Chapter 10 Physiologic Lipids for Barrier Repair in Dermatology
INTRODUCTION
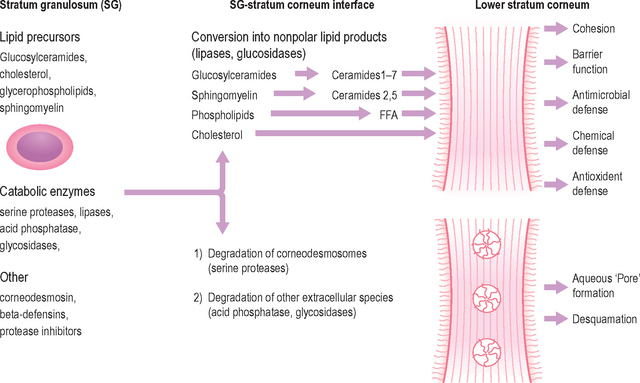
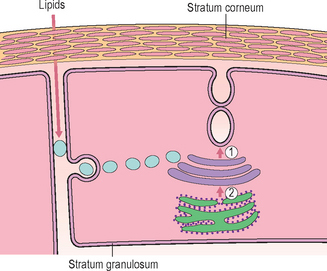
Although the stratum corneum (SC) serves many defensive functions (Table 10.1), none is as important as its ability to prevent excess loss of fluids and electrolytes, i.e. the permeability barrier. Permeability barrier function is mediated by the organization of the extracellular lipids of the SC into a series of parallel lamellar membranes, which mediate not only permeability barrier function, but also additional, key protective functions of the epidermis (Fig. 10.1). The functions of the SC can be further localized to either the cellular (corneocyte) compartment or extracellular matrix (Table 10.1).
Table 10.1 Protective functions of mammalian stratum corneum
| Function | Localization |
| Permeability barrier* | Extracellular |
| Initiation of inflammation (cytokine activation)* | Corneocyte (and granular cell) |
| Cohesion (Integrity) → Desquamation* | Extracellular |
| Antimicrobial barrier (innate immunity)* | Extracellular |
| Mechanical (impact and shear resistance) | Corneocyte |
| Toxic chemical/antigen exclusion | Extracellular |
| Selective absorption | Extracellular |
| Hydration | Corneocyte |
| UV barrier | Corneocyte |
| Psychosensory interface | Unknown |
| Thermal barrier | Unknown |
DYNAMICS OF BARRIER RECOVERY
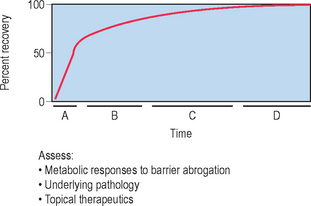
The skin barrier is assaulted frequently in daily life by hot water, detergents, solvents, mechanical trauma, and occupation-related chemicals. If these insults are frequently repeatedly and/or insufficiently repaired, they threaten the organism with desiccation due to accelerated transepidermal water loss (TEWL). To avoid this outcome, the underlying epidermis mounts a coordinated metabolic response, ranging from increased lipid synthesis to accelerated lipid secretion, aimed at rapidly restoring normal function. This response is elicited by any type of barrier insult (e.g. organic solvents, detergents, tape stripping) that depletes the SC of its complement of lipids. Although the total time required for barrier recovery varies according to age, there is an initial, rapid recovery phase that leads to 50–60% recovery in young humans in about 12 hours, with full recovery requiring about 3 days (Fig. 10.2). But in aged humans (> 75 years), complete recovery from comparable insults is prolonged to about 1 week. Restoration of barrier function is accompanied by reaccumulation of lipids, visible with either oil red O staining or Nile red fluorescence, and by the reappearance of membrane structures within the SC interstices, as early as 2 hours after acute disruption. Because artificial restoration of the barrier with vapor-impermeable membranes inhibits barrier recovery, as well as all of the metabolic processes linked to it, the entire metabolic response represents a response that is aimed specifically at restoring normal permeability barrier homeostasis.
CLINICAL APPLICATIONS OF THE CUTANEOUS STRESS TEST
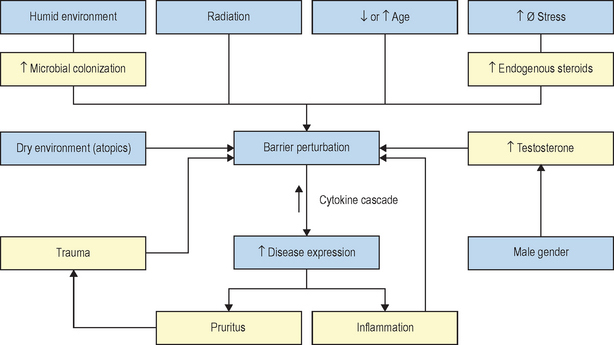
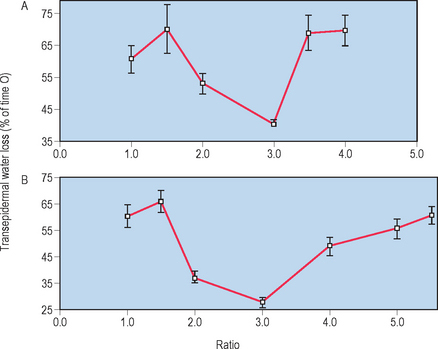
The kinetics of barrier recovery after acute perturbations (also called the ‘cutaneous stress test’) can discern abnormal function or underlying pathology, even when basal parameters are normal (Fig. 10.2), analogous to the cardiac treadmill exam. Indeed, the cutaneous stress test reveals deficient barrier function both in aged and in neonatal skin, despite deceptively normal function in both age groups under basal conditions. Further, it amplifies differences between other ‘normal’ groups (Fig. 10.3):
 testosterone-replete versus testosterone-deficient individuals
testosterone-replete versus testosterone-deficient individuals
Finally, individuals subjected to increased psychological stress reveal a defect in barrier recovery, explaining the propensity for these factors to exacerbate inflammatory dermatoses, such as psoriasis and atopic dermatitis, which already display barrier abnormalities. We have proposed that these factors further amplify the cytokine cascade that characterizes these disorders (Fig. 10.3).
LIPID COMPOSITION OF LAMELLAR MEMBRANES
The extracellular lipids in the SC derive primarily from the secreted contents of epidermal lamellar bodies (LB), which are enriched in cholesterol, glucosylceramide, phospholipids, as well as their respective hydrolytic enzymes (Fig. 10.1). These lipid-processing enzymes catalyze the extracellular degradation of sphingomyelin and glucosylceramides to ceramides (Cer), and phospholipids to free fatty acids (FFA) that, along with cholesterol (Chol), form the lamellar membranes required for permeability barrier function. Thus, the SC lamellar membrane comprises unique biologic membranes that mediate the barrier because:
 they are extremely hydrophobic
they are extremely hydrophobic
 their total lipid weight percentage in SC (= number of lamellae in the extracellular spaces)
their total lipid weight percentage in SC (= number of lamellae in the extracellular spaces)
 their presence in an equimolar mixture
their presence in an equimolar mixture
 the presence of certain lipids, such as acylceramides, bearing linoleic acid.
the presence of certain lipids, such as acylceramides, bearing linoleic acid.
Together, these characteristics largely explain the barrier properties of the SC.
LIPID SYNTHESIS AND REQUIREMENTS FOR THE BARRIER
• Equimolar distribution of the three key SC lipids
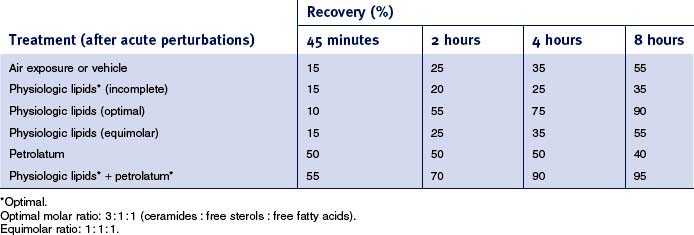
Whereas the above-described studies clearly demonstrate the individual requirement for Chol, FFA, and Cer for the permeability barrier, when these lipids are applied topically, they must be supplied in approximately equimolar proportions for normal barrier recovery to occur (Table 10.2). For example, topical applications of any one or two of the three key lipids to acutely perturbed skin actually delays barrier recovery (Table 10.2). Both incomplete and complete mixtures of the three key lipids rapidly traverse the SC, internalize within the granular cell layer, targeting the trans-Golgi network, where LB are formed (Fig. 10.4). Exogenous and endogenous lipids mix within nascent LB, producing normal or abnormal LB contents and derived lamellar membrane structures, depending on the molar distribution of the applied lipids. Barrier recovery can be further accelerated by increasing the proportion of any one of the three key lipids to a 3 : 1 : 1 ratio (Table 10.2 and Fig. 10.5). Thus, physiologic mixtures of topical lipids influence barrier function, not by partially occluding the SC, as do nonphysiologic lipids (see below), but rather by contributing to the epidermal lipid pool that is delivered to the SC interstices.
NONPHYSIOLOGIC LIPIDS: MECHANISM OF ACTION
In contrast to physiologic lipids, classic nonphysiologic lipids, such as petrolatum, do not enter the lipid-secretory pathway of the granular cell, and, in fact, they do not penetrate beneath the SC. They do, however, fully infiltrate the extracellular domains of the SC, where they form a hydrophobic, nonlamellar phase that largely displaces the lamellar bilayers. These lipids, which include not only petrolatum, but also agents such as beeswax, lanolin, squalane, and a variety of other hydrocarbons, function largely as a vapor-permeable membrane, i.e. they reduce water loss immediately (Table 10.2) but not completely. In contrast, physiologic lipids display a lag time that reflects the time necessary for SC transport, endocytosis, secretion, and lamellar membrane formation (Table 10.2—compare petrolatum to physiologic lipids; Fig. 10.4). Nonphysiologic lipids have the further advantage that they do not discriminate among types of barrier abnormalities, and the same degree of correction is achieved, regardless of the nature of the barrier disturbances. Further, it should be noted that the nonphysiologic lipids, though not components of the lamellar membranes, in some cases display a host of other, potentially beneficial properties, including anti-inflammatory, hydrating, waterproofing, and insulating characteristics. Because of the fundamental differences in their mechanisms, the two classes of molecules are complementary in improving barrier function (e.g. Table 10.2—nonphysiologic plus physiologic).
RATIONALE FOR BARRIER REPAIR THERAPY
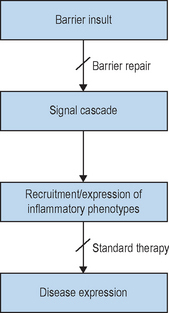
The contrasting features of nonphysiologic and physiologic lipids further dictate the clinical settings where each should be uniquely useful. While nonphysiologic lipids such as petrolatum function like vapor-permeable membranes at the surface of the SC, physiologic lipids augment or supplement the epidermis’s own lipid biosynthetic machinery. As noted above, many skin diseases are associated with barrier abnormalities, and other pathophysiologic insults, such as psychological stress or aging, can further aggravate these processes (Fig. 10.3). In some of these cases, e.g. psychological stress or glucocorticoid therapy, there is an equivalent, global reduction in lipid production. In others, e.g. aging and atopic dermatitis, the global reduction is aggravated by a further reduction in one of the three key species (see below).
Logically then, in these studies, bolstering epidermal barrier status should decrease susceptibility not only to these skin diseases, but also to others that are triggered, sustained, or exacerbated by external perturbations, most notably contact dermatitis and psoriasis (Fig. 10.6). In fact, in all skin disorders characterized by a barrier abnormality, the extent of the barrier abnormality parallels the extent of clinical severity, a further indication of the importance of the barrier abnormality for disease pathogenesis.
DEPLOYMENT OF BARRIER REPAIR THERAPY
The recent emergence of ‘barrier repair’ therapy represents a set of pathophysiologically based strategies that should decrease the susceptibility to these and other disorders, characterized by barrier abnormalities. These repair approaches can be classified into three subcategories (Table 10.3):
 Optimized mixtures of the three physiologic lipids (ceramides, cholesterol, and free fatty acids) in appropriate molar ratios that correct underlying biochemical abnormalities in the targeted disease.
Optimized mixtures of the three physiologic lipids (ceramides, cholesterol, and free fatty acids) in appropriate molar ratios that correct underlying biochemical abnormalities in the targeted disease.
 One or more nonphysiologic lipids (e.g. petrolatum, lanolin), which restore function transiently without correcting specific abnormalities.
One or more nonphysiologic lipids (e.g. petrolatum, lanolin), which restore function transiently without correcting specific abnormalities.
 Dressings, either vapor-permeable, which allow metabolic (repair) processes to continue in the underlying epidermis, or vapor-impermeable, which shut down metabolic responses in the underlying epidermis.
Dressings, either vapor-permeable, which allow metabolic (repair) processes to continue in the underlying epidermis, or vapor-impermeable, which shut down metabolic responses in the underlying epidermis.
Table 10.3 Logical barrier repair strategies—clinical indications
| Repair strategy | Clinical indication |
| Dressings | |
| Vapor-permeable | Healing wounds |
| Vapor-impermeable | Keloids |
| Nonphysiologic lipids (NPL) | |
| Petrolatum or lanolin | Radiation dermatitis or severe sunburn |
| Premature infants (aged <34 weeks) | |
| Physiologic lipids (PL): | |
| Optimal molar ratio | |
| Cholesterol-dominant | Aging or photoaging |
| Ceramide-dominant | Atopic dermatitis |
| Free fatty acid-dominant | Neonatal skin, including psoriasis, diaper dermatitis (with added NPL) |
| Cholesterol-, ceramide-, or free fatty acid-dominant | Irritant contact dermatitis (with added NPL) |
| Glucocorticoid-treated (vehicle), psychological stress |
Modified from Elias PM, Feingold KR 2001 Does the tail wag the dog? Role of the barrier in the pathogenesis of inflammatory dermatoses and therapeutic implications. Archives of Dermatology 137:1079–1081
Nevertheless, all of these strategies have their appropriate use and clinical indications. In fact, we can now choose an appropriate barrier strategy for a specific clinical indication based upon knowledge of disease pathogenesis (Table 10.3). For example, atopic dermatitis (AD) is characterized by a global decrease in SC lipids with a steep reduction in ceramides attributable to increased sphingomyelin/glucosylceramide deacylase activity in affected epidermis. Hence, the logic, and recent apparent success, of a ceramide-dominant mixture of the three physiologic lipids as ancillary therapy in AD. In contrast, aged and photoaged epidermis exhibits a global reduction in SC lipids, with a further decrease in cholesterol synthesis—hence the success of a cholesterol-dominant mixture of physiologic lipids in this setting. So critical is the choice of a proper formulation that substitution of an FFA-dominant mixture for the cholesterol-dominant version drastically delays barrier recovery in aged skin (Table 10.4). Yet, there are several clinical situations where physiologic lipids might not be effective, if deployed alone, due to an impaired LB secretory system. These examples include radiation dermatitis (both UVB and x-irradiation), very premature infants (i.e. < 33 weeks), and perhaps the initial stages of wound healing. In such situations, nonphysiologic lipids or vapor-permeable dressing alone, with or without added physiologic lipids, would become the most logical choice.
Table 10.4 Effects of physiologic lipid mixtures on barrier recovery in young versus aged human skin
| Physiologic lipids | Young | Aged |
| Single lipid | Delays | Accelerates (cholesterol only) |
| Triple lipids (equimolar) | No change | Accelerates |
| Triple lipids (optimized): | ||
Physiologic lipids = free fatty acids, cholesterol, ceramides.
Modified from Zettersten EM, Ghadially R, Feingold KR, Crumrine D, Elias PM 1997 Optimal ratios of topical stratum corneum lipids improve barrier recovery in chronologically aged skin. Journal of the American Academy of Dermatology 37:403–408
SUMMARY
This chapter has addressed some of the new understandings regarding barrier function and the role of ceramides. The ideas are nicely summarized in Table 10.4 where the effects of physiologic lipid mixtures on barrier recovery in young versus aged skin are compared. Note that the relationship between the components of the intercellular lipids, consisting of fatty acids, ceramides, and cholesterol, may affect the ability of the skin to effect barrier repair. In contrast, ceramide-containing lipid mixtures represent an interesting cosmeceutical that may have an important role in atopic dermatitis (Table 10.3).
Altemus M, Rao B, Dhabhar FS, Ding W, Granstein RD. Stress-induced changes in skin barrier function in healthy women. Journal of Investigative Dermatology. 2001;117:309–317.
Behne MJ, Barry NP, Hanson KM, et al. Neonatal development of the stratum corneum pH gradient: localization and mechanisms leading to emergence of optimal barrier function. Journal of Investigative Dermatology. 2003;120:998–1006.
Chamlin SL, Kao J, Frieden IJ, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. Journal of the American Academy of Dermatology. 2002;47:198–208.
Denda M, Sato J, Masuda Y, et al. Exposure to a dry environment enhances epidermal permeability barrier function. Journal of Investigative Dermatology. 1998;111:858–863.
Elias PM, Feingold KR. Does the tail wag the dog? Role of the barrier in the pathogenesis of inflammatory dermatoses and therapeutic implications. Archives of Dermatology. 2001;137:1079–1081.
Elias PM, Feingold KR. Skin as an organ of protection. In: Freedberg IM, Eisen AZ, Wolff K, et al, editors. Fitzpatrick’s dermatology in general medicine. Philadelphia: McGraw-Hill; 2003:164–174.
Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Advances in Lipid Research. 1991;24:1–26.
Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. American Journal of Contact Dermatitis. 1999;10:119–126.
Feingold KR. The regulation and role of epidermal lipid synthesis. Advances in Lipid Research. 1991;24:57–82.
Garg A, Chren MM, Sands LP, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Archives of Dermatology. 2001;137:53–59.
Ghadially R, Halkier-Sorensen, Elias PM. Effects of petrolatum on stratum corneum structure and function. Journal of the American Academy of Dermatology. 1992;26:387–396.
Ghadially R, Brown BE, Sequeira-Martin SM, Feingold KR, Elias PM. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. Journal of Clinical Investigations. 1995;95:2281–2290.
Ghadially R, Reed JT, Elias PM. Stratum corneum structure and function correlates with phenotype in psoriasis. Journal of Investigative Dermatology. 1996;107:558–564.
Grubauer G, Elias PM, Feingold KR. Transepidermal water loss: the signal for recovery of barrier structure and function. Journal of Lipid Research. 1989;30:323–333.
Halkier-Sorensen L, Menon GK, Elias PM, Thestrup-Pedersen K, Feingold KR. Cutaneous barrier function after cold exposure in hairless mice: a model to demonstrate how cold interferes with barrier homeostasis among workers in the fish-processing industry. British Journal of Dermatology. 1995;132:391–401.
Hara J, Higuchi K, Okamoto R, Kawashima M, Imokawa G. High-expression of sphingomyelin deacylase is an important determinant of ceramide deficiency leading to barrier disruption in atopic dermatitis. Journal of Investigative Dermatology. 2000;115:406–413.
Holleran WM, Uchida Y, Halkier-Sorensen L, et al. Structural and biochemical basis for the UVB-induced alterations in epidermal barrier function. Photodermatology, Photoimmunology and Photomedicine. 1997;13:117–128.
Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? Journal of Investigative Dermatology. 1991;96:523–526.
Kao JS, Garg A, Mao-Qiang M, et al. Testosterone perturbs epidermal permeability barrier homeostasis. Journal of Investigative Dermatology. 2001;116:443-451.
Man MM, Feingold KR, Thornfeldt CR, Elias PM. Optimization of physiological lipid mixtures for barrier repair. Journal of Investigative Dermatology. 1996;106:1096–1101.
Man MQ, Feingold KR, Elias PM. Exogenous lipids influence permeability barrier recovery in acetone-treated murine skin. Archives of Dermatology. 1993;129:728–738.
Mao-Qiang M, Brown BE, Wu-Pong S, Feingold KR, Elias PM. Exogenous nonphysiologic versus physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Archives of Dermatology. 1995;131:809–816.
Proksch E, Jensen JM, Elias PM. Skin lipids and epidermal differentiation in atopic dermatitis. Clinics in Dermatology. 2003;21:134–144.
Reed JT, Ghadially R, Elias PM. Skin type, but neither race nor gender, influence epidermal permeability barrier function. Archives of Dermatology. 1995;131:1134–1138.
Schmuth M, Sztankay A, Weinlich G, et al. Permeability barrier function of skin exposed to ionizing radiation. Archives of Dermatology. 2001;137:1019–1023.
Sugarman JL, Fluhr JW, Fowler AJ, Bruckner T, Diepgen TL, Williams ML. The objective severity assessment of atopic dermatitis score: an objective measure using permeability barrier function and stratum corneum hydration with computer-assisted estimates for extent of disease. Archives of Dermatology. 2003;139:1417–1422.
Williams ML, Elias PM. Enlightened therapy of the disorders of cornification. Clinics in Dermatology. 2003;21:269–273.
Zettersten EM, Ghadially R, Feingold KR, Crumrine D, Elias PM. Optimal ratios of topical stratum corneum lipids improve barrier recovery in chronologically aged skin. Journal of the American Academy of Dermatology. 1997;37:403–408.