Critical Care Medicine in Pregnancy
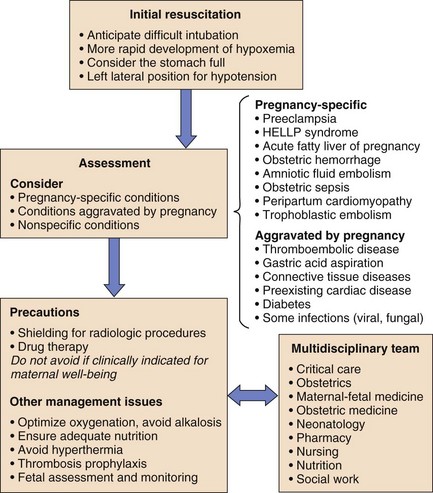
Management of the critically ill pregnant patient is a situation with which few intensive care physicians gain significant expertise. The usual clinical approach may be altered by the physiologic changes induced by pregnancy, by the relatively uncommon pregnancy-specific conditions, and by perceived limitations on therapy produced by the presence of a fetus (Fig. 81.1).
Physiologic Changes in Pregnancy
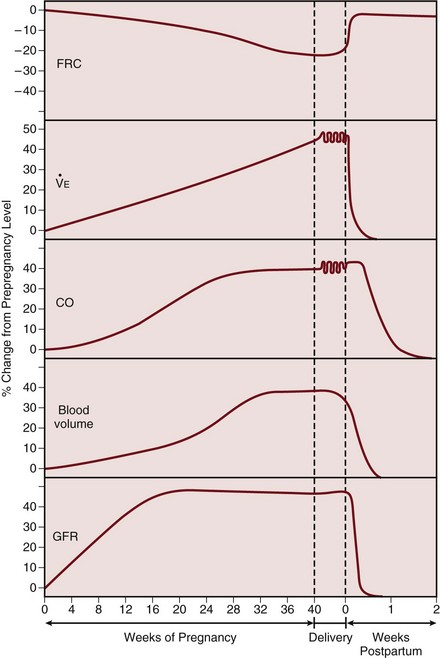
The pregnant woman undergoes a number of physiologic changes affecting various systems relevant to critical care management. From a respiratory perspective, the upper airways develop edema and hyperemia, which may be relevant during endotracheal intubation. Changes in lung volumes occur, with a 10% to 25% decrease in functional residual capacity (FRC), whereas total lung capacity decreases only minimally as the thoracic cage widens to compensate.1 Forced expiratory volume in 1 second (FEV1) is not altered by the pregnant state. Lung compliance remains unchanged, but chest wall and total respiratory compliance are reduced.2
The rising progesterone level stimulates an increase in ventilation. Tidal volume and minute ventilation increase from the first trimester, reaching 20% to 40% above baseline by term (Table 81.1).3 A mild respiratory alkalosis is produced with compensatory renal excretion of bicarbonate (PaCO2 28 to 32 mm Hg; HCO3− 18 to 21 mEq/L). Oxygen consumption increases because of the demands of the fetus and maternal metabolic processes, reaching levels up to 33% above baseline by term. Arterial PO2 remains normal throughout pregnancy, but mild hypoxemia caused by an increased alveolar-arterial oxygen tension difference may develop in the supine position, as FRC diminishes near term.
Table 81.1
Physiologic Changes in Late Pregnancy
| Parameter | Change |
| Respiratory | |
| Functional residual capacity | Decreased 10-25% |
| Minute ventilation | Increased 20-40% |
| Arterial partial pressure of oxygen | No change |
| Arterial partial pressure of carbon dioxide | Reduced to 28-32 mm Hg |
| Serum bicarbonate | Reduced to 18-21 mEq/L |
| Cardiac | |
| Heart rate | Increased 10-30% |
| Pulmonary capillary wedge pressure | No change |
| Cardiac output | Increased 30-50% |
| Systemic vascular resistance | Decreased 20-30% |
| Pulmonary vascular resistance | Decreased 20-30% |
| Renal | |
| Glomerular filtration rate | Increased 50% |
| Creatinine | Decreased (24-68 µmol/L; 0.29-0.77 mg/dL) |
Maternal blood volume and cardiac output increase through pregnancy, reaching a peak at 30% to 50% above baseline levels by about 28 weeks (Fig. 81.2).4 Hemodynamic measurements by pulmonary artery catheter in the near-term patient demonstrate the increased cardiac output, with a reduced systemic vascular resistance and pulmonary vascular resistance (see Table 81.1).5 During labor and continuing into the immediate postpartum period, cardiac output is further augmented by the return of 300 to 500 mL of blood to the central circulation.6
Oxygen delivery to the fetus is dependent on the maternal arterial oxygen content and the uterine blood flow. Maternal hypotension, alkalosis (e.g., hyperventilation), and endogenous or exogenous catecholamines can vasoconstrict the uterine artery and adversely affect fetal oxygenation.7 Uterine blood flow is also reduced transiently by uterine contractions. Although umbilical venous blood returning to the fetus has a relatively low oxygen tension, a high oxygen content is maintained by the left shift of the oxygen dissociation curve of fetal hemoglobin.
Glomerular filtration rate increases early in pregnancy, reaching a value 50% above prepregnancy levels in the second trimester, and remains elevated throughout pregnancy (see Fig. 81.2).8 The normal serum creatinine level is therefore in the range of 0.5 to 0.7 mg/dL (45 to 60 µmol/L). As pregnancy progresses, mild ureteric dilation and mild hydronephrosis may occur as a result of uterine compression and smooth muscle relaxation.
The increase in plasma volume is associated with a lesser increase in red blood cell mass causing a physiologic anemia, with a hematocrit of 32% to 34% by the third trimester. A mild leukocytosis occurs with white blood cell count rising further during labor. Platelet counts are usually unchanged, although a condition of benign mild thrombocytopenia may occur.9 Procoagulant factors rise, contributing to the hypercoagulable state of pregnancy. The erythrocyte sedimentation rate (ESR) rises related to increased levels of plasma globulins.
Critical Care Management
General Care
Positioning
In the supine position the gravid uterus produces mechanical effects on the vena cava and aorta, reducing central venous return. This results in a decrease in cardiac output and hypotension. This “supine hypotensive syndrome” should be considered in hemodynamically unstable patients.10 Pregnant patients should be positioned on their left side, or at least with the right hip slightly elevated.
Radiologic Procedures and Fetal Risk
Radiologic investigations are often essential for the assessment and management of the critically ill pregnant patient. Although there are potential risks of exposing the fetus to ionizing radiation, fetal well-being depends on maternal recovery, and appropriate radiologic procedures should not be avoided. Techniques such as shielding the abdomen with lead and using a well-collimated x-ray beam can effectively reduce exposure. With these precautions, estimated fetal radiation exposure can be limited to safe levels for most procedures, although investigations such as abdominal-pelvic computed tomography (CT) will obviously cause significant fetal radiation exposure (Table 81.2).11
Table 81.2
| Investigation | Fetal Radiation Exposure (mGy) |
| Chest radiograph (with abdomen shielded) | 0.01 |
| Ventilation-perfusion scan | |
| Perfusion | 0.1-1 |
| Ventilation | 0.1-0.4 |
| CT pulmonary angiogram | 0.1-4 |
| CT scan of pelvis and abdomen | 30-50 |
| Radiation effect on fetus | |
| Teratogenicity | 50-100 |
| Oncogenicity | 20-50 |
The adverse effects of fetal exposure to radiation include oncogenicity and teratogenicity. A twofold increased risk of childhood leukemia may result from fetal exposure in the range of 20 to 50 mGy (2 to 5 rads). Most intensive care unit (ICU) procedures can be carried out well below these levels of exposure to the fetus (see Table 81.2). Teratogenicity does not appear to occur at these radiation doses, requiring radiation exposure greater than 50 to 100 mGy (5 to 10 rads). Nonetheless, every effort should be made to minimize uterine exposure, particularly in the first trimester.
Drug Therapy in Pregnancy
Pharmacotherapy during pregnancy requires consideration of the altered drug clearance, metabolism, and volume of distribution of drugs in pregnancy, and the potential pharmacologic and teratogenic effects on the embryo. A detailed description of drug therapy in pregnancy is beyond the scope of this chapter. Consultation with an obstetrician and pharmacist is essential, and several excellent resources are available.12,13 Some common ICU drugs are discussed briefly as follows.
Sedation, Analgesia, and Neuromuscular Blockade
Little data exist on the preferred drugs for prolonged sedation, analgesia, or neuromuscular blockade in pregnancy. Benzodiazepine use in early pregnancy has been associated with a small risk of congenital malformations, mainly cleft lip and palate. Midazolam crosses the placenta to a lesser degree than diazepam, which can accumulate in the fetus at levels greater than in the mother. Propofol has been used as an induction agent for cesarean section, but a single case report describes the development of non–anion gap acidosis in two pregnant women receiving propofol infusion for neurosurgical procedures.14 Congenital malformations have not been demonstrated with use of narcotic analgesics such as morphine, meperidine, and fentanyl. The majority of nondepolarizing neuromuscular blocking agents have been shown to cross the placenta including pancuronium, vecuronium, and atracurium, but transfer is unlikely to have clinical effects on the fetus in the short term. Nevertheless, if sedative or paralyzing agents are used in the pregnant woman, this information must be communicated to the neonatologist, who should anticipate the need for ventilatory support for the fetus.
Fetal Oxygenation and Monitoring
Attention to interventions aimed at optimizing fetal oxygenation is important in the management of any critically ill pregnant patient. Depending on gestational age, delivery of the fetus may be the most appropriate intervention. Uteroplacental oxygen delivery can be optimized by increasing oxygen carrying capacity—by blood transfusion, improving maternal cardiac output, and optimizing maternal oxygenation. The simple maneuver of tilting the patient to the left lateral position to increase cardiac output should always be considered.10
Ventilatory Support
Airway Management
Failed intubation is more common in the obstetric population than in other anesthetic intubations.15 The reduced oxygen reserve caused by diminished FRC and increased oxygen consumption produces rapid desaturation in response to apnea or hypoventilation.16 Preoxygenation with 100% oxygen is beneficial, but respiratory alkalosis must be avoided. In view of the delayed gastric emptying and elevated intra-abdominal pressure, the pregnant patient should always be considered to have a full stomach and appropriate precautions should be taken. Upper airway hyperemia and edema may reduce visualization and necessitate use of a smaller endotracheal tube. The risk of bleeding is increased and nasal intubation should be avoided.
Mechanical Ventilation
The normal PaCO2 of about 30 mm Hg in late pregnancy should be considered in the interpretation of arterial blood gases when decisions are made to institute ventilatory support. Data on the prolonged mechanical ventilation of pregnant patients are limited. Hyperventilation should be avoided because this adversely affects uterine blood flow17 because of the resulting alkalemia as well as the effect of positive-pressure ventilation in reducing cardiac output. The current ventilatory approach of avoiding excessive lung stretch by pressure limitation and permissive hypercapnia has not been assessed in pregnancy. The usual pressure limits (e.g., plateau pressure of 35 cm H2O) may not be applicable in the near-term patient, in whom chest wall compliance is reduced. Transpulmonary pressures may not be elevated at a plateau pressure of 35 cm H2O, and higher ventilatory pressures may be acceptable in pregnant patients near term. Although late pregnancy is associated with a mild respiratory alkalosis, maternal hypercapnia up to 60 mm Hg in the presence of adequate oxygenation does not appear to be detrimental to the fetus.18 Maternal hypercapnia and respiratory acidosis may produce fetal acidosis, but this does not have the same ominous implications as fetal acidosis due to lactic acidosis resulting from fetal hypoxia.19 However, acidosis produces a right shift of the hemoglobin oxygen dissociation curve, which may negate the beneficial oxygen-carrying characteristics of fetal hemoglobin. If marked respiratory acidosis results from permissive hypercapnia, treatment with bicarbonate may improve maternal and fetal acidemia.
It may be considered that, because of the effects of pregnancy on respiratory physiology, delivery of the pregnant patient will result in improvement in the mother’s respiratory status.20 Limited case series addressing this issue have not found a significant benefit to the mother, and delivery carries a risk of harm.21,22 Some improvement in oxygenation has been noted, but without improvement in respiratory system compliance or reduction in positive end-expiratory pressure (PEEP) level.20 Delivery is therefore not indicated purely in the hope of improving “maternal condition.” If the fetus is considered viable but is at risk due to severe maternal hypoxia, consultation with a neonatologist may identify a benefit to the fetus in being delivered. Obstetric indications should always determine the mode of delivery.
Cardiopulmonary Resuscitation
Cardiopulmonary resuscitation requires only minor modifications from standard protocols.23 Intravenous access should be established above the diaphragm owing to potentially impeded inferior vena caval blood flow. Electrical cardioversion and defibrillation may be performed in pregnancy, but fetal monitoring leads should be removed to prevent electrical arcing. Management in the supine position may cause aortocaval compression, resulting in impaired venous return and inadequate cardiac output. Manual displacement of the uterus to the left should be used, or alternatively a 30-degree left lateral tilt, which may impede cardiac compressions. Appropriate pharmacologic therapy should not be withheld if clinically indicated. Perimortem cesarean section may save the fetus when initial attempts at resuscitation have failed in a woman with a fetus at a viable gestation. Data suggest that infant survival without neurologic sequelae is highest if the postmortem cesarean section is initiated within 4 minutes of cardiac arrest, aiming for delivery within 5 minutes of arrest.
Pregnancy-Specific Conditions Requiring Intensive Care Unit Care
Preeclampsia
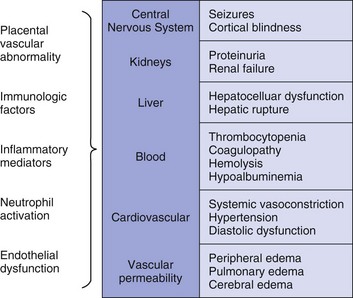
Preeclampsia is a pregnancy-induced condition usually occurring after 20 weeks’ gestation, characterized by hypertension and proteinuria.24 The cause remains unknown but is likely related to a placental abnormality producing a diffuse maternal endothelial effect, which leads to vasospasm and reduced organ perfusion (Fig. 81.3). Preeclampsia and its complications account for 20% to 50% of obstetric admissions to the ICU.25–27 Less common multisystem diseases should be considered in the differential diagnosis including systemic lupus erythematosus (SLE), thrombotic thrombocytopenic purpura (TTP), and hemolytic-uremic syndrome (HUS) (Table 81.3). Complications of preeclampsia may result in critical illness including pulmonary edema, cerebral edema, renal failure, hypertensive crisis, seizures (eclampsia), and the HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome (microangiopathic hemolytic anemia, thrombocytopenia, and hepatic involvement).28
The only specific treatment is delivery of the fetus and placenta. Delivery is always in the best interest of the mother, but timing may depend on fetal maturity. The role of antihypertensive therapy is to prevent maternal hypertensive complications, and it does not alter the natural history of the condition or benefit the fetus.29 Commonly used agents include intravenous hydralazine or labetalol, as well as oral calcium antagonists.24 Rapid reduction in blood pressure should usually be avoided because of the risk of reducing uteroplacental perfusion. Magnesium sulfate is used for seizure prophylaxis and treatment, giving an initial intravenous bolus (2 to 4 g) followed by an infusion of 1 to 3 g/hour. Toxic magnesium levels may occur (>3.5 mmol/L), particularly in the presence of renal dysfunction, producing respiratory muscle weakness and cardiac conduction defects. Hypocalcemia may be noted during magnesium infusion and should not be corrected routinely because this would negate the therapeutic effects of magnesium. Fluid management in preeclampsia usually requires careful volume expansion, but excessive fluid administration can cause pulmonary or cerebral edema.
HELLP Syndrome
The HELLP syndrome is a condition associated with preeclampsia, characterized by the development of a microangiopathic hemolytic anemia and a consumptive thrombocytopenia.28 Reduced hepatic perfusion results in periportal and focal parenchymal necrosis, elevated liver enzymes, and rarely hepatic hemorrhage or rupture. The clinical presentation is often with epigastric or right upper quadrant pain, nausea, vomiting, or evidence of bleeding. Features of preeclampsia do not occur in all patients, and approximately 30% of patients with HELLP develop the disease only in the postpartum period.
Management includes early delivery if the fetus is viable, blood product support, and management of associated preeclampsia. Dexamethasone initiated prior to delivery has been reported to produce more rapid recovery of thrombocytopenia in some studies but this was not confirmed in the most recent study.30,31 Delivery may require platelet support, and if cesarean section is necessary, hemorrhage should be anticipated and adequate drains should be inserted. Epidural anesthesia is usually contraindicated in the presence of thrombocytopenia.
Significant overlap exists between HELLP and other multisystem conditions and thrombotic microangiopathies (see Table 81.3). A response to plasmapheresis may be noted in some patients, possibly representing those with TTP or HUS rather than HELLP. Plasmapheresis is recommended for patients with delayed postpartum resolution when severe thrombocytopenia, hemolysis, or organ dysfunction persist more than 72 hours following delivery. The maternal mortality rate for women with HELLP syndrome is reported between 1% and 3% (with isolated reports as high as 25%) and with a significant perinatal mortality rate at 8% to 60%.
Acute Fatty Liver of Pregnancy
AFLP is an uncommon condition affecting about 1 in 15,000 pregnancies. Although early reports described acute fulminant hepatic failure with high maternal and fetal mortality rates, the condition is now usually recognized at an earlier stage, allowing early delivery and a significantly improved outcome.32 Maternal mortality rate has been reported at 0% to 18% with fetal loss of 23% to 60%. The cause of this condition is unknown, but an association with a fetal inborn error of metabolism (L-CHAD [long-chain 3-hydroxyacyl-coenzyme A dehydrogenase] deficiency) has been described.33 Pathologically, diffuse fatty infiltration of the liver occurs with necrosis and inflammation being mild or absent.
The differential diagnosis of liver disease in pregnancy is wide, and the multisystemic effects of AFLP may resemble systemic lupus, TTP, or HUS (see Table 81.3).
Amniotic Fluid Embolism
Amniotic fluid embolism is a catastrophic cardiopulmonary complication of pregnancy, carrying a mortality rate of 10% to 86%.34 It usually occurs in association with labor and delivery but may be precipitated by uterine trauma or manipulations. Amniotic fluid enters the venous circulation and particulate contents or humoral factors produce acute pulmonary hypertension, as well as acute left ventricular dysfunction.35 The pregnant woman develops sudden onset of dyspnea, hypoxemia, and cardiovascular collapse. The presenting feature may sometimes be seizures related to cerebral hypoxia or acute fetal hypoxia and distress. Many patients with amniotic fluid embolism die within the first hour. No specific diagnostic test is available, and the diagnosis of amniotic fluid embolism is based on a typical clinical picture and exclusion of other conditions such as septic shock, pulmonary thromboembolism, abruptio placentae, tension pneumothorax, or a myocardial ischemic event.
Treatment involves routine resuscitative and supportive measures including mechanical ventilation and inotropic therapy. A role for corticosteroids has been suggested on the basis of the hypothesis that the process may involve an anaphylactoid reaction to amniotic fluid contents.34 Survivors of the initial process may develop disseminated intravascular coagulation (DIC) and ARDS. Neurologic damage caused by the initial hypotension and hypoxemia is common.
Obstetric Hemorrhage
Supportive management of obstetric hemorrhage is similar to that for any cause of hemorrhage and involves rapid volume replacement, supplemental oxygen administration, and red blood cell and blood product support for an associated dilutional coagulopathy. Various pharmacologic agents are utilized to control uterine bleeding. Intramuscular administration of methylergonovine (0.2 mg) may be useful in the presence of uterine atony but is contraindicated in the presence of hypertension.36 Oxytocin is infused intravenously in a dose greater than that used for augmentation of labor, up to 100 mU/minute (e.g., 40 U in 1000 mL normal saline at 150 mL/hour). This dose may produce an antidiuretic effect and cause hyponatremia. A prostaglandin F2a analog (carboprost tromethamine—Hemabate) given intramuscularly (0.25 mg, repeated every 15 to 90 minutes to maximum 2 mg) or intramyometrially, is effective in controlling hemorrhage.36,37 Side effects include vomiting, hypertension, bronchoconstriction, and increased intrapulmonary shunt. Obstetricians are increasingly using intrauterine balloon devices to tamponade bleeding.38 Antibiotic prophylaxis is often given while the balloon is in place and analgesia is essential.
Isolated reports suggest that recombinant factor VIIa may be useful in the management of severe obstetric hemorrhage.39 If the above-mentioned methods fail to control bleeding, radiologic transcatheter embolization of the internal iliac or uterine artery is usually effective.40 Surgical exploration with arterial ligation or hysterectomy may become necessary.
Peripartum Cardiomyopathy
Cardiac failure may occur in the absence of preexisting heart disease because of peripartum cardiomyopathy. This idiopathic condition presents in the last month of pregnancy or within 5 months of delivery, with an incidence of approximately 1 in 3500 live births.41 The clinical presentation is usually after 36 weeks’ gestation (in contrast to women with preexisting cardiac disease) and involves the gradual onset of symptoms of heart failure. The diagnosis is made by the demonstration of left ventricular systolic dysfunction and by the exclusion of other causes of cardiomyopathy. During labor and the early postpartum period, tachycardia and increased cardiac output may precipitate acute pulmonary edema.
Treatment is similar to other patients with cardiac failure, although angiotensin-converting enzyme (ACE) inhibitors should be avoided before delivery. Anticoagulation is essential because of the hypercoagulable state of pregnancy and the high incidence of thrombotic complications. A subset of patients may have an inflammatory myocarditis, and immunosuppressive therapy may be considered in those who do not improve after 2 weeks of standard treatment.41 Although a high mortality rate has been described (10% to 50%), about half of patients recover normal ventricular function.42
Tocolytic Pulmonary Edema
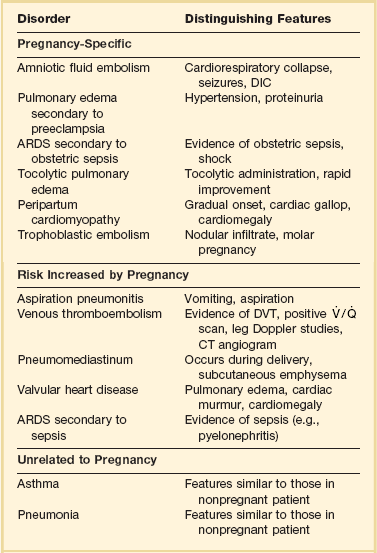
β-Adrenergic blockers can be used to inhibit uterine contractions in preterm labor, although this is less frequently used because studies have demonstrated a lack of fetal benefit from this practice. A complication of this β-agonist therapy in pregnancy is the development of acute pulmonary edema.43 Treatment involves discontinuing the β-agonist and supportive treatment including diuresis and oxygen therapy. Failure of the pulmonary edema to resolve within 24 hours should prompt a search for an alternative diagnosis (Table 81.4).
Gestational Trophoblastic Disease
Pulmonary hypertension and pulmonary edema may occur in the setting of benign hydatidiform mole, as a result of trophoblastic pulmonary embolism. This most commonly occurs during evacuation of the uterus, with a higher incidence of pulmonary complications in the woman later in pregnancy.44 With supportive treatment resolution occurs within 48 to 72 hours. Molar pregnancy may also be associated with choriocarcinoma, which can produce multiple, discrete, pulmonary metastases.
Conditions Not Specific to Pregnancy
Septic Shock
Changes occur in the immune status in pregnant women, facilitating tolerance to paternally derived fetal antigens. An altered TH1/TH2 balance occurs, with the maternal immune response favoring humoral immunity (TH2 response), and suppressing cell-mediated immunity (TH1 response), which could be harmful to the fetus.45 This altered immune response may predispose pregnant women to increased incidence or severity of certain infections, including Listeria monocytogenes infection, disseminated herpesvirus infections, varicella, and coccidioidomycosis infections. Human immunodeficiency virus infections should always be considered. Obstetric sepsis in the antepartum period produces chorioamnionitis, but most obstetric sepsis occurs in the postpartum period. The most common location of infection is the placental site, causing endometritis, which can spread to become a peritonitis. Episiotomy sites or cesarean section wounds may also become infected. Infections often involve a mixed flora, with microorganisms originating from the vagina (e.g., anaerobes, group B streptococci), intestine (gram-negative organisms, enterococci, anaerobes), sexual transmission (e.g., Neisseria gonorrhoeae), or hematogenous spread (e.g., Listeria, group A streptococci).46
Initial empiric antibiotic therapy should provide broad gram-negative, gram-positive, and anaerobic cover. Common regimens use drugs such as ampicillin, gentamicin, and clindamycin; ampicillin/sulbactam; ticarcillin/clavulanate; piperacillin and gentamicin; or carbapenems.47 Urgent delivery may be required for management of chorioamnionitis with sepsis syndrome.
Group A streptococcal necrotizing fasciitis and toxic shock syndrome have been reported to occur unexpectedly following an uncomplicated pregnancy and delivery.48 Management includes antibiotic therapy with penicillin and clindamycin and early surgical intervention. Intravenous immunoglobulin (2 g/kg) may be beneficial.
Acute Respiratory Distress Syndrome in Pregnancy
The pregnant patient is at risk of developing acute lung injury from pregnancy-associated complications and other conditions (see Table 81.4).49 The pregnant patient appears to be at increased risk of developing pulmonary edema because of the cardiovascular changes, the reduced albumin level occurring in pregnancy, increased capillary leak, and possibly an upregulation of components of the acute inflammatory response.
Management is similar to that in the nonpregnant patient. Adequate maternal oxygenation is essential for fetal well-being, and delivery may benefit both the mother and the fetus.50 Survival from ARDS is as good as or better than that in the general population, likely because of these patients’ young age, lack of comorbid conditions, and the reversibility of many of the predisposing conditions.
Pulmonary Thromboembolic Disease
Investigation of suspected pulmonary embolism is not different from that in the nonpregnant patient, with duplex ultrasound as the initial investigation. False positives may occur with Doppler alone because of venous obstruction by the gravid uterus. Ventilation-perfusion scanning and CT pulmonary angiography can be performed with low risk of fetal radiation exposure (see Table 81.2).11
Warfarin is generally avoided in pregnancy because of the risk of a first trimester embryopathy and central nervous system abnormalities with second- and third-trimester exposure. Heparin does not cross the placenta and can be readily reversed, and low-molecular-weight heparins are safe and effective in pregnancy although less easy to reverse acutely.51 Thrombolysis has been used successfully in pregnancy but should be limited to life-threatening situations. Transvenous inferior vena caval filters have been used in the pregnant patient, although there is a risk of dislodgement because of venous dilation and pressure effects.
Asthma
The hormonal changes of pregnancy can affect asthma variably, with worsening, improvement, or no substantial change occurring. Asthma is a common condition, and acute asthmatic attacks are therefore an important cause of respiratory compromise in pregnancy. In assessment of the patient with a severe acute attack, the reduced PaCO2 occurring in late pregnancy should be considered; a normal PaCO2 level associated with acidosis may imply respiratory failure. Treatment is similar to the nonpregnant patient, including β-agonists and corticosteroids. Although there is a natural reluctance to prescribe drug therapy in these patients, pregnancy is not an absolute contraindication to systemic corticosteroid therapy.52 Uncontrolled asthma is more dangerous to the fetus than appropriate drug therapy, and management should highlight the importance of adequate oxygenation.
Cardiac Disease
Overt or occult preexisting heart disease may produce acute cardiac decompensation as the blood volume and cardiac output increase during pregnancy. Women with prior cardiac events, cyanotic disease, or pulmonary hypertension are at particular risk. Patients with mitral and aortic stenosis are at risk of developing hemodynamic deterioration as the physiologic changes peak at about 28 weeks, but mild to moderate regurgitant valvular disease is generally well tolerated.53 The onset of atrial fibrillation or severe hypertension can precipitate sudden hemodynamic deterioration even in those women with less severe cardiac disease.
Patients with moderate to severe mitral stenosis are likely to experience hemodynamic deterioration during the third trimester or during labor. Treatment is similar to the nonpregnant patient using digoxin and beta blockers to control heart rate and diuretics to reduce left atrial pressure.54 ACE inhibitors should be avoided. Most patients with mitral stenosis can undergo vaginal delivery with consideration to invasive hemodynamic monitoring during labor and the early postpartum period. Epidural anesthesia is usually better tolerated hemodynamically than general anesthesia during labor and delivery. Electrical cardioversion can be performed safely if indicated.
Severe aortic stenosis is associated with significant risk during pregnancy,54 with symptoms such as dyspnea, angina, or syncope appearing from late in the second trimester. Percutaneous aortic balloon valvuloplasty can be performed during pregnancy. Spinal and epidural anesthesia during labor may adversely affect hemodynamics because of the vasodilatory effects.
Ischemic heart disease is uncommon in pregnancy but may be missed because of masking of the symptoms, signs, and cardiac enzyme levels by pregnancy. Coronary artery dissection may occur, particularly in the immediate postpartum period. Aortic dissection may occur related to hypertension, aortic coarctation, or Marfan syndrome and is associated with significant maternal and fetal death.55
Women with cyanotic congenital heart disease, particularly those with associated pulmonary hypertension, are at significant risk during pregnancy. Because of the inability of the right ventricle to tolerate the increases in cardiac output, primary or secondary pulmonary hypertension is associated with a high mortality rate in pregnancy.56 New specific treatments are now available, and although clinical trials in pregnancy are lacking, mortality rate appears to be decreasing to some degree.57 Vasodilators such as prostacyclin (inhaled or intravenous) or inhaled nitric oxide, as well as phosphodiesterase-5 inhibitors such as sildenafil, have been used successfully in pregnancy.58,59 Endothelin receptor antagonists (e.g., bosentan) are avoided because of potential teratogenicity, and anticoagulation is recommended for most patients. Patients with pulmonary hypertension should be managed in a center with expertise and experience in this area.
Trauma
The anatomic and physiologic changes of pregnancy may alter the manifestations and severity of traumatic injury. Uterine injury can produce severe hemorrhage because of the high uterine blood flow and may precipitate placental abruption or rarely uterine rupture. Uterine rupture manifests with maternal shock, abdominal pain, and palpable fetal parts. Penetrating injury to the abdomen predominantly affects the uterus in later pregnancy, but because intra-abdominal viscera are compressed in the upper abdomen, minor injury may result in significant damage.60 Fractures of the pelvis can produce severe retroperitoneal hemorrhage because of dilation of pelvic veins.
Maternal trauma is associated with a significant increase in fetal loss, due to maternal shock or hypoxia, placental injury, or direct fetal injury.61 Direct fetal injury resulting from blunt trauma usually involves head injury related to maternal pelvic fracture. Other fetal injuries may occur following blunt or penetrating trauma, sometimes with minimal maternal injury. A high fetal mortality rate occurs in the presence of maternal burns to greater than 30% of the body surface area. Placental abruption is an important cause of fetal demise,60 and may present with vaginal bleeding, abdominal cramps, uterine tenderness, amniotic fluid leakage, and unexplained fetal distress or maternal hypovolemia. Placental abruption is often complicated by DIC due to release of thromboplastin into the maternal circulation.
Evaluation of the pregnant trauma patient should include a detailed abdominal examination, but physical signs may be affected by changing organ position and the reduced peritoneal sensitivity that occurs in pregnancy.62 Initial investigations should include blood type including Rh status. Ultrasound is useful for evaluation of the fetus for injury and biophysical profile and to assess intra-abdominal organ damage. Ultrasound-guided paracentesis or diagnostic peritoneal lavage (by open technique, above the uterus) may aid in detecting bowel perforation or intraperitoneal hemorrhage. Transplacental hemorrhage of fetal blood into the maternal circulation may occur and can result in fetal exsanguination and maternal Rh sensitization. Fetomaternal hemorrhage is detected by the Kleihauer-Betke test, which identifies fetal cells in the maternal blood smear, and can estimate the volume of fetal hemorrhage by the percentage of red blood cells of fetal origin. Fetal evaluation includes heart rate assessment by auscultation (possible from 20 weeks) or Doppler probe. Obstetric consultation and continuous fetal cardiotocography are important when the fetus is at a viable gestation.
Care of the severely injured pregnant patient requires a multidisciplinary approach involving the emergency physician, trauma surgeon, obstetrician, intensivist, and neonatologist. Initial resuscitation follows usual principles with efforts directed primarily at stabilizing the mother. Fluid replacement may need to be given more rapidly than in nonpregnant women because of the physiologic increase in plasma volume, and maternal blood pressure and heart rate may not be reliable predictors of the degree of hemorrhage.62 Left lateral positioning to prevent supine hypotensive syndrome is an important consideration in the hypotensive patient. Rh-negative mothers with abdominal trauma should receive Rh immune globulin even in the presence of a negative Kleihauer-Betke test. Higher doses of Rh immune globulin will be necessary in the presence of significant fetomaternal hemorrhage.
References
1. Elkus, R, Popovich, J. Respiratory physiology in pregnancy. Clin Chest Med. 1992; 13:555–565.
2. Marx, GF, Murthy, PK, Orkin, LR. Static compliance before and after vaginal delivery. Br J Anaesth. 1970; 42:1100–1104.
3. Rees, GB, Pipkin, FB, Symonds, EM, Patrick, JM. A longitudinal study of respiratory changes in normal human pregnancy with cross-sectional data on subjects with pregnancy-induced hypertension. Am J Obstet Gynecol. 1990; 162:826–830.
4. Mabie, WC, DiSessa, TG, Crocker, LG, et al. A longitudinal study of cardiac output in normal human pregnancy. Am J Obstet Gynecol. 1994; 170:849–856.
5. Clark, SL, Cotton, DB, Lee, W, et al. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol. 1989; 161:1439–1442.
6. Robson, SC, Dunlop, W, Boys, RJ, Hunter, S. Cardiac output during labour. BMJ. 1987; 295:1169–1171.
7. Assali, NS. Dynamics of the uteroplacental circulation in health and disease. Am J Perinatol. 1989; 6:105–109.
8. Baylis, C. Glomerular filtration rate in normal and abnormal pregnancies. Semin Nephrol. 1999; 19:133–139.
9. Schwartz, KA. Gestational thrombocytopenia and immune thrombocytopenias in pregnancy. Hematol Oncol Clin North Am. 2000; 14:1101–1116.
10. Kinsella, SM, Lohmann, G. Supine hypotensive syndrome. Obstet Gynecol. 1994; 83:774–788.
11. Ratnapalan, S, Bentur, Y, Koren, G. “Doctor, will that x-ray harm my unborn child?”. CMAJ. 2008; 179:1293–1296.
12. Briggs GG, Freeman RK, Yaffe SJ, eds. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk, 9th ed, Baltimore: Lippincott Williams & Wilkins, 2011.
13. Hospital for Sick Children, Toronto. Available at http://www.motherisk.org, 2012. [Accessed March 9].
14. Hilton, G, Andrzejowski, JC. Prolonged propofol infusions in pregnant neurosurgical patients. J Neurosurg Anesthesiol. 2007; 19:67–68.
15. King, TA, Adams, AP. Failed tracheal intubation. Br J Anaesth. 1990; 65:400–414.
16. Archer, GW, Marx, GF. Arterial oxygen tension during apnoea in parturient women. Br J Anaesth. 1974; 46:358–360.
17. Levinson, G, Shnider, SM, deLorimier, AA, Steffenson, JL. Effects of maternal hyperventilation on uterine blood flow and fetal oxygenation and acid-base status. Anesthesiology. 1974; 40:340–347.
18. Ivankovic, AD, Elam, JO, Huffman, J. Effect of maternal hypercarbia on the newborn infant. Am J Obstet Gynecol. 1970; 107:939–946.
19. Low, JA, Panagiotopoulos, C, Derrick, EJ. Newborn complications after intrapartum asphyxia with metabolic acidosis in the term fetus. Am J Obstet Gynecol. 1994; 170:1081–1087.
20. Daily, WH, Katz, AR, Tonnesen, A, Allen, SJ. Beneficial effect of delivery in a patient with adult respiratory distress syndrome. Anesthesiology. 1990; 72:383–386.
21. Tomlinson, MW, Caruthers, TJ, Whitty, JE, Gonik, B. Does delivery improve maternal condition in the respiratory-compromised gravida? Obstet Gynecol. 1998; 91:108–111.
22. Mabie, WC, Barton, JR, Sibai, BM. Adult respiratory distress syndrome in pregnancy. Am J Obstet Gynecol. 1992; 167(4 Pt 1):950–957.
23. Vanden Hoek, TL, Morrison, LJ, Shuster, M, et al. Part 12: Cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010; 122:S829–S861.
24. Lindheimer, MD, Taler, SJ, Cunningham, FG. Hypertension in pregnancy. J Am Soc Hypertens. 2008; 2:484–494.
25. Lapinsky, SE, Kruczynski, K, Seaward, G, et al. Critical care management of the obstetric patient. Can J Anaesth. 1997; 44:325–329.
26. Hazelgrove, JF, Price, C, Pappachan, VJ, et al. Multicenter study of obstetric admissions to 14 intensive care units in southern England. Crit Care Med. 2001; 29:770–775.
27. Karnad, DR, Lapsia, V, Krishnan, A, Salvi, VS. Prognostic factors in obstetric patients admitted to an Indian intensive care unit. Crit Care Med. 2004; 32:1294–1299.
28. Weinstein, L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: A severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982; 142:159–167.
29. von Dadelszen, P, Ornstein, MP, Bull, SB, et al. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: A meta-analysis. Lancet. 2000; 355:87–92.
30. Matchaba, P, Moodley, J. Corticosteroids for HELLP syndrome in pregnancy. Cochrane Database Syst Rev. (1):2004.
31. Fonseca, JE, Mendez, F, Catano, C, Arias, F. Dexamethasone treatment does not improve the outcome of women with HELLP syndrome: A double-blind, placebo-controlled, randomized clinical trial. Am J Obstet Gynecol. 2005; 193:1591–1598.
32. Knox, TA, Olans, LB. Liver disease in pregnancy. N Engl J Med. 1996; 335:569–576.
33. Ibdah, JA, Bennett, MJ, Rinaldo, P, et al. A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women. N Engl J Med. 1999; 340:1723–1731.
34. Conde-Agudelo, A, Romero, R. Amniotic fluid embolism: An evidence-based review. Am J Obstet Gynecol. 2009; 201:445.
35. Clark, SL, Cotton, DB, Gonik, B, et al. Central hemodynamic alterations in amniotic fluid embolism. Am J Obstet Gynecol. 1988; 158:1124–1126.
36. Shevell, T, Malone, FD. Management of obstetric hemorrhage. Semin Perinatol. 2003; 27:86–104.
37. Hayashi, RH, Castillo, MS, Noah, ML. Management of severe postpartum hemorrhage with a prostaglandin F2a analogue. Obstet Gynecol. 1984; 63:806–814.
38. Georgiou, C. Balloon tamponade in the management of postpartum haemorrhage: A review. Br J Gynaecol. 2009; 116:748.
39. Segal, S, Shemesh, IY, Blumenthal, R. Treatment of obstetric hemorrhage with recombinant activated factor VII (rFVIIa). Arch Gynecol Obstet. 2003; 268:266–267.
40. Hansch, E, Chitkara, U, McAlpine, J, et al. Pelvic arterial embolization for control of obstetric hemorrhage: A five-year experience. Am J Obstet Gynecol. 1999; 180:1454–1460.
41. Pearson, GD, Veille, JC, Rahimtoola, S, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000; 283:1183–1188.
42. O’Connell, JB, Costanzo-Nordin, MR, Subramanian, R, et al. Peripartum cardiomyopathy: Clinical, hemodynamic, histologic and prognostic characteristics. J Am Coll Cardiol. 1986; 8:52–56.
43. Pisani, RJ, Rosenow, EC, III. Pulmonary edema associated with tocolytic therapy. Ann Intern Med. 1989; 110:714–718.
44. Twiggs, LB, Morrow, CP, Schlaerth, JB. Acute pulmonary complications of molar pregnancy. Am J Obstet Gynecol. 1979; 135:189–194.
45. Szekeres-Bartho, J. Immunological relationship between the mother and the fetus. Intern Rev Immunol. 2002; 21:471–495.
46. Lapinsky, SE, Seaward, PGR. Sepsis in the gynecological and obstetric patient. In: Fein A, Abraham EM, Balk R, et al, eds. Sepsis and Multiorgan Failure: Mechanisms for Treatment Strategies. Baltimore: Williams & Wilkins; 1997:419–430.
47. Ledger, WJ. Postpartum endomyometritis diagnosis and treatment: A review. J Obstet Gynaecol Res. 2003; 29:364–373.
48. Silver, RM, Heddleston, LN, McGregor, JA, Gibbs, RS. Life-threatening puerperal infection due to group A streptococci. Obstet Gynecol. 1992; 79:894–896.
49. Bandi, VD, Munnur, U, Matthay, MA. Acute lung injury and acute respiratory distress syndrome in pregnancy. Crit Care Clin. 2004; 20:577–607.
50. Daily, WH, Katz, AR, Tonnesen, A, Allen, SJ. Beneficial effect of delivery in a patient with adult respiratory distress syndrome. Anesthesiology. 1990; 72:383–386.
51. Shannon, M, Bates, SM, Greer, IA, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141(2 Suppl):e691S–e736S.
52. Guy, ES, Kirumaki, A, Hanania, NA. Acute asthma in pregnancy. Crit Care Clin. 2004; 20:731–745.
53. Ray, P, Murphy, GJ, Shutt, LE. Recognition and management of maternal cardiac disease in pregnancy. Br J Anaesth. 2004; 93:428–439.
54. Siu, SC, Colman, JM. Heart disease and pregnancy. Heart. 2001; 85:710–715.
55. Zeebregts, CJ, Schepens, MA, Hameeteman, TM, et al. Acute aortic dissection complicating pregnancy. Ann Thorac Surg. 1997; 64:1345–1348.
56. Weiss, BM, Zemp, L, Seifert, B, Hess, OM. Outcome of pulmonary vascular disease in pregnancy: A systematic overview from 1978 through 1996. J Am Coll Cardiol. 1998; 31:1650–1657.
57. Bédard, E, Dimopoulos, K, Gatzoulis, MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J. 2009; 30:256–265.
58. Easterling, TR, Ralph, DD, Schmucker, BC. Pulmonary hypertension in pregnancy: Treatment with pulmonary vasodilators. Obstet Gynecol. 1999; 93:494–498.
59. Higton, AM, Whale, C, Musk, M, Gabbay, E. Pulmonary hypertension in pregnancy: Two cases and review of the literature. Intern Med J. 2009; 39:766–770.
60. Pearlman, MD, Tintinalli, JE, Lorenz, RP. A prospective controlled study of outcome after trauma during pregnancy. Am J Obstet Gynecol. 1990; 162:665–671.
61. Drost, TF, Rosemurgy, AS, Sherman, HF, et al. Major trauma in pregnant women: Maternal/fetal outcome. J Trauma. 1990; 30:574–578.
62. Pearlman, MD, Tintinalli, JE, Lorenz, RP. Blunt trauma during pregnancy. N Engl J Med. 1990; 323:1609–1613.

 , minute ventilation.
, minute ventilation. 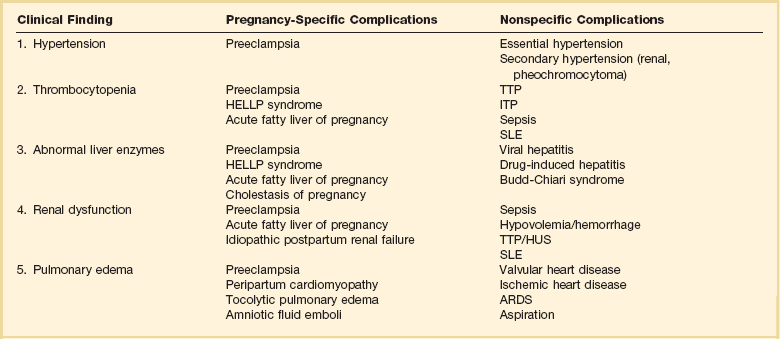
 , ventilation-perfusion.
, ventilation-perfusion.




