Chapter 118 Cystoid Macular Edema and Vitreomacular Traction
Introduction
Macular edema is defined as an abnormal thickening of the macula associated with accumulation of fluid in the extracellular space of the outer plexiform layer and the inner nuclear layer, and occasionally in the intracellular space.1,2 It contributes to vision loss by altering the functional cell relationship in the retina and promoting an inflammatory reparative response. Macular edema associated with vitreous traction represents a particular tissue reaction due to the mechanical distortion of the retina. However, it is often difficult to differentiate whether edema is linked to the fact that the vitreous is pulling actively on the retina or to the fact that it is simply adherent. Furthermore, in certain circumstances, the changes at the vitreoretinal interface may represent the cause and in some others, the effect of vitreoretinal traction.3
The therapeutic approaches to macular edema depend on its pathophysiological mechanisms related to the underlying etiology, and they may be purely surgical or in combination with medial treatments. Therapies have evolved dramatically over the last years, as research has led to enhanced understanding of its causes as well as to the development of new pharmacologic agents and surgical approaches.3 Cystoid macular edema (CME) is considered a leading cause of central vision loss in the developed world, and it is therefore of enormous medical and socioeconomic importance.4
Historical discovery of macular edema
One of the first reports, after the advent of the direct ophthalmoscope, on what would be described as diabetic macular edema today was published by Eduard Jaeger in 1856.5 In 1872, Edward Nettleship provided the first histopathological proof for a “cystoid degeneration of the macula” in patients with diabetes,6 while histological observations on a cystic degeneration unrelated to diabetes were further documented by the Russian ophthalmologist Iwanoff.7
The first description of macular edema with documented vitreomacular traction was published by Irvine in his classical paper on CME after intra- and extracapsular cataract extraction characterizing the vitreous tug syndrome after incarcerated vitreous in the corneal wound.8
Anatomy and pathophysiology of macular edema
Anatomy of cystoid macular edema
Cystoid spaces in macular edema are intracellular or extracellular, or both according to different pathologic studies.9–11 The central area of the retina is predisposed to develop edema due to its unique anatomy, which is characterized by an extremely high cell count with increased metabolic activity and a central avascular zone, creating a watershed arrangement between the choroidal and retinal circulation which decreases resorption of extracellular fluid.12
The classic understanding has been that cystoid spaces form within Henle’s layer, which courses laterally away from the central fovea. In the foveal region, these fibers of the outer plexiform layer demonstrate a loose arrangement allowing accumulation of fluid leaking from perifoveal capillaries. However, it has been shown that the cystoid spaces can also form in the outer nuclear, inner nuclear, inner plexiform, and even the ganglion cell layer.13
The leakage from the perifoveal capillaries may explain the presence of cystoid spaces in the inner nuclear layer and Henle fiber layer. The presence of cystoid spaces in the ganglion cell layer might be explained with the assumption that epiretinal membranes, thickened internal limiting membrane (ILM), and/or adherence of the vitreal cortex to the retina disturbs the water movement between the vitreous and retina.14
Pathophysiology of tractional macular edema
In a normal steady state, several mechanisms prevent an accumulation of extracellular and intraretinal fluid and proteins. These mechanisms are able to maintain a balance of osmotic forces, hydrostatic forces, capillary permeability, and tissue compliance. The result is that the rate of capillary filtration equals the rate of fluid removal from the extracellular retinal tissue. Therefore, the interstitial spaces of the retina can be kept “dry” in physiological conditions.2
Fluid accumulation can be caused by increased water influx into the retinal tissue by decreased fluid clearance through glial and retinal pigment epithelium (RPE) cells.15 The former can be a result of an increase in the hydrostatic pressure (as occurring, e.g., during increased retinal blood flow and vasodilation), and of osmotic imbalances (hypo-osmolarity of the blood/vitreous and/or hyperosmolarity of the retinal tissue, as occurring, e.g., in diabetic retinopathy and cases of hepatic and renal failures). A decreased water clearance through glial and RPE cells occurs when inflammatory conditions impair the transcellular transport of osmolytes, or when the fluid influx through the disrupted blood–retinal barrier (BRB) exceeds the fluid clearance capacity of the cells.
The vitreous has been implicated as a cause of macular edema via several mechanical and physiologic mechanisms. One of the most constructive hypotheses on how vitreomacular traction (VMT) may result in macular edema was given by Schubert in 1989, and was summarized by Bringmann and Wiedemann in 2009.16,17 Vitreous fibers, which adhere to Müller cell end-feet at sites of vitreoretinal attachments after partial detachment of the vitreous, exert tractional forces onto the cells; this activates Müller cells and results in cell hypertrophy, proliferation, and vascular leakage.16,17 Furthermore, long-lasting mechanical stress of astrocytes and Müller cells induced by vitreal fibers adhering to the cells can stimulate the release of inflammatory factors such as basic fibroblast growth factor (bFGF), inducing local inflammation and BRB breakdown promoting vascular leakage and macular edema.18
Vitreoretinal traction can also exert forces at the level of the RPE, which can eventually result in morphological RPE changes.19,20 Furthermore, VMT has been shown to induce pigment epithelial detachment and RPE tearing.21 It is a well-known phenomenon in many retinal vascular diseases that direct traction on the macula or on the RPE may induce not only local inflammation but also increase vascular endothelial growth factor (VEGF) locally, with subsequent macular edema formation.22–24
Mechanical traction can also cause macular edema by direct distortion of the surrounding intraretinal vessels, resulting in leakage due to disturbance of the macular microcirculation with reduced capillary blood flow and a loss of apposition between the retina and the RPE pump.25
Sebag and others have found enzyme-mediated vitreous cross-linking and nonenzymatic glycation in the diabetic vitreous, and they have suggested that the abnormal cross-linking might affect the collagen structure and destabilize the attached vitreous gel strengthening the adhesion of the posterior vitreous cortex to the ILM, which in turn produces stronger vitreoretinal attachment with subsequent macular edema.26,27
Furthermore, the vitreous in various pathologic conditions may act as a sink for factors influencing macular edema themselves. The breakdown of the BRB usually leads to an increased concentration of intravitreal serum-derived chemoattractants, which may provide a stimulus for cellular migration into the attached premacular posterior hyaloid. Cellular contraction may potentially lead to tangential traction with consequent leakage and macular edema.28,29 In turn, leakage from the vascular bed aggravates chemoattractant outflow, thus creating an inexorable vicious circle.
It has also been shown that several growth factors such as VEGF, IL-6, platelet-derived growth factor (PDGF), and others are secreted in large amounts into the vitreous during proliferative vasculopathies such as diabetic retinopathy or retinal vein occlusion30–32 and these factors may increase vasopermeability and promote macular edema. Furthermore, during aging, VEGF is increasingly bound by altered vitreous collagen fibrils at the interface between retina and posterior vitreous cortex potentiating the effects of these growth factors.33
Clinical signs of cystoid macular edema
Clinically, macular edema can be best detected using the slit lamp and either a contact lens (e.g., Goldmann lens) or a handheld, noncontact lens (e.g., +78 D, +60 D). Ophthalmoscopically, the cysts are characterized by an altered light reflex. These changes may be better visible using green light, while retroillumination can help to delineate the polycystic spaces. The lack of sensitivity of the clinical examination for detection of mild edema has been demonstrated for eyes with a foveal center thickness between 201 and 300 µm. Only 14% of eyes were noted to have foveal edema by contact lens biomicroscopy. The term “subclinical foveal edema” describes such cases.34,35 A thorough examination of the anterior segment should always complement the clinical evaluation as incarcerated vitreous or a badly placed intraocular lens may be the underlying cause of macular edema.14 Symptoms range from loss in distance visual acuity, contrast sensitivity, color vision, reading acuity to a reduction in reading speed.36–38 Accompanying symptoms may include metamorphopsia and micropsia. Additionally, a marked reduction in central retinal sensitivity with either a relative or absolute scotoma during active macular edema, but also after the edema has resolved, has been reported.39 Retinal thickness appears to be most closely correlated with visual acuity,40 whereas increased leakage on fluorescein angiography is not directly correlated with reduced visual acuity.
Imaging of cystoid macular edema
Angiography
The fundus fluorescein angiogram has for many years been one of the most useful tests in detecting macular edema of various etiologies.41
In the early phase of the angiogram, capillary dilation can be detected in the perifoveal region. In the late phase, fluorescein pools in cystoid spaces located in the outer plexiform layer (Henle’s layer) displayed as the classic petaloid staining pattern.35,41 The fusiform cystoid spaces are arranged in a radial pattern. In long-standing macular edema, the cystoid spaces enlarge and may merge, representing irreversible damage to the retina. When cystoid spaces occur outside the perifoveal area, fluorescein angiography shows a honeycomb appearance, rather than a petaloid pattern.41,42 The amount of fluorescein leakage depends on the dysfunction of the retinal vascular endothelium and there is a significant correlation between visual acuity and the area covered by these cystoid changes.11,43 So-called “silent” angiograms have also been reported, which correspond to the presence of clinical macular edema, which shows, however, no leakage on fluorescein angiography. One reason for this may be very old changes within the retina, which are characterized by intraretinal cysts, which have become impermeable to the fluorescein dye diffusion.
Furthermore, one should consider that macular edema can also occur without vascular leakage, when the fluid clearance through glial and RPE cells is impaired.15 This may also explain the presence of edema in cases without significant angiographic vascular leakage.
Indocyanine green angiography is not considered a very useful tool for detecting macular edema.35 However, in some cases, particularly for laser scanning, it may provide additional direct signs of macular edema for the delimitation of cystoid spaces progressively filled with the dye and also for precise analysis of RPE alterations.41 The autofluorescence can also depict the cysts as hyperautofluorescent because of the displacement of macular pigments that naturally attenuate the autofluorescent signal.35
Optical coherence tomography
Optical coherence tomography (OCT) is able to accurately measure the retinal thickness, allowing a more precise and reproducible assessment than fluorescein angiography,44–46 with a particular efficacy in the volumetric analysis of macular edema. Several authors have proposed different patterns and classifications of macular edema, according to the underling pathology based on OCT findings.35,47–52
OCT and diabetic macular edema
In diabetic macular edema (DME), there are several broad nonexclusive categories: diffuse retinal thickening; cystoid macular edema; serous retinal detachment, and vitreomacular interface abnormality. It has been demonstrated that the amount of reflectivity within these diabetic cystoid spaces is due to higher concentration of protein associated with the breakdown of the inner blood–retinal barrier in diabetic macular edema.53 OCT identified vitreomacular interface abnormalities including the presence of epiretinal membranes (ERM) and/or VMT. An ERM can be manifested on OCT by the presence of a macular pseudohole, a hyperreflective band along the inner aspect of the retina, or a visible hyperreflective membrane tuft or edge. Vitreomacular traction is identified by a hyperreflective band that is in apposition with the inner surface of the retina at discrete site(s) and elevated above the surface of the retina elsewhere.47,51
Ghazi et al.54 demonstrated that in eyes with persistent DME, vitreomacular interface abnormalities may be found in up to 52–67% on OCT, and concluded that OCT was nearly twice as sensitive as traditional techniques in detecting vitreomacular interface abnormalities.
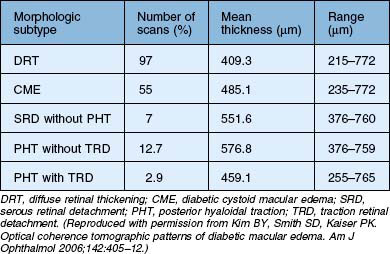
Kim et al.47 proposed five different morphologic patterns at OCT evaluation of diabetic macular edema in relation to VMT: Pattern I is a diffuse increased retinal thickening, with areas of reduced intraretinal reflectivity; Pattern II is CME as described above; Pattern III shows posterior hyaloidal traction, which appears as a highly reflective band over the retinal surface; Pattern IV exhibits serous retinal detachment not associated with posterior hyaloidal traction, which appears as a dark accumulation of subretinal fluid beneath a highly reflective dome-like elevation of detached retina; Pattern V shows posterior hyaloidal traction and tractional retinal detachment, which appear as a peak-shaped detachment with a highly reflective signal arising from the inner retinal surface and with an area of low signal beneath the highly reflective border of detached retina (Table 118.1). More recent reports have also shown – using immunohistochemistry – unequivocal proof of proliferative changes on the retinal and vitreal surface of the diabetic ILM,55 while others have quantified the frequency of vitreomacular interface pathologies in diabetic macular edema in general.56
A very recent publication showed furthermore, that in eyes with DME, ERM and incomplete posterior vitreous detachment, the posterior cortical vitreous and the membrane appeared as one membrane in most eyes and were typically associated with vitreopapillary adhesion.57
OCT in vitreomacular traction syndrome
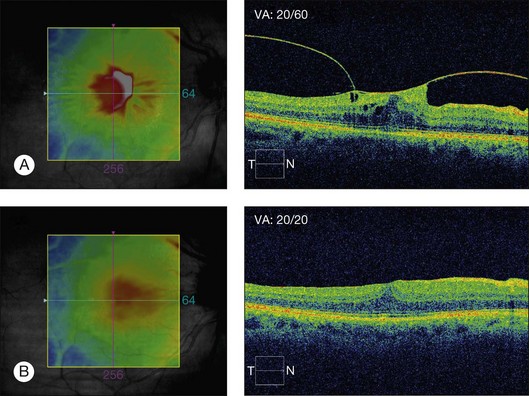
Vitreomacular traction syndrome (VMTS) can sometimes be characterized by a particular type of macular edema and OCT represents the most helpful tool for the diagnosis and follow-up of these pathologies. OCT can show perifoveal vitreous detachment, a thickened hyperreflective posterior hyaloid and ERM (Fig. 118.1).35 Koizumi H et al.52 described two different OCT patterns of vitreous attachment in VMT using spectral-domain OCT: foveal cavitation defined as the formation of cystoid cavity located in the inner part of the central fovea secondary to mechanical forces and CME that was defined as intraretinal cyst-like cavities extending beyond the foveal region. Sometimes, a neurosensory retinal detachment occurs while the macular traction may resolve in the formation of a lamellar-macular hole.43
Odrobina et al.50 also advocated that vitreous surface adhesion and persistence of ERM on OCT may be the prognostic factors for the natural course of VMT. They observed spontaneous resolution of VMT in eyes with less vitreous surface adhesion and without ERMs, while in eyes with higher vitreous surface adhesion or coexisting ERM they suggested a surgical intervention.50 Similar observations have been made on VMT in retinal vein occlusion.58
OCT for vitreomacular traction in age-related macular degeneration
The initial observations were made using B-scan ultrasound which showed that ARMD patients have a high rate of persisting vitreous attachment using ultrasound59 and other means of diagnosis.60–62 But more recently, using high-resolution OCT, vitreomacular adhesions and traction have also been associated with the presence of ARMD. OCT-aided studies confirmed a high incidence of attached vitreous of up to 79%.24
Surgical treatment of tractional macular edema
Rationale for vitrectomy
Tractional origin of macular edema
The initial rationale for using vitrectomy in cases of macular edema was entirely structural, i.e. aimed at the removal of vitreous traction on the macula.63,64 The latter becomes understandable by looking at Newton’s third law: to any action there is always an equal reaction in the opposite direction. The force of vitreoretinal traction will thus be met by an equal and opposite force in the retina, resulting in the manifold pathological reactions described previously. Vitrectomy may thus conceptually relieve traction on Müller and RPE cells that had resulted in vascular leakage, and it may also suppress the release of inflammatory factors previously induced by the mechanical stress on these cells. Additionally, vitrectomy may reduce the tractional disturbance of the macular microcirculation and it may restore the apposition between the retina and the RPE pump.
Nontractional origin of macular edema
Recent discoveries have shown that vitrectomy may not only be beneficial in the presence of macular traction, but also in cases where no particular deformation of the macula can be identified. This is particularly true for macular edema of vascular origin, such as diabetes or retinal vein occlusion. The beneficial effect of vitrectomy is thought to be based – at least in part – on two mechanisms. First, it has been found that oxygenation of the posterior segment of the eye is increased after vitrectomy.65–68 Others have shown that pharmacologic vitreolysis also improves vitreal O2 levels and that it increases the rate of O2 exchange within the vitreous cavity.69,70 This may also be the mechanism behind the observation that vitrectomy may reduce the extent of the foveal avascular zone as seen on fluorescein angiography.71 Second, it has been shown that several growth factors such as VEGF, IL-6, platelet-derived growth factor, and others are secreted in large amounts into the vitreous during proliferative vasculopathies such as diabetic retinopathy or retinal vein occlusion31,32,72,73 and it is conceivable that a complete vitrectomy will remove this excess of growth factors mechanically with the desired effect of a restitution of the blood–retinal barrier.19 The rapid clearance of VEGF and other cytokines may thus help to prevent macular edema and retinal neovascularization in ischemic retinopathies, such as diabetic retinopathy and retinal vein occlusions. Vitreous clearance of growth factors may indeed have the same effect as the presence of, e.g., VEGF antibodies in the vitreous cavity.74–76
Rationale for internal limiting membrane peeling
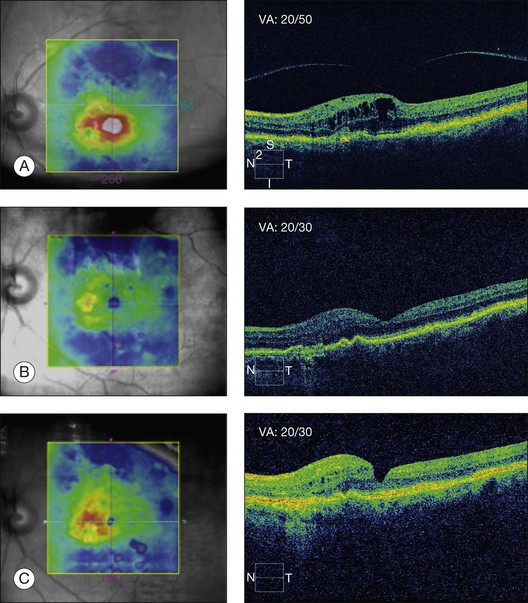
ILM may thicken due to an increased content of extracellular matrices and cellular proliferation on the vitreous surface in cases of diabetic macular edema.77 It has been hypothesized that ILM changes contribute to the structural and functional disturbance of water movement between the vitreous and the retina77 and eventually retain proteins in the interstitial space avoiding diffusion of proteins to the vitreous space leading to macular edema.78 Additionally, the diffusion of oxygen from the fluid in the vitreous cavity into the retina would be retarded by a thickened ILM.79 Also, the absence of the vitreous gel would increase the transport of cytokines, such as VEGF, from the retina into the vitreous cavity, and the absence of ILM would further speed up this clearance of cytokines from the retina.75 The efficacy of ILM delamination may be caused by the removal of a growth factor reservoir, which may have accumulated in the ILM and in cellular elements on its vitreous side. Vitreous remnants may be present even after surgical vitreous separation80 and ILM delamination allows a more complete removal of vitreous elements.77,80 In uveitis and in VMT complicating ARMD, it has been speculated that intraocular inflammation, through the release of chemokines and cytokines into the vitreous, may result in a firmer attachment of the posterior hyaloid to the macula and/or contraction of the ILM creating tangential traction on the retina and the development of macular edema. In such cases, macular edema may be refractive to medical therapy – corticosteroids and anti-VEGF agents – and it may only be relieved by vitrectomy with separation of the posterior hyaloid and/or peeling of the ILM (Fig. 118.2).81
Clinical entities with cystoid macular edema associated with vitreomacular traction
Diabetic macular edema (DME)
Role of vitrectomy in DME
In 1992 Lewis et al. were the first to report the resolution of macular edema in 80% of cases after vitrectomy for diabetic edema associated with posterior hyaloidal traction.63 Other studies have also suggested a beneficial result of vitrectomy for tractional DME.82,83 The functional prognosis was considered to be better when vitrectomy is performed at an early stage.84
In 2010, the Diabetic Retinopathy Clinical Research Network evaluated vitrectomy for diabetic macular edema in eyes with at least moderate vision loss and vitreomacular traction. They found retinal thickening to be reduced in most eyes postoperatively. The median change in visual acuity increased on average at 6 months after surgery by three letters, with visual acuity improving by ≥10 letters from baseline to 6 months in 38% and worsening by ≥10 letters in 22%. Reduction in central macular thickness as shown by OCT to be less than 250 µm occurred in almost half, and most eyes had a thickness reduction of ≥50%.85 Since 1990, several other authors have also reported that vitrectomy is beneficial for diffuse DME combined with a taut thickened posterior hyaloid presumably with tangential hyaloidal traction.86 Pendergast et al.87 reported stability or improvement in 91% of eyes and complete disappearance in 92% of patients after vitrectomy for diffuse DME associated with a taut posterior hyaloid.
Reports in the literature on vitrectomy for DME, in the absence of traction, include mixed visual acuity results. Some studies suggested positive outcomes; others have shown anatomic but not visual improvement after surgery, while some studies suggested that vitrectomy is not beneficial in eyes with DME without traction.83–85
Role of internal limiting membrane peel in DME
Despite several clinical studies over the past few years, the role of ILM peeling in DME is still far from clear. Stolba et al. and Stefaniotou et al. reported a favorable outcome following vitrectomy and ILM peeling opposed to the natural course of DME.88,89 In contrast, others found good anatomical but less impressive visual results. Bahadir et al. found similar degrees of improvement in visual acuity after vitrectomy without ILM peel compared to vitrectomy with ILM peeling in the treatment of DME.90 Patel et al. in a comparative, prospective study of vitrectomy with and without ILM peeling for diffuse clinically significant macular edema, reported structural improvement but with limited visual improvement after ILM peeling.91 Kumar et al. compared the effectiveness of vitrectomy and ILM peeling with grid laser photocoagulation in patients with diffuse DME and found that vitrectomy with ILM peeling was beneficial by inducing a statistically significant reduction of macular thickness and macular volume. However, the comparative visual acuity outcome analysis between the two groups was not significantly different.92
Gentile et al. advocated that a taut ILM can still cause diffuse DME after vitrectomy, and that its removal can restore the normal foveal contour and improve visual acuity.93 In a recent randomized controlled study, Hoerauf et al.79 demonstrated a favorable effect of additional ILM removal in vitrectomy for cystoid DME without evident VMT. They concluded that in the long term, while posterior vitreous detachment (PVD) alone slowly improves the anatomical results, it is markedly less effective than additional ILM removal. However, even though the morphological results were substantial, visual results were unsatisfactory.
Surgical technique
Pars plana vitrectomy
A standard three-port pars plana vitrectomy is performed. A PVD is induced if not present by suction over the optic nerve disc until the Weiss ring is identified. Simple suction may not result in complete PVD in case of taut posterior hyaloid and the membrane on the retinal surface needs to be engaged initially with a pick or a bent microvitreoretinal blade and formally removed using membrane forceps. Following this, the peripheral vitrectomy is completed and fibrovascular membranes, if present, need to be delaminated.94
Role of triamcinolone
The injection of intravitreal triamcinolone can be used to better visualize the posterior hyaloid and thus facilitate PVD induction. Furthermore, triamcinolone acetate has been shown to facilitate fluid absorption from the edematous retinal tissue by both stimulating endogenous adenosine signaling in Müller cells,95 and by downregulating VEGF production.96 It can thus be used as an adjuvant also at the end of the surgery to reduce macular edema and postsurgical inflammation in general.
Internal limiting membrane peel
The ILM peel may be initiated with a slight incision of the ILM temporal to the fovea, or the ILM may be grasped directly with the forceps. In this area, it is less likely to slice nerve fibers and cause small paracentral scotoma. Alternatively, a diamond-dusted brush can be used. Once the ILM is lifted, it is peeled tangentially and circumferentially like a capsulorhexis.97 Visibility of the ILM can be greatly improved using brilliant blue G (BBG) that demonstrates a high biocompatibility and appears to be safe as shown in clinical and experimental trials.98 Preliminary studies showed promising results using a heavier variation namely heavy that can be handled in a very controlled way as it can be applied directly over the region of interest and can be removed easily in a very controlled fashion.99
Cystotomy for diabetic CME
Long-standing macular edema may be characterized by large macrocysts. Tachi et al.100 described the surgical technique of cystotomy as an adjunct to vitrectomy in the treatment of CME in diabetic patients. They described four techniques of cystotomy: (1) direct puncture and aspiration; (2) deroofing; (3) cystectomy, and (4) lateral puncture. They reported visual acuity improvement in 32% of the studied cases that included 22 eyes. The long-term role of cystotomy in the treatment of CME is, however, not clear to-date.
Central retinal vein occlusion
Role of vitrectomy in CRVO
Pars plana vitrectomy has been reported as a potential treatment for macular edema related to CRVO.101 Noma et al.102 described significant functional and anatomic improvement after vitrectomy for macular edema in patients with central retinal vein occlusion. Interestingly, they found that the vitreous level of VEGF was significantly higher in CRVO patients with less improvement of best-corrected visual acuity (BCVA) after vitrectomy. They hypothesized that high VEGF levels may be associated with permanent photoreceptor cell damage due to macular ischemia.
Leizaola-Fernández et al.103 found moderate improvement of BCVA in 60% of eyes and stabilization of BCVA in 40% after vitrectomy, with complete posterior hyaloid removal in patients with ischemic CRVO. Additionally, in this study, a decrease in macular thickness and of the P1 wave amplitude after vitrectomy was documented. A more recent retrospective study has characterized extrafoveal vitreoretinal traction in a large proportion of retinal vein occlusion patients as documented by OCT video clips, causing macular edema and macular detachment. The authors also addressed the important question of when adhesion becomes traction. They regarded it to be evidence of traction where the retina was elevated or thickened and where there was deformity at the traction site, and where the posterior hyaloid or vitreous strands changed angle. Conversely, vitreous adherence without traction was defined as vitreous attachment not associated with inner retinal deformity.104
Role of internal limiting membrane peel in CRVO
Park et al. in a study of long-term effects of vitrectomy and ILM peeling for macular edema secondary to CRVO and hemiretinal vein occlusion (HRVO) reported an anatomical improvement, which persists up to 5 years, and BCVA improvement, at least in perfused CRVO and HRVO.105 DeCroos et al. reported that vitrectomy, ILM peel, and panretinal endophotocoagulation for macular edema secondary to CRVO reduced foveal thickness at final follow-up; however, anatomic improvement did not correlate with a statistically significant improvement in VA.106
Radial optic neurotomy (RON)
Based on the assumption that CRVO is a compartment-like syndrome resulting from increased pressure on the central retinal vein within the confined space of the scleral ring, Opremcak et al. proposed in 2001 a surgical technique to dissect the lamina cribrosa transvitreally via a radial incision on the nasal side of the optic nerve to improve venous outflow.107 In a study of 117 cases of CRVO treated with RON, they reported anatomical resolution of CRVO in 95% patients and improved visual function in 71%.108 Improvements in BCVA have been reported by several studies.109–112 However, other studies did not demonstrate significant function improvement of RON in CRVO,113 especially for the ischemic type.114 Arevalo et al. in a retrospective, uncontrolled, multicenter case series, reported that 32% of eyes had improvement in the ischemic CRVO, while 50% of eyes had an improvement in BCVA in the non-ischemic CRVO after RON. They concluded that although RON may improve BCVA in some eyes, surgery by itself did not seem to improve the outcome of CRVO when compared with its natural history.113 Since these publications, several studies have reported conflicting results and the role of RON remains controversial.
Branch retinal vein occlusion (BRVO)
Role of vitrectomy in BRVO
Pars plana vitrectomy has also been reported as a potential treatment for macular edema secondary to BRVO.115 Noma et al. reported significant improvement of BCVA from a mean logMAR 0.81 ± 0.42, to logMAR 0.52 ± 0.41 and the retinal thickness 6 months after vitrectomy for BRVO-related macular edema. Additionally, they found that vitreous levels of VEGF, soluble intercellular adhesion molecule-1 (ICAM-1), and pigment epithelium-derived factor (PEDF) may influence the visual prognosis and the response of macular edema after vitrectomy. More precisely, they found that VEGF and ICAM-1 were significantly lower in patients with more marked improvement of BCVA after vitrectomy, while PEDF was significantly higher. VEGF and ICAM-1 levels were significantly higher in patients with greater postoperative improvement of macular edema, while PEDF was significantly lower.116
Yamasaki et al. also reported both a significant improvement of BCVA and retinal thickness after vitrectomy for BRVO-related macular edema and a significant positive correlation between the vitreous levels of VEGF and improvement of macular edema.117
Adventitial sheathotomy in BRVO
Pars plana vitrectomy combined with arteriovenous adventitial sheathotomy using a microvitreoretinal blade has also been proposed as a potential therapy for macular edema in BRVO. This was based on the assumption that cutting the sheath and separating the retinal artery from the vein at the occlusion site may relieve pressure on the underlying vein and improve blood flow with subsequent reoxygenation of the retina.118
Mason et al.119 performed a nonrandomized, controlled trial of 20 eyes that underwent vitrectomy with arteriovenous sheathotomy in comparison to grid laser photocoagulation or observation and found significantly better visual outcomes in the surgical group. Kumagai et al. however, found that while vitrectomy may improve the long-term functional and anatomic outcomes for patients with macular edema secondary to BRVO, additional arteriovenous sheathotomy did not lead to a distinct functional benefit.120 Oh et al. have even questioned the effect of vitrectomy alone in a very small series of eight patients, in whom visual outcomes were better in observed control eyes after 36 months of follow-up than in the operated eyes.121 In the era of less-invasive treatment modalities for macular edema associated with BRVO such as anti-VEGF therapy or dexamethasone, whose efficacy has been documented in large phase III randomized studies, it may thus be difficult to justify a surgical approach based on the existing conflicting data unless there is an obvious coexistent tractional component to the macular edema.
Uveitic macular edema
Role of vitrectomy in uveitic macular edema
The value of vitreous surgery in uveitic macular edema is thought to be based on the assumption that many inflammatory mediators accumulate in the vitreous and a removal of these mediators may have a beneficial effect on macular edema. In addition, inflammation often results in the formation of ERM and vitrectomy associated with an ERM peel could thus be of benefit.122
There have been few reports on the outcome of pars plana vitrectomy in uveitis cases that have proved unresponsive to medical treatment.123–131 Dugel et al.129 investigated the efficacy of vitrectomy in 11 eyes of nine patients with intraocular inflammation-related CME that was unresponsive to corticosteroids. They reported improvement of BCVA and regression of macular edema.
Wiechens et al.132 investigated the role of vitrectomy in refractive uveitic CME and found that the response to vitrectomy was variable according to the type of underlying form of uveitis. The lowest success rate could be observed in eyes with multifocal chorioretinitis. Postoperative regression rate of CME was satisfactory in eyes with juvenile rheumatoid arthritis (JRA), however, long-term BCVA results were disappointing due to secondary complications of JRA. Best results were achieved in patients with intermediate uveitis though statistically not significant. Stavrou et al.133 studied the effect of vitrectomy without ILM peel in 37 eyes with intermediate uveitis and reported resolution of CME in 32.4% of the cases and they concluded that vitrectomy combined with simultaneous cataract surgery can improve the visual outcome in these patients. Clinical improvement allowed discontinuation of immunosuppressive treatment in 16% of patients. Kiryu et al.126 reported visual improvement of two or more Snellen lines in 56% of 18 studied eyes within 12 months after vitrectomy for CME secondary to sarcoid uveitis. Tranos et al.123 found angiographic improvement in one third of the patients that underwent vitrectomy for macular oedema secondary to chronic uveitis in a randomized, controlled pilot study.
Role of internal limiting membrane peel in uveitic CME
Gutfleisch et al.134 performed a vitrectomy with ILM peeling and injection of 4 mg triamcinolone in patients with refractory uveitic CME and found in 44% of the eyes a decrease of macular edema on fluorescein angiography, but a worsening of BCVA in 22% of the patients. Schaal et al. reported a positive effect of vitrectomy and ILM peel on retinal thickness and BCVA.81
In summary, vitrectomy may be a useful therapeutic alternative in selective cases.135 Such cases could include cases unresponsive to medical treatment, cases with significant inflammatory vitreous debris that compromise clinical assessment, cases in whom reduction or discontinuation of immunosuppressive treatment is imperative and cannot be achieved otherwise; and cases associated with ERMs and/or an obvious tractional component.
Postoperative macular edema
Postoperative CME is thought to be an inflammatory process136 and is the most frequent cause of decreased vision in patients following cataract surgery.137 Clinically significant CME has a reported incidence of 1–2% after cataract surgery,138 while angiographic CME is more common and has been reported to occur after about 20% of cataract surgeries.139,140 Its incidence is higher when associated with complicated surgery that may include capsule rupture, vitreous loss, vitreous wick, and retained lens fragments.
The Vitrectomy-Aphakic-Cystoid Macular Edema Study, a prospective, multicenter study of patients with vitreous adherent to the corneoscleral wound and with chronic aphakic cystoid macular edema showed significant improvement in visual acuity following vitrectomy.64,141
Harbour et al. reported vitrectomy results of 24 eyes with chronic pseudophakic macular edema, unresponsive to medical treatment, and evidence of either vitreous adhesions to anterior segment structures or iris capture of the intraocular lens. Visual acuity improved in all patients with 71% of subjects experiencing postoperative visual improvement of three or more lines. Postoperative visual outcome in this study was not associated with the duration of macular edema or the preoperative levels of visual acuity.142 The authors also commented that the visual outcome was better when a complete vitrectomy was carried out compared to a limited effect after a mere anterior vitrectomy. Pendergast et al.143 reported a significant improvement in visual acuity after vitrectomy in cases of pseudophakic CME, even without vitreous incarceration in the wound.
Vitreomacular traction syndrome and epiretinal membrane
Vitrectomy with peeling of any tangential tractional components usually results in resolution of retinal thickening and visual improvement in patients with VMTS.3 Hikichi et al.144 in a study regarding the natural history of VMT in the pre-OCT era, reported that 64% of the eyes lost two lines of vision over the course of follow-up. Since a report by Smiddy et al.145 described successful surgery for nondiabetic eyes with macular traction and visual decrease, many surgeons have elected to operate rather than to follow patients with evident VMT and significant visual impairment.85 Davis et al.146 in a recent study, reported improvement of more than two lines in 50% of eyes after vitrectomy for vitreofoveal traction syndrome and complete resolution of traction on OCT in all eyes while cystic changes improved markedly or resolved in 86% of eyes. Patients with symptoms for less than a 6-month duration (P = 0.048) were more likely to obtain a visual acuity of 20/40 or better, postoperatively. Vitrectomy surgery with peeling of the ERM and the posterior hyaloid is able to relieve all of the traction and has been shown to be associated with good visual outcome and resolution of the CME.147–149 Konstantinidis et al.150 reported that the concomitant administration of intravitreal triamcinolone acetonide after pars plana vitrectomy may speed up and improve the anatomic and functional outcome considerably.
Vitrectomy for vitreomacular traction in age-related macular degeneration
There have been several hypotheses linking VMT with ARMD, although it is still debated whether this relationship is causal or not. Chronic traction on the retina may cause degeneration or alteration of the RPE or Bruch’s membrane, and traction has also been shown to induce low-grade inflammation and VEGF release, which in turn may influence the progression of ARMD. Furthermore, an adhesion between the vitreous and the retina may create anatomical structures which facilitate a sustained contact of free radicals or other angiogenic cytokines in the vitreous gel with the retina, thus promoting the evolution of ARMD. In addition, an attached posterior vitreous face may also prevent oxygen diffusion or nutrient exchange.151
In some recent publications, it has thus been proposed that vitrectomy for vitreous adhesion and traction on the macula in patients with exudative ARMD has a therapeutic effect on the neovascular portion of the disease (Fig. 118.2). In a series of 12 eyes of 11 patients and a follow-up of 6 months, six eyes showed regression of choroidal neovascularization (CNV). In two eyes, the CNV disappeared completely. VA improved vision in four eyes; vision was unchanged in four eyes and there was worsening of vision in four eyes.152 Roller et al.,153 in a recent pilot study, found that vitrectomy was associated with a reduced progression of geographic atrophy or CNV.
Retinitis pigmentosa
Garcia-Arumi et al.154 has evaluated the role of vitrectomy accompanied by ILM peeling in 12 eyes with retinitis pigmentosa and CME refractory to medical therapy and reported anatomic and functional improvement. Their results showed a decrease in macular thickness of >40% in ten eyes (83.3%), while the mean BCVA increased from 20/115 to 20/45, with an average of three lines of improvement. These positive results were not confirmed in a case report by Hagiwara et al.155
Pharmacologic vitreolysis
In the absence of PVD, the vitreous cortex is adherent to the ILM of the retina. This junction is thought to participate in the pathophysiology of macular edema. For example, the risk of developing diffuse DME may be 3.4-fold lower in the group of eyes with complete PVD or complete vitreoretinal separation compared with the eyes with incomplete PVD.156 Moreover, a small prospective study by Hikichi and colleagues strongly suggested that vitreomacular separation can cause spontaneous resolution of DME.157
Pharmacologic vitreolysis can potentially relieve vitreoretinal traction and increases vitreal O2 levels and the rate of O2 exchange within the vitreous cavity.70,158 Different substances have been investigated, including chondroitinase, dispase, hyaluronidase, plasmin, and microplasmin.
Plasmin is a nonspecific serine protease that may promote PVD acting on a variety of glycoproteins and activating endogenous metalloproteinases. Gandorfer et al. showed, in two different studies on human donor eyes, that plasmin facilitates the induction of PVD and eliminates cortical vitreous remnants,159 with no evidence of damage to the retina.160 Sakuma et al. recently reported significant macular thickness improvement and BCVA improvement of two lines or more in about 88% of the cases after intravitreal injection of autologous plasmin enzyme for macular edema associated with BRVO.161 Similarly, Udaondo et al. reported anatomic and functional improvement after intravitreal injection of autologous plasmin enzyme for macular edema associated with BRVO.162 When used as an adjunct to vitrectomy, plasmin showed promising results as an adjunct to vitrectomy in DME facilitating PVD induction.163 The main drawback of plasmin is that it is not easily available for clinical use. Autologous plasminogen has to be isolated from the patient’s own blood and then converted in vitro to plasmin by streptokinase. This procedure has to be done immediately before surgery, as it yields a highly unstable product.164 Microplasmin is currently the agent that shows the greatest clinical potential. It is a recombinant product that contains only the catalytic domain of human plasmin and shares all of its catalytic properties. It is much more stable than the original molecule which greatly simplifies storage and administration.164 Phase II trials have shown that intravitreal microplasmin is well tolerated in patients with DME and VMTS.165 Ease of PVD induction during surgery was found to be dose- and time-dependent. A 125 µg dose that was repeated up to three times, released adhesion in 58% of patients with VMTS, 28 days after the final injection.166
The MIVI-IIT trial – a Phase IIb, randomized, double-masked, placebo-controlled, dose ranging trial – assessed the safety and efficacy of microplasmin after intravitreal injection given 7 days prior to the patient’s planned vitrectomy. They found that 125 µg of microplasmin was able to resolve the VMT in 28% of patients without the need for vitrectomy. Successfully treated patients also did not show any signs of recurrence and continued to see an improvement in their BCVA at 6 months.167 Preliminary results of the Phase III MIVI-TRUST (Traction Release without Surgical Treatment) trial, which is a Phase III multicenter, randomized, placebo-controlled trial evaluating 125 µg of microplasmin alone for the treatment of focal vitreomacular adhesion (VMA) associated with subjective visual dysfunction, indicated a statistically significant improvement in the rate of pharmacological resolution of symptomatic vitreomacular adhesion in the microplasmin group compared with placebo.168
1 Coscas G, Cunha-Vaz J, Soubrane G. Macular edema: definition and basic concepts. Dev Ophthalmol. 2010;47:1–9.
2 Scholl S, Kirchhof J, Augustin AJ. Pathophysiology of macular edema. Ophthalmologica. 2010;224:S8–S15.
3 Johnson MW. Etiology and treatment of macular edema. Am J Ophthalmol. 2009;147:11–21. e1
4 Hogan P, Dall T, Nikolov P, American Diabetes Association. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932.
5 Wolfensberger TJ, Hamilton AM. Diabetic retinopathy – an historical review. Semin Ophthalmol. 2001;16:2–7.
6 Nettleship E. On oedema or cystic disease of the retina. Roy Ophth Lond Hosp Rep. 1872;VII(3):343–351.
7 Iwanoff A. Das oedem der netzhaut. Graefes. Arch Ophthalmol. 1869;15(2):88–105.
8 Irvine SR. A newly defined vitreous syndrome following cataract surgery. Am J Ophthalmol. 1953;36:499–619.
9 Fine BS, Brucker AJ. Macular edema and cystoid macular edema. Am J Ophthalmol. 1981;92:466–481.
10 Yanoff M, Fine BS, Brucker AJ, et al. Pathology of human cystoid macular edema. Surv Ophthalmol. 1984;28:S505–S511.
11 Gass JD, Anderson DR, Davis EB. A clinical, fluorescein angiographic, and electron microscopic correlation of cystoid macular edema. Am J Ophthalmol. 1985;100:82–86.
12 Scholl S, Augustin A, Loewenstein A, et al. General pathophysiology of macular edema. Eur J Ophthalmol. 2010;21:10–19.
13 Tso MO. Pathology of cystoid macular edema. Ophthalmology. 1982;89:902–915.
14 Antcliff RJ, Hussain AA, Marshall J. Hydraulic conductivity of fixed retinal tissue after sequential excimer laser ablation: barriers limiting fluid distribution and implications for cystoid macular edema. Arch Ophthalmol. 2001;119:539–544.
15 Bringmann A, Reichenbach A, Wiedemann P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004;36:241–249.
16 Schubert HD. Cystoid macular edema: the apparent role of mechanical factors. Prog Clin Biol Res. 1989;312:277–291.
17 Bringmann A, Wiedemann P. Involvement of Muller glial cells in epiretinal membrane formation. Graefes Arch Clin Exp Ophthalmol. 2009;247:865–883.
18 Lindqvist N, Liu Q, Zajadacz J, et al. Retinal glial (Muller ) cells: sensing and responding to tissue stretch. Invest Ophthalmol Vis Sci. 2010;51:1683–1690.
19 Wolfensberger TJ, Gregor ZJ. Macular edema – rationale for therapy. Dev Ophthalmol. 2010;47:49–58.
20 Smiddy WE, Green WR, Michels RG, et al. Ultrastructural studies of vitreomacular traction syndrome. Am J Ophthalmol. 1989;107:177–185.
21 Meyer CH, Toth CA. Retinal pigment epithelial tear with vitreomacular attachment: a novel pathogenic feature. Graefes Arch Clin Exp Ophthalmol. 2001;239:325–333.
22 Kuiper EJ, de Smet MD, van Meurs JC, et al. Association of connective tissue growth factor with fibrosis in vitreoretinal disorders in the human eye. Arch Ophthalmol. 2006;124:1457–1462.
23 Donoso LA, Kim D, Frost A, et al. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51:137–152.
24 Krebs I, Brannath W, Glittenberg C, et al. Posterior vitreomacular adhesion: a potential risk factor for exudative age-related macular degeneration? Am J Ophthalmol. 2007;144:741–746.
25 Augustin A, Loewenstein A, Kuppermann BD. Macular edema. General pathophysiology. Dev Ophthalmol. 2010;47:10–26.
26 Bhagat N, Grigorian RA, Tutela A, et al. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54:1–32.
27 Sebag J, Buckingham B, Charles MA, et al. Biochemical abnormalities in vitreous of humans with proliferative diabetic retinopathy. Arch Ophthalmol. 1992;110:1472–1476.
28 Jumper JM, Embabi SN, Toth CA, et al. Electron immunocytochemical analysis of posterior hyaloid associated with diabetic macular edema. Retina. 2000;20:63–68.
29 Lewis H. The role of vitrectomy in the treatment of diabetic macular edema. Am J Ophthalmol. 2001;131:123–125.
30 Praidou A, Papakonstantinou E, Androudi S, et al. Vitreous and serum levels of vascular endothelial growth factor and platelet-derived growth factor and their correlation in patients with non-proliferative diabetic retinopathy and clinically significant macula oedema. Acta Ophthalmol. 2011;89:248–254.
31 Praidou A, Klangas I, Papakonstantinou E, et al. Vitreous and serum levels of platelet-derived growth factor and their correlation in patients with proliferative diabetic retinopathy. Curr Eye Res. 2009;34:152–161.
32 Noma H, Funatsu H, Mimura T, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology. 2009;116:87–93.
33 Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005;25:111–118.
34 Brown JC, Solomon SD, Bressler SB, et al. Detection of diabetic foveal edema: contact lens biomicroscopy compared with optical coherence tomography. Arch Ophthalmol. 2004;122:330–335.
35 Staurenghi G, Invernizzi A, de Polo L, et al. Macular edema. Diagnosis and detection. Dev Ophthalmol. 2010;47:27–48.
36 Kiss CG, Barisani-Asenbauer T, Maca S, et al. Reading performance of patients with uveitis-associated cystoid macular edema. Am J Ophthalmol. 2006;142:620–624.
37 Valentincic NV, Berendschot TT, Hawlina M, et al. Effect of tinted optical filters on visual acuity and contrast sensitivity in patients with inflammatory cystoid macular edema. Retina. 2007;27:483–489.
38 Pinckers A, van Aarem A, Keunen JE. Colour vision in retinitis pigmentosa. Influence of cystoid macular edema. Int Ophthalmol. 1993;17:143–146.
39 Kiss CG, Barisani-Asenbauer T, Simader C, et al. Central visual field impairment during and following cystoid macular oedema. Br J Ophthalmol. 2008;92:84–88.
40 Nussenblatt RB, Kaufman SC, Palestine AG, et al. Macular thickening and visual acuity. Measurement in patients with cystoid macular edema. Ophthalmology. 1987;94:1134–1139.
41 Cunha-Vaz J, Coscas G. Diagnosis of macular edema. Ophthalmologica. 2010;224:S2–S7.
42 Shetty N. Cystoid macular edema. Atlas of fundus fluorescein angiography. New York: Informa Healthcare; 2004. p. 130–4
43 Tranos PG, Wickremasinghe SS, Stangos NT, et al. Macular edema. Surv Ophthalmol. 2004;49:470–490.
44 Hee MR, Puliafito CA, Duker JS, et al. Topography of diabetic macular edema with optical coherence tomography. Ophthalmology. 1998;105:360–370.
45 Abouzeid H, Wolfensberger T. Optical coherence tomography assessment of macular oedema. In: Holz RS, ed. Medical retina. Berlin: Springer; 2005:1–16.
46 Antcliff RJ, Stanford MR, Chauhan DS, et al. Comparison between optical coherence tomography and fundus fluorescein angiography for the detection of cystoid macular edema in patients with uveitis. Ophthalmology. 2000;107:593–599.
47 Kim BY, Smith SD, Kaiser PK. Optical coherence tomographic patterns of diabetic macular edema. Am J Ophthalmol. 2006;142:405–412.
48 Markomichelakis NN, Halkiadakis I, Pantelia E, et al. Patterns of macular edema in patients with uveitis: qualitative and quantitative assessment using optical coherence tomography. Ophthalmology. 2004;111:946–953.
49 Soliman W, Sander B, Jorgensen TM. Enhanced optical coherence patterns of diabetic macular oedema and their correlation with the pathophysiology. Acta Ophthalmol Scand. 2007;85:613–617.
50 Odrobina D, Michalewska Z, Michalewski J, et al. Long-term evaluation of vitreomacular traction disorder in spectral-domain optical coherence tomography. Retina. 2011;31:324–331.
51 Baskin DE. Optical coherence tomography in diabetic macular edema. Curr Opin Ophthalmol. 2010;21:172–177.
52 Koizumi H, Spaide RF, Fisher YL, et al. Three-dimensional evaluation of vitreomacular traction and epiretinal membrane using spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;145:509–517.
53 Barthelmes D, Sutter FK, Gillies MC. Differential optical densities of intraretinal spaces. Invest Ophthalmol Vis Sci. 2008;49:3529–3534.
54 Ghazi NG, Ciralsky JB, Shah SM, et al. Optical coherence tomography findings in persistent diabetic macular edema: the vitreomacular interface. Am J Ophthalmol. 2007;144:747–754.
55 Kenawy N, Wong D, Stappler T, et al. Does the presence of an epiretinal membrane alter the cleavage plane during internal limiting membrane peeling? Ophthalmology. 2010;117:320–323. e1
56 Thomas D, Bunce C, Moorman C, et al. Frequency and associations of a taut thickened posterior hyaloid, partial vitreomacular separation, and subretinal fluid in patients with diabetic macular edema. Retina. 2005;25:883–888.
57 Ophir A, Martinez MR. Epiretinal membranes and incomplete posterior vitreous detachment in diabetic macular edema using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011.
58 Ophir A, Trevino A, Martinez MR. Extrafoveal vitreous traction associated with branch retinal vein occlusion. Eur J Ophthalmol. 2010;20:733–739.
59 Weber-Krause B, Eckardt U. Incidence of posterior vitreous detachment in eyes with and without age-related macular degeneration. An ultrasonic study. Ophthalmologe. 1996;93:660–665.
60 Ondes F, Yilmaz G, Acar MA, et al. Role of the vitreous in age-related macular degeneration. Jpn J Ophthalmol. 2000;44:91–93.
61 Hayreh SS, Jonas JB. Posterior vitreous detachment: clinical correlations. Ophthalmologica. 2004;218:333–343.
62 Lambert HM. The management of subfoveal choroidal neovascular membranes and hemorrhage. Semin Ophthalmol. 2000;15:92–99.
63 Lewis H, Abrams GW, Blumenkranz MS, et al. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992;99:753–759.
64 Fung WE. Vitrectomy for chronic aphakic cystoid macular edema. Results of a national, collaborative, prospective, randomized investigation. Ophthalmology. 1985;92:1102–1111.
65 Williamson TH, Grewal J, Gupta B, et al. Measurement of PO2 during vitrectomy for central retinal vein occlusion, a pilot study. Graefes Arch Clin Exp Ophthalmol. 2009;247:1019–1023.
66 Stefansson E, Novack RL, Hatchell DL. Vitrectomy prevents retinal hypoxia in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 1990;31:284–289.
67 Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139:302–310.
68 Siegfried CJ, Shui YB, Holekamp NM, et al. Oxygen distribution in the human eye: relevance to the etiology of open-angle glaucoma after vitrectomy. Invest Ophthalmol Vis Sci. 2010;51:5731–5738.
69 Giblin FJ, Quiram PA, Leverenz VR, et al. Enzyme-induced posterior vitreous detachment in the rat produces increased lens nuclear pO2 levels. Exp Eye Res. 2009;88:286–292.
70 Quiram PA, Leverenz VR, Baker RM, et al. Microplasmin-induced posterior vitreous detachment affects vitreous oxygen levels. Retina. 2007;27:1090–1096.
71 Kadonosono K, Itoh N, Ohno S. Perifoveal microcirculation before and after vitrectomy for diabetic cystoid macular edema. Am J Ophthalmol. 2000;130:740–744.
72 Noma H, Funatsu H, Mimura T, et al. Pigment epithelium-derived factor and vascular endothelial growth factor in branch retinal vein occlusion with macular edema. Graefes Arch Clin Exp Ophthalmol. 2010;248:1559–1565.
73 Matsunaga N, Chikaraishi Y, Izuta H, et al. Role of soluble vascular endothelial growth factor receptor-1 in the vitreous in proliferative diabetic retinopathy. Ophthalmology. 2008;115:1916–1922.
74 Stefansson E. The therapeutic effects of retinal laser treatment and vitrectomy. A theory based on oxygen and vascular physiology. Acta Ophthalmol Scand. 2001;79:435–440.
75 Stefansson E. Physiology of vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2009;247:147–163.
76 Stefansson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006;51:364–380.
77 Matsunaga N, Ozeki H, Hirabayashi Y, et al. Histopathologic evaluation of the internal limiting membrane surgically excised from eyes with diabetic maculopathy. Retina. 2005;25:311–316.
78 Saravia M. Persistent diffuse diabetic macular edema. The role of the internal limiting membrane as a selective membrane: the oncotic theory. Med Hypotheses. 2011;76:858–860.
79 Hoerauf H, Bruggemann A, Muecke M, et al. Pars plana vitrectomy for diabetic macular edema. Internal limiting membrane delamination vs posterior hyaloid removal. A prospective randomized trial. Graefes Arch Clin Exp Ophthalmol. 2011;249:997–1008.
80 Gandorfer A, Rohleder M, Grosselfinger S, et al. Epiretinal pathology of diffuse diabetic macular edema associated with vitreomacular traction. Am J Ophthalmol. 2005;139:638–652.
81 Schaal S, Tezel TH, Kaplan HJ. Surgical intervention in refractory CME – role of posterior hyaloid separation and internal limiting membrane peeling. Ocul Immunol Inflamm. 2008;16:209–210.
82 Harbour JW, Smiddy WE, Flynn HW, et al. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol. 1996;121:405–413.
83 Massin P, Duguid G, Erginay A, et al. Optical coherence tomography for evaluating diabetic macular edema before and after vitrectomy. Am J Ophthalmol. 2003;135:169–177.
84 Bandello F, Battaglia Parodi M, Lanzetta P, et al. Diabetic macular edema. Dev Ophthalmol. 2010;47:73–110.
85 Diabetic Retinopathy Clinical Research Network Writing CommitteeHaller JA, Qin H, Apte RS, et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117:1087–1093. e3
86 Capone A, Jr., Panozzo G. Vitrectomy for refractory diabetic macular edema. Semin Ophthalmol. 2000;15:78–80.
87 Pendergast SD, Hassan TS, Williams GA, et al. Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloid. Am J Ophthalmol. 2000;130:178–186.
88 Stolba U, Binder S, Gruber D, et al. Vitrectomy for persistent diffuse diabetic macular edema. Am J Ophthalmol. 2005;140:295–301.
89 Stefaniotou M, Aspiotis M, Kalogeropoulos C, et al. Vitrectomy results for diffuse diabetic macular edema with and without inner limiting membrane removal. Eur J Ophthalmol. 2004;14:137–143.
90 Bahadir M, Ertan A, Mertoglu O. Visual acuity comparison of vitrectomy with and without internal limiting membrane removal in the treatment of diabetic macular edema. Int Ophthalmol. 2005;26:3–8.
91 Patel JI, Hykin PG, Schadt M, et al. Pars plana vitrectomy with and without peeling of the inner limiting membrane for diabetic macular edema. Retina. 2006;26:5–13.
92 Kumar A, Sinha S, Azad R, et al. Comparative evaluation of vitrectomy and dye-enhanced ILM peel with grid laser in diffuse diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2007;245:360–368.
93 Gentile RC, Milman T, Eliott D, et al. Taut internal limiting membrane causing diffuse diabetic macular edema after vitrectomy: clinicopathological correlation. Ophthalmologica. 2011;226:64–70.
94 Lyndon da Cruz ZJ. Surgery in the treatment of cystoid macular edema, 4th edn. In: Ryan SJ, ed. Retina. St Louis: Mosby, 2005.
95 Reichenbach A, Wurm A, Pannicke T, et al. Muller cells as players in retinal degeneration and edema. Graefes Arch Clin Exp Ophthalmol. 2007;245:627–636.
96 Zhang X, Bao S, Lai D, et al. Intravitreal triamcinolone acetonide inhibits breakdown of the blood-retinal barrier through differential regulation of VEGF-A and its receptors in early diabetic rat retinas. Diabetes. 2008;57:1026–1033.
97 Williamson T. Macular disorders. Vitreoretinal surgery. Berlin: Springer-Verlag; 2008. p. 120
98 Haritoglou C, Thaler S, Kampik A, et al. Vital dyes in vitreoretinal surgery. Current application concepts. Ophthalmologe. 2009;106:7–10.
99 Haritoglou C, Schumann RG, Kampik A, et al. Heavy brilliant blue G for internal limiting membrane staining. Retina. 2011;31:405–407.
100 Tachi N, Hashimoto Y, Ogino N. Cystotomy for diabetic cystoid macular edema. Doc Ophthalmol. 1999;97:459–463.
101 Aref AA, Scott IU. Management of macular edema secondary to central retinal vein occlusion: an evidence-based. Adv Ther. 2011;28:40–50.
102 Noma H, Funatsu H, Mimura T, et al. Visual acuity and foveal thickness after vitrectomy for macular edema. Ophthalmologica. 2010;224:367–373.
103 Leizaola-Fernandez C, Suarez-Tata L, Quiroz-Mercado H, et al. Vitrectomy with complete posterior hyaloid removal for ischemic central retinal vein occlusion: series of cases. BMC Ophthalmol. 2005;5:10.
104 Martinez MR, Ophir A. Extrafoveal traction in retinal vein occlusion using spectral domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2011;249:811–820.
105 Park DH, Kim IT. Long-term effects of vitrectomy and internal limiting membrane peeling for macular edema secondary to central retinal vein occlusion and hemiretinal vein occlusion. Retina. 2010;30:117–124.
106 DeCroos FC, Shuler RK, Jr., Stinnett S, et al. Pars plana vitrectomy, internal limiting membrane peeling, and panretinal endophotocoagulation for macular edema secondary to central retinal vein occlusion. Am J Ophthalmol. 2009;147:627–633.
107 Opremcak EM, Bruce RA, Lomeo MD, et al. Radial optic neurotomy for central retinal vein occlusion: a retrospective pilot study of 11 consecutive cases. Retina. 2001;21:408–415.
108 Opremcak EM, Rehmar AJ, Ridenour CD, et al. Radial optic neurotomy for central retinal vein occlusion: 117 consecutive cases. Retina. 2006;26:297–305.
109 Zambarakji HJ, Ghazi-Nouri S, Schadt M, et al. Vitrectomy and radial optic neurotomy for central retinal vein occlusion: effects on visual acuity and macular anatomy. Graefes Arch Clin Exp Ophthalmol. 2005;243:397–405.
110 Weis E, Gan KD, Hinz BJ, et al. A retrospective cohort study of radial optic neurotomy for severe central retinal vein occlusions. Can J Ophthalmol. 2008;43:73–78.
111 Nagpal M, Nagpal K, Bhatt C, et al. Role of early radial optic neurotomy in central retinal vein occlusion. Indian J Ophthalmol. 2005;53:115–120.
112 Binder S, Aggermann T, Brunner S. Long-term effects of radial optic neurotomy for central retinal vein occlusion consecutive interventional case series. Graefes Arch Clin Exp Ophthalmol. 2007;245:1447–1452.
113 Arevalo JF, Garcia RA, Wu L, et al. Radial optic neurotomy for central retinal vein occlusion: results of the Pan-American Collaborative Retina Study Group (PACORES). Retina. 2008;28:1044–1052.
114 Martinez-Jardon CS, Meza-de Regil A, Dalma-Weiszhausz J, et al. Radial optic neurotomy for ischaemic central vein occlusion. Br J Ophthalmol. 2005;89:558–561.
115 Aref AA, Scott IU. Management of macular edema secondary to branch retinal vein occlusion: an evidence-based update. Adv Ther. 2011;28:28–39.
116 Noma H, Funatsu H, Mimura T, et al. Visual prognosis and vitreous molecules after vitrectomy for macular edema with branch retinal vein occlusion. Clin Ophthalmol. 2011;5:223–229.
117 Yamasaki M, Noma H, Funatsu H, et al. Changes in foveal thickness after vitrectomy for macular edema with branch retinal vein occlusion and intravitreal vascular endothelial growth factor. Int Ophthalmol. 2009;29:161–167.
118 Avci R, Inan UU, Kaderli B. Evaluation of arteriovenous crossing sheathotomy for decompression of branch retinal vein occlusion. Eye (Lond). 2008;22:120–127.
119 Mason J, 3rd., Feist R, White M, Jr., et al. Sheathotomy to decompress branch retinal vein occlusion: a matched control study. Ophthalmology. 2004;111:540–545.
120 Kumagai K, Furukawa M, Ogino N, et al. Long-term outcomes of vitrectomy with or without arteriovenous sheathotomy in branch retinal vein occlusion. Retina. 2007;27:49–54.
121 Oh IK, Kim S, Oh J, et al. Long-term visual outcome of arteriovenous adventitial sheathotomy on branch retinal vein occlusion induced macular edema. Korean J Ophthalmol. 2008;22:1–5.
122 Ossewaarde-van Norel A, Rothova A. Clinical review: Update on treatment of inflammatory macular edema. Ocul Immunol Inflamm. 2011;19:75–83.
123 Tranos P, Scott R, Zambarakji H, et al. The effect of pars plana vitrectomy on cystoid macular oedema associated with chronic uveitis: a randomised, controlled pilot study. Br J Ophthalmol. 2006;90:1107–1110.
124 Aylward GW. The place of vitreoretinal surgery in the treatment of macular oedema. Doc Ophthalmol. 1999;97:433–438.
125 Radetzky S, Walter P, Fauser S, et al. Visual outcome of patients with macular edema after pars plana vitrectomy and indocyanine green-assisted peeling of the internal limiting membrane. Graefes Arch Clin Exp Ophthalmol. 2004;242:273–278.
126 Kiryu J, Kita M, Tanabe T, et al. Pars plana vitrectomy for cystoid macular edema secondary to sarcoid uveitis. Ophthalmology. 2001;108:1140–1144.
127 Androudi S, Ahmed M, Fiore T, et al. Combined pars plana vitrectomy and phacoemulsification to restore visual acuity in patients with chronic uveitis. J Cataract Refract Surg. 2005;31:472–478.
128 Wiechens B, Nolle B, Reichelt JA. Pars-plana vitrectomy in cystoid macular edema associated with intermediate uveitis. Graefes Arch Clin Exp Ophthalmol. 2001;239:474–481.
129 Dugel PU, Rao NA, Ozler S, et al. Pars plana vitrectomy for intraocular inflammation-related cystoid macular edema unresponsive to corticosteroids. A preliminary study. Ophthalmology. 1992;99:1535–1541.
130 Heiligenhaus A, Bornfeld N, Foerster MH, et al. Long-term results of pars plana vitrectomy in the management of complicated uveitis. Br J Ophthalmol. 1994;78:549–554.
131 Heiligenhaus A, Bornfeld N, Wessing A. Long-term results of pars plana vitrectomy in the management of intermediate uveitis. Curr Opin Ophthalmol. 1996;7:77–79.
132 Wiechens B, Reichelt JA, Urbat C, et al. Pars plana vitrectomy in cystoid macular edema of different forms of chronic uveitis. Ophthalmologe. 2003;100:33–43.
133 Stavrou P, Baltatzis S, Letko E, et al. Pars plana vitrectomy in patients with intermediate uveitis. Ocul Immunol Inflamm. 2001;9:141–151.
134 Gutfleisch M, Spital G, Mingels A, et al. Pars plana vitrectomy with intravitreal triamcinolone: effect on uveitic cystoid macular oedema and treatment limitations. Br J Ophthalmol. 2007;91:345–348.
135 Konstantinidis L, Wolfensberger TJ. Inflammatory macular edema. In: Gupta A, Herbort CP, Khairallah M. Uveitis text and imaging. New Delhi: Jaypee Brothers; 2009:809–822.
136 Henderson BA, Kim JY, Ament CS, et al. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg. 2007;33:1550–1558.
137 Rossetti L, Autelitano A. Cystoid macular edema following cataract surgery. Curr Opin Ophthalmol. 2000;11:65–72.
138 Ray S, D’Amico DJ. Pseudophakic cystoid macular edema. Semin Ophthalmol. 2002;17:167–180.
139 Wright PL, Wilkinson CP, Balyeat HD, et al. Angiographic cystoid macular edema after posterior chamber lens implantation. Arch Ophthalmol. 1988;106:740–744.
140 Ursell PG, Spalton DJ, Whitcup SM, et al. Cystoid macular edema after phacoemulsification: relationship to blood-aqueous barrier damage and visual acuity. J Cataract Refract Surg. 1999;25:1492–1497.
141 Fung WE. The national, prospective, randomized vitrectomy study for chronic aphakic cystoid macular edema. Progress report and comparison between the control and nonrandomized groups. Surv Ophthalmol. 1984;28:S569–S575.
142 Harbour JW, Smiddy WE, Rubsamen PE, et al. Pars plana vitrectomy for chronic pseudophakic cystoid macular edema. Am J Ophthalmol. 1995;120:302–307.
143 Pendergast SD, Margherio RR, Williams GA, et al. Vitrectomy for chronic pseudophakic cystoid macular edema. Am J Ophthalmol. 1999;128:317–323.
144 Hikichi T, Yoshida A, Trempe CL. Course of vitreomacular traction syndrome. Am J Ophthalmol. 1995;119:55–61.
145 Smiddy WE, Michels RG, Glaser BM, et al. Vitrectomy for macular traction caused by incomplete vitreous separation. Arch Ophthalmol. 1988;106:624–628.
146 Davis RP, Smiddy WE, Flynn HW, Jr., et al. Surgical management of vitreofoveal traction syndrome: optical coherence tomographic evaluation and clinical outcomes. Ophthalmic Surg Lasers Imaging. 2010;41:150–156.
147 Fine HF, Iranmanesh R, Iturralde D, et al. Outcomes of 77 consecutive cases of 23-gauge transconjunctival vitrectomy surgery for posterior segment disease. Ophthalmology. 2007;114:1197–1200.
148 Tewari A, Shah GK, Fang A. Visual outcomes with 23-gauge transconjunctival sutureless vitrectomy. Retina. 2008;28:258–262.
149 Kinoshita T, Kovacs KD, Wagley S, et al. Morphologic differences in epiretinal membranes on ocular coherence tomography as a predictive factor for surgical outcome. Retina. 2011;31:1692–1698.
150 Konstantinidis L, Berguiga M, Beknazar E, et al. Anatomic and functional outcome after 23-gauge vitrectomy, peeling, and intravitreal triamcinolone for idiopathic macular epiretinal membrane. Retina. 2009;29:1119–1127.
151 Green-Simms AE, Bakri SJ. Vitreomacular traction and age-related macular degeneration. Semin Ophthalmol. 2011;26:137–138.
152 Ikeda T, Sawa H, Koizumi K, et al. Pars plana vitrectomy for regression of choroidal neovascularization with age-related macular degeneration. Acta Ophthalmol Scand. 2000;78:460–464.
153 Roller AB, Mahajan VB, Boldt HC, et al. Effects of vitrectomy on age-related macular degeneration. Ophthalmology. 2010;117:1381–1386.
154 Garcia-Arumi J, Martinez V, Sararols L, et al. Vitreoretinal surgery for cystoid macular edema associated with retinitis pigmentosa. Ophthalmology. 2003;110:1164–1169.
155 Hagiwara A, Yamamoto S, Ogata K, et al. Macular abnormalities in patients with retinitis pigmentosa: prevalence on OCT examination and outcomes of vitreoretinal surgery. Acta Ophthalmol. 2011;89:e122–e125.
156 Lopes de Faria JM, Jalkh AE, Trempe CL, et al. Diabetic macular edema: risk factors and concomitants. Acta Ophthalmol Scand. 1999;77:170–175.
157 Hikichi T, Fujio N, Akiba J, et al. Association between the short-term natural history of diabetic macular edema and the vitreomacular relationship in type II diabetes mellitus. Ophthalmology. 1997;104:473–478.
158 Stefansson E. Microplasmin-induced posterior vitreous detachment affects vitreous oxygen levels. Retina. 2008;28:1175–1176. author reply 1176
159 Gandorfer A, Ulbig M, Kampik A. Plasmin-assisted vitrectomy eliminates cortical vitreous remnants. Eye (Lond). 2002;16:95–97.
160 Gandorfer A, Priglinger S, Schebitz K, et al. Vitreoretinal morphology of plasmin-treated human eyes. Am J Ophthalmol. 2002;133:156–159.
161 Sakuma T, Mizota A, Inoue J, et al. Intravitreal injection of autologous plasmin enzyme for macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2010;150:876–882.
162 Udaondo P, Diaz-Llopis M, Garcia-Delpech S, et al. Intravitreal plasmin without vitrectomy for macular edema secondary to branch retinal vein occlusion. Arch Ophthalmol. 2011;129:283–287.
163 Sakuma T, Tanaka M, Inoue J, et al. Use of autologous plasmin during vitrectomy for diabetic maculopathy. Eur J Ophthalmol. 2006;16:138–140.
164 Rheaume MA, Vavvas D. Pharmacologic vitreolysis. Semin Ophthalmol. 2010;25:295–302.
165 de Smet MD, Gandorfer A, Stalmans P, et al. Microplasmin intravitreal administration in patients with vitreomacular traction scheduled for vitrectomy: the MIVI I trial. Ophthalmology. 2009;116:1349–1355.
166 Stalmans P, Delaey C, de Smet MD, et al. Intravitreal injection of microplasmin for treatment of vitreomacular adhesion: results of a prospective, randomized, sham-controlled phase II trial (the MIVI-IIT trial). Retina. 2010;30:1122–1127.
167 Benz MS, Packo KH, Gonzalez V, et al. A placebo-controlled trial of microplasmin intravitreous injection to facilitate posterior vitreous detachment before vitrectomy. Ophthalmology. 2010;117:791–797.
168 Dugel PU, Group M-TS. A single injection of microplasmic for the treatment of symptomatic vitreomacular adhesion (sVMA): results of the Phase III MIVI-TRUST Program [abstract]. Invest Ophthalmol Vis Sci (suppl.). 2011;52:6628.