Chapter 70 Cranial Neuropathies
Olfactory Nerve (Cranial Nerve I)
Optic Nerve (Cranial Nerve II)
Oculomotor Nerve (Cranial Nerve III)
Trochlear Nerve (Cranial Nerve IV)
Trigeminal Nerve (Cranial Nerve V)
Abducens Nerve (Cranial Nerve VI)
Facial Nerve (Cranial Nerve VII)
Vestibulocochlear Nerve (Cranial Nerve VIII)
Glossopharyngeal Nerve (Cranial Nerve IX)
Oculomotor Nerve (Cranial Nerve III)
Anatomy
Paired oculomotor nuclei are located in the dorsal midbrain ventral to the periaqueductal gray matter at the level of the superior colliculus (Donzelli et al, 1998). Each is composed of a superior rectus subnucleus providing innervation to the contralateral superior rectus; inferior rectus, medial rectus, and inferior oblique subnuclei providing ipsilateral innervation; and an Edinger-Westphal nucleus supplying preganglionic parasympathetic output to the iris sphincter and ciliary muscles. A single midline caudal central subnucleus provides innervation to both levator palpebrae superioris muscles.
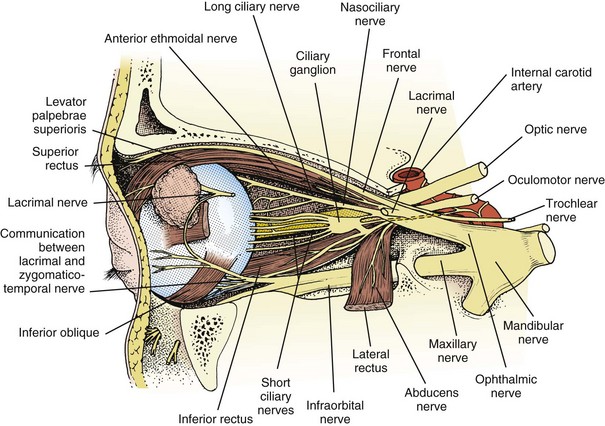
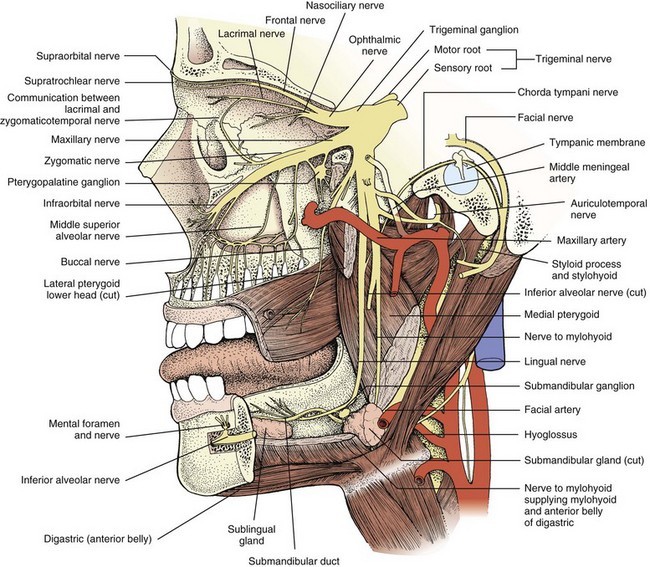
A third nerve fascicle originates from the ventral surface of each nucleus and traverses the midbrain, passing through the red nucleus and in close proximity to the cerebral peduncles before emerging ventrally as rootlets in the lateral interpeduncular fossa. In the interpeduncular fossa, the rootlets converge into a third nerve trunk that continues ventrally through the subarachnoid space toward the cavernous sinus, passing between the superior cerebellar artery and the posterior cerebral artery. It travels parallel to the posterior communicating artery (PCOM) and is very near to the vessel at its junction with the intracranial internal carotid artery. In the cavernous sinus, the third nerve is located within the dural sinus wall, just lateral to the pituitary gland. From the cavernous sinus, the third nerve enters the orbit via the superior orbital fissure. Just prior to entry, the nerve anatomically divides into superior and inferior divisions in the anterior cavernous sinus, although careful evaluation of brainstem lesions and their corresponding patterns of pupil and muscle involvement suggests that functional division occurs in the midbrain (Saeki et al., 2000). Within the orbit, the superior division innervates the superior rectus and the levator palpebrae superioris, and the inferior division innervates the inferior and medial recti, the inferior oblique, and the iris sphincter and ciliary muscles (Fig. 70.1). Prior to innervating the ciliary and sphincter muscles as the short ciliary nerves, parasympathetic third nerve fibers synapse in the ciliary ganglion within the orbit (see Fig. 70.1).
Clinical Lesions
Oculomotor Nucleus
In addition to potentially causing ipsilateral weakness of the medial rectus, inferior rectus, and inferior oblique muscles, an oculomotor nuclear lesion may result in bilateral superior rectus weakness. Ipsilateral subnucleus involvement affects the contralateral superior rectus. This occurs because of the completely crossed nature of superior rectus innervation, with a single superior rectus subnucleus containing fibers destined to decussate to the contralateral side. A unilateral oculomotor nuclear lesion may affect these unilateral originating fibers destined for decussation and those fibers that originated contralaterally and have already decussated. If the single midline levator palpebrae superioris subnucleus is involved in an oculomotor nuclear lesion, bilateral ptosis results. Isolated bilateral ptosis or isolated paresis of a single extraocular muscle are also possible from a small focal nuclear lesion, given the functional division of the subnuclei (Rabadi and Beltmann, 2005). Involvement of the rostral and dorsally located Edinger-Westphal nucleus will lead to pupil involvement. Common brainstem lesions include ischemia, hemorrhage, demyelination, infectious and noninfectious inflammation, and neoplasm.
Oculomotor Palsy Appearance
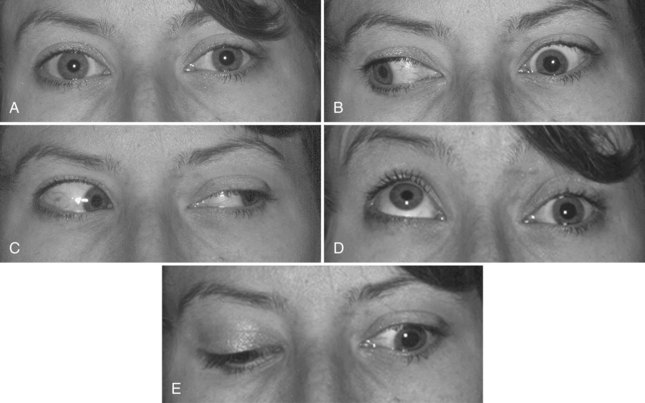
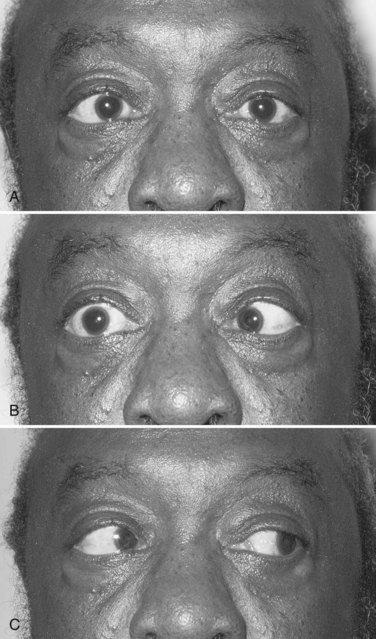
Identification of a complete third nerve palsy is straightforward on examination, with an ipsilateral eye that is deviated inferiorly and laterally, ipsilateral ptosis, and an ipsilateral enlarged and nonreactive pupil (Fig. 70.2). Great emphasis is placed on the presence of pupil involvement versus pupil sparing with regard to potential lesion etiology. Pupillary fibers are located superomedially near the surface of the nerve and are particularly prone to compression by a PCOM aneurysm. An enlarged, poorly reactive pupil is a key diagnostic feature helpful in distinguishing this entity from an intrinsic nerve lesion such as microvascular ischemia. Relative pupil involvement with an average of 0.8 mm of anisocoria may be seen in up to a third of patients with microvascular oculomotor nerve ischemia, but the pupil generally remains reactive in this scenario (Jacobson, 1998). In rare patients with microvascular ischemia, anisocoria of up to 2 mm may be present, prompting urgent evaluation (Chou et al., 2004). Relative pupil involvement is also commonly seen with mass lesions compressing the nerve (Jacobson, 2001).
Brainstem Fascicle
Claude syndrome is the combination of an ipsilateral oculomotor nerve palsy and contralateral ataxia (Liu et al., 1994) (Table 70.1 and ![]() Videos 70.1 and 70.2 [available at www.ExpertConsult.com]). Although historically thought to involve the oculomotor fascicle and the red nucleus, the ataxia is likely due to involvement of the superior cerebellar peduncle at the caudal end of the red nucleus (Seo et al., 2001). Nothnagel syndrome is the combination of ipsilateral oculomotor nerve palsy and ipsilateral cerebellar ataxia from involvement of the oculomotor fascicle and superior cerebellar peduncle (see Table 70.1). Weber syndrome is the combination of an ipsilateral fascicular oculomotor nerve palsy and contralateral hemiparesis from cerebral peduncle involvement (see Table 70.1). Benedikt syndrome involves the oculomotor fascicle and red nucleus, causing an ipsilateral oculomotor nerve palsy and contralateral chorea or tremor (see Table 70.1). Common brainstem lesions include ischemia, hemorrhage, demyelination, infectious and noninfectious inflammation, and neoplasm.
Videos 70.1 and 70.2 [available at www.ExpertConsult.com]). Although historically thought to involve the oculomotor fascicle and the red nucleus, the ataxia is likely due to involvement of the superior cerebellar peduncle at the caudal end of the red nucleus (Seo et al., 2001). Nothnagel syndrome is the combination of ipsilateral oculomotor nerve palsy and ipsilateral cerebellar ataxia from involvement of the oculomotor fascicle and superior cerebellar peduncle (see Table 70.1). Weber syndrome is the combination of an ipsilateral fascicular oculomotor nerve palsy and contralateral hemiparesis from cerebral peduncle involvement (see Table 70.1). Benedikt syndrome involves the oculomotor fascicle and red nucleus, causing an ipsilateral oculomotor nerve palsy and contralateral chorea or tremor (see Table 70.1). Common brainstem lesions include ischemia, hemorrhage, demyelination, infectious and noninfectious inflammation, and neoplasm.
| Syndrome* | Symptoms and Signs | Involved Structures |
|---|---|---|
| Claude | Ipsilateral III | III—brainstem fascicle |
| Contralateral ataxia | Red nucleus/superior cerebellar peduncle | |
| Nothnagel | Ipsilateral III | III—brainstem fascicle |
| Ipsilateral ataxia | Superior cerebellar peduncle | |
| Weber | Ipsilateral III | III—brainstem fascicle |
| Contralateral hemiparesis | Cerebral peduncle | |
| Benedikt | Ipsilateral III | III—brainstem fascicle |
| Contralateral chorea or tremor | Red nucleus | |
| Tolosa-Hunt | Ipsilateral III, IV, VI | Cavernous sinus |
| Ipsilateral 1st and 2nd branches of V | III, IV, VI, 1st and 2nd branches of V | |
| Ipsilateral Horner syndrome | Sympathetic nerves | |
| Wallenberg lateral medullary syndrome | Ipsilateral facial numbness and ↓ pinprick | Spinal trigeminal tract and nucleus |
| Contralateral hemibody ↓ pain and temperature | Spinothalamic tract | |
| Dysphagia and ↓ gag reflex | Nucleus ambiguus | |
| Limb ataxia | Inferior cerebellar peduncle | |
| Horner syndrome | Sympathetic nerves | |
| Vertigo | Vestibular nuclei | |
| Opalski syndrome | Wallenberg in addition to ipsilateral hemiparesis | Corticospinal fibers caudal to pyramidal decussation |
| Gradenigo | Facial and mastoid area pain | Petrous apex—temporal bone V, VI, VII |
| Ipsilateral 1st branch of V | ||
| Ipsilateral VI and VII | ||
| Foville | Ipsilateral VI and VII | VI and VII—brainstem |
| Contralateral ataxia | Mid-cerebellar peduncle | |
| Ipsilateral Horner syndrome | Sympathetic nerves | |
| Ipsilateral deafness | Vestibulocochlear nerve/fascicle | |
| Ipsilateral ↓ taste and facial sensation | Spinal trigeminal tract/nucleus solitarius | |
| Millard-Gubler | Ipsilateral VI and VII | VI and VII—brainstem |
| Contralateral hemiparesis | Pyramidal tract | |
| Raymond | Ipsilateral VI | VI—brainstem |
| Contralateral hemiparesis | Pyramidal tract | |
| Bell palsy | VII | VII—intratemporal nerve and geniculate ganglion |
| Ramsay Hunt | VII | VII |
| Vesicular otic or oral rash ± ↓ hearing | ± VIII | |
| Melkersson-Rosenthal | VII | VII—distal branches |
| Facial edema | ||
| Fissured tongue | ||
| Eagle | IX | XI—elongated styloid process or ossified stylohyoid ligament |
| Vernet | IX, X, XI | Jugular foramen |
| Villaret | IX, X, XI | Jugular foramen |
| XII | Hypoglossal canal | |
| Horner syndrome | Sympathetic nerves | |
| Collet-Sicard | IX, X, XI | Jugular foramen |
| XII | Hypoglossal canal | |
| Avellis | X | X—brainstem or peripheral |
| Contralateral hemiparesis | Pyramidal tract | |
| Tapia | X and XII | X and XII—brainstem or peripheral |
| Dejerine medial medullary syndrome | Ipsilateral XII | XII—nuclei or fascicle |
| Contralateral hemiparesis | Pyramidal tract | |
| Contralateral hemisensory deficit | Medial lemniscus | |
| Babinski-Nageotte | Combined symptoms and signs of Wallenbergand Dejerine syndromes | Hemi-medullary: lateral and medial medulla |
↓, Decreased.
* Descriptions of named syndromes vary slightly depending on reference used.
Interpeduncular Fossa and Subarachnoid Space: PCOM Aneurysm
The most common etiology of oculomotor dysfunction in this region is compression by a PCOM aneurysm (Trobe, 2009). Identification of a pupil-involving oculomotor palsy requires urgent evaluation for a PCOM aneurysm, given the high risk for subarachnoid hemorrhage and mortality if left undiagnosed and untreated. Involvement of all oculomotor-supplied muscles in the setting of a normal pupil is very unlikely to result from aneurysmal compression; however, the presence of incomplete or partial impairment of the oculomotor muscles even in the setting of a normal pupil should prompt investigation for an aneurysm (Chou et al., 2004). Some patients with an aneurysmal incomplete third nerve palsy will lack pupillary involvement at initial presentation, but the majority will progress to pupillary involvement within 1 week. Spontaneous improvement in third nerve function preceding aneurysm treatment is reported and should not preclude diagnostic testing. Onset of oculomotor dysfunction following minor trauma should also prompt investigation for an underlying aneurysm (Levy et al., 2005; Walter et al., 1994) (see Fig. 70.2). Magnetic resonance angiography (MRA) and computed tomographic angiography (CTA) have largely supplanted conventional cerebral angiography as the preferred diagnostic tests for aneurysm detection, but expert interpretation of these studies requires a high level of experience, skill, and time (Chaudhary et al., 2009; Elmalem et al., 2011). Neither MRA nor CTA is known to be 100% sensitive, and the risks of conventional angiogram are still warranted when strong clinical suspicion for a PCOM aneurysm is present.
In the subarachnoid space, the oculomotor nerve passes in close proximity to the medial temporal lobe. Herniation of the temporal lobe uncus secondary to increased intracranial pressure may result in compression of the oculomotor nerve, manifested clinically as sudden enlargement and poor reactivity of the ipsilateral pupil—the Hutchison pupil. Oculomotor nerve involvement in the interpeduncular fossa and subarachnoid space may also occur secondary to inflammatory or neoplastic meningitis. Enlargement and enhancement of the nerve are usually present on MRI in ophthalmoplegic migraine (see Chapter 69). Oculomotor palsy in meningitis may occur in isolation or be accompanied by signs of meningeal inflammation such as meningismus or additional cranial nerve palsies.
Cavernous Sinus: Tolosa-Hunt Syndrome
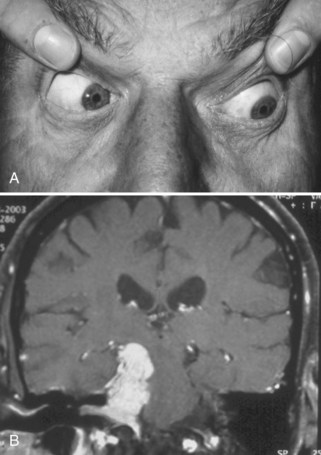
An oculomotor palsy in this location may occur in isolation or accompanied by dysfunction of other structures located here, including the abducens and trochlear nerves, the first and second divisions of the trigeminal nerve, and sympathetic fibers. Tolosa-Hunt is a painful syndrome of idiopathic self-limited inflammation of the cavernous sinus, typically responsive to corticosteroids (see Table 70.1 and ![]() Videos 70.3 and 70.4 [available at www.ExpertConsult.com]). Cavernous sinus infiltration by metastatic disease is clinically and radiographically identical to Tolosa-Hunt syndrome and should be suspected in older patients. Cavernous sinus lymphoma is typically steroid responsive and should be considered, especially if disease recurs with corticosteroid taper. Inflammation associated with systemic rheumatological disease, nasopharyngeal neoplastic infiltration, carotid-cavernous fistulas, and compression from an intracavernous internal artery aneurysm or meningioma may also cause a cavernous sinus syndrome (Nallasamy et al., 2010). Pituitary apoplexy should be considered in the differential diagnosis for sudden-onset painful unilateral or bilateral oculomotor palsies, with or without accompanying visual loss (Dubuisson et al., 2007).
Videos 70.3 and 70.4 [available at www.ExpertConsult.com]). Cavernous sinus infiltration by metastatic disease is clinically and radiographically identical to Tolosa-Hunt syndrome and should be suspected in older patients. Cavernous sinus lymphoma is typically steroid responsive and should be considered, especially if disease recurs with corticosteroid taper. Inflammation associated with systemic rheumatological disease, nasopharyngeal neoplastic infiltration, carotid-cavernous fistulas, and compression from an intracavernous internal artery aneurysm or meningioma may also cause a cavernous sinus syndrome (Nallasamy et al., 2010). Pituitary apoplexy should be considered in the differential diagnosis for sudden-onset painful unilateral or bilateral oculomotor palsies, with or without accompanying visual loss (Dubuisson et al., 2007).
Isolated Oculomotor Nerve Palsy
Isolated oculomotor dysfunction may occur from any lesion along the course of the nerve (Brazis, 2009). A pupil-sparing oculomotor palsy may represent microvascular ischemia to the nerve, especially in older patients with vascular risk factors. The actual location of ischemia may be anywhere along the course of the nerve but is typically peripheral and often considered to be in the cavernous sinus, although it is not visible with neuroimaging. Pain in the ipsilateral brow and eye is present in two-thirds of patients and may be severe (Wilker et al., 2009). Spontaneous resolution over 8 to 12 weeks is typical. A small percentage of such patients will ultimately be found to have an underlying structural lesion, and neuroimaging should be considered (Chou et al., 2004). In the absence of complete spontaneous resolution, neuroimaging is essential.
Elevation of the eyelid or constriction of the pupil during adduction or depression of the eye is suggestive of aberrant regeneration (anomalous axon innervation). When aberrant regeneration develops following an acute oculomotor palsy, a PCOM artery aneurysm or traumatic etiology is highly probable (see Fig. 70.2, B). When it develops spontaneously without a preexisting acute palsy, a cavernous sinus meningioma or internal carotid artery aneurysm is likely, although this may rarely occur with an unruptured PCOM aneurysm (Carrasco et al., 2002). Aberrant regeneration should not occur following a microvascular oculomotor palsy.
Trochlear Nerve (Cranial Nerve IV)
Anatomy
Paired trochlear nuclei lie very close to the dorsal surface of the midbrain just inferior to the inferior colliculus. The fascicles emerge from the nuclei and course dorsally only 3 to 9 mm before exiting the brainstem (Yousry et al., 2002). The trochlear nerves are the only cranial nerves to emerge from the dorsal brainstem surface. After emergence, the nerves decussate within the anterior medullary velum and wrap around the surface of the midbrain to travel ventrally within the subarachnoid space toward the cavernous sinus. In the cavernous sinus, the trochlear nerve is located in the lateral dural wall, inferior to the oculomotor nerve. From the cavernous sinus, the nerve passes into the superior orbital fissure and ultimately innervates the superior oblique muscle contralateral to the nucleus of origin (see Fig. 70.1).
Clinical Lesions
Trochlear Nucleus and Fascicle
It is difficult to differentiate a trochlear nuclear lesion from a fascicular lesion because of the short course of the trochlear fascicle in the brainstem and the predecussation location of both structures. Both locations will result in paresis of the contralateral superior oblique muscle. Isolated nuclear or fascicular involvement occurs rarely; other brainstem signs are usually present (Thomke and Hopf, 2000). Brainstem lesions include ischemia, hemorrhage, demyelination, infectious and noninfectious inflammation, and neoplasm (Jacobson et al., 1999).
Trochlear Palsy Appearance
Trochlear nerve dysfunction results in elevation of the affected eye (hypertropia) and vertical diplopia. The diplopia and hypertropia are worse with downgaze when the eye is in an adducted position, as this is the direction of action of the superior oblique muscle. Impaired depression of the affected eye in the adducted position may be seen but is often subtle (Fig. 70.3). A resting head tilt in the direction away from the paretic eye (e.g., right trochlear palsy, left head tilt) may be present and is considered a sign of chronicity. Because the superior oblique is an intortor of the eye, diplopia is minimized when a contralateral head tilt places the affected eye in an extorted position. Congenital trochlear palsy is relatively common, and identification of a long-standing head tilt in old photographs of the patient can help confirm this diagnosis.
Subarachnoid Space
At the point of trochlear nerve decussation, the nerves are near the tentorium cerebelli and are prone to unilateral or bilateral traumatic injury (Keane, 2005). Unlike traumatic oculomotor neuropathy, which usually occurs following severe head trauma with loss of consciousness, the trochlear nerves are injured more easily by minor degrees of head trauma (Dhaliwal et al., 2006).
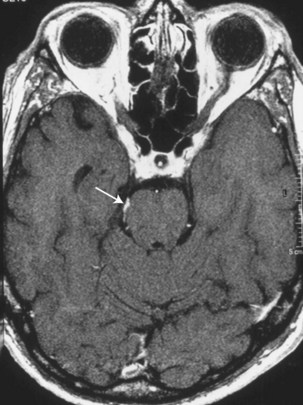
Trochlear nerve involvement in isolation or accompanied by signs of meningeal inflammation (e.g., meningismus, additional cranial nerve palsies) may occur secondary to inflammatory or neoplastic meningitis. Enhancement of the nerve as it travels dorsally to ventrally around the midbrain may be present on MRI. An increasingly recognized cause of such enhancement in the setting of normal cerebrospinal fluid is trochlear nerve schwannoma (Elmalem et al., 2009; Feinberg and Newman, 1999) (Fig. 70.4).
Cavernous Sinus
Trochlear palsy in this location is generally accompanied by dysfunction of other structures including the abducens and oculomotor nerves, the first and second divisions of the trigeminal nerve, and sympathetic fibers. Idiopathic inflammation, inflammation from systemic rheumatological disease, metastatic or nasopharyngeal neoplastic infiltration, carotid-cavernous fistulas, and compression from an intracavernous internal artery aneurysm or meningioma are common causes. Pituitary apoplexy very rarely results in isolated trochlear paresis (Petermann and Newman, 1999).
Trigeminal Nerve (Cranial Nerve V)
Anatomy
The trigeminal nerve consists of afferent sensory, efferent motor, and parasympathetic fibers. The ophthalmic (V1), maxillary (V2), and mandibular (V3) trigeminal sensory nerve branches emerge from the anterior surface of the trigeminal (gasserian) ganglion in the cave of Meckel (a dural cavity overlying the apex of the petrous bone) and innervate the facial skin, mucous membranes of the nose and mouth, teeth, orbital contents, and supratentorial meninges (Yousry et al., 2005) (Fig. 70.5; also see Fig. 70.1). The ophthalmic division courses in the lateral wall of the cavernous sinus inferior to the trochlear nerve and exits the skull via the superior orbital fissure. It is the anatomical substrate for the afferent limb of the corneal reflex. The maxillary division also courses in the lateral wall of the cavernous sinus, exiting the skull via the foramen rotundum to enter the sphenopalatine fossa and the inferior orbital fissure. The mandibular division exits the skull through the foramen ovale. Sensory input from these three branches travels centrally from the trigeminal ganglion via the trigeminal sensory root in the prepontine subarachnoid cistern to the trigeminal sensory nucleus, which is composed of mesencephalic, principal (main) sensory, and descending spinal nuclei (nucleus caudalis) that descend to the cervical spinal cord. Sensory information ultimately ascends to the contralateral thalamus.
Clinical Lesions
Trigeminal Nucleus
The extension of the trigeminal nuclear complex throughout the entire brainstem renders it susceptible to involvement from any pathological brainstem process. Trigeminal lesions are particularly common with demyelinating disease, may involve either the nuclei or sensory root, and may be clinically silent or symptomatic (Mills et al., 2010). Ischemia, hemorrhage, infectious and noninfectious inflammation, and neoplasm are other causes of trigeminal brainstem involvement. Additional brainstem signs are frequently present. Wallenberg syndrome from lateral medullary ischemia typically causes ipsilateral facial numbness and impaired pinprick sensation secondary to descending spinal tract and nucleus involvement (see Table 70.1).
Sensory and Motor Roots: Trigeminal Neuralgia
Classic trigeminal neuralgia is characterized by seconds-long stereotyped episodes of intensely painful electric-like shocks along one or more of the trigeminal nerve branches. Pain is often elicited by sensory stimuli on the face, such as shaving or wind. There is no accompanying sensory loss in the affected distribution on examination. The second and third branches are most commonly affected, with less than 5% of patients experiencing involvement of the ophthalmic division. Many cases are attributable to compression of the sensory nerve root by a vascular structure, most often the superior cerebellar artery, and are amenable to surgical decompression (Lorenzoni et al., 2009). Because vascular contact with the sensory or motor nerve roots is a relatively common finding in cadavers and asymptomatic controls, vascular contact alone may be insufficient to cause trigeminal neuralgia (Masur et al., 1995; Yousry et al., 2004). Compression or indentation of the nerve root is likely necessary, with resultant axonal loss and demyelination (Miller et al., 2009). See Chapter 69 for additional discussion of trigeminal neuralgia and other facial pain syndromes. Table 70.2, available in the online version of this chapter at www.ExpertConsult.com, outlines current treatments for facial palsy and trigeminal and glossopharyngeal neuralgia.
Table 70.2 Facial Palsy, Trigeminal Neuralgia, and Glossopharyngeal Neuralgia Treatments
| Condition | Treatments | Considerations |
|---|---|---|
| Facial palsy | Lubrication and taping | Corneal protection during temporary facial weakness with minimal corneal risk |
| External upper eyelid weights, Botox, temporary tarsorrhaphy | Corneal protection during temporary facial weakness with corneal exposure | |
| Permanent gold upper-eyelid weights or permanent tarsorrhaphy | Corneal protection with permanent facial weakness | |
| Facial physical therapy or neuromuscular facial retraining | May be enhanced by electromyographic feedback, may be helpful for synkinetic dysfunction | |
| Brow elevation | For visual dysfunction or discomfort from brow ptosis | |
| Proximal facial nerve grafting with sural or greater auricular nerve; hypoglossal or spinal accessory to facial nerve anastomosis; microneurovascular free muscle flap transfer (e.g., with temporalis or latissimus dorsi muscle) | Facial mobilization procedures; most commonly used after permanent iatrogenic surgical facial nerve section or for neoplastic facial dysfunction | |
| Trigeminal and glossopharyngeal neuralgia | Pharmacologic treatment with phenytoin, carbamazepine, gabapentin, lamotrigine, or topiramate | Prophylactic pain therapy; often the mainstay of therapy |
| Microvascular decompression | Typically the preferred treatment for young patients with medically refractory pain; low pain recurrence rate | |
| Percutaneous radiofrequency thermocoagulation; glycerol rhizolysis; or balloon microcompression | May be the preferred treatment for older patients at high surgical risk with medically refractory pain |
Trigeminal Ganglion: Herpes Zoster Ophthalmicus
Middle cranial fossa malignant infiltration, compressive neoplastic lesions, and autoimmune inflammation are among the most common causes of trigeminal ganglion pathology. The trigeminal ganglion is a location in which the varicella-zoster virus (VZV) often lies latent, sometimes reactivating along the course of the trigeminal ophthalmic branch later in life to cause herpes zoster ophthalmicus. Identification of skin lesions along the side of the nose (Hutchinson sign) corresponding to the nasociliary branch of the ophthalmic division strongly predict subsequent ocular complications (Nithyanandam et al., 2010).
Trigeminal Nerve Branches
Lesions of a single branch may result from inflammation, compression, or neoplasm. Damage to small distal branches may occur after dental interventions. The ophthalmic branch may be involved in Gradenigo syndrome in combination with the abducens and facial nerves from a lesion at the petrous apex, most common clinically as inflammation following otitis media in children (see Table 70.1). Both the ophthalmic and maxillary branches may be affected by a cavernous sinus lesion, in which setting dysfunction of the oculomotor, trochlear, and abducens nerves is often present.
Two particularly ominous clinical scenarios are (1) insidious development of facial numbness or paresthesias in a patient with a history of facial skin malignancy and (2) the “numb chin” or “numb cheek” syndromes. In the first situation, perineural invasion of the trigeminal branches is likely (Chang et al., 2004; Warden et al., 2009). This is most frequently seen with head and neck adenoid cystic carcinoma, squamous cell carcinoma, and melanoma (Boerman et al., 1999). The numb chin syndrome results from involvement of the mental nerve branches of the mandibular division and is most commonly due to focal metastatic nerve infiltration within the mandible, most often from breast, lung, prostate, or hematological malignancies. This syndrome may rarely be the presenting manifestation of malignancy but more often represents recurrence or progression of known disease (Laurencet et al., 2000). The numb cheek syndrome occurs when branches of the maxillary division are involved.
Abducens Nerve (Cranial Nerve VI)
Anatomy
Paired abducens nuclei are located in the dorsal pons in the floor of the fourth ventricle in close proximity to the fascicle of the facial nerve. Each nucleus contains abducens motoneurons that form the abducens nerve, and interneurons that decussate at the nuclear level and ascend in the medial longitudinal fasciculus (MLF) to the contralateral oculomotor medial rectus subnucleus to facilitate conjugate horizontal gaze in the direction ipsilateral to the abducens nuclear origin of the interneurons. The abducens fascicle arises from the ventral surface of the nucleus, traverses the brainstem, emerges from the ventral pontomedullary sulcus or caudal pontine surface, and travels in the subarachnoid space, where it ascends near the clivus. It pierces the dura and passes under the petroclinoid (Gruber) ligament in the Dorello canal, then travels through the body of the cavernous sinus lateral to the internal carotid artery (unlike the oculomotor, trochlear, and trigeminal nerves, which are housed in the lateral dural wall), and ultimately into the superior orbital fissure to innervate the ipsilateral lateral rectus muscle (Ono et al., 2004) (see Fig. 70.1).
Clinical Lesions
Abducens Nucleus
Because the abducens nucleus contains the interneurons destined to ascend in the contralateral MLF to the contralateral oculomotor medial rectus subnucleus to permit conjugate horizontal gaze, abducens nuclear lesions cause conjugate horizontal gaze palsy toward the side of the lesion (Muri et al., 1996). Rare cases of isolated horizontal gaze palsy are reported (Miller et al., 2002), but accompanying ipsilateral facial palsy with lower motor neuron facial weakness is typically present. Lesions involving both the abducens nucleus and ipsilateral MLF cause the one-and-a-half syndrome, with an ipsilateral conjugate gaze palsy and impaired adduction of the contralateral eye (see Chapter 19). Brainstem lesions include ischemia, hemorrhage, demyelination, infectious and noninfectious inflammation, and neoplasm (Barr et al., 2000). Periventricular necrosis from Wernicke encephalopathy also frequently results in horizontal gaze or abducens palsy from involvement of the abducens nucleus or fascicle, respectively.
Abducens Palsy Appearance
Abducens nerve dysfunction results in impaired ipsilateral abduction of the eye and deviation of the eyes toward one another (esotropia) (Fig. 70.6 and ![]() Videos 70.5 and 70.6 [available at www.ExpertConsult.com]). Binocular horizontal diplopia and the esotropia are worse with gaze in the direction of the abduction deficit.
Videos 70.5 and 70.6 [available at www.ExpertConsult.com]). Binocular horizontal diplopia and the esotropia are worse with gaze in the direction of the abduction deficit.
Brainstem Fascicle
The original Foville syndrome was the combination of an ipsilateral abducens palsy, ipsilateral lower motor neuron facial palsy, and contralateral hemiparesis from corticospinal tract involvement (see Table 70.1). It is now more commonly applied to ipsilateral abducens and facial palsies with contralateral ataxia, ipsilateral Horner syndrome, ipsilateral deafness, and ipsilateral loss of taste and facial sensation. Millard-Gubler syndrome is the combination of ipsilateral abducens and facial palsies with contralateral hemiparesis (see Table 70.1). Raymond syndrome is the combination of ipsilateral abducens palsy and contralateral hemiparesis (see Table 70.1). Brainstem lesions include ischemia, hemorrhage, demyelination, infectious and noninfectious inflammation, and neoplasm. Isolated abducens palsy is rarely the presenting manifestation of demyelination or paraneoplastic brainstem encephalitis (Barr et al., 2000; Hammam et al., 2005).
Quantitative assessment of the velocity of abducting saccades via eye movement recordings may help differentiate a central fascicular abducens lesion from a peripheral abducens palsy. With an acute abducens palsy (<1 month), saccadic velocities are reduced in both fascicular and peripheral lesion locations. However, after 2 months, saccadic velocities return to normal with peripheral lesions and remain impaired with fascicular lesions (Wong et al., 2006).
Subarachnoid Space
An abducens palsy may occur in isolation or in combination with other cranial nerve palsies from infectious, inflammatory, or neoplastic meningitis. The abducens nerve is in close approximation with the clivus and the basilar and vertebral arteries in this location and may be affected by a neoplastic or inflammatory clivus process or compressed by an aneurysm or dolichoectatic artery (Zhu et al., 2005).
The abducens nerves are particularly prone to dysfunction from alterations in intracranial pressure. Abducens palsies may be seen with spontaneous or post–lumbar puncture intracranial hypotension and with intracranial hypertension from any cause, the latter often accompanied by papilledema. It has been suggested historically that the abducens nerve is prone to such injury because of its long intracranial course; however, it has a shorter course than the trochlear nerve, which is the longest intracranial nerve and not prone to injury from raised intracranial pressure. Rather, it is probably the tethered nature of the abducens nerve at the pontomedullary sulcus and at the point of entry into the Dorello canal that predisposes the abducens subarachnoid portion to stretch and distortion injury with alterations in intracranial pressure (Hanson et al., 2004).
Dorello Canal
Abducens palsy is common in Gradenigo syndrome in combination with trigeminal ophthalmic division and facial nerve involvement from a lesion at the petrous apex (see Table 70.1). This is most commonly seen clinically as inflammation following otitis media in children.
Cavernous Sinus
An abducens palsy in this location may occur in isolation or accompanied by dysfunction of the oculomotor and trochlear nerves, the first and second divisions of the trigeminal nerve, and sympathetic fibers. The combination of an abducens palsy and ipsilateral Horner syndrome is highly suggestive of an ipsilateral cavernous sinus lesion, because the sympathetic fibers join the abducens nerve briefly in the posterior cavernous sinus (Tsuda et al., 2005). Inflammation, neoplasms, carotid-cavernous fistulas, and compression from an intracavernous internal carotid artery aneurysm or meningioma may occur.
Isolated Abducens Palsy
An isolated painful abducens palsy may represent microvascular ischemia, especially in older patients with vascular risk factors (Brazis, 2009) (see Fig. 70.6). Spontaneous resolution over 8 to 12 weeks is typical. In the absence of complete spontaneous resolution, neuroimaging is essential (Chi and Bhatti, 2009). Head trauma, even if mild, is another relatively common cause of abducens palsy (Dhaliwal et al., 2006). Impaired ability to abduct the eye past midline or bilateral presentation predict poor spontaneous recovery (Holmes et al., 2001). The abducens nerve is the cranial nerve most commonly affected bilaterally in isolation. This occurs most often from trauma and increased intracranial pressure (Keane, 2005).
Facial Nerve (Cranial Nerve VII)
Anatomy
The facial nerve comprises afferent gustatory, afferent sensory, efferent motor, and parasympathetic fibers. The facial nerve innervates taste buds in the anterior two-thirds of the tongue (Fig. 70.7). Unipolar neurons with cell bodies in the geniculate ganglion within the temporal bone carry taste information from the taste buds via the chorda tympani facial nerve branch, which is joined by the lingual branch of the trigeminal nerve. The chorda tympani nerve branch joins the main trunk of the facial nerve just proximal to the stylomastoid foramen. From the geniculate ganglion, taste information travels proximally to enter the solitary tract and ultimately the rostral solitary (or gustatory) nucleus in the rostral medulla via the nervus intermedius, which passes through the internal auditory canal. Afferent sensory information from the soft palate, middle ear, tympanic membrane, and external auditory canal travel in the facial nerve.
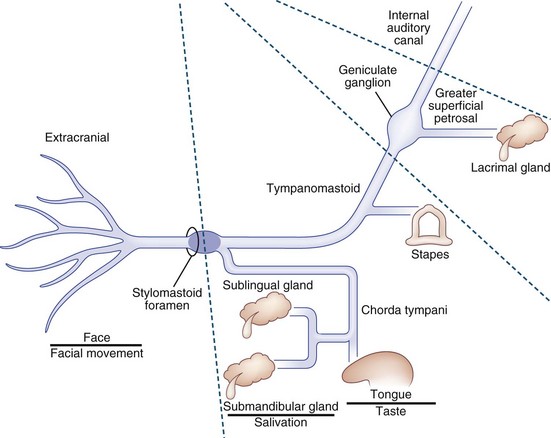
Fig. 70.7 Facial nerve schematic showing the major subdivisions and their principal functions.
(Courtesy Patrick Sweeney, MD.)
The motor efferents originate in the motor facial nucleus in the lateral tegmentum of the caudal pons. Supranuclear innervation is bilateral to the rostral portion of the nucleus, which innervates the upper facial muscles, but unilateral to the caudal portion, which innervates the lower facial muscles. Prior to exiting the brainstem, these motor efferent fibers ascend rostrally and wrap around the abducens nucleus as the genu of the facial nerve fasciculus that protrudes into the floor of the fourth ventricle to form the facial colliculus. Fascicular fibers then traverse the brainstem to exit the lateral pons. The motor fibers travel through the cerebellopontine angle to enter the petrous temporal bone via the internal auditory canal, travel through the geniculate ganglion without synapsing, and exit the stylomastoid foramen after a tortuous course through the temporal bone and facial canal. Prior to exiting the skull via the stylomastoid foramen, a small branch is given off to the stapedius muscle (see Fig. 70.7). After exiting the stylomastoid foramen, the motor nerve enters the substance of the parotid gland before branching into temporal, zygomatic, buccal, mandibular, and cervical branches to innervate the muscles of facial expression, stylohyoid, and posterior belly of the digastric. Muscles of facial expression include the orbicularis oculi, orbicularis oris, buccinator, and platysma. Efferent motor fibers to the orbicularis oculi are the anatomical substrate for the efferent limb of the corneal reflex.
The facial nerve carries efferent preganglionic parasympathetic innervation for the lacrimal gland in the greater superficial petrosal nerve, the first branch off of the peripheral facial nerve near the geniculate ganglion (see Fig. 70.7). This is joined by the deep petrosal nerve carrying sympathetic fibers from the internal carotid artery plexus to form the nerve of the pterygoid canal (Vidian nerve) that proceeds to the pterygopalatine ganglion. Preganglionic parasympathetic innervation for the salivary glands originates in the brainstem superior salivatory nucleus and travels via the nervus intermedius and chorda tympani to the submandibular ganglion (see Fig. 70.7). Postganglionic fibers are carried in trigeminal nerve branches.
Clinical Lesions
Facial Palsy Appearance
Facial nerve dysfunction results in unilateral upper and lower facial weakness manifested by flattening of the nasolabial fold, an asymmetrical smile, poor eyebrow elevation, decreased forehead wrinkling, and weak eye closure (Fig. 70.8 and ![]() Video 70.7 [available at www.ExpertConsult.com]). The palpebral fissure is widened on the affected side, and deviation of the eye upward and laterally with attempted eye closure (Bell phenomenon) may become visible because of orbicularis oculi weakness. Attention to adequacy of corneal coverage with provision of appropriate lubrication and protection will minimize the risk for permanent corneal damage from exposure due to incomplete eye closure. Lesions proximal to the greater superficial petrosal nerve increase the risk for corneal damage by impairing lacrimal secretion (for treatments, see Table 70.2, available in the online version of this chapter at www.ExpertConsult.com).
Video 70.7 [available at www.ExpertConsult.com]). The palpebral fissure is widened on the affected side, and deviation of the eye upward and laterally with attempted eye closure (Bell phenomenon) may become visible because of orbicularis oculi weakness. Attention to adequacy of corneal coverage with provision of appropriate lubrication and protection will minimize the risk for permanent corneal damage from exposure due to incomplete eye closure. Lesions proximal to the greater superficial petrosal nerve increase the risk for corneal damage by impairing lacrimal secretion (for treatments, see Table 70.2, available in the online version of this chapter at www.ExpertConsult.com).
Although it is theoretically possible to localize the facial nerve lesion to a specific nerve portion with specialized tests of tear production, stapedial muscle reflex, salivation, and taste, the primary clinical manifestation of facial nerve dysfunction is facial weakness. This must be distinguished from upper motor neuron facial weakness that causes only lower facial weakness because of the bilateral supranuclear innervation to the upper facial muscles, which may originate from upper facial cortical motor representation outside the primary motor cortex in the cingulate gyrus (Morecraft et al., 2001). Upper motor neuron facial weakness may also result in selective facial weakness for either volitional or emotional facial movements, whereas lower motor neuron facial weakness from facial nerve palsy affects both equally.
Facial Nucleus and Fascicle
Although isolated lower motor neuron weakness occasionally occurs from a brainstem lesion, accompanying brainstem signs, such as horizontal gaze palsy from sixth nerve nuclear involvement, are typically present. The original Foville syndrome was the combination of an ipsilateral abducens palsy, ipsilateral lower motor neuron facial palsy, and contralateral hemiparesis from corticospinal tract involvement (see Table 70.1). It is now more commonly applied to ipsilateral abducens and facial palsies with contralateral ataxia, ipsilateral Horner syndrome, ipsilateral deafness, and ipsilateral loss of taste and facial sensation. Millard-Gubler syndrome is the combination of ipsilateral abducens and facial palsies with contralateral hemiparesis (see Table 70.1). Brainstem lesions include ischemia, hemorrhage, demyelination, infectious and noninfectious inflammation, and neoplasm. Continuous twitching of individual facial muscles, called facial myokymia, is most commonly seen with demyelination and brainstem gliomas.
Nerve Root: Hemifacial Spasm
Hemifacial spasm (HFS) is characterized by involuntary episodic contractions of the muscles innervated by the facial nerve (see ![]() Video 70.7, available at www.ExpertConsult.com). It is most often unilateral and may be caused by compression and indentation of the facial motor nerve root at its brainstem exit by an aberrant vascular loop or dolichoectatic artery (Ho et al., 1999). HFS infrequently results from neoplastic compression or inflammation of the nerve root or from intrapontine lesions such as tumor or demyelination (Han et al., 2009). Facial weakness in isolation or in combination with other cranial nerve palsies may result from infectious, inflammatory, or neoplastic meningitis. Cerebellopontine angle mass lesions such as meningioma or acoustic schwannoma may cause facial nerve involvement. The facial nerve and vestibulocochlear nerves form a complex as they exit the brainstem, and sensorineural hearing loss is nearly always the primary feature of acoustic schwannomas. Acoustic schwannomas may be difficult to differentiate from facial nerve schwannomas in the cerebellopontine angle, but the latter tend to have earlier clinical facial weakness and the radiological appearance of a “labyrinthine tail” as the facial schwannoma extends into the facial canal in the temporal bone (Marzo et al., 2009; Wiggins et al., 2006).
Video 70.7, available at www.ExpertConsult.com). It is most often unilateral and may be caused by compression and indentation of the facial motor nerve root at its brainstem exit by an aberrant vascular loop or dolichoectatic artery (Ho et al., 1999). HFS infrequently results from neoplastic compression or inflammation of the nerve root or from intrapontine lesions such as tumor or demyelination (Han et al., 2009). Facial weakness in isolation or in combination with other cranial nerve palsies may result from infectious, inflammatory, or neoplastic meningitis. Cerebellopontine angle mass lesions such as meningioma or acoustic schwannoma may cause facial nerve involvement. The facial nerve and vestibulocochlear nerves form a complex as they exit the brainstem, and sensorineural hearing loss is nearly always the primary feature of acoustic schwannomas. Acoustic schwannomas may be difficult to differentiate from facial nerve schwannomas in the cerebellopontine angle, but the latter tend to have earlier clinical facial weakness and the radiological appearance of a “labyrinthine tail” as the facial schwannoma extends into the facial canal in the temporal bone (Marzo et al., 2009; Wiggins et al., 2006).
Intratemporal Facial Nerve and Geniculate Ganglion: Bell Palsy
Bell palsy is a self-limited, typically monophasic facial nerve palsy of acute-subacute onset (see Fig. 70.8 and Table 70.1). Pain accompanies facial weakness in 60% of patients, impaired lacrimation in 60%, taste changes in 30% to 50%, and hyperacusis in 15% to 30%. Ipsilateral facial sensory symptoms are not uncommon and are hypothetically explained by extension of inflammation from the facial nerve to the trigeminal nerve via the greater superficial petrosal nerve (Vanopdenbosch et al., 2005). Internal auditory canal MRI often reveals enhancement of the facial nerve, most commonly at the geniculate ganglion. Eighty-five percent of patients spontaneously recover normal facial function in 3 weeks (Peitersen, 2002). Bell palsy occurs with increased frequency during pregnancy, in which setting it is associated with a poorer recovery rate (Gillman et al., 2002). Residual abnormalities after acute Bell palsy occur in 12% of patients and include persistent severe facial weakness in 4% and synkinetic contraction and twitching of the upper and lower facial muscles in 17% (Beurskens et al., 2010; Kanaya et al., 2009) (see ![]() Video 70.7, available at www.ExpertConsult.com). Acutely, the nasolabial fold is flattened and the palpebral fissure is widened on the affected side; however, with chronicity, the affected side often becomes hypercontracted, with a deepened and prominent nasolabial fold and a narrowed palpebral fissure. Aberrant regeneration involving the lacrimal gland may result in tearing with facial muscle contraction (syndrome of “crocodile tears”), particularly during eating. Electromyographic presence of spontaneous fibrillation in facial muscles 10 to 14 days after onset of facial weakness is predictive of poor outcome (Sittel and Stennert, 2001). Bell palsy may be recurrent, but alternative causes such as Lyme disease or sarcoidosis must be considered. Bilateral facial weakness is also common with acute inflammatory demyelinating polyneuropathy, Lyme disease, sarcoidosis, and Epstein-Barr virus infection (Coddington et al., 2010; Morris et al., 2002).
Video 70.7, available at www.ExpertConsult.com). Acutely, the nasolabial fold is flattened and the palpebral fissure is widened on the affected side; however, with chronicity, the affected side often becomes hypercontracted, with a deepened and prominent nasolabial fold and a narrowed palpebral fissure. Aberrant regeneration involving the lacrimal gland may result in tearing with facial muscle contraction (syndrome of “crocodile tears”), particularly during eating. Electromyographic presence of spontaneous fibrillation in facial muscles 10 to 14 days after onset of facial weakness is predictive of poor outcome (Sittel and Stennert, 2001). Bell palsy may be recurrent, but alternative causes such as Lyme disease or sarcoidosis must be considered. Bilateral facial weakness is also common with acute inflammatory demyelinating polyneuropathy, Lyme disease, sarcoidosis, and Epstein-Barr virus infection (Coddington et al., 2010; Morris et al., 2002).
Although Bell palsy is considered idiopathic, herpes simplex virus and VZV reactivation in the geniculate ganglion have been implicated in its pathogenesis. High-quality evidence of treatment effect was lacking until recently, but early administration of corticosteroids and antiviral agents was common practice. Evidence now demonstrates a clear benefit in preventing unsatisfactory recovery for oral corticosteroids alone. Treatment with antiviral agents alone is not associated with a reduced risk of unsatisfactory recovery, although co-administration of corticosteroids and antiviral agents may provide added benefit over corticosteroids alone (Almeida et al., 2009; Thaera et al., 2010).
Ramsay Hunt syndrome consists of facial nerve palsy accompanied by a vesicular otic or oral rash due to VZV, with or without vestibulocochlear symptoms (see Table 70.1). The vesicular outbreak may occur before, after, or simultaneously with the facial weakness. Diagnosis is confirmed by a rise in serological VZV titers or positive VZV polymerase chain reaction (PCR). Serological detection or positive VZV PCR in facial palsy without a vesicular rash suggests zoster sine herpete. Facial weakness is more severe and spontaneous recovery less common in Ramsey Hunt and zoster sine herpete compared to idiopathic Bell palsy. Early diagnosis and initiation of corticosteroid and antiviral treatment significantly improves recovery (Furuta et al., 2000; Murakami et al., 1997).
Intratemporal facial nerve schwannomas within the facial canal, traumatic fractures and occult skull-based neoplasms of the temporal bone, and complicated otitis media with mastoiditis may also affect the facial nerve and should be considered when the onset of facial weakness is insidious and the course is progressive and accompanied by hearing loss (i.e., because of the proximity of the vestibulocochlear nerve). Gradenigo syndrome results from inflammation of the petrous apex and causes facial nerve palsy in combination with trigeminal and abducens nerve impairment (see Table 70.1). Traumatic temporal bone fractures may cause immediate or delayed facial nerve palsy (Nash et al., 2010).
Facial Nerve Branches
Parotid neoplasms, surgical procedures, and infiltration of facial skin cancers along facial motor nerve branches may result in weakness of individual facial nerve innervated muscles. Taste changes and hyperacusis do not typically occur with these lesions, given their distal nature, and either the upper or lower facial muscles may be affected in isolation. This may lead to diagnostic confusion with an upper motor neuron lesion when only the lower facial musculature is involved. Peripheral branches may also be affected by Lyme disease and sarcoidosis in the absence of meningeal inflammation. Melkersson-Rosenthal syndrome is a rare granulomatous disease with a triad of facial nerve palsy, facial edema, and tongue fissures (see Table 70.1). The complete triad occurs in only a quarter of patients.
Glossopharyngeal Nerve (Cranial Nerve IX)
Clinical Lesions
Nerve Root: Glossopharyngeal Neuralgia
Glossopharyngeal neuralgia is characterized by paroxysmal severe episodes of unilateral stabbing pain in the tongue base, tonsilar fossa, pharynx, or middle ear. It is often triggered by oropharyngeal movements such as chewing, swallowing, or yawning. It may very rarely be associated with syncope when vascular compression of the glossopharyngeal and vagus nerve roots together result in dysfunction of the glossopharyngeal-vagal reflex arc, with precipitation of bradycardia or asystole (Takaya et al., 2003). Glossopharyngeal neuralgia is much less common than trigeminal neuralgia, but like trigeminal neuralgia, neurovascular compression of the nerve root is likely a common etiology, and surgical neurovascular decompression may result in symptom relief (see Chapter 69) (Spengos et al., 2005) (for treatments, see Table 70.2, available at www.ExpertConsult.com).
Compressive lesions of the nerve root (e.g., cerebellopontine angle neoplasms, Chiari I malformations) may also cause glossopharyngeal neuralgia. Brainstem pathology such as tumor and demyelination is an infrequent cause (Minagar and Sheremata, 2000). Eagle syndrome from compression of the glossopharyngeal nerve by an elongated styloid process or ossified stylohyoid ligament may mimic glossopharyngeal neuralgia, but the pain tends to be more persistent and dull in nature (Mendelsohn et al., 2006; Piagkou et al., 2009) (see Table 70.1).
Petrosal Ganglion and Jugular Foramen
Neoplasia, blunt and penetrating trauma, inflammation, and internal carotid artery dissections may affect glossopharyngeal function at the jugular foramen. Vernet syndrome is a pure jugular foramen syndrome with involvement of the glossopharyngeal, vagus, and spinal accessory nerves (see Table 70.1). Since the jugular foramen is in close proximity to the hypoglossal canal that houses the hypoglossal nerve, simultaneous involvement of cranial nerves IX through XII is not uncommon. When accompanied by Horner syndrome from sympathetic involvement, the combination is called Villaret syndrome (posterior retropharyngeal) and is most commonly due to internal carotid artery dissection or neoplasm (see Table 70.1). In the absence of Horner syndrome, the combination is called Collet-Sicard syndrome (see Table 70.1), which may occur with carotid dissection, neoplasm, inflammation, or trauma (Rees et al., 1997).
Glomus jugulare tumors of the jugular bulb are the most common tumors in the jugular foramen (Fayad et al., 2010). They are slow-growing, hypervascular, benign paragangliomas that present with a neck mass, pulsatile tinnitus, lower cranial nerve dysfunction, and hearing loss from extension into the middle ear (Karaman et al., 2009). Examination of the lower cranial nerves reveals the deficits to be strictly unilateral, and skull-base imaging will reveal the lesion. Glomus tumors may also occur in the middle ear tympanic plexus, on the vagus nerve, and in the carotid body. Schwannomas, meningiomas, and metastases also occur in the jugular foramen. Glossopharyngeal schwannomas more often present with vestibulocochlear dysfunction than glossopharyngeal involvement (Vorasubin et al., 2009).
Glossopharyngeal Nerve Branches
Direct pressure or stretch injury of the branches may occur from surgical intervention, particularly with suspension laryngoscopy and tonsillectomy (Ford and Cruz, 2004). Postoperative taste changes and impaired swallowing are usually transient (Rosen et al., 2005). Reflex otalgia, or referred middle ear pain, occurs with oropharyngeal neoplasms owing to the anatomical neural connections between the oropharyngeal and tympanic branches. It is generally a poor prognostic sign that increases the likelihood of tumor recurrence after treatment (Thoeny et al., 2004). Syncope in a patient with a history of head and neck cancer may be an ominous harbinger of recurrent disease (Nakahira et al., 2002). Carotid hypersensitivity syndrome (syncope provoked by carotid massage), glossopharyngeal neuralgia with syncope, and parapharyngeal space-lesion syncope syndrome (syncope in the absence of neuralgia or carotid massage) are all occasionally seen from focal neoplastic infiltration of the glossopharyngeal nerve and the glossopharyngeal-vagal reflex arc.
Vagus Nerve (Cranial Nerve X)
Clinical Lesions
Vagus Nucleus
Nuclear lesions may result from ischemia, hemorrhage, infectious and noninfectious inflammation, neoplasm, and demyelination. Wallenberg syndrome from lateral medullary ischemia affects the nucleus ambiguus, causing dysphagia with bilateral impairment of pharyngeal and laryngeal function, likely explained by involvement of bilateral cortical prenuclear inputs into each nucleus (Aydogdu et al., 2001) (see Table 70.1). Swallowing impairment is accompanied by hoarseness of the voice, decreased gag reflex, facial pain from trigeminal involvement, limb ataxia from cerebellar peduncle involvement, Horner syndrome, contralateral body impaired pain and temperature sensation from spinothalamic tract involvement, and vertigo from vestibular involvement. Opalski syndrome is a variant of the lateral medullary syndrome accompanied by ipsilateral hemiparesis due to extension of the lesion into the cervical spinal cord, affecting corticospinal fibers caudal to the pyramidal decussation. Ipsilateral palatolaryngeal paresis in combination with contralateral hemiparesis (Avellis syndrome) occurs most commonly from medullary pathology, although the initial description of the syndrome was secondary to extramedullary vagus nerve involvement, presumably from a large lesion causing ventral brainstem compression with hemiparesis (Krasnianski et al., 2003) (see Table 70.1). Nuclear involvement in multisystem atrophy and Lewy body disease may explain the cardiovagal failure and gastrointestinal symptoms in these diseases (Benarroch et al., 2006). Motor neurons in the nucleus ambiguus are preferentially affected in amyotrophic lateral sclerosis.
Nodose Ganglion and Jugular Foramen
Neoplasm, blunt and penetrating trauma, inflammation, and internal carotid artery dissection may affect vagus function at the jugular foramen. Vernet syndrome is a pure jugular foramen syndrome with involvement of the glossopharyngeal, vagus, and spinal accessory nerves (see Table 70.1). Simultaneous involvement of cranial nerves IX through XII is common because of the proximity of the jugular foramen and hypoglossal canal. As noted earlier, when accompanied by Horner syndrome, the combination is called Villaret syndrome (posterior retropharyngeal) (Rees et al., 1997); in the absence of Horner syndrome, the combination is called Collet-Sicard syndrome; and the combination of vagus and hypoglossal nerve lesions is called Tapia syndrome (see Table 70.1), although the original description was of a patient with a brainstem lesion and contralateral hemiparesis.
Spinal Accessory Nerve (Cranial Nerve XI)
Anatomy
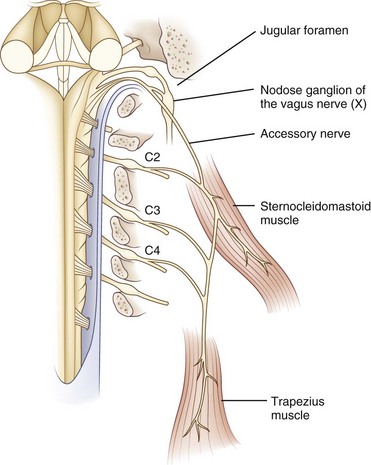
The spinal accessory nucleus is located in the dorsolateral ventral horn of the cervical spinal cord. Motor neurons from this nucleus exit the cervical cord as distinct nerve rootlets that converge and ascend through the foramen magnum to form the main trunk of the spinal accessory nerve, which exits the cranium via the jugular foramen with the glossopharyngeal and vagus nerves (Fig. 70.9). After exiting the jugular foramen, nerve branches innervate the ipsilateral sternocleidomastoid and trapezius muscles. Contralateral epileptic head turning and sternocleidomastoid function following hemispheric strokes indicate that each cerebral hemisphere innervates the ipsilateral sternomastoid muscle, seemingly after a double decussation in the brainstem (DeToledo and Dow, 1998).
According to classic anatomical teaching, fibers originating in the caudal nucleus ambiguus travel through a cranial accessory branch to join the spinal accessory branch as it travels through the jugular foramen. These cranial accessory fibers then join the vagus nerve to innervate palatal and laryngeal muscles, but anatomical studies call into question the existence of this cranial branch (Lachman et al., 2002).
Clinical Lesions
Spinal Accessory Palsy Appearance
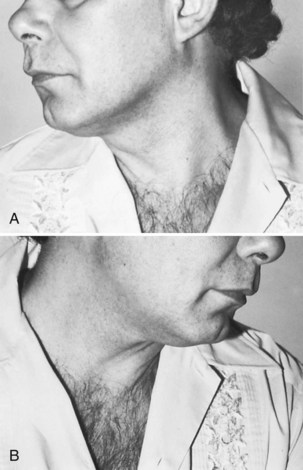
Spinal accessory nerve dysfunction results in weakness of contralateral head turning and ipsilateral shoulder elevation. Unilateral atrophy of the sternocleidomastoid and trapezius occurs with chronic lesions (Fig. 70.10). Scapular winging with active external shoulder rotation may be seen (Chan and Hems, 2006). Pain in the neck or shoulder is common.
Jugular Foramen
Vernet, Villaret, and Collet-Sicard syndromes are described earlier and in Table 70.1.
Spinal Accessory Nerve Branches
Iatrogenic injury during lymph node biopsy or dissection for head and neck cancers is the most common cause of spinal accessory nerve dysfunction. Variations in individual branch anatomy make the nerve particularly prone to difficult identification and traction or ischemic injury. Involvement of the dominant arm, scapular winging, and limited arm elevation are poor prognostic indicators (Friedenberg et al., 2002). Spinal accessory branches may be intentionally injured in treatment for cervical dystonia. Trauma and isolated peripheral neoplasms occur rarely.
Hypoglossal Nerve (Cranial Nerve XII)
Anatomy
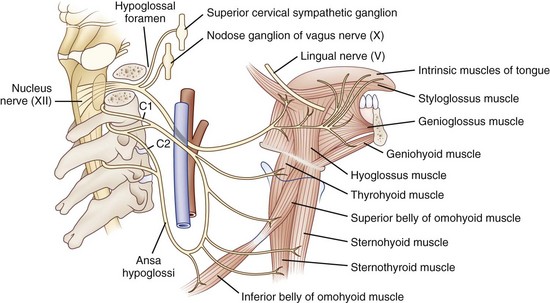
The hypoglossal nucleus is located in the medial medulla. Supranuclear inputs are generally considered to be bilateral and symmetrical, but evaluation of tongue weakness in patients with hemispheric strokes suggests asymmetry with a greater contralateral innervation (Umapathi et al., 2000). Motor neurons emerge from the ventral surface of the nucleus, forming the hypoglossal fasciculus that traverses the brainstem ventrolaterally, to exit as rootlets anterior to the inferior olive. The hypoglossal nerve exits the skull via the hypoglossal canal in the occipital condyle of the occipital bone. It then descends near the extracranial internal carotid artery, ultimately branching to innervate the styloglossus, hyoglossus, and intrinsic tongue muscles (Fig. 70.11). Fibers from cervical motor nerve roots form the ansa cervicalis, which has multiple connections with the hypoglossal nerve.
Clinical Lesions
Hypoglossal Palsy Appearance
Hypoglossal nerve dysfunction results in atrophy and fasciculation of the ipsilateral tongue muscles and ipsilateral deviation of the tongue upon protrusion from the mouth (Fig. 70.12).
Hypoglossal Nucleus and Fasciculus
The medial medullary syndrome of Dejerine consists of ipsilateral tongue weakness, contralateral hemiplegia from pyramidal tract involvement, and contralateral hemisensory loss from medial lemniscal involvement (see Table 70.1) (Bassetti et al., 1997). More common than isolated medial medullary syndrome is the hemi-medullary syndrome of Babinski-Nageotte that combines Dejerine and Wallenberg syndromes: swallowing impairment, hoarseness of the voice, decreased gag reflex, facial pain, limb ataxia, Horner syndrome, contralateral hemibody impaired pain and temperature sensation, and vertigo (see Table 70.1). As with the other cranial nerves, there are multiple etiologies, including amyotrophic lateral sclerosis. Although neurovascular compression as the hypoglossal nerve roots exit the brainstem is not classically considered a cause of hypoglossal palsy, recently reported cases suggest this as a diagnostic possibility (Osburn et al., 2010; Straube and Linn, 2008).
Hypoglossal Canal
Neoplasm, blunt and penetrating trauma with occipital condyle fractures, inflammation, and postradiation injury may affect hypoglossal function at the hypoglossal canal. Since the hypoglossal canal is in close proximity to the jugular foramen, simultaneous involvement of cranial nerves IX through XII is not uncommon. Villaret, Collet-Sicard, and Tapia syndromes are described earlier and in Table 70.1.
Hypoglossal Peripheral Branches
Mechanisms of hypoglossal branch disease are similar to those in the hypoglossal canal. Hypoglossal dysfunction is an ominous clinical finding; 50% of cases are caused by neoplasm, typically malignant (Keane, 1996). Nasopharyngeal carcinoma and skull-based metastatic disease are among the most common malignancies affecting the hypoglossal nerve. The combination of hypoglossal dysfunction with an abducens palsy is highly suggestive of an aggressive clival malignancy (Keane, 2000). Retropharyngeal mass lesions other than malignancy include granulomatous inflammation and abscess. Hypoglossal palsy may be the presenting manifestation of carotid artery dissection or aneurysm and may occur iatrogenically after carotid endarterectomy, occasionally disclosing underlying hereditary neuropathy with liability to pressure palsy (Corwin and Girardet, 2003; Mokri et al., 1996).
Almeida J.R., Al Khabori M., Guyatt G.H., et al. Combined corticosteroid and antiviral treatment for bell palsy: a systematic review and meta-analysis. JAMA. 2009;302:985-993.
Aydogdu I., Ertekin C., Tarlaci S., et al. Dysphagia in lateral medullary infarction (Wallenberg’s syndrome): an acute disconnection syndrome in premotor neurons related to swallowing activity? Stroke. 2001;32:2081-2087.
Barr D., Kupersmith M.J., Turbin R., et al. Isolated sixth nerve palsy: an uncommon presenting sign of multiple sclerosis. J Neurol. 2000;247:701-704.
Bassetti C., Bogousslavsky J., Mattle H., et al. Medial medullary stroke: report of seven patients and review of the literature. Neurology. 1997;48:882-890.
Benarroch E.E., Schmeichel A.M., Sandroni P., et al. Involvement of vagal autonomic nuclei in multiple system atrophy and Lewy body disease. Neurology. 2006;6:378-383.
Beurskens C.H.G., Oosterhof J., Nijhuis-van der Sanden M.W.G. Frequency and location of synkinesis in patients with peripheral facial paresis. Otol Neuroltol. 2010;31:671-675.
Boerman R.H., Maassen E.M., Joosten J., et al. Trigeminal neuropathy secondary to perineural invasion of head and neck carcinomas. Neurology. 1999;53:213-216.
Brazis P.W. Isolated palsies of cranial nerves III, IV, and VI. Semin Neurol. 2009;29:14-28.
Carrasco J.R., Savino P.J., Bilyk J.R. Primary aberrant oculomotor nerve regeneration from a posterior communicating artery aneurysm. Arch Ophthalmol. 2002;120:663-665.
Chan P.K., Hems T.E. Clinical signs of accessory nerve palsy. J Trauma. 2006;60:1142-1144.
Chang P.C., Fischbein N.J., McCalmont T.H., et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradiol. 2004;25:5-11.
Chaudhary N., Davagnanam I., Ansari S.A., et al. Imaging of intracranial aneurysms causing isolated third cranial nerve palsy. J Neuroophthalmol. 2009;29:238-244.
Chi S.L., Bhatti M.T. The diagnostic dilemma of neuro-imaging in acute isolated sixth nerve palsy. Curr Opin Ophthalmol. 2009;20:423-429.
Chou K.L., Galetta S.L., Liu G.T., et al. Acute ocular motor mononeuropathies: prospective study of the roles of neuroimaging and clinical assessment. J Neurol Sci. 2004;219:35-39.
Coddington C.T., Isaacs J.D., Siddiqui A.Q., et al. Neurological picture. Bilateral facial nerve palsy with Epstein-Barr virus infection. J Neurol Neurosurg Psychiatry. 2010;81:1155-1156.
Corwin H.M., Girardet R.E. Hereditary neuropathy with liability to pressure palsies mimicking hypoglossal nerve injuries. Neurology. 2003;61:1457-1458.
DeToledo J.C., Dow R. Sternomastoid function during hemispheric suppression by Amytal: insights into the inputs to the spinal accessory nerve nucleus. Mov Disord. 1998;13:809-812.
Dhaliwal A., West A.L., Trobe J.D., et al. Third, fourth, and sixth cranial nerve palsies following closed head injury. J Neuroophthalmol. 2006;26:4-10.
Donzelli R., Marinkovic S., Brigante L., et al. The oculomotor nuclear complex in humans. Microanatomy and clinical significance. Surg Radiol Anat. 1998;20:7-12.
Dubuisson A.S., Beckers A., Stevenaert A. Classical pituitary tumour apoplexy: clinical features, management and outcomes in a series of 24 patients. Clin Neurol Neurosurg. 2007;109:63-70.
Elmalem V.I., Hudgins P.A., Bruce B.B., et al. Underdiagnosis of posterior communicating artery aneurysms in noninvasive brain vascular studies. J Neuroopthalmol. 2011;31:103-109.
Elmalem V.I., Younge B.R., Biousse V., et al. Clinical course and prognosis of trochlear nerve schwannomas. Ophthalmology. 2009;116:2011-2016.
Fayad J.N., Keles B., Brackmann D.E. Jugular foramen tumors: clinical characteristics and treatment outcomes. Otol Neurotol. 2010;31:299-305.
Feinberg A.S., Newman N.J. Schwannoma in patients with isolated unilateral trochlear nerve palsy. Am J Ophthalmol. 1999;127:183-188.
Ford L.C., Cruz R.M. Bilateral glossopharyngeal nerve paralysis after tonsillectomy: case report and anatomic study. Laryngoscope. 2004;114:2196-2199.
Friedenberg S.M., Zimprich T., Harper C.M. The natural history of long thoracic and spinal accessory neuropathies. Muscle Nerve. 2002;25:535-539.
Furuta Y., Ohtani F., Mesuda Y., et al. Early diagnosis of zoster sine herpete and antiviral therapy for the treatment of facial palsy. Neurology. 2000;55:708-710.
Gillman G.S., Schaitkin B.M., May M., et al. Bell’s palsy in pregnancy: a study of recovery outcomes. Otolaryngol Head Neck Surg. 2002;126:26-30.
Hammam T., McFadzean R.M., Ironside J.W. Anti-hu paraneoplastic syndrome presenting as bilateral sixth cranial nerve palsies. J Neuroophthalmol. 2005;25:101-104.
Han I.B., Chang J.H., Chang J.W., et al. Unusual causes and presentations of hemifacial spasm. Neurosurgery. 2009;65:130-137.
Hanson R.A., Ghosh S., Gonzalez-Gomez I., et al. Abducens length and vulnerability? Neurology. 2004;62:33-36.
Ho S.L., Cheng P.W., Wong W.C., et al. A case-controlled MRI/MRA study of neurovascular contact in hemifacial spasm. Neurology. 1999;53:2132-2139.
Holmes J.M., Beck R.W., Kip K.E., et al. Predictors of nonrecovery in acute traumatic sixth nerve palsy and paresis. Ophthalmology. 2001;108:1457-1460.
Jacobson D.M. Pupil involvement in patients with diabetes-associated oculomotor nerve palsy. Arch Ophthalmol. 1998;116:723-727.
Jacobson D.M. Relative pupil-sparing third nerve palsy: Etiology and clinical variables predictive of a mass. Neurology. 2001;56:797-798.
Jacobson D.M., Moster M.L., Eggenberger E.R., et al. Isolated trochlear nerve palsy in patients with multiple sclerosis. Neurology. 1999;53:877-879.
Kanaya K., Ushio M., Kondo K., et al. Recovery of facial movement and facial synkinesis in Bell’s palsy patients. Otol Neurotol. 2009;30:640-644.
Karaman E., Isildak H., Yilmaz M., et al. Management of paragangliomas in otolaryngology: review of a 7-year experience. J Craniofac Surg. 2009;20:1294-1297.
Keane J.R. Twelfth-nerve palsy: analysis of 100 cases. Arch Neurol. 1996;53:561-566.
Keane J.R. Combined VIth and XIIth cranial nerve palsies: a clival syndrome. Neurology. 2000;54:1540-1541.
Keane J.R. Bilateral involvement of a single cranial nerve: Analysis of 578 cases. Neurology. 2005;65:950-952.
Krasnianski M., Neudecker S., Schluter A., et al. [Avellis’ syndrome in brainstem infarctions]. Fortschr Neurol Psychiatr. 2003;71:650-653.
Lachman N., Acland R.D., Rosse C. Anatomical evidence for the absence of a morphologically distinct cranial root of the accessory nerve in man. Clin Anat. 2002;15:4-10.
Laurencet F.M., Anchisi S., Tullen E., et al. Mental neuropathy: report of five cases and review of the literature. Crit Rev Oncol Hematol. 2000;34:71-79.
Levy R.L., Geist C.E., Miller N.R. Isolated oculomotor palsy following minor head trauma. Neurology. 2005;65:169.
Liu G.T., Crenner C.W., Logigian E.L., et al. Midbrain syndromes of Benedikt, Claude, and Nothnagel: setting the record straight. Neurology. 1994;42:1820-1822.
Lorenzoni J., David P., Devriendt D., et al. Patterns of neurovascular compression in patients with classic trigeminal neuralgia: a high resolution MRI-based study. Eur J Radiol. 2009. Oct 9. [Epub ahead of print]
Marzo S.J., Zender C.A., Leonetti J.P. Facial nerve schwannoma. Curr Opin Otolaryngol Head Neck Surg. 2009;17:346-350.
Masur H., Papke K., Bongartz G., et al. The significance of three-dimensional MR-defined neurovascular compression for the pathogenesis of trigeminal neuralgia. J Neurol. 1995;242:93-98.
Mendelsohn A.H., Berke G.S., Chhetri D.K. Heterogeneity in the clinical presentation of Eagle’s syndrome. Otolaryngol Head Neck Surg. 2006;134:389-393.
Miller J.P., Acar F., Hamilton B.E.W., et al. Radiographic evaluation of trigeminal neurovascular compression in patients with and without trigeminal neuralgia. J Neurosurg. 2009;110:627-632.
Miller N.R., Biousse V., Hwang T., et al. Isolated acquired unilateral horizontal gaze paresis from a putative lesion of the abducens nucleus. J Neuroophthalmol. 2002;22:204-207.
Mills R.J., Young C.A., Smith E.T. Central trigeminal involvement in multiple sclerosis using high-resolution MRI at 3T. Br J Radiol. 2010;83:493-498.
Minagar A., Sheremata W.A. Glossopharyngeal neuralgia and MS. Neurology. 2000;54:1368-1370.
Mokri B., Silbert P.L., Schievink W.I., et al. Cranial nerve palsy in spontaneous dissection of the extracranial internal carotid artery. Neurology. 1996;46:356-359.
Morecraft R.J., Louie J.L., Herrick J.L., et al. Cortical innervation of the facial nucleus in the non-human primate: a new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain. 2001;124:176-208.
Morris A.M., Deeks S.L., Hill M.D., et al. Annualized incidence and spectrum of illness from an outbreak investigation of Bell’s palsy. Neuroepidemiology. 2002;21:255-261.
Murakami S., Hato N., Horiuchi J., et al. Treatment of Ramsay Hunt syndrome with acyclovir-prednisone: significance of early diagnosis and treatment. Ann Neurol. 1997;41:353-357.
Muri R.M., Chermann J.F., Cohen L., et al. Ocular motor consequences of damage to the abducens nucleus area in humans. J Neuroophthalmol. 1996;16:191-195.
Nakahira M., Nakatani H., Takeda T. Syncope as a sign of occult malignant recurrence in the retropharyngeal and parapharyngeal space: CT and MR imaging findings in four cases. AJNR Am J Neuroradiol. 2002;23:1257-1260.
Nallasamy S., Lesniak S., Volpe N., et al. Unusual presentations of cavernous carotid aneurysms: further evidence for topographic organization of the oculomotor nerve. J Neurol Sci. 2010;295:82-86.
Nash J.J., Friedland D.R., Boorsma K.J., et al. Management and outcomes of facial paralysis from intratemporal blunt trauma: a systematic review. Laryngoscope. 2010;120:1397-1404.
Nithyanandam S., Joseph S.J., Dabir S. Factors affecting visual outcome in herpes zoster ophthalmicus: a prospective study. Clin Experiment Ophthalmol. 2010;38:845-850.
Ono K., Arai H., Endo T., et al. Detailed MR imaging anatomy of the abducent nerve: evagination of CSF into Dorello canal. AJNR Am J Neuroradiol. 2004;25:623-626.
Osburn L.L., Moller A.R., Bhatt J.R., et al. Hemilingual spasm: defining a new entity, its electrophysiological correlates and surgical treatment through microvascular decompression. Neurosurgery. 2010;67:192-196.
Peitersen E. Bell’s palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol Suppl. 2002;549:4-30.
Petermann S.H., Newman N.J. Pituitary macroadenoma manifesting as an isolated fourth nerve palsy. Am J Ophthalmol. 1999;127:235-236.
Piagkou M., Anagnostopoulou S., Kouladouros K., et al. Eagle’s syndrome: a review of the literature. Clin Anat. 2009;22:545-558.
Rabadi M.H., Beltmann M.A. Midbrain infarction presenting isolated medial rectus nuclear palsy. Am J Med. 2005;118:836-837.
Rees J.H., Valentine A.R., Llewelyn J.G. Spontaneous bilateral carotid and vertebral artery dissection presenting as a Collet-Sicard syndrome. Br J Radiol. 1997;70:856-858.
Rosen C.A., Andrade Filho P.A., Scheffel L., et al. Oropharyngeal complications of suspension laryngoscopy: A prospective study. Laryngoscope. 2005;115:1681-1684.
Saeki N., Yamaura A., Sunami K. Bilateral ptosis with pupil sparing because of a discrete midbrain lesion: magnetic resonance imaging evidence of topographic arrangement within the oculomotor nerve. J Neuroophthalmol. 2000;20:130-134.
Seo S.W., Heo J.H., Lee K.Y., et al. Localization of Claude’s syndrome. Neurology. 2001;57:2304-2307.
Sittel C., Stennert E. Prognostic value of electromyography in acute peripheral facial nerve palsy. Otol Neurotol. 2001;22:100-104.
Spengos K., Tsivgoulis G., Stouraitis G., et al. Neurological picture. Hemifacial spasm, neuralgia, and syncope due to cranial nerve compression in a patient with vertebral artery ectasia. J Neurol Neurosurg Psychiatry. 2005;76:1500.
Straube A., Linn J. Unilateral headache attacks and ipsilateral atrophy of the tongue due to neurovascular compression of the hypoglossal nerve. Cephalalgia. 2008;28:996-998.
Takaya N., Sumiyoshi M., Nakata Y. Prolonged cardiac arrest caused by glossopharyngeal neuralgia. Heart. 2003;89:381.
Thaera G.M., Wellik K.E., Barrs D.M., et al. Are corticosteroids and antiviral treatments effective for Bell palsy? Neurologist. 2010;16:138-140.
Thoeny H.C., Beer K.T., Vock P., et al. Ear pain in patients with oropharynx carcinoma: how MRI contributes to the explanation of a prognostic and predictive symptom. Eur Radiol. 2004;14:2206-2211.
Thomke F., Hopf H.C. Isolated superior oblique palsies with electrophysiologically documented brainstem lesions. Muscle Nerve. 2000;23:267-270.
Trobe J. Searching for brain aneurysm in third cranial nerve palsy. J Neuroophthalmol. 2009;29:171-172.
Tsuda H., Ishikawa H., Asayama K., et al. Abducens nerve palsy and Horner syndrome due to metastatic tumor in the cavernous sinus. Intern Med. 2005;44:644-646.
Umapathi T., Venketasubramanian N., Leck K.J., et al. Tongue deviation in acute ischaemic stroke: a study of supranuclear twelfth cranial nerve palsy in 300 stroke patients. Cerebrovasc Dis. 2000;10:462-465.
Vanopdenbosch L.J., Verhoeven K., Casselman J.W. Bell’s palsy with ipsilateral numbness. J Neurol Neurosurg Psychiatry. 2005;76:1017-1018.
Vorasubin N., Sang H., Mafee M., et al. Glossopharyngeal schwannomas: a 100 year review. Laryngoscope. 2009;119:26-35.
Walter K.A., Newman N.J., Lessell S. Oculomotor palsy from minor head trauma: initial sign of intracranial aneurysm. Neurology. 1994;44:148-150.
Warden K.F., Parmar H., Trobe J.D. Perineural spread of cancer along the three trigeminal divisions. J Neuroophthalmol. 2009;29:300-307.
Wiggins R.H., Harnsberger H.R., Salzman K.L., et al. The many faces of facial nerve schwannoma. AJNR Am J Neuroradiol. 2006;27:694-699.
Wilker S.C., Rucker J.C., Newman N.J., et al. Pain in ischaemic ocular motor cranial nerve palsies. Br J Ophthalmol. 2009;93:1657-1659.
Wong A.M., McReelis K., Sharpe J.A. Saccade dynamics in peripheral vs central sixth nerve palsies. Neurology. 2006;66:1390-1398.
Yousry I., Moriggl B., Dieterich M., et al. MR anatomy of the proximal cisternal segment of the trochlear nerve: Neurovascular relationships and landmarks. Radiology. 2002;223:31-38.
Yousry I., Moriggl B., Holtmannspoetter M., et al. Detailed anatomy of the motor and sensory roots of the trigeminal nerve and their neurovascular relationships: A magnetic resonance imaging study. J Neurosurg. 2004;101:427-434.
Yousry I., Moriggl B., Schmid U.D., et al. Trigeminal ganglion and its divisions: detailed anatomic MR imaging with contrast-enhanced 3D constructive interference in the steady state sequences. AJNR Am J Neuroradiol. 2005;26:1128-1135.
Zhu Y., Thulborn K., Curnyn K., et al. Sixth cranial nerve palsy caused by compression from a dolichoectatic vertebral artery. J Neuroophthalmol. 2005;25:134-135.