9 Cranial Nerves IX and X
Glossopharyngeal and Vagus
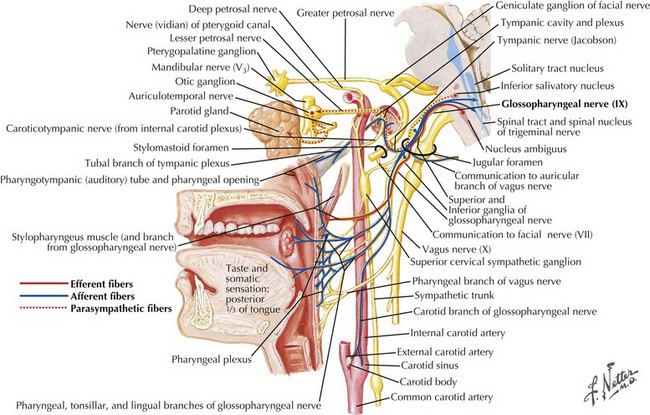
Cranial Nerve IX: Glossopharyngeal Nerve and Swallowing
Physiology
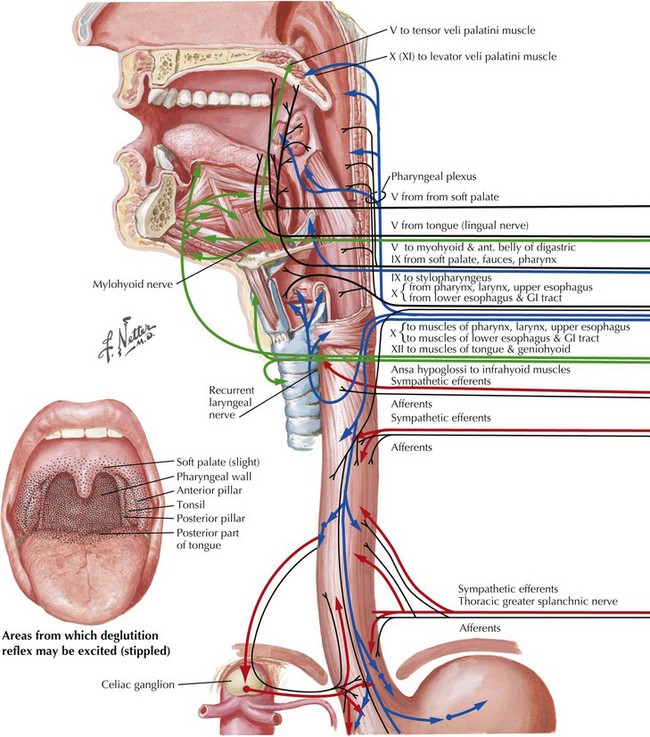
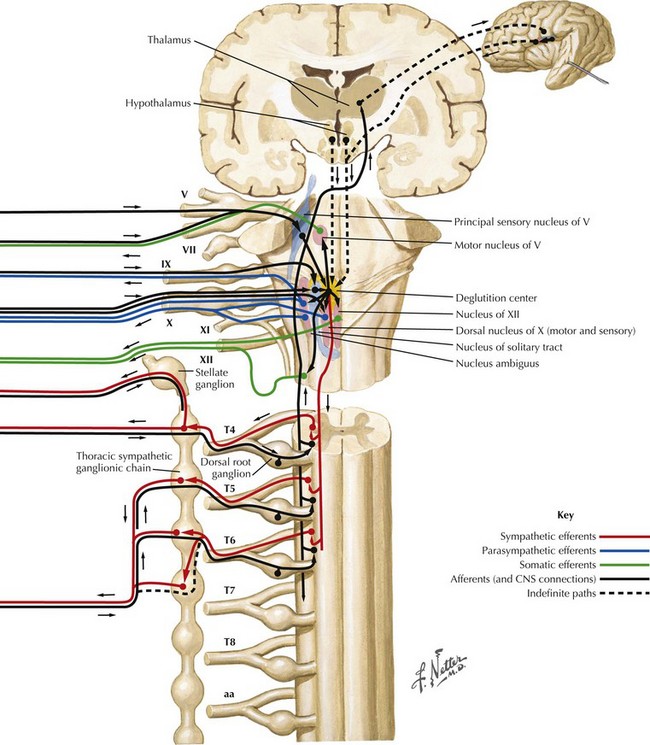
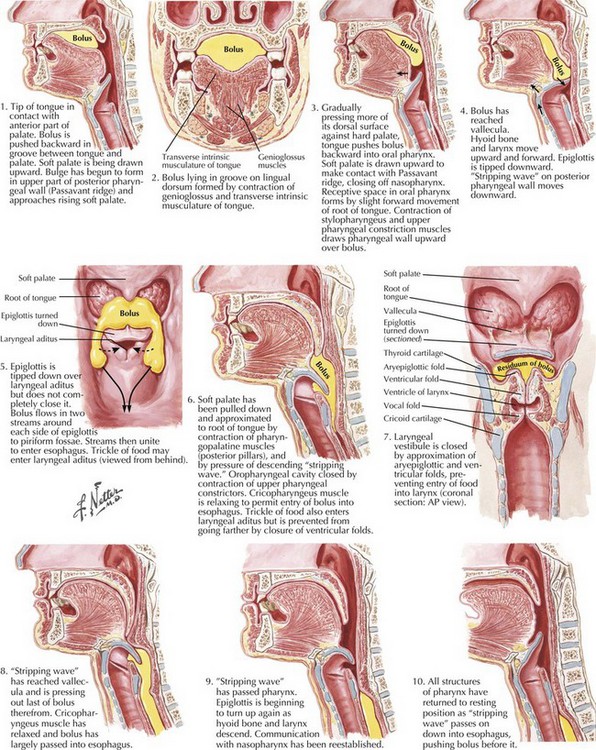
Swallowing is a complex process involving motor control with sensory feedback from many anatomic structures within the oral cavity, pharynx, larynx, and esophagus (Fig. 9-1). The trigeminal (CN-V), facial (CN-VII), glossopharyngeal (CN-IX; Fig. 9-2), vagus (CN-X; Fig. 9-3), and hypoglossal (CN-XII) cranial nerves are involved. A “normal swallow” comprises two major components, bolus transport and airway protection. The swallowing process is typically classified into four phases (Fig. 9-4).
1. The oral preparatory phase involves voluntary motor function during which food or liquid is taken into the mouth, masticated (CN-V), and mixed with saliva to form a cohesive bolus (Fig. 9-4, nos. 1 and 2). This phase requires coordination of several cranial nerves and corresponding structures. Tension in the labial and buccal musculature closes the anterior and lateral sulci (CN-VII) while rotary mandible motion produces chewing (CN-V3). Lateral rolling tongue motion (CN-XII) and bulging of the soft palate forward (widening the nasal airway, and sealing the posterior oral cavity) properly positions the bolus for the swallowing (CN-IX). Tongue mobility is the most important neuromuscular function involved in this first phase. The mid and lower divisions of CN-V provide sensory feedback for positioning the bolus. Saliva derived from the parotid, sublingual, and submandibular glands (innervated by secretomotor fibers of CN-IX and -VII) contain digestive enzymes that act as an emollient to soften and shape the bolus.
2. The oral swallowing phase is initiated when the tongue (CN-XII) sequentially squeezes the bolus posteriorly against the hard palate and initiates propulsion into the oropharynx (Fig. 9-4, nos. 3 and 4). Lips and buccal muscles contract (CN-V and CN-VII) with elevation of the velum (CN-V and CN-X) providing the valving process that generates pressure to seal the nasopharynx, preventing reflux and nasal regurgitation. CN-V is responsible for the afferent (sensory) feedback for the entire oral cavity and tongue. The soft palate (CN-IX), critical to containing the bolus within the oral cavity during the oral preparatory phase, now moves posteriorly to allow the bolus to pass through the faucial arches and simultaneously prevent the bolus from entering the nasopharynx. The swallowing reflex is triggered as the bolus passes the anterior tonsillar pillars, which initiates the pharyngeal phase.
3. The pharyngeal phase begins with the bolus passing into the throat, triggering the swallowing reflex and causing several pharyngeal physiologic actions to occur simultaneously, allowing food to pass into the esophagus (Fig. 9-4, nos. 5–7). Once pharyngeal swallowing is elicited, essential functions of airway protection occur. Intrinsic laryngeal muscles innervated by CN-X close the larynx at the aryepiglottic, false vocal, and at the true vocal folds, creating a seal that separates the airway from the digestive tract protecting the laryngeal vestibule from foreign material aspiration. The tongue (CN XII) is the major force pushing the bolus through the pharynx. Synergistic actions with CN-X produce pharyngeal peristalsis as it innervates the pharyngeal constrictors and carries afferents from the lower pharynx.
Poor airway protection and delayed triggering of pharyngeal swallow may cause aspiration. When swallowing is inefficient and aspiration occurs, a reflexive cough needs to occur as a respiratory defense against foreign matter. The cough reflex is induced by irritation of afferent CN-IX and CN-X sensory fibers in the larynx, trachea, and larger bronchi (Figs. 9-2 and 9-3). If a reflexive cough is not elicited in response to foreign material within the airway, silent aspiration results; it is radiographically documented in 50% of aspiration cases.
4. The esophageal phase occurs with the passage of the bolus through the cricopharyngeal sphincter, moving over the closed airway and passing the pharyngoesophageal segment into the esophagus via the cricopharyngeal sphincter at the proximal esophagus (Fig. 9-4, nos. 8–10). This area contains the cricopharyngeus muscle that normally keeps the esophagus closed. CN-X mediates the action of the cricopharyngeus, which relaxes to allow food to pass from the hypopharynx into the esophagus.
Cranial Nerve X, Vagus: Voice Disorders
Anatomy of the Larynx
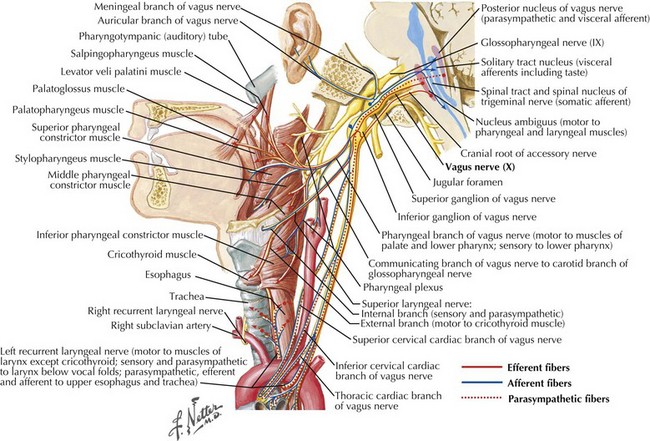
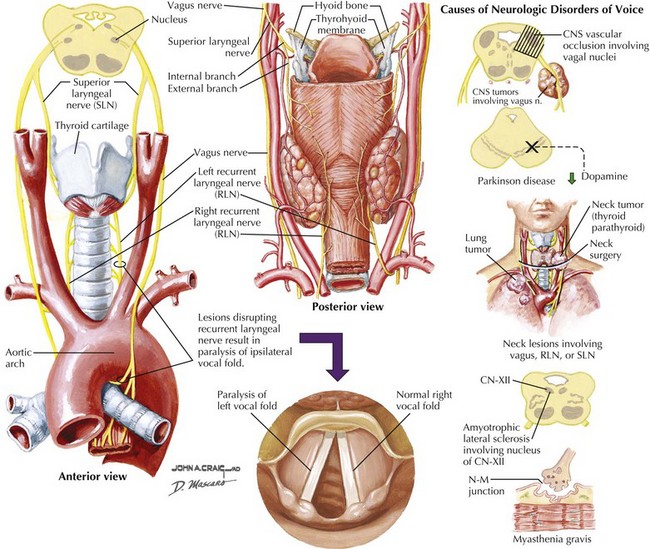
The framework of the larynx consists of thyroid and cricoid cartilages. The arytenoid cartilages articulate with the posterior portion of the cricoid. Vocal ligaments stretch from the arytenoids to the thyroid cartilage. Muscles inserting on the arytenoids move the arytenoids and vocal folds together for speech and swallowing, and apart for respiration. Although the arytenoids’ motion is multidimensional, knowledge of the intrinsic laryngeal muscles and their functions is important for diagnosis (Table 9-1; Fig. 9-3). Note that the cricothyroid muscle is the only intrinsic laryngeal muscle innervated by the superior laryngeal nerve (SLN), and the posterior cricoarytenoid is the only vocal fold abductor.
The motor supply of the laryngeal muscles begins in the nucleus ambiguus (see Fig. 9-3). These fibers travel within the vagus nerve (CN-X) as it exits the cranium via the jugular foramen, traveling through the neck within the carotid sheath (Fig. 9-5). High in the neck, the SLN splits from CN-X and travels medially and inferiorly. It splits again into internal and external branches. The internal branch pierces the thyrohyoid membrane and provides sensory innervation to the pharynx and larynx. The external branch travels lower in the neck past the superior pole of the thyroid gland to innervate the cricothyroid muscle.
Disorders of Voice
Recurrent Laryngeal Nerve
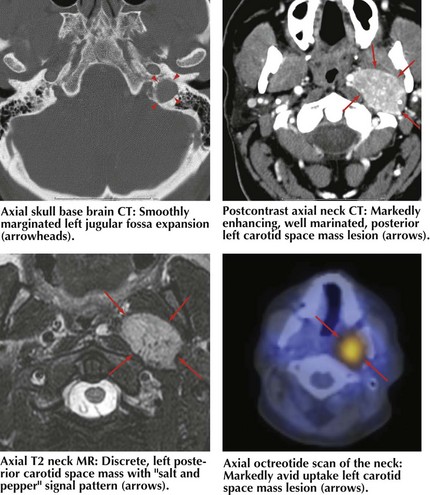
Common causes of vocal fold paralysis include thyroid, lung, or neck tumors; cerebrovascular accidents; CN-X tumors (paragangliomas or glomus vagale [Fig. 9-6]); and surgery near CN-X or the RLN. Less common causes include thyroiditis (causing inflammation of the RLN), infectious diseases, diabetes, or other neuropathies.
Hughes TAT, Wiles CM. Clinical measurement of swallowing in health and in neurogenic dysphagia. Q J Med. 1996;89:109-116.
Langmore SE, Skarupski KA, Park PS, et al. Predictors of aspiration pneumonia in nursing home residents. Dysphagia. 2002;17:298-307.
Larson C. Neurophysiology of speech and swallowing. Semin Speech Lang. 1985;6:275-289.
Logemann JA. Evaluation and Treatment of Swallowing Disorders, 2nd ed. Austin, Tex: Pro-Ed; 1998.
McConnell FMS, Cerenko D, Mendelson MS. Manofluorgraphic analysis of swallowing. Otolaryngol Clin N Am. 1988;21:625-635.
Zemlin WR. Speech and Hearing Science: Anatomy and Physiology, 4th ed. Boston, Mass: Allyn and Bacon; 1998.
Hill AN, Jankovic J, Vuong KD, et al. Treatment of hypophonia with collagen vocal cord augmentation in patients with parkinsonism. Mov Disord. 2003 Oct;18(10):1190-1192.
Lorenz RR, Esclamado RM, Teker AM, et al. Ansa cervicalis-to-recurrent laryngeal nerve anastomosis for unilateral vocal fold paralysis: experience of a single institution. Ann Otol Rhinol Laryngol. 2008 Jan;117(1):40-45.
Mao VH, Abaza M, Spiegel JR, et al. Laryngeal myasthenia gravis: report of 40 cases. J Voice. 2001;15:122-130. The largest series of patients with laryngeal manifestations of myasthenia gravis
Paniello RC, Barlow J, Serna JS. Longitudinal follow-up of adductor spasmodic dysphonia patients after botulinum toxin injection: quality of life results. Laryngoscope. 2008 Mar;118(3):564-568.
Rosen CA, Gartner-Schmidt J, Casiano R, et al. Vocal fold augmentation with calcium hydroxylapatite: twelve-month report. Laryngoscope. 2009 May;119(5):1033-1041.
Roy N, Smith ME, Dromey C, et al. Exploring the phonatory effects of external superior laryngeal nerve paralysis: an in vivo model. Laryngoscope. 2009 Apr;119(4):816-826.
Rubin AD, Sataloff RT. Vocal fold paresis and paralysis. Otolaryngol Clin North Am. 2007 Oct;40(5):1109-1131. viii-ix
Sulica L. The natural history of idiopathic unilateral vocal fold paralysis: evidence and problems. Laryngoscope. 2008 Jul;118(7):1303-1307. Meta-analysis of 717 cases of idiopathic unilateral vocal fold paralysis regarding duration and outcome of the paralysis