Chapter 8 Current Surgical Management of High-Grade Gliomas
The malignant glioma has been the neurosurgeon’s eternal hydra, continually growing despite treatment. The category “high-grade glioma” (HGG) is heterogeneous, including mainly anaplastic astrocytoma (AA), glioblastoma multiforme (GBM), gliosarcoma, and anaplastic oligodendroglioma (AO). The incidence of new primary brain tumors in the United States is estimated to be 18 per 100,000, resulting in approximately 40,000 new primary brain tumors per year, 22,000 of which are high grade.1 A total of 12,920 deaths in 2008 were attributed to primary malignant brain tumors.2 Despite continually renewed efforts at treating HGGs, the odds of significant long-term survival have remained poor and stable for the past three decades with 2% to 4% of patients with GBM surviving to the 5-year point.3
Preoperative Workup
Magnetic resonance imaging (MRI) with and without gadolinium is essential for preliminary differential diagnosis, decision for surgery, and operative planning. Thallium SPECT scan, PET scan, or MR spectroscopy may help in determination of high grade versus low grade tumor, although none of these studies is definitive, and differentiation between HGGs and metastases is difficult.4 These studies are more helpful in cases with previous surgery and radiation in determining recurrent tumor vs. radiation effect, especially MR spectroscopy and MR perfusion. For selected patients who cannot undergo MRI (e.g., patients with a cardiac pacemaker), CT with and without contrast provides similar, albeit less detailed information. CT perfusion may be an aid in better defining the tumor from cerebral edema as well as help with the potential for post-treatment radiation effects.
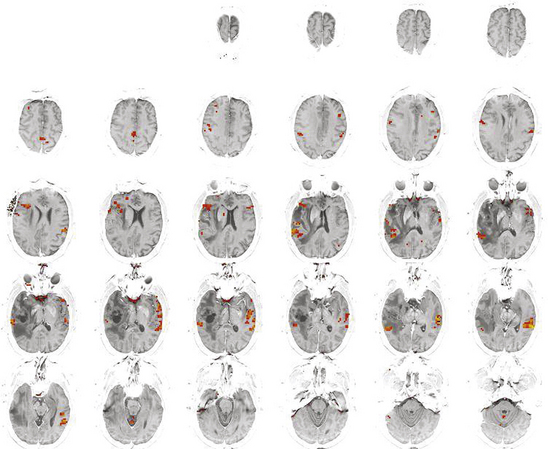
A Wada (intracarotid amobarbital) test is the definitive, albeit invasive, method to establish cerebral hemispheric dominance for language and memory. It is required for procedures in patients with a seizure disorder with tumor in whom a formal temporal lobectomy is planned. A Wada may be useful in selected other patients, such as patients with a dominant hemisphere temporal lobe tumor in whom tumor resection without temporal lobectomy is planned. Although both functional MRI and a Wada test offer similar information about cerebral dominance,5,6 a Wada test does not offer anatomic localization of critical areas for language as MRI does nor truly investigates the potential bilaterality of language.7,8 Functional MRI is more useful than a Wada test for lesions of the dominant hemisphere near the motor cortex, frontal lobe pars triangularis and opercularis (Broca’s area), or Wernicke’s area. With changes in metabolic activity and blood flow demand, an active area of the brain during a silent speech or motor task becomes infused with more oxygenated blood; this change can be detected since oxygenated blood carries a different paramagnetic signal than deoxygenated (blood oxygen level–dependent or BOLD signal) (Fig. 8-1).9,10 One limitation of functional MRI is that it becomes less useful in patients with a recurrent tumor because of altered vascular patterns and MR artifacts from the previous surgery. Some patients require both a Wada and a functional MRI as part of preoperative planning.
Cytoreduction
Although decompression of mass effect is a surgical goal and influences symptomatic survival, controversy exists over whether the extent of resection influences survival or time to progression for HGGs. Dandy originally proposed hemispherectomy for selected patients with malignant tumors, but there was no significant effect on mortality.11 Next, there were data suggesting that for GBMs, biopsy and resection were equivalent in terms of survival, and that it was really postoperative radiation that had a meaningful effect on survival. More recently, the Glioma Outcomes Project reported a statistically significant extension of survival for patients with HGGs who undergo resection over biopsy (median survival 51.6 weeks vs. 27.1 weeks, respectively).12 This study was limited by lack of central pathologic review, lack of quantification of amount of resection, sampling error from a biopsy, and selection bias in biopsy vs. resection. Other surgeons have also reported extension of survival for patients with 90% or better resection; resection better than 98% was associated with a median survival of 13 months versus 8.8 months with less than 98% in one study.13 In a recent series focused on the GBM population for patients given aggressive (but not always gross total) resection, Gliadel chemotherapy wafers, radiation, and Temodar, median survival was 20.7 months, and the 2-year survival rate was 36%.14 Additionally, subtotal resection, the volume of residual tumor at the time of first recurrence, may negatively influence response to chemotherapy in terms of time to progression and overall survival.15 Thus, it is not clear that only a biopsy should be performed if a surgeon is facing a tumor that cannot totally be resected. However, even gross total resection does not truly address the diffuse nature of malignant gliomas.
Intraoperative Imaging
Frameless stereotactic navigation is very helpful in a craniotomy for tumor resection—some would say essential. This technology incorporates a preoperatively obtained MRI with fiducial markers that are left in place on the scalp. In the operating room, these markers or contours of the face can be registered in reference to a frame that is visualized by a computer via an optical apparatus, electromagnetic waves, or mechanical arms. This technology allows the surgeon to visualize points on the scalp and skull and compare them to the MRI, aiding in planning of a small, localized incision and craniotomy as well as ensuring that the exposure of the lesion is adequate. Surgical navigation can be performed intracranially with a localizing probe or with image fusion into the operating microscope based on focus depth. Because frameless navigation is based on a preoperative set of images without updating in the operating room, the surgeon needs to account for brain shift during the procedure. Brain shift up to 2 cm can occur, and is more common with increased patient age, cortical rather than subcortical structures, larger tumor volume, and lesions far from some point of tethering such as the skull base or falx.16 Once brain shift is taken into account, resection to the imaging abnormality borders (when safe) assists in the goal of cytoreduction.
Intraoperative MRI systems are available as well. Low-field (<0.5-T) systems allow most normal operating room equipment to be used throughout the case except right at the point of imaging.17,18 Because the imaging can be updated, the surgeon does not need to account for brain shift. For craniotomies, once resection is deemed complete by the surgeon, an MRI can be performed to assess whether there is occult residual tumor, aiding in the aim of cytoreduction. These systems offer a smaller field of view, less detailed images, longer acquisition time, and fewer types of imaging options than conventional diagnostic MRIs.
High-field (1.5-T) systems exist, which offer all the imaging capabilities of a standard, diagnostic MRI.19 A biopsy needle can be watched in near–real time as it is passed to target and verified at the target before samples are taken. With craniotomies, intraoperative imaging can confirm completeness of tumor resection, which is especially helpful in cases of low-grade gliomas when the distinction between tumor and normal brain is less apparent. At closure, an MRI can be performed to exclude hemorrhage; for patients with a biopsy or simple craniotomy, excluding the hemorrhage may allow a patient to be transferred to a step-down unit instead of the ICU. These systems, however, require construction of an operating room suite specifically designed for an intraoperative MRI to provide adequate shielding and safety measures. The high-field strength requires a larger magnet than the low-field systems, limiting access to the patient. Normal operating room equipment can be used outside the 5-gauss line (several feet from the center of the bore of the magnet); inside that line, only MRI-compatible (titanium or surgical-grade stainless steel) instruments can be used.
Motor Strip Mapping
Surgery in the parietal lobe or the posterior frontal lobe may require motor strip mapping. Short-acting muscle relaxants are used during induction, and anesthetics are lightened for the mapping, but the patient does not need to emerge from anesthesia fully. Rather than identification of the motor cortex itself, this technique relies on identification of the sensory cortex by looking for somatosensory-evoked potentials (SSEPs). A 1 × 8 or other sized subdural electrode is used. By noting the electrodes with a positive (precentral) as opposed to a negative (postcentral) amplitude and noting the two electrodes between which there is phase reversal, the primary motor cortex can be identified and protected. This technique has good correlation to magnetoencephalography when integrated into the surgical navigation system.20
Frontal Lobe
High-grade gliomas are most common in the frontal lobe, as it occupies one third of the surface of the brain and is the largest lobe.21 The frontal lobe tolerates unilateral surgical resection very well as long as the motor cortex and Broca’s area are respected. Frontal lobe tumors are often amenable to image-complete resection. For tumors with significant growth into the corpus callosum and across the midline, surgical resection is unlikely to provide significant cytoreduction, and therefore a biopsy may be the more prudent choice.
Broca’s Area
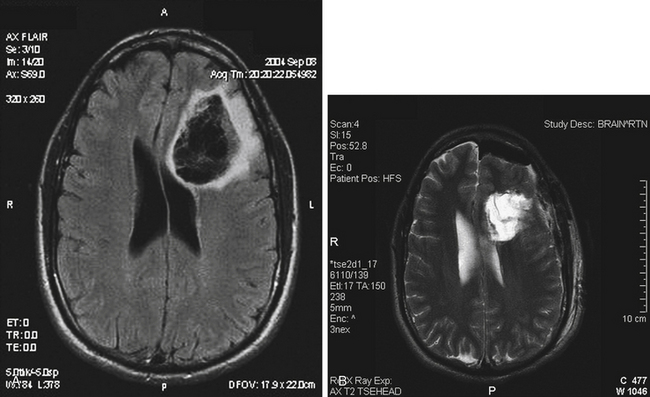
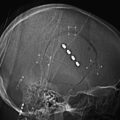
Approximately 95% of right-handed, 85% of ambidextrous, and 75% of left-handed persons will have left-sided cerebral dominance for language.22 Broca’s area encompasses the middle and posterior parts of the inferior frontal gyrus, that is, the pars opercularis and the pars triangularis. Protection of language area via awake craniotomy and intraoperative corticography or subdural electrode placement for cortical mapping is essential for tumors adjacent to Broca’s area (Fig. 8-2).
Supplementary Motor Area
The supplementary motor area (SMA) occupies the posterior one third of the superior frontal gyrus and is responsible for planning of complex movements of contralateral extremities but ipsilateral planning to a small effect.23 The full “SMA syndrome” involves speech arrest, contralateral weakness, and near-total recovery in weeks to months. For tumors involving the SMA, functional MRI shows ipsilateral decreased SMA activity compensated by increased contralateral activity.24 After resection in the SMA, the motor deficit is further compensated by recruitment of activity in the contralateral SMA and premotor cortex. Typically, leg weakness improves followed by the arm and then speech. There are patients who have reported even 6 months of significant speech trouble before returning to almost normal speech.
Temporal Lobe
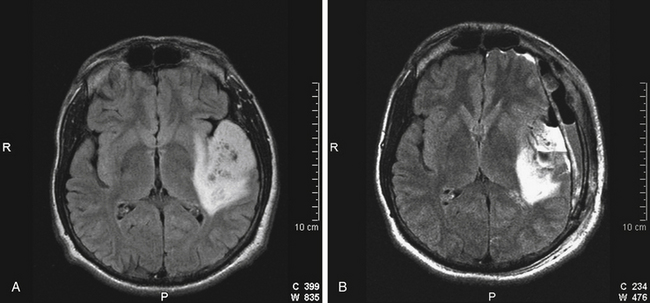
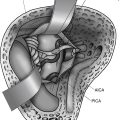
Anterior temporal lobe lesions are amenable to surgical resection. Decompression of mass effect in this area is especially important due to proximity to the brainstem. Resection is generally safe back to 4 cm back from the temporal tip on the dominant hemisphere and 6 cm back on the nondominant side. Removal of temporal lobe back to 6 cm is often associated with at least a rim of visual defect in the contralateral superior quadrant, but this deficit is generally well tolerated. On the dominant side, resection at or behind 4 cm back from the tip of the middle cranial fossa requires either intraoperative electrocorticography or subdural grid placement for identification of Wernicke’s area (Fig. 8-3). When a lesion involves the hippocampus, it is most likely that the contralateral hippocampus is compensating for function, but for the dominant hemisphere, this is best proven with a Wada test before surgery.
Parietal Lobe
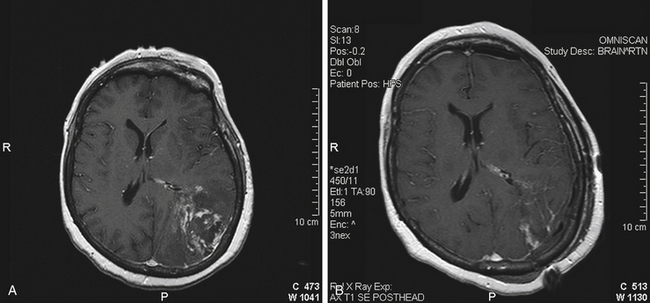
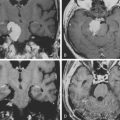
Complete lesions of the dominant parietal lobe can be characterized by Gerstmann’s syndrome, which consists of left/right confusion, digit agnosia, acalculia, and agraphia.25 Even with a large parietal lobe neoplasm, the deficit is usually incomplete. Lesions of the dominant superior parietal lobule alone rarely cause the full syndrome; the angular gyrus has to be involved.26 Low-grade gliomas are more likely than HGGs to have infiltrated into still functioning cortical areas; HGGs tend more to displace and destroy function. Restoration of a preoperative dominant parietal deficit is unlikely unless there is a cystic component, a hematoma to evacuate, or significant mass effect from edema that resolves with surgery and radiation (Fig. 8-4). Surgery within the nondominant parietal lobe is generally tolerated well as long as there is not a large parietal stroke that can lead to significant neglect of the contralateral side. Motor strip mapping adds an extra layer of safety as described above.
Prognosis
Outcome from diagnosis for an HGG depends on several factors. Precise pathologic diagnosis contributes a significant impact, as approximately 30% of patients with GBM survive to the 1-year point versus 60% of patients with anaplastic astrocytoma.27 However, glioblastoma and the rare gliosarcoma do not differ significantly in behavior, response to therapy, or cytogenetics.28 According to recently published data from the Glioma Outcomes Project, resection instead of biopsy, age less than or equal to 60, and Karnofsky Performance Scale of 70 or greater were all significantly correlated with outcome.12
New Directions
Certainly, surgery alone for HGGs will not provide a cure or have the largest long-term effect for patients with an HGG. Surgeons also have impact on radiation and chemotherapy delivery to the brain. Besides local chemotherapy with BCNU-impregnated wafers,29 other avenues proposed to effect change in the local tumor environment include convection-enhanced delivery (CED) of chemotherapy or targeted toxins.30 An option for increasing the local dose of radiation is an implantable balloon system for radioactive iodine,31 but it is unclear if this method of radiation delivery is clearly better than focused conventional radiation to the area. To date, there have been no convincing data that have driven an intracavity therapy to be widely accepted. Overall, these local delivery approaches to HGGs raise interesting questions but also battle with the notion over whether malignant glioma is a focal or diffuse disease.
An approach that attacks the diffuse nature of gliomas is immunotherapy. One option involves creation of a subcutaneous vaccine specific to the patient’s resected tumor using tumor lysate–pulsed dendritic cells.32,33 Vaccinated patients demonstrate an antigen-specific T-cell response and survival benefit as well as increased responsiveness to chemotherapy.34 Other potential strategies include interleukin gene introduction via viral vectors,35 vaccination with dendritic–glioma cell fusions using interleukin-12,36 or scores of other targets to immunotherapy. What additional therapies will be useful over the next 10 years are unpredictable.
Barnholtz-Sloan J.S., Sloan A.E., Schwartz A.G. Relative survival rates and patterns of diagnosis analyzed by time period for individuals with primary malignant brain tumor, 1972-1997. J Neurosurg. 2003;99:458-466.
Benbadis S.R., Binder J.R., Swanson S.J., et al. Is speech arrest during wada testing a valid method for determining hemispheric representation of language? Brain Lang. 1998;65(3):441-446.
Binder J.R., Swanson S.J., Hammeke T.A., et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978-984.
Chu R.M., Tummala R.P. Hall WA: intraoperative magnetic resonance imaging-guided neurosurgery. Neurosurg Q. 2003;13:234-250.
Galanis E., Buckner J.C., Dinapoli R.P., et al. Clinical outcome of gliosarcoma compared with glioblastoma multiforme: north Central Cancer Treatment Group results. J Neurosurg. 1998;89:425-430.
Keles G.E., Lamborn K.L., Chang S.M., et al. Volume of residual disease as a predictor of outcome in adult patients with recurrent supratentorial glioblastomas multiforme who are undergoing chemotherapy. J Neurosurg. 2004;100:41-46.
Knecht S., Drager B., Deppe M. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512-2518.
Krainik A., Duffau H., Capelle L., et al. Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology. 2004;62:1323-1332.
Kunwar S. Convection-enhanced delivery of IL13-PE38QQR for treatment of malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl. 2003;88:105-111.
Lacroix M., Abi-Said D., Fourney D.R., et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190-198.
Laws E.R., Parney I.F., Huang W., et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99(3):467-473.
McGirt M.J., Than K.D., Weingart J.D., et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110:583-588.
McLendon R.E., Halperin E.C. Is the long-term survival of patients with intracranial glioblastoma multiforme overstated? Cancer. 2003;98:1745-1748.
Reinges M.H., Nguyen H.H., Krings T., et al. Course of brain shift during microsurgical resection of supratentorial cerebral lesions: limits of conventional neuronavigation. Acta Neurochir (Wien). 2004;146(4):369-377.
Romstock J., Fahlbusch R., Ganslandt O., et al. Localisation of the sensorimotor cortex during surgery for brain tumors: feasibility and waveform patterns of somatosensory evoked potentials. J Neurol Neurosurg Psychiatry. 2002;72:221-229.
Roux F., Boetto S., Sacko O., et al. Writing, calculating, and finger recognition in the region of the angular gyrus: a cortical stimulation study of Gerstmann syndrome. J Neurosurg. 2003;99:716-727.
Russell S.M., Kelly P.J. Incidence and clinical evolution of postoperative deficits after volumetric stereotactic resection of glial neoplasms involving the supplementary motor area. Neurosurgery. 2003;52:506-516.
Sabsevitz D.S., Swanson S.J., Hammeke T.A., et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60:1788-1792.
Steinmeier R., Fahlbusch R., Ganslandt O., et al. Intraoperative magnetic resonance imaging with the Magnetom open scanner: concepts, neurosurgical indications, and procedures: a preliminary report. Neurosurgery. 1998;43:739-748.
Tatter S.B., Shaw E.G., Rosenblum M.L., et al. An inflatable balloon catheter and liquid 125I radiation source (GliaSite Radiation Therapy System) for treatment of recurrent malignant glioma: multicenter safety and feasibility trial. J Neurosurg. 2003;99:297-303.
Westphal M., Hilt D.C., Bortey E., et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-Oncology. 2003;5(2):79-88.
Wheeler C.J., Das A., Liu G., et al. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10:5316-5326.
Yu J.S., Liu G., Yong W.H., et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973-4979.
1. Central Brain Tumor Registry of the United States. Primary Brain Tumors in the United States Statistical Report 2002-2006. CBTRUS. 2009-2010.
2. American Cancer Society. Cancer Facts and Figures 2009. Atlanta, GA: American Cancer Society, Inc. Surveillance Research; 2009.
3. McLendon R.E., Halperin E.C. Is the long-term survival of patients with intracranial glioblastoma multiforme overstated? Cancer. 2003;98:1745-1748.
4. Majos C., Alonso J., Aguilera C., et al. Proton magnetic resonance spectroscopy ((1)H MRS) of human tumours: assessment of differences between tumour types and its applicability in brain tumour categorization. Eur Radiol. 2003;13(3):582-591.
5. Desmond J.E., Sum J.M., Wagner A.D., et al. Functional MRI measurement of language lateralization in Wada-tested patients. Brain. 1995;118:1411-1419.
6. Binder J.R., Swanson S.J., Hammeke T.A., et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978-984.
7. Benbadis S.R., Binder J.R., Swanson S.J., et al. Is speech arrest during Wada testing a valid method for determining hemispheric representation of language? Brain Lang. 1998;65(3):441-446.
8. Sabsevitz D.S., Swanson S.J., Hammeke T.A., et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60:1788-1792.
9. Bandettini P.A., Wong E.C., Hinks R.S., et al. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390-397.
10. Logothetis N.K., Pauls J., Augath M., et al. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150-157.
11. Dandy W.E. Removal of right cerebral hemisphere for certain tumors with hemiplegia: preliminary case report. JAMA. 1928;90:823-825.
12. Laws E.R., Parney I.F., Huang W., et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99(3):467-473.
13. Lacroix M., Abi-Said D., Fourney D.R., et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190-198.
14. McGirt M.J., Than K.D., Weingart J.D., et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110:583-588.
15. Keles G.E., Lamborn K.L., Chang S.M., et al. Volume of residual disease as a predictor of outcome in adult patients with recurrent supratentorial glioblastomas multiforme who are undergoing chemotherapy. J Neurosurg. 2004;100:41-46.
16. Reinges M.H., Nguyen H.H., Krings T., et al. Course of brain shift during microsurgical resection of supratentorial cerebral lesions: limits of conventional neuronavigation. Acta Neurochir (Wien). 2004;146(4):369-377.
17. Tronnier V.M., Wirtz C.R., Knauth M., et al. Intraoperative diagnostic and interventional magnetic resonance imaging in neurosurgery. Neurosurgery. 1997;40:891-902.
18. Steinmeier R., Fahlbusch R., Ganslandt O., et al. Intraoperative magnetic resonance imaging with the Magnetom open scanner: concepts, neurosurgical indications, and procedures. A preliminary report. Neurosurgery. 1998;43:739-748.
19. Chu R.M., Tummala R.P., Hall W.A. Intraoperative magnetic resonance imaging-guided neurosurgery. Neurosurg Q. 2003;13:234-250.
20. Romstock J., Fahlbusch R., Ganslandt O., et al. Localisation of the sensorimotor cortex during surgery for brain tumors: feasibility and waveform patterns of somatosensory evoked potentials. J Neurol Neurosurg Psychiatry. 2002;72:221-229.
21. Carpenter M.B. Core Text of Neuroanatomy, 4th ed. Baltimore, MD: Williams and Wilkins; 1991.
22. Knecht S., Drager B., Deppe M. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512-2518.
23. Russell S.M., Kelly P.J. Incidence and clinical evolution of postoperative deficits after volumetric stereotactic resection of glial neoplasms involving the supplementary motor area. Neurosurgery. 2003;52:506-516.
24. Krainik A., Duffau H., Capelle L., et al. Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology. 2004;62:1323-1332.
25. Gerstmann J. Syndrome of finger agnosia, disorientation for right and left, agraphia, and acalculia. Arch Neurol Psychiatry. 1940;44:398-408.
26. Roux F., Boetto S., Sacko O., et al. Writing, calculating, and finger recognition in the region of the angular gyrus: A cortical stimulation study of Gerstmann syndrome. J Neurosurg. 2003;99:716-727.
27. Barnholtz-Sloan J.S., Sloan A.E., Schwartz A.G. Relative survival rates and patterns of diagnosis analyzed by time period for individuals with primary malignant brain tumor, 1972-1997. J Neurosurg. 2003;99:458-466.
28. Galanis E., Buckner J.C., Dinapoli R.P., et al. Clinical outcome of gliosarcoma compared with glioblastoma multiforme: North Central Cancer Treatment Group results. J Neurosurg. 1998;89:425-430.
29. Westphal M., Hilt D.C., Bortey E., et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-Oncology. 2003;5(2):79-88.
30. Kunwar S. Convection-enhanced delivery of IL13-PE38QQR for treatment of malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl. 2003;88:105-111.
31. Tatter S.B., Shaw E.G., Rosenblum M.L., et al. An inflatable balloon catheter and liquid 125I radiation source (GliaSite Radiation Therapy System) for treatment of recurrent malignant glioma: multicenter safety and feasibility trial. J Neurosurg. 2003;99:297-303.
32. Yamanaka R., Abe T., Yajima N., et al. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 2003;89(7):1172-1179.
33. Yu J.S., Liu G., Yong W.H., et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973-4979.
34. Wheeler C.J., Das A., Liu G., et al. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10:5316-5326.
35. Ren H, Boulikas T, Lundstrom K, et al. Immunogene therapy of recurrent glioblastoma multiforme with liposomally encapsulated replication-incompetent Semliki forest virus vector carrying the human interleukin-12 gene-A phase I/II clinical protocol. J Neurooncol 64(1-2): 147-154.
36. Kikuchi T., Akasaki Y., Abe T. Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother. 2004;27(6):452-459.