Croup versus epiglottitis
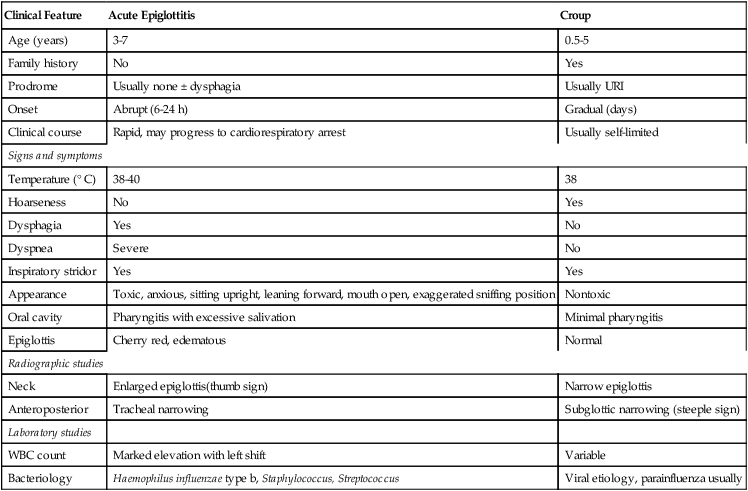
Because of the possibility of rapid clinical progression to complete obstruction, acute epiglottitis requires early and prompt intervention. To provide the appropriate therapeutic interventions, one must be able to differentiate between acute epiglottitis and laryngotracheobronchitis. Table 205-1 compares these two causes of severe stridor.
Table 205-1
Acute Epiglottitis Versus Croup
| Clinical Feature | Acute Epiglottitis | Croup |
| Age (years) | 3-7 | 0.5-5 |
| Family history | No | Yes |
| Prodrome | Usually none ± dysphagia | Usually URI |
| Onset | Abrupt (6-24 h) | Gradual (days) |
| Clinical course | Rapid, may progress to cardiorespiratory arrest | Usually self-limited |
| Signs and symptoms | ||
| Temperature (° C) | 38-40 | 38 |
| Hoarseness | No | Yes |
| Dysphagia | Yes | No |
| Dyspnea | Severe | No |
| Inspiratory stridor | Yes | Yes |
| Appearance | Toxic, anxious, sitting upright, leaning forward, mouth open, exaggerated sniffing position | Nontoxic |
| Oral cavity | Pharyngitis with excessive salivation | Minimal pharyngitis |
| Epiglottis | Cherry red, edematous | Normal |
| Radiographic studies | ||
| Neck | Enlarged epiglottis(thumb sign) | Narrow epiglottis |
| Anteroposterior | Tracheal narrowing | Subglottic narrowing (steeple sign) |
| Laboratory studies | ||
| WBC count | Marked elevation with left shift | Variable |
| Bacteriology | Haemophilus influenzae type b, Staphylococcus, Streptococcus | Viral etiology, parainfluenza usually |
Management
Croup
Acute epiglottitis
In cases with a more toxic presentation (see Table 205-1) or imminent respiratory collapse, a diagnosis of epiglottitis (supraglottitis) must be suspected. Consider the four “Ds” of epiglottitis—drooling, dysphagia, dysphonia, and dyspnea. The child with acute epiglottitis should be disturbed as little as possible (e.g. by radiography examinations or phlebotomy). Transport the child to the operating room in the sitting position with airway equipment readily available for possible ventilatory support. Do not attempt to visualize the pharynx, which may cause acute obstruction. The operating room should be set up for direct laryngoscopy, emergency bronchoscopy, and possible tracheostomy. Monitoring should include blood pressure, electrocardiography, precordial stethoscope, and pulse oximetry. Induction of anesthesia with O2 and an inhalation anesthetic agent (sevoflurane or halothane, if it is available), with the child seated, should be performed. Because of the unpredictable variation in the amount of edema, and the potential anatomic distortion and difficulty with ventilation, the use of neuromuscular blocking agents and barbiturates should be avoided.




