Chapter 85B Cryoablation for liver tumors
Overview
Hepatic resection is the standard of care for the majority of resectable primary and metastatic liver tumors confined to the liver (Cha et al, 2002; Fong et al, 1999; Scheele et al, 1995; Wei et al, 2006; see Chapters 80 and 81A). In patients with impaired liver function or small hepatocellular tumors, ablation is sometimes employed as first-line treatment (Chen et al, 2006; Livraghi et al, 2008); however, most patients with malignant liver tumors are not amenable to potentially curative resection because of anatomic, functional, or prognostic factors (see Chapter 2). Even when the malignancy is considered unresectable, local control of primary and isolated metastatic hepatic malignancy may change the natural history of these diseases. Several prospective and retrospective analyses have shown liver failure to be a common cause of death in patients with unresected liver tumors (Couto et al, 2007; Nagorney & Gigot, 1996). Ablative techniques can be combined with surgical resections to decrease hepatic tumor load and possibly eliminate it. They also expand the treatment options for disease recurrence in patients whose liver remnant is not optimized or amenable to surgical resection. With ablation, it is possible to prolong survival and increase the disease-free interval in patients. Cryotherapy was one of the original ablative techniques, but it has increasingly been replaced by radiofrequency ablation (RFA) and more recently microwave ablation (MWA) because of high complication rates and high local recurrence rates, somewhat cumbersome technology, and higher complication rates with cryoablation, although its use continues to be described for treating close or positive margins after resection.
Patient Population
Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world with more than 1 million cases occurring each year worldwide (see Chapter 80; Nordenstedt et al, 2010). It is well known that HCC is the most common malignancy in Southeast Asia and in sub-Saharan Africa owing to endemic viral hepatitis, especially hepatitis B. Since the 1990s the incidence of HCC has increased significantly in the United States and other Western countries as hepatitis C rates have risen. In the United States, the mortality rate for HCC has improved over the past 2 decades, as screening programs have identified patients with tumors at earlier stages. Of patients presenting with HCC, only 15% to 25% are operable, and less than 10% are resectable with curative intent (Bolondi et al, 2001; Farmer et al, 1994); however, most patients with unresectable disease have tumor confined to the liver. At a regional referral center for liver cancer, curative treatments can be applied to 30% to 40% of patients (Bruix & Llovet, 2002). More than half of patients presenting with primary liver cancer are potential candidates for hepatic regional therapy, of which a significant number may be amenable to ablative techniques.
Metastatic Disease
The liver is one of the most common sites of metastatic spread, particularly from gastrointestinal malignancies (see Chapter 81A). Most malignancies that recur in the liver do so in combination with extrahepatic metastases, making resection or ablative regional therapies unsuitable management strategies. In contrast, recurrences from colorectal primary tumors are localized to the liver in approximately one third of patients, allowing use of potentially curative regional interventions. Only a small proportion of patients with metastases confined to the liver are operable, and an even smaller fraction are resectable, owing to technical and prognostic factors. By evaluating all patients with hepatic colorectal metastases at a single center, Scheele and colleagues (1995) found 21% of patients with synchronous disease and 51% of patients with metachronous disease to be potentially resectable.
Most patients with metastatic disease confined to the liver are not candidates for resection therapies but are potentially eligible for regional ablative treatments. Systemic and regional chemotherapy for metastatic colorectal cancer has improved dramatically, with median survivals of greater than 20 months now commonly reported (see Chapters 86 and 87). Additionally, response rates for systemic chemotherapy are generally greater than 50%, and combined with regional chemotherapy, they approach 90%. Survival at 5 years for patients after resection of colorectal liver metastases in modern series ranges from 46% to 58% (Karanjia et al, 2009; Pawlik et al, 2005). Resectional and ablative therapies now must be interpreted in the context of effective chemotherapy, opening the door for downstaging of unresectable tumors and adjuvant therapy for borderline resectable tumors.
General Indications for Nonresectional Therapy of Liver Tumors
Numerous patient- and tumor-related factors conspire to make liver tumors “inoperable” or “unresectable.” General and liver-related comorbidities are common contraindications to hepatic resection and are often independent of the extent of disease or proposed operation. Most patients with HCC have limited hepatic reserve secondary to cirrhosis or portal hypertension or both, which often precludes a safe liver resection (see Chapters 2 and 70A). Many technical factors make a hepatic malignancy unresectable. Insufficient future liver remnant, involvement of all three hepatic veins, and involvement of portal inflow to both lobes of the liver are examples of such technical issues. Many techniques to deal with technically unresectable tumors have been developed, such as portal vein embolization, parenchymal-sparing segmental resections, and two-stage operations (see Chapters 92, 93A, and 93B); nonetheless, most liver tumors are still unresectable.
Pathophysiology of Cryoablation
The methods by which subzero temperatures destroy tumors are not tissue specific, and both normal and neoplastic tissues are sensitive to extreme cold. Cryotherapy causes cell death by a variety of physical and chemical mechanisms that depend on the rate of cooling, absolute depth of hypothermia, rate of thawing, number of freeze-thaw cycles used, and delayed effects of ischemia after thawing. When a cryoprobe is inserted into the liver, three overlapping zones of injury develop within the ice ball. Rapid tissue freezing occurs closest to the cryoprobe, and the rate of freezing decreases in proportion to the distance from the probe, creating zones of intermediate and slow cooling. Similarly, a gradient of temperature develops in the ice ball, decreasing 3° C/mm to 10° C/mm from −170° C near the probe to just below 0° C at the periphery of the cryolesion. The dynamics of the freezing process cause different mechanisms of injury in these three idealized zones (Gage & Baust, 1998; Mascarenhas & Ravikumar, 1998).
Thawing Process
Repeated Freeze-Thaw Cycles
Repeated freeze-thaw cycles in animal models have been shown to move the margin of reliable complete cell kill outward by producing larger ice balls more rapidly (Poppendiek, 1967). This movement is due to increased thermoconductivity of the previously frozen liver. Tissue near the cryoprobe is ablated adequately with one freeze-thaw cycle, because low enough temperatures are achieved to ensure complete cellular destruction. The added benefit of multiple cycles occurs in the periphery of the tumor, where the depth of hypothermia is unpredictable, and the cell kill is unreliable. Early histologic signs of cellular damage in lesions undergoing multiple freeze-thaw cycles are more dramatic, confirming the added benefit of such cycles.
Microvascular Effects
In addition to the acute physicochemical and structural damage to the cell and its environment, the lethal effects of cryoablation are potentiated by disruption of vascular structures, which causes delayed hypoxia and necrosis (Rubinsky et al, 1990). The vascular effects are acute and chronic. Freezing at slow cooling rates causes the radius of the sinusoids to increase by a factor of 2, which is equivalent to increasing the volume in the intravascular space by a factor of 4. The expansion of the vascular space tears the endothelium, exposing and disrupting the underlying basement membrane. Platelet thrombi develop, and permeability increases, leading to swelling and microcirculatory failure. The tissues supplied by these damaged vessels become ischemic and necrotic. This mechanism of injury is more important in the intermediate and slow cooling zones, where direct cellular injury by intracellular ice formation or dehydration is not reliable. For maximal benefit, cryosurgery should be performed so that there is rapid freeze, slow thaw, and repeated freeze-thaw cycles; however, experimental evidence has shown that high-flow venules at the periphery of an ice ball are crucial in shutting down the microcirculation of the tumor, and repeated cycles do not seem to improve this effect (Richter et al, 2005). It also has been shown in experimental models that the serine protease inhibitor aprotinin decreases platelet trapping and improves tissue destruction in cryolesions (Kollmar et al, 2004). The effects of cryosurgery on tissues are a result of direct cellular damage secondary to physicochemical effects and indirect cell damage secondary to loss of cellular integrity and destruction of vascular channels.
Immunologic Effects of Cryoablation
In early studies of cryotherapy, anecdotal reports of regression of untreated tumor sites suggested a generalized, tumor-specific immunologic response to the cryoablated lesion (Faraci et al, 1975). These effects have not been substantiated in animal or human models, although it is unlikely that an antitumor immune response enhances the effect of cryotherapy.
Operative Technique
Preoperative Preparation
Two devastating complications associated with hepatic cryotherapy are the cryoshock syndrome, a complex of coagulopathy and renal and pulmonary failure, and intraoperative hypothermia (Seifert & Morris 1998); preoperative hydration is important to decrease the incidence of renal dysfunction (Bagia et al, 1998). Hypothermia is another significant complication that can be alleviated using heated fluid and airway circuits and a Bair Hugger (Arizant Healthcare, Eden Prairie, MN) warming system (Onik et al, 1993). Blood and blood products should be made available for the infrequent cases of intraoperative bleeding.
Cryotherapy at Laparotomy: Open Cryotherapy
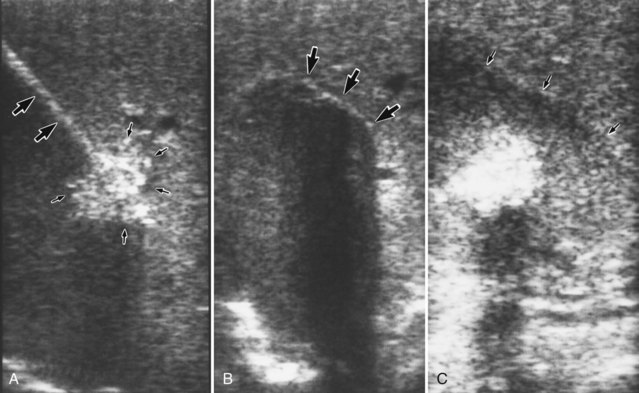
Depending on the depth of the lesion, direct introduction of the probe into the tumor or wire-guided, Seldinger-style localization may be used. The Seldinger technique is best used for deep intraparenchymal and nonpalpable tumors. First, the tumor is identified by intraoperative US. An echogenic needle is passed into the center of the lesion under US guidance and subsequently is exchanged for a J-wire (Fig. 85B.1A). When the J-wire is in place, the tract of the wire is dilated with a coaxial dilator and peel-away sheath. The dilator is withdrawn, and the cryoprobe is inserted through the sheath into the center of the tumor. The position of the cryoprobe is evaluated again using US in two or three different axes. The probe must be placed through the center of the tumor with the tip near the opposite margin to encompass the tumor completely within the cryolesion.
It is crucial to tailor the type of cryoprobe and freezing technique to the size and location of the tumor. The temperature gradient within the ice ball significantly affects the efficacy of the procedure. The temperature near the probe approximates that of liquid nitrogen, whereas at the periphery of the ice ball, the tissue may be only a few degrees below 0° C. In addition, the rate of cooling is determined by the size of the probe, the flux of refrigerant, and the thermoconductivity of the uninsulated portion of the probe. To achieve maximum tumor ablation, the operator must understand the physical properties of the cryotherapy system. The system we use employs vacuum insulated probes and supercooled liquid nitrogen refrigerant. It permits the use of four simultaneous and completely independent probe placements. Two probes are available with this system, accommodating different size tumors. The 3-mm blunted probe creates a cryolesion 4 cm in diameter, whereas the 8-mm trocar point probe creates a 6-cm freeze zone. Two 8-mm probes, used in tandem, create a 10-cm cryolesion (Ravikumar et al, 1991b). Other systems have flat-faced probes that can be applied to a flat surface to create a hemispheric cryolesion of 3 to 4 cm, which can be helpful to treat close margins (Gruenberger et al, 2001).
Maximal cooling is started, and real-time US monitoring of the freezing process is carried out. The freeze front is seen as a hyperechoic rim with posterior acoustic shadowing (Fig. 85B.1B). Cooling continues until the freeze front extends 1 cm beyond the US margin of the tumor. Typically, the freezing process takes 8 to 15 minutes to complete, depending on the efficiency of the cryotherapy system and the size of the tumor. The freeze zone shown by intraoperative US closely approximates the pathologic and histologic volume of cryonecrosis.
After completion of the second freeze-thaw cycle, the probe is actively rewarmed, allowing it to disengage before the tumor completely thaws. The probe tract is packed with absorbable knitted fabric, such as Surgicel or another hemostatic material, and gentle pressure is applied to the liver, preventing delayed hemorrhage from the probe tract. Rough handling of the thawing ice ball may cause a cleavage plane to develop at the interface of the ice ball and the normal liver parenchyma, which can cause massive bleeding. Generally, the probe insertion tract stops bleeding promptly, as the coagulation cascade activates at body temperature. After the two freeze-thaw cycles, the treated volume usually remains hyperechoic on US, whereas the surrounding normal liver becomes hypoechoic owing to edema; this gives the treated tumor a “halo” appearance on imaging studies (Fig. 85B.1C).
Laparoscopic Cryotherapy
The reported experience with laparoscopic cryotherapy is scant, and the patients are highly selected. With the expansion of RFA and its laparoscopic application, laparoscopic cryoablation has become less popular, and further publications have not been forthcoming. Reports have been limited to small case series, generally fewer than 10 patients, but the technique seems to be safe and effective in these highly selected patients (Seifert & Junginger, 2004). Despite current technologic limitations, the laparoscopic approach offers several advantages: it is minimally invasive, it eliminates the morbidity associated with a laparotomy incision, and it decreases the length of hospitalization and overall recovery.
Percutaneous Cryotherapy Approaches
Percutaneous approaches for cryotherapy are established, although they have been relatively slow to develop; this is likely related to the explosion in popularity of percutaneous RFA. This approach originally was limited by the large diameter of current cryoprobes, location of the tumors, and accurate radiologic localization. Despite these problems, several moderate-sized series have been published, which confirm the feasibility and safety of this approach. Guidance most typically was performed with CT or with US, but more recently, techniques using magnetic resonance imaging (MRI) have been developed (Wu et al, 2010).
Follow-Up of Patients Treated by Cryotherapy
Although cryotherapy is an accepted procedure for HCC and liver metastases, prospective and comparative data supporting its efficacy are limited. It is important for a reasonably standardized follow-up to be collected on all patients that includes careful documentation of recurrence patterns and disease-specific outcome. Follow-up of patients after cryotherapy comprises history and physical examination, contrast-enhanced CT scans, and tumor marker determinations. The patient should undergo clinical evaluation, serial liver tests, and tumor marker determinations every 3 to 4 months for 2 years and then every 6 months. carcinoembryonic antigen (CEA) and α-fetoprotein (AFP) levels usually reach their nadir at 4 to 8 weeks after ablation (Steele et al, 1990).
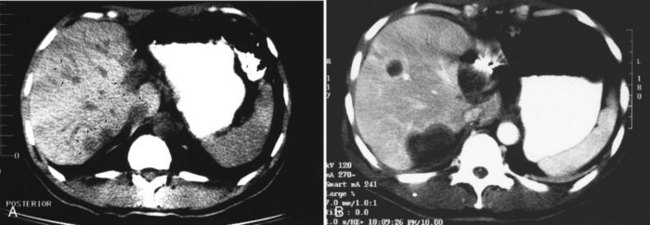
CT scans of the liver should be obtained at 4- to 6-month intervals for the first 2 years. It is important to obtain an early CT scan within a few weeks after treatment to be used as a baseline. Successfully treated tumors initially appear larger than the original lesion because of swelling and because of the margin of normal tissue also treated (Fig. 85B.2). Subsequently, the lesion decreases in size and may disappear completely. Gas bubbles may be seen within the necrotic tumor and may be a result of the packed probe tract. These bubbles rarely indicate hepatic abscess and should not be acted on unless other signs of sepsis are evident. In 3 to 6 months, the lesions shrink and leave behind a persistent area of fibrosis and architectural distortion.
Technical Considerations
Inflow Occlusion
The flow of warm blood through vessels adjacent to the tumor may act as a heat sink that changes the conformation of the ice ball and alter tumor ablation near the vessel. Some groups have used inflow occlusion with a Pringle maneuver to diminish these effects. Experimental studies have not confirmed these assertions in the porcine model, in which clamping the hepatoduodenal ligament did not alter the size of the ice ball that formed, the amount of liver necrosis, or the incidence of large vessel infarction (Kahlenberg et al, 1998). The relative contraindication to cryoablation near vessels may be only theoretic, and further evaluation of this problem is necessary.
Complications of Cryotherapy
The complications of hepatic cryotherapy can be broadly classified into three types of problems: 1) operative, 2) technical, and 3) late onset. The operative complications include complications that are not specific to cryotherapy, whereas the technical and late-onset complications are directly related to the procedure (Table 85B.1). The overall morbidity rate ranges from 10% to 40%, and the mortality rate ranges from 0% to 5% (Seifert & Junginger, 2004).
Table 85B.1 Complications After Hepatic Cryotherapy: Summary of 869 Patients in 20 Series
| Complication | Incidence (%) | Range (%) |
|---|---|---|
| Cracking of ice ball | 19* | 1-25 |
| Hemorrhage | 3.7 | 0-13 |
| Coagulopathy | 3.8 | 0-8 |
| Acute renal failure | 1.4 | 0-17 |
| Bile fistula/biloma | 2.9 | 0-10 |
| Intraabdominal abscess | 1.7 | 0-9 |
| Pleural effusion | 6.3 | 4-18 |
| Death | 1.6 | 0-8 |
* Reported in only 5 of 20 studies
Modified from Seifert JK, et al, 1998: A collective review of the world literature on hepatic cryotherapy. J R Coll Surg Edinb 43:141-154.
Bile Fistula and Bile Collections
Bile collections are reported after cryotherapy, affecting approximately 3% of patients in collected series (Seifert & Morris, 1998). Bile collections and fistulae are most common when superficial lesions are treated. Late strictures of the major bile ducts owing to injury during the freezing process may occur and predispose patients to cholangitis. The risk is increased for cryoablated tumors located near the hepatic hilum or the bifurcation of the major biliary ducts, although no long-term studies have confirmed this risk of bile duct injury and late stricture (see Chapters 42A and 42B).
Cryoshock
Cryoshock is a complex of multisystem organ failure, renal failure, and DIC after cryotherapy, and it is potentially lethal. The cause of this symptom complex is unknown, but it is responsible for 18% of deaths after hepatic cryotherapy. In a survey of all groups using cryotherapy, cryoshock was reported in only 21 of 2173 patients undergoing hepatic cryotherapy for tumor (Seifert & Morris, 1998). The cause of this phenomenon is likely related to systemic release of proinflammatory cytokines, such as tumor necrosis factor, interleukin (IL)-1, IL-2, and IL-6. Experimental studies have correlated the risk of this syndrome with larger ablations and with higher systemic levels of these cytokines (Ng et al, 2004; Seifert et al, 2002).
Mortality
The overall early mortality rate for cryotherapy is low at 1.5% (range, 0% to 8%) (Seifert & Junginger, 2004). In expert hands, the operative mortality for major hepatic resection is similar. The most common causes of death after hepatic cryotherapy are not specific to the cryotherapy but rather to cardiac and pulmonary comorbidities. The more common treatment-specific causes of death include cryoshock, liver failure, and hemorrhage.
Long-Term Results of Cryoablation
Cryoablation of Hepatocellular Carcinoma
For primary liver cancer patients treated with cryotherapy alone, the 5-year survival is approximately 30%. As expected, survival correlates well with the biologic characteristics of the tumor, of which size seems to be the most important factor. Zhou and colleagues (1996) showed that 5-year survival was 48% for tumors less than 5 cm, whereas it was only 25% for larger tumors (Zhou et al, 1996). The pattern of recurrence is predominantly in the liver after cryotherapy, but the reports typically do not describe whether the cryotherapy was complete, or what the intended margin was. In addition, it is often not reported whether the tumor recurred in the treated area, or whether these recurrences were a separate, intrahepatic, distant tumor resulting from intrahepatic metastases or new primaries. Few studies contain longitudinal survival information, and these results must be corroborated by larger multicenter studies controlling for tumor size, number of lesions treated, and adequacy of treatment.
It is difficult to compare the results of resection with cryotherapy, because the reported series generally are not comparable in terms of tumor characteristics. The operative mortality for resection of HCC historically has been 6% to 8% in most series, but now with better selection criteria and improved techniques, mortality is generally less than 5%. The median survival is approximately 30 months, with 5-year survivals of 30% to 40%. Similar survivals are reported in selected patients who undergo percutaneous ethanol injection (PEI); however, these are performed in highly selected patients (see Chapter 85A). Livraghi and colleagues (1995) reported a multicenter trial of PEI for HCC that included 746 patients (Livraghi et al, 1995). Survival was related to the size of the lesion, number of tumors, and Child-Turcotte-Pugh (CTP) class of the associated cirrhosis. For unifocal tumors less than 5 cm, the 5-year survival rates were 47%, 29%, and 0% for class A, B, and C cirrhosis, respectively. Even in the presence of multifocal disease, 36% of patients with class A cirrhosis survived 5 years after PEI. Similar results have been reported by Castells and associates (1993) in a comparative study of resection and PEI for HCC less than 4 cm. At 4 years, survival was equivalent for the two modalities.
Cryoablation of Liver Metastases
Liver metastases arising from colorectal, lung, pancreas, and stomach primary tumors constitute the most common malignant tumors of the liver (see Chapter 81A). Most cancers that metastasize to the liver do so in combination with extrahepatic dissemination. The natural history and pattern of recurrence for colorectal cancer is different. Of the approximately 160,000 new cases each year, half develop liver metastases within 5 years of diagnosis. Approximately 20% of these patients (16,000) develop metastatic disease confined to the liver. Only a quarter (4000 to 5000 patients) are amenable to hepatic resection with curative intent; the remaining 12,000 patients with liver-only colorectal metastases are candidates for regional therapies to the liver. The number of patients being considered for liver resection is now increasing because of improved resection techniques and dramatic improvements in adjuvant chemotherapy.
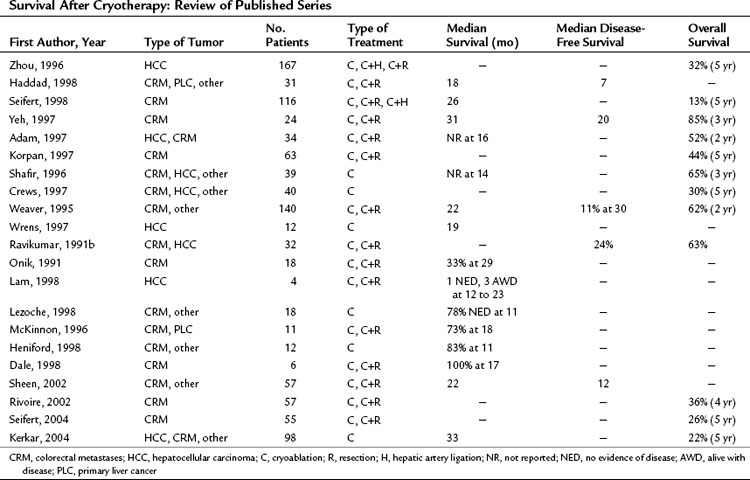
The ultimate goal of regional treatment of liver metastases is increased survival, but long-term follow-up of patients after cryotherapy for liver metastases is inadequate. Most published studies do not have follow-up exceeding 2 years, and few groups have treated enough patients to draw valid conclusions regarding the efficacy of cryotherapy in this setting. Series with adequate follow-up reporting 3- and 5-year survival have been published, albeit with relatively small numbers. In addition, cryotherapy has been used primarily as a salvage procedure or in combination with other treatment modalities, including resection or hepatic artery ligation or infusion, which obscures the results for cryotherapy alone. Ravikumar and associates (1991a) reported overall and disease-free survivals of 78% and 39% at 5 years for 18 patients undergoing complete cryoablation of liver metastases. These encouraging results have not been duplicated in more recent studies, probably because of less stringent selection criteria. Most series (Table 85B.2) report median overall survival of about 2 years after cryoablation of liver metastases. In 24 patients treated with cryotherapy, regardless of whether the ablation was complete or not, Ravikumar and associates (1991b) reported disease-free and overall survival rates of 24% and 63%, respectively. Similarly, Weaver and colleagues (1995) found that 11% of patients were disease-free at a mean follow-up of 30 months, and 62% of treated patients were alive 24 months after cryoablation. Seifert and Morris (1998), in a series of 116 patients with colorectal metastases, showed a median survival of 26 months, and 13% of patients were alive at 5 years. More recent publications have reported 4- and 5-year actuarial survival ranging from 22% to 36% (Kerkar et al, 2004; Rivoire et al, 2002; Seifert & Junginger, 2004). Despite the wide variation in outcome with cryotherapy, some patients in each of the series achieved durable survival with this treatment. Actual follow-up is greater than 5 years for individual patients, suggesting that cryotherapy can be curative in some cases. Based on the worldwide data, it seems that durable local control is achieved in about 20% of patients treated with cryotherapy.
Because the series reporting survival are generally small and include patients treated with cryotherapy, in combination with resection for control of inadequate margins, and patients receiving hepatic artery infusion chemotherapy (see Chapter 86), the results cannot be combined for a proper meta-analysis. Because there are no randomized trials of cryotherapy, Tandan and coworkers (1997) undertook a critical comparative review of resection and cryoablation of colorectal liver metastases. This analysis reviewed 178 studies published from 1973 to 1995. Only studies with survival data exceeding 2 years and data for liver resection if the series included more than 60 patients were included; four cryotherapy and nine resection studies met these minimum inclusion criteria. The median follow-up times for the two procedures were 12 to 28.8 months for cryotherapy and 21 to 69 months for liver resection. The data on liver resection were found to be more valid and consistent, but this review supported a role for cryotherapy in the management of liver metastases. Based on this evaluation, the authors concluded that cryosurgery offers potential advantages in the treatment of liver metastases, it should not be used in cases of resectable disease outside of a clinical trial, and randomized trials comparing the two modalities are needed. Survival seems to be related to the size of the lesions, the volume of liver replaced by tumor, a low pretreatment serum CEA level, absence of extrahepatic metastases, and whether the lesion underwent complete ablation. Normalization of an elevated precryotherapy CEA has been a favorable prognostic factor. Failure to achieve this normalization of CEA probably results from residual unrecognized micrometastatic disease.
Complete response at the treated site ranges from 60% to 90%, and most recurrences are at sites away from the cryolesion. The pattern of recurrence in these patients is primarily in the liver, with or without synchronous extrahepatic disease. This finding underscores the need for additional therapy to further reduce the incidence of hepatic recurrence. With the improvements in regional and systemic chemotherapy, the utility of cryotherapy combined with chemotherapy has been shown even in grossly unresectable patients (Kemeny et al, 2001). An additional use for cryoablation is in the treatment of close or inadequate margins. Edge cryotherapy in these situations has resulted in good local tumor control (Gruenberger et al, 2001; Hou et al, 2007; Yan et al, 2006). Survival after hepatic resection of liver metastases is substantially better (20% to 58%) than after cryotherapy, but the patient populations are not comparable. Cryotherapy generally has been reserved for patients who are unable to undergo resection and who might have a poorer prognosis.
Comparison with Radiofrequency Ablation
Over the past 10 years, RFA has replaced cryotherapy in many centers (see Chapter 85C). Pearson and colleagues (1999) prospectively studied the complication rates and early local recurrence rates in patients with primary or metastatic liver tumors who were treated with cryotherapy or RFA. With a total of 54 patients treated with cryotherapy and 92 patients treated with RFA, a 41% complication rate was reported in the cryotherapy group compared with a 3% complication rate in the RFA group (P < .001). The majority of complications in the cryotherapy group were symptomatic pleural effusions and intrahepatic abscesses. Recurrence rates with a median follow-up of 15 months also favored RFA, with a 2% recurrence rate compared to 14% in the cryoablation group (P < .01). Percutaneous cryotherapy and RFA were compared in a retrospective study by Adam and colleagues (2002) with a group of 64 patients. They reported complication rates of 29% for cryotherapy patients and 24% for those who underwent RFA (P = .66), but local recurrence rates were 53% in the cryotherapy group, compared with 18% in the RFA group (P = .003).
Adam R, et al. Place of cryotherapy in the treatment of malignant liver tumors. Ann Surg. 1997;225:39-50.
Adam R, et al. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch Surg. 2002;137(12):1332-1339. discussion 1340
Bagia JS, et al. Renal impairment in hepatic cryotherapy. Cryobiology. 1998;36(4):263-267.
Bolondi L, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48(2):251-259.
Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35(3):519-524.
Castells A, et al. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology. 1993;18(5):1121-1126.
Cha C, et al. Surgery and ablative therapy for hepatocellular carcinoma. J Clin Gastroenterol. 2002;35(5 Suppl 2):S130-137.
Chen MS, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321-328.
Couto OF, et al. Causes of death in patients with unresectable hepatocellular carcinoma. Dig Dis Sci. 2007;52(11):3285-3289.
Crews KA, et al. Cryosurgical ablation of hepatic tumors. Am J Surg. 1997;174:614-618.
Faraci RP, et al. The effect of curative cryosurgery on the tumor-specific immune response of C57 mice. Cryobiology. 1975;12(2):175-179.
Farmer DG, et al. Current treatment modalities for hepatocellular carcinoma. Ann Surg. 1994;219(3):236-247.
Fong Y, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309-318. discussion 318-321
Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37(3):171-186.
Gruenberger T, et al. Reduction in recurrence risk for involved or inadequate margins with edge cryotherapy after liver resection for colorectal metastases. Arch Surg. 2001;136(10):1154-1157.
Haddad FF, et al. Clinical experience with cryotherapy for advanced hepatobiliary tumors. J Surg Res. 1998;75:103-108.
Heniford BT, et al. Laparoscopic cryoablation of hepatic metastases. Semin Surg Oncol. 1998;15:194-201.
Hou RM, et al. The effects of surgical margin and edge cryotherapy after liver resection for colorectal cancer metastases. HPB (Oxford). 2007;9(3):201-207.
Kahlenberg MS, et al. Clinicopathologic effects of cryotherapy on hepatic vessels and bile ducts in a porcine model. Ann Surg Oncol. 1998;5(8):713-718.
Karanjia ND, et al. A comparison of right and extended right hepatectomy with all other hepatic resections for colorectal liver metastases: a ten-year study. Eur J Surg Oncol. 2009;35(1):65-70.
Kemeny N, et al. Phase I study of hepatic arterial infusion of floxuridine and dexamethasone with systemic irinotecan for unresectable hepatic metastases from colorectal cancer. J Clin Oncol. 2001;19(10):2687-2695.
Kerkar S, et al. Long-term follow up and prognostic factors for cryotherapy of malignant liver tumors. Surgery. 2004;136(4):770-779.
Kollmar O, et al. Aprotinin inhibits local platelet trapping and improves tissue destruction in hepatic cryosurgery. Surgery. 2004;136(3):624-632.
Korpan NN. Hepatic cryosurgery for liver metastases. Ann Surg. 1997;225:193-201.
Lam C, et al. Hepatic cryotherapy for recurrent hepatocellular carcinoma after hepatectomy. J Surg Oncol. 1998;68:104-106.
Lezoche E, et al. Ultrasound guided laparoscopic cryoablation of heopatic tumors: preliminary report. World J Surg. 1998;22:829-836.
Livraghi T, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197(1):101-108.
Livraghi T, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47(1):82-89.
Mascarenhas BA, Ravikumar TS. Experimental basis for hepatic cryotherapy. Semin Surg Oncol. 1998;14(2):110-115.
McKinnon JG, et al. Cryosurgery for malignant tumours of the liver. Can J Surg. 1996;39:401-406.
Nagorney DM, Gigot JF. Primary epithelial hepatic malignancies: etiology, epidemiology, and outcome after subtotal and total hepatic resection. Surg Oncol Clin N Am. 1996;5(2):283-300.
Ng KK, et al. Comparison of systemic responses of radiofrequency ablation, cryotherapy, and surgical resection in a porcine liver model. Ann Surg Oncol. 2004;11(7):650-657.
Nordenstedt H, et al. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42(Suppl 3):S206-S214.
Onik G, et al. Ultrasound guided hepatic cryosurgery in the treatment of metastatic colon carcinoma. Cancer. 1991;67:901-907.
Onik GM, et al. Hepatic cryosurgery with and without the Bair Hugger. J Surg Oncol. 1993;52(3):185-187.
Pawlik TM, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715-722. discussion 722-714
Pearson AS, et al. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg. 1999;178(6):592-599.
Poppendiek D. Thermal conductivity measurements and predictions for biological fluids and tissues. Cryobiology. 1967;4:318-327.
Ravikumar TS, et al. A 5-year study of cryosurgery in the treatment of liver tumors. Arch Surg. 1991;126(12):1520-1523. discussion 1523-1524
Ravikumar TS, et al. Experimental and clinical observations on hepatic cryosurgery for colorectal metastases. Cancer Res. 1991;51(23 Pt 1):6323-6327.
Richter S, et al. Arteriolovenular shunting critically determines shutdown of microcirculation upon cryotherapy in tumor-bearing rat liver. Ann Surg Oncol. 2005;12(4):303-312.
Rivoire M, et al. Combination of neoadjuvant chemotherapy with cryotherapy and surgical resection for the treatment of unresectable liver metastases from colorectal carcinoma. Cancer. 2002;95(11):2283-2292.
Rubinsky B, et al. The process of freezing and the mechanism of damage during hepatic cryosurgery. Cryobiology. 1990;27(1):85-97.
Scheele J, et al. Resection of colorectal liver metastases. World J Surg. 1995;19(1):59-71.
Seifert JK, Junginger T. Cryotherapy for liver tumors: current status, perspectives, clinical results, and review of literature. Technol Cancer Res Treat. 2004;3(2):151-163.
Seifert JK, Morris DL. Prognostic factors after cryotherapy for hepatic metastases from colorectal cancer. Ann Surg. 1998;228(2):201-208.
Seifert JK, et al. Large volume hepatic freezing: association with significant release of the cytokines interleukin-6 and tumor necrosis factor a in a rat model. World J Surg. 2002;26(11):1333-1341.
Shafir M, et al. Cryoablation of unresectable malignant liver tumors. Am J Surg. 1996;171(1):27-31.
Sheen AJ, et al. Cryotherapeutic ablation of liver tumors. Br J Surg. 2002;89:1396-1402.
Steele GJr, et al. New surgical treatments for recurrent colorectal cancer. Cancer. 1990;65(3 Suppl):723-730.
Tandan VR, et al. Long-term survival after hepatic cryosurgery versus surgical resection for metastatic colorectal carcinoma: a critical review of the literature. Can J Surg. 1997;40(3):175-181.
Weaver ML, et al. Hepatic cryosurgery in treating colorectal metastases. Cancer. 1995;76(2):210-214.
Wei AC, et al. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol. 2006;13(5):668-676.
Wrens SM, et al. Is cryosurgical ablation appropriate for treating hepatocellular carcinoma? Arch Surg. 1997;132:599-604.
Wu B, et al. Magnetic resonance imaging-guided percutaneous cryoablation of hepatocellular carcinoma in special regions. Hepatobiliary Pancreat Dis Int. 2010;9(4):384-392.
Yan TD, et al. Management of involved or close resection margins in 120 patients with colorectal liver metastases: edge cryotherapy can achieve long-term survival. Am J Surg. 2006;191(6):735-742.
Yeh KA, et al. Cryosurgical ablation of hepatic metastases from colorectal carcinomas. Am Surg. 1997;63:63-68.
Zhou XD, et al. Ablative approach for primary liver cancer: Shanghai experience. Surg Oncol Clin N Am. 1996;5(2):379-390.