Chapter 79B Cystic hepatobiliary neoplasia
Overview
The vast majority of nonparasitic cystic lesions of the liver are simple cysts. They are unilocular, do not communicate with the biliary tree, have a serous content, and although the epithelium does divide to account for volume expansion, they are not considered a tumor and have no malignant potential. Furthermore, they do not tend to recur following partial resection. These lesions are described in detail elsewhere in this textbook (see Chapter 69A). Although primary or secondary solid hepatic malignancies may become cystic as a result of tumor necrosis, it is extremely rare for them to be totally cystic, although this has been described in case reports for embryonic liver sarcoma, malignant fibrous histiocytoma, or metastases from a variety of primaries, most including neuroendocrine tumors or even colorectal primaries (Yang et al, 2009; Kim et al, 2009; Ding et al, 2006; Sugawara et al, 2000).
Primarily cystic hepatobiliary neoplasms that constitute another group of cystic tumors are extremely rare. They are of biliary origin and comprise both benign (with malignant potential) and malignant tumors that have initially been given the names of cystadenoma and cystadenocarcinoma (Ishak & Kamal, 1994); however, increasing evidence, in particular from Asian authors, suggests that biliary cystic neoplasia comprises a wider group of entities that resemble those described in the pancreas as being either mucinous cystic neoplasms (MCNs) or intraductal papillary mucinous neoplasm (IPMNs) (Zen et al, 2006a, 2006b). The main difference relates to the presence or absence of an ovarian stroma (OS) and whether a luminal communication exists between the tumor and the bile ducts.
No international consensus has been reached, and detailed characteristics of these tumors are not always described in the literature, casting some confusion on previous descriptions and reports of cystadenoma. Although the same confusion has been observed for cystic tumors of the pancreas, the clinical and pathologic differentiations of these two tumors are now accepted worldwide, now that a consensus on the definition of pancreatic MCNs has been reached (Kloppel et al, 2000; Suzuki et al, 2004). A second problem in analyzing the literature is that because of the rarity of the tumor, the number of large series is very limited, and most references are case reports.
Definition
The initial definitions of cystadenoma by Edmondson in 1958 and by Wheeler and Edmondson in 1985 were strict and included three distinctive features: the lesion should be 1) multilocular (composed of multiloculated cysts) 2) lined by a columnar epithelium, and 3) accompanied by a densely cellular ovarian-like stroma.
Hepatobiliary Cystadenoma with Ovarian Stroma
The most classic primary cystic tumors of the liver are hepatobiliary cystadenoma. Being of biliary origin, these tumors may occur anywhere along the biliary tree, including the common hepatic duct, cystic duct, or gallbladder; but the most frequent location, found in 83% to 94% of the patients, is the liver (Devaney et al, 1994; Buetow et al, 1995). These tumors are well known because of their inherent risk of malignant transformation, common to all adenomatous lesions, although this risk has not been precisely quantified.
Incidence
Cystadenomas are exceedingly rare. No accurate incidence estimates are available, but the largest series included 52 patients (Devaney et al, 1994), and most studies since then have comprised fewer than 10 patients; in the early 2000s, it was estimated that fewer than 200 patients had been reported (Duchini, 2001). It is frequently quoted that intrahepatic cystadenomas constitute fewer than 5% of cystic lesions of the liver based on an old report (Ishak et al, 1977), but this figure is likely overestimated; it has since become obvious that the prevalence of simple cysts of the liver in the adult population is higher than previously thought.
Pathology (See Chapter 78)
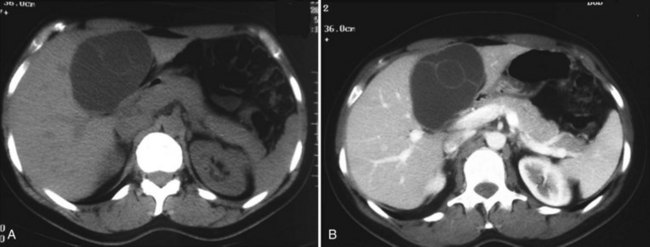
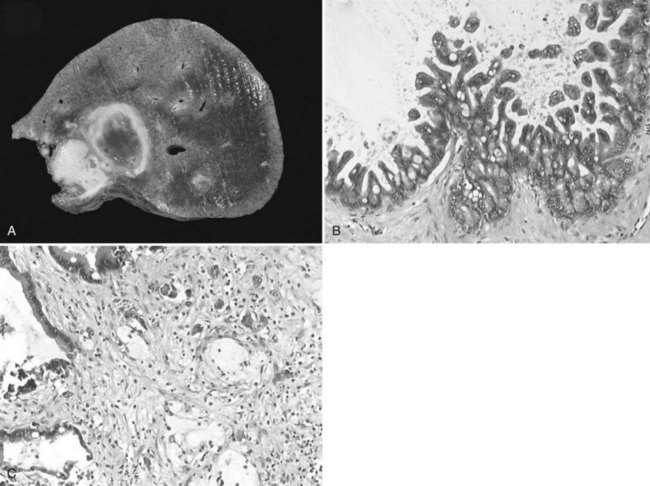
Cystadenomas are almost always solitary, and they may range in size from less than 1 to 40 cm; they are generally large, with a mean diameter of 15 cm (Buetow et al, 1995). Cystadenomas have been reported to develop equally in the right and left liver or to involve both lobes (approximately 33% each) (Devaney et al, 1994; Akwari et al, 1990); however, a striking feature in our experience, as well as in recent series, is the very high proportion of tumors occurring in the left paramedian section (segment IV) (Daniels et al, 2006; Lewin et al, 2006; Seo et al, 2010). Pictures of cystadenoma provided in the literature also show tumors in this location almost exclusively; an example is shown in Figure 79B.1.
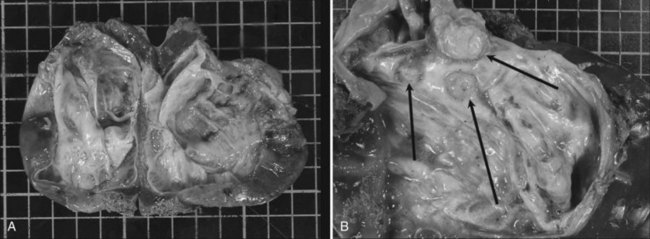
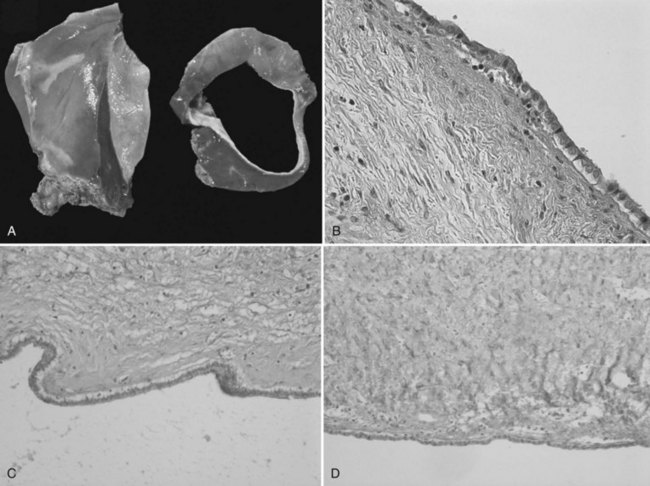
Cystadenomas are grossly lobulated and multiloculated and usually contain clear to mucinous fluid (Buetow et al, 1995). Hemorrhagic fluid may be present (Lewin et al, 2006), but this is very rare and should raise the suspicion of malignancy (Buetow et al, 1995). A bilious content is similarly very unusual (Buetow et al, 1995), as lack of communication with the biliary tree is a distinctive feature; however, fistulization of cystadenoma in the biliary tree has been reported (Hanazaki, 1996; Yi et al, 2009), the same as for pancreatic mucinous cystadenoma, which may explain the bilious content.
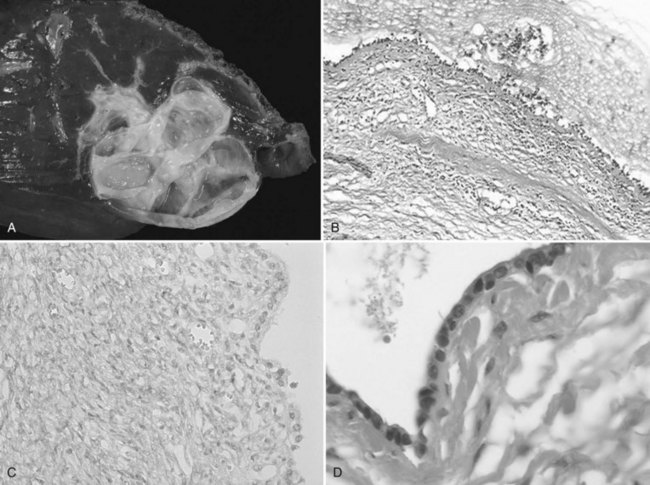
The internal lining is generally smooth but may contain microscopic, or more rarely macroscopic, polypoid lesions projecting into the lumen (Fig. 79B.2). This internal lining has three distinct layers: 1) an epithelial layer, 2) a mesenchymal stroma, and 3) an outer layer of collagen connective tissue that separates it from the adjacent parenchyma (Fig. 79B.3).
The epithelial lining, as for pancreatic cystadenoma, consists of glandular, nonciliated cells arranged in a single, flat row with occasional papillary or polypoid projections, pseudostratifications, and cryptlike invaginations. In two thirds of the patients, the epithelium is columnar, but in one third, it consists of a combination of columnar and cuboidal epithelium. The epithelial lining may show denuded areas with chronic inflammation and hemorrhage. The columnar cells contain histologically benign appearing, basally located nuclei and intracellular mucin. The lining epithelium occasionally shows papillary infoldings, and mild epithelial atypia with a slight increase in the size of the basally located nuclei has been reported to be at least focally present in most patients. More severe dysplasia consisting of a multilayer appearance of hyperchromatic cells with loss of polarity appears much less frequently. Foci of intestinal metaplasia are also rare, seen in the form of numerous goblet cells. Atypia and metaplasia are suggestive of a high risk of malignant transformation (Devaney et al, 1994).
Phenotype has been further characterized in a limited number of patients. The epithelium shows strong and diffuse cytoplasmin staining with antibodies against CEA and CA19-9, whereas CA-125 staining is focal or absent (Subramony et al, 1993; Logroño et al, 2002). It also stains positive for CK-7, CK-19, CK-8, and CK-18 (Abdul Al et al, 2007), which supports a biliary origin, and it is negative for inhibin-α (Owono et al, 2001; Abdul Al et al, 2007), vimentin (Owono et al, 2001), and estrogen or progesterone receptors (Daniels, 2006), and CD56 is only expressed focally (Gütgemann et al, 2006).
The stroma consists of a compact arrangement of bland, spindle-shaped cells with round to oval nuclei. This appearance is reminiscent of OS, hence the name given to these tumors; but it has also been found to resemble the primitive mesenchymal elements associated with the developing biliary system in the fetus. This stroma may be abundant in some patients and focal in others, but mitotic activity is not prominent. It expresses estrogen and progesterone receptors, as does the stroma of pancreatic cystadenoma (Daniels et al, 2006; Weihing et al, 1997); α-inhibin, a gonadal protein that has a more limited expression in the sex cord–stromal tissue, is also expressed in both hepatic and pancreatic mucinous cystadenoma (Ridder et al, 1998; Lam et al, 2008) and in sex cord–stromal tumors. They are immunoreactive with α–smooth muscle actin, vimentin, but also desmin. There is no immune reaction with CEA, CA19-9, or CA-125 (Devaney et al, 1994; Logroño et al, 2002).
Despite the similarities between hepatic and pancreatic mucinous cystadenomas, few studies have compared the phenotypes of both lesions. One reported denser and more abundant stroma, more intense estrogen or progesterone receptors, and more α-inhibin in hepatic than in pancreatic cystadenomas, but the significance of this is unclear (Lam et al, 2008).
Risk Factors and Origin
There are no clearly identified risk factors apart from gender, as cystadenomas with OS are exclusively observed in women. However, a history of previous oral contraceptive use does not appear to be a distinctive feature (Devaney et al, 1994). Several hypotheses have been raised based on some pathologic features of the tumor, most notably the presence of an ovarian-like stroma or the occasional presence of eosinophilic or endocrine cells. It is still unclear whether cystadenomas are of congenital origin or are acquired neoplastic lesions.
The resemblance is striking among cystadenomas with OS located in the liver, pancreas, retroperitoneum, and ovary (Bakker et al, 2007; Nelson et al, 1988; Turbiner et al, 2007). The particular OS common to these locations contains estrogen and progesterone receptors and α-inhibin (Lam et al, 2008). These similarities suggest a common pathway of tumor development, although simultaneous occurrences of hepatic cystadenoma with pancreatic (Brachet et al, 2007) or ovarian cystadenoma (Skopelitou et al, 1996) are exceedingly rare.
One hypothesis is that during embryonic development, ectopic ovarian cells would have migrated to the liver or pancreas, released hormones and growth factors, and caused endodermally derived epithelium to proliferate and finally to form a tumor (Zamboni et al, 1999). The right and left primordial gonads are indeed located directly under the diaphragm prior to their descent, at the level of the dorsocranial side of the liver and the tail of the pancreas respectively. Furthermore, the cells covering the gonads show an activated morphology in contrast to the peritoneal epithelium elsewhere (Erdogan et al, 2006), which suggests that they have the ability to detach from the gonadal surface, cross the peritoneal cleft, and attach to the peritoneal surface of nearby organs. This hypothesis would explain the predominance of cystadenoma in the body or tail of the pancreas, rather than in the head, as well as the apparent predominance of liver cystadenoma in segment IV. Splenogonadal fusion has also been documented (Duncan & Barraza, 2005), and demonstration of endocrine cells in about 50% of cystadenomas is a distinctive feature (Terada et al, 1997) compatible with the hypothesis of these tumors developing from peribiliary glands.
The OS resembles the primitive embryonic mesenchyma of embryonic gallbladder and large bile ducts that contribute to the connective tissue surrounding the bile ducts (Subramony et al, 1993), and it has therefore also been suggested that these tumors could derive from ectopic embryonal tissue destined to form the gallbladder (Subramony et al, 1993) or from ectopic embryonic rests of primitive foregut sequestered within the liver (Wheeler & Edmondson, 1985).
Presentation
Intrahepatic biliary cystadenoma with an OS is exclusively observed in women. Age at diagnosis is highly variable, between 1 (Beasley et al, 1986) and 70 years, but it peaks in the fourth or early fifth decade. In most patients, the tumor is discovered during the workup of epigastric or right upper quadrant pain or vague abdominal complaints that include abdominal discomfort or swelling. Palpation of an abdominal mass that moves freely with respiration is also a classic circumstance of diagnosis. As the tumor may grow to a considerable size, a gradual increase in abdominal girth and/or compression of the stomach or duodenum may also occur; however, because of the slow progression of the neoplasm, the onset of symptoms tends to be insidious and to have evolved for a prolonged period of time before treatment (Akwari et al, 1990; Thomas et al, 2005). This may explain why most reported cystadenomas are large tumors. With the increasingly liberal use of imaging to investigate even the most minor symptoms, cystadenomas are often discovered incidentally, and it is possible that a significant proportion are not identified or are mistaken for simple cysts (Hara et al, 2001).
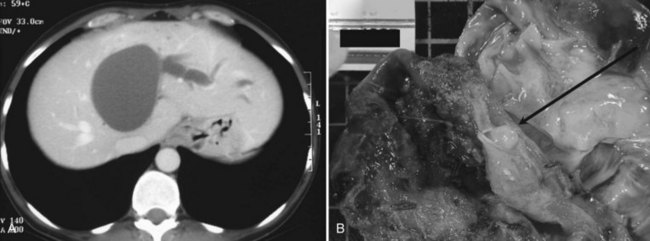
More acute episodes of pain have been reported in up to 35% of the patients, and most of these are related to biliary obstruction (Akwari et al, 1990; Erdogan et al, 2010). This is biologically witnessed by concomitant cytolysis or cholestasis. Jaundice or itching (Siriwardana & Pathirana, 2009) may also be present, whereas cholangitis is rare. Typically, such episodes are transient, and any jaundice tends to resolve spontaneously, which is compatible with the migration of mucous material or tumor embolus from the cyst into the bile duct (Fig. 79B.4). Such tumor protrusion in the bile duct lumen has been reported even for small cystadenomas less than 4 cm in diameter (Erdogan et al, 2006). An alternative mechanism of obstruction is simple compression of the biliary confluence by the cystadenoma. Other causes of acute presentation have included tumor rupture (Lempinen et al, 2005), superinfection, bleeding (Lewis et al, 1988), and caval compression (Catto et al, 1999), but these are rare.
Diagnosis
Differential Diagnosis
Apart from the other cystic tumors addressed in this chapter, most notably cystadenocarcinoma, the main differential diagnosis of cystadenoma is simple cysts that have become atypical on imaging studies as a result of intracystic bleeding (Kitajima et al, 2003) or previous sclerotherapy (Takayasu et al, 2003; see Chapter 69A). The risk of mistaking a cystadenoma for a benign cyst is that this lesion may be treated by simple unroofing, which is an inappropriate treatment associated with a high risk of recurrence (see Management below); to mistake a simple or atypical cyst for a cystadenoma is to perform an occasionally risky and unwarranted resection, because simple cysts do not require complete resection. Considering the very high incidence of simple cysts, the latter is probably much more common, and it has been shown that a cystadenoma-like lesion is almost as likely to be a simple cyst as to be a cystadenoma (Shimada et al, 1998; Teoh et al, 2006; Koea et al, 2008; Seo et al, 2010). Clinical history is totally unreliable in the diagnosis of hemorrhagic cysts, as intracystic hemorrhage can occur in the absence of any symptoms (Kitajima et al, 2003), and the diagnosis mainly relies on imaging.
Alternative differential diagnosis must include nonsuppurative granulomatous infection of a simple cyst (Kawashita et al, 2006), hepatic abscess (Yamamoto et al, 2009), echinococcal cysts (Ramacciato et al, 2006), mesenchymal hamartoma (Mori et al, 2008), ciliated hepatic foregut cysts, intrahepatic lymphangioma, cystic hemangioma, cystic forms of hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma, mucin-producing metastases from thyroid or colon carcinoma and cystic metastases of the ovary, of melanoma, and of kidney and neuroendocrine tumors (Del Poggio & Buonocore, 2008). They have also been occasionally confused with pancreatic cysts (Logroño et al, 2002).
Imaging
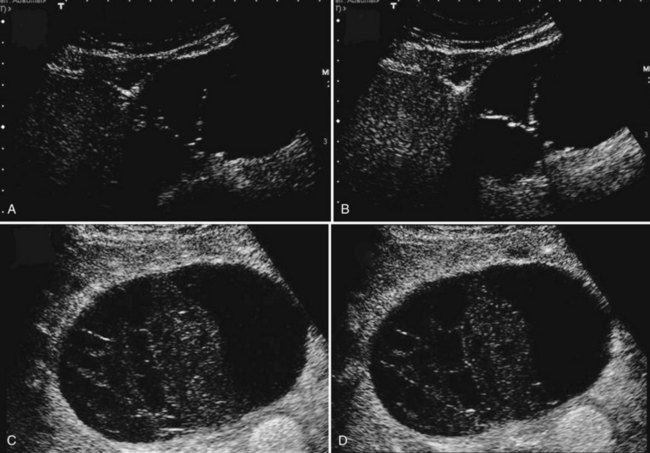
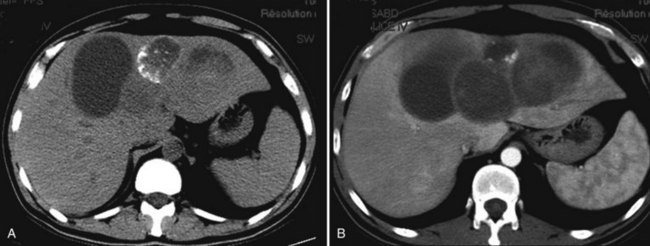
The characteristic ultrasonographic (US) finding for biliary cystadenoma is an anechoic mass with echogenic internal septations (Fig. 79B.5). Papillary projections into the cystic space may also be seen. On CT scans, the lesions appear as multiloculated hypodense masses with a well-defined wall. The content demonstrates fluid attenuation because of the presence of mucin, blood, or bile. Fine septal calcifications may occasionally be seen, and both the wall and the septations enhance following administration of contrast material (see Fig. 79B.1).
With magnetic resonance imaging (MRI), cyastadenoma is typically seen as a fluid-containing multilocular cyst with homogeneous high signal intensity on T2-weighted images and homogeneous low or isosignal intensity on T1-weighted images (Lewin et al, 2006). These signals may vary depending on the content of the cystic fluid. Mucinous fluid will appear with an isosignal, serous fluid with a hyposignal, and hemorrhagic fluid with an hyperintense signal that can only be seen in the lower part of a fluid-fluid level. Thin internal septal structures separate fluid-filled spaces. These, along with the wall of the lesion, are contrast enhanced (Fig. 79B.6).
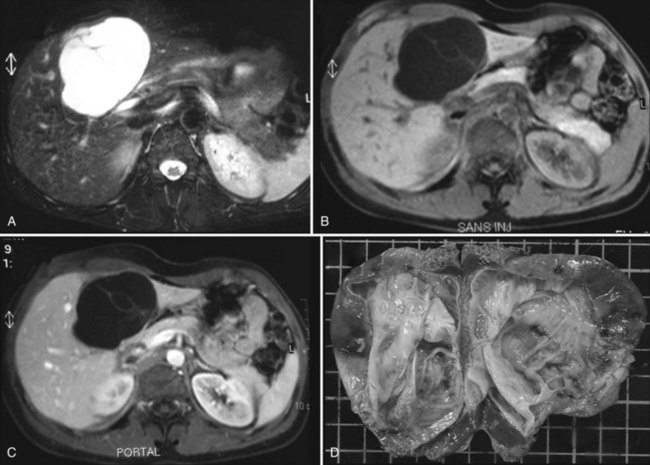
FIGURE 79B.6 Magnetic resonance imaging of a cystadenoma with ovarian-like stroma (A–C) and macroscopic view (D). Specimen is the same as in Figure 79B.1. A, The tumor is hyperintense on T2-weighted images. B, Septa are visible on T1-weighted images. C, Tumors enhance after injection of gadolinium.
Contrast US is a promising exploration that allows analysis of the vascularization of the cyst wall and septa, but it has been used infrequently. An intracystic structure of complicated simple cysts corresponds to clots and is not enhanced (Akiyama et al, 2008). Although the periphery of the cyst may show enhancement, this corresponds to compressed adjacent liver, and this should be differentiated from enhancement of the cyst wall of cystadenoma (see Fig. 79B.5).
As for other cystic lesions, CT scan is less reliable and accurate than US or MRI. Cystadenoma in particular may wrongly appear unilocular on CT scan, whereas US (Korobkin et al, 1989) and MRI (Lewin et al, 2006; Williams et al, 2001) will visualize the internal septa. Two additional features besides multilocularity may help in the differential diagnosis of simple cysts: they are frequently multiple (Vuillemin-Bodaghi et al, 1997), whereas cystadenomas are single lesions; and although biliary dilation has been considered uncommon, a mild enlargement upstream of the tumor is probably more frequent than previously thought—using higher resolution imaging, enlargement was observed in one to two thirds of the patients in recent series (Lim et al, 2007; Seo et al, 2010). Along the same line, an increase in serum alkaline phosphatase levels has recently been shown to be a feature distinctive from simple cysts (Seo et al, 2010).
Cytology and Tumor Markers
Cytology
Aspiration cytology is either nonspecific or it shows chronic inflammatory exudates with large numbers of neutrophils, lymphocytes, and macrophages. A few groups of nonatypical bland cuboidal to columnar epithelial cells, occasionally arranged in papillary clusters, may be seen (Logroño et al, 2002).
Tumor Markers
Unlike for the pancreas, measurement of tumor markers in hepatobiliary cystic lesions has been infrequently performed, and results are confusing. Early case reports have pointed to a link between cystadenoma and CA19-9 by showing its expression by epithelial lining and by revealing increased intracystic and serum concentrations (Thomas et al, 1992; Lee et al, 1996). However, CA19-9 is also expressed by normal biliary epithelial cells, and very high levels have occasionally been measured in the bile of patients with various nontumoral conditions (Horsmans et al, 1997; Shimada et al, 1998; Ker et al, 1991). It is also expressed by the epithelium lining simple cysts of the liver (Park et al, 2006) and in the cystic fluid of simple cysts (Park et al, 2006; Choi et al, 2010). An increased serum concentration of CA19-9 in these patients has even been reported and can occasionally be very high, probably as a result of communication of the cyst with the circulation. Three studies have shown that cyst concentration (Choi et al, 2010; Seo et al, 2010) and serum concentration of CA19-9 (Park et al, 2006; Choi et al, 2010) are comparable in patients with cystadenoma and simple cysts. As will be discussed below, CA19-9 is also inaccurate in differentiating cystadenoma from cystadenocarcinoma (Horsmans et al, 1997).
It has also been suggested that a CEA concentration greater than 600 ng/mL in the supernatant of cystic liver lesions could accurately differentiate cystadenoma or cystadenocarcinoma from benign, nonmucinous cysts (Pinto & Kaye, 1989). The epithelial lining of cystadenoma indeed stains positive for CEA (Logroño et al, 2002); however, this has not been confirmed in subsequent studies, in which concentrations in cystadenoma and simple cysts were comparable (Shimada et al, 1998; Choi et al, 2010; Seo et al, 2010).
Complications
Cystadenoma with an OS is considered a precancerous lesion, as will be detailed below. Malignancy arises in most cases from the epithelium lining (cystadenocarcinoma), but sarcomatous transformation of the OS has also been reported (Akwari et al, 1990). No specific feature has been clearly linked to a higher risk of malignancy, apart from the presence of epithelial atypia (dysplasia) or intestinal metaplasia. The odds ratio for their association with malignancy has been calculated to be 8 (95% confidence interval [CI], 2.4 to 27.0) and 2.4 (95% CI, 1.2 to 3.5), respectively (Devaney et al, 1994).
Management
Cystadenomas require complete excision to prevent recurrence of symptoms and malignant transformation. Evidence that partial excision, aspiration, and external or internal drainage are ineffective is that recurrence has been noted very early on (Ishak et al, 1977; Wheeler & Edmondson, 1985; Lewis et al, 1988; Devaney et al, 1994) and that 40% to 50% of the patients culled by tertiary referral centers had undergone such previous treatments prior to referral (Hansman et al, 2001; Thomas et al, 2005; Vogt et al, 2005; Daniels et al, 2006; Delis et al, 2008). Updated evidence is shown in Table 79B.1. Recurrence occurs at a mean of 21 months but may be delayed up to 4 years (Vogt et al, 2005). This may explain why recurrence following incomplete resection has not been systematically observed, as most studies only have short-term follow-up (Barabino et al, 2004; Lewis et al, 1988; Manouras et al, 2008). Development of a cystadenoma following partial resection of a cystadenocarcinoma has also been documented (Woods, 1981; Devine et al, 1985; Lei & Howard, 1992; Akwari et al, 1990).
Fenestration of the cystadenoma with fulguration of the internal cystic lining has been attempted with occasionally adequate long-term success (Thomas et al, 2005), but experience is too limited to recommend this strategy. Although not documented, it is likely that some small cystadenomas mistaken for simple cysts have also been successfully treated with percutaneous ethanol injection. Ethanol is indeed effective in the management of simple cysts, hydatid disease, and hepatocellular carcinoma; however, this cannot be currently recommended, especially as most cystadenomas are large, and ethanol injection induces morphologic changes that prevent accurate follow-up of the cyst wall. In any case, the clinician should not rely on intraoperative frozen-section biopsies to differentiate simple cysts and cystadenoma, as these can be falsely negative even when repeated (Vogt et al, 2005; Manouras et al, 2008).
Surgery can consist of partial hepatectomy or enucleation, as there is a dissection plane between the cystadenoma and the adjacent parenchyma. Care should be taken when the cyst lies in segment IV so as not to injure the biliary bifurcation. IOC should be performed to document a potential biliary communication and exclude the presence of mucus or tumor material in the bile duct. Treatment of extrahepatic cystadenoma should include bile duct resection and bilioenteric reconstruction rather than simple enucleation from the bile duct wall. As for other tumors, laparoscopic resection can be an option for trained surgeons (Koffron et al, 2004; Veroux et al, 2005). A single case of recurrence following complete resection has been reported (Wheeler & Edmondson 1985).
Hepatobiliary Cystadenoma without Ovarian Stroma
Some cystadenomas have been reported that do not have OS. Before considering this diagnosis, several samples of the specimen should be analyzed, as in some patients, the OS may be only focally present (Devaney et al, 1994). Although cystadenoma with and without an OS have very similar clinical and morphologic features, a distinctive characteristic is that the former only develops in women, whereas the latter may develop in both genders. It is unclear, however, whether cystadenoma without an OS is a discrete entity, or whether it comprises a heterogeneous group of cystic tumors. For this reason, some pathologists would not retain the diagnosis of cystadenoma if the OS were lacking.
Incidence
The largest surgical series of cystadenoma, with or without an OS, published over the past 15 years have, as a rule, included a higher number of tumors with OS than without in a proportion of 5 to 10 to 1 (Table 79B.2; Buetow et al, 1995; Devaney et al, 1994; Erdogan et al, 2010; Regev et al, 2001); however, others have reported equal numbers (Owono et al, 2001; Vogt et al, 2005), and in one study, virtually all cystadenoma lacked OS (Koffron et al, 2004). This discordance is puzzling but probably speaks to the lack of strict pathologic definition of this entity. It is likely that a number of tumors previously diagnosed as cystadenoma without an OS were in fact simple cysts of the liver that had a multilocular appearance as a result of intracystic bleeding, or because several cysts were juxtaposed. They might also have included cystic forms of IPMN-B, which is addressed separately in this chapter.
Pathology
Cystadenomas without OS are typically multilocular and have a mucinous content (Fig. 79B.7). The epithelium is phenotypically similar to the classic cystadenoma (see above). Devaney and colleagues (1994) did not observe areas of atypia or dysplasia in this variant, but others have (Owono et al, 2001). These tumors also tend to more frequently communicate with the biliary tree than cystadenoma with an OS. In particular, a higher proportion of patients are seen with bilious fluid within their cyst (Buetow et al, 1995); however, data are scarce, and ERCP and IOC have not been routinely performed. If confirmed this feature would strengthen the parallel between some, at least cystadenoma without an OS and IPMN-B.
The phenotype of the epithelium lining cystadenoma without an OS has been very inconsistently characterized but has been reported to stain positive for CK-7, CK-8, CK-18, CK-19, MUC1, MUC5, and MUC6 but not for CEA, CA 19-9, or MUC3 (Kazama et al, 2005; Qu et al, 2009). This phenotype is close to that of large bile ducts (see Chapter 78).
Presentation and Diagnosis
Unlike those with OS, cystadenomas without OS have been described in both genders, and the sex ratio is actually close to 1 based on the rare available data (Owono et al, 2001; Buetow et al, 1995; Devaney et al, 1994). Otherwise, age (peaking between 40 and 60 years), symptoms, and size of the cyst are comparable (Buetow et al, 1995). Imaging studies have up to now been unable to differentiate between cystadenoma with and without an OS (Buetow et al, 1995; Owono et al, 2001; Lewin et al, 2006). In the cystic fluid, CA19-9 appears always increased, and CEA levels are also increased in most patients (Dixon et al, 2001; Koffron et al, 2004).
Natural History and Management
Very little data are available on the natural history of cystadenoma without an OS. Case reports suggest that it may undergo morphologic changes with a progressive increase in diameter, mural thickening, development of papillary nodules, and subsequent malignant transformation (Akiyoshi et al, 2003; Fukunaga et al, 2008). These changes have been observed over a prolonged period of time (10 years). Whether cystadenoma with and without an OS have the same malignant potential is unclear. It is accepted, however, that both require complete resection. IOC should be performed to document a potential biliary communication. If present, the diagnosis of IPMN-B should be considered and ruled out by frozen-section analysis.
Cystadenocarcinoma
Definition and Incidence
Cystadenocarcinomas are very rare primary cystic tumors that do not primarily communicate with the biliary tree; they are lined by a tubulopapillary malignant epithelium (Fig. 79B.8). Single case reports exist in which malignancy was an adenosquamous carcinoma (Devaney et al, 1994) or it developed in the stroma (Akwari et al, 1990). Cystadenocarcinoma was first described in 1943 by Willis, but by 2000, only 100 had been reported in the literature (Lauffer et al, 1998; Bardin et al, 2004), and they were considered to account for only 0.4% of hepatic malignancies (Takayasu et al, 1988). Most have been found in the liver, with only anecdotal reports of locations in the extrahepatic bile duct and the gallbladder in particular (Waldmann et al, 2006). As for cystadenomas, these are usually stratified in two groups depending on the presence or absence of an OS (Ishak et al, 1977; Lee et al, 2009); however, some pathologists are reluctant to consider the diagnosis of cystadenocarcinoma when the OS is lacking, as these may correspond to cystic variants of mass-forming intrahepatic cholangiocarcinoma or to malignant IPMN-B (Zen et al, 2006). The distinction is not purely academic, as these tumors have distinct modes of spread and prognoses that could influence management. This should be taken into account when analyzing the literature, and even when reading this section.
Epidemiology and Presentation
Overall female to male ratio is 2 : 1, but cystadenocarcinomas with OS are exclusively observed in women, whereas those without OS are twice as frequent in men (Bardin et al, 2004). Although the age range is wide—from 18 to 88 years—it peaks in the late 50s and 60s (Lauffer et al, 1998; Seo et al, 2010), approximately 15 to 20 years later than cystadenomas. This difference is in especially obvious for those lesions with OS (Akwari et al, 1990; Buetow et al, 1995; Devaney et al, 1994; Wheeler & Edmondson, 1985).
Although usually nonspecific, symptoms are virtually always present and are initially rather indolent. They are common to cystadenoma and include abdominal swelling, discomfort, pain, or palpation of an abdominal mass. Biliary obstruction with jaundice is reported in 20% of the patients, related to biliary compression or migration of mucus or tumor material. Acute symptoms may otherwise occur as a result of intracystic hemorrhage or tumor rupture. As a rule, diagnosis is delayed, and the mean time interval between first symptoms and treatment is 29 months (Lauffer et al, 1998); one case was reported of a cystadenocarcinoma being discovered 1 year after bone metastasis (Berjian et al, 1981).
Origin and the Cystadenoma-Cystadenocarcinoma Sequence
Cystadenocarcinomas have no identified risk factors, and their origin is unknown, but it is usually assumed that they are the malignant counterpart of cystadenoma. The proliferating epithelial cells indeed resemble those observed in cystadenoma, both express a biliary-type phenotype, and typical areas of cystadenoma (benign columnar epithelium) coexist with malignant papillary epithelium in most patients (up to 90%; Lauffer et al, 1998). Longitudinal morphologic studies of single patients whose cystic lesions were followed for more than a decade have also shown the progressive development of typical cystadenocarcinoma (Akiyoshi et al, 2003; Kubota et al, 2003). As a whole, cystadenocarcinomas also tend to be larger and to be discovered 5 to 10 years later than cystadenoma (Lauffer et al, 1998). The transition from one to the other is thought to occur through dysplasia or metaplasia that may be observed within cystadenoma (see Pathology above). This applies to both intrahepatic (Ishak et al, 1977; Akiyoshi et al, 2003) and extrahepatic cystadenoma (Davies et al, 1995; Coulter & Baxter, 1989) as well to cystadenomas both with and without OS (Ishibashi et al, 2007).
The risk of malignant transformation of cystadenoma into cystadenocarcinoma is unknown, as the tumor is rare, and limited data are available. A rough estimate can be made by comparing the relative proportion of “cystadenoma” and cystadenocarcinoma in published series (see Table 79B.2). Among resected patients, 15% of tumors with OS were malignant. Although biased, this estimate is consistent with that reported for choledochal cysts, also premalignant lesions. In contrast, the proportion of cystadenocarcinoma is apparently much higher among tumors without OS or an undefined stroma (see Table 79B.2). The reason for this discrepancy is unclear, which does not necessarily imply that cystadenomas without OS are at greater risk of malignant transformation. These could also include intrahepatic cholangiocarcinoma mistaken for cystadenocarcinoma or malignant IPMN-B (papillary cholangiocarcinoma; see Cystic Variants section below).
The time necessary for a cystadenoma, with or without OS, to undergo malignant change and subsequently become invasive in the cyst wall, the adjacent parenchyma, or neighboring organs is unknown. Case reports have shown that a lesion may remain morphologically unchanged for years, but that once mural nodules appear, these may progress within a few months (Akiyoshi et al, 2003). The propensity of, and time necessary for, the malignant epithelial proliferation to become invasive is also unknown. It has been reported that in approximately one third (Devaney et al, 1994) to one half (Lauffer et al, 1998; Nakajima et al, 1992) of the patients who had been operated, the tumor was confined to the cyst, which may explain the very high survival rates reported after complete resection. However, parenchymal extension appearing as satellite nodules or perineural and lymphatic invasion for tumors located close to a glissonian pedicle does exist, and distant metastases are present at the time of diagnosis in 20% of the patients (Lauffer et al, 1998).
Diagnosis
Gross Morphology and Imaging
Cystadenocarcinoma shares most of the morphologic and radiologic features of cystadenoma. The lesions are solitary (Seo et al, 2010) and usually large, with an average diameter of 12 cm but growing up to 40 cm (Lauffer et al, 1998); they can also be small, and cystadenocarcinoma of less than 5 cm have been reported, including one of 2 cm (Lauffer et al, 1998; Williams et al, 2001; Lewin et al, 2006). Therefore a small tumor is not evidence that a lesion is benign. Macroscopically, they more frequently have a hemorrhagic or bilious content (Buetow et al, 1995), and hemorrhage or nodularity within the cyst wall is evident (Buetow et al, 1995; Lewin et al, 2006; Seo et al, 2010).
On imaging, cystadenocarcinomas are also more frequently associated with larger septa; intrahepatic cysts debris; bile duct dilation (Seo et al, 2010); enhancement of mural nodules on CT scan, MRI, or contrast US (Korobkin et al, 1989; Pojchamarnwiputh et al, 2008; Ren et al, 2010); and coarse calcifications along the wall or septa (Buetow et al, 1995; Korobkin et al, 1989; Fig. 79B.9). However, all of these features can also be observed in nonmalignant cystadenoma (Fukunaga et al, 2008; Choi et al, 2010), and differentiating both tumors on macroscopy or imaging alone has up to now proven very difficult (Buetow et al, 1995; Hai et al, 2003). Case reports exist of fluorodeoxyglucose positron emission tomography (FDG-PET) CT positivity (Takanami et al, 2009), but the accuracy of this exploration has not been evaluated.
Biology
An increase in serum tumor markers CEA and CA19-9 is very rare and, when present, is usually mild (Lauffer et al, 1998; Hai et al, 2003). This has also been described, in particular for CA19-9, in cystadenoma both with and without an OS (Hai et al, 2003; Horsmans et al, 1996; Kim et al, 1998; Thomas et al, 1992), and serum levels greater than 50,000 have been reported (Scoggins et al, 2004).
Cyst Content
Cystic fluid analysis does not provide discriminant information. The content is usually hemorrhagic, bilious, or mixed. Although in earlier case reports, fine needle aspiration cytology was found to be useful (Wee et al, 1993; Iemoto et al, 1983), more recent studies have shown that malignant or even atypical cells are very infrequently retrieved (Hai et al, 2003; Seo et al, 2010).
Although inconsistently measured, CA19-9 and CEA concentrations are usually increased in the cystic fluid; but this increase is comparable to that observed in cystadenomas, and CA19-9 is present at very high concentrations in most patients (Hai et al, 2003; Seo et al, 2010), although it has been reported to be normal in a patient whose cystadenocarcinoma did not stain positive for this tumor marker (Horsmans et al, 1997). Although some patients may have CEA levels higher than those observed in cystadenoma or simple cysts, most have normal or mildly elevated concentrations that are therefore not discriminant (Seo et al, 2010). This is consistent with the observation that the epithelium of both cystadenoma and cystadenocarcinoma stain positive for CEA (Tomioka et al, 1986).
Cyst sampling should in any case be performed with caution, as cystadenocarcinomas—and biliary tumors in general—have a high propensity for peritoneal seeding that has been observed following this procedure (Iemoto et al, 1981; Nakajima et al, 1992). Pleural effusion, presumed to be related to aspiration cytology, has also been reported (Hai et al, 2003).
Treatment
Surgery should consist of complete resection, and IOC should be considered, as some of these tumors may communicate with the biliary tree. As tumor extension cannot be reliably assessed on preoperative imaging, it is advisable to perform a formal resection with a wide margin rather than an enucleation. Resection of the biliary confluence followed by a hepaticojejunostomy may be required if the tumor lies close to the biliary confluence. Every effort should be made not to open the cyst, as peritoneal carcinomatosis has been observed after accidental cyst rupture despite negative cytology for tumor cells in cystic fluid (Kosuge et al, 1992). Partial resection of cystadenocarcinoma with an OS is associated with a dismal prognosis.
Following complete resection, the prognosis appears better overall than that of patients with hepatocellular carcinoma (see Chapter 80) or cholangiocarcinoma (see Chapter 50A). Cystadenocarcinomas associated with OS tend to have a more favorable course than those not associated with OS (Devaney et al, 1994; Asahara et al, 1999) with less frequent parenchymal, vascular, and lymphatic invasion (Hai et al, 2003). It is unclear, however, whether this relates directly to the presence of an OS; to gender (cystadenocarcinomas with an OS are exclusively observed in women), as shown for other malignancies; or to tumor invasiveness.
Cystic Variants of Intraductal Papillary Mucinous Neoplasm of the Bile Duct
IPMN-B is a relatively new, distinct pathology characterized by intraluminal papillary proliferation in the bile ducts that produce mucin (Nakanuma et al, 2002). They almost exclusively occur in the intrahepatic bile ducts or the left or right bile ducts, do not have an OS, and probably correspond to entities previously described using the terms biliary papilloma, mucin-producing ICC, intraductal mucosal-spreading mucin-producing peripheral cholangiocarcinoma, intraductal growth-type peripheral cholangiocarcinoma, and intraductal variant of peripheral cholangiocarcinoma. These tumors have striking clinical, histologic, and phenotypic similarities with IPMN of the pancreas (IPMN-P; Kim et al, 2000; Zen et al, 2006a; Fukushima & Mukia, 2000; Kloppel & Kosmahl, 2006), and both may occasionally coexist in the same patient (Ishida et al, 2002; Zalinski et al, 2007; see Chapters 56 and 57).
They may macroscopically be divided into two types: the ductectatic type evidences diffusely dilated bile ducts; the cystic type appears as a large cystic mass (Sakamoto et al, 1997). A parallel has therefore been drawn between branch duct cystic IPMN-P and cystic variants of IPMN-B that are thought to develop from small bile ducts. Because these variants of IPMN-B have both cystic (mucin) and solid (papillary projection) compartments, they may resemble cystadenoma or cystadenocarcinoma (Terada & Taniguchi, 2004; Aoki et al, 2005; Lim et al, 2007) and might have been mistaken for them in the past.
Definition and Etiology
IPMN-B, including its cystic variants, has mainly been described in the East, initially in patients with hepatolithiasis or Clonorchiasis infection (Chen et al, 2001). It develops in the bile duct lumen and is characterized by innumerable frondlike papillary infoldings, which consist of columnar epithelial cells surrounding slender fibrovascular stalks supported by connective tissue from the lamina propria. Further stratification is based on the presence and severity of atypia of the biliary epithelia: type 1 is low-grade dysplasia, type 2 is high-grade dysplasia, type 3 is in situ and microinvasive adenocarcinoma, and type 4 is invasive adenocarcinoma. Types 3 and 4 are associated with papillary tumor tissue and mucobilia in the large, dilated ducts containing stones; whereas this is frequently absent in type 1 lesions, intraductal spreading is virtually constant. This description was somewhat confusing, as these tumors had been observed in a specific context of chronic biliary inflammation; however, it has subsequently been described in the absence of such conditions (Shibahara et al, 2004; Zen et al, 2006a; Hayashi et al, 2008), although etiology remains unknown.
Pathology
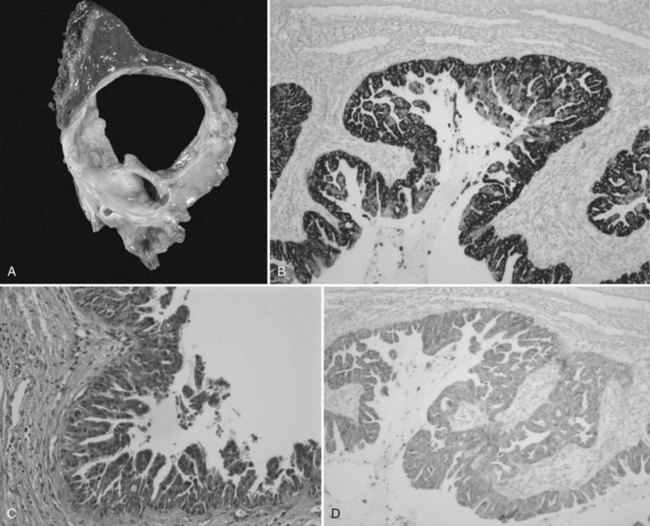
Cystic IPMN-B morphologically is multilocular and frequently contains mucinous fluid (Fig. 79B.10). It may also occasionally be hemorrhagic (Zen et al, 2006a). Papillary mural nodules within an otherwise smooth or finely granular wall are virtually constant except in totally benign lesions (Paik et al, 2008). In situ or invasive adenocarcinomas are particularly prevalent in IPMN-B and are present overall in up to 60% to 70% of the patients, and even higher rates have been found in the cystic variant (Yeh et al, 2006; Paik et al, 2008). This may either be a mucinous carcinoma, associated with profuse mucous secretion; a tubular adenocarcinoma, invading the bile duct wall with abundant fibrous stroma at the base of the papillary lesions and commonly associated with vascular, lymphatic, and perineural invasion; or a combination of these (Zen et al, 2006a, 2006b).
As for IPMN-P, the lining epithelia of IPMN-B has been classified into a pancreatobiliary type, intestinal type, gastric type, and oncocytic type (Zen et al, 2006b). More simply it can be categorized as a columnar type, which resembles the intestinal type of IPMN-P, and a cuboidal type, which resembles the pancreatobiliary type or oncocytic variant of IPMN-P (Shibahara et al, 2004). In one study, both were observed in the cystic form of IPMN-B, whereas only the columnar type was observed in the ductectatic form (see Chapter 78).
A parallel has been established between the cell type and the incidence of malignant changes. Cuboidal pancreatobiliary oncocytic-type epithelium is more frequently—actually, almost always—associated with the presence of carcinoma than the columnar type, but this is more frequently a superficial (in situ) tumor than an invasive one (Shibahara et al, 2004; Zen et al, 2006a).
The epithelium stains positive for CK-7 (biliary phenotype) and occasionally and inconsistently for CK-20 (intestinal phenotype). CA19-9 is observed diffusely, and CEA is restricted to areas of high-grade dysplasia and sometimes carcinoma (Zen et al, 2006a, 2006b).
Although MUC5AC is expressed in virtually all patients (gastric phenotype), MUC2 (intestinal phenotype) is more frequent in the columnar type, and MUC6 is more often seen in the cuboidal type (Shibahara et al, 2004; Zen et al, 2006a, 2006b). MUC1 is in contrast not expressed except in some invasive areas of tubular cholangiocarcinomas, in which MUC2 expression may disappear (Shibahara et al, 2004; Zen et al, 2006a, 2006b).
Diagnosis
Cystic variants of IPMN-B develop almost equally in both genders and peak in the sixth decade, which is 10 to 20 years older than for cystadenoma with an OS (Shibahara et al, 2004; Zen et al, 2006a). Many patients are asymptomatic, with their tumor discovered during a general health examination, but epigastralgia, jaundice, and cholangitis may occur. Cystic variants cannot be differentiated from cystadenoma or cystadenocarcinoma by their diameter, which may range between 2 and 17 cm (Lim et al, 2007; Yamashita et al, 2007), nor can it be discerned by thickness of septa or presence of calcifications (Lim et al, 2007); however, cystic variants are more frequently associated with prominent or large (>1 cm) mural nodules and bile duct dilation distal to the tumor. The latter is due to the issue of mucus from the cyst in the bile ducts. Communication with the biliary lumen is indeed a key feature (Kazama et al, 2005), but CT and MRI are insufficient to show the luminal communication (Lim et al, 2007; Yamashita et al, 2007), probably because it is too narrow. Preoperative ERCP or IOC is more reliable; in contrast, macroscopic examination of the specimen is inaccurate (Zen et al, 2006a).
Complications
The two main complications of cystic variants of IPMN-B relate to the migration of mucus or tumor material in the bile ducts and to malignant transformation. Mucobilia may result in transient symptoms of biliary obstruction, and biliary obstruction by mucus has occasionally resulted in bile duct rupture (Lim et al, 2004). Malignant transformation is particularly frequent, although the stage of malignancy is less advanced than for other biliary tumors (Zen et al, 2006b). As most of these tumors show malignant changes at the time of diagnosis, they are likely slow growing.
Management and Prognosis
Cystic IPMN-B requires resection, and an IOC should be performed to document the biliary communication and rule out the presence of mucus in the bile duct. As IPMN-B may spread superficially along the lumen of the bile ducts or be present in adjacent ducts, simple enucleation of the cyst may be insufficient. Formal hepatectomy should be performed, occasionally with extrahepatic bile duct resection and lymph node sampling. Even in patients with associated carcinoma, the prognosis is better than after resection of intrahepatic or hilar cholangiocarcinoma (Shibahara et al, 2004; Zen et al, 2006a, 2006b; Paik et al, 2008). Five-year survival is 61% and may be as high as 80% for those patients with the cuboidal cell type. Survival following resection of malignant IPMN-B is adversely impacted by the presence of the columnar cell type (Shibahara et al, 2004) and MUC1 expression (Higashi et al, 1999; Shibahara et al, 2004).
Abdul-Al HM, Makhlouf HR, Goodman ZD. Expression of estrogen and progesterone receptors and inhibin-alpha in hepatobiliary cystadenoma: an immunohistochemical study. Virchows Arch. 2007;450:691-697.
Akiyama T, et al. Levovist ultrasonography imaging in intracystic hemorrhage of simple liver cyst. World J Gastroenterol. 2008;14:805-807.
Akiyoshi T, et al. Cystadenocarcinoma of the liver without mesenchymal stroma: possible progression from a benign cystic lesion suspected by follow-up imagings. J Gastroenterol. 2003;38:588-592.
Akwari OE, et al. Hepatobiliary cystadenoma with mesenchymal stroma. Ann Surg. 1990;211:18-27.
Ammori BJ, et al. Surgical strategy for cystic diseases of the liver in a Western hepatobiliary center. World J Surg. 2002;26:462-469.
Aoki S, et al. Intrahepatic biliary papilloma morphologically similar to biliary cystadenoma. J Gastroenterol Hepatol. 2005;20:321-324.
Asahara T, et al. A case of biliary cystadenocarcinoma of the liver. Hiroshima J Med Sci. 1999;48:45-48.
Bakker RF, et al. Primary retroperitoneal mucinous cystadenoma with sarcoma-like mural nodule: a case report and review of the literature. Virchows Arch. 2007;451:853-857.
Barabino M, et al. Hepatobiliary cystadenoma: diagnostic uncertainty. HPB (Oxford). 2004;6:52-54.
Bardin RL, et al. Oncocytic biliary cystadenocarcinoma: a case report and review of the literature. Arch Pathol Lab Med. 2004;128:e25-e28.
Beasley SW, et al. Biliary cystadenoma in a young child. Pediatr Surg Int. 1986;1:72-73.
Berjian RA, et al. Biliary cystadenocarcinoma: report of a case presenting with osseous metastasis and a review of the literature. J Surg Oncol. 1981;18:305-316.
Brachet D, et al. The simultaneous occurrence of mucinous cystadenomas in liver and pancreas. Eur J Gastroenterol Hepatol. 2007;19:801-804.
Buetow PC, et al. Biliary cystadenoma and cystadenocarcinoma: clinical-imaging-pathologic correlations with emphasis on the importance of ovarian stroma. Radiology. 1995;196:805-810.
Catto JW, Madan M, Alexander DJ. Hepatobiliary cystadenoma presenting with intermittent inferior vena caval obstruction. J Hepatobiliary Pancreat Surg. 1999;6:324-326.
Chen TC, et al. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001;34:651-658.
Choi HK, et al. Differential diagnosis for intrahepatic biliary cystadenoma and hepatic simple cyst: significance of cystic fluid analysis and radiologic findings. J Clin Gastroenterol. 2010;44:289-293.
Coulter GN, Baxter JN. Cystadenoma of the common bile duct with malignant transformation. Aust N Z J Surg. 1989;59:291-294.
Daniels JA, et al. Biliary cystadenomas: hormone receptor expression and clinical management. Dig Dis Sci. 2006;51:623-628.
Davies W, Chow M, Nagorney D. Extrahepatic biliary cystadenomas and cystadenocarcinoma: report of seven cases and review of the literature. Ann Surg. 1995;222:619-625.
Delis SG, et al. Intrahepatic biliary cystadenoma: a need for radical resection. Eur J Gastroenterol Hepatol. 2008;20:10-14.
Del Poggio P, Buonocore M. Cystic tumors of the liver: a practical approach. World J Gastroenterol. 2008;14:3616-3620.
Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma: a light microscopic and immunohistochemical study of 70 patients. Am J Surg Pathol. 1994;18:1078-1091.
Devine P, Ucci AA. Biliary cystadenocarcinoma arising in a congenital cyst. Hum Pathol. 1985;16:92-94.
Ding GH, et al. Primary hepatic malignant fibrous histiocytoma mimicking cystadenocarcinoma: a case report. Hepatobiliary Pancreat Dis Int. 2006;5:620-623.
Dixon E, et al. Cystadenoma of the liver without mesenchymal stroma in a female following hormonal therapy for acne. HPB (Oxford). 2001;3:183-186.
Duchini A. Hepatic cystadenomas. eMedicine Journal. Available at http://www.emedicine.com/med/topic492.htm, 2001.
Duncan WLJr, Barraza MA. Splenogonadal fusion: a case report and review of literature. J Pediatr Surg. 2005;40:e5-e7.
Edmondson HA. Tumors of the liver and intrahepatic bile ducts, ed 1. Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology. 1958.
Erdogan D, et al. Cystadenomas with ovarian stroma in liver and pancreas: an evolving concept. Dig Surg. 2006;23:186-191.
Erdogan D, et al. Mucinous cystadenomas in liver: management and origin. Dig Surg. 2010;27:19-23.
Fiamingo P, et al. Incidental cystadenoma after laparoscopic treatment of hepatic cysts: which strategy? Surg Laparosc Endosc Percutan Tech. 2004;14:282-284.
Fukunaga N, et al. Hepatobiliary cystadenoma exhibiting morphologic changes from simple hepatic cyst shown by 11-year follow up imagings. World J Surg Oncol. 2008;6:129.
Fukushima N, Mukai K. Differential diagnosis between intraductal papillary-mucinous tumors and mucinous cystic tumors of the pancreas. Int J Surg Pathol. 2000;8:271-278.
Gadzijev E, Ferlan-Marolt V, Grkman J. Hepatobiliary cystadenomas and cystadenocarcinoma: report of five cases. HPB Surg. 1996;9:83-92.
Gütgemann I, et al. CD56 expression aids in the differential diagnosis of cholangiocarcinomas and benign cholangiocellular lesions. Virchows Arch. 2006;448:407-411.
Hai S, et al. Surgical management of cystic hepatic neoplasms. J Gastroenterol. 2003;38:759-764.
Hanazaki K. Intrahepatic biliary cystadenoma demonstrated by intraoperative cholangiography. Hepatogastroenterology. 1996;43:1024-1028.
Hansman MF, et al. Management and long-term follow-up of hepatic cysts. Am J Surg. 2001;181:404-410.
Hara H, et al. Hepatobiliary cystadenoma combined with multiple liver cysts: report of a case. Surg Today. 2001;31:651-654.
Hayashi J, et al. A case of asymptomatic intraductal papillary neoplasm of the bile duct without hepatolithiasis. World J Gastroenterol. 2008;14:1625-1629.
Higashi M, et al. Expression of MUC1 and MUC2 mucin antigens in intrahepatic bile duct tumors: its relationship with a new morphological classification of cholangiocarcinoma. Hepatology. 1999;30:1347-1355.
Horsmans Y, et al. Serum and cystic fluid CA 19-9 determinations as a diagnostic help in liver cysts of uncertain nature. Liver. 1996;16:255-257.
Horsmans Y, et al. Hepatobiliary cystadenocarcinoma without ovarian stroma and normal CA 19-9 levels: unusually prolonged evolution. Dig Dis Sci. 1997;42:1406-1408.
Iemoto Y, Kondo Y, Fukamachi S. Biliary cystadenocarcinoma with peritoneal carcinomatosis. Cancer. 1981;48:1664-1667.
Iemoto Y, et al. Biliary cystadenocarcinoma diagnosed by liver biopsy performed under ultrasonographic guidance. Gastroenterology. 1983;84:399-403.
Ishak KG, Kamal G. Epithelial tumors. In Ishak KG, et al (eds): Histological Typing of Tumours of the Liver, ed 2. Berlin: Springer-Verlag; 1994.
Ishak KG, et al. Biliary cystadenoma and cystadenocarcinoma: report of 14 cases and review of the literature. Cancer. 1977;39:322-338.
Ishibashi Y, et al. Invasive biliary cystic tumor without ovarian-like stroma. Pathol Int. 2007;57:794-798.
Ishida M, et al. Intraductal mucinous tumors occurring simultaneously in the liver and pancreas. J Gastroenterol. 2002;37:1073-1078.
Kawashita Y, et al. Destructive granuloma derived from a liver cyst: a case report. World J Gastroenterol. 2006;12:1798-1801.
Kazama S, et al. Giant intrahepatic biliary cystadenoma in a male: a case report, immunohistopathological analysis, and review of the literature. Dig Dis Sci. 2005;50:1384-1389.
Ker CG, et al. Assessment of serum and bile levels of CA19-9 and CA125 in cholangitis and bile duct carcinoma. J Gastroenterol Hepatol. 1991;6:505-508.
Kim HJ, et al. Mucin-hypersecreting bile duct tumor characterized by a striking homology with an intraductal papillary mucinous tumor (IPMT) of the pancreas. Endoscopy. 2000;32:389-393.
Kim HS, et al. Undifferentiated pleomorphic sarcoma of the liver presenting as a unilocular cyst. Hepatobiliary Pancreat Dis Int. 2009;8:541-543.
Kim K, et al. Biliary cystadenoma of the liver. J Hepatobiliary Pancreat Surg. 1998;5:348-352.
Kitajima Y, et al. Intracystic hemorrhage of a simple liver cyst mimicking a biliary cystadenoma. J Gastroenterol. 2003;38:190-193.
Kloppel G, Kosmahl M. Is the intraductal papillary mucinous neoplasia of the biliary tract a counterpart of pancreatic papillary mucinous neoplasm? J Hepatol. 2006;44:249-250.
Kloppel G, et al. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000.
Koea JB. Cystic lesions of the liver: 6 years of surgical management in New Zealand. N Z Med J. 2008;121:61-69.
Koffron A, et al. Intrahepatic biliary cystadenoma: role of cyst fluid analysis and surgical management in the laparoscopic era. Surgery. 2004;136:926-936.
Korobkin M, et al. Biliary cystadenoma and cystadenocarcinoma: CT and sonographic findings. AJR Am J Roentgenol. 1989;153:507-511.
Kosuge T, et al. Surgical management of biliary cystadenocarcinoma. Hepatogastroenterology. 1992;39:417-419.
Kubota E, et al. Biliary cystadenocarcinoma followed up as benign cystadenoma for 10 years. J Gastroenterol. 2003;38:278-282.
Lam MM, et al. Ovarian-type stroma in hepatobiliary cystadenomas and pancreatic mucinous cystic neoplasms: an immunohistochemical study. Am J Clin Pathol. 2008;129:211-218.
Lauffer JM, et al. Biliary cystadenocarcinoma of the liver: the need for complete resection. Eur J Cancer. 1998;34:1845-1851.
Lee JH, et al. Mucinous biliary cystadenoma with mesenchymal stroma: expressions of CA 19-9 and carcinoembryonic antigen in serum and cystic fluid. J Gastroenterol. 1996;31:732-736.
Lee JH, et al. Biliary cystadenoma and cystadenocarcinoma of the liver: 10 cases of a single center experience. Hepatogastroenterology. 2009;56:844-849.
Lei S, Howard JM. Biliary cystadenocarcinoma arising from benign cystadenoma. Arch Surg. 1992;127:1478.
Lempinen M, et al. Spontaneous rupture of a hepatic cystadenoma and cystadenocarcinoma: report of two cases. J Hepatobiliary Pancreat Surg. 2005;12:409-414.
Lewin M, et al. Assessment of MRI and MRCP in diagnosis of biliary cystadenoma and cystadenocarcinoma. Eur Radiol. 2006;16:407-413.
Lewis WD, et al. Surgical treatment of biliary cystadenoma: a report of 15 cases. Arch Surg. 1988;123:563-568.
Lim JH, et al. Intraductal papillary mucinous tumor of the bile ducts. Radiographics. 2004;24:53-66.
Lim JH, et al. Biliary cystic intraductal papillary mucinous tumor and cystadenoma/cystadenocarcinoma: differentiation by CT. Abdom Imaging. 2007;32:644-651.
Logroño R, Rampy BA, Adegboyega PA. Fine needle aspiration cytology of hepatobiliary cystadenoma with mesenchymal stroma. Cancer. 2002;96:37-42.
Manouras A, et al. Laparoscopic fenestration of multiple giant biliary mucinous cystadenomas of the liver. World J Gastroenterol. 2008;14:4257-4259.
Mori R, et al. Giant mesenchymal hamartoma of the liver in an adult. J Hepatobiliary Pancreat Surg. 2008;15:667-669.
Nakajima T, et al. Biliary cystadenocarcinoma of the liver: a clinicopathologic and histochemical evaluation of nine cases. Cancer. 1992;69:2426-2432.
Nakanuma Y, et al. Biliary papillary neoplasm of the liver. Histol Histopathol. 2002;27:851-861.
Nelson H, Benjamin B, Alberty R. Primary retroperitoneal mucinous cystadenocarcinoma. Cancer. 1988;61:2117-2121.
Oh TH, et al. Thirteen cases of intrahepatic biliary cystadenoma and cystadenocarcinoma: a single center experience. Korean J Gastroenterol. 2006;47:379-385.
Owono P, et al. Hepatobiliary cystic tumors: clinical, radiological and histopathological study of 7 cases. Gastroenterol Clin Biol. 2001;25:414-421.
Paik KY, et al. Intraductal papillary neoplasm of the bile ducts: the clinical features and surgical outcome of 25 cases. J Surg Oncol. 2008;97:508-512.
Park KH, et al. Significances of serum level and immunohistochemical stain of CA19-9 in simple hepatic cysts and intrahepatic biliary cystic neoplasms. Korean J Gastroenterol. 2006;47:52-58.
Pinto MM, Kaye AD. Fine needle aspiration of cystic liver lesions: cytologic examination and carcinoembryonic antigen assay of cyst contents. Acta Cytol. 1989;33:852-856.
Pojchamarnwiputh S, et al. Computed tomography of biliary cystadenoma and biliary cystadenocarcinoma. Singapore Med J. 2008;49:392-396.
Qu ZW, et al. Giant hepatobiliary cystadenoma in a male with obvious convex papillate. World J Gastroenterol. 2009;15:1906-1909.
Ramacciato G, et al. Emergency laparotomy for misdiagnosed biliary cystadenoma originating from caudate lobe. World J Surg Oncol. 2006;4:76.
Regev A, et al. Large cystic lesions of the liver in adults: a 15-year experience in a tertiary center. J Am Coll Surg. 2001;193:36-45.
Ren XL, et al. Biliary cystadenocarcinoma diagnosed with real-time contrast-enhanced ultrasonography: report of a case with diagnostic features. World J Gastroenterol. 2010;16:131-135.
Ridder GJ, et al. Ovarian-like stroma in an invasive mucinous cystadenocarcinoma of the pancreas positive for inhibin: a hint concerning its possible histogenesis. Virchows Arch. 1998;432:451-454.
Sakamoto E, et al. Clinicopathological studies of mucin-producing cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 1997;4:157-162.
Scoggins CR, et al. Supra-elevated CA 19-9 in a benign hepatic cyst adenoma. HPB (Oxford). 2004;6:43-44.
Seo JK, et al. Appropriate diagnosis of biliary cystic tumors: comparison with atypical hepatic simple cysts. Eur J Gastroenterol Hepatol. 2010;22:989-996.
Shibahara H, et al. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2004;28:327-338.
Shimada M, et al. Treatment strategy for patients with cystic lesions mimicking a liver tumor: a recent 10-year surgical experience in Japan. Arch Surg. 1998;133:643-646.
Siriwardana PN, Pathirana A. Episodic biliary obstruction due to an intrahepatic biliary cystadenoma: a case report. J Med Case Reports. 2009;3:9032.
Skopelitou A, Hadjiyannakis M. Hepatobiliary cystadenoma with mesenchymal stroma (CMS) in association with unilateral mucinous cystadenoma of the ovary (MCO): case report and review of the literature. Eur J Gynaecol Oncol. 1996;17:234-240.
Subramony C, Herrera GA, Turbat-Herrera EA. Hepatobiliary cystadenoma: a study of five cases with reference to histogenesis. Arch Pathol Lab Med. 1993;117:1036-1042.
Sugawara Y, et al. Cystic liver metastases from colorectal cancer. J Surg Oncol. 2000;74:148-152.
Suzuki Y, et al. Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor: cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas. 2004;28:241-246.
Takanami K, et al. F-18 FDG PET/CT scan in biliary cystadenocarcinoma. Clin Nucl Med. 2009;34:470-472.
Takayasu K, et al. Imaging diagnosis of bile duct cystadenocarcinoma. Cancer. 1988;61:941-946.
Takayasu K, et al. Late complication of a large simple cyst of the liver mimicking cystadenocarcinoma after sclerotherapy. AJR Am J Roentgenol. 2003;181:464-466.
Tan YM, et al. Does laparoscopic fenestration provide long-term alleviation for symptomatic cystic disease of the liver? Aust N Z J Surg. 2002;72:743-745.
Teoh AY, et al. Biliary cystadenoma and other complicated cystic lesions of the liver: diagnostic and therapeutic challenges. World J Surg. 2006;30:1560-1566.
Terada T, Taniguchi M. Intraductal oncocytic papillary neoplasm of the liver. Pathol Int. 2004;54:116-123.
Terada T, et al. Endocrine cells in hepatobiliary cystadenomas and cystadenocarcinomas. Virchows Arch. 1997;430:37-40.
Thomas JA, et al. Elevated serum CA 19-9 levels in hepatobiliary cystadenoma with mesenchymal stroma. Cancer. 1992;70:1841-1846.
Thomas KT, et al. Effective treatment of biliary cystadenoma. Ann Surg. 2005;241:769-773.
Tomioka T, et al. Cystadenoma and cystadenocarcinoma of the liver: localization of carcinoembryonic antigen. Surg Today. 1986;16:62-67.
Tsiftsis D, et al. Primary intrahepatic biliary cystadenomatous tumors. J Surg Oncol. 1997;64:341-346.
Turbiner J, et al. Cystic nephroma and mixed epithelial and stromal tumor of kidney: a detailed clinicopathologic analysis of 34 cases and proposal for renal epithelial and stromal tumor (REST) as a unifying term. Am J Surg Pathol. 2007;31:489-500.
Veroux M, et al. Cystadenoma and laparoscopic surgery for hepatic cystic disease: a need for laparotomy? Surg Endosc. 2005;19:1077-1081.
Vogt DP, Henderson JM, Chmielewski E. Cystadenoma and cystadenocarcinoma of the liver: a single center experience. J Am Coll Surg. 2005;200:727-733.
Vuillemin-Bodaghi V, et al. Imaging of atypical cystic lesions of the liver: review of 26 surgical cases. Gastroenterol Clin Biol. 1997;21:394-399.
Waldmann J, et al. Cystadenocarcinoma of the gallbladder. J Hepatobiliary Pancreat Surg. 2006;13:594-599.
Wee A, et al. Biliary cystadenocarcinoma arising in a cystadenoma: report of a case diagnosed by fine needle aspiration cytology. Acta Cytol. 1993;37:966-970.
Weihing RR, et al. Hepatobiliary and pancreatic mucinous cystadenocarcinomas with mesenchymal stroma: analysis of estrogen receptors/progesterone receptors and expression of tumor-associated antigens. Mod Pathol. 1997;10:372-379.
Wheeler DA, Edmondson HA. Cystadenoma with mesenchymal stroma (CMS) in the liver and bile ducts: a clinicopathologic study of 17 cases, 4 with malignant change. Cancer. 1985;56:1434-1445.
Williams DM, Vitellas KM, Sheafor D. Biliary cystadenocarcinoma: seven year follow-up and the role of MRI and MRCP. Magn Reson Imaging. 2001;19:1203-1208.
Willis RA. Carcinoma arising in congenital cysts of the liver. J Pathol Bacteriol. 1943;50:492-495.
Woods GL. Biliary cystadenocarcinoma: case report of hepatic malignancy originating in benign cystadenoma. Cancer. 1981;47:2936-2940.
Yamamoto T, et al. Huge liver abscess radiologically mimicking cystadenocarcinoma. Osaka City Med J. 2009;55:53-59.
Yamashita Y, et al. Mucin-hypersecreting bile duct neoplasm characterized by clinicopathological resemblance to intraductal papillary mucinous neoplasm (IPMN) of the pancreas. World J Surg Oncol. 2007;5:98.
Yang L, et al. Clinical features and spiral computed tomography analysis of undifferentiated embryonic liver sarcoma in adults. J Dig Dis. 2009;10:305-309.
Yeh TS, et al. Cholangiographic spectrum of intraductal papillary mucinous neoplasm of the bile ducts. Ann Surg. 2006;244:248-253.
Yi B, et al. A special growth manner of intrahepatic biliary cystadenoma. World J Gastroenterol. 2009;15:6134-6136.
Zacherl J, et al. Long-term results after laparoscopic unroofing of solitary symptomatic congenital liver cysts. Surg Endosc. 2000;14:59-62.
Zalinski S, et al. Intraductal papillary mucinous tumors of both biliary and pancreatic ducts. J Hepatol. 2007;46:978-979.
Zamboni G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410-422.
Zen Y, et al. Biliary cystic tumors with bile duct communication: a cystic variant of intraductal papillary neoplasm of the bile duct. Mod Pathol. 2006;19:1243-1254.
Zen Y, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333-1343.