Chapter 7 Cosmeceutical Vitamins: Vitamin E
TERMINOLOGY AND DEFINITIONS
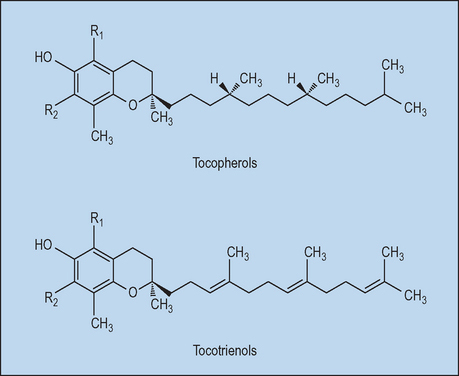
Vitamin E is the major lipophilic antioxidant in plasma, membranes, and tissues. The term ‘vitamin E’ collectively refers to the eight naturally occurring molecules (four tocopherols and four tocotrienols) which exhibit vitamin E activity. Tocotrienols differ from tocopherols in that they have an isoprenoid instead of a phytyl side chain; the four forms of tocopherols and tocotrienols differ in the number of methyl groups on the chromanol nucleus (α- has three, β- and γ- have two, and δ- has one; Fig. 7.1). In humans, α-tocopherol is the most abundant vitamin E homolog, followed by γ-tocopherol. To compare the potency of different vitamin E derivates, their biologic activities are measured and compared to RRR-α-tocopherol. The potency is expressed as international units (IU) α-tocopherol equivalents (α-TE) and was developed for oral supplementation.
INDICATIONS AND BIOLOGIC ACTIVITY
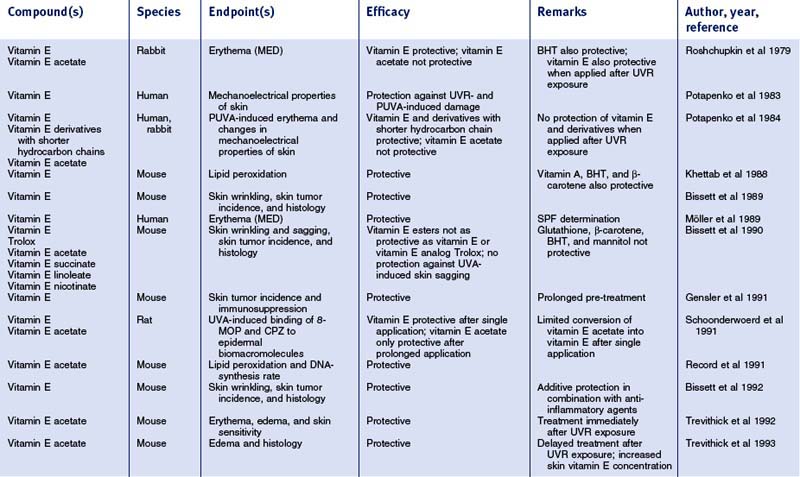
After more than half a century of research there is still little convincing evidence of vitamin E’s effectiveness in treating specific dermatologic disorders. In trials and case reports, oral vitamin E supplementation is recommended in the therapy of yellow nail syndrome, vibration disease, epidermolysis bullosa, cancer prevention, claudication, cutaneous ulcers, collagen synthesis, and wound healing. Clearly, with vitamin E not being a pharmaceutical, there is a lack of placebo-controlled studies for treatment of these conditions. However, in the field of skin care, which includes cosmeceuticals, there is a large body of experimental evidence pointing to, in particular, photoprotective effects (for review, see Table 7.1). Moreover, recent studies indicate that vitamin E may reveal dermatologic benefits that exceed the purpose of cosmetics and may extend into an area that has recently been termed ‘cosmeceuticals’.
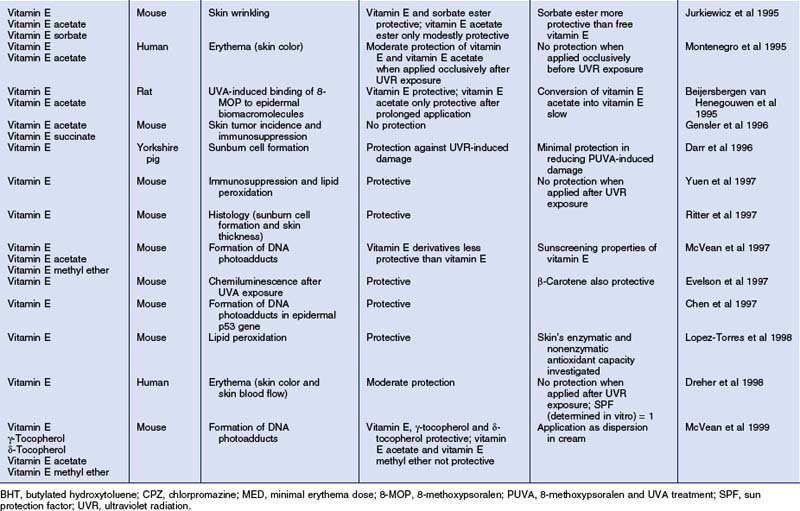
Table 7.1 Photoprotective effects of topically applied vitamin E (α-tocopherol) and derivatives in vivo
Recently, Tsoureli-Nikita et al performed a clinical single-blind, placebo-controlled study in which 96 atopic dermatitis patients were treated with either placebo or oral vitamin E (400 IU/day) for 8 months. They found an improvement and near remission of atopic dermatitis and a 62% decrease in serum IgE levels based on initial conditions in the vitamin E-treated group. The correlation between α-tocopherol intake, IgE levels, and the clinical manifestations of atopy indicates that oral vitamin E could be an excellent therapeutic adjunct for atopic dermatitis (Tsoureli-Nikita et al 2002). Other multi-clinical double-blinded studies revealed a significant improvement of chloasma and pigmented contact dermatitis lesions using topical vitamins E and C, with the combination clearly proving superior to the single vitamin treatment groups. Topical formulations used for depigmentation that contain, besides the commonly used hydroquinone and sunscreen, vitamins C and E, appear to be safe and efficient. Further research in well-designed controlled trials is needed to clarify the role of vitamin E and its derivates in the above-mentioned and further skin disorders.
MECHANISMS OF ACTION
Vitamin E is among the early recognized biologic antioxidants, and its redox and free radical chemistry are well documented. The major antioxidant role of vitamin E is generally considered to be the arrest of chain propagation by scavenging lipid peroxyl radicals. The initial oxidation product of tocopherol is the metastable tocopheroxyl radical, which can be either reduced to tocopherol by co-antioxidants, or reacts with another lipid peroxyl radical, yielding tocopherol-quinone. Thus, one molecule of tocopherol has the ability to scavenge two peroxyl radical molecules. Since the physiologic molar ratio of tocopherols to polyunsaturated phospholipids, first-line targets of oxidative attack, is less than about 1 : 1000 in most biologic membranes, regeneration of tocopherol is essential for its high antioxidant efficacy in vivo. Several hydrophilic co-antioxidants, such as ascorbate and glutathione, can regenerate vitamin E from the tocopheroxyl radical and thus enhance the antioxidant capacity of vitamin E. Furthermore, there is some in vitro evidence that ubiquinol-10 (‘coenzyme Q’) protects α-tocopherol from photo-oxidation by recycling mechanisms. Hence, the lack of such ‘co-antioxidants’ from the antioxidant network may diminish the antioxidant properties of vitamin E and result in limited antioxidant protection of lipid bilayers or other lipophilic domains.
A series of studies investigating nonenzymatic stratum corneum antioxidants have demonstrated that, in human skin, vitamin E is the predominant physiologic antioxidant of the skin barrier (Thiele et al 2001). When compared to nucleated epidermal layers, there is a lack of important co-antioxidants such as vitamin C in the stratum corneum as well as in the dermis. Taken together, these findings suggest that the skin barrier as well as the upper dermis reveal a lack of antioxidant protection. Accordingly, upon solar ultraviolet (UV) exposure, these are the cutaneous sites exhibiting the most pronounced oxidative protein damage (Sander et al 2002). Thus, antioxidant supplementation with vitamin E as well as synergistically active co-antioxidants, such as vitamin C, may enhance photoprotective strategies using sunscreens. While single studies have demonstrated significant penetration of topical vitamin E into dermal layers in vitro and in animals, there is still controversy about the efficacy of such strategies for dermal targets in human skin.
• Photoprotection by vitamin E
The largest body of scientific evidence for a beneficial role of topical vitamin E exists for photoprotection (summarized in Table 7.1). Numerous topical studies demonstrated significantly reduced acute skin responses when vitamin E was applied before UV radiation exposure, such as erythema and edema, sunburn cell formation, lipid peroxidation, DNA-adduct formation, immunosuppression, as well as chemiluminescence. Chronic skin reactions due to prolonged UVR exposure, such as skin wrinkling and skin tumor incidence (Burke et al 2000), were also diminished by topical vitamin E formulations. Vitamin E esters, particularly vitamin E acetate, were also shown to be promising agents in reducing UVR-induced skin damage. However, their photoprotective effects appeared to be less pronounced as compared to vitamin E; moreover, some studies failed to detect photoprotection provided by vitamin E esters. Since the free aromatic hydroxyl group is responsible for the antioxidant properties of vitamin E, vitamin E esters need to be hydrolyzed during skin absorption to show activity.
Vitamin E acetate was shown to be absorbed and penetrate skin. A skin bioavailability study demonstrated that vitamin E and vitamin E acetate behave similarly with regard to penetration of rat epidermis. The difference between physicochemical parameters determining skin transport for vitamin E and its esters seems negligible. Notably, the bioconversion of vitamin E acetate to its active antioxidative form, α-tocopherol, was found to be slow and to occur only to a minor extent in vivo. As demonstrated in recent studies with viable micro-Yucatan pig skin or viable human skin (Baschong et al 2001) ex vivo, vitamin E acetate was not found to be hydrolyzed in the skin penetration limiting layer, the stratum corneum. In the nucleated epidermis, however, the bioconversion of vitamin E acetate into vitamin E occurs, but seems to be dependent on formulation. Some evidence exists, however, that the bioconversion of vitamin E acetate into vitamin E might be enhanced due to UVR exposure. UVB exposure was demonstrated to cause an increase in esterase activity in murine epidermis.
• Vitamin E and skin barrier protection
Vernix caseosa is a complex, proteolipid material synthesized in part by fetal sebaceous glands during the last trimester of pregnancy. The strategic location of vernix on the fetal skin is thought to protect the skin after birth from exposure to a pro-oxidative (high oxygen tension, UV exposure) environment. Recently, it was shown that vernix contains very high amounts of vitamin E, which was suggested to bolster the antioxidant and barrier function of neonatal skin (Visscher et al 2005).
Besides its antioxidant properties, topically applied vitamin E appears to benefit the skin barrier function by nonantioxidative mechanisms. It is postulated that vitamin E acts as a penetration enhancer by intercalating within the lipid layer region of the stratum corneum, thus altering the characteristics of the membrane affecting permeability. Unlike other well-known enhancers, vitamin E is thought to be nonirritating and to possess emollient properties (Trivedi et al 1995).
DOSAGE AND PRACTICAL USAGE REGIMENS
• Topical supplementation
Vitamin E is one of the most frequently used ingredients in cosmetic and skin care formulations. While products with concentrations of less than 0.1% and up to 20% have been developed and marketed in Europe and the US, there is a surprisingly evident lack of published data on dose–response studies defining the optimal dosage of vitamin E. In addition to the relative lack of efficacy control requirements for over-the-counter (OTC) products, this might also be attributed to ill-defined endpoints as well as to the difficulty of measuring oxidative stress in vivo. Recent advances in biophysical (e.g. ultraweak photon emission, near-infrared/Raman spectroscopy, electron paramagnetic resonance) and biochemical research (e.g. the recent identification of highly sensitive and specific skin surface lipid photo-oxidation products/SqmOOH; Ekanayake-Mudiyanselage et al 2003) have led to the development of non-invasive assays (e.g. the ‘sebum photo-oxidation test’) that will help to better define relevant dose–response curves of antioxidants such as vitamin E.
Using this approach, we have recently demonstrated that even the use of rinse-off products containing α-tocopherol in concentrations of less than 0.5% leads to significantly increased levels of vitamin E in the stratum corneum of human skin (Ekanayake-Mudiyanselage et al 2005). Therefore, if the product claim refers to improved antioxidant protection of the skin barrier, topical formulations with α-tocopherol at concentrations ranging from 0.1 to 1%, but not vitamin E esters, are very likely to be efficient. According to the antioxidant network theory outlined earlier, combinations with co-antioxidants such as vitamin C may help to improve antioxidant effects and the stability of vitamin E.
• Systemic/dietary supplementation
While topical vitamin E has been studied extensively, little is known on the oral bioavailability of this antioxidant in skin. Ongoing studies by our laboratory indicate that a daily intake of as low as 400 IU vitamin E functions to increase cutaneous vitamin E levels. Conflicting results published on this issue are due to the fact that differing analytical methods and compartments of the skin have been investigated. While earlier studies have investigated full skin thickness, recent research efforts have focused on vitamin E delivery via sebaceous gland secretion, which would primarily lead to increased vitamin E levels in skin surface lipids and the upper epidermis/stratum corneum. Our recent results (Ekanayake-Mudiyanselage et al 2004) indicate that:
 sebaceous gland secretion is a major mechanism leading to site-specific differences in vitamin E increases
sebaceous gland secretion is a major mechanism leading to site-specific differences in vitamin E increases
 the bioavailability of 400 mg RRR-α-tocopherol acetate (derived from natural vitamin E) or 400 mg all-rac-α-tocopherol acetate (derived from synthetic vitamin E) is comparable
the bioavailability of 400 mg RRR-α-tocopherol acetate (derived from natural vitamin E) or 400 mg all-rac-α-tocopherol acetate (derived from synthetic vitamin E) is comparable
 possible protective effects in the skin require a supplementation period of 2–3 weeks.
possible protective effects in the skin require a supplementation period of 2–3 weeks.
These results are important in that they suggest that no improvement of antioxidant protection of skin barrier lipids and proteins can be obtained in the first 2 weeks of oral vitamin E supplementation.
CAUTIONS, CONTRAINDICATIONS, AND ADVERSE EFFECTS
Although vitamin E is widely used in many topical cosmetic products, reports of side effects such as allergic or irritant skin reactions are rare. Nevertheless, clinical side effects have been described after topical application of vitamin E-containing products, e.g. local and generalized contact dermatitis, contact urticaria, and erythema multiforme-like eruptions. In 1992, an ‘epidemic outbreak’ of about 1000 cases of allergic papular and follicular contact dermatitis caused by α-tocopherol linoleate in a cosmetic line was reported in Switzerland. The authors found that this compound was easily oxidized under the storage conditions used. Therefore, secondary or tertiary oxidation products of α-tocopherol linoleate, rather than the reduced vitamin E ester, are likely to have caused irritation or even the oxidation of proteins and subsequent hapten formation. Furthermore, positive patch test reactions were reported in several cases after application of α-tocopherol acetate, a widely used water-soluble derivate of α-tocopherol.
In conclusion, reported positive patch test results due to α-tocopherol are rare and need to be critically reviewed. Investigators used the oil of vitamin E capsules for patch testing without further evaluating the contained tocopherol derivates, source or further components of these capsules. However, it could not be excluded in many cases that the symptoms were caused by soybean oil, glycerin or gelatin, all of which were also present in the topically applied vitamin E capsules (Harris & Taylor 1997).
Doses of 50 IU up to 1000 IU α-tocopherol per day have been tolerated in humans with no or minimal side effects. Vitamin E supplements for pregnancy usually contain smaller doses of vitamin E, but adverse effects have not been observed even with higher doses. Theoretically, however, due to the involvement of the cytochrome P450 system in the metabolism of orally supplemented RRR-α-tocopherol, drug interactions have to be taken into account when supranutritional dosages of vitamin E are provided. Since tocopherols and their oxidation products are able to inhibit platelet aggregation, simultaneous supplementation of anticoagulants and vitamin E is not recommended, which has implications for surgical procedures (Thiele et al 2005).
CURRENT RESEARCH AND POSSIBLE FUTURE APPLICATIONS
As indicated above, topical strategies alone may not be sufficient to bolster the skin’s antioxidative defense in the dermis and thus to prevent or lessen photoaging in this skin compartment. Therefore, current research on vitamin E focuses on systemic delivery of vitamin E to the various compartments of human skin. It was recently discovered that human sebum contains high amounts of α-tocopherol and that sebaceous gland secretion is a relevant physiological route of α-tocopherol delivery to sebaceous gland-rich skin regions, such as facial skin (Thiele et al 1999). Similarly, orally administered drugs have been reported to be transported to the skin surface and the stratum corneum by the sebaceous gland secretion route (Ekanayake-Mudiyanselage et al 2004). Ongoing studies are investigating the relevance and time course of this delivery pathway for increasing the levels of vitamin E in human skin. A further ongoing study is focusing on the relevance of this mechanism for the physiologic vitamin E levels in skin surface lipids in different age groups. The outcome of these studies will certainly have implications for conditions of sebostatic, dry skin (e.g. as in atopic dermatitis), as well as for the skin of prepubertal children, who have a low activity of sebaceous glands.
Baschong W, Artmann C, Hueglin D, Roeding J. Direct evidence for bioconversion of vitamin E acetate into vitamin E: an ex vivo study in viable human skin. Journal of Cosmetic Science. 2001;2:155–161.
Burke KE, Clive J, Combs GFJr, Commisso J, Keen CL, Nakamura RM. Effects of topical and oral vitamin E on pigmentation and skin cancer induced by ultraviolet irradiation in Skh:2 hairless mice. Nutrition and Cancer. 2000;38:87–97.
Ekanayake-Mudiyanselage S, Hamburger M, Elsner P, Thiele JJ. Ultraviolet A induces generation of squalene monohydroperoxide isomers in human sebum and skin surface lipids in vitro and in vivo. Journal of Investigative Dermatology. 2003;120:915–922.
Ekanayake-Mudiyanselage S, Kraemer K, Thiele JJ. Oral supplementation with all-rac- and RRR-alpha-tocopherol increases vitamin E levels in human sebum after a latency period of 14–21 days. Annals of the New York Academy of Sciences. 2004;1031:184–194.
Ekanayake-Mudiyanselage S, Tavakkol A, Polefka TG, Nabi Z, Elsner P, Thiele JJ. Vitamin E delivery to human skin by a rinse-off product: penetration of alpha-tocopherol versus wash-out effects of skin surface lipids. Skin Pharmacology and Physiology. 2005;18:20–26.
Harris BD, Taylor JS. Contact allergy to vitamin E capsules: false negative patch tests to vitamin E. Contact Dermatitis. 1997;36:273.
Sander CS, Chang H, Salzmann S, et al. Photoaging is associated with protein oxidation in human skin in vivo. Journal of Investigative Dermatology. 2002;118:618–625.
Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Molecular Aspects of Medicine. 2007;28:646–667.
Thiele JJ, Weber SU, Packer L. Sebaceous gland secretion is a major physiologic route of vitamin E delivery to skin. Journal of Investigative Dermatology. 1999;113:1006–1010.
Thiele JJ, Schroeter C, Hsieh SN, Podda M, Packer L. The antioxidant network of the stratum corneum. Current Problems in Dermatology. 2001;29:26–42.
Thiele JJ, Hsieh SN, Ekanayake-Mudiyanselage S. Vitamin E: a critical review on its current use in cosmetic and clinical dermatology. Dermatologic Surgery. 2005;31:805–813.
Trivedi JS, Krill SL, Fort JJ. Vitamin E as a human skin penetration enhancer. European Journal of Pharmaceutical Sciences. 1995;3:241–243.
Tsoureli-Nikita E, Hercogova J, Lotti T, Menchini G. Evaluation of dietary intake of vitamin E in the treatment of atopic dermatitis: a study of the clinical course and evaluation of the immunoglobulin E serum levels. International Journal of Dermatology. 2002;41:146–150.
Visscher MO, Narendran V, Pickens WL, et al. Vernix caseosa in neonatal adaptation. Journal of Perinatology. 2005;25:440–446.