Rittierodt M., Lorenz M., Heissler H.E. Traumatic brain swelling and operative decompression: a prospective investigation. Acta Neurochir Suppl (Wien). 1990;51:326-328.
Honeybul S., Ho K.M. Long-term complications of decompressive craniectomy for head injury. J Neurotrauma. 2011;28:929-935.
Howard J.L., Cipolle M.D., Anderson M., et al. Outcome after decompressive craniectomy for the treatment of severe traumatic brain injury. J Trauma. 2008;65:380-386.
Hutchinson P.J., Corteen E., Czosnyka M., et al. Decompressive craniectomy in traumatic brain injury: the randomized multicenter RESCUEicp study, www.RESCUEicp.com Acta Neurochir Suppl., 2006;96:17-20
Jagannathan J., Okonkwo D.O., Dumont A.S., et al. Outcome following decompressive craniectomy in children with severe traumatic brain injury: a 10-year single-center experience with long-term follow up. J Neurosurg. 2007;106:268-275.
Jiang J.Y., Xu W., Li W.P., et al. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: a multicenter, prospective, randomized controlled study. J Neurotrauma. 2005;22:623-628.
Kan P., Amini A., Hansen K., et al. Outcomes after decompressive craniectomy for severe traumatic brain injury in children. J Neurosurg. 2006;105:337-342.
Kleist-Welch Guerra W., Gaab M.R., Dietz H., et al. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999;90:187-196.
Kontopoulos V., Foroglou N., Patsalas J., et al. Decompressive craniectomy for the management of patients with refractory hypertension: should it be reconsidered? Acta Neurochir (Wien). 2002;144:791-796.
Kunze E., Meixensberger J., Janka M., et al. Decompressive craniectomy in patients with uncontrollable intracranial hypertension. Acta Neurochir Suppl.. 1998;71:16-18.
Meier U., Grawe A. The importance of decompressive craniectomy for the management of severe head injuries. Acta Neurochir Suppl. 2003;86:367-371.
Münch E., Horn P., Schurer L., et al. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery. 2000;47:315-323.
Polin R.S., Shaffrey M.E., Bogaev C.A., et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84-94.
Sahuquillo J., Arikan F.. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev, 2006;1:CD003983
Taylor A., Butt W., Rosenfeld J., et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst. 2001;17:154-162.
Timofeev I., Kirkpatrick P.J., Corteen E., et al. Decompressive craniectomy in traumatic brain injury: outcome following protocol-driven therapy. Acta Neurochir Suppl.. 2006;96:11-16.
Ucar T., Akyuz M., Kazan S., Tuncer R. Role of decompressive surgery in the management of severe head injuries: prognostic factors and patient selection. J Neurotrauma. 2005;22:1311-1318.
Whitfield P.C., Patel H., Hutchinson P.J., et al. Bifrontal decompressive craniectomy in the management of posttraumatic intracranial hypertension. Br J Neurosurg. 2001;15:500-507.
1. Faul M., Xu L., Wald M.M., Coronado V.G.. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006, 2010, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Atlanta, GA http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf Accessed December 16, 2011
2. Bullock M.R., Povlishock J.T., Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, AANS/CNS Joint Section on Neurotrauma and Critical Care. Guidelines for the management of severe traumatic brain injury. 3rd ed, J Neurotrauma, 2007;24(suppl 1):S1-S106 https://www.braintrauma.org/pdf/protected/Guidelines_Management_2007w_bookmarks.pdf Accessed December 16, 2011
3. Bullock M.R., Chesnut R., Ghajar J., et al. Guidelines for the surgical management of traumatic brain injury author group: acknowledgments. Neurosurgery. 2006;58(suppl 3):S2.
4. Sahuquillo J., Arikan F.. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev, 2006;1:CD003983
5. Bakay L. An Early History of Craniotomy: from antiquity to the napoleonic era. Springfield, IL: Thomas; 1985.
6. Kocher T. Die Therapie des Hirndruckes. In: Hölder A., editor. Hirnerschütterung, Hirndruck und chirurgische Eingriffe bei Hirnkrankheiten. Vienna, Austria: A. Hölder; 1901:262-266.
7. Cushing H. The establishment of cerebral hernia as a decompression measure for inaccessible brain tumor; with the description of intramuscular methods of making the bone defect in temporal and occipital regions. Surg Gyn Obstet. 1905;1:297-314.
8. Cushing H. Subtemporal decompressive operations for the intracranial complications associated with bursting fractures of the skull. Ann Surg. 1908;47:641-644.
9. Jamieson K.G., Yelland J.D. Extradural hematoma: report of 167 cases. J Neurosurg. 1968;29:13-23.
10. Jamieson K.G., Yelland J.D. Surgically treated traumatic subdural hematomas. J Neurosurg. 1972;37:137-149.
11. Jamieson K.G., Yelland J.D. Traumatic intracerebral hematoma. Report of 63 surgically treated cases. J Neurosurg. 1972;37:528-532.
12. Kjellberg R.N., Prieto A.Jr. Bifrontal decompressive craniotomy for massive cerebral edema. J Neurosurg. 1971;34:488-493.
13. Venes J.L., Collins W.F. Bifrontal decompressive craniectomy in the management of head trauma. J Neurosurg. 1975;42:429-433.
14. Ransohoff J., Benjamin M.V., Gage E.L.Jr., Epstein F. Hemicraniectomy in the management of acute subdural hematoma. J Neurosurg. 1971;34:70-76.
15. Cooper P.R., Rovit R.L., Ransohoff J. Hemicraniectomy in the treatment of acute subdural hematoma: a re-appraisal. Surg Neurol. 1976;5:25-28.
16. Britt R.H., Hamilton R.D. Large decompressive craniotomy in the treatment of acute subdural hematoma. Neurosurgery. 1978;2:195-200.
17. Yamaura A., Uemura K., Makino H. Large decompressive craniectomy in management of severe cerebral contusion: a review of 207 cases. Neurol Med Chir (Tokyo). 1979;19:717-728.
18. Morantz R.A., Abad R.M., George A.E., Rovit R.L. Hemicraniectomy for acute extracerebral hematoma: an analysis of clinical and radiographic findings. J Neurosurg. 1973;39:622-628.
19. Hase U., Reulen H.J., Meinig G., Schurmann K. The influence of the decompressive operation on the intracranial pressure and the pressure-volume relation in patients with severe head injuries. Acta Neurochir (Wien). 1978;45:1-13.
20. Moody R.A., Ruamsuke S., Mullan S.F. An evaluation of decompression in experimental head injury. J Neurosurg. 1968;29:586-590.
21. Cooper P.R., Hagler H., Clark W.K., Barnett P. Enhancement of experimental cerebral edema after decompressive craniectomy: implications for the management of severe head injuries. Neurosurgery. 1979;4:296-300.
22. Becker D.P., Miller J.D., Ward J.D., et al. The outcome from severe head injury with early diagnosis and intensive management. J Neurosurg. 1977;47:491-502.
23. Teasdale G., Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2:81-84.
24. Jennett B., Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480-484.
25. Gower D.J., Lee K.S., McWhorter J.M. Role of subtemporal decompression in severe closed head injury. Neurosurgery. 1988;23:417-422.
26. Gaab M.R., Rittierodt M., Lorenz M., Heissler H.E. Traumatic brain swelling and operative decompression: a prospective investigation. Acta Neurochir Suppl (Wien). 1990;51:326-328.
27. Polin R.S., Shaffrey M.E., Bogaev C.A., et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84-94.
28. Kleist-Welch Guerra W., Gaab M.R., Dietz H., et al. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999;90:187-196.
29. Münch E., Horn P., Schurer L., et al. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery. 2000;47:315-323.
30. Aarabi B., Hesdorffer D.C., Ahn E.S., et al. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104:469-479.
31. Albanese J., Leone M., Alliez J.R., et al. Decompressive craniectomy for severe traumatic brain injury: evaluation of the effects at one year. Crit Care Med. 2003;31:2535-2538.
32. Chibbaro S., Tacconi L. Role of decompressive craniectomy in the management of severe head injury with refractory cerebral edema and intractable intracranial pressure: our experience with 48 cases. Surg Neurol. 2007;68:632-638.
33. Coplin W.M., Cullen N.K., Policherla P.N., et al. Safety and feasibility of craniectomy with duraplasty as the initial surgical intervention for severe traumatic brain injury. J Trauma. 2001;50:1050-1059.
34. Danish S.F., Barone D., Lega B.C., Stein S.C. Quality of life after hemicraniectomy for traumatic brain injury in adults: a review of the literature. Neurosurg Focus. 2009;26:E2.
35. Flint A.C., Manley G.T., Gean A.D., et al. Post-operative expansion of hemorrhagic contusions after unilateral decompressive hemicraniectomy in severe traumatic brain injury. J Neurotrauma. 2008;25:503-512.
36. Goettler C.E., Tucci K.A. Decreasing the morbidity of decompressive craniectomy: the Tucci flap. J Trauma. 2007;62:777-778.
37. Jaeger M., Soehle M., Meixensberger J. Improvement of brain tissue oxygen and intracranial pressure during and after surgical decompression for diffuse brain oedema and space occupying infarction. Acta Neurochir Suppl. 2005;95:117-118.
38. Jagannathan J., Okonkwo D.O., Dumont A.S., et al. Outcome following decompressive craniectomy in children with severe traumatic brain injury: a 10-year single-center experience with long-term follow up. J Neurosurg. 2007;106:268-275.
39. Jiang J.Y., Xu W., Li W.P., et al. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: a multicenter, prospective, randomized controlled study. J Neurotrauma. 2005;22:623-628.
40. Kan P., Amini A., Hansen K., et al. Outcomes after decompressive craniectomy for severe traumatic brain injury in children. J Neurosurg. 2006;105:337-342.
41. Kontopoulos V., Foroglou N., Patsalas J., et al. Decompressive craniectomy for the management of patients with refractory hypertension: should it be reconsidered? Acta Neurochir (Wien). 2002;144:791-796.
42. Leitgeb J., Erb K., Mauritz W., et al. Severe traumatic brain injury in Austria V: CT findings and surgical management. Wien Klin Wochenschr. 2007;119:56-63.
43. Meier U., Grawe A. The importance of decompressive craniectomy for the management of severe head injuries. Acta Neurochir Suppl.. 2003;86:367-371.
44. Messing-Junger A.M., Marzog J., Wobker G., et al. Decompressive craniectomy in severe brain injury. Zentralbl Neurochir. 2003;64:171-177.
45. Pompucci A., De Bonis P., Pettorini B., et al. Decompressive craniectomy for traumatic brain injury: patient age and outcome. J Neurotrauma. 2007;24:1182-1188.
46. Potts M.B., Chi J.H., Meeker M., et al. Predictive values of age and the Glasgow Coma Scale in traumatic brain injury patients treated with decompressive craniectomy. Acta Neurochir Suppl.. 2008;102:109-112.
47. Rubiano A.M., Villarreal W., Hakim E.J., et al. Early decompressive craniectomy for neurotrauma: an institutional experience. Ulus Travma Acil Cerrahi Derg. 2009;15:28-38.
48. Schneider G.H., Bardt T., Lanksch W.R., Unterberg A. Decompressive craniectomy following traumatic brain injury: ICP, CPP and neurological outcome. Acta Neurochir Suppl. 2002;81:77-79.
49. Skoglund T.S., Eriksson-Ritzen C., Jensen C., Rydenhag B. Aspects on decompressive craniectomy in patients with traumatic head injuries. J Neurotrauma. 2006;23:1502-1509.
50. Timofeev I., Czosnyka M., Nortje J., et al. Effect of decompressive craniectomy on intracranial pressure and cerebrospinal compensation following traumatic brain injury. J Neurosurg. 2008;108:66-73.
51. Timofeev I., Kirkpatrick P.J., Corteen E., et al. Decompressive craniectomy in traumatic brain injury: outcome following protocol-driven therapy. Acta Neurochir Suppl. 2006;96:11-16.
52. Ucar T., Akyuz M., Kazan S., Tuncer R. Role of decompressive surgery in the management of severe head injuries: prognostic factors and patient selection. J Neurotrauma. 2005;22:1311-1318.
53. Whitfield P.C., Patel H., Hutchinson P.J., et al. Bifrontal decompressive craniectomy in the management of posttraumatic intracranial hypertension. Br J Neurosurg. 2001;15:500-507.
54. Woertgen C., Rothoerl R.D., Schebesch K.M., Albert R. Comparison of craniotomy and craniectomy in patients with acute subdural haematoma. J Clin Neurosci. 2006;13:718-721.
55. Ziai W.C., Port J.D., Cowan J.A., et al. Decompressive craniectomy for intractable cerebral edema: experience of a single center. J Neurosurg Anesthesiol. 2003;15:25-32.
56. Cooper D.J., Rosenfeld J.V., Murray L., et al. DECRA Trial Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493-1502. A published erratum appears in N Engl J Med. 2011;365:2040
57. Kunze E., Meixensberger J., Janka M., et al. Decompressive craniectomy in patients with uncontrollable intracranial hypertension. Acta Neurochir Suppl. 1998;71:16-18.
58. Aarabi B., Hesdorffer D.C., Simard J.M., et al. Comparative study of decompressive craniectomy after mass lesion evacuation in severe head injury. Neurosurgery. 2009;64:927-940.
59. Akyuz M., Ucar T., Acikbas C., et al. Effect of early bilateral decompressive craniectomy on outcome for severe traumatic brain injury. Turk Neurosurg. 2010;20:382-389.
60. Eberle B.M., Schnuriger B., Inaba K., et al. Decompressive craniectomy: surgical control of traumatic intracranial hypertension may improve outcome. Injury. 2010;41:934-938.
61. Howard J.L., Cipolle M.D., Anderson M., et al. Outcome after decompressive craniectomy for the treatment of severe traumatic brain injury. J Trauma. 2008;65:380-386.
62. Williams R.F., Magnotti L.J., Croce M.A., et al. Impact of decompressive craniectomy on functional outcome after severe traumatic brain injury. J Trauma. 2009;66:1570-1576.
63. Vollmer D.G., Torner J.C., Jane J.A., et al. Age and outcome following traumatic coma: why do older patients fare worse? J Neurosurg. 1991;75:S37-S49.
64. De Bonis P., Pompucci A., Mangiola A., et al. Post-traumatic hydrocephalus after decompressive craniectomy: an underestimated risk factor. J Neurotrauma. 2010;27:1965-1970.
65. Marshall L.F., Klauber G.T., Eisenberg H.M. The outcome of severe closed head injury. J Neurosurg. 1991;75:S28-S36.
66. Teasdale G., Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir (Wien). 1976;34:45-55.
67. Marion D.W., Carlier P.M. Problems with initial Glasgow Coma Scale assessment caused by prehospital treatment of patients with head injuries: results of a national survey. J Trauma. 1994;36:89-95.
68. Weiner G.M., Lacey M.R., Mackenzie L., et al. Decompressive craniectomy for elevated intracranial pressure and its effect on the cumulative ischemic burden and therapeutic intensity levels after severe traumatic brain injury. Neurosurgery. 2010;66:1111-1119.
69. Honeybul S., Ho K.M., Lind C.R., Gillett G.R. Surgical intervention for severe head injury: ethical considerations when performing life-saving but non-restorative surgery. Acta Neurochir (Wien). 2011;153:1105-1110.
70. Quinn T.M., Taylor J.J., Magarik J.A., et al. Decompressive craniectomy: technical note. Acta Neurol Scand. 2011;123:239-244.
71. Ragel B.T., Klimo P.Jr., Martin J.E., et al. Wartime decompressive craniectomy: technique and lessons learned. Neurosurg Focus. 2010;28:E2.
72. Li G., Wen L., Yang X.F., et al. Efficacy of large decompressive craniectomy in severe traumatic brain injury. Chin J Traumatol. 2008;11:253-256.
73. Wagner S., Schnippering H., Aschoff A., et al. Suboptimum hemicraniectomy as a cause of additional cerebral lesions in patients with malignant infarction of the middle cerebral artery. J Neurosurg. 2001;94:693-696.
74. Csokay A., Egyud L., Nagy L., Pataki G. Vascular tunnel creation to improve the efficacy of decompressive craniotomy in post-traumatic cerebral edema and ischemic stroke. Surg Neurol. 2002;57:126-129.
75. Ban S.P., Son Y.J., Yang H.J., et al. Analysis of complications following decompressive craniectomy for traumatic brain injury. J Korean Neurosurg Soc. 2010;48:244-250.
76. Honeybul S., Ho K.M. Long-term complications of decompressive craniectomy for head injury. J Neurotrauma. 2011;28:929-935.
77. Yang X.F., Wen L., Shen F., et al. Surgical complications secondary to decompressive craniectomy in patients with a head injury: a series of 108 consecutive cases. Acta Neurochir (Wien). 2008;150:1241-1248.
78. Wang HK, Lu K, Liang CL, et al. Contralateral subdural effusion related to decompressive craniectomy performed in patients with severe traumatic brain injury. Injury. In press.
79. Mazzini L., Campini R., Angelino E., et al. Posttraumatic hydrocephalus: a clinical, neuroradiologic, and neuropsychologic assessment of long-term outcome. Arch Phys Med Rehabil. 2003;84:1637-1641.
80. Wen L., Wan S., Zhan R.Y., et al. Shunt implantation in a special sub-group of post-traumatic hydrocephalus: patients have normal intracranial pressure without clinical representations of hydrocephalus. Brain Inj. 2009;23:61-64.
81. Sun Y., Wang J., Wu X., et al. Validating the incidence of coagulopathy and disseminated intravascular coagulation in patients with traumatic brain injury: analysis of 242 cases. Br J Neurosurg. 2011;25:363-368.
82. Tian H.L., Chen H., Wu B.S., et al. D-dimer as a predictor of progressive hemorrhagic injury in patients with traumatic brain injury: analysis of 194 cases. Neurosurg Rev. 2010;33:359-366.
83. Schiffer J., Gur R., Nisim U., Pollak L. Symptomatic patients after craniectomy. Surg Neurol. 1997;47:231-237.
84. Stiver S.I., Wintermark M., Manley G.T. Motor trephine syndrome: a mechanistic hypothesis. Acta Neurochir Suppl. 2008;102:273-277.
85. Fodstad H., Love J.A., Ekstedt J., et al. Effect of cranioplasty on cerebrospinal fluid hydrodynamics in patients with the syndrome of the trephined. Acta Neurochir (Wien). 1984;70:21-30.
86. Suzuki N., Suzuki S., Iwabuchi T. Neurological improvement after cranioplasty: analysis by dynamic CT scan. Acta Neurochir (Wien). 1993;122:49-53.
87. Taylor A., Butt W., Rosenfeld J., et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst. 2001;17:154-162.
88. Hutchinson P.J., Corteen E., Czosnyka M., et al. Decompressive craniectomy in traumatic brain injury: the randomized multicenter RESCUEicp study, www.RESCUEicp.com Acta Neurochir Suppl., 2006;96:17-20