Chapter 181 Craniovertebral Abnormalities and Their Neurosurgical Management
Craniovertebral junction abnormalities can be developmental, genetic, or acquired in origin.1–3 To effectively treat these disorders when they are symptomatic, the clinician must have a knowledge of the embryology and the functional anatomy of the area. A myriad of abnormal neurologic findings may be present that are secondary to compression or ischemia of neural tissue. The surgical management of these disorders depends on precise identification of the underlying pathophysiologic condition as determined by appropriate radiologic studies. The operative treatment includes anterior, lateral, and posterior approaches to the craniovertebral junction with and without bony fusion. Our database and experience with more than 6000 patients who had symptomatic craniovertebral abnormalities is the basis for management of these complex disorders. The disorders can be classified as listed in Table 181-1.
TABLE 181-1 Classification of Craniovertebral Junction Abnormalities
| Developmental Anomalies of the Craniovertebral Junction |
| Malformations of Occipital Bone |
| Clivus segmentations |
| Remnants around foramen magnum |
| Basilar invagination |
| Condylar hypoplasia |
| Abnormal occipitoatlantal alignment |
| Malformations of Atlas |
| Failure of atlas segmentation from occiput (assimilation) |
| Atlantoaxial fusion |
| Aplasia of atlas arches |
| Malformations of Axis |
| Irregular atlantoaxial segmentation |
| Dens dysplasias |
| Ossiculum terminale persistens |
| Os odontoideum |
| Hypoplasia-aplasia |
| Segmentation failure of C2-C3 |
| Neural Dysgenesis |
| Genetic and Acquired Abnormalities of Craniovertebral Junction |
| Abnormalities at the Foramen Magnum |
| Basilar impression (secondary basilar invagination) |
| Foraminal stenosis |
| Traumatic occipitoatlantal dislocation |
| Os odontoideum |
| Tumors |
| Atlantoaxial Instability |
| Errors of metabolism |
| Down’s syndrome |
| Infections |
| Inflammatory |
| Traumatic atlantoaxial dislocations |
| Miscellaneous |
History
Subsequent to the first description of spontaneous atlantoaxial dislocation in 1830 by Bell,4 lesions affecting the cervicomedullary junction have emerged from being medical curiosities to being conditions that can be effectively managed. Except for acute dislocations, the treatment of occipitoatlantoaxial joint pathologic entities was marked with failure before the era of skeletal traction. Subsequently, it was apparent that most acute and chronic dislocations could be reduced even years after the initial injury.
The early operative procedures were posterior decompression of the cervicomedullary junction with and without fusion for stabilization. Posterior decompression in patients with irreducible ventral compression of neural tissue at the craniovertebral area is often associated with a high operative risk and a low incidence of improvement; most patients are unchanged or have increased neurologic deficit.5
More recently, transpalatine-transoral1,2,6–11 and extrapharyngeal12–15 ventral operations were described for fractures, tumors,16 congenital abnormalities, infection, and inflammatory conditions at the craniocervical junction.17,18 Stabilization of the atlantoaxial occipital joints usually has been performed by fusing the spinal column posteriorly. No single anterior or posterior surgical procedure can be used for all patients with craniovertebral abnormalities; the operation or combination of operations must be selected on an individual basis to correct the pathologic process responsible for the neurologic deficit.1–319
Embryology and Anatomy
By definition, the craniovertebral junction includes the basiocciput, the foramen magnum, the atlas, and the axis vertebra. The occipital bone is formed by fusion of four sclerotomes. The proatlas is the most caudal of the sclerotomes and loses its identity in humans. The neural arch of the primitive proatlas divides into ventral rostral and dorsal caudal segments.20 The ventral rostral segment gives rise to the occipital condyles and the alar and cruciate ligaments. The dorsal caudal segment forms the posterior arch of the atlas and the lateral atlantal masses to help form its rostral articular facets. If the posterior segment of the proatlas remains separate, the atlas has bipartite cranial articular facets, a rare anomaly that can result in horizontal instability of this joint. Finally, the proatlas also gives rise to the apex of the dens.21 Synchondrosial growth between the four occipital sclerotomes occurs until age 18 years. During this time, the clivus elongates and the volume of the posterior fossa expands. Insufficient volume of the posterior fossa as a result of insufficient growth results in tonsillar ectopia as the hindbrain herniates through the foramen magnum. Therefore, extended follow-up is critical for children with craniocervical abnormalities while growth continues into adolescence.22
An occipital vertebra is a bony structure that is separate from the foramen magnum and incorporates the occipital condyles. The anterior arch may be partially or completely fused to the anterior margin of the foramen magnum, and the transverse process, if present, does not have a foramen for the vertebral artery. In contrast, an atlanto-occipital fusion is characterized by ankylosis between the atlas and the skull base, usually with persistence of the normal joints. 22
The atlas is derived from the first cervical sclerotome as well as the proatlas. The body of the atlas as such disappears and gives origin to the dens. The anterior arch of the atlas has one center of ossification, although at times two centers may be present. The atlas has two robust lateral masses situated at the anterolateral portion of the ring. Lateral to these is located the transverse foramen, which transmits the vertebral artery. The artery then courses within a groove along the posterior arch parallel to the first spinal nerve before piercing the dura. The lateral mass extends below the posterior arch to contact the axis. Normally, the ring of the atlas becomes ossified within the first 3 to 4 years of life. If the atlas does not fuse and a persistent bifid anterior and posterior arch persists, a condition similar to a Jefferson fracture can occur with lateral displacement of the lateral masses. In children younger than 3 years, use of a custom molded brace will allow fusion of the atlas, and stability can be restored with conservative management alone. If the atlas remains bifid after the age of 4 years with abnormal motion, arthrodesis is necessary.22
Failure of the segmentation between the proatlas and the first cervical sclerotome results in atlas assimilation. Often identified in patients with Klippel-Feil syndrome, this abnormality is commonly associated with segmentation failure at C2–3 as well, which places great stress on the atlantoaxial interface. In a review of 30 patients with occipitalization of the atlas, 57% were found to have atlantoaxial instability on dynamic imaging and 47% had an associated C2–3 fusion.23 Initially, a pannus develops around the odontoid. As the instability progresses, cranial settling occurs, causing the odontoid to migrate through the foramen magnum, resulting in basilar invagination. In the early stages, the lesion is still reducible. However, in severe cases, bone remodeling occurs, resulting in a shortened horizontal clivus and a flattened occiput. The posterior fossa volume is reduced and an acquired hindbrain herniation syndrome develops.22
The axis is developed from four primary ossification centers. The facets and posterior arch of the axis all originate from the second cervical sclerotome. The dens originates from three different sclerotomes. As discussed, the apex arises from the proatlas. The C1 sclerotome gives rise to the body of the odontoid process, and the inferior portion of the C2 body is formed by the second cervical sclerotome. At birth, a cartilaginous band persists between the odontoid and the inferior body of the axis, termed the neural central synchondrosis. By age 8 years, the neural central synchondrosis is no longer seen. The tip of the dens is not visible at birth and first appears at age 3 years as a separate ossification center. Typically, the odontoid becomes fully ossified by age 12 years.21 The complex embryologic origin of the odontoid process explains several important abnormalities resulting from dysgenesis of the dens. Failure of the proatlas and the dens to fuse results in ossiculum terminale. Os odontoideum occurs as a result of trauma between the ages of 1 and 4 years or in nontraumatic cases associated with Down’s24 and Morquio’s syndrome and is actually a fracture separation of the dens tip. Hypoplasia and agenesis of the dens is the result of developmental failure of the distal ossification centers. The common pathophysiology that produces neurologic deficit with agenesis or hypoplasia of the dens is instability between the first and second cervical vertebrae that results from ligamentous incompetence.22
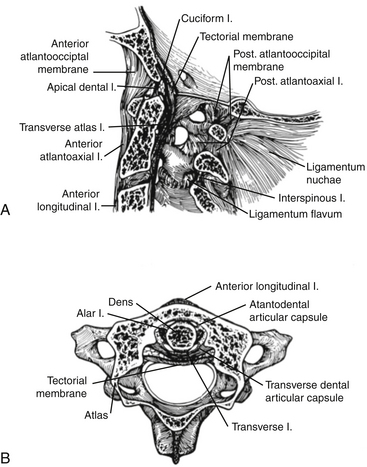
To accurately assess the stability of the craniocervical junction, a working knowledge of the complex ligamentous anatomy is required and is demonstrated in Figure 181-1. The dens is approximated to the anterior arch of the atlas by the transverse ligament, which is anchored to the tubercle on the mesial aspect of each lateral mass of the atlas. This ligament is responsible for the stability of the atlantoaxial joint. The axis is connected to the occiput by (1) the alar ligaments that course obliquely upward from the posterior lateral surface of the dens to the anterior and medial surface of the occipital condyles; (2) the apical dens ligament, which continues from the medial aspect of the foramen magnum to the tip of the dens; (3) the tectorial membrane (an extension of the deep layer of the posterior longitudinal ligament); and (4) the cruciate ligament, which consists of the transverse ligament plus triangular ascending and descending slips to the anterior rim of the foramen magnum and the axis, respectively (see Fig. 181-1A, B).25 Finally, the tectorial membrane is the rostral extension of the posterior longitudinal ligament and courses posterior to the cruciate ligament and inserts into the occiput. The integrity of the ligamentous structures, particularly the cruciate ligament, can be clearly identified on high-resolution magnetic resonance imaging (MRI) and helps determine the stability of the craniocervical junction.
The occipitoatlantoaxial joints are complex, both anatomically and kinematically.26,27 Anatomically, two occipitoatlantal articulations exist. There are four atlantoaxial joints, with a common synovial lining between the dens and the anterior arch of the atlas, the dens, and the transverse ligament, and between the four lateral masses. Flexion-extension occurs at both the occipitoatlantal and atlantoaxial articulations and accounts for approximately 25% of the flexion and extension movement in the neck. During flexion, the cruciate ligament limits the drift of the odontoid away from the anterior arch of C1 (predental space) to less than 5 mm in children and 3 mm in adults. If the cruciate ligament is disrupted, the alar and apical ligaments ultimately fail and instability ensues. The atlantoaxial joints are responsible for the largest degree of rotation in the cervical spine. Total rotation of the neck is up to 90 degrees, with up to 30 degrees being accounted for at C1–2. With rotation beyond 30 degrees at C1–2, stretching and compression of the contralateral vertebral artery can occur.25
The development of the neck musculature is inadequate to supplement joint stability until the age of 8 years. Before this age, laxity of the ligamentous tissue permits excessive movement of the occipitoatlantoaxial articulations.28,29 Forward gliding of the skull in relation to the spine occurs if hypoplastic occipital condyles are present. This is the mechanism for the development of neurologic deficit in children who have spondyloepiphyseal dysplasia, Conradi’s syndrome, or Morquio’s syndrome. Further, several disorders are associated with even greater ligamentous laxity such as Down’s syndrome,24 juvenile rheumatoid arthritis, and Klippel-Feil syndrome, which place these children at risk for severe injuries after seemingly insignificant trauma.
The lymphatic drainage to the occipitoatlantoaxial joints is through retropharyngeal glands to the deep cervical lymphatic chain. In children, nasopharyngeal infections can cause an inflammatory reaction to the synovial joints of the craniovertebral junction and weaken the ligaments within the craniocervical junction. Because the neck musculature is not fully developed and is unable to compensate for the ligamentous laxity, C1–2 subluxation can develop and has been referred to as Grisel’s syndrome.30
In the osseoligamentous destruction caused by rheumatoid arthritis, the synovial bursa and associated ligaments that surround the odontoid process are damaged and result in loss of stability.31 Subluxation can occur secondary to the atlas moving anteriorly on the axis (caused by insufficiency of the cruciate ligament or fracture of the odontoid process), secondary to the atlas moving posteriorly on the axis (from erosion or fracture of the odontoid process), or by telescoping of the skull on the axis (from destruction of the axis lateral masses or apophyseal joints).31 Chronic subluxation often results in ligamentous hypertrophy and the accumulation of granulation tissue behind the odontoid process from the hypertrophied soft tissue. Even though normal bone alignment is present on roentgenograms, there may be ventral compression of the cervicomedullary junction by soft tissue.
Signs and Symptoms
Craniocervical junction abnormalities produce a myriad of symptoms and signs, including an abnormal general physical appearance; myelopathy; brain stem, cranial nerve, and cervical root dysfunction; vascular insufficiency; or any combination of these.1–3,32,33 The head may be tilted to one side as a result of rotatory subluxation of the atlas on the axis. Patients with Klippel-Feil syndrome classically present with a triad of findings including limited neck movement, a short neck, and a low posterior hairline. In addition, patients with various types of dwarfism, including spondyloepiphyseal dysplasia and achondroplasia, have an increased incidence of craniovertebral disorders.34,35
Neck and occipital pain is the most common complaint, occurring in 85% of affected children. The pain is classically described as originating in the suboccipital area and extending up toward the vertex in the sensory distribution of the greater occipital nerve. The dysesthesias are from irritation of the second cervical nerve as it traverses the lateral atlantoaxial joint capsule. In those with basilar invagination, a “basilar migraine” is a common complaint occurring in up to 25% of these patients. Fortunately, these headaches often regress after surgery.36
Myelopathy was the most common neurologic deficit in our series, occurring in 98% of the patients. The initial symptoms, such as lack of physical endurance, may be subtle, particularly in younger patients. The severity of myelopathy is variable and can manifest as different degrees of weakness in the upper or lower extremities. False localizing signs were common, and motor deficits included monoparesis, hemiparesis, paraparesis, tetraparesis, and quadriparesis. A myelopathy mimicking the central cord syndrome was often present in patients with basilar invagination.36 The pathophysiology of motor myelopathy has been attributed to repetitive trauma on the pyramidal tracts secondary to chronic compression.37 In addition, instability at the craniocervical junction can lead to repeated compression to the anterior spinal artery, leading to spasm and occlusion. The false localizing signs have been attributed to stagnant hypoxia of the cervical spinal cord from venous stasis.38
Cranial nerve deficits are also often observed. Loss of the gag reflex, unilaterally or bilaterally, is often observed and can lead to aspiration pneumonia. Respiratory arrest, sleep apnea, and snoring were associated with both anterior and posterior compression of the cervicomedullary junction,39 and they often resolve after decompression. Basilar invagination can also lead to internuclear ophthalmoplegia. Nystagmus to lateral gaze is not uncommon, and downbeat nystagmus is associated with hindbrain herniation syndrome.40 Interestingly, hearing loss, tinnitus, or both, was observed in 23% of patients and was particularly common in those with Klippel-Feil syndrome.
Diagnostic Investigations
Several reference lines are used to evaluate plain roentgenograms in the assessment of the cervicobasilar relationships.41 McRae’s line measures the sagittal diameter of the foramen magnum from its anterior margin to its posterior margin (average, 35 mm). Towne’s projection is useful for determining the transverse diameter of the foramen magnum (35 mm ± 4 mm). Chamberlain’s line is a diagonal from the hard palate to the posterior margin of the foramen magnum (Fig. 181-2). The odontoid process should not extend more than one third of its length above this line or no more than 5 mm. Wackenheim’s clivus-canal line is drawn along the posterior surface of the clivus, and basilar invagination is present if this line is intersected by the odontoid process (see Fig. 181-2). The clivus-canal line consists of an angle formed by Wackenheim’s line and a line along the posterior margin of the dens. Normally, this angle is 120 to 140 degrees, and ventral compression can occur at more-acute angles. Fishgold’s digastric line is measured on the frontal projection and connects the digastric grooves. The line is normally 11 mm ± 4 mm above the atlantoidooccipital junction. The digastric line is the upper limit of position for the odontoid tip.42 Patients with abnormalities of the craniovertebral junction become symptomatic when the effective diameter of the spinal canal at the foramen magnum (from the posterior surface of the odontoid process to the posterior margin of the foramen magnum) is less than 19 mm.43
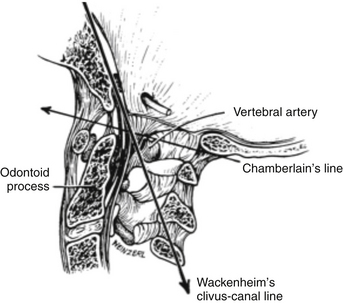
FIGURE 181-2 A midsagittal section of the craniovertebral junction illustrating Chamberlain’s and Wackenheim’s clivus-canal lines.
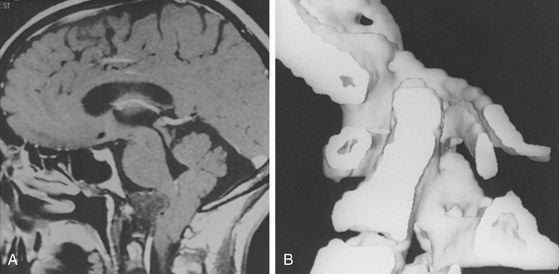
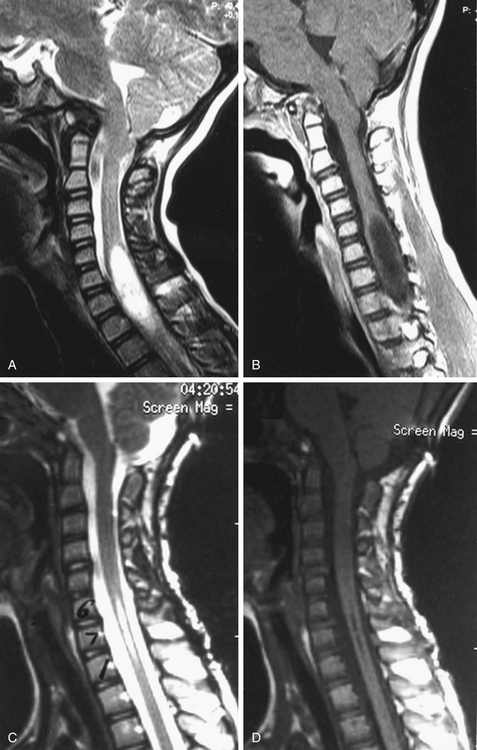
Special radiologic procedures are necessary for the clarification of the etiology and pathophysiology of the craniovertebral abnormalities.1–3 These examinations of the cervicomedullary junction include MRI (Fig. 181-3A), computed tomography (CT), single- or three-dimensional CT (see Fig. 181-3B), and plain radiographs. These studies provide complementary information.
The most significant examination of the posterior fossa contents and spinal cord is provided by MRI, which demonstrates the medulla and cervical spinal cord with great clarity. Abnormality in size and position of the cervicomedullary junction, abnormality of cerebellar tonsil position, and presence or absence of hydromyelia are demonstrated without the use of ionizing radiation (see Fig. 181-3A). Diagnostic studies of the craniovertebral junction in the flexion-extension positions (with or without contrast material) can be performed with plain radiographic studies and with MRI. MRI protocols have also been developed to provide high-resolution imaging of the ligamentous structures of the craniocervical junction. The alar ligaments, transverse ligament, accessory atlantoaxial ligament, and posterior atlanto-occipital membrane can be clearly identified.44,45
• A predental space greater than 5 mm in children younger than 8 years or greater than 3 mm in patients older than 8 to 10 years is seen.
• On open-mouth odontoid views or coronal CT images, the lateral displacement of the two atlas lateral masses is greater than 6 mm.
• The vertical translation between the clivus and the odontoid is greater than 2 mm.
• Excessive motion on flexion and extension views of the craniocervical junction is observed.
• Disruption of the transverse cruciate ligament, alar ligament, or tectorial membrane and bony malalignment are seen on high-resolution MRI.
Careful preoperative evaluation of the bony relationships on static and dynamic imaging, as well as careful inspection of the ligamentous anatomy, can help determine the need for internal fixation.46
Treatment Algorithm for Craniovertebral Abnormalities
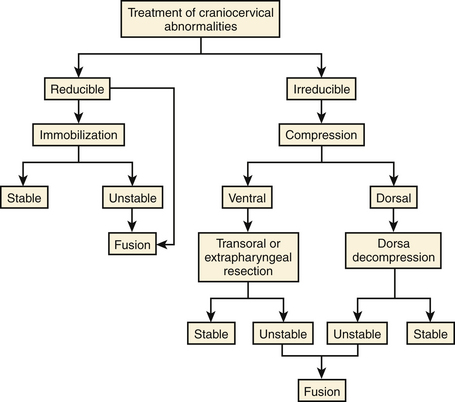
The following algorithm has been proposed (Fig. 181-4) to guide clinical decision making based on the etiology of the lesion, the reducibility of the deformity with traction, direction of encroachment, presence of abnormal ossification centers and growth plates, and stability of the craniocervical junction.47 Based on these criteria, all patients with abnormalities of the craniocervical junction can be divided into six surgical categories.46
The first determination to make for patients with compressive lesions at the craniocervical junction is if the lesion is reducible by positioning or traction. One should start with a low weight (5 to 7 lb) and slowly add additional weight as needed up to 12 to 15 lb (in adults). The alignment can be assessed with radiographs, and MRI during traction provides critical information regarding the need for additional decompression after adequate alignment is achieved. If the lesion is reducible and additional decompression is not required, stabilization alone is necessary to maintain alignment.
Certain situations require an initial conservative approach. For young infants with conditions such as spondyloepiphyseal dysplasia and Goldenhar’s syndrome, a custom-built orthosis, to allow continued growth, can be used when instability is identified. If adequate bone growth occurs over time, the brace can be gradually discontinued. However, if after 4 years sufficient bone growth has not occurred, and craniocervical instability still exists, arthrodesis is required. In children with Grisel’s syndrome, a firm cervical collar is required while the infection is being treated and until ligamentous laxity resolves. Our experience has confirmed that fusion is rarely required in these patients.46
Operative Techniques
Reducible Pathologic Conditions Requiring External Immobilization Only
We have purposely omitted from this discussion odontoid and atlantoaxial fractures that require fixation only because the causes and treatment of these entities are well documented elsewhere.48,49 Although some reducible pathologic conditions can be realigned by positioning alone, most require up to 12 to 15 lb of skeletal traction with a halo ring.
The halo ring with pin fixation has the advantage over other traction devices in that changing the apparatus attached to the skull is unnecessary when the patient is placed in a body brace. An acrylic vest lined with lamb’s wool is preferable, and lightweight metals or alloys have replaced the stainless-steel rings, pins, and vertical support bars. Metals such as aluminum, titanium, or graphite alloy do not distort CT or MRI scans of patients who are immobilized in such a brace. The halo brace fixation was preferred for stabilizing the craniovertebral junction but has been supplanted by the custom-built occipitocervical orthosis.50
Reducible Lesions Requiring Internal Fixation
A variety of fixation techniques have been described to achieve arthrodesis of the occiput and the cervical spine, and several of these are described here. In general, rod and screw constructs are more rigid than wire constructs, and biomechanical studies have demonstrated that it is mechanically advantageous to include as many fixation points as possible.51,52 However, no one strategy is best for all patients with craniocervical junction instability. Ultimately, the age of the patient, bone anatomy, and alignment dictate which technique is best for a particular patient.
Occipitoatlantoaxial Posterior Fusion with Autograft and Wiring
Before anesthesia is administered, the patient is intubated while awake by use of regional block and topical anesthesia to the pharynx and larynx. After intubation, the patient is positioned on the operating table in the prone position. The head is placed in the cerebellar headrest, with the clinician ensuring that no pressure is placed about the eyes. Skeletal traction is maintained throughout the operation at between 5 and 7 lb, which is sufficient to maintain satisfactory alignment of the craniocervical junction.
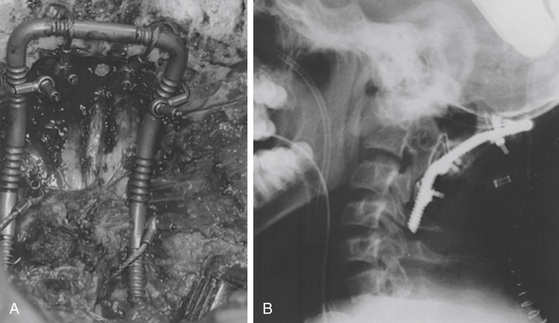
In patients with an unstable atlantoaxial articulation, fusion includes only the rostral two or three cervical vertebrae. In patients with occipitoatlantoaxial instability, the fusion includes the occiput in addition to the atlantoaxial vertebrae. A notch is placed inferiorly and superiorly on each lamina, and a hole is drilled through each side of the occipital bone lateral to the foramen magnum. Titanium cables are passed in a sublaminar fashion and through the occipital bones (Fig. 181-5).53 The advantage of these cables is that they are more pliable yet have equivalent strength when compared with the wire.
Bone donor sites are either the rib or iliac crest.54 Rib grafts are preferred owing to a lower complication rate, improved strength secondary to the cortical-cancellous composition, and less pain at the donor site. Rib grafts also are naturally contoured to match the normal cervical lordosis. The bone graft is notched adjacent to the lamina, and a notch or a hole is placed in the rostral end for the occipital cable. The graft is secured to the opposing laminar surface, or the occipital bone, or both, and the cables are tightened (see Fig. 181-5). Bone chips may be placed along the fusion area for additional strength.
Occipitoatlantoaxial Posterior Fusion with Rod and Wire Instrumentation
The exposure is as previously described for rib graft fusion. The titanium loop is placed against the dorsal occipitocervical articulation, and the contour of the loop is custom fitted to the patient. Cables secure the loop at the points of fixation to the occiput as well as the dorsal aspects of the laminae from C1 to C2 (Fig. 181-6A). The construct should extend to two levels below the area of instability, and in cases of axial instability, the loop can incorporate C3. In most patients, the titanium cables can be cinched to 30 lb of torque pressure (maximum) at the occiput and C2. At C1 and C3, the torque pressure is between 15 and 20 lb. Rib grafts are placed medial to the instrumentation so as to have contact with the bony surface of the occiput and laminae of the cervical spine and can be secured in place with suture (see Fig. 181-6).
Occipitoatlantoaxial Posterior Fusion with Polyaxial Screw and Occipital Plate Instrumentation
Recent studies have demonstrated that instrumentation with polyaxial screws and occipital plating systems provides the most rigid construct available. Rod and screw instrumentation has also been reported to have fewer hardware failures and higher fusion rates than wire constructs. In recent years, occipital plating systems have undergone many changes in design to improve the ease of use and to lower the complication rate compared to earlier versions. Early systems consisted of occipital extensions of rods and lateral mass plates or occipital screw connectors applied to the end of a rod, which generally attached to the thin squamous portion of the occipital bone. Modern occipital plates now allow multiple screws to be placed in the midline keel of the occipital bone and can allow more than 10 mm of bicortical purchase to increase pullout strength. In addition, polyaxial screw heads are located more laterally on the plate to easily accommodate either bent or jointed rods. Given the superior stability of the screw and plate systems and improved ease of use, these systems are being used more commonly.55
After the occipital plate has been secured, a number of posterior screw placement options have been described to allow rigid occipitocervical screw fixation. C1 lateral mass screws, a C1–2 transarticular screw, a C2 pars screw, or C2 laminar screws can all be used to provide rigid fixation. Screw placement is selected depending on the patient’s anatomy as determined by preoperative imaging and the surgeon’s preference. The entry point of a C1–2 transarticular screw is located at the inferior edge of the inferior articular process of C2. The medial edge of the spinal canal provides a medial reference point, and intraoperative fluoroscopy guides the rostrocaudal angle. A K-wire is inserted and passed toward the anterior arch tubercle of C1, to ensure passage through the C2 pars interarticularis. A threaded tap is then inserted over the guidewire, and after the tap is removed, a screw is advanced over the guidewire. The screw is usually 40 to 46 mm in length and should be measured so that when fully inserted the head is positioned flush against the bone (Fig. 181-7).
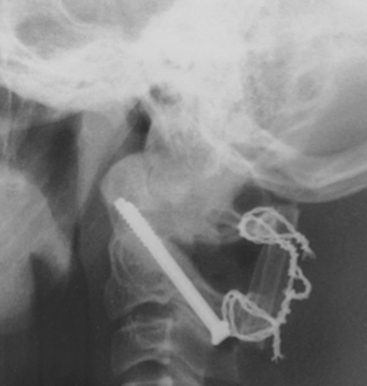
FIGURE 181-7 Lateral roentgenogram illustrates placement of atlas-axis transarticular screws with bone fusion.
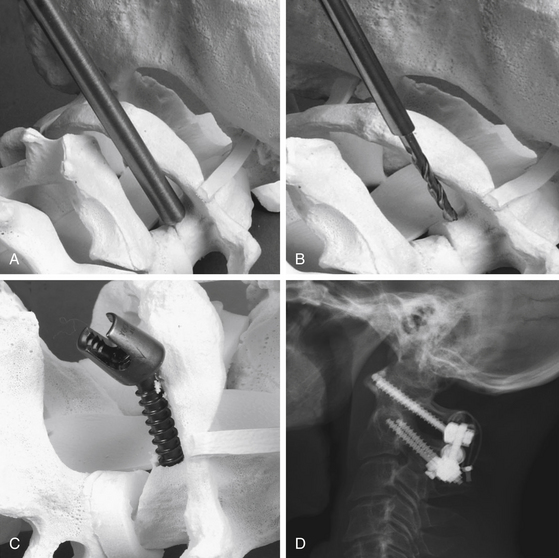
If the anatomy prevents a C1–2 transarticular screw, C1 lateral mass screws can be used to incorporate C1 into the fusion construct (Figure 181-8). A meticulous subperiosteal dissection is performed along the inferior border of the posterior arch of C1 to prevent profuse bleeding from the local epidural venous plexus. To improve visualization of the lateral mass of C1, a small amount of the trailing edge of the posterior arch of C1 must sometimes be removed. The C2 nerve root is depressed inferiorly and can be resected if required to allow adequate visualization. The entry point is located within the center of the atlantal lateral mass; a small emissary vein is often located at the desired entry point. A K-wire or awl is typically used to create an entry point, and then a manual drill is used with a trajectory parallel to the plane of C1 toward the anterior arch of C1 using fluoroscopic guidance (Fig. 181-8A and B). It is critical to avoid the vertebral artery laterally, the internal carotid ventrally, and the thecal sac medially while drilling. The hole is then tapped with a 3.5 mm tap. Though the C1 lateral mass provides only 10 to 15 mm of bony purchase, a 35- to 45-mm screw is used to allow the screw head to be located posterior to the C1 lateral mass to allow fixation rod access (see Fig. 181-8C and D).55
If a C1 lateral mass screw is placed, C2 pars screws or C2 laminar screws can be used. The entry point for a C2 pars screw is similar to a C1–2 transarticular screw and is located 3 mm rostral and 3 mm lateral to the inferomedial aspect of the inferior articular surface. The trajectory is parallel to the C2 pars angle and is guided by fluoroscopy and stops short of the C1–2 joint. The medial border of the C2 pars is directly visualized during placement to ensure a medial breach does not occur. The appropriate screw length is determined based on preoperative imaging and is typically between 12 to 18 mm. In some patients, the course of the vertebral artery prevents the safe placement of a C2 pars screw. If imaging demonstrates robust C2 lamina and a C2 laminectomy is not required, C2 laminar screws are also an option. The entry point for C2 laminar screws is selected near the rostral half of the spinous process on one side and near the caudal half on the contralateral side to prevent the screws from contacting as they cross. A drill is used to slowly manually drill into the lamina, and a ball-tip feeler is used to confirm cortical break has not occurred and to determine the appropriate length of the screw. A lateral connector generally is required to secure the C2 laminar screw to the rod during assembly of the final construct. A C3 lateral mass screw is then placed using a modified Magerl screw trajectory. An entry point is selected 2 mm medial and inferior to the center of the lateral mass and an awl is used to pierce the outer cortex. A drill is angled 30 degrees cephalad and 30 degrees laterally to allow for adequate bony purchase and to avoid the vertebral artery. A polyaxial screw is secured in place. Finally, a rod is bent to the appropriate contour and secured in place with set screws.55 Naturally, the length of the construct and the screw locations are selected based on the extent of the instability and the patient’s bony anatomy.
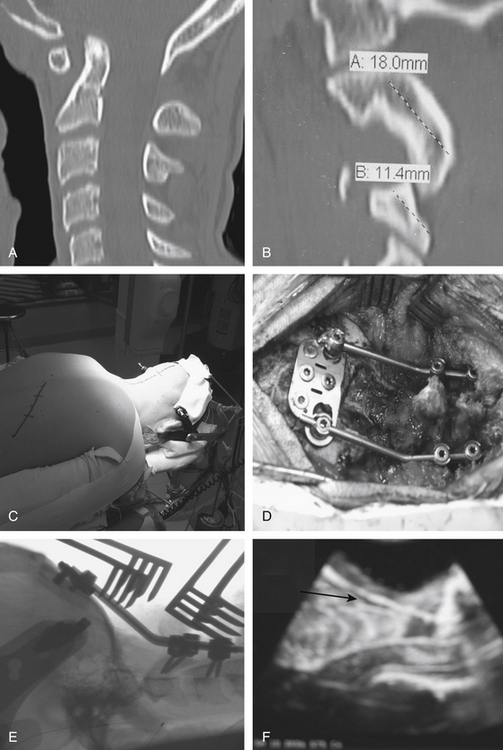
In Figure 181-9, we illustrate a representative case of a child with basilar invagination. Preoperative imaging demonstrated an increased predental space and anatomy that would allow us to use occipital, C2 pars, and C3 lateral mass screws (Fig. 181-9A and B). The patient was positioned prone in traction as described earlier (Fig. 181-9C). After decompression and adequate placement of hardware (Fig. 181-9D and E), the construct was used to perform additional distraction to achieve improved alignment. Intraoperative ultrasound (Fig. 181-9F) demonstrated ascent of the tonsils with cerebrospinal fluid (CSF) signal posteriorly. The patient did well postoperatively without complication.
Dorsal Compression
Suboccipital Craniectomy and Upper Cervical Laminectomy
The patient’s positioning on the operating table, the anesthesia-induction technique, and the operative exposure of the spinous process and lamina are identical to those outlined in the discussion of posterior cervical fusion. A suboccipital craniectomy is performed that includes the posterior and lateral bone surrounding the foramen magnum. The laminae and spinous processes of C1 and C2 (and C3 if necessary) are removed in a rostrocaudal direction. The laminectomy should extend laterally to the medial portion of the facets. It is important to excise all compressive soft tissue, including the constricting dural band that is often present in the Chiari malformation at the level of the foramen magnum.
Ventral Compression
Transoral-Transpharyngeal Approach
In children with ventral compression of the cranial cervical junction, in more than 80% of the patients reduction was successful and adequate decompression was achieved with cervical traction alone. However, when the ventral compression is irreducible, a transoral-transpharyngeal operation is used to decompress the cervicomedullary junction. A wide variety of pathologies can cause ventral compression and be successfully treated through a transoral-transpharyngeal approach. We recently reviewed our experience of 786 patients who underwent a transoral-transpalatopharyngeal approach from 1977 to 2008 and have listed the pathology in Table 181-2. Reports have described endoscopic approaches to decompress the anterior craniocervical junction.56,57 Though these reports demonstrate exciting new advances in skull base surgery, we continue to prefer the open approach described here, which allows a wide exposure and the ability to achieve a watertight closure. After anterior decompression, the patients are then turned prone and an occipitocervical fusion is then performed using one of the techniques described earlier.
TABLE 181-2 Pathology in 786 Patients Who Underwent Transoral-Transpalatopharyngeal Approach to the Clivus and Upper Cervical Spine (1977-2008) University of Iowa Hospitals and Clinics
| Primary basilar invagination and congenital anomalies | 356 |
| Rheumatoid irreducible dislocation or cranial settling | 163 |
| Basilar invagination after trauma including odontoid malunion, O-C1 dislocation, previous fusion procedure | 50 |
| Dystopic os odontoideum complex | 52 |
| Tumors: | |
| Pseudogout | 45 |
| Chordoma | 39 |
| Osteoblastoma | 3 |
| Plasmacytoma | 8 |
| Fibrous dysplasia | 7 |
| Granulation masses | 14 |
| Miscellaneous (Down’s syndrome, regional ileitis, psoriasis, osteogenesis imperfecta, osteomyelitis, ankylosing spondylitis fetal warfarin syndrome, Goldenhar’s syndrome, eosinophilic granuloma, metaphyseal dysplasia | 49 |
| 786 |
Because of the anticipated postoperative pharyngeal swelling and the need to perform both an anterior and posterior approach successively, airway protection is of utmost importance. Previously, a tracheostomy was performed in each patient to ensure an adequate airway after surgery. Currently, a malleable endotracheal tube is placed, often fiberoptically, and is left in place until the pharyngeal swelling has subsided several days after surgery.58 After the patient is intubated and the tube is meticulously secured, gauze packing is used to occlude the pharynx to prevent blood leakage into the stomach.
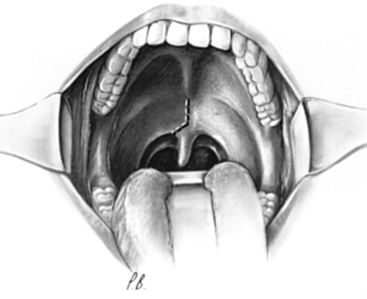
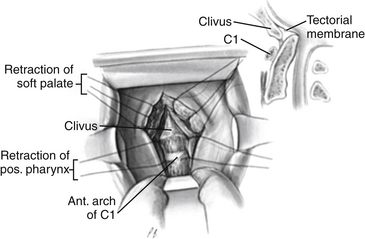
The surgical approach has been revisited and described in detail.59,60 The mouth is kept open with a Dingman retractor, with placement of the previously molded custom mouth guards over the teeth. Self-retaining retractors are attached to the frame of this instrument to depress the tongue. The retraction on the tongue should be loosened intermittently during the operation to prevent lingual congestion.
The soft palate is infiltrated with 1% lidocaine (Xylocaine) with 1:200,000 epinephrine or normal saline, and a midline incision is made that extends from the hard palate and diverts from the midline at the base of the uvula (Fig. 181-10). This incision ensures minimal bleeding and unrestricted healing to the soft palate, because the palatine artery and its accompanying palatine nerve enter the soft palate laterally and terminate in the midline. Stay sutures for retraction are placed on the soft palate flaps to allow maximal exposure through the pharynx to the caudal portion of the clivus. For situations in which surgery is necessary through the clivus or in patients with a foreshortened clivus and platybasia, the hard palate is exposed and the posterior 7 to 10 mm is resected. This allows high nasopharyngeal exposure without splitting the mandible or doing a median glossotomy.
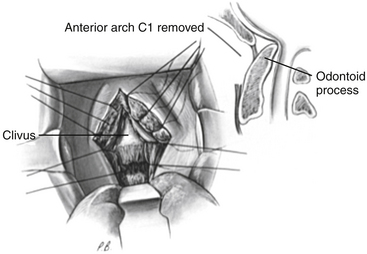
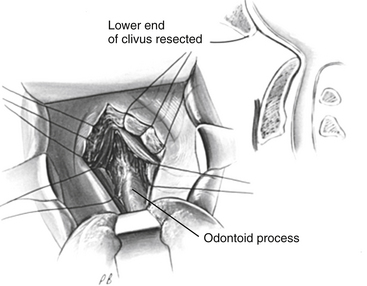
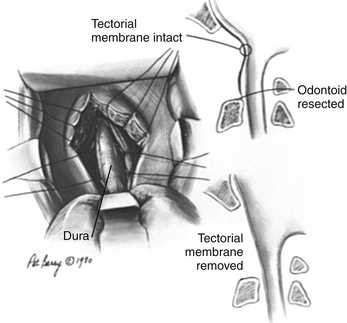
After exposure of the anterior longitudinal ligament, the operating microscope is used to provide magnification and a concentrated light source. The ligament is coagulated to reduce bleeding, and the anterior body of the axis, anterior arch of the atlas, and caudal anterior clivus are exposed in the subperiosteal plane by use of a periosteal elevator (Fig. 181-11). The ventral atlantoaxial articulation is separated and the anterior atlas arch is removed with a 4-mm diamond bur on a high-speed drill along with a 45-degree-angled punch rongeur to expose the caudal odontoid process (Fig. 181-12). The apical ligament with its attachment to the caudal clivus is removed, and if the odontoid invagination is severe, resecting a portion of the caudal clivus may be necessary. If resection of the caudal clivus is required, the tissue posterior to the clivus must be carefully separated from the bone because the dura can be easily penetrated, and troublesome bleeding might occur from the marginal sinus. The surgeon should next identify the distal tip of the odontoid process by subperiosteal dissection of ligamentous tissue from its osseous ventral surface (Fig. 181-13). The bulk of the odontoid process is then removed with a steel cutting bur. A diamond bur is then substituted to remove the tip and the thin dorsal bony shell of the dens to avoid tearing the posterior soft tissue. We prefer to use a 45-degree-angled handpiece attachment to the drill to provide unrestricted visualization of the surgical field. After the posterior tissue plane at the odontoid tip is identified, the odontoid process and body of the axis are removed in a rostral-to-caudal direction (Fig. 181-14). Finally, extensive ligamentous hypertrophy and an inflammatory pannus are sometimes present and need to be resected. Resection is accomplished with meticulous dissection and bipolar cautery in a piecemeal fashion in a rostral to caudal direction. In children, the cruciate ligament and tectorial membrane should be preserved. This measure allows new bone formation, and spontaneous ventral fusion has occurred in several of our patients. The surgeon can be assured that adequate cervicomedullary decompression has been accomplished when the pulsatile dura protrudes ventrally into the decompression site.
If the dura is torn or CSF is identified, closure of the dura can be difficult.61,62 In our experience, repair of the fistula can be accomplished by placing two or three layers of fascia over the rent. The fascia is harvested from the external oblique aponeurosis or fascia lata from the anterior lateral thigh. This fascia graft is reinforced with a fat pad before closure of the operative wound. In this situation, a lumbar CSF drain is inserted and maintained for 7 to 10 days after surgery.
If a tumor such as a chordoma is encountered, direct visualization of its extent is possible.63 Piecemeal removal is done without undue traction in case of dural penetration. In situations when an intradural lesion is encountered, a midline incision in the ventral dura is made beginning inferiorly and proceeding rostrally. If the circular sinus is prominent, the linear incision is converted into a cruciform one just caudal to the sinus to avoid troublesome bleeding. The tumor resection is facilitated by a previously placed spinal subarachnoid drain to relieve CSF turgidity. When the intradural operation is completed, the dural leaves are approximated in as watertight a closure as possible. A fascia and fat graft is placed over the closure as previously described.
It was initially felt that approximately 20% of patients demonstrated craniovertebral stability based on flexion-extension polytomes obtained 5 to 8 days postoperatively. Subsequently, the craniovertebral instability has been found to exceed 97%, and a posterior fusion is now recommended following transoral surgery.60
A representative case is illustrated in Figure 181-15. Preoperative imaging demonstrated severe basilar invagination with proatlas segmentation abnormalities and an accompanying cervicothoracic syrinx. The ventral bony abnormality was not reducible. A transpalatopharyngeal decompression was performed followed by a posterior foramen magnum decompression and fusion. Postoperative imaging reveals complete decompression of the cervicomedullary junction and a marked decrease in the size of the syrinx.
Careful postoperative management is critical to allow wound healing and minimize complications.59,64 After surgery, intravenous fluids and enteral feedings are continued for 5 to 6 days, followed by gradually increased feedings to a regular diet by the 15th day after surgery. Intravenous antibiotics are discontinued 48 hours after surgery if the dura is intact. If the dura is violated and a fascial graft has been used for repair, intravenous antibiotics and spinal drainage are continued for 10 days after surgery. If a tracheostomy is present, it is discontinued as soon as the patient’s status permits.
Median Labiomandibular Glossotomy Approach
In cases where the oral opening between incisors is less than 2.5 cm or the pathology not only includes the craniocervical junction but also extends as far inferiorly as C5, mandibular split approaches have been developed.65,66 Such pathology is most commonly observed in the setting of congenital malformations including Klippel-Feil anomaly and secondary to neoplasms including chordoma, chondroma, chondrosarcoma, osteogenic sarcoma, and osteoblastoma. We prefer to use a median labiomandibular glossotomy (MLG) approach. Generally performed as a combined procedure with both neurosurgeons and head and neck surgeons, the MLG approach provides full access to the anterior spine from the clivus down to C5. The approach also carries with it a number of unique complications that, though rare, need to be fully discussed preoperatively. These include facial scarring, injury to dentition, dysphagia, impaired tongue mobility and sensation, mandibular duct injury, and velopalatine incompetence. Furthermore, a tracheostomy is essential in all patients. Nevertheless, in selected patients, the MLG approach provides outstanding surgical access to pathology not accessible by other means.65
The surgical approach has been described in detail.65 The identical precautions are taken as previously described in the transoral procedure regarding anesthesia and intubation. A crown halo is then placed and traction is applied. A tracheostomy is performed. A tracheostomy allows unimpeded access to the anterior cervical spine during surgery and provides a secure airway in the postoperative period. A midline incision is then made through the lower lip, around the mental protuberance, and along the midline to the level of the hyoid. The dissection is carried down to expose the mandible. A lateral incisor is extracted and the planned mandibular osteotomy is marked. Titanium plates are contoured to match the intact mandible and the screw holes are marked, which allows precise realignment of the mandible during closure. An osteotomy is performed, the dissection is carried posteriorly between the mandibular ducts, and the tongue musculature is divided. After the tongue is divided, the epiglottis is exposed down to the level of the hyoid. The posterior mucosa and paraspinal musculature are divided in the midline, and the inferior clivus down to C5 can be exposed. A ventral decompression can then be performed. In cases where a multilevel corpectomy is performed, an anterior fusion can be achieved by using costal or fibular grafts.
Transcervical Extrapharyngeal Approach
The anterior retropharyngeal approach to the upper cervical spine has been described by several authors.12–1519 This approach provides anterior access to the neural elements from the clivus to the third cervical vertebra without entrance into the oral cavity and allows a ventral fusion. I have used a variation of these procedures for the extrapharyngeal approach.19
The procedure used is similar to that described previously.67 The identical precautions are taken as previously described in the transoral procedure regarding anesthesia and intubation. The cervical incision is initiated behind the ear and extends over the mastoid process to 2 cm below the angle of the mandible toward the midline rostral to the hyoid bone. At the level of the omohyoid muscle, the incision is extended vertically over the sternomastoid muscle, converting the transverse incision into a T. The extent of this vertical limb depends on the level of cervical spine that must be exposed. After dissection through the subcutaneous tissue and platysma muscle, the inferior division of the facial nerve is identified and retracted. The dissection is continued anterior to the sternomastoid muscle and carotid sheath. The submandibular salivary gland is elevated, and if resection is done, it is important to ligate the salivary duct to prevent a fistula. The posterior belly of the digastric muscle is followed to its tendinous insertion and is transected after first transfixing it with a suture for subsequent reapproximation. The stylohyoid muscle is divided to allow medial retraction of the laryngopharynx. The hypoglossal nerve is identified in its path between the external and internal carotid arteries at the hyoid bone and mobilized superiorly, taking care to preserve the descendens hypoglossi branch. After entering the retropharyngeal space, the prevertebral fascia is vertically incised and the longus colli muscles are exposed. These latter are detached from their medial origin to expose the anterior arch of the atlas and clivus. Orientation to the midline must be maintained at all times and care must be taken with retraction to prevent injury to the hypoglossal nerve as it emerges from the condylar foramen.
Anterior decompression is done using a high-speed drill, and the technique is similar to that described for the transoral operation. After decompression of the neural elements, bone fusion is completed using a tricorticate iliac crest graft or fibular or rib strut between the caudal clivus and vertebra.
Ahmed R., Traynelis V.C., Menezes A.H. Fusions at the craniovertebral junction. Childs Nerv Syst. 2008;24:1209-1224.
Dastur D.K., Wadia N.H., Desai A.D., Sinh G. Medullospinal compression due to atlanto-axial dislocation and sudden haematomyelia during decompression. Pathology, pathogenesis and clinical correlations. Brain. 1965;88:897-924.
Dolan K.D. Cervicobasilar relationships. Radiol Clin North Am. 1977;15:155-166.
Gholve P.A., Hosalkar H.S., Ricchetti E.T., et al. Occipitalization of the atlas in children. Morphologic classification, associations, and clinical relevance. J Bone Joint Surg Am. 2007;89:571-578.
Maktabi M. Anesthetic management of CVJ surgical procedures, particularily transoral procedures. Operative Techniques in Neurosurgery. 2005;8:143-149.
Menezes A. Pathology encountered at the craniocervical junction. Operative Techniques in Neurosurgery. 2005;8:116-124.
Menezes A.H. Acquired abnormalities of the craniovertebral junction. In: Winn H.R., editor. Youman’s Neurological Surgery. Philadelphia: WB Saunders, 2003. 4569–4585
Menezes A.H. Craniovertebral junction. In: Albright A.G., Pollack E.F., Addison F.D. Principles and Practice of Pediatric Neurosurgery. New York: Thieme, 1999. 363–386
Menezes A.H. Surgical approaches: Postoperative care and complications “posterolateral-far lateral transcondylar approach to the ventral foramen magnum and upper cervical spinal canal”. Childs Nerv Syst. 2008;24:1203-1207.
Menezes A.H., Folz G. Transoral approach to the ventral craniocervical border. Operative Techniques in Neurosurgery. 2008;8:150-157.
Menezes A.H., Traynelis V.C. Anatomy and biomechanics of normal craniovertebral junction (a) and biomechanics of stabilization (b). Childs Nerv Syst. 2008;24:1091-1100.
Moore L.J., Schwartz H.C. Median labiomandibular glossotomy for access to the cervical spine. J Oral Maxillofac Surg. 1985;43:909-912.
Sawin P.D., Traynelis V.C., Menezes A.H. A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg. 1998;88:255-265.
Smoker W.R., Khanna G. Imaging the craniocervical junction. Childs Nerv Syst. 2008;24:1123-1145.
Taylor A.R., Byrnes D.P. Foramen magnum and high cervical cord compression. Brain. 1974;97:473-480.
Vishteh A.G., Beals S.P., Joganic E.F., et al. Bilateral sagittal split mandibular osteotomies as an adjunct to the transoral approach to the anterior craniovertebral junction. Technical note. J Neurosurg. 1999;90:267-270.
Werne S. The possibilities of movement in the craniovertebral joints. Acta Orthop Scand. 1959;28:165-173.
1. Menezes A.H., VanGilder J.C. Platybasia, basilar invagination, and cranial settling. In: Apuzzo M.L.J., editor. Brain surgery complication, avoidence and management. New York: Churchill Livingstone, 1993. 2029–2049
2. Menezes A.H., VanGilder J.C.. Anomalies of the craniovertebral junction, Youmans J.R., editor. Neurological Surgery, 3rd ed, Philadelphia: WB Saunders, 1990. 1359–1420
3. Menezes A.H. Craniovertebral junction. In: Albright A.G., Pollack E.F., Addison F.D. Principles and Practice of Pediatric Neurosurgery. New York: Thieme, 1999. 363–386
4. Bell C. The Nervous System of the Human Body. London: Longman, Rees, Orme, Brown, & Green; 1830.
5. Dastur D.K., Wadia N.H., Desai A.D., Sinh G. Medullospinal compression due to atlanto-axial dislocation and sudden haematomyelia during decompression. Pathology, pathogenesis and clinical correlations. Brain. 1965;88:897-924.
6. Hall J.E., Denis F., Murray J. Exposure of the upper cervical spine for spinal decompression by a mandible and tongue-splitting approach. Case report. J Bone Joint Surg Am. 1977;59:121-123.
7. Menezes A.H., VanGilder J.C. Transoral-transpharyngeal approach to the anterior craniocervical junction. Ten-year experience with 72 patients. J Neurosurg. 1988;69:895-903.
8. Crockard H.A., Calder I., Ransford A.O. One-stage transoral decompression and posterior fixation in rheumatoid atlanto-axial subluxation. J Bone Joint Surg Br. 1990;72:682-685.
9. Greenberg A.D., Scoville W.B., Davey L.M. Transoral decompression of atlanto-axial dislocation due to odontoid hypoplasia. Report of two cases. J Neurosurg. 1968;28:266-269.
10. Di Lorenzo N. Transoral approach to extradural lesions of the lower clivus and upper cervical spine: an experience of 19 cases. Neurosurgery. 1989;24:37-42.
11. Moore L.J., Schwartz H.C. Median labiomandibular glossotomy for access to the cervical spine. J Oral Maxillofac Surg. 1985;43:909-912.
12. de Andrade J.R., Macnab I. Anterior occipito-cervical fusion using an extra-pharyngeal exposure. J Bone Joint Surg Am. 1969;51:1621-1626.
13. McAfee P.C., Bohlman H.H., Riley L.H.Jr., et al. The anterior retropharyngeal approach to the upper part of the cervical spine. J Bone Joint Surg Am. 1987;69:1371-1383.
14. Whitesides T.E.Jr., McDonald A.P. Lateral retropharyngeal approach to the upper cervical spine. Orthop Clin North Am. 1978;9:1115-1127.
15. Kawashima M., Tanriover N., Rhoton A.L.Jr., et al. Comparison of the far lateral and extreme lateral variants of the atlanto-occipital transarticular approach to anterior extradural lesions of the craniovertebral junction. Neurosurgery. 2003;53:662-674.
16. Menezes A.H. Craniovertebral junction neoplasms in the pediatric population. Childs Nerv Syst. 2008;24:1173-1186.
17. Menezes A. Pathology encountered at the craniocervical junction. Operative Techniques in Neurosurgery. 2005;8:116-124.
18. Menezes A.H., Vogel T.W. Specific entities affecting the craniocervical region: syndromes affecting the craniocervical junction. Childs Nerv Syst. 2008;24:1155-1163.
19. Menezes A.H. Surgical approaches to the craniocervical junction. In: Weinstein S.L., editor. The pediatric spine: principles and practice. New York: Raven Press, 1994. 1311–1327
20. Ganguly D.N., Roy K.K. A Study on the cranio-vertebral joint in the man. Anat Anz. 1964;114:433-452.
21. Bailey D.K. The normal cervical spine in infants and children. Radiology. 1952;59:712-719.
22. Menezes A.H. Craniocervical developmental anatomy and its implications. Childs Nerv Syst. 2008;24:1109-1122.
23. Gholve P.A., Hosalkar H.S., Ricchetti E.T., et al. Occipitalization of the atlas in children. Morphologic classification, associations, and clinical relevance. J Bone Joint Surg Am. 2007;89:571-578.
24. Menezes A.H. Specific entities affecting the craniocervical region: Down’s syndrome. Childs Nerv Syst. 2008;24:1165-1168.
25. Menezes A.H., Traynelis V.C. Anatomy and biomechanics of normal craniovertebral junction (a) and biomechanics of stabilization (b). Childs Nerv Syst. 2008;24:1091-1100.
26. White A.A.3rd, Panjabi M.M. The clinical biomechanics of the occipitoatlantoaxial complex. Orthop Clin North Am. 1978;9:867-878.
27. Werne S. The possibilities of movement in the craniovertebral joints. Acta Orthop Scand. 1959;28:165-173.
28. Holmes J.C., Hall J.E. Fusion for instability and potential instability of the cervical spine in children and adolescents. Orthop Clin North Am. 1978;9:923-943.
29. Gilles F.H., Bina M., Sotrel A. Infantile atlantooccipital instability. The potential danger of extreme extension. Am J Dis Child. 1979;133:30-37.
30. Sullivan A.W. Subluxation of the atlanto-axial joint, sequel to inflammatory processes of the neck. J Pediatr. 1949;35:451-464.
31. Clark C.R., Menezes A.H. Rheumatoid arthritis: Surgical considerations. In: Rothman R.H., Simeone F.A. The Spine. Philadelphia: WB Saunders, 1999. pp 1281–1301
32. VanGilder J.C., Menezes A.H. Craniovertebral junction abnormalities. In: Wilkins R.H., Rengachery. Neurosurgery. New York: McGraw-Hill, 1996. pp 3587–3392
33. Michie I., Clark M. Neurological syndromes associated with cervical and craniocervical anomalies. Arch Neurol. 1968;18:241-247.
34. Manaligod J.M., Bauman N.M., Menezes A.H., Smith R.J. Cervical vertebral anomalies in patients with anomalies of the head and neck. Ann Otol Rhinol Laryngol. 1999;108:925-933.
35. Klippel M.F.A. Un cas d’absence des vertebres cervicales avec cage throacique remmtant jusqu’a la base du craine. Nouv Icon Soipetriene. 1912;25:223-228.
36. Menezes A.H. Craniovertebral junction database analysis: incidence, classification, presentation, and treatment algorithms. Childs Nerv Syst. 2008;24:1101-1108.
37. Menezes A.H. Acquired abnormalities of the craniovertebral junction. In: Winn H.R., editor. Youman’s Neurological Surgery. Philadelphia: WB Saunders, 2003. pp 4569–4585
38. Taylor A.R., Byrnes D.P. Foramen magnum and high cervical cord compression. Brain. 1974;97:473-480.
39. Botelho R.V., Bittencourt L.R., Rotta J.M., Tufik S. A prospective controlled study of sleep respiratory events in patients with craniovertebral junction malformation. J Neurosurg. 2008;99:1004-1009.
40. Cogan D.G., Barrows L.J. Platybasia and the Arnold-Chiari malformation. AMA Arch Ophthalmol. 1954;52:13-29.
41. Dolan K.D. Cervicobasilar relationships. Radiol Clin North Am. 1977;15:155-166.
42. Khanna G.Y.S. Imaging of the craniovertebral junction. Operative Techniques in Neurosurgery. 2005;8:131-142.
43. Smoker W.R., Khanna G. Imaging the craniocervical junction. Childs Nerv Syst. 2008;24:1123-1145.
44. Krakenes J., Kaale B.R., Rorvik J., Gilhus N.E. MRI assessment of normal ligamentous structures in the craniovertebral junction. Neuroradiology. 2001;43:1089-1097.
45. Yuksel M., Heiserman J.E., Sonntag V.K. Magnetic resonance imaging of the craniocervical junction at 3-T: observation of the accessory atlantoaxial ligaments. Neurosurgery. 2006;59:888-892.
46. Menezes A.H. Decision making. Childs Nerv Syst. 2008;24:1147-1153.
47. Menezes A. Decision making for management of craniovertebral junction pathology. Operative Techniques in Neurosurgery. 2005;8:125-130.
48. Apuzzo M.L., Heiden J.S., Weiss M.H., et al. Acute fractures of the odontoid process. An analysis of 45 cases. J Neurosurg. 1978;48:85-91.
49. Bohlman HHDT, Levine A.M., et al. Spine trauma in adults. In: Rothman R.H., Simeone F.A. The Spine. Philadelphia: WB Saunders, 1992. pp 889–1070
50. Johnson R.M., Hart D.L., Simmons E.F., et al. Cervical orthoses. A study comparing their effectiveness in restricting cervical motion in normal subjects. J Bone Joint Surg Am. 1977;59:332-339.
51. Puttlitz C.M., Melcher R.P., Kleinstueck F.S., et al. Stability analysis of craniovertebral junction fixation techniques. J Bone Joint Surg Am. 2004;86:561-568.
52. Naderi S., Crawford N.R., Song G.S., et al. Biomechanical comparison of C1–C2 posterior fixations. Cable, graft, and screw combinations. Spine. 1998;23:946-955.
53. Menezes A.H. Craniovertebral junction congenital abnormalities. In: Kaye A.H., Black P. Operative Neurosurgery. London: Harcourt International, 2000. pp 1755–1770
54. Sawin P.D., Traynelis V.C., Menezes A.H. A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg. 1998;88:255-265.
55. Ahmed R., Traynelis V.C., Menezes A.H. Fusions at the craniovertebral junction. Childs Nerv Syst. 2008;24:1209-1224.
56. Husain M., Rastogi M., Ojha B.K., et al. Endoscopic transoral surgery for craniovertebral junction anomalies. Technical note. J Neurosurg Spine. 2006;5:367-373.
57. Kassam A.B., Snyderman C., Gardner P., et al. The expanded endonasal approach: a fully endoscopic transnasal approach and resection of the odontoid process: technical case report. Neurosurgery. 2005;57:E213.
58. Maktabi M. Anesthetic management of CVJ surgical procedures, particularly transoral procedures. Operative Techniques in Neurosurgery. 2005;8:143-149.
59. Menezes A.H., Folz G. Transoral approach to the ventral craniocervical border. Operative Techniques in Neurosurgery. 2008;8:150-157.
60. Menezes A.H. Surgical approaches: postoperative care and complications “transoral-transpalatopharyngeal approach to the craniocervical junction”. Childs Nerv Syst. 2008;24:1187-1193.
61. Guity A., Young P.H. A new technique for closure of the dura following transsphenoidal and transclival operations. Technical note. J Neurosurg. 1990;72:824-828.
62. Yamaura A., Makino H., Isobe K., et al. Repair of cerebrospinal fluid fistula following transoral transclival approach to a basilar aneurysm. Technical note. J Neurosurg. 1979;50:834-838.
63. Menezes A.H., Greenlee J.D.W. Transoral approach. In: Harsh G., Janecka I., Mankin H., et al. Chordomas and Chondrosarcomas of the Skull Base and Spine. New York: Thieme, 2003. pp 139–145
64. Menezes A. Avoiding complications with transoral surgical procedures to the craniocervical junction. Operative Techniques in Neurosurgery. 2008;8:160-163.
65. Brookes J.T., Smith R.J., Menezes A.H., Smith M.C. Median labiomandibular glossotomy approach to the craniocervical region. Childs Nerv Syst. 2008;24:1195-1201.
66. Vishteh A.G., Beals S.P., Joganic E.F., et al. Bilateral sagittal split mandibular osteotomies as an adjunct to the transoral approach to the anterior craniovertebral junction. Technical note. J Neurosurg. 1999;90:267-270.
67. Menezes A.H. Surgical approaches: postoperative care and complications “posterolateral-far lateral transcondylar approach to the ventral foramen magnum and upper cervical spinal canal”. Childs Nerv Syst. 2008;24:1203-1207.